Abstract
Background & Aims
Liver X receptors (LXRs) are lipid-activated nuclear receptors with important roles in cholesterol transport, lipogenesis, and anti-inflammatory signaling. Hepatic stellate cells (HSCs) activate during chronic liver injury and mediate the fibrotic response. These cells are also major repositories for lipids, but the role of lipid metabolism during stellate cell activation remains unclear. Here we show that LXR signaling is an important determinant of stellate cell activation and susceptibility to fibrotic liver disease.
Methods
Immortalized and primary stellate cells purified from mice were treated with highly specific LXR ligands. Carbon tetrachloride (CCl4) and methionine choline deficiency (MCD) were used as chronic liver injury models. Reciprocal bone marrow transplants were performed to test the importance of hematopoietically-derived cells to the fibrotic response.
Results
LXR ligands suppressed markers of fibrosis and stellate cell activation in primary mouse stellate cells. Lxrαβ −/− stellate cells produce increased levels of inflammatory mediators and conditioned media from Lxrαβ−/− cells increases the fibrogenic program of wild-type cells. Furthermore, Lxrαβ−/− stellate cells exhibit altered lipid morphology and increased expression of fibrogenic genes, suggesting they are primed for activation. In vivo, Lxrαβ−/− mice have marked susceptibility to fibrosis in two injury models. Bone marrow transplants point to altered stellate cell function, rather than hematopoietic cell inflammation, as the primary basis for the Lxrαβ−/− phenotype.
Conclusions
These results reveal an unexpected role for LXR signaling and lipid metabolism in the modulation of hepatic stellate cell function.
Keywords: Nuclear receptors, LXRs, hepatic stellate cells, liver fibrosis
INTRODUCTION
Hepatic fibrosis is the reversible accumulation of extracellular matrix (ECM) in the liver following chronic injury. Unchecked fibrosis can progress to cirrhosis, an end-stage lesion with significant morbidity and mortality. Fibrosis is mediated primarily by hepatic stellate cells, resident mesenchymal cells located in the subendothelial space of Disse1, 2. These cells were formerly known as hepatic ‘lipocytes’3 because of characteristic lipid droplets containing retinyl esters (vitamin A), triglycerides, and cholesterol esters4, 5. The importance of stellate cells to liver fibrosis is firmly established 6–8. Upon liver injury, stellate cells undergo a major cellular transformation—“activation”—during which they lose their lipid content, proliferate, and express a fibrogenic program1. Although there are no known specific markers for quiescent stellate cells, the production of collagen α1(I) and α-smooth muscle actin are specific markers of activated stellate cells9. It has long been appreciated that stellate cells are the major storehouse for vitamin A (retinol and retinyl esters), but it is unknown whether loss of lipid / retinoid is required for, or merely coincident with, activation1, 2, 4. Stellate cell activation is heavily influenced by inflammatory cues and interactions with multiple types of hematopoietically-derived cells, including Kupffer, NKT, B and T cells7, 8, 10–13.
LXRs sit at the fulcrum of lipid metabolism and inflammation. These nuclear receptors function as whole body cholesterol sensors, promoting reverse cholesterol transport and cholesterol excretion into bile 14. They are key regulators of de novo lipogenesis in the liver and Lxrαβ −/− mice show reduced expression of lipogenic genes, including sterol regulatory element binding protein 1c (Srebp-1c) and stearoyl-CoA desaturase 1 (Scd1)15, 16. LXRs are also negative regulators of inflammatory gene expression in macrophages17. In response to inflammatory stimuli, LXR signaling inhibits the production of several inflammatory mediators, including interleukin 6 (IL-6) and monocyte chemotattractant protein (MCP-1). The ability of LXRs to modulate immune responses is also apparent in vivo. LXR ligands attenuate inflammation in mouse models of atherosclerosis and contact dermatitis, and limit the proliferation of antigen-stimulated T cells 18–20.
Previous work has implicated hepatocyte LXR expression in protection from cholesterol and bile acid toxicity 21, but a role for LXRs in stellate cell activation or fibrogenesis has not been elucidated. Here we examine the role of LXRs in lipid metabolic and inflammatory signaling in stellate cells and find that Lxrαβ−/− mice are more susceptible to chronic fibrosis in two models of liver injury. There are intrinsic differences in the fibrogenic and inflammatory programs of purified stellate cells from Lxrαβ−/− mice. Surprisingly, hematopoietically-derived cells do not influence this susceptibility to fibrosis as determined by reciprocal bone marrow transplantation studies. Our data show that LXR signaling influences the activation of hepatic stellate cells and contributes to the pathogenesis of liver fibrosis. We suggest that modulation of LXR or lipid metabolism in these cells may have therapeutic potential for the treatment of fibrosing diseases of the liver.
RESULTS
LXRs have reciprocal anti-inflammatory and lipid metabolic effects in activated stellate cells
To characterize nuclear receptor expression in hepatic stellate cells, we performed quantitative real-time PCR on two immortalized, fully activated stellate cell lines: rat HSC-T6 cells22, and human LX-2 cells23. These lines are supposed to model stellate cells in their fully activated, myofibroblast-like state. Supplementary Table 1 lists the relative abundance of RXR heterodimers found in LX-2 cells, with LXRβ, vitamin D, thyroid, retinoic acid (RARα) and retinoid X (RXRα) receptors being predominant. LXRα, PPARα, and PPARδ were weakly expressed. We observed no significant expression of PPARγ or FXR, and no appreciable induction of their specific target genes by selective agonists (Supplementary Figure 1A). By contrast, in primary stellate cells activating on plastic, the expression of LXRβ is robust and does not diminish over time (Supplementary Figure 1B). Our data are consistent with prior work suggesting that PPARγ expression diminishes with activation24, 25, but differ from work suggesting that FXR may play a role in stellate cell activation26.
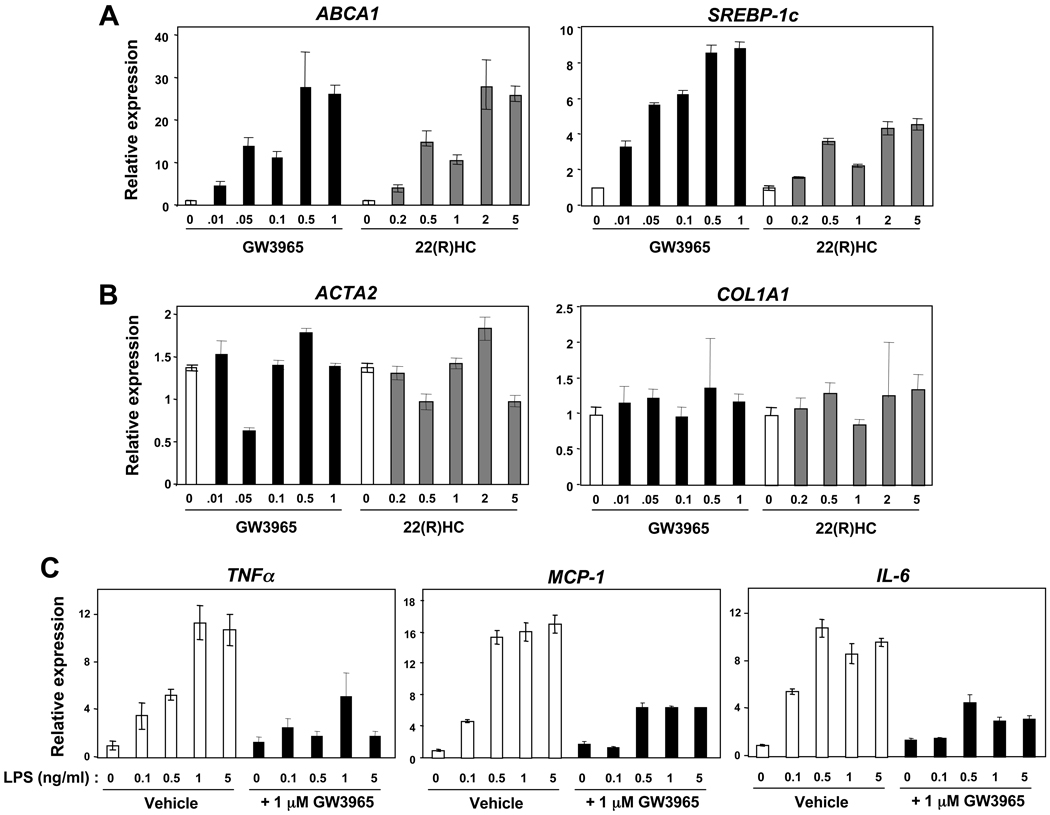
LXRs are functional in LX-2 cells, inducing the expression of canonical target genes (ABCA1, SREBP-1c) in a dose-responsive fashion with both synthetic (GW3965) and endogenous (22R-hydroxycholesterol) ligands (Figure 1A). Although ligands had no effect on the expression of collagen α1(I) (COL1AI) or α-smooth muscle actin (ACTA2) (Figure 1B) in immortalized cells, they had a marked inhibitory effect on the LPS-induced expression of TNFα, MCP-1, and IL-6 (Figure 1C). Similar results were obtained in the rat HSC-T6 cell line (data not shown). Unfortunately, there are no reliable antibodies available for the detection of murine LXRα or LXRβ. Nevertheless, these data establish that LXRs are abundant and functional in activated stellate cell lines. Although LXR agonists did not appear to affect typical fibrosis related genes in immortalized cells, we considered the possibility that immortalized cell lines might not adequately model the importance of lipid signaling during stellate cell activation. Primary stellate cells have robust expression of LXRs (Supplementary Figure 1B), so we focused on LXR signaling in freshly isolated stellate cells from mice or in vivo responses for all subsequent experiments.
Figure 1. Reciprocal lipogenic / anti-inflammatory action of LXRs in immortalized stellate cells.
Gene expression in human LX-2 stellate cells by qPCR. (A–B) LX-2 cells were cultured to near confluence and treated with increasing amounts of ligand for 12–18 hours: GW3965 (0 → 1 µM), 22R-hydroxycholesterol (0 → 5 µM). (C) LX-2 stellate cells were pretreated for 12 hours with 1 µM GW3965 or vehicle (DMSO), then exposed to LPS (0 → 5 ng/mL) for another 6 hours.
Primary stellate cells from LXR null mice exhibit altered lipid distribution and increased markers of activation
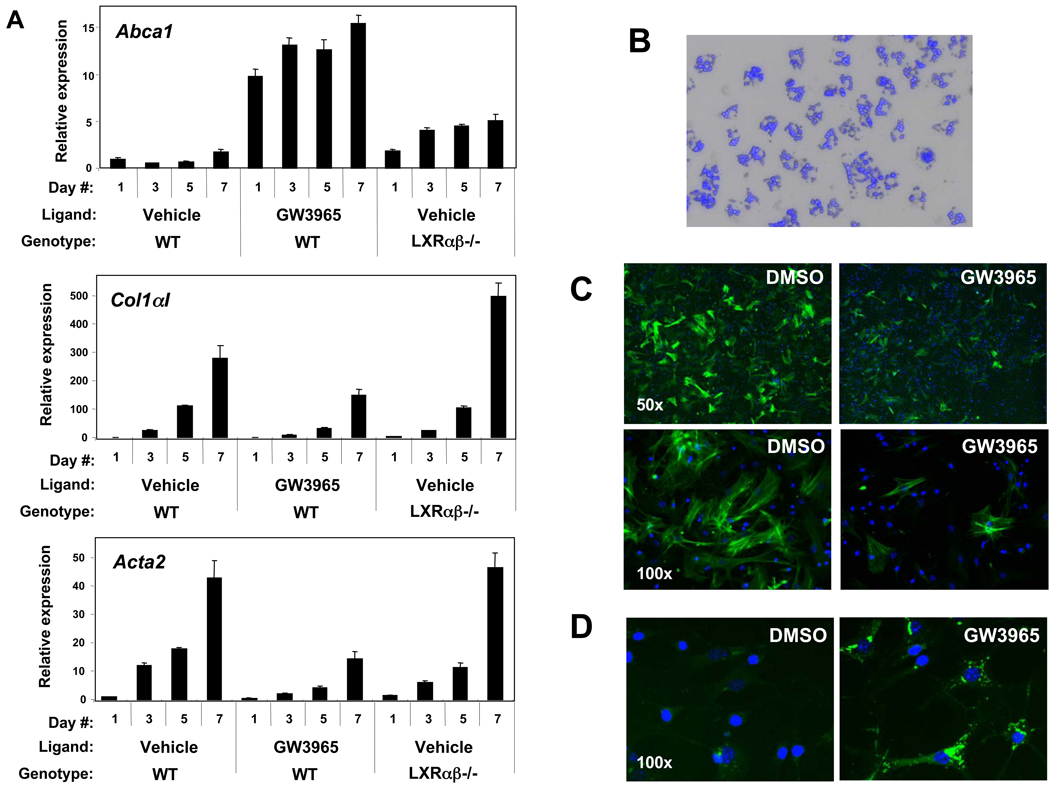
To test the role of LXR signaling in a primary culture model, we obtained stellate cells from mice by in situ sequential digestion with Pronase and collagenase6, 27, 28. When the non-parenchymal fraction was simply cultured on plastic in the presence of LXR ligand, collagen α1(I) and α-smooth muscle actin expression was suppressed in WT cells, while these transcripts were significantly increased in Lxrαβ−/− cells (Figure 2A). Increased basal expression of ABCA1 is an expected consequence of LXR deletion in macrophages and is likely due to the presence of macrophages in the preparation 29, 30. Ligand-treated cells appeared healthy throughout the course of treatment and showed no evidence of toxicity. The proliferation of the non-parenchymal fraction was also inhibited by LXR ligands in WT, but not Lxrαβ−/−, stellate cells (Supplementary Figure 2). These findings most likely reflect direct effects of LXRs in stellate cells, but it remained possible that secondary anti-inflammatory effects from contaminating Kupffer cells may have contributed.
Figure 2. LXR agonism suppresses myofibroblastic gene expression and promotes neutral lipid accumulation in stellate cells.
(A) Gene expression from hepatic non-parenchymal fractions of WT and Lxrαβ −/− mice treated for 7 days with 1 µM GW3965 or vehicle (DMSO). (B) Freshly isolated, highly purified primary stellate cells from WT mice are shown in phase contrast overlaid with autofluorescence (blue DAPI excitation) of the retinoid content in each cell, 200×. (C) WT stellate cells (day 5 of culture activation) stained with a FITC-conjugated, α-smooth muscle actin monoclonal antibody (green), 50× and 100×. (D) Neutral lipid staining by BODIPY (green) in WT primary stellate cells (day 5), incubated with 1 µM GW3965 or DMSO for 36 hours, 400×. Nuclei are stained with DAPI (C & D).
We therefore further purified murine stellate cells by ultracentrifugation over a discontinuous density gradient. This approach separates non-parenchymal cells based on their relative buoyancy, a property that is largely determined by the presence of lipid/retinoid droplets in stellate cells6, 27. The purity of these fractions was assessed by morphology, the absence of other cell types, gene expression and the characteristic autofluorescence of stellate cells that fades rapidly upon DAPI excitation (Figure 2B). During early culture activation, LXR ligands suppress α-smooth muscle actin protein in WT stellate cells (Figure 2C). In parallel, they promote the accumulation of neutral lipids as detected by BODIPY staining (Figure 2D), correlating with the increased lipogenic gene expression we observed in immortalized cells (Figure 1A).
LXR-deficient stellate cells exhibit increased fibrogenic and inflammatory capacity
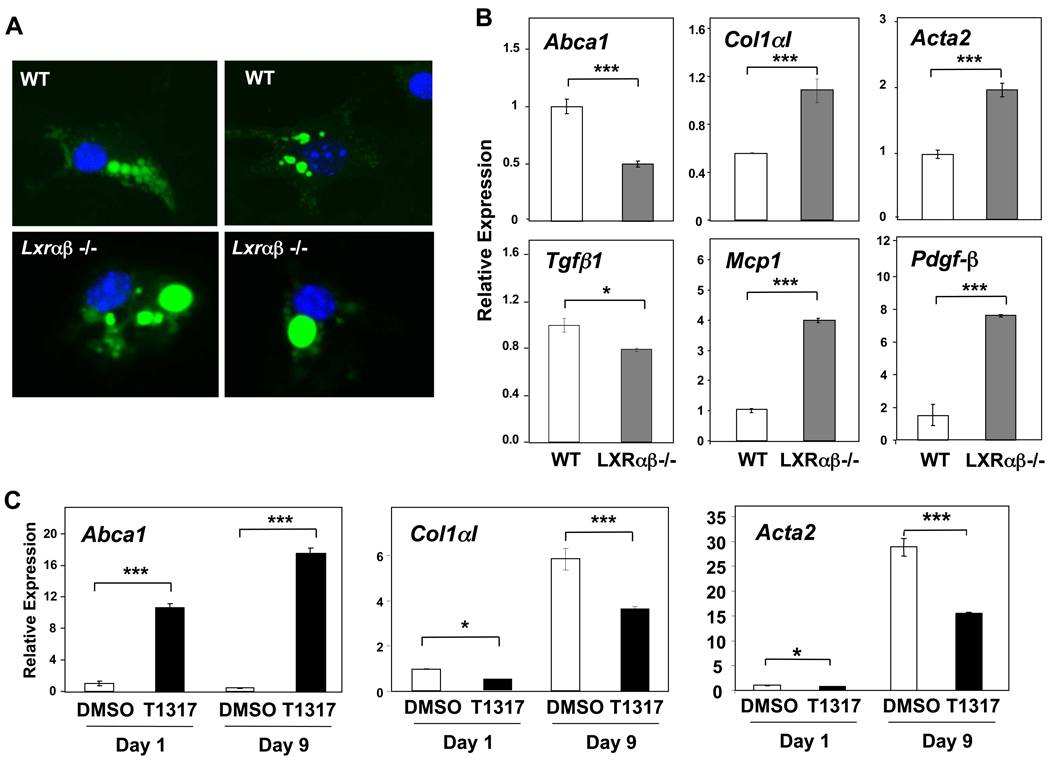
Surprisingly, we found that purified stellate cells from Lxrαβ−/− mice predominantly contain one large lipid droplet, a marked difference from WT stellate cells (Figure 3A). Additionally, Lxrαβ−/− stellate cells migrate slightly lower during ultracentrifugation, suggestive of increased mass. Purified Lxrαβ−/− stellate cells express more collagen α1(I) than WT cells, and have increased expression of several other activation-related genes: Pdgfβ, Acta2, and Mcp1 (Figure 3B). LXR agonism in primary WT stellate cells suppresses myofibroblastic genes (Col1aI and Acta2) while targets related to cholesterol transport (Abca1, Abcg1) and de novo lipogenesis (Srebp1c, Scd1) are induced (Figures 3C and Supplementary Figures 3,4). LXR stimulation negatively regulates Mcp1 and Il6 expression in primary stellate cells (Supplementary Figures 3,4), while deletion of LXRs increases inflammatory gene expression (Figure 3B). As expected, ligand regulation was lost in Lxrαβ−/− cells (data not shown). These results suggest a direct effect of LXR signaling on establishing the fibrogenic program in primary stellate cells, independently of the pro-fibrogenic cytokine, Tgfβ1, which was not increased in the knockout cells (Figure 3B and Supplementary Figure 4). Oxysterols appear to suppress Klf6 (Supplementary Figure 4), and growth-promoting isoforms of this factor have previously been shown to be upregulated in early culture activation of stellate cells1. Collectively, these observations implicate LXRs as physiologic suppressors of stellate cell activation that are important for maintaining quiescence through transrepression of an inflammatory cascade or modulation of lipid pathways.
Figure 3. LXR null stellate cells have marked alterations in lipid / retinoid distribution and increased fibrotic and inflammatory gene expression.
(A) High-power (630×) micrographs of BODIPY stained (green), purified stellate cells from WT and Lxrαβ −/− mice one day after isolation. Stellate cells from wild-type mice have characteristic multiple, small droplets while Lxrαβ −/− cells typically have a particularly large droplet, almost the same size as the nucleus (blue DAPI). Autofluorescence of retinoid overlaps entirely with the lipid droplets (not shown). (B) Gene expression in primary WT and Lxrαβ −/− stellate cells early in culture activation (day 3). (C) Gene expression in primary WT stellate cells treated continuously with either 1 µM T0901317 (abbreviated T1317) or vehicle (DMSO) throughout culture activation.
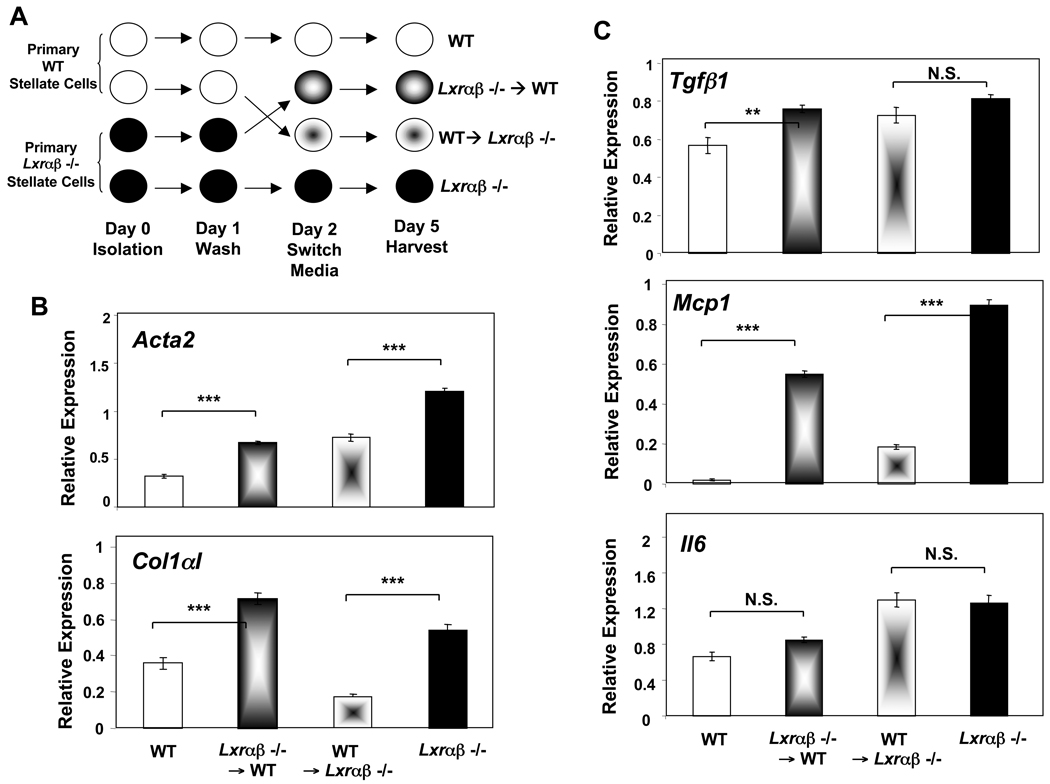
To further explore the functional differences between WT and Lxrαβ−/− stellate cells during culture activation, we purified stellate cells from each genotype and performed a media exchange assay (schematized in Figure 4A). We hypothesized that increased mediator production from LXR-deficient cells might alter the fibrogenic capacity of neighboring cells in a paracrine fashion. The conditioned media from Lxrαβ−/− stellate cells increases expression of myofibroblastic (Acta2, Col1aI) and inflammatory genes (Mcp1) in WT cells (Figure 4B, 4C). Conversely, the conditioned media from WT stellate cells dampened the expression of these genes in Lxrαβ−/− cells. Together, these data show that the increased fibrogenic capacity of Lxrαβ−/− cells results, at least in part, from the induction of secreted inflammatory mediators.
Figure 4. Conditioned media from LXR null stellate cells increases the fibrotic and inflammatory program in WT cells.
Purified, primary stellate cells from each genotype were isolated in parallel (cf. Methods) and plated on plastic for spontaneous culture activation. (A) Experimental design. (B,C) Gene expression by qPCR from day 5 culture activated cells showing myofibroblastic (B) and inflammatory (C) genes.
LXR null mice are susceptible to acute and chronic liver injury
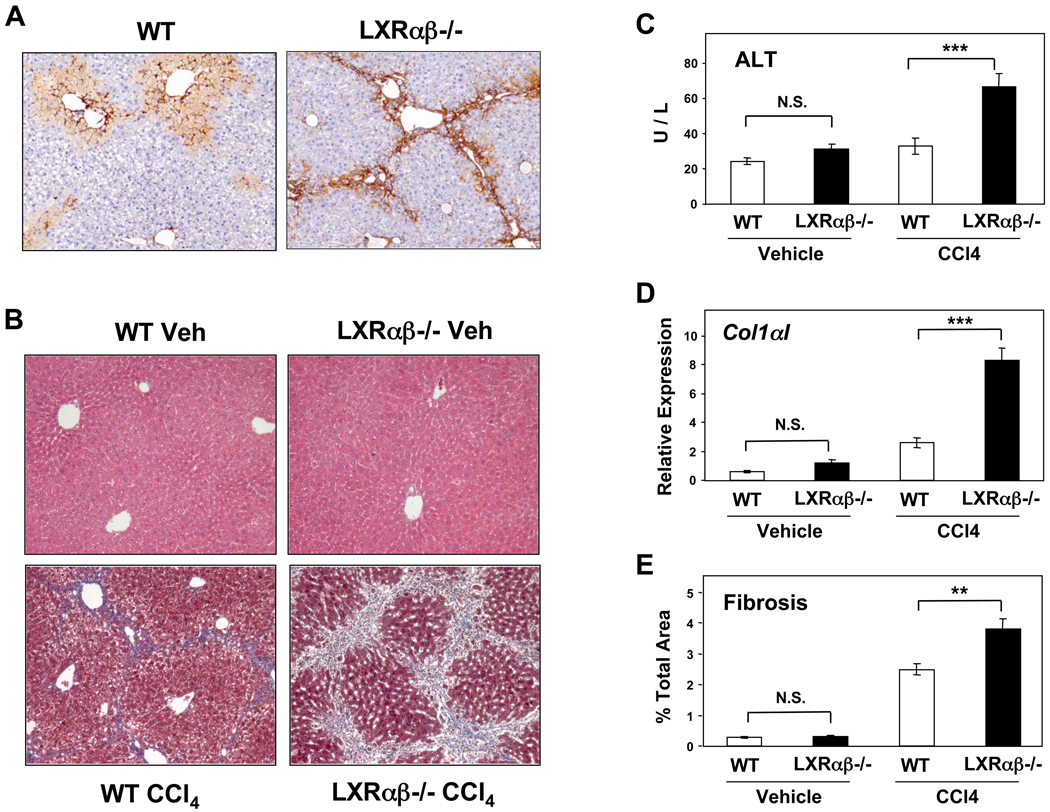
The observation that LXR signaling modulates stellate cell activation suggested that loss of LXR expression might render mice susceptible to liver injury and fibrosis. To test this possibility, we induced liver injury by intraperitoneal injection of carbon tetrachloride (CCl4). CCl4 is a well-characterized liver toxin, metabolized in hepatocytes into an unstable trichloromethyl free radical. Its downstream effects include lipid peroxidation, intracellular protein damage, and alterations in calcium homeostasis that lead to hepatocellular necrosis and the activation of stellate and Kupffer cells31. The initial necroinflammatory activity is followed by a fibrotic response, mediated by stellate cells. We assessed acute injury and stellate cell activation by staining for α-smooth muscle actin protein 72 hours after a single dose of CCl4. Compared to WT, Lxrαβ−/− livers show increased amounts of dense, α-smooth muscle actin expression and a greater tendency to form bridging septae, even after a single injury stimulus (Figure 5A).
Figure 5. LXR null mice are susceptible to liver injury from CCl4.
(A) α-smooth muscle actin expression (dark brown) by immunohistochemistry, 72 hours after a single dose of CCl4. N=10 per genotype, 100×. (B) WT and Lxrαβ−/− mice were challenged biweekly for one month with intraperitoneal CCl4 or vehicle (cf Methods). Liver sections of intraparenchymal fibrotic septae (blue Masson’s trichrome) after chronic CCl4 injury are shown, 100×. (C) ALT levels from CCl4-treated mice. (D) Collagen α1(I) gene expression by qPCR from CCl4-treated mice. (E) Hepatic fibrosis as quantified by digital scanning of entire liver sections (cf. Methods). N = 6–8 mice per group, repeated on 2 separate occasions.
To assess the influence of LXR expression on chronic liver injury, we performed biweekly intraperitoneal injections of CCl4 for a month. Serum and livers were harvested for analysis of serum transaminases, gene expression, and Masson’s trichrome staining for fibrotic septae. Neither WT nor Lxrαβ−/− livers were affected by injection of the vehicle (Figures 5B). WT mice treated with CCl4 developed mild bridging liver fibrosis as expected. Comparatively, CCl4-treated Lxrαβ−/− mice exhibited substantially more damage in their livers, as assessed by measuring the release of hepatic enzymes, collagen α1(I) gene expression, and digital assessment of the fibrotic tissue (Figure 5C–E). Intriguingly, we also found that non-injured Lxrαβ−/− livers showed almost twice the amount of basal fibrotic tissue compared to WT controls (Supplementary Figure 5). There was no difference in expression of Cyp2e1, which metabolizes CCl4, between genotypes (Supplementary Figure 6A).
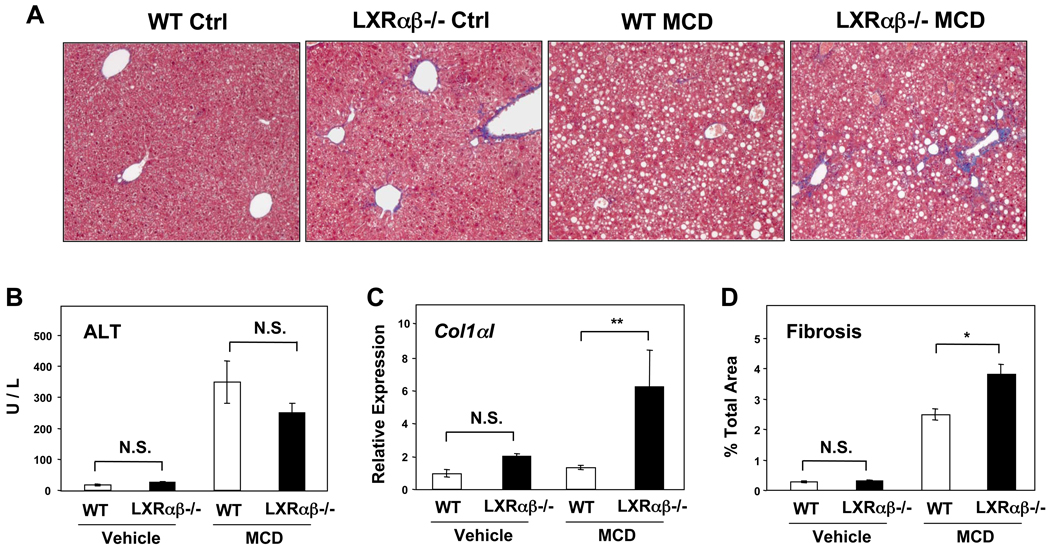
We also tested the response of LXR-deficient mice in a second model of liver injury, methionine choline deficiency, and found increased fibrosis in the absence of LXRs (Figure 6A). We found similar elevations of the alanine aminotransferase (ALT) in both genotypes, but substantially increased expression of collagen α1(I) transcript and more quantifiable fibrosis only in Lxrαβ−/− mice (Figure 6B–D). These results indicate that the genetic loss of LXRs renders mice susceptible to fibrotic liver disease triggered by different mechanisms.
Figure 6. LXR null mice develop more fibrosis on MCD diet.
(A) Masson’s trichrome staining of liver sections from WT and Lxrαβ−/− mice after a month of MCD or control diets, 100×. (B) ALT levels from mice fed an MCD diet. (C) Collagen α1(I) gene expression by qPCR from mice fed the MCD diet. (D) Quantified hepatic fibrosis after MCD diet (cf. Methods). N = 6–10 per group.
Hematopoietic compartment does not confer the susceptibility of LXR null mice to CCl4 injury
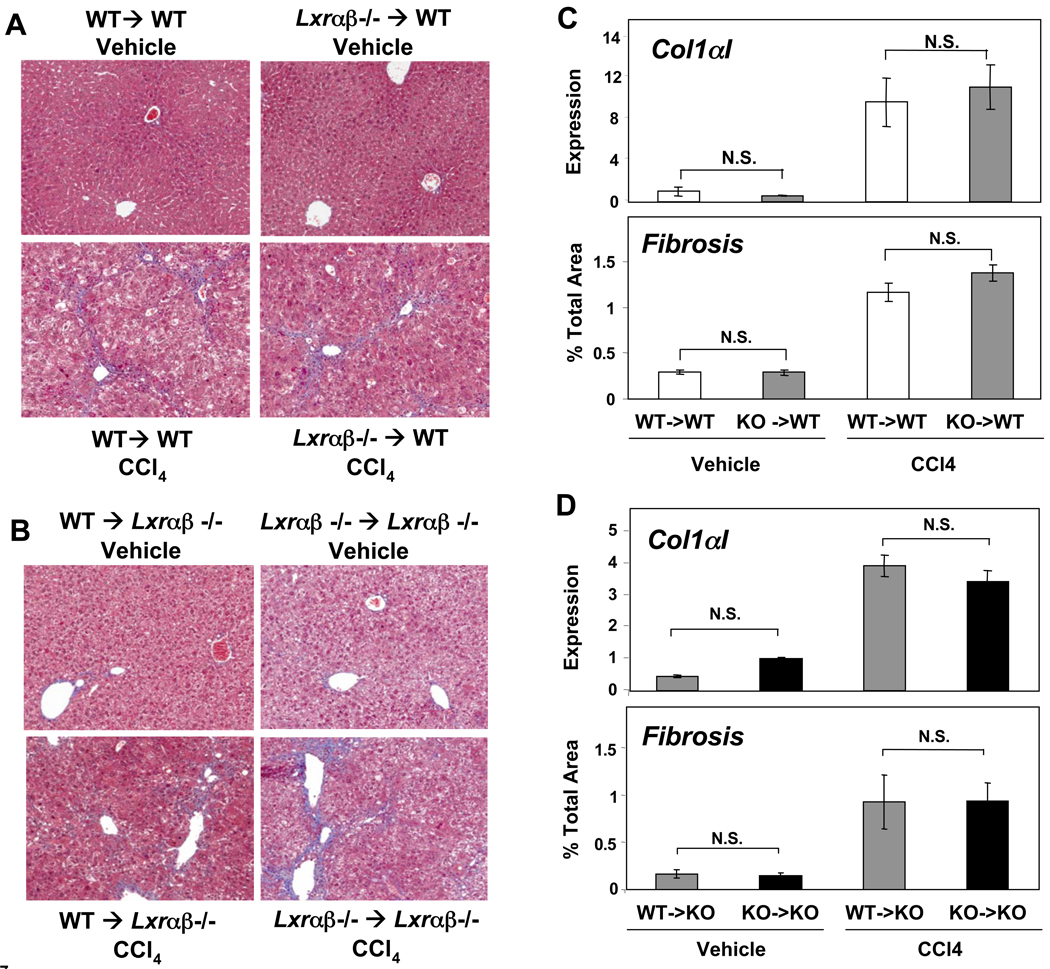
The hematopoietic compartment is a source of immune cells important for liver fibrosis, including macrophages (Kupffer cells), NKT cells, and B and T lymphocytes 1, 2, 10–13. Thus, the ability of LXRs to inhibit inflammatory signaling in bone marrow-derived cells such as Kupffer cells could potentially explain the enhanced susceptibility of LXR null mice to fibrosis. Most authorities believe that hepatic stellate cells are the major precursors of activated myofibroblasts during liver injury and that these cells have their origins principally within the liver itself, not the bone marrow1, 2. Although strategies for selective gene inactivation in stellate cells have not been developed, elimination of bone marrow expression represents an alternative strategy to address the relative contribution of bone-marrow-derived cells and stellate cells to the fibrotic phenotype of LXR null mice. We performed reciprocal bone marrow transplants into WT and Lxrαβ−/− recipients and then subjected them to chronic CCl4 liver injury. In contrast to our results with mice globally lacking LXRs, transplantation of Lxrαβ−/− marrow did not worsen liver fibrosis in WT recipients (Figure 7A,C). Conversely, transplanting WT marrow into Lxrαβ−/− recipients did not lessen fibrosis (Figure 7B,D). Importantly, the genotype of the marrow did not influence the degree of hepatocellular damage (ALT levels) or expression of Cyp2e1, which metabolizes CCl4 (Supplementary Figure 6B,C). Correlating with the fibrosis, the levels of collagen α1(I) expression were also not significantly different between WT and LXR-deficient donors (Figure 7C,D). These data strongly suggest that altered stellate cell function, rather than increased hematopoietic cell inflammation, is the primary basis for the phenotype of Lxrαβ−/− mice.
Figure 7. Reciprocal bone marrow transplants between WT and LXR null mice.
Lethally irradiated WT or Lxrαβ−/− (KO) recipient mice were reconstituted with bone marrow from WT or KO donors (cf. Methods). Eight weeks after transplantation the mice were then challenged with intraperitoneal CCl4 injections as before. (A) Masson’s trichrome staining of liver sections from vehicle and CCl4-treated chimeric animals. N = 8–10 per group, magnification 100×. (B) As in (A), except all recipient mice were Lxrαβ−/−, N = 4–7 per group, magnification 100×. (C) Col1αI gene expression by qPCR and quantified fibrosis in WT recipients after chronic CCl4 administration. (D) Col1αI gene expression by qPCR and quantified fibrosis in Lxrαβ−/− recipients after chronic CCl4 administration.
DISCUSSION
LXRs are key regulators of cholesterol homeostasis and hepatic lipogenesis 16, but they also transrepress inflammatory genes in macrophages17 and mediate anti-proliferative effects in T cells during the adaptive immune response20. We have shown here that LXRs are among the most highly expressed nuclear receptors in stellate cells and that LXR signaling regulates the expression of genes linked to metabolism, inflammation, and fibrogenesis in primary cells. Consistent with the ability of LXRs to affect stellate cell function, Lxrαβ−/− mice are susceptible to developing fibrosis in two distinct models of liver injury. We considered that this phenotype could result from alterations in either stellate cells or hepatic macrophages (Kupffer cells) since the anti-atherogenic effects of LXRs are mediated by vascular macrophages32. However, reciprocal bone marrow transplants into both WT and Lxrαβ−/− recipients did not alter the susceptibility to liver fibrosis, consistent with the involvement of a non-hematopoietic cell type such as stellate cells. The relative importance of stellate and immune cells to hepatic fibrosis is hotly debated1, 2, but our results clearly support the argument that the principle cells responsible for fibrosis are derived exclusively from the liver.
In contrast to hepatocytes and macrophages, in which LXRα plays a major role, stellate cells express constant, high levels of only LXRβ. Hepatic pathologies ascribed to the in vivo deletion of LXRα can therefore be attributed to effects in other cell types, such as hepatocytes and Kupffer cells. The LXR signaling pathway unexpectedly suppresses inflammatory gene expression and the fibrogenic capacity of primary murine stellate cells. Conversely, Lxrαβ−/− stellate cells show increased expression of several markers of activation when cultured on plastic. LXR-deficient stellate cells produce increased soluble mediators of inflammation that promote the fibrotic capacity of neighboring cells in a paracrine manner. We have not yet determined which factor(s) (e.g. MCP1, IL-6, or other) might be responsible for these paracrine effects (Figure 4). Antibody neutralization of individual cytokines and knockdown of their receptors should be informative in establishing which factors are critical.
Interestingly, not all activation-related genes are increased in Lxrαβ−/− cells. For example, we do not detect an increase in TGFβ1, a pro-fibrogenic cytokine associated with the initiation and perpetuation of stellate cell activation1, 2. Even though we did not find changes in the expression of TGFβ mRNA, it will be important to determine, in future experiments, whether Lxrαβ−/− stellate cells have alterations in TGFβ protein production or in the activity of the components of the TGFβ signaling pathway. It is also notable that immortalized stellate cell lines are poor model systems for the study of LXR signaling, because, although effects on metabolic gene expression are preserved, these cells are refractory to the anti-fibrotic effects of LXRs. Once established, activated stellate cells have an autonomous program for fibrosis, which may explain why immortalized cell lines do not model the activation program with complete fidelity. Our work emphasizes the importance of using purified, primary stellate cells and in vivo models whenever possible. Further work is needed to determine exactly which aspect(s) of LXR signaling are critical in stellate cell activation and how these interact with TGFβ signaling.
Another interesting observation is that LXR null stellate cells have marked differences in retinoid / lipid distribution in conjunction with their propensity for activation. Lxrαβ−/− stellate cells predominantly contain a single large lipid droplet, while wild-type cells have multiple smaller ones (Figure 3A). No clear relationship has been established between droplet size and the activation state of stellate cells, although sub-populations of lipid droplets have been identified previously4. A functional role for retinoic acid in stellate cells remains controversial, with several reports that retinoids exacerbate fibrosis, and others reporting an inhibitory effect33–35. It will be important to determine the distinguishing features of the lipid droplets in Lxrαβ−/− stellate cells and whether there are also alterations in retinoid metabolism.
Few transcriptional pathways affecting stellate cell activation have been characterized in detail. Deletion of the homeobox gene, Lhx2, has been reported to produce spontaneous fibrosis and stellate cell activation in mice 36. Similar to Lxrαβ−/− animals, Lhx2−/− mice exhibit increased basal expression of collagen α1(I) and α-smooth muscle actin in their hepatic stellate cells. But that developmental defect was also associated with an architectural distortion of the liver, a feature that is not observed in Lxrαβ−/− mice. We, and others21, have not observed any obvious developmental differences between WT and Lxrαβ−/− livers, nor have we observed increased histologic evidence of hepatocyte injury, death, or turnover. This is an important point given that hepatocyte injury / death is an accepted stimulus for liver fibrosis1. Future work on the impact of LXR signaling in liver regeneration and hepatocyte turnover should be revealing. Whether Lhx2−/− mice have increased basal differences in hepatocyte injury / death was not reported36. Notably, however, basal levels of hepatic fibrosis are subtly increased in Lxrαβ−/− mice (Supplementary Figure 5), consistent with our hypothesis that Lxrαβ−/− stellate cells are poised for activation. Whether this is present in the prenatal state remains to be determined.
Our data are consistent with the hypothesis that increased inflammatory gene expression within stellate cells drives the increased fibrogenic potential of Lxrαβ−/− cells. We would predict that loss of LXR-dependent trans-repression of inflammatory genes is the primary mechanism involved. By using LXR mutants that separate the trans-repressive from the trans-activating functions37, it should be possible to directly test the importance of inflammatory gene repression to stellate cell activation. It is also possible that lipid sensing (sterols, triglycerides, and / or retinoids) within stellate cells is mechanistically tied to activation and proliferation, but further studies are needed to elucidate this. The anti-proliferative effect of LXR ligands on stellate cells (Supplementary Figure 2) may be mediated through suppression of growth promoting splice isoforms of KLF61 (Supplementary Figure 4), but this effect would likely be indirect, as we do not suspect KLF6 is a direct LXR target gene. Unfortunately, we are not currently able to specifically determine the relative contribution of LXR signaling in stellate cells to the in vivo susceptibility to fibrosis, as there are no known methods to specifically delete genes in stellate cells. A liver-specific knockout for LXRβ may be helpful in dissecting this since stellate cells express only this LXR isotype.
Since LXR activation suppresses genes linked to activation and fibrosis in stellate cells, an obvious question is whether treatment of mice with synthetic LXR activators might have a beneficial effect in models of fibrotic liver disease. Unfortunately, preliminary studies indicate that the answer to this question is “no.” The main side effect of currently available pan-LXR agonists (that activate both LXRα and LXRβ) is hepatotoxicity. Mice treated with LXR agonists develop marked hepatic steatosis due to the induction of de novo lipogenesis 15. In our studies, combining this insult with a fibrotic stimulus led to a worsening of overall hepatic pathology, despite any beneficial effects that LXR agonist may have had on stellate cells (data not shown). It is likely that methods for the specific targeting of LXR activity to stellate cells in vivo would be required to separate stellate cell effects from undesirable effects in hepatocytes.
MATERIALS AND METHODS
(See Supplementary Materials and Methods for Additional Details)
Mice and liver injury models
Male Lxrαβ−/− mice (12–24 weeks old) were obtained by backcrossing ≥ 10 generations on a pure C57/Bl6 background. Animal housing was temperature-controlled, with a 12 hour light / dark cycle, pathogen-free conditions, and ad lib access to water and standard chow. The following specialized diets (Research Diets) were used in Figure 6: control = A02082003B; MCD = A02082002B. Both diets differ only in the presence or absence of DL-Methionine (3 gm) and choline bitartrate (2 gm). Chronic liver injury with carbon tetrachloride was induced by intraperitoneal injection of a 10% solution of CCl4 in sterile olive oil (0.5 µl pure CCl4/g body weight) two times per week for four weeks, with harvesting 72 hours after the last dose. For single dose acute injury, 3.5 µL pure CCl4/g body weight was used. For bone marrow transplantation, 12-week-old recipient mice were lethally irradiated with 900 rads and transplanted with ~3 × 106 bone marrow cells isolated from the long bones of 16- to 18-week-old male donors via tail vein injection as previously described32. Eight weeks after transplantation, chronic injury was induced as above. All animal experiments were approved by the Institutional Animal Care and Research Advisory Committee of the University of California, Los Angeles.
Cell culture and Immunocytochemistry
Immortalized stellate cells were cultured in Dubecco’s Modified Eagle Media (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin / streptomycin. Primary stellate cells were cultured in DMEM, low glucose (1 g/L), with 1% penicillin, streptomycin, and amphotericin. Additional media for stellate cell isolation and purification, Eagles Minimal Essential Medium (EMEM), and DMEM / F12, were from Biowhittaker Lonza. Immunocytochemistry was performed on primary stellate cells (day 4–5 of culture activation) by fixing in 10% formalin for 30 minutes, washing in PBS, and then incubating with a FITC-conjugated α-smooth muscle actin MAb (Sigma F3777, clone 1A4) or a matched isotype control (Sigma F6522) for 2 hours at 1:500 titer. BODIPY staining for neutral lipids was similarly performed on formalin-fixed primary stellate cells, incubated with the BODIPY reagent (Invitrogen) at 1:1000 for 1 hour. Cells were also stained with DAPI to mark nuclei (1:10,000).
Primary stellate cell isolation
Isolation and purification of murine stellate cells was performed as previously described27 and outlined in the Supplementary Methods.
Real-Time Quantitative PCR
RNA from cells or liver tissues was extracted using TRIzol (Invitrogen). One microgram of total RNA was reverse transcribed using iScript cDNA Synthesis Kit (Biorad). Sybergreen (Diagenode) real-time quantitative PCR assays were performed using an Applied Biosystems 7900HT sequence detector. Results are normalized to 36B4 and expressed as mean fold induction over controls +/− SEM. Specific primer sequences are available on request.
Statistics
All data shown as mean +/− SEM. Differences between two groups were compared with a 2-tailed unpaired t test. Differences between multiple groups were compared by 1-way or 2-way ANOVA with either Newman-Keuls or Bonferroni post tests (GraphPad Prism 4.0a, California). *, P < .05; **, P < .01; ***, P < .001; NS, P > .05.
Supplementary Material
ACKNOWLEDGEMENTS
We thank David Mangelsdorf for LXR null mice, Tim Willson for GW3965, Scott Friedman for the HSC-T6 and LX-2 cell lines, and Sam French, Sr. for assistance with α-smooth muscle actin immunohistochemistry. We also thank Clara Magyar for digital quantification of fibrosis and Jon Salazar for his assistance with mouse husbandry. P. Tontonoz is an investigator of the Howard Hughes Medical Institute. This work was supported by an NIH Training Grant T32 DK07180-30 and a UCLA Center for Ulcer Research and Education (CURE) Pilot and Feasibility Study Grant 441349-BB-39108 (to S.W. Beaven) and grants HL66088 and HL30568 and DK063491 (to P. Tontonoz).
Grant support: Additional support for cell isolation (H. Tsukamoto) was from R24AA12885 (Non-Parenchymal Liver Cell Core) and P50AA11999 (Southern California Research Center for ALPD and Cirrhosis).
Abbreviations
- CCl4
carbon tetrachloride
- ECM
extracellular matrix
- HSCs
hepatic stellate cells
- LXRs
liver X receptors
- MCDs
methionine choline deficient diet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare that no conflict of interest exists and have no other disclosures to make.
Contributions: SB: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; PT: study concept and design; analysis and interpretation of data; drafting of the manuscript; obtained funding; HT: study concept and design; analysis and interpretation of data; obtained funding; SB: acquisition of data; analysis and interpretation of data; KW: technical support, acquisition of data; JW: technical support, acquisition of data; CH: technical support, acquisition of data.
REFERENCES
- 1.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bronfenmajer S, Schaffner F, Popper H. Fat-storing cells (lipocytes) in human liver. Arch Pathol. 1966;82:447–453. [PubMed] [Google Scholar]
- 4.Blaner WS, O'Byrne SM, Wongsiriroj N, Kluwe J, D'Ambrosio DM, Jiang H, Schwabe RF, Hillman EM, Piantedosi R, Libien J. Hepatic stellate cell lipid droplets: A specialized lipid droplet for retinoid storage. Biochim Biophys Acta. 2009;1791:467–473. doi: 10.1016/j.bbalip.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wake K. "Sternzellen" in the liver: perisinusoidal cells with special reference to storage of vitamin A. Am J Anat. 1971;132:429–462. doi: 10.1002/aja.1001320404. [DOI] [PubMed] [Google Scholar]
- 6.Friedman SL, Roll FJ, Boyles J, Bissell DM. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gressner AM, Bachem MG. Cellular sources of noncollagenous matrix proteins: role of fat-storing cells in fibrogenesis. Semin Liver Dis. 1990;10:30–46. doi: 10.1055/s-2008-1040455. [DOI] [PubMed] [Google Scholar]
- 8.Matsuoka M, Tsukamoto H. Stimulation of hepatic lipocyte collagen production by Kupffer cell-derived transforming growth factor beta: implication for a pathogenetic role in alcoholic liver fibrogenesis. Hepatology. 1990;11:599–605. doi: 10.1002/hep.1840110412. [DOI] [PubMed] [Google Scholar]
- 9.Rockey DC, Boyles JK, Gabbiani G, Friedman SL. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992;24:193–203. [PubMed] [Google Scholar]
- 10.Novobrantseva TI, Majeau GR, Amatucci A, Kogan S, Brenner I, Casola S, Shlomchik MJ, Koteliansky V, Hochman PS, Ibraghimov A. Attenuated liver fibrosis in the absence of B cells. J Clin Invest. 2005;115:3072–3082. doi: 10.1172/JCI24798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 13.Muhanna N, Doron S, Wald O, Horani A, Eid A, Pappo O, Friedman SL, Safadi R. Activation of hepatic stellate cells after phagocytosis of lymphocytes: A novel pathway of fibrogenesis. Hepatology. 2008;48:963–977. doi: 10.1002/hep.22413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol. 2003;17:985–993. doi: 10.1210/me.2003-0061. [DOI] [PubMed] [Google Scholar]
- 15.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaven SW, Tontonoz P. Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annu Rev Med. 2006;57:313–329. doi: 10.1146/annurev.med.57.121304.131428. [DOI] [PubMed] [Google Scholar]
- 17.Castrillo A, Tontonoz P. Nuclear receptors in macrophage biology: at the crossroads of lipid metabolism and inflammation. Annu Rev Cell Dev Biol. 2004;20:455–480. doi: 10.1146/annurev.cellbio.20.012103.134432. [DOI] [PubMed] [Google Scholar]
- 18.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 19.Fowler AJ, Sheu MY, Schmuth M, Kao J, Fluhr JW, Rhein L, Collins JL, Willson TM, Mangelsdorf DJ, Elias PM, Feingold KR. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J Invest Dermatol. 2003;120:246–255. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- 20.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uppal H, Saini SP, Moschetta A, Mu Y, Zhou J, Gong H, Zhai Y, Ren S, Michalopoulos GK, Mangelsdorf DJ, Xie W. Activation of LXRs prevents bile acid toxicity and cholestasis in female mice. Hepatology. 2007;45:422–432. doi: 10.1002/hep.21494. [DOI] [PubMed] [Google Scholar]
- 22.Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, Blaner WS. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000;41:882–893. [PubMed] [Google Scholar]
- 23.Xu L, Hui AY, Albanis E, Arthur MJ, O'Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsukamoto H, She H, Hazra S, Cheng J, Miyahara T. Anti-adipogenic regulation underlies hepatic stellate cell transdifferentiation. J Gastroenterol Hepatol. 2006;21 Suppl 3:S102–S105. doi: 10.1111/j.1440-1746.2006.04573.x. [DOI] [PubMed] [Google Scholar]
- 25.Miyahara T, Schrum L, Rippe R, Xiong S, Yee HF, Jr, Motomura K, Anania FA, Willson TM, Tsukamoto H. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000;275:35715–35722. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- 26.Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pellicciari R, Morelli A. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Han YP, Yan C, Zhou L, Qin L, Tsukamoto H. A matrix metalloproteinase-9 activation cascade by hepatic stellate cells in trans-differentiation in the three-dimensional extracellular matrix. J Biol Chem. 2007;282:12928–12939. doi: 10.1074/jbc.M700554200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 29.Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 30.Wagner BL, Valledor AF, Shao G, Daige CL, Bischoff ED, Petrowski M, Jepsen K, Baek SH, Heyman RA, Rosenfeld MG, Schulman IG, Glass CK. Promoter-specific roles for liver X receptor/corepressor complexes in the regulation of ABCA1 and SREBP1 gene expression. Mol Cell Biol. 2003;23:5780–5789. doi: 10.1128/MCB.23.16.5780-5789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalf GF, Post GB, Snyder R. Solvent toxicology: recent advances in the toxicology of benzene, the glycol ethers, and carbon tetrachloride. Annu Rev Pharmacol Toxicol. 1987;27:399–427. doi: 10.1146/annurev.pa.27.040187.002151. [DOI] [PubMed] [Google Scholar]
- 32.Tangirala RK, Bischoff ED, Joseph SB, Wagner BL, Walczak R, Laffitte BA, Daige CL, Thomas D, Heyman RA, Mangelsdorf DJ, Wang X, Lusis AJ, Tontonoz P, Schulman IG. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci U S A. 2002;99:11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellemans K, Verbuyst P, Quartier E, Schuit F, Rombouts K, Chandraratna RA, Schuppan D, Geerts A. Differential modulation of rat hepatic stellate phenotype by natural and synthetic retinoids. Hepatology. 2004;39:97–108. doi: 10.1002/hep.20015. [DOI] [PubMed] [Google Scholar]
- 34.Okuno M, Moriwaki H, Imai S, Muto Y, Kawada N, Suzuki Y, Kojima S. Retinoids exacerbate rat liver fibrosis by inducing the activation of latent TGF-beta in liver stellate cells. Hepatology. 1997;26:913–921. doi: 10.1053/jhep.1997.v26.pm0009328313. [DOI] [PubMed] [Google Scholar]
- 35.Senoo H, Wake K. Suppression of experimental hepatic fibrosis by administration of vitamin A. Lab Invest. 1985;52:182–194. [PubMed] [Google Scholar]
- 36.Wandzioch E, Kolterud A, Jacobsson M, Friedman SL, Carlsson L. Lhx2−/− mice develop liver fibrosis. Proc Natl Acad Sci U S A. 2004;101:16549–16554. doi: 10.1073/pnas.0404678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.