Abstract
BACKGROUND & AIMS
Interleukin (IL)-17–producing CD4+ helper T cells (Th17) mediate mucosal immunity and are involved in the pathogenesis of inflammatory bowel disease (IBD). They are believed to arise from the same precursor population as regulatory T (Treg) cells, but little is known about how these T-cell subsets interact under chronic inflammatory conditions. We studied Th17 cells and Treg cells isolated from intestinal lamina propria of patients with inflammatory bowel disease (IBD) to investigate their role in pathogenesis.
METHODS
FoxP3 expression (a marker of Treg cells) and IL-17 production were assessed in CD4+ lamina propria lymphocytes (LPLs) isolated from IBD patients and healthy subjects. IL17+FoxP3+ and IL-17+ CD4+ T-cell clones were generated by limiting dilution. An in vitro suppression assay was performed to assess the functional capacity of derived T-cell clones.
RESULTS
IL17+FoxP3+ T cells were identified in inflamed intestinal mucosa of patients with Crohn’s disease (CD), but not in patients with ulcerative colitis (UC) or healthy controls. These cells shared phenotypic characteristics of Th17 and Treg cells, and showed potent suppressor activity in vitro. Transforming growth factor-® was necessary and sufficient to induce development of a IL17+ FoxP3+ cell population in CD4+ LPLs derived from patients with UC.
CONCLUSIONS
The inflammatory environment in the intestinal mucosa of patients with CD contributes to the generation of a distinct population of Treg cells that are FoxP3+ and produce IL-17. These cells are likely to arise during differentiation of Th17 and Treg cells. Specific microenvironmental cues from tissues are likely to determine their commitment to either lineage and affect the balance between regulation and inflammation in the intestine.
Keywords: Immune system, T-cell development, TGF-®, immune suppression
INTRODUCTION
Interleukin (IL)-17-producing CD4+ T lymphocytes, known as Th17 cells, constitute a subset of T-helper cells that has been linked to the pathogenesis of several chronic inflammatory disorders, including inflammatory bowel disease (IBD) 1. At mucosal surfaces, Th17 cells are thought to protect the host from infection, whereas several types of regulatory T (Treg) cells keep effector T cell populations in check and prevent T cell-mediated destruction of intestinal tissue 2–10. The development of both cell types requires transforming factor-β (TGF-β), which suggests that Treg and Th17 cells may be related and may arise from the same precursor in distinct cytokine milieus 4, 11–13. Indeed, a series of recent studies have provided cumulative evidence of such an intimate link between Treg and Th17 cell differentiation programs both in vitro and in vivo 4, 14–16. Exposure of antigen-activated naïve CD4+ T cells to TGF-β results in the transcriptional upregulation of both FoxP3 and RORγt, transcription factors that direct Treg and Th17 cell differentiation, respectively 9, 15, 17, 18. T cells that co-express FoxP3 and RORγt have been identified in vivo in both mice and humans 15, 19. Even though it has been suggested that FoxP3 inhibits expression of IL-17, FoxP3+IL-17+ CD4+ T cells have been observed both in vitro, after long-term polarization in the presence of TGF-β and IL-6, and in vivo in human tonsil 14, 15, 19. While recent evidence shows a great degree of flexibility in Th17 and Treg differentiation programs, the physiological relevance of such plasticity has yet to be determined. In addition, the interplay between Treg and Th17 cells under chronic inflammatory conditions that potentially can contribute to the pathogenesis of human diseases has not been addressed.
Crohn’s disease (CD), one of the forms of IBD, has been shown to be driven by Th17-mediated inflammation; there is higher expression of IL-17 in the inflamed intestinal mucosa of patients with CD 20–22. Furthermore, genome-wide analyses have identified several uncommon Il23r variants inversely correlated with susceptibility to IBD 23, 24. At the same time, an increased number of Tregs in the lamina propria (LP), mesenteric lymph nodes (MLN) and inflamed intestinal mucosa of CD patients has been reported 25–27. CD is believed to be the result of an aberrant response of the gut-associated lymphoid tissue to bacterial and dietary antigens. Although the presence of Th17 cells and Tregs in the inflamed intestine has been demonstrated 20, 25, their dynamics and contribution to the disease process has remained elusive.
Here we report the identification of a FoxP3+ IL-17-producing CD4+ Treg cell population in the intestinal lamina propria of CD patients, but not in patients with ulcerative colitis (UC) or healthy controls. Notably, this population was found to be significantly increased in inflamed CD gut mucosa when compared to its slightly or non-inflamed counterparts. FoxP3+ IL-17-producing cells also produced large amounts of another effector cytokine, IFNγ. They shared phenotypic characteristics of both Th17 and Treg cells, and showed potent suppressor activity in vitro. Together, our data demonstrate the ability of human tissue-resident Tregs to display a certain degree of plasticity in vivo reflected in their capacity to produce inflammatory cytokines in the specific inflammatory environment. Thus, selective intestinal microenvironmental cues regulate the balance between Th17 and Treg cells and are likely to influence intestinal immunity and tolerance.
MATERIALS AND METHODS
Cell purification
Surgical specimens from patients undergoing bowel resection for IBD or colorectal cancer at the Mount Sinai Medical Center were used as the source for lamina propria mononuclear cells. Institutional review board approval was obtained prior to involving patients in the study. Lamina propria lymphocytes (LPLs) were isolated according to an established protocol using Dispase II (Roche Diagnostics) and collagenase (Sigma) treatment. Blood samples were obtained from healthy donors and IBD patients undergoing treatment at the Mount Sinai Medical Center. When possible, paired samples of peripheral blood (PB) and gut-draining mesenteric lymph nodes (MLN) were acquired from Crohn’s disease (CD) patients undergoing bowel resection. LP, MLN and PB CD4+ T cells were isolated by negative selection using a human CD4+ T-cell Enrichment Kit (StemCell Technologies). IL17-producing CD4+ T cells were enriched using IL-17 Secretion Assay-Cell Enrichment and Detection Kit (PE) (Miltenyi Biotec) according to the manufacturer’s instructions. CD4+ IL-17+ and CD4+ IL-17− fractions were sorted by flow cytometry after enrichment with the IL-17 Secretion Assay-Cell Enrichment and Detection Kit (PE) (Miltenyi Biotec).
Cell culture and expansion
Gut-, MLN- and PB-derived CD4+ T lymphocytes were cultured in RPMI 1640 medium supplemented with penicillin-streptomycin-glutamine (Invitrogen), 0.5 µg/ml fungizone (Invitrogen) and 10% FBS (ATCC). Purified CD4+ LPLs were expanded with αCD2/αCD3/αCD28 beads (Miltenyi Biotec) according to the manufacturer’s instructions. After 3 d of priming, cells were supplemented with rIL-2 (20 IU/ml; Novartis). For long-term experiments, cells were split as needed in the presence of rIL-2. Where indicated, IL-1β (10ng/ml; BioLegend), IL-6 (10ng/ml; eBioscience), IL-21 (10ng/ml; eBioscience), IL-23 (10ng/ml; R&D Systems), various concentrations of TGF-β1 (eBioscience) and neutralizing antibody to TGF-β1 (1µg/ml; R&D Systems) were added to the cultures at day 0 and were maintained throughout the experiment. Cells were collected on day 6 for intracellular staining.
Surface and intracellular staining
For basal cytokine detection, CD4+ LPLs were cultured in triplicate at 1×106 cells/ml in a 48-well plate and stimulated with 50 ng/ml PMA (Sigma) and 1µg/ml ionomycin (Sigma) in the presence of brefeldin A (GolgiPlug, BD Biosciences) for 4 hours. Cell surfaces were stained by incubation for 30 min on ice with fluorochrome-conjugated antibodies against CCR6 (53103; R&D Systems), CD103 (Ber-ACT8; BD Bioscience), CD161 (HP-3G10; eBioscience), CD101 (BB27; eBioscience), CD127 (RDR5; eBioscience), integrin α4 (9F10; eBioscience), integrin β7 (FIB504; eBioscience), Vβ TCR (Immunotech) and isotype controls: rat IgG1 (eBRG1; eBioscience), rat IgG2a (eBR2a; eBioscience), mouse IgG1 (MOPC-21; BD Biosciences) and mouse IgG2B (eBioscience) in FACS buffer containing 2% FBS. The FoxP3 staining buffer set (eBioscience) was used for intracellular staining. Cells were fixed and made permeable for 30 min at 21°C and were stained for 30 min on ice with antibodies specific for FoxP3 (236A/E7; eBioscience), RORγt (eBioscience), IL-17 (eBio64Dec17; eBioscience), IL-22 (22URT1; eBioscience), IL-21 (eBio3A3-N2; eBioscience), IL-10 (JES3-9D7; eBiosceince) and IFNγ (4S.B3; eBioscience) in permeabilization buffer. FACSCalibur (BD Biosciences), LSRII (BD Biosciences) and CELLQuest™ software (BD Biosciences) were used for flow cytometry.
Cell cloning
Gut-derived IL-17+ and FoxP3+IL-17+ T-cell clones were generated from IL-17-enriched CD-derived LP CD4+ T cells by limiting dilution (0.3 cell/well) in 96-well U-bottom plates using 105 irradiated (30Gy) allogeneic feeder cells (PBMCs) and rIL-2 (20 U/ml). After 4–5 weeks of expansion, each clone from outgrowth wells was tested for FoxP3 expression and IL-17 production. The clonality of T-cell clones was determined using monoclonal antibodies against specific Vβ TCRs. FoxP3+/IL-17+ and IL-17+ CD4+ T-cell clones were expanded as described above and were tested for suppressive activity.
Suppression assay
In a suppression assay, CD4+ PBMCs were labeled with 5µM CFSE (Invitrogen) in PBS containing 0.1% BSA for 10 min at 37°C. Cells were washed twice in media containing 10% FBS. 5×104 CFSE-labeled CD4+ PBMCs were cultured with αCD3/αCD28 activation beads (Dynabeads Human T-activator CD3/CD28; Invitrogen) at a 1:1 ratio in the absence or presence of increasing amounts of FoxP3+IL-17+ or IL-17+ CD4+ T-cell clones (at the indicated ratios). The proliferation of responder T cells (PBMCs) assessed by CFSE dilution was analyzed by flow cytometry on day 4. The percentage of suppression was calculated based on an equation comparing the percentage of proliferating cells in medium alone to the percentage of cells proliferating in the presence of regulatory cells.
Statistical analysis
Groups were compared with Prism software (GraphPad) using the unpaired or paired Student’s t test and Kruskal-Wallis test. All P values of 0.05 or less were considered significant.
RESULTS
Ex vivo identification of IL-17-producing FoxP3+ regulatory CD4+ T cells in inflamed Crohn’s disease (CD) tissues
To study the contribution, phenotype and function of Th17 and Treg cells during normal and inflammatory mucosal immune responses, IL-17 production and FoxP3 expression were assessed by flow cytometry on CD4+ T cells freshly isolated from the lamina propria (LP) of normal and disease-affected areas in IBD patients, after their stimulation with PMA and ionomycin. As a control, CD4+ T cells derived from the apparently healthy intestine of individuals who underwent colectomy because of colon carcinoma were assessed. In accordance with previous reports 1, we found that the proportion of IL-17-producing CD4+ T cells was significantly higher in disease-affected bowel from CD patients (8.6%±4.5%) than from patients with ulcerative colitis (UC) (data not shown) or in the apparently healthy intestine of subjects with colon carcinoma (2.3%±0.7%; Supplementary Figure 1A). The expression of FoxP3 was also slightly increased in CD-derived LP CD4+ T cells (22%±6.9%) compared to UC patients (data not shown) and normal controls (18.5%±7.2%; Supplementary Figure 1B). Notably, during the course of defining the repertoire of Tregs in normal and inflamed intestines, we identified a distinct population of FoxP3+ IL-17-co-expressing CD4+ T cells in the lamina propria of CD patients (3.6%±0.52%; Figure 1A, upper panel), but not in UC patients or healthy controls (Figure 1A, middle and lower panels, respectively). Intriguingly, these FoxP3+IL-17-producing cells were virtually undetectable in the peripheral blood of CD and UC patients, as well as in healthy controls (Supplementary Figure 2).
Figure 1.
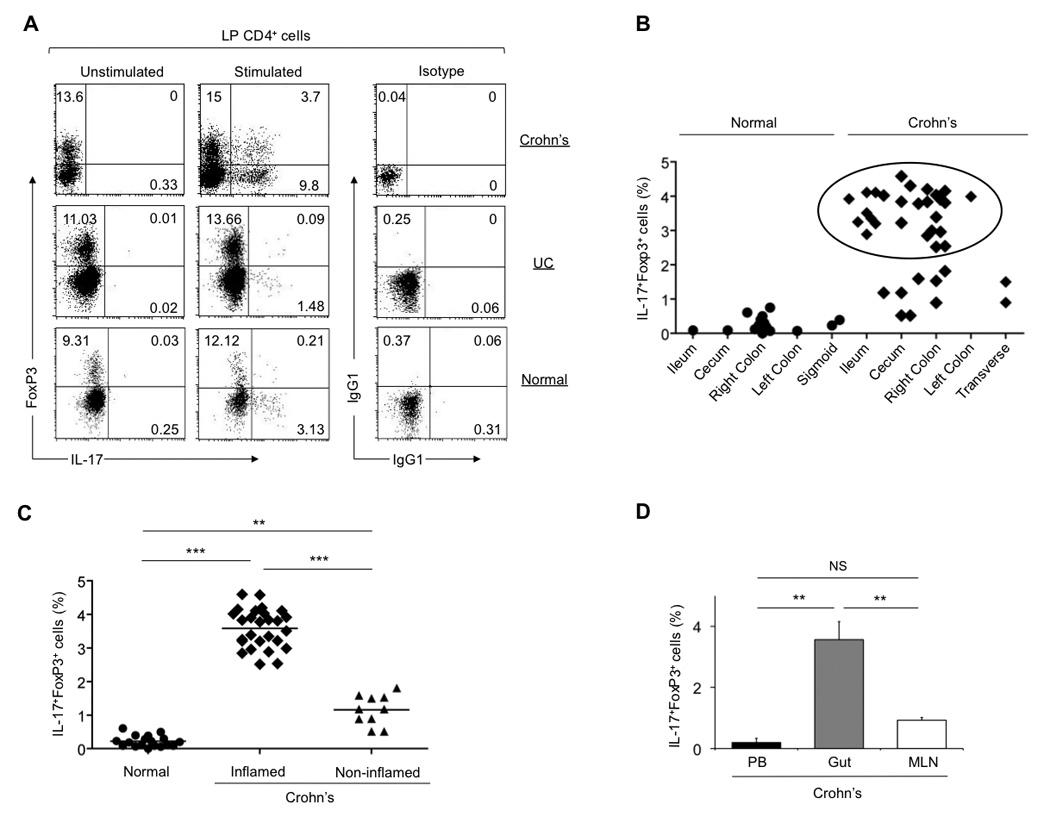
A distinct population of FoxP3+ IL-17-producing CD4+ T cells is present in the inflamed intestinal mucosa of CD patients, but not UC patients or healthy controls. (A) Flow cytometry analyzing the expression of FoxP3 and IL-17 by lamina propria (LP) CD4+ T lymphocytes freshly derived from disease-affected and apparently healthy gut areas of IBD patients and healthy individuals, respectively. Numbers in the quadrants indicate the percent of cells in each. Data are representative of at least twenty independent experiments. (B) Flow cytometric analysis of the distribution of FoxP3+IL17+ CD4+ T cells within the bowel in CD patients and healthy controls. The frequencies of FoxP3+ IL17-producing T cells derived from inflamed CD tissues are indicated in the circle. (C) Summary figure: flow cytometric analysis of the frequencies of FoxP3+IL17+ CD4+ T cells in inflamed, slightly- or non-inflamed gut areas of CD patients and healthy gut areas of normal controls. Means (small horizontal bars): healthy controls (n=18), 0.3 (s.d., 0.2); inflamed gut areas from CD patients (n=27), 3.6 (s.d., 0.52); slightly or non-inflamed gut areas from CD patients (n=10), 1.2 (s.d., 0.32). **, P≤0.002. ***, P≤0.0001. Each symbol represents the percent of cells co-expressing IL-17 and FoxP3 for a single donor (B, C). (D) Comparative assessment of FoxP3+IL17+ cell frequencies in the peripheral blood (PB), inflamed gut and gut-draining mesenteric lymph node (MLN) compartments derived from the same CD patient (error bars, s.d.; n=10). **, P≤0.001. Intracellular staining for IL-17 production and FoxP3 expression was performed on sorted LP CD4+ T cells 4h post stimulation with PMA/ionomycin (A, B, C, D).
Further analysis of the distribution of FoxP3+IL17+ double expressing cells within different segments of the CD intestine revealed no preference for distinct sites in the bowel (Figure 1B). Notably, FoxP3+IL-17-producing cells were found in higher numbers when derived from inflamed CD mucosal tissues (Figure 1B, shown in circle). To further probe the potential link between intestinal inflammation and the ability of CD FoxP3+ Tregs to produce IL-17, we looked for the presence of FoxP3+ IL-17-producing cells in inflamed and slightly or non-inflamed gut areas derived from the same CD patients. Intriguingly, we found that the proportion of FoxP3+ IL-17-producing cells was significantly lower in slightly or non-inflamed tissues compared to their inflamed counterparts (1.2%±0.32% and 3.6%±0.52%, respectively; Figure 1C and Supplementary Figure 3).
In order to determine whether FoxP3+ IL-17-producing T cells are present in both effector and inductive sites, we examined the expression of IL-17 and FoxP3 in the intestine, PB and MLN derived from the same CD patients. A significant fraction of FoxP3+IL-17+ cells was present in the lamina propria, whereas this population was absent in the MLN and PB (Figure 1D).
FoxP3+ IL-17-producing CD-derived LP CD4+ T cells exhibit phenotypic characteristics of both conventional Th17 and Treg cells
To further characterize FoxP3+ IL-17-producing LP CD4+ T cells identified in disease-affected gut areas of CD patients, we assessed the expression of surface markers known to be associated with either Th17 or Treg cells, and tested their cytokine producing capacity. We found that a vast majority of FoxP3+ IL-17-producing LP CD4+ T cells expressed CCR6 (95%±1.3%; Figure 2A), a receptor that mediates homing to skin and mucosal tissues 28 and has recently been shown to be expressed by Th17 cells 1, 7. LP FoxP3+ IL-17-producing cells also expressed high levels of the integrin α4β7 and CD103 (96%±1.5% and 22.4%±1.8%, respectively; Figure 2A), suggesting that these cells are imprinted for gut homing. Furthermore, FoxP3+ IL-17-producing LP CD4+ T cells shared some additional phenotypic features of conventional Th17 cells, expressing high levels of the Th17 lineage-specific transcriptional factor RORγt, as well as CD161, purportedly a human Th17 cell marker 29 (77.8%±10.5% and 62%±1.1%, respectively; Figure 2B). However, unlike conventional Th17 but similar to Treg cells, FoxP3+ IL-17-producing cells expressed high levels of CD101 and low levels of CD127 (24.7%±3.8% and 1.3%±0.1%, respectively; Figure 2B).
Figure 2.
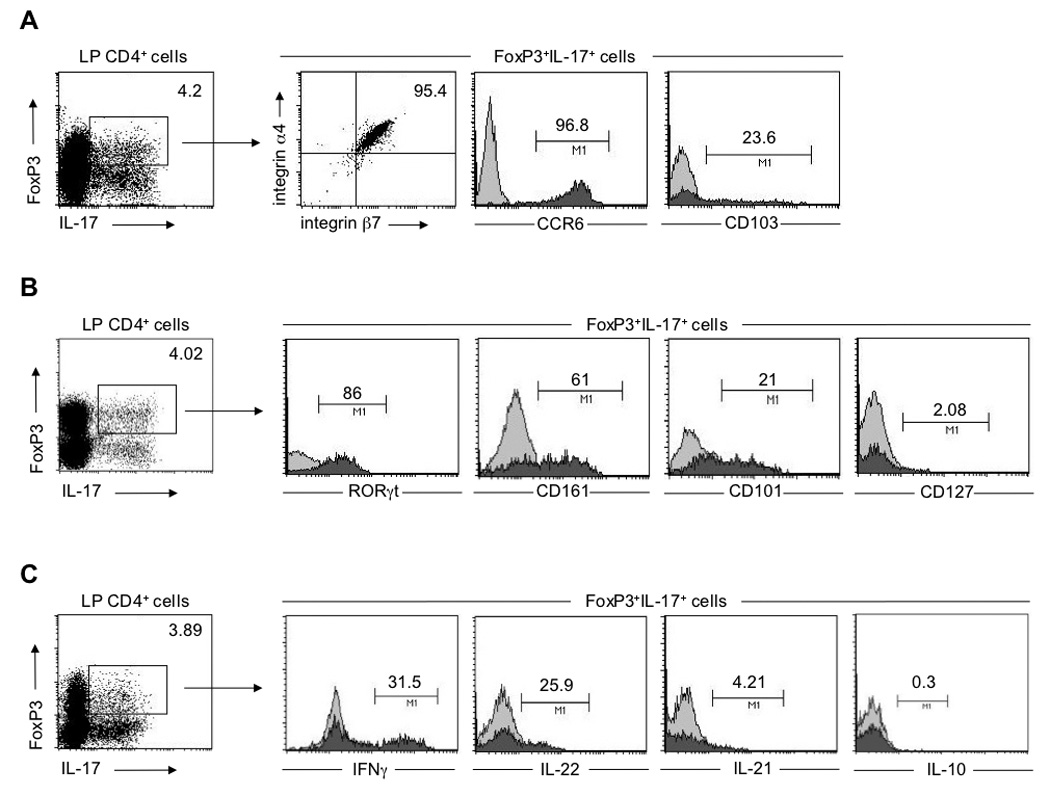
CD-derived FoxP3+ IL-17-producing CD4+ T cells display phenotypic similarities to both Th17 and Treg cells. (A, B, C) Surface and intracellular staining of CD-derived LP CD4+ T cells 4h post stimulation with PMA/ionomycin. (A) Surface expression of integrin α4β7, CCR6 and CD103 by LP FoxP3+IL-17+ cells. (B) Expression of RORγt, CD161, CD101 and CD127 by LP FoxP3+IL-17+ cells (C) Cytokine secretion profile of LP FoxP3+IL-17+cells. (A, B, C) LP CD4+ T cells were co-stained with anti-IL-17 and anti-FoxP3. Gating on FoxP3+IL-17+ cells: filled histograms, specific staining (antibodies to markers below the plots); open histograms, isotype-matched control antibodies. Numbers adjacent to outlined areas and above lines indicate the percent of cells in the gate. Data are representative of at least seven independent experiments.
The Th17 phenotype was also reflected in the cytokine production pattern of IL-17-producing FoxP3+ Treg cells. Similar to conventional Th17 cells, FoxP3+ IL-17-producing cells also produced IL-22 and to a lesser extent IL-21 (28.5%±2.6% and 4.8%±0.8%, respectively), but not IL-10 (0.4%±0.1%; Figure 2C). Moreover, CD-derived LP FoxP3+IL-17+ Treg cells also acquired the capacity to produce significant amounts of another effector cytokine, IFN-γ (38%±6.4%; Figure 2C), and to express high levels of T-bet, but not GATA 3 or IL-4 (Supplementary Figure 4). In addition, FoxP3+ IL-17-producing cells did not express granzyme B, perforin or CTLA4 (Supplementary Figure 5). These results collectively raise the possibility that, under the appropriate inflammatory environment, a significant proportion of Treg cells may acquire effector properties that could, in turn, further contribute to the pathological process.
Similar TCR β chain variable region usage by FoxP3+ IL-17-producing and FoxP3+ LP CD4+ T cells suggest that these cells share a common ancestry
Analysis of the TCR repertoire revealed that CD-derived FoxP3+IL-17+, similar to FoxP3+, predominantly expressed Vβ2, Vβ5.1 and Vβ9 (Figure 3, middle and right panels, respectively; Supplementary Figure 6). Notably, the TCR Vβ repertoire of FoxP3+IL-17+ and FoxP3+ cells was distinct from that used by the Th17 cells derived from the same CD patients (Figure 3 and Supplementary Figure 6). Altogether, these results suggest that FoxP3+ IL-17-producing cells may arise from FoxP3+ Treg cells exposed to the unique environment present in CD tissues.
Figure 3.
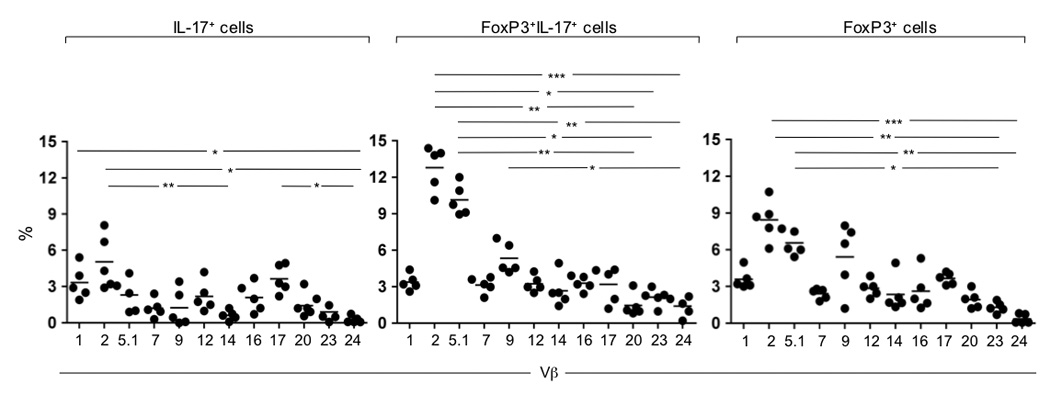
TCR repertoire analysis of CD-derived FoxP3+ IL-17-producing LP CD4+ T cells. Sorted LP CD4+ T cells were stained with anti-IL-17 and anti-FoxP3 4h post stimulation with PMA/ionomycin. The TCR β-chain variable region usage of IL-17+, FoxP3+IL-17+ and FoxP3+ T cell subsets was defined by flow cytometry using specific anti-Vβ TCR antibodies. Mean values are shown for six different CD donors. *, P≤0.05. **, P≤0.01. ***, P≤0.001
FoxP3+ IL-17-producing CD-derived T cell clones retain their in vitro suppressive function
To better investigate the functional features of IL-17-producing FoxP3+ Treg cells, LP CD4+ T cells derived from diseased areas of CD patients were expanded in vitro by stimulation for 6 days with αCD2/αCD3/αCD28 beads in the presence of rIL-2. Notably, under these conditions, in agreement with the aforementioned ex vivo findings, LP CD4+ T cells demonstrated the ability to produce IL-17 and express FoxP3 at comparable levels (data not shown). Expanded LP CD4+ T cells were further enriched for IL-17 and cloned by limiting dilution in the presence of allogeneic irradiated feeder cells and rIL-2. By this process FoxP3+IL-17+ and IL-17+ T-cell clones were generated and their in vitro suppressor activity was assessed. Intriguingly, FoxP3+IL-17+ T-cell clones strongly inhibited the proliferation of responder CD4+ T cells activated by αCD3/αCD28 (54%±0.5% suppression), whereas IL-17+ T-cell clones did not exhibit suppressive properties (Figure 4A). Furthermore, the suppression mediated by FoxP3+IL-17+ T-cell clones was dose-dependent (Figure 4B).
Figure 4.
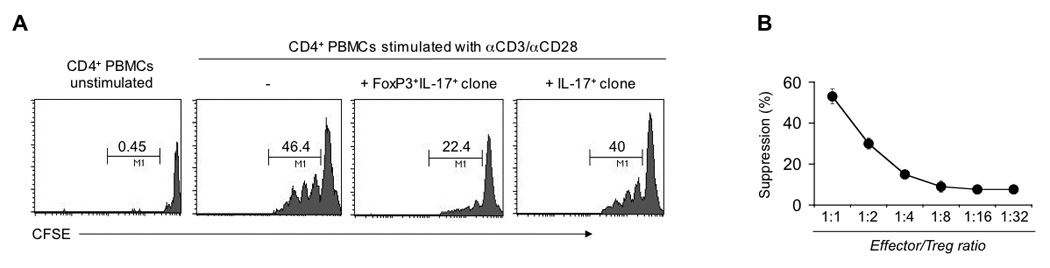
CD-derived FoxP3+IL-17+ T cell clones suppress proliferation of responder CD4+ T cells. (A) In vitro suppression assay with CFSE-labeled PB-derived CD4+ T cells cultured with αCD3/αCD28 beads in the absence or presence of conventional CD4+CD25+ Tregs, CD-derived FoxP3+IL-17+ or IL-17+ T cell clones at effector/Treg ratios of 1:1. The proliferation of responder CD4+ T cells accompanied by CFSE dilution was analyzed by flow cytometry on day 4. Numbers above the lines indicate percent proliferating CD4+ T cells. (B) Suppression of responder CD4+ T cell proliferation in the presence of CD-derived FoxP3+IL-17+ T cell clones incubated at the indicated effector/Treg ratios. Data are representative of eight (A) or three (B) independent experiments.
TGF-β promotes the induction of the FoxP3+IL-17+ population in LP CD4+ T cells derived from UC patients
We next sought to determine whether the FoxP3+IL-17+ T cell subset could be skewed either towards a Th17 or Treg cell phenotype in vitro. LP CD4+ T cells derived from IBD patients and normal controls were stimulated for 6 days with αCD2/αCD3/αCD28 beads in the presence of blocking antibodies against IFN-γ and IL-4 and TGF-β alone or with different combinations of IL-6, IL-1β and IL-23 and, the cytokines that have been shown to promote Treg and human Th17 cell differentiation, respectively 4, 11, 30–34. We allowed the cells to proliferate and analyzed their capacity to express FoxP3 and produce IL-17 by intracellular staining. Interestingly, we observed no enhancing effect of the addition of any combination of cytokines to the cultures of CD and normal LP CD4+ T cells (Figure 5A, upper and lower panels, respectively). In contrast, the addition of TGF-β resulted in a striking induction of a FoxP3+IL-17+ double positive population in UC-derived LP CD4+ T cells (Figure 5A, middle panel). The induction of this population was blocked by addition of neutralizing anti-TGF-β antibody to the cultures (Figure 5B). To address the origin of FoxP3+IL-17+ cells induced by TGF-β, we sorted UC-derived LP CD4+ T cells into CD4+ IL-17− and CD4+IL-17+ fractions and assessed the effect of TGF-β alone or in combination with IL-6, IL-1β and IL-23 on the development of FoxP3+IL-17+ cells in vitro (Supplementary Figure 7). We were unable to analyze the CD4+ IL-17+ fraction due to the small number of IL-17-producing cells recovered from UC. However, the addition of TGF-β to CD4+ IL-17- fraction resulted in the generation of FoxP3+IL-17+ double positive population from FoxP3+ cells. These results indicate that TGF-β is an essential factor in the development of FoxP3+IL-17+ cells, and that this population originates from FoxP3+ precursor cells in a distinct inflammatory microenvironment. At the same time, the ability of TGF-β to activate FoxP3+ IL-17-producing cells in UC, but not normal LP CD4+ T cells provides additional evidence for the requirement of selective microenvironmental cues, constitutively present in CD tissues, to allow for their growth and differentiation.
Figure 5.
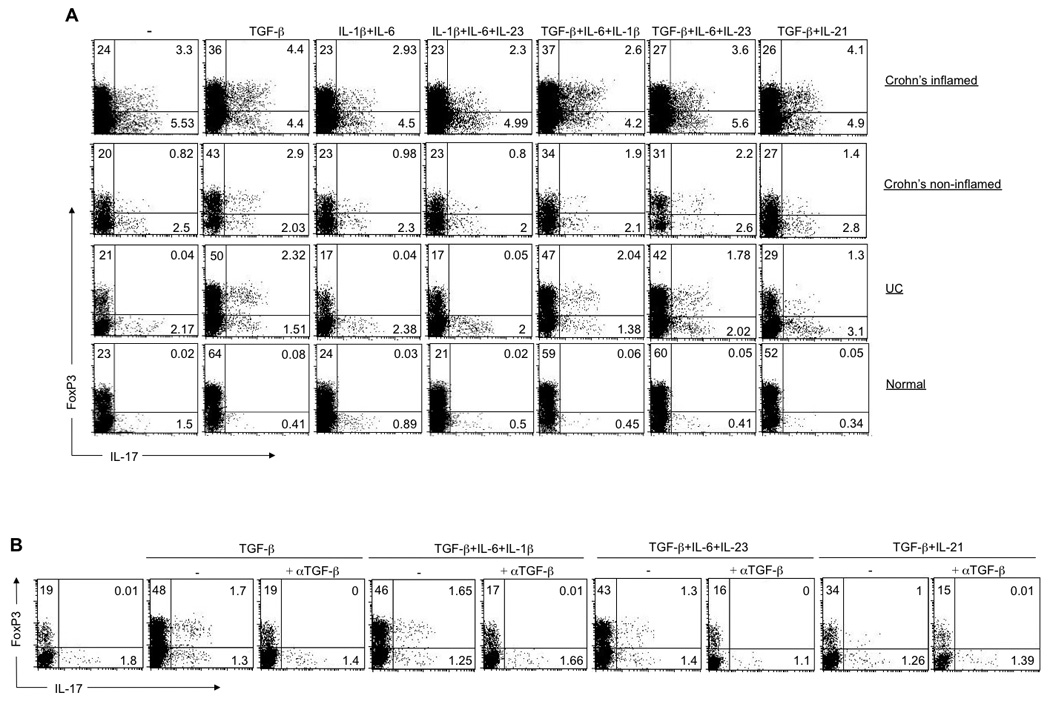
TGF-β is necessary and sufficient for the induction of FoxP3+IL-17+ population in UC-derived LP CD4+ T cells. (A) Flow cytometry analyzing the expression of IL-17 and FoxP3 by LP CD4+ T cells derived from IBD patients and normal controls primed with αCD2/αCD3/αCD28 coated beads without cytokines or in the presence of various combinations of IL-1β, IL-6, IL-21, IL-23 and TGF-β (above the graph), with IL-2 added on day 3 and analyzed on day 6 of culture. Numbers in the quadrants indicate the percent of cells in each. (B) Flow cytometry analyzing the expression of IL-17 and FoxP3 by UC-derived LP CD4+ T cells primed with αCD2/αCD3/αCD28 coated beads in the absence or presence of various combinations of indicated cytokines and neutralizing anti-TGF-β (above the graph), with IL-2 added on day 3 and analyzed on day 6. Data are representative of four (A) and three (B) independent experiments.
DISSCUSSION
Higher IL-17 expression and greater numbers of FoxP3+ Tregs in the gut during intestinal inflammation led us to investigate the interplay between gut-resident Th17 and Treg cells in a disease setting, and its contribution, if any, to the pathogenesis of IBD. Here we report the identification of human FoxP3+ IL-17-co-expressing Treg cells in the intestinal lamina propria of CD patients, but not in UC patients or healthy controls, which indicates that these cells are associated with, or arise as a consequence of, CD pathology. Furthermore, notably, tissue resident FoxP3+IL-17+ cells also exhibited the ability to produce large quantities of IFNγ. These cells shared phenotypic characteristics of Th17 cells with secretion of IL-17, IL-22, IL-21, while expressing high levels of CCR6, CD161 and RORγt. However, unlike conventional Th17 cells and similar to FoxP3+ Tregs, FoxP3+IL-17+ cells express high levels of CD101 and low levels of CD127 and functionally retain their suppressive activity in in vitro co-culture systems. Analysis of the TCR repertoire further revealed a similar TCR β chain variable region usage by FoxP3+IL-17+ and FoxP3+ CD4+ T cells suggesting that FoxP3+ IL-17-producing cells may arise from FoxP3+ Tregs when exposed to the unique signals present in inflamed CD tissues.
In light of recent studies suggesting that there is a great deal of plasticity in the differentiation pathways of Treg and Th17 cells 14–16, 19, our data imply that FoxP3+ IL-17-producing Tregs sit at the crossroads between Treg and Th17 cells and that further commitment to either lineage results from microenvironmental cues present in the tissues. Interestingly, a similar cell population was previously observed in another mucosal site associated with chronic inflammation, the tonsil 19, supporting the concept that FoxP3+IL-17+ cells are generated at mucosal sites during inflammation driven by specific microenvironmental cues. Our findings also raise the possibility that in the presence of specific inflammatory mediators, present in CD tissues, gut-resident Tregs may also convert to inflammatory-cytokine-producing cells and thus potentially contribute to the pathogenesis of the disease. Indeed, higher frequencies of FoxP3+ IL-17-producing cells were observed in the inflamed gut mucosa of CD patients, but not in UC patients, providing further evidence for selective microenvironmental cues, constitutively present in CD tissues and required for the growth and differentiation of FoxP3+IL-17+ cells in vivo. One strong candidate for this factor may be TGF-β, which is essential for the development of both Th17 and Treg cells 11–13, 33. The actual tissue concentration of this cytokine may dictate the path of cellular differentiation. Notably, when added alone or in combination with various cytokines that have been shown to be sufficient to induce human Th17 and Treg cell differentiation, TGF-β potently promoted the induction of FoxP3+IL-17+ cells in UC but not in normal LP CD4+ T cells. Moreover, TGF-β induced the generation of FoxP3+IL-17+ population from FoxP3+ cells within the UC-derived LP CD4+ IL-17− fraction, suggesting that FoxP3+IL-17+ cells originate from FoxP3+ precursor cells. Overall, these results are not unexpected, given that elevated expression levels of TGF-β, as well as of IL-6, have been detected in the inflamed intestinal mucosa of patients with CD, but not in UC 35, 36. It is conceivable that these proinflammatory cytokines might promote the conversion of Treg cells into inflammatory effector T cells, while retaining their suppressive properties. In this manner, they could contribute to the inflammatory environment seen in CD. However, since we were unable to generate FoxP3+IL-17+ cells from normal LP or PB CD4+ T cells derived from any source despite the presence of a combination of cytokines known to promote Th17 and Treg cell differentiation, additional factors required for the induction of these cells must be present in vivo. Intriguingly, we were unable to further expand FoxP3+IL-17+ cell population in LP CD4+ T cells derived from CD tissues.
Our data clearly point towards a unique population of cells that sit at the crossroads of Th17 and Treg (and possibly Th1) cell differentiation. Given specific microenvironmental cues, the commitment toward a given lineage likely dictates the balance between regulation and inflammation. Further elucidation of specific intestinal cues that influence the differentiation of FoxP3+IL-17+ Treg subset promises to provide a better understanding of immune responses in the intestine.
Supplementary Material
ACKNOWLEDGMENTS
Grant support: This work was supported by the NIH grants (AI044236, AI084952, DK072201, DK086605).
We thank S. Lira and J. Magarian Blander for critical reading of the manuscript and comments.
Abbreviations
- CD
Crohn’s disease
- CFSE
carboxyfluoroscein succinimidyl ester
- IBD
inflammatory bowel disease
- IFN
interferon
- IL
interleukin
- LP
lamina propria
- LPL
lamina propria lymphocytes
- MLN
mesenteric lymph node
- PB
peripheral blood
- PMA
phorbol 12-myristate 13-acetate
- TCR
T cell receptor
- Th17
T helper interleukin-17-producing cell
- Treg
regulatory T cell
- UC
ulcerative colitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflict of interests to disclose.
Author contributions:
Z.H. and L.M. designed experiments; Z.H. did all experiments; J.T. assisted in the isolation of lamina propria lymphocytes from surgical specimens; D.L. provided input into experimental design; and Z.H. and L.M. wrote the manuscript.
REFERENCES
- 1.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 3.Aujila SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19:377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 5.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 6.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-21-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang W, Kools JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. FoxP3+ Treg cell functioning in suppression of effector T cells is essential in maintenance of peripheral tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, Rudensky AY. FoxP3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 10.Wan Y, et al. TGF-β and regulatory T cell in immunity and autoimmunity. J Clin Immunol. 2008;28:647–659. doi: 10.1007/s10875-008-9251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor-β induces development of the Th17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 12.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-β1 maintains supressor function and FoxP3 expression in CD4+CD25+ regulatory T cells. J Epx Med. 2005;201 doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcriptional factor FoxP3. J Epx Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang XO, Nurieva R, Martinez GJ, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cells programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou L, Lopes JE, Chong MM, et al. TGF-β-induced FoxP3 inhibits Th17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koenen HJ, Smeets RL, Vink PM, et al. Human CD25high FoxP3+ regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 17.Eberl G, Marmon S, Sunshine MJ, et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 18.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor ROR-gammat dirrects the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Voo KS, Wang YH, Santori FR, et al. Identification of IL-17-producing FoxP3+ regulatory T cells in humans. Proc Natl Acad Sci. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujino S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen OH, Kirman I, Rüdiger N, et al. Upregulation of interleukin-12 and -17 in active inflammatory bowel disease. Scand J Gastroenterol. 2003;38:180–185. doi: 10.1080/00365520310000672. [DOI] [PubMed] [Google Scholar]
- 22.Seiderer J, Elben I, Diegelmann J, et al. Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): upregulated colonic IL-17F expression in active Crohn's disease and analysis of the IL-17F p.His161Arg polymorphism in IBD. Inflamm Bowel Dis. 2008;14:437–445. doi: 10.1002/ibd.20339. [DOI] [PubMed] [Google Scholar]
- 23.Duerr RH, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubinsky MC, Wang D, Picornell Y, et al. IL-23 receptor (IL-23R) gene protects against pediatric Crohn's disease. Inflamm Bowel Dis. 2007;13:511–515. doi: 10.1002/ibd.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+CD25high T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 26.Saruta M, Yu QT, Fleshner PR, et al. Characterization of FoxP3+ CD4+ regulatory T cells in Crohn's disease. Clinical Immunology. 2007;125:281–290. doi: 10.1016/j.clim.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Makita S, Kanai T, Oshima S, et al. CD4+CD25+ bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173:3119–3130. doi: 10.4049/jimmunol.173.5.3119. [DOI] [PubMed] [Google Scholar]
- 28.Liao Fea. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsivenedd to macrophage inflammatory protein 3 a. J Immunol. 1999;162:186–194. [PubMed] [Google Scholar]
- 29.Kleinschek MA, Boniface K, Sadekova S, et al. Circulating and gut-resistant human Th17 cells express CD161 and promote intestinal inflammation. J Epx Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)-17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Epx Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 33.Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Zhou L, Ivanov II, Spolski R, et al. IL-6 program T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 35.Del Zotto B, Mumolo G, Pronio AM, et al. TGF-β1 production in inflammatory bowel disease:differing production patterns in Crohn's disease and ulcerative colitis. Clin Exp Immunol. 2003;134:120–126. doi: 10.1046/j.1365-2249.2003.02250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.León AJ, Gómez E, Garrote JA, et al. High levels of proinflammatory cytokines, but not markers of tissue injury, in unaffected intestinal areas from patients with IBD. Mediators Inflamm. 2009;2009:580450. doi: 10.1155/2009/580450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


