Abstract
Solving how insulin regulates glucose transport into skeletal muscle and adipose tissue remains a fundamental challenge in biology and a significant issue in medicine. A central feature of this process is the coordinated accumulation of the glucose transporter GLUT4 into the plasma membrane. New signaling and cytoskeletal mechanisms of insulin-stimulated GLUT4 exocytosis are of emerging interest, particularly those at or just beneath the plasma membrane. This review examines signals that functionally engage GLUT4 exocytosis, considers cytoskeletal regulation of the stimulated GLUT4 itinerary, and appraises involvement of plasma membrane parameters in GLUT4 control. We also explore how these newly defined signaling, cytoskeletal, and membrane mechanisms may be of therapeutic interest in the treatment and/or prevention of GLUT4 dysregulation in disease.
Recruiting GLUT4
Under normal insulin responsiveness, insulin promotes the removal of excess glucose from the circulation by stimulating the exocytic recruitment of intracellular GLUT4 storage vesicles (GSVs) to the plasma membrane (PM) of skeletal muscle and fat cells [1, 2]. This stimulated redistribution of intracellular GSVs results in PM GLUT4 accrual that facilitates cellular glucose uptake (Figure 1). Activation of GSVs by insulin requires a phosphatidylinositol 3-kinase (PI3K) signal involving the upstream insulin receptor (IR) and insulin receptor substrate (IRS) activators and the downstream Akt2 target enzyme [1–3].
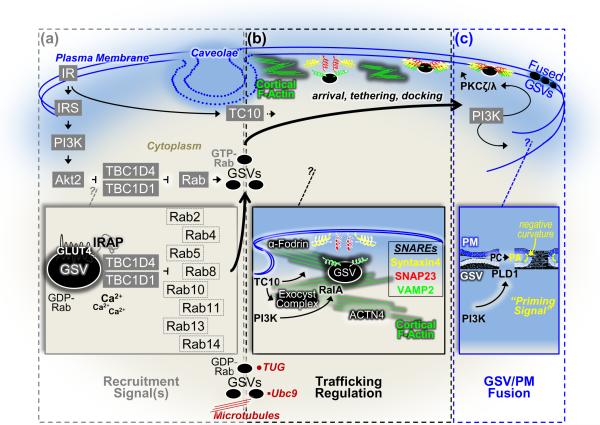
Figure 1.
Schematic illustration of putative signals, cytoskeletal mechanisms, and plasma membrane parameters involved in insulin-stimulated GLUT4-storage vesicle (GSV) exocytosis. (a) Activation of GSVs by insulin requires a PI3K signal involving the upstream IR and IRS activators and the downstream Akt2 target enzyme. TBC1D4 and TBC1D1, substrates of Akt2, have been suggested to couple the PI3K/Akt2 signal to GSVs via its action on one or more critical Rab proteins. The basal intracellular pool of GDP-Rab GSVs shown associated with several putative anchoring systems (e.g., microtubules, Ubc9, TUG) are activated by the suppression of the Rab-GAP activity of TBC1D4/TBC1D1 by Akt2. Several putative Rab proteins, the existence of possible calcium regulation, and mechanisms associating TBC1D4 (and presumably TBC1D1) to the GSV via IRAP have been suggested (see inset). (b) Cortical F-actin, likely originating at the neck region of caveolae PM microdomains, plays a critical role in GSV trafficking. Reorganization of the cortical F-actin meshwork by insulin signaling to TC10 allows GSV/PM arrival, tethering, and docking. A large number of proposed insulin-regulated processes occur in this PM vicinity such as TC10-regulated formation of the exocyst complex and cortical F-actin remodeling, PI3K/RalA-stimulated transition of trafficking GSVs to tethered GSVs, a role of ACTN4 and/or the exocyst complex in tethering, and an α-fodrin-mediated rearrangement of cortical actin filaments in the area of syntaxin 4 to facilitate GSV/PM SNARE protein interaction and docking (see inset). (c) Insulin signaling, through two putative PI3K signals that activate PKCζ/λ and PLD1, prepares GSVs for fusion with the PM. The first PKCζ/λ signal has been implicated in promoting the dissociation of Munc18c from syntaxin4, contributing to the fusion-competent SNARE complex. The second PLD1 signal primes the GSV and PM for fusion by generating PA, which has been suggested to act as a fusogenic lipid in biophysical modeling studies by lowering the activation energy for membrane bending (i.e., negative membrane curvature) during generation and expansion of fusion pores (see inset).
Until the discovery of AS160 (a 160-kDa substrate of Akt) in 2002 [4], it remained unclear how the IR/IRS1/PI3K/Akt2 signal coupled to GSVs. This protein, also known as TBC1D4 (Tre-2 BUB2 CDC16, 1 domain family member 4), contains a GTPase activating domain (GAP) for Rabs, small G proteins implicated in vesicle trafficking [5, 6]. In the basal state, the Rab-GAP function of TBC1D4 is thought to contribute to the intracellular retention of GSVs by promoting the inactive GDP-bound state of Rabs; whereas insulin-stimulated Akt2 suppresses the Rab-GAP activity of the TBC1D4 and thus increases the active GTP-bound form of Rabs on GSVs to promote exocytosis (Figure 1a). Consistent with this localized functionality, TBC1D4 associates with GSVs via binding to the insulin-responsive amino peptidase (IRAP), a GSV cargo protein [7, 8]. Another Rab-GAP known as TBC1D1 with identical Rab specificity as TBC1D4 [9] also displays similar regulation of GLUT4 in 3T3-L1 adipocytes [9], skeletal muscle myotubes [10], and mouse skeletal muscle [11]. Interestingly, expression of a TBC1D1 genetic variant (R125W, linked with human obesity [12]), impairs insulin-stimulated glucose transport in mouse skeletal muscle [11]. However, expression of R125W in 3T3-L1 adipocytes displays a similar inhibitory effect as wild-type TBC1D1 [10]. Moreover, GLUT4 regulation in these cells is intact following TBC1D1 knockdown [13]. Together these findings raise questions on the importance of TBC1D1 and R125W in health and disease. Nevertheless, the high expression levels of TBC1D1 in skeletal muscle compared to adipocytes [13] supports the need for future attention on TBC1D1 functionality in GLUT4 regulation. Another important area of current investigation is aimed at identifying which Rab protein(s) are targeted by TBC1D4 and TBC1D1 Rab-GAP activity (Figure 1a, inset).
Using immunoblotting and mass spectrometry techniques to analyze GLUT4-containing intracellular vesicles, the Rab proteins Rabs 4, 5, and 11 were found to associate with GSVs [5, 6]. Additional evidence from proteomic analysis [14] and mass spectrometry [15] demonstrates that Rabs 2, 8, 10, and 14 are associated with GSVs, raising questions as to which Rabs are important. Historically, Rab4 has been a major Rab of focus in GLUT4 regulation [6]; however, new evidence suggests that other Rabs may play roles in regulating GLUT4. The highly homologous nature of Rab proteins, lack of antibodies specific for Rabs, and potential false positives represent ongoing challenges in dissecting specific Rabs associated with GSVs in subcellular fractionation and immunlocalization studies [6]. Moreover, establishing a functional significance for one or more Rabs in GSV trafficking has been challenging. Functionally, Rabs 2, 8A, 10, and 14 have been identified as putative targets of TCB1D4 in vitro [15]. Recent studies have utilized siRNA knockdown of Rabs to more precisely dissect roles in GLUT4 regulation, as interpretation of data from overexpression-based analyses are complicated by potential off target effects on other Rabs and Rab effectors. Knockdown of Rab10 in adipocytes has suggested a role for Rab10 in insulin-stimulated GLUT4 translocation [16]. These results are supported by findings showing that only knockdown of Rab10, not that of Rabs 8A, 8B, and 14, prevented insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes [17]. In line with earlier findings regarding Rab4 [6], Rab 4B knockdown in adipocytes supports its role in regulating GLUT4 translocation [18]. In muscle cells, Rabs 8A and 14, but not Rabs 8B and 10, rescue the inhibition of GLUT4 translocation by a constitutively-active TBC1D4 [19]. Consistent with these findings, knockdown of Rabs 8A and 14 inhibits insulin-stimulated GLUT4 translocation [20] implicating these Rabs in muscle GLUT4 regulation. Although Rab13 was not reported in previous screens of Rab association with GSVs, recent findings now implicate Rab13 in regulating GLUT4 translocation in muscle [21]. For example, in L6 myotubes insulin transiently promotes GTP loading of both Rab13 and Rab8A, with Rab8A GTP loading preceding that of Rab13. Interestingly, knockdown of Rab13 inhibits insulin-stimulated GLUT4 translocation, and this inhibition is rescued by re-expression of Rab13, not Rab8A. Together these data and the observation that Rab13, not Rab8A, co-localizes with GLUT4 at or near the PM following insulin stimulation suggests that Rab13 functions distal to Rab8A representing a novel distal Rab signal regulating muscle GLUT4 translocation [21]. Although Rab specificity is not completely dissected, together these studies begin to establish tissue-specific roles for Rab proteins in GLUT4 translocation and highlight the crucial need for future efforts to fill this gap in our understanding.
While it was hoped that the discovery of TBC1D4 would lead to the precise identification of the GSV-regulatory Rab protein, the finding of TBC1D1 and several GSV-associated Rabs that are targeted by TBC1D4 and TBC1D1 have provided additional advances in our understanding that require further study. In this regard it is intriguing that calmodulin binds to a small domain that is near the GAP domain of TBC1D4 [22], and this association is calcium dependent. However, study of a point mutant of TBC1D4 lacking calmodulin binding did not seem to indicate a requirement for calmodulin/TBC1D4 binding in GLUT4 regulation. Perhaps this calmodulin-binding domain regulates contraction-, but not insulin-, stimulated GLUT4 regulation [23]. Despite these data fitting a calcium-independent model of insulin-regulated GLUT4 translocation, intermittent study through the years seems to support a role for calcium [24]. Interestingly, new studies have identified requirements for inositol 1,4,5-triphosphate-receptor and calcium/calmodulin-dependent protein kinase II pathways in GLUT4 regulation by insulin [25, 26]. These new insights support the need for future investigation into calcium-based aspects of GLUT4 control.
In summary, new additions to our understanding of GSV recruitment have been made. Namely, data implicate that insulin-mediated suppression of the Rab-GAP activity of GSV-localized TBC1D4 (and presumably TBC1D1) activates a critical GSV-regulatory Rab protein. Although the precise identification of the Rab protein or proteins involved in GSV recruitment needs continued delineation, data from several studies have framed key cytoskeletal events distal to Rab functionality in the itinerary of the activated GSV.
Motoring GSVs
A long-standing view has been that microtubules coordinate long-range, whereas actin orchestrates short-range, GSV movement [27–29]. Findings implicate microtubules in mediating basal subcellular distribution of GSVs, but not the accelerated rate of GLUT4 translocation stimulated by insulin [30]. For example, basally GSVs display long-range movements beneath the PM, with their trajectories extensively spread on the entire PM [31]. This is consistent with findings that insulin stimulation halts this long-range GSV basal itinerary and stimulates GSV/PM tethering, docking, and fusion in rat primary adipocytes [32]. Recent evidence supports the theory that this insulin-stimulated switch from a basal GSV trajectory to an insulin-regulated PM-bound track occurs at a microtubule/actin junction beneath the PM.
It is well-documented that insulin elicits a rapid, dynamic remodeling of actin filaments into a cortical mesh, and this mesh is necessary for GLUT4 translocation in both cultured and primary skeletal muscle and fat cells [28]. At a functional level, new studies by several groups continue to point to important roles of several recognized and new insulin-regulated proteins (e.g., TC10 [33], actin-related proteins 2/3 (Arp2/3) and Cofilin [34], Myo1c [26], Rac1 [35], focal adhesion kinase (FAK) [36]) that control actin dynamics to influence GLUT4 translocation. Data also place GSVs in the meshwork and suggest a functionality of the actin mesh in GLUT4 translocation (Figure 1b). For example, alpha-actinin-4 (ACTN4), a protein responsible for linking actin filaments to intracellular structures, is required for insulin-stimulated GLUT4 translocation in L6 myotubes [37]. Mechanistically, ACTN4 co-precipitated and co-localized with GLUT4 along actin filaments induced by insulin stimulation, suggesting that ACTN4 may play a role in tethering GSVs to the actin cytoskeleton (Figure 1b, inset). The tethering function of the cortical actin mesh likely plays a critical role in the final steps of GSV/PM docking and fusion regulated by syntaxin 4 and synaptosomal-associated protein 23 (SNAP23) target (t-) soluble N-ethylmaleimide sensitive factor attachment protein receptors (SNAREs) and the GSV vesicle (v-) SNARE vesicle-associated membrane protein 2 (VAMP2) [38]. Together these findings provide evidence that ACTN4 may direct GSVs to the insulin-organized cortical actin meshwork to facilitate GSV delivery to appropriate PM-localized SNARE-mediated docking machinery. With other putative tethering mechanisms existing, the importance of ACTN4 remains unclear.
In cultured and primary adipocytes, an evolutionarily conserved tethering complex termed the exocyst complex has been described for GSV/PM targeting [39, 40]. This complex, comprised of eight subunits, mediates the initial recognition of the exocytic vesicle and target membranes for fusion. The assembly of the adipocyte GSV exocyst complex occurs at PM caveolae/raft regions and requires insulin activation of the Rho family member GTPase TC10, which mediates recruitment of exocyst components Exo70, Sec6, and Sec8 [39]. Interestingly, TC10 activation also appears necessary for the cortical filamentous actin (F-actin) reorganization (Figure 1b, inset). Insulin not only activates a Rab GTPase for GSV recruitment and a Rho GTPase for GSV/PM arrival, but it also activates a GSV-associated Ral GTPase termed RalA that induces GSV/exocyst association juxtaposed to the PM (Figure 1b, inset) [41]. The observation that RalA interacts with Myo1c, a molecular motor implicated in GSV trafficking, implicates a convergence of vesicle trafficking to tethering [26, 41].
Of interest are the insulin signals to each of the GTPases. Similar to Rab activation, insulin signaling to RalA is PI3K-mediated [41]. Whereas the PI3K/Rab signal involves Akt2 signaling to TBC1D4 and/or TBC1D1, it remains unclear if PI3K signaling to RalA involves Akt2 stimulation and distal signaling to TBC1D4, TBC1D1, or a yet to be determined substrate. Unlike Rab and RalA activation, insulin is appreciated to use a PI3K-independent signal to activate the Rho GTPase TC10. Although initial studies delineated several component proteins (e.g., APS, c-Cbl, CAP, CrkII/C3G) involved in this TC10 activation, subsequent siRNA knockdown studies challenged their proximal importance in TC10-regulated GLUT4 translocation [42]. Subsequent study revealed that TC10 has two isoforms (TC10α and TC10β), and that insulin-stimulated TC10α, not TC10β, regulates GLUT4 translocation [33, 43]. Studies with pharmacological inhibitors and siRNA-mediated knockdown show that proximal insulin signaling to TC10α-, but not TC10β-, involves the activation of cyclin-dependent kinase-5 (CDK5) in caveolae/raft domains via proximal non-receptor tyrosine kinase Fyn activation by insulin [43]. Furthermore, active CDK5 maintains TC10α in caveolae/rafts where it functions to disrupt cortical F-actin.
With regards to actin, identification of α- and β-fodrin isoforms as abundant components of rat primary adipocyte PM caveolae/rafts is of interest [44]. Fodrin is a nonerythroid spectrin that forms filamentous α-β heterodimers and binds to actin at both ends, forming a repeating “corral”-like network beneath the PM. Interestingly, α-fodrin and syntaxin 4 co-localize and interact in rat adipocytes, and insulin enhances this interaction. In contrast, disruption of cortical actin by latrunculin A reduces the α-fodrin-syntaxin 4 interaction, blocks α-fodrin remodeling, and inhibits GLUT4 translocation. In this case, the regulated remodeling of the fodrin-actin network apparently plays a key role in permitting GSV/PM fusion by allowing GSV-VAMP2 access to syntaxin 4 (Figure 1b, inset). Once insulin-stimulated α-fodrin remodeling occurs, GLUT4 translocation became insensitive to latrunculin A, suggesting that only the exocytic step of GLUT4 translocation at or near the PM requires this aspect of cortical F-actin remodeling [44].
In summary, these data are consistent with the concept that the insulin-stimulated motoring of the GSV, subsequent to its Rab recruitment signal, requires insulin signaling to RalA by an undefined PI3K mechanism and to TC10α via a Fyn/CDK5 pathway. Whereas RalA activation stimulates the trafficking GSV to tether with the caveolae/raft-localized exocyst complex, active TC10α regulates the formation of the exocyst complex for GSV tethering and also reorganizes the cortical F-actin for GSV docking. Together these studies suggest that fodrin potentially permits the transition of the exocyst-tethered GSV to the appropriate PM-localized SNARE docking machinery by coupling syntaxin 4 to caveolae-localized F-actin.
Bilayering GSVs
It is clear that the regulated meshwork of actin filaments beneath the PM plays a critical role in several steps of the GSV itinerary, particularly GSV arrival, tethering, and docking. In addition to a likely role of bilayer parameters in GSV recruitment and mobilization steps, new data indicate that insulin-regulated changes in PM lipids promote GSV/PM fusion. As lipids are key pathophysiological players in disorders of glucose metabolism, studies demonstrating an impact of PM lipids on insulin action and GLUT4 translocation warrant consideration. Indeed, several microscopy-based explorations of PM functionality provide new insight into PM/F-actin coupling and GSV/PM fusion regulation by insulin.
Caveolae represent specialized, morphologically distinct sphingolipid-cholesterol microdomains of the PM, which are stabilized by caveolin proteins [45]. Through the years many functions for caveolae have been postulated in insulin and GLUT4 action. Although caveolae functionality needs to be cautiously interpreted because problems are associated with each of the numerous strategic approaches used to study these structures, a caveolae-based TC10α/exocyst complex tethering and cortical F-actin dispersion mechanism in this vicinity is consistent with a caveolae-actin association [46, 47]. Particularly, fluorescence confocal labeling of caveolae and cortical F-actin revealed actin filaments emanating from caveolae microdomains [46]. Disruption of this caveolin-associated F-actin, termed Cav-actin structure, with latrunculin B, Clostridium difficile toxin B, or a dominant-interfering TC10 mutant (TC10/T31N), did not affect the organization of clustered caveolae; however, disruption of the clustered caveolae with methyl-beta-cyclodextrin dispersed the Cav-actin structure [46]. Quantitative electron microscopy and freeze-fracture analyses later revealed that cytoskeletal components, including actin, are highly enriched in the membrane area underlying the neck part of caveolae [47]. Together, these findings assign caveolae a critical functionality in cortical F-actin organization. Given the unequivocal importance of cortical F-actin in insulin-regulated GLUT4 translocation, these findings also emphasize the importance of caveolae in GLUT4 regulation. Of interest to our understanding of Cav-actin structure regulation are new electron microscopic data showing high concentrations of phosphatidylinositol 4,5 bisphosphate (PIP2) at the rim of caveolae [48]. This localization of PIP2 is consistent with its regulation of the cytoskeleton where this lipid's availability is recognized to modulate membrane/cytoskeleton interaction, the stability of cortical F-actin, and the turnover of cytoplasmic stress fibers [49]. Interestingly, reduced PM PIP2 and cortical F-actin structure are observed in hyperinsulinemia-induced insulin-resistant 3T3-L1 adipocytes and L6 myotubes where insulin-stimulated GLUT4 translocation is impaired, but corrected with exogenous PIP2 addition to the PM by provoking a restoration of cortical F-actin structure [50, 51].
Given the emerging evidence of PM functionality in GLUT4 regulation, recent studies have employed total internal reflection microscopy (TIRFM) to critically examine GSV/GLUT4 regulation. This microscopy uses an evanescent wave to selectively illuminate and excite fluorophores in a restricted region of the cell immediately adjacent to the PM. This further experimental scrutiny has expanded upon previous findings from cell-free reconstitution assays showing that prior insulin activation of the PM fraction, which was then reconstituted in the cell-free assay, is essential for GSV/PM fusion [52]. For example, several TIRFM analyses have provided strong evidence for a critical PM signal that appears to prime the PM and/or the GSVs for fusion [53–55]. With a combination of live cell and steady-state TIRFM analyses with PI3K and Akt inhibition, Akt was suggested to be a crucial regulator of insulin-stimulated GSV/PM pre-fusion (i.e., recruitment, tethering, docking) events [53]. In contrast, insulin-stimulated GSV/PM fusion seemed to occur independently of Akt activity. That is, although GSV/PM pre-fusion was inefficient with Akt blockade, fusion of this lower level of docked GSVs with the PM was properly stimulated by insulin. However, wortmannin inhibits GSV/PM fusion, suggesting this postdocking step is PI3K-dependent. Dynamic tracking of single GSVs with computational analysis of thousands of events lends strong support to this model whereby insulin uses a PI3K/Akt signal to accelerate GSV/PM pre-fusion and another signal to prepare GSVs and/or the PM for GSV/PM fusion [54]. Although the steady-state analysis suggested this second signal required PI3K, this conclusion could not be made with live cell GSV tracking [53, 54].
Certainly, the putative role of PI3K in priming GSV/PM fusion requires confirmation; still, it is of great interest since PI3K signaling to PKCζ/λ/Munc18c [56, 57] and/or PLD1 [58] could promote GSV/PM fusion (Figure 1c). Whereas insulin signaling through PKCζ/λ has been proposed to dissociate Munc18c from syntaxin 4, a necessary postdocking/prefusion event [56, 57], PLD1 activation by insulin has been implicated in generating fusion-competent membranes [59]. Mechanistically, phospholipase D1 (PLD1) generates the lipid phosphatidic acid (PA), which has been suggested to act as a fusogenic lipid in biophysical modeling studies by lowering the activation energy for membrane bending (i.e., negative membrane curvature) during generation and expansion of fusion pores (Figure 1c, inset) [60]. Together these data suggest PI3K may use two distinct signals to regulate critical mechanisms of GSV/PM fusion post GSV/PM docking.
Clinical perspective
New additions to the molecular details of GLUT4 regulation by insulin attest to the great progress being made in our mechanistic understanding of insulin-stimulated glucose transport in health and disease. Despite the increase in knowledge, global prevalence of diabetes in 2010 was 284 million people worldwide, constituting around 6.4% of the world population. Projections for 2030 estimate the prevalence reaching 439 million individuals, comprising ~7.7% of the world population [61]. This is attributed in large part to the rising incidence of obesity worldwide, which makes it essential to focus attention on molecular mechanisms underlying insulin resistance that are fueled by obesity. In this regard, a large number of endocrine, inflammatory, neural, and cell-intrinsic pathways have been shown to be dysregulated in obesity and impair insulin signaling by increasing inhibitory serine phosphorylation of IRS1, which can have a direct impact on GLUT4 regulation [62].
However, several studies showing GLUT4 dysregulation without apparent defects in proximal insulin signaling point to the existence of other disabling factors of insulin-regulated GLUT4 exocytosis. A major goal now is to precisely and accurately assess the functional status of the GLUT4 regulatory system. Whether critical flaws in cytoskeletal F-actin organization, lipid bilayer composition, and/or GSV/PM fusion priming contribute to insulin resistance is not known; yet, a clear association has been demonstrated between the diabetic milieu, cytoskeletal disorganization, and bilayer abnormalities. For example, isolated neutrophils from patients with type 2 diabetes display decreased actin polymerization compared to neutrophils from non-diabetic control subjects [63]. This impairment is associated with persistent expression of the endothelial adhering beta 2-integrin CD11b/CD18, potentially exacerbating vascular dysfunction in diabetic patients. Also, diabetic rat retinal endothelial cells have a prominent reduction in F-actin integrity, a finding closely linked to vascular leakage [64]. Loss of rat mesangial cell F-actin has also been reported after exposure to early-diabetic state-like conditions [65], possibly causing diabetic hyperfiltration. Further support has been derived from examination of erythrocytes from overweight, insulin-resistant individuals which show marked changes in the phospholipid composition of the PM [66].
With regards to insulin action and glucose transport, cholesterol complexing drug experiments have demonstrated that removal of cholesterol from the PM augments basal glucose uptake and metabolism [67, 68]. Consistent with cholesterol causing highly-ordered gel-like states, moderate increases in PM fluidity increase glucose transport in adipocytes [69]. In direct support of this observation, insulin-stimulated glucose transport declined when fluidity was diminished [69]. Mechanistically, it is now appreciated that non-physiological PM cholesterol depletion greater than 50% reduces the rate of internalization of PM GLUT4 by more than 85% [70]. Although this certainly explains the gain in PM GLUT4, it is an artificial, experimentally-induced gain. In contrast, reductions of PM cholesterol less than 50% do not affect the rate of endocytosis, but do increase PM GLUT4 content [71]. These later findings suggest moderate PM cholesterol lowering augments GSV exocytosis. How these PM cholesterol-based aspects of GLUT4 regulation intermingle with insulin signaling and/or GSV regulation is unknown, yet coordinated signaling events and/or F-actin reorganization are areas of interest [28]. Perhaps PM cholesterol toxicity contributes to impaired PIP2-regulated cortical F-actin organization observed in insulin-induced insulin resistant 3T3-L1 adipocytes and L6 myotubes.
Although speculative, we view this as a possible indication that membrane/cytoskeletal defects in skeletal muscle and/or adipose tissue could negatively impinge on GLUT4 translocation, perhaps before defects are induced in proximal insulin signaling. Supporting this view are studies using various cell model systems of insulin resistance that demonstrate insulin signaling to Akt2/TBC1D4 is not impaired [50, 51, 72–75]. Collectively these data point to the notion that some insulin-resistant states may result from a membrane/cytoskeletal-based mechanical defect in GSV/PM arrival, tethering, docking, and/or fusion. In summary, several new essential features of insulin-stimulated GLUT4 regulation have been revealed. From IR activation to GSV/PM fusion, multiple signals and a large number of processes apparently occur. Recent data also suggest that insulin continues to control the post-fusion dispersal of GLUT4 in the PM [76]. Although complete discussion of this new putative level of insulin regulation is out of the scope of this review, it exemplifies the fact that other unknown signals and processes likely await discovery. In the context of insulin resistance, it becomes excruciatingly apparent why the failure of insulin-regulated glucose transport into skeletal muscle and adipose tissue is such an extremely difficult problem to solve. In conjunction with pinning down the complete mechanisms of the Akt2/GSV recruitment signal, the TC10/GSV tethering and actin reorganization system, and several putative PI3K transition signals (i.e., the RalA GSV trafficking-to-tethering signal; the PKCζ/λ docking disassembly signal; and the PLD priming GSV/PM fusibility signal), important additional advances should include delineating the spatial and temporal aspects of all these signals and associated processes. Such additions will create a parallel opportunity to define the molecular events responsible for GLUT4 dysregulation in insulin resistance.
Acknowledgements
This work was supported by National Institutes of Health: AT001846 (JSE) and DK082773 (JSE); and the Indiana University Diabetes and Obesity Research Training Program: DeVault Fellowship (NJH) and T32-DK064466 (NJH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Klip A. The many ways to regulate glucose transporter 4. Appl Physiol Nutr Metab. 2009;34:481–487. doi: 10.1139/H09-047. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez E, McGraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009;8:2502–2508. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kane S, et al. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem. 2002;277:22115–22118. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- 5.Ishikura S, et al. Small G proteins in insulin action: Rab and Rho families at the crossroads of signal transduction and GLUT4 vesicle traffic. Acta Physiol (Oxf) 2008;192:61–74. doi: 10.1111/j.1748-1716.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaddai V, et al. Rab proteins in endocytosis and Glut4 trafficking. Acta Physiol (Oxf) 2008;192:75–88. doi: 10.1111/j.1748-1716.2007.01787.x. [DOI] [PubMed] [Google Scholar]
- 7.Stockli J, et al. Regulation of glucose transporter 4 translocation by the Rab guanosine triphosphatase-activating protein AS160/TBC1D4: role of phosphorylation and membrane association. Mol Endocrinol. 2008;22:2703–2715. doi: 10.1210/me.2008-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peck GR, et al. Interaction of the Akt substrate, AS160, with the glucose transporter 4 vesicle marker protein, insulin-regulated aminopeptidase. Mol Endocrinol. 2006;20:2576–2583. doi: 10.1210/me.2005-0476. [DOI] [PubMed] [Google Scholar]
- 9.Roach WG, et al. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J. 2007;403:353–358. doi: 10.1042/BJ20061798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peck GR, et al. Insulin-stimulated phosphorylation of the Rab GTPase-activating protein TBC1D1 regulates GLUT4 translocation. J Biol Chem. 2009;284:30016–30023. doi: 10.1074/jbc.M109.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An D, et al. TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle. Diabetes. 2010;59:1358–1365. doi: 10.2337/db09-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone S, et al. TBC1D1 is a candidate for a severe obesity gene and evidence for a gene/gene interaction in obesity predisposition. Hum Mol Genet. 2006;15:2709–2720. doi: 10.1093/hmg/ddl204. [DOI] [PubMed] [Google Scholar]
- 13.Chavez JA, et al. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating protein abundant in skeletal muscle, is partially relieved by AMP-activated protein kinase activation. J Biol Chem. 2008;283:9187–9195. doi: 10.1074/jbc.M708934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larance M, et al. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem. 2005;280:37803–37813. doi: 10.1074/jbc.M503897200. [DOI] [PubMed] [Google Scholar]
- 15.Miinea CP, et al. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J. 2005;391:87–93. doi: 10.1042/BJ20050887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sano H, et al. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007;5:293–303. doi: 10.1016/j.cmet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Sano H, et al. Rab10 in insulin-stimulated GLUT4 translocation. Biochem J. 2008;411:89–95. doi: 10.1042/BJ20071318. [DOI] [PubMed] [Google Scholar]
- 18.Kaddai V, et al. Rab4b is a small GTPase involved in the control of the glucose transporter GLUT4 localization in adipocyte. PLoS One. 2009;4:e5257. doi: 10.1371/journal.pone.0005257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikura S, et al. Rabs 8A and 14 are targets of the insulin-regulated Rab-GAP AS160 regulating GLUT4 traffic in muscle cells. Biochem Biophys Res Commun. 2007;353:1074–1079. doi: 10.1016/j.bbrc.2006.12.140. [DOI] [PubMed] [Google Scholar]
- 20.Ishikura S, Klip A. Muscle cells engage Rab8A and myosin Vb in insulin-dependent GLUT4 translocation. Am J Physiol Cell Physiol. 2008;295:C1016–1025. doi: 10.1152/ajpcell.00277.2008. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, et al. Rab8A and Rab13 are activated by insulin and regulate GLUT4 translocation in muscle cells. Proc Natl Acad Sci U S A. 2010;107:19909–19914. doi: 10.1073/pnas.1009523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kane S, Lienhard GE. Calmodulin binds to the Rab GTPase activating protein required for insulin-stimulated GLUT4 translocation. Biochem Biophys Res Commun. 2005;335:175–180. doi: 10.1016/j.bbrc.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 23.Kramer HF, et al. Calmodulin-binding domain of AS160 regulates contraction- but not insulin-stimulated glucose uptake in skeletal muscle. Diabetes. 2007;56:2854–2862. doi: 10.2337/db07-0681. [DOI] [PubMed] [Google Scholar]
- 24.Lanner JT, et al. Ca(2+) and insulin-mediated glucose uptake. Curr Opin Pharmacol. 2008;8:339–345. doi: 10.1016/j.coph.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Contreras-Ferrat AE, et al. An inositol 1,4,5-triphosphate (IP3)-IP3 receptor pathway is required for insulin-stimulated glucose transporter 4 translocation and glucose uptake in cardiomyocytes. Endocrinology. 2010;151:4665–4677. doi: 10.1210/en.2010-0116. [DOI] [PubMed] [Google Scholar]
- 26.Yip MF, et al. CaMKII-mediated phosphorylation of the myosin motor Myo1c is required for insulin-stimulated GLUT4 translocation in adipocytes. Cell Metab. 2008;8:384–398. doi: 10.1016/j.cmet.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Eyster CA, Olson AL. Compartmentalization and regulation of insulin signaling to GLUT4 by the cytoskeleton. Vitam Horm. 2009;80:193–215. doi: 10.1016/S0083-6729(08)00608-0. [DOI] [PubMed] [Google Scholar]
- 28.Brozinick JT, Jr., et al. “Actin”g on GLUT4: membrane & cytoskeletal components of insulin action. Curr Diabetes Rev. 2007;3:111–122. doi: 10.2174/157339907780598199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaid H, et al. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem J. 2008;413:201–215. doi: 10.1042/BJ20080723. [DOI] [PubMed] [Google Scholar]
- 30.Eyster CA, et al. Microtubule network is required for insulin signaling through activation of Akt/protein kinase B: evidence that insulin stimulates vesicle docking/fusion but not intracellular mobility. J Biol Chem. 2006;281:39719–39727. doi: 10.1074/jbc.M607101200. [DOI] [PubMed] [Google Scholar]
- 31.Xu YK, et al. Bi-directional transport of GLUT4 vesicles near the plasma membrane of primary rat adipocytes. Biochem Biophys Res Commun. 2007;359:121–128. doi: 10.1016/j.bbrc.2007.05.075. [DOI] [PubMed] [Google Scholar]
- 32.Lizunov VA, et al. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J Cell Biol. 2005;169:481–489. doi: 10.1083/jcb.200412069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang L, et al. TC10alpha is required for insulin-stimulated glucose uptake in adipocytes. Endocrinology. 2007;148:27–33. doi: 10.1210/en.2006-1167. [DOI] [PubMed] [Google Scholar]
- 34.Ting Chiu T, et al. Arp2/3- and Cofilin-coordinated Actin Dynamics Is Required for Insulin-mediated GLUT4 Translocation to the Surface of Muscle Cells. Mol Biol Cell. 2010 doi: 10.1091/mbc.E10-04-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueda S, et al. Crucial role of the small GTPase Rac1 in insulin-stimulated translocation of glucose transporter 4 to the mouse skeletal muscle sarcolemma. FASEB J. 2010;24:2254–2261. doi: 10.1096/fj.09-137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bisht B, Dey CS. Focal Adhesion Kinase contributes to insulin-induced actin reorganization into a mesh harboring Glucose transporter-4 in insulin resistant skeletal muscle cells. BMC Cell Biol. 2008;9:48. doi: 10.1186/1471-2121-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talior-Volodarsky I, et al. Alpha-actinin-4 is selectively required for insulin-induced GLUT4 translocation. J Biol Chem. 2008;283:25115–25123. doi: 10.1074/jbc.M801750200. [DOI] [PubMed] [Google Scholar]
- 38.Watson RT, Pessin JE. GLUT4 translocation: the last 200 nanometers. Cell Signal. 2007;19:2209–2217. doi: 10.1016/j.cellsig.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Inoue M, et al. Compartmentalization of the exocyst complex in lipid rafts controls Glut4 vesicle tethering. Mol Biol Cell. 2006;17:2303–2311. doi: 10.1091/mbc.E06-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoue M, et al. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422:629–633. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- 41.Chen XW, et al. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev Cell. 2007;13:391–404. doi: 10.1016/j.devcel.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Mitra P, et al. RNAi-based analysis of CAP, Cbl, and CrkII function in the regulation of GLUT4 by insulin. J Biol Chem. 2004;279:37431–37435. doi: 10.1074/jbc.C400180200. [DOI] [PubMed] [Google Scholar]
- 43.Okada S, et al. CDK5-dependent phosphorylation of the Rho family GTPase TC10(alpha) regulates insulin-stimulated GLUT4 translocation. J Biol Chem. 2008;283:35455–35463. doi: 10.1074/jbc.M806531200. [DOI] [PubMed] [Google Scholar]
- 44.Liu L, et al. Role of insulin-dependent cortical fodrin/spectrin remodeling in glucose transporter 4 translocation in rat adipocytes. Mol Biol Cell. 2006;17:4249–4256. doi: 10.1091/mbc.E06-04-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 46.Kanzaki M, Pessin JE. Caveolin-associated filamentous actin (Cav-actin) defines a novel F-actin structure in adipocytes. J Biol Chem. 2002;277:25867–25869. doi: 10.1074/jbc.C200292200. [DOI] [PubMed] [Google Scholar]
- 47.Foti M, et al. The neck of caveolae is a distinct plasma membrane subdomain that concentrates insulin receptors in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 2007;104:1242–1247. doi: 10.1073/pnas.0610523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujita A, et al. Quantitative electron microscopy for the nanoscale analysis of membrane lipid distribution. Nat Protoc. 2010;5:661–669. doi: 10.1038/nprot.2010.20. [DOI] [PubMed] [Google Scholar]
- 49.Kwik J, et al. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci U S A. 2003;100:13964–13969. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen G, et al. Protective effect of phosphatidylinositol 4,5-bisphosphate against cortical filamentous actin loss and insulin resistance induced by sustained exposure of 3T3-L1 adipocytes to insulin. J Biol Chem. 2004;279:39705–39709. doi: 10.1074/jbc.C400171200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCarthy AM, et al. Loss of cortical actin filaments in insulin-resistant skeletal muscle cells impairs GLUT4 vesicle trafficking and glucose transport. Am J Physiol Cell Physiol. 2006;291:C860–868. doi: 10.1152/ajpcell.00107.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koumanov F, et al. Insulin signaling meets vesicle traffic of GLUT4 at a plasma-membrane-activated fusion step. Cell Metab. 2005;2:179–189. doi: 10.1016/j.cmet.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and - independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell. 2006;17:4484–4493. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai L, et al. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 2007;5:47–57. doi: 10.1016/j.cmet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 55.Jiang L, et al. Direct quantification of fusion rate reveals a distal role for AS160 in insulin-stimulated fusion of GLUT4 storage vesicles. J Biol Chem. 2008;283:8508–8516. doi: 10.1074/jbc.M708688200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thurmond DC, et al. Munc18c function is required for insulin-stimulated plasma membrane fusion of GLUT4 and insulin-responsive amino peptidase storage vesicles. Mol Cell Biol. 2000;20:379–388. doi: 10.1128/mcb.20.1.379-388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hodgkinson CP, et al. Protein kinase-zeta interacts with munc18c: role in GLUT4 trafficking. Diabetologia. 2005;48:1627–1636. doi: 10.1007/s00125-005-1819-y. [DOI] [PubMed] [Google Scholar]
- 58.Lee JS, et al. Phosphatidylinositol (3,4,5)-trisphosphate specifically interacts with the phox homology domain of phospholipase D1 and stimulates its activity. J Cell Sci. 2005;118:4405–4413. doi: 10.1242/jcs.02564. [DOI] [PubMed] [Google Scholar]
- 59.Huang P, et al. Insulin-stimulated plasma membrane fusion of Glut4 glucose transporter-containing vesicles is regulated by phospholipase D1. Mol Biol Cell. 2005;16:2614–2623. doi: 10.1091/mbc.E04-12-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kooijman EE, et al. Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic. 2003;4:162–174. doi: 10.1034/j.1600-0854.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 61.Farag YM, Gaballa MR. Diabesity: an overview of a rising epidemic. Nephrol Dial Transplant. 2010 doi: 10.1093/ndt/gfq576. [DOI] [PubMed] [Google Scholar]
- 62.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21:1443–1455. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 63.Advani A, et al. Impaired neutrophil actin assembly causes persistent CD11b expression and reduced primary granule exocytosis in Type II diabetes. Diabetologia. 2002;45:719–727. doi: 10.1007/s00125-002-0802-0. [DOI] [PubMed] [Google Scholar]
- 64.Yu PK, et al. Endothelial F-actin cytoskeleton in the retinal vasculature of normal and diabetic rats. Curr Eye Res. 2005;30:279–290. doi: 10.1080/02713680590923230. [DOI] [PubMed] [Google Scholar]
- 65.Zhou X, et al. High glucose alters actin assembly in glomerular mesangial and epithelial cells. Lab Invest. 1995;73:372–383. [PubMed] [Google Scholar]
- 66.Candiloros H, et al. Hyperinsulinemia is related to erythrocyte phospholipid composition and membrane fluidity changes in obese nondiabetic women. J Clin Endocrinol Metab. 1996;81:2912–2918. doi: 10.1210/jcem.81.8.8768851. [DOI] [PubMed] [Google Scholar]
- 67.Akhtar RA, Perry MC. The effect of digitonin of the stimulation by insulin of glucose uptake by isolated fat cells. Biochim Biophys Acta. 1975;411:30–40. doi: 10.1016/0304-4165(75)90282-2. [DOI] [PubMed] [Google Scholar]
- 68.Kuo JF. Stimulation of glucose utilization and inhibition of lipolysis by polyene antibiotics in isolated adipose cells. Arch Biochem Biophys. 1968;127:406–412. doi: 10.1016/0003-9861(68)90243-9. [DOI] [PubMed] [Google Scholar]
- 69.Pilch PF, et al. Coordinate modulation of D-glucose transport activity and bilayer fluidity in plasma membranes derived from control and insulin-treated adipocytes. Proc Natl Acad Sci U S A. 1980;77:915–918. doi: 10.1073/pnas.77.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blot V, McGraw TE. GLUT4 is internalized by a cholesterol-dependent nystatin-sensitive mechanism inhibited by insulin. EMBO J. 2006;25:5648–5658. doi: 10.1038/sj.emboj.7601462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu P, et al. Sphingomyelinase activates GLUT4 translocation via a cholesterol-dependent mechanism. Am J Physiol Cell Physiol. 2004;286:C317–329. doi: 10.1152/ajpcell.00073.2003. [DOI] [PubMed] [Google Scholar]
- 72.Xiong W, et al. GLUT4 is sorted to vesicles whose accumulation beneath and insertion into the plasma membrane are differentially regulated by insulin and selectively affected by insulin resistance. Mol Biol Cell. 2010;21:1375–1386. doi: 10.1091/mbc.E09-08-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoehn KL, et al. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 2008;7:421–433. doi: 10.1016/j.cmet.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.JeBailey L, et al. Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells. Diabetes. 2007;56:394–403. doi: 10.2337/db06-0823. [DOI] [PubMed] [Google Scholar]
- 75.Strawbridge AB, Elmendorf JS. Endothelin-1 impairs glucose transporter trafficking via a membrane-based mechanism. J Cell Biochem. 2006;97:849–856. doi: 10.1002/jcb.20687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stenkula KG, et al. Insulin Controls the Spatial Distribution of GLUT4 on the Cell Surface through Regulation of Its Postfusion Dispersal. Cell Metab. 2010;12:250–259. doi: 10.1016/j.cmet.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]