Abstract
Background
In atopic individuals, food ingestion drives the production of IgE antibodies that can trigger hypersensitivity reactions. The IL-4 pathway plays critical roles in this response and genetic polymorphisms in its components have been linked to allergy.
Objective
To test whether an activating mutation in the IL-4 receptor (IL-4R) α chain enhances allergic responses to a food antigen.
Methods
F709 mice, in which the IL-4Rα immuno-tyrosine inhibitory motif (ITIM) motif is inactivated, were gavage fed with ovalbumin (OVA). Reactions to OVA challenge and immune responses including antibody production and Th2 responses were assessed.
Results
F709 mice, but not wild-type (WT) controls, sensitized by gavage with OVA and either cholera toxin (CT) or Staphylococcal enterotoxin B (SEB), displayed mast cell activation and systemic anaphylaxis upon enteral challenge. Anaphylaxis was elicited even in F709 mice enterally sensitized with OVA alone. Bone marrow chimera experiments established that the increased sensitivity conferred by the F709 genotype was mediated mostly by hematopoietic cells but that nonhematopoietic cells also contributed. F709 mice exhibited increased intestinal permeability to macromolecules. The F709 genotype conferred increased OVA-specific IgE but not IgG1 responses, local and systemic Th2 responses and intestinal mast cell hyperplasia as compared with WT mice. Anaphylaxis was abrogated in F709 mice lacking IgE or the high affinity receptor for IgE (FcεRI).
Conclusion
Augmented IL-4Rα signaling confers increased intestinal permeability and dramatically enhanced sensitivity to food allergens. Unlike anaphylaxis to injected antigens, which in rodents can be mediated by either IgE or IgG antibodies, the food-induced response in F709 mice is solely IgE-dependent.
Keywords: Food allergy, IL-4, tolerance, anaphylaxis, IgE
Introduction
Food-mediated allergic reactions are a growing health problem with an estimated prevalence of 6% in children and 3–4% in the adult population1–3. The cytokines IL-4 and IL-13 play important roles in the induction and effector phases of allergic responses. They bind to and signal via two receptors which share the IL-4Rα chain paired with one of two alternative subunits (γc or IL-13Rα1) to form the type I or type II IL-4 receptors respectively (reviewed in 4, 5). IL-4R type I, which exclusively binds IL-4, is present predominantly in hematopoietic cells. The type II receptor is expressed on multiple cell types and recognizes both IL-4 and IL-13. Both play distinct roles in the development of immune responses.6–8
A large assortment of independent human studies, including work from our group, has linked polymorphisms in the IL-4/IL-13 axis with atopy.4, 9–11 We have used knockin mouse models to directly test the effects of such mutations on allergic responses in a homogenous genetic background. Targeted disruption of the I4R site of IL-4Rα in mice amplifies IgE responses and elicits enhanced allergic responses to inhaled allergens. 12 Mice with a mutation of the immunoreceptor tyrosine-based inhibitory motif (ITIM) at position 709 (Y → F) of IL-4Rα also have an allergic phenotype with markedly elevated IgE levels and susceptibility to allergen-induced airway inflammation13. There is no human equivalent for the F709 mutation. However, the mutation is prototypic of a number of human IL-4Rα polymorphisms that promote receptor signaling and are associated with atopy including IL-4Rα I75V and IL-4Rα Q576R4,11. The effects of IL-4Rα mutations on cell activation by IL-4 and IL-13 extend to nonhematopoietic cells, which do not express the γc chain of the Type I receptor, suggesting that the allergic phenotype of animals harboring these mutations derives from enhanced signaling via both the Type I and II receptors.
In the most severely affected patients, food ingestion can provoke systemic anaphylaxis. In animal models, both IL-4 and IL-13 can prime end organs for enhanced anaphylactic responses.14 A number of studies have demonstrated that anaphylaxis in mice parenterally immunized with antigen and adjuvant and then challenged intravenously can arise by both IgE- and IgG-mediated mechanisms.15–18 Food allergen ingestion by mice drives robust IgE responses as demonstrated by Li and colleagues using peanut extracts 19 Similarly, Brandt et al. have shown that repeated enteral administration of OVA, in mice previously primed intraperitoneally with OVA and alum, leads to OVA-IgE production and IgE dependent mast cell activation with increased intestinal permeability and diarrhea 20. The animals in this “allergic diarrhea” model do not exhibit hypothermia following enteral challenge (parenteral antigen injection is required to elicit systemic responses) 20–23. These responses are IgE- and mast cell-dependent. However, assessment of the relative contribution of IgE antibodies to food hypersensitivity in purely enterally-sensitized and enterally challenged animals has been prevented by the fact that ingestion has a tolerizing effect so that it has been generally difficult to induce robust allergic sensitization by intestinal immunization alone.
We hypothesized that activating mutations of IL-4Rα would enhance the susceptibility of mice to gastrointestinal allergic responses. To test this hypothesis we studied the responses of F709 mice enterally exposed to OVA either with or without adjuvants (CT or SEB) over nine weeks and then challenged by gavage. OVA gavage of sensitized F709 mice triggered intense systemic anaphylaxis. Bone marrow chimera experiments identified hematopoietic cell IL-4R function as the major driver of this effect. These animals had elevated serum levels of OVA-specific IgE. Anaphylactic responses could be elicited even in F709 mice sensitized to OVA in the absence of any adjuvant. Unlike active systemic anaphylaxis following immunization by injection, which can be IgG-mediated and elicited in mice lacking IgE or FcεRI, the allergic reactions of F709 mice to ingested OVA were completely IgE-dependent. Allergen exposed F709 mice exhibited a Th2-biased systemic OVA specific response along with increased gut expression of Th2 cytokine transcripts. They had marked intestinal mastocytosis along with elevations in intestinal IL-9 transcripts and increased intestinal permeability to macromolecules. Our findings suggest that amplification of IL-4Rα signals facilitate allergic sensitization to ingested antigens, impair tolerance, support intestinal mast cell expansion and drive IgE-dependent anaphylactic responses.
Materials and methods
Animals
Wild-type (Y709) BALB/c mice were purchased from Taconic Farms (Germantown, NY). Igh-7−/− (IgE−/−)16 and C.129.Il4raF709/F709 (F709) mice were each bred onto a BALB/c background (ten generations). F709 mice have been deposited at JAX lab (Strain name: C.129X1-Il4ra<tm3.1Tch>/J; Stock Number 012709). F709/IgE−/− mice were generated by crossing F709 mice with IgE−/−. All mice were housed in a specific pathogen-free environment and were 6 to 12 weeks old. All experiments were carried out in accordance with the IACUC policies and procedures of Children’s Hospital.
Sensitization of mice
For sensitization, Y709, F709, IgE−/− and F709/IgE−/− were treated intragastrically (i.g.) with sterile PBS or 5 mg OVA (Sigma) and 20 μg CT in 250 μl sterile PBS (saline) on days 0, 1, 2 and 7, followed by weekly sensitization with OVA and CT for 8 more weeks. In the 9th week, mice were challenged with a large dose (150 mg) of OVA. Responses to OVA alone were studied in mice exposed to 5 mg OVA as described above in the absence of adjuvant.
Measurement of anaphylaxis, symptom scores and diarrhea
Anaphylaxis was assessed in challenged mice by measuring changes in body temperature and recording symptom scores. Temperature changes were measured using transponders placed subcutaneously two days prior to challenge (Implantable Programmable Temperature Transponder™) (Biomedic Data Systems, Seaford, DE). Symptom scores were determined according to criteria shown in Table 1 “(see Table E1 in the Online Repository).”
Constructon of bone marrow chimeras
Recipient mice were irradiated 2 doses of 600 rads 3 hrs apart and were infused immediately with 2.5 ×106 bone marrow cells from congenic donor mice. Mice were treated with Bactrim during a 6 week period of reconstitution and were then subjected to OVA sensitization. Thy1 congenic mice were used and flow cytometry was used to confirm the effectiveness of bone marrow reconstitution.
Passive anaphylaxis studies
Mice were sensitized i.v. with 4 μg DNP-IgE. 24h later, they were challenged i.v. with DNP-HSA and core body temperature was recorded.
ELISA’s for murine mast cell protease- 1(mMCP-1), total and OVA-specific IgE and IgG1
mMCP-1, total IgE, OVA-IgE and OVA-IgG1 ELISAa were performed as previously described.24
Measurement of intestinal permeability
Intestinal permeability was determined as previously described 25. Briefly, mice were gavaged with 20 ml/kg of 22 mg/ml fluorescein isothiocyanate conjugated dextran (FITC-dextran, molecular mass 4 kDa; Sigma, St. Louis, MO) and 1 mg/ml horseradish peroxidase (HRP, MW 44 kDa; Sigma). Blood was obtained 4h later and the concentration of fluorescein in plasma determined by spectrophotofluorometry, using serially diluted samples of the marker as standard. HRP captured anti-HRP on a plate was measured using the TMB substrate system.
Splenocyte stimulation and ELISA’s for IL-4, IL-5, IL-13 and IFN-γ
One million spleen cells were cultured in triplicate with 200 μg/ml OVA or 0.2 μg/ml CD3 and CD28 in 1ml complete RPMI 1640 with 10% fetal calf serum. Supernatants were collected at 24, 48 and 96 hr and levels of IL-4, IL-5, IL-13 and IFN-γ determined by ELISA according to the manufacturer’s instructions (IL-4, IL-5 and IFN-γ from BD Biosciences; IL-13 from R&D Systems).
Histological analysis and enumeration of mast cells
Intestinal mast cells were enumerated by microscopic examination of sections of paraffin-embedded jejunal tissue. Tissue sections were stained with chloroacetate esterase. Mast cells were counted in 4–5 complete cross-sections of jejunum as specified in the figure labels.
Quantitative PCR analysis
Quantitative real-time PCR was performed as previously described26. Expression of IL-4, IL-5, IL-13, IFN-γ, IL-33, IL-10 and IL-9 was calculated relative to GAPDH transcripts.
Results
Exposure to oral antigen induces systemic anaphylaxis in F709 mutants but not wild-type Y709 mice
We hypothesized that the F709 mutants, which have enhanced IL-4R signaling, would have exaggerated responses to food allergens. We tested this hypothesis by subjecting F709 mice to enteral sensitization with OVA followed by a gavage challenge. Anaphylaxis was detected by noninvasive measurement of body temperature and observation of symptoms (Fig. 1A, B). A dramatic and rapid drop in core body temperature was observed in F709 mice sensitized with OVA and CT. Enteral challenge of wild-type (Y709) BALB/c mice sensitized by this protocol did not elicit significant responses. Similar reactions were elicited in F709 mice sensitized using SEB in place of CT, as recently described by Ganeshan and colleagues27 “(see Figure E1 in the Online Repository).”
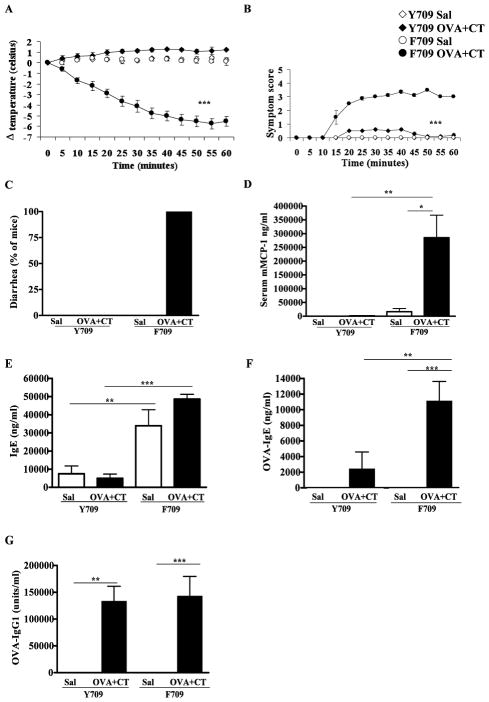
Figure 1. Allergic responses in F709 mice challenged with OVA following enteral senstitization with OVA with CT.
Systemic anaphylaxis was evaluated in orally gavaged mice. (A) Core body temperature, (B) symptom score, (C) diarrhea, (D) serum mMCP-1 levels, (E) Total IgE, (F) OVA-IgE and (G) OVA-IgG1 are shown. n=5–7 mice; data are representative of 3 experiments. * = p<0.05, ** = p<0.01 (student’s t-test); *** = p<0.001 (ANOVA).
OVA-challenged F709 mice exhibited very obvious signs of anaphylaxis including decreased activity and periods of inactivity, with no response to prodding or touching. Some mice also exhibited trembling and convulsions (Fig. 1B). All F709 animals and none of the controls developed profuse diarrhea (Fig. 1C). Y709 mice had only minimal symptoms. mMCP-1, a marker of mast cell degranulation, was increased in OVA-challenged F709 but not Y709 mice suggesting that mast cell activation drove the physiologic responses (Fig. 1D).
Enterally sensitized F709 mice develop elevated levels of OVA-specific IgE
Anaphylactic sensitivity to food allergens in humans is strongly correlated with the production of high-titer IgE antibodies. Total IgE levels were markedly elevated in both control and experimental F709 mice as compared to Y709 mice (Fig. 1E). OVA-IgE responses were detected only in antigen-exposed mice with levels of 2,459±2136 ng/ml in Y709 mice and 11,159±2458ng/ml in F709 mice respectively (Fig. 1F). In contrast, and somewhat unexpectedly, anti-OVA IgG1 responses, which are also Th2-dependent, were comparable in Y709 and F709 mice treated with OVA and CT (Fig. 1G).
Enhanced anaphylactic sensitivity in F709 mice is conferred primarily by IL-4R effects on hematopoietic cells with a modest contribution from nonhematopoietic cells
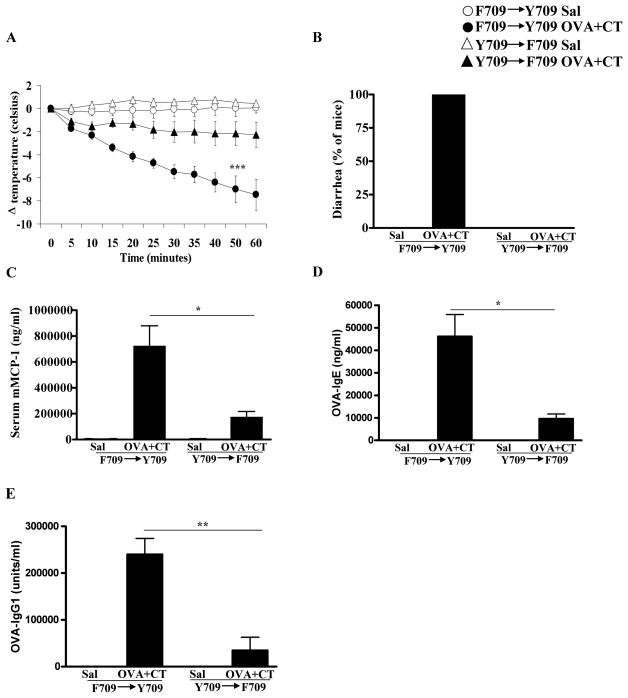
Both bone marrow-derived and nonhematopoietic cells including epithelial cells express IL-4R. In order to determine the relative contributions of IL-4R function on hematopoietic vs. nonhematopoietic cells to the F709 allergic phenotype, we assessed the responses of BM chimeras. Irradiated Y709 recipients reconstituted with F709 BM developed robust anaphylactic responses, similar to those observed in F709 animals (Fig. 2A). The induction of anaphylaxis was fatal in at least 2 animals and was accompanied by severe symptoms (data not shown). All of the animals exhibited diarrhea (Fig. 2B) and had elevated levels of mMCP-1 (Fig. 2C). In contrast, the development of anaphylaxis in F709 recipients receiving BM from Y709 donors was modest, and only 1 animal had significant decreases in core body temperature (Fig. 2A). Moreover, the presence of diarrhea and the levels of mMCP-1 were also attenuated in these animals. Determination of antibody responses revealed the presence of enhanced anti-OVA IgE and anti-OVA IgG1 responses in F709 → Y709 mice (Fig. 2D & 2E). These observations indicate that the augmentation of anaphylactic responses in the IL-4Rα mutant, F709, is largely mediated by hematopoietic cells with a modest additional contribution from nonhematopoietic cell types.
Figure 2. Oral anaphylaxis in bone marrow chimera mice.
Y709/F709 chimeric mice were enterally sensitized and challenged with OVA (Sal) plus CT. (A) Core body temperature, (B) diarrhea, (C) serum mMCP-1, (D) OVA-IgE and (E) OVA-IgG1 are shown. n=5–7 mice; * = p<0.05, ** = p<0.01 (ttest); *** = p<0.001 (ANOVA).
The development of anaphylaxis in F709 mice is IgE-dependent
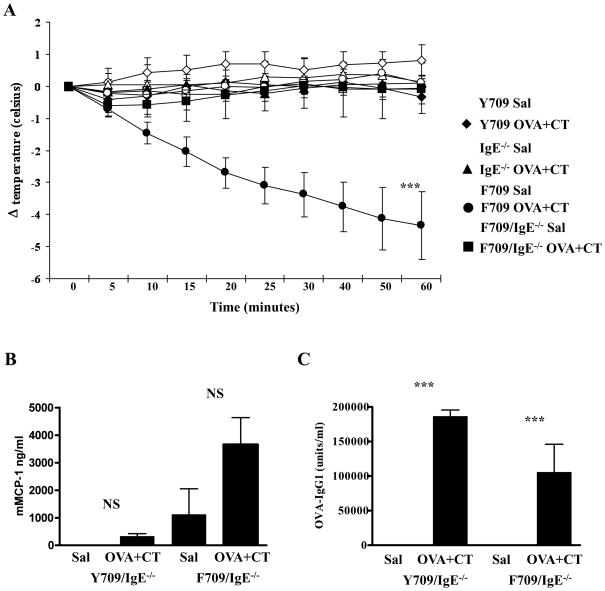
In rodents immunized by parenteral administration of antigen and adjuvant, systemic anaphylaxis to intravenous antigen challenge can occur in the absence of IgE in a reaction driven by IgG antibodies.17, 28 In order to determine the role of IgE in the anaphylactic responses of F709 mice to food antigens we compared the responses of F709 mice with those of double mutants lacking IgE (F709/IgE−/−). As expected, F709 mice in this experiment again exhibited robust responses to intragastric OVA challenge (Fig. 3A). Surprisingly, the development of anaphylaxis was completely abrogated in the absence of IgE in F709/IgE−/− mice (Fig. 3A). In contrast to F709 mice, these mice displayed no clinical symptoms and did not exhibit any diarrhea (data not shown). Their mMCP-1 levels (Fig. 3B) were only about 1% of those observed in F709/IgE+/+ mice (Fig. 1D) and were not significantly different from saline controls. The dependence of the anaphylactic response on IgE was confirmed in separate experiments, in which F709/FcεRI−/− double mutant mice were sensitized with OVA and SEB “(see Fig. E2 in Online Repository).” Examination of the levels of OVA-specific IgG1 showed that both groups involved exhibited similar IgG1 responses (Fig. 3C). These findings indicate that the presence of a strong IL-4R signal during enteral sensitization has a stronger effect on IgE than on IgG responses and that IgE is required for the expression of systemic anaphylaxis to ingested antigens in animals that have been sensitized enterally.
Figure 3. Anaphylaxis and antibody responses in OVA challenged Y709/IgE−/− and F709/IgE−/− mice enterally sensitized with OVA and CT.
(A) Anaphylaxis was assessed by measuring changes in body temperature. Following sacrifice, the levels of serum mMCP-1 (B), and anti-OVA IgG1 (C) were determined by ELISA. n=5–7 mice; data are representative of 3 experiments. NS = Not Significant, *** = p<0.001 (ANOVA)
Sensitization to food allergens in the absence of adjuvant in F709 mice
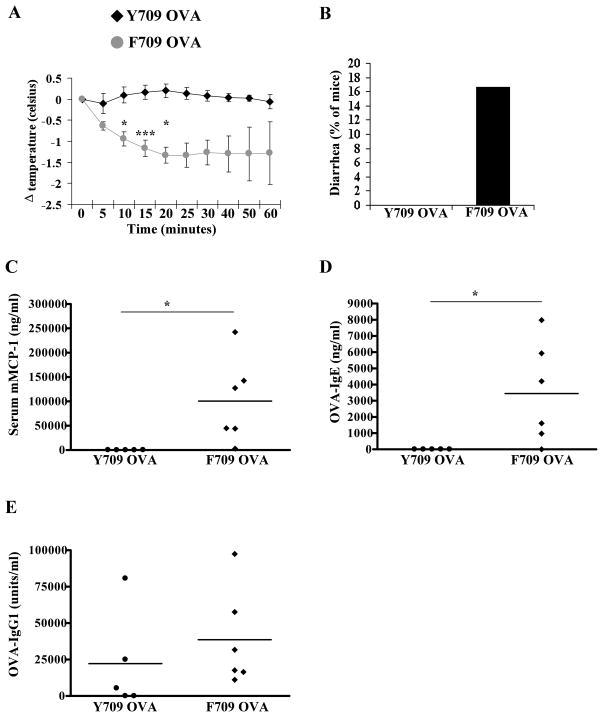
We hypothesized that enhanced signaling by the IL-4R might enhance allergic sensitization in F709 mice and investigated whether ingestion of OVA alone would provoke responses. Unlike Y709 mice, F709 animals fed OVA alone showed significant responsiveness upon challenge (Fig. 4A). Diarrhea occurred in some of these animals (Fig. 4B) and detectable elevations in mMCP-1 were present in five out of six mice studied (Fig. 4C). OVA-specific IgE responses were also detectable in the majority of animals fed with OVA alone (Fig. 4D). None of the Y709 mice fed OVA alone generated mMCP-1 or IgE responses. In contrast, both Y709 and F709 mice exhibited enhanced OVA-IgG1 levels (Fig. 4E). Such allergic responses to protein antigens ingested in the absence of any adjuvant represents a remarkable and unique feature of the F709 model and strongly indicates that activation of IL-4Rα signaling can dampen normal tolerogenic mechanisms.
Figure 4. Allergic responses in F709 mice enterally sensitized to OVA in the absence of cholera toxin.
Mice were sensitized with OVA in the absence of CT and then challenged with OVA. (A) Core body temperature, (B) diarrhea, (C) serum mMCP-1, (D) OVA-IgE, and (E) OVA-IgG1 are shown. n=5–7 mice; data are representative of 3 experiments. * = p<0.05; *** = p<0.001 by t-test.
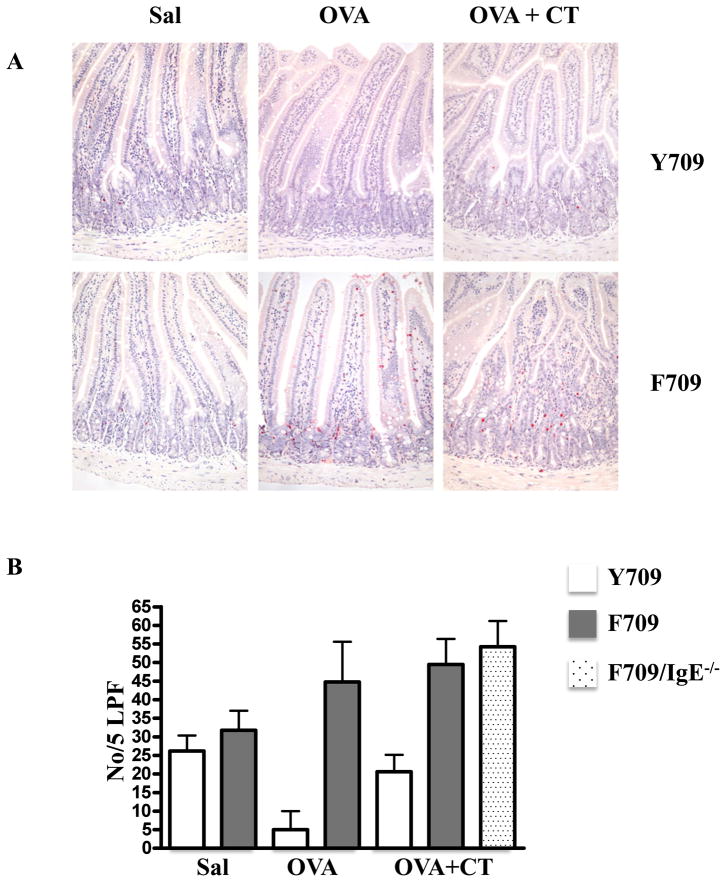
Allergen-fed F709 mice have elevated numbers of intestinal mast cells
Inspection for chloroacetate esterase reactivity in sections of jejunum revealed the presence of numerous mucosal mast cells both in mice treated with OVA and CT and those receiving OVA alone (Fig. 5A). Enumeration of mast cells showed that OVA and CT-treated F709 mice had about twice as many mast cells (41±12cells/5 LPF) as Y709 mice (21±4cells/5 LPF) (Fig. 5B). Similarly, F709 mice receiving OVA alone had 45±15cells/5 LPF vs. 5±5cells/5 LPF for Y709 mice. The F709 animals not only exhibited more mast cells but also had mucosal mast cells high up in the villi, a site which might favor interaction with absorbed food proteins. These results indicate that IL-4Rα signaling supports mast cell differentiation in the gut following food allergen exposure and that the intense anaphylactic responses of F709 mice might, in part, be due to the presence of large numbers of these key effector cells. Mast cell expansion was also observed in F709/IgE−/− mice (Fig. 5D), suggesting that IgE antibodies are not required for the recruitment and expansion of intestinal mast cells as we have previously reported for allergen-induced airway mast cells 26 (see also Fig. E4 in the Online Repository). The jejunal mast cells in F709/IgE−/− mice had a consistently unactivated morphology whereas many of the mast cells in IgE-sufficient mice exhibited features of degranulation. Levels of mMCP-1 were much higher in F709 than in F709/IgE−/− mice (Fig. 3B vs. Fig. 1D) consistent with ongoing IgE-mediated activation.
Figure 5. Mast cells in the intestines of OVA treated F709 mice and Y709 controls.
Mice were treated with OVA and CT or OVA alone. Chloroacetate esterase-reactive cells were identified in sections of jejunum. (A) Jejunal sections. Mast cells appear red. (B) Mast cell counts per 5 low power fields. n=5–7 mice. Data are representative of 3 independent experiments.
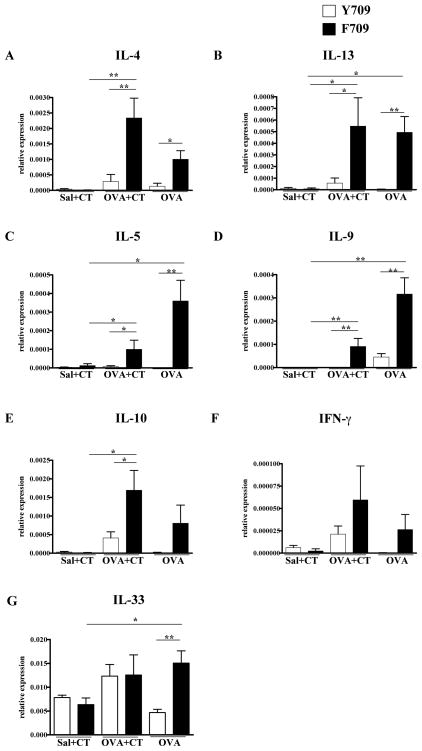
Enhanced expression of Th2 cytokines in the intestines of food allergen-exposed F709 mice
We hypothesized that F709 mutants would have enhanced local and systemic Th2 responses to ingested OVA. Except for IL-10, analysis of jejunal RNA for levels of IL-4, IL-13, IL-5, IL-9, IL-33 and IFN-γ transcripts in Y709 mice exposed to OVA and CT revealed no statistically significant increases in expression of any of these cytokines. In contrast, the relative levels of IL-4, IL-5, IL-13, IL-9 and IL-10 (Fig. 6A–F) were all significantly increased in F709 mice gavaged with OVA and CT in comparison with respective controls. Similar increases were observed for these cytokines as well as IL-33 in the group treated with OVA alone. (Fig. 6A–G). These results indicate that enhanced signaling via IL-4R is permissive for the induction of a local intestinal Th2 response to food allergens. The intense induction of IL-9, a cytokine which also supports mast cell development and recruitment, suggests a possible mechanism for the mast cell responses to food allergen observed in the F709 mice.
Figure 6. Cytokine transcripts in the intestines of mice enterally sensitized to OVA.
Mice were treated with OVA+CT or OVA alone. RNA from jejunum was analyzed for cytokine transcripts by RT-PCR. Expression of the following genes was measured. (A) IL-4 (B) IL-13 (C) IL-5 (D) IL-9 (E) IL-10 (F) IFN-γ (G) IL-33.n=5–7 mice; * = p<0.05; ** = p<0.01 by t-test.
Systemic Th2 skewing and T cell activation in food-allergen treated F709 mice
In order to test systemic Th2 responses to food allergens, we measured IL-4, IL-13 and IL-5 in the supernatants of spleen cells from saline and CT, OVA and CT, and OVA alone treated mice stimulated with OVA “(see Fig. E3 in the Online Repository)”. As expected, F709 mice mounted strong systemic Th2 anti-OVA responses with markedly more elevated splenocyte production of IL-4, IL-13 and IL-5 than either saline-sensitized controls or OVA-sensitized Y709 mice. Similarly, the levels of serum IL-4 were also significantly enhanced in these animals. These observations confirm that the local Th2 response to OVA observed in the intestines of F709 mice translates into a strong systemic response.
Target tissues of anaphylaxis of F709 mice exhibit similar sensitivity to mediators of anaphylaxis as do those of Y709 controls
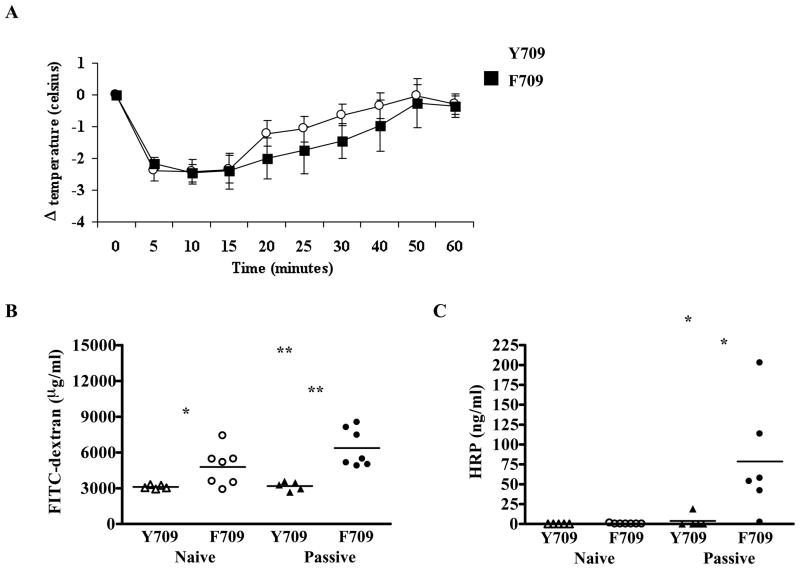
The abrogation of anaphylaxis in F709/IgE−/− mice as well as the enhanced levels of OVA-IgE in F709 vs. Y709 controls implicate the intensity of IgE responses as the major determinant of anaphylactic sensitivity. However, an alternative explanation for heightened sensitivity might be an increased sensitivity of target tissues expressing the F709 variant of IL-4Rα to mast cell-derived mediators. In order to distinguish these possibilities, we examined the intensity of passive antigen-specific IgE-mediated anaphylactic responses in Y709 and F709 mice. Sensitization with DNP-specific IgE, followed by challenge with antigen elicited robust responses with a similar magnitude in Y709 and F709 animals (Fig. 7A) suggesting that the increased sensitivity to anaphylaxis in animals harboring the disinhibited IL-4Rα variant, F709 is linked to IgE production rather than target tissue sensitivity.
Figure 7. Passive IgE-mediated anaphylaxis and intestinal permeability in F709 mice.
(A) Core body temperature in mice treated with anti-DNP-IgE and challenged with DNP-HSA. Plasma levels of FITC-dextran (B) and HRP (C) in naïve mice and mice as treated in (A). n=5–7 mice; data are representative of 3 independent experiments. * = p<0.05; ** = p<0.01 by t-test.
Expression of the F709 form of IL-4Rα confers enhanced intestinal permeability in F709 mice
Both the relative ease of sensitization to food allergens delivered enterally and the robust anaphylactic responses exhibited by F709 mice following oral challenge suggested that these animals might have altered intestinal barrier function. This could affect antigen uptake during sensitization as well as exposure of mast cells to antigen upon challenge. Both IL-4 and mast cells have been shown to affect intestinal permeability29–31. To determine whether alterations in IL4R signaling might affect intestinal permeability we gavaged F709 mutants as well as control Y709 mice with two macromolecules FITC-dextran (4 kDa) and HRP (~44 kDa) and followed their appearance in the blood. F709 mice exhibited enhanced absorption of FITC-dextran compared with Y709 animals (Fig. 7B), consistent with increased intestinal uptake, likely via a paracellular path. In contrast, HRP was barely detectable in the plasma of either Y709 or F709 mice (Fig. 7C) suggesting limited permeability for this protein which likely penetrates the intestinal epithelium via both paracellular and transcellular transport mechanisms. As mast cell mediators are known to enhance gut permeability31, we next determined whether uptake of these two tracers might be further altered following mast cell activation by IgE:antigen. We therefore followed levels of FITC-dextran and HRP in the blood of mice whose mast cells had been activated by i.v. infusion of anti-DNP IgE followed by DNP-HSA. Both FITC-dextran and HRP uptake were significantly enhanced in F709 animals but not Y709 mice following mast cell activation (Figs. 7B & 7C), suggesting that enhanced IL-4R signaling renders both paracellular and transcellular pathways of intestinal epithelial antigen uptake more sensitive to mast cell-derived mediators.
Discussion
The findings reported here indicate that augmented IL-4 receptor signaling is singularly effective in promoting both the development of allergic sensitization to ingested protein antigens and the induction of anaphylactic responses upon enteral challenge. Studies in humans and mice have previously established that IL-4 and IL-13 play central roles in the pathogenesis of gastrointestinal allergic responses.32–38 Genetic polymorphisms affecting the IL-4/IL-13 axis are reported to be correlated with asthma susceptibility4, 9–11 and risk associations between polymorphisms in the IL-4, IL-4Rα and IL-13 genes and food allergy have been reported.39, 40 We therefore hypothesized that augmented IL-4 signaling could predispose to food allergy and tested this in F709 mice in which the sensitivity of IL-4Rα signaling is enhanced resulting in increased IL-4 induced STAT6 phosphorylation, Th2 induction and IgE production13.
Our results demonstrate that the F709 genotype confers a very dramatic sensitivity to food allergen and that this phenotype is mediated primarily by IL-4R signaling effects in hematopoietic cells. Mice with this mutation exhibited not only increased susceptibility to immune sensitization, producing both specific IgE antibodies and Th2 responses, but also a marked intestinal mast cell expansion, enhance intestinal permeability and a capacity to mount intense anaphylactic responses upon enteral challenge. Perhaps the single most notable phenotype of these mice with respect to food allergy was their susceptibility both to immune sensitization and to enteral allergen-induced anaphylaxis even when exposed only to a protein antigen, OVA, in the absence of any immune stimulating adjuvant. Taken together, these observations indicate that IL-4 signaling plays critical roles in food allergy by enhancing allergic sensitization, promoting mast cell expansion and anaphylactic sensitivity and by interfering with the physiologic induction of tolerance to new protein antigens introduced via the gastrointestinal tract.
A number of groups have developed murine models to study food-allergen mediated hypersensitivity reactions in mice.19, 20, 27, 41–47. Studies by Rothenberg and colleagues using an “allergic diarrhea” have established a central role for the IL-4/IL-13 axis in the development of food allergy. Our findings confirm and advance this observation by demonstrating that enhanced IL-4R signaling is permissive for purely enteral immune sensitization in the absence of parenteral priming with adjuvant as is used in the allergic diarrhea protocol. Furthermore, we find that the IL-4R effect also results in the manifestation of vascular responses (hypothermia) upon purely enteral challenge (as opposed to i.v. challenge as in the allergic diarrhea system)..
Recently it was shown that IL-4 can divert developing Treg to a cytokine profile dominated by IL-9.48 IL-9, produced by epithelial and other cells, is an important mast cell growth and differentiation factor and has been implicated in intestinal anaphylaxis in an IgE-dependent manner.21, 49–51 Forbes and colleagues have elegantly demonstrated that IL-9-driven mast cell expansion in the intestine leads to enhanced intestinal permeability21. This finding suggests a mechanism whereby the enhanced IL-4Rα signaling of F709 mice, which is accompanied by high levels of intestinal IL-9 transcripts and markedly enhanced mast cell numbers, might increase the intestinal permeability in these mice. We and others have observed that recruitment of mast cell progenitors to allergen challenged lung in sensitized mice is IL-9 dependent.52–54 Dramatically increased jejunal IL-9 levels were observed in the F709 mutants in our study (Fig 6), suggesting pro-amplifying effects of the intestinal epithelium on mast cell accumulation. This is further borne out by the finding, that although the sensitivity to anaphylaxis is largely mediated by hematopoietic cells such as mast cells, non-hematopoietic cells also contribute.
IgE antibodies, which in combination with mast cells serve as exquisitely sensitive biological sensors for allergen, have long been implicated as the key trigger of anaphylactic reactions in allergic individuals. Osterfeld and colleagues have shown that reactions to food allergens in parenterally sensitized mice are IgE dependent23. Thus, at first glance, our finding that the robust anaphylactic responses expressed by F709 mice in response to food allergen challenge were abrogated in IgE−/−F709 animals might not seem surprising. It is important to note though that anaphylaxis in rodents can be driven by completely IgE-independent mechanisms and that the IgG isotype can substitute for IgE.16, 17, 28 Our data show for the first time that IgE is critical in the development of active anaphylaxis to food antigens using exclusively oral routes for both sensitization as and challenge. This is in contrast to our previous findings which showed that parenterally mediated systemic active anaphylaxis could occur in the absence of IgE 16.
Strait and colleagues have shown that while the IgE pathway is more sensitive, requiring lower threshold levels of antigen for full activation, IgG-mediated responses, occurring at appropriate antibody to antigen ratios can nevertheless be lethal. Our observations regarding anaphylactic responses in response to enteral OVA in F709 mice provide important information regarding the relative contributions of IgE and IgG isotypes in the gut. Although OVA specific IgE responses were markedly higher in F709 mice than in Y709 controls, IgG1 antibodies to OVA were similar in the two groups. This finding indicates a preferential effect of IL-4Rα signaling on IgE vs. IgG production in intestinal immune responses to food allergens. More interesting though, was our finding that, in the absence of IgE, the presence of IgG1 responses in both Y709 and F709 animals was insufficient to drive anaphylactic reactions. Neither IgE−/− mice nor F709/IgE−/− double mutants displayed physiologic signs of anaphylaxis upon enteral allergen challenge despite having significant anti-OVA IgG1 titers. This observation suggests that either the amount of absorbed antigen in the gut is insufficient to trigger non-IgE pathways or that the local effector cells of anaphylaxis, likely the markedly expanded mast cells, are preferentially IgE responsive. This observation certainly lends support to the concept currently being evaluated in clinical trials that anti-IgE therapy might block food allergy responses.
Our bone marrow chimera data suggest a partial role of heightened target organ responsiveness to mast cell mediators in the anaphylaxis sensitivity of F709 mice. Finkelman and colleagues have demonstrated that administration of IL-4 immediately prior to anaphylactic challenge results in markedly stronger physiologic reactions because of enhanced tissue sensitivity to platelet activating factor, histamine, serotonin, and cysteinyl leukotrienes.14, 55 Nonhematopoietic cells express predominantly the Type II IL-4/13 receptor (IL-4Rα and IL-13Rα1).
In addition to providing important information regarding potential effects of amplified IL-4 signaling on immune sensitization, mast cell responses and anaphylaxis, we believe that the F709 mice will provide a powerful tool for investigators studying food allergy. Furthermore, the failure of enteral tolerance in these mice will permit not only investigations of physiologic tolerogenic mechanisms but may additionally provide a practical model for evaluating allergenicity of food proteins in the absence of any immune stimulating adjuvants. We anticipate that the observations presented in this report, along with the increasing availability of mouse strains with alterations in IL-4 receptor function will lead to fruitful future investigations of the pathogenesis of food allergy.
Acknowledgments
We thank Ms. Diana Kombe, Mr. Benjamin Caplan and Ms. Alanna Darling for expert technical assistance.
This project was supported by National Institutes of Health grants: NIAID R01-AI054471 and 1R21AI087666 (HCO) (HCO), 5T32AI007512-24 (CBM), 2R01AI065617 and 1R21AI080002 (TAC), AI083516 (MFG)
Abbreviations
- OVA
Ovalbumin
- CT
Cholera toxin
- SEB
Staphylococcal enteroxin B
- WT
Wild-type
- IgE−/−
IgE-deficient
Footnotes
Clinical implications
Therapeutic interventions that target the IL-4R pathway may be beneficial in restoring oral tolerance in food-allergic individuals.
Conflict of Interest Statement
None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eigenmann PA, Beyer K, Wesley Burks A, Lack G, Liacouras CA, Hourihane JO, et al. New visions for food allergy: an iPAC summary and future trends. Pediatr Allergy Immunol. 2008;19 (Suppl 19):26–39. doi: 10.1111/j.1399-3038.2008.00765.x. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Sampson HA. 9. Food allergy. J Allergy Clin Immunol. 2006;117:S470–5. doi: 10.1016/j.jaci.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med. 2009;60:261–77. doi: 10.1146/annurev.med.60.042407.205711. [DOI] [PubMed] [Google Scholar]
- 4.Chatila TA. Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends Mol Med. 2004;10:493–9. doi: 10.1016/j.molmed.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 6.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci U S A. 2008;105:7240–5. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, Wilson MS, et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wills-Karp M, Finkelman FD. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci Signal. 2008;1:pe55. doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hershey GK, Friedrich MF, Esswein LA, Thomas ML, Chatila TA. The association of atopy with a gain-of-function mutation in the alpha subunit of the interleukin-4 receptor. N Engl J Med. 1997;337:1720–5. doi: 10.1056/NEJM199712113372403. [DOI] [PubMed] [Google Scholar]
- 10.Shirakawa I, Deichmann KA, Izuhara I, Mao I, Adra CN, Hopkin JM. Atopy and asthma: genetic variants of IL-4 and IL-13 signalling. Immunol Today. 2000;21:60–4. doi: 10.1016/s0167-5699(99)01492-9. [DOI] [PubMed] [Google Scholar]
- 11.Tachdjian R, Mathias C, Al Khatib S, Bryce PJ, Kim HS, Blaeser F, et al. Pathogenicity of a disease-associated human IL-4 receptor allele in experimental asthma. J Exp Med. 2009 doi: 10.1084/jem.20091480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaeser F, Bryce PJ, Ho N, Raman V, Dedeoglu F, Donaldson DD, et al. Targeted inactivation of the IL-4 receptor alpha chain I4R motif promotes allergic airway inflammation. J Exp Med. 2003;198:1189–200. doi: 10.1084/jem.20030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tachdijian R, Al Khatib S, Schwinglshackl A, Kim HS, Chen A, Blasioli J, et al. In vivo Regulation of the Allergic Response by the Interleukin 4 Receptor Alpha Chain Immunoreceptor Tyrosine-based Inhibitory Motif. J Allergy Clin Immunol. 2010 doi: 10.1016/j.jaci.2010.01.054. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strait R, Morrist SC, Finkelman FD. Cytokine enhancement of anaphylaxis. Novartis Found Symp. 2004;257:80–91. discussion -100, 276–85. [PubMed] [Google Scholar]
- 15.Nussenzweig RS, Merryman C, Benacerraf B. Electrophoretic separation and properties of mouse anti-hapten antibodies involved in passive cutaneous anaphylaxis and passive hemolysis. Journal of Experimental Medicine. 1964;120:315–28. doi: 10.1084/jem.120.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–70. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 17.Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol. 2002;109:658–68. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 18.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–15. doi: 10.1016/j.jaci.2007.07.033. quiz 16–7. [DOI] [PubMed] [Google Scholar]
- 19.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106:150–8. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 20.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–77. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandt EB, Munitz A, Orekov T, Mingler MK, McBride M, Finkelman FD, et al. Targeting IL-4/IL-13 signaling to alleviate oral allergen-induced diarrhea. J Allergy Clin Immunol. 2009;123:53–8. doi: 10.1016/j.jaci.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osterfeld H, Ahrens R, Strait R, Finkelman FD, Renauld JC, Hogan SP. Differential roles for the IL-9/IL-9 receptor alpha-chain pathway in systemic and oral antigen-induced anaphylaxis. J Allergy Clin Immunol. 125:469–76. e2. doi: 10.1016/j.jaci.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryce PJ, Mathias CB, Harrison KL, Watanabe T, Geha RS, Oettgen HC. The H1 histamine receptor regulates allergic lung responses. J Clin Invest. 2006;116:1624–32. doi: 10.1172/JCI26150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, Fang CH, Hasselgren PO. Intestinal permeability is reduced and IL-10 levels are increased in septic IL-6 knockout mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1013–23. doi: 10.1152/ajpregu.2001.281.3.R1013. [DOI] [PubMed] [Google Scholar]
- 26.Mathias CB, Freyschmidt EJ, Caplan B, Jones T, Poddighe D, Xing W, et al. IgE influences the number and function of mature mast cells, but not progenitor recruitment in allergic pulmonary inflammation. J Immunol. 2009;182:2416–24. doi: 10.4049/jimmunol.0801569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol. 2009;123:231–8. e4. doi: 10.1016/j.jaci.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol. 2005;115:449–57. doi: 10.1016/j.jaci.2004.12.1125. quiz 58. [DOI] [PubMed] [Google Scholar]
- 29.Berin MC, Yang PC, Ciok L, Waserman S, Perdue MH. Role for IL-4 in macromolecular transport across human intestinal epithelium. Am J Physiol. 1999;276:C1046–52. doi: 10.1152/ajpcell.1999.276.5.C1046. [DOI] [PubMed] [Google Scholar]
- 30.Ceponis PJ, Botelho F, Richards CD, McKay DM. Interleukins 4 and 13 increase intestinal epithelial permeability by a phosphatidylinositol 3-kinase pathway. Lack of evidence for STAT 6 involvement. J Biol Chem. 2000;275:29132–7. doi: 10.1074/jbc.M003516200. [DOI] [PubMed] [Google Scholar]
- 31.Groschwitz KR, Ahrens R, Osterfeld H, Gurish MF, Han X, Abrink M, et al. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci U S A. 2009;106:22381–6. doi: 10.1073/pnas.0906372106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schade RP, Van Ieperen-Van Dijk AG, Van Reijsen FC, Versluis C, Kimpen JL, Knol EF, et al. Differences in antigen-specific T-cell responses between infants with atopic dermatitis with and without cow’s milk allergy: relevance of TH2 cytokines. J Allergy Clin Immunol. 2000;106:1155–62. doi: 10.1067/mai.2000.110802. [DOI] [PubMed] [Google Scholar]
- 33.Beyer K, Castro R, Birnbaum A, Benkov K, Pittman N, Sampson HA. Human milk-specific mucosal lymphocytes of the gastrointestinal tract display a TH2 cytokine profile. J Allergy Clin Immunol. 2002;109:707–13. doi: 10.1067/mai.2002.122503. [DOI] [PubMed] [Google Scholar]
- 34.Morafo V, Srivastava K, Huang CK, Kleiner G, Lee SY, Sampson HA, et al. Genetic susceptibility to food allergy is linked to differential TH2-TH1 responses in C3H/HeJ and BALB/c mice. J Allergy Clin Immunol. 2003;111:1122–8. doi: 10.1067/mai.2003.1463. [DOI] [PubMed] [Google Scholar]
- 35.Turcanu V, Maleki SJ, Lack G. Characterization of lymphocyte responses to peanuts in normal children, peanut-allergic children, and allergic children who acquired tolerance to peanuts. J Clin Invest. 2003;111:1065–72. doi: 10.1172/JCI16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe T, Katsukura H, Shirai Y, Yamori M, Chiba T, Kita T, et al. Helper CD4+ T cells for IgE response to a dietary antigen develop in the liver. J Allergy Clin Immunol. 2003;111:1375–85. doi: 10.1067/mai.2003.1466. [DOI] [PubMed] [Google Scholar]
- 37.Tiemessen MM, Van Ieperen-Van Dijk AG, Bruijnzeel-Koomen CA, Garssen J, Knol EF, Van Hoffen E. Cow’s milk-specific T-cell reactivity of children with and without persistent cow’s milk allergy: key role for IL-10. J Allergy Clin Immunol. 2004;113:932–9. doi: 10.1016/j.jaci.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 38.Scott-Taylor TH, Hourihane JB, Harper J, Strobel S. Patterns of food allergen-specific cytokine production by T lymphocytes of children with multiple allergies. Clin Exp Allergy. 2005;35:1473–80. doi: 10.1111/j.1365-2222.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, Beaty TH, Deindl P, Huang SK, Lau S, Sommerfeld C, et al. Associations between specific serum IgE response and 6 variants within the genes IL4, IL13, and IL4RA in German children: the German Multicenter Atopy Study. J Allergy Clin Immunol. 2004;113:489–95. doi: 10.1016/j.jaci.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 40.Zitnik SE, Ruschendorf F, Muller S, Sengler C, Lee YA, Griffioen RW, et al. IL13 variants are associated with total serum IgE and early sensitization to food allergens in children with atopic dermatitis. Pediatr Allergy Immunol. 2009 doi: 10.1111/j.1399-3038.2008.00815.x. [DOI] [PubMed] [Google Scholar]
- 41.Li XM, Schofield BH, Huang CK, Kleiner GI, Sampson HA. A murine model of IgE-mediated cow’s milk hypersensitivity. J Allergy Clin Immunol. 1999;103:206–14. doi: 10.1016/s0091-6749(99)70492-6. [DOI] [PubMed] [Google Scholar]
- 42.Kweon MN, Yamamoto M, Kajiki M, Takahashi I, Kiyono H. Systemically derived large intestinal CD4(+) Th2 cells play a central role in STAT6-mediated allergic diarrhea. J Clin Invest. 2000;106:199–206. doi: 10.1172/JCI8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Wijk F, Hartgring S, Koppelman SJ, Pieters R, Knippels LM. Mixed antibody and T cell responses to peanut and the peanut allergens Ara h 1, Ara h 2, Ara h 3 and Ara h 6 in an oral sensitization model. Clin Exp Allergy. 2004;34:1422–8. doi: 10.1111/j.1365-2222.2004.02062.x. [DOI] [PubMed] [Google Scholar]
- 44.Sun J, Arias K, Alvarez D, Fattouh R, Walker T, Goncharova S, et al. Impact of CD40 ligand, B cells, and mast cells in peanut-induced anaphylactic responses. J Immunol. 2007;179:6696–703. doi: 10.4049/jimmunol.179.10.6696. [DOI] [PubMed] [Google Scholar]
- 45.Knight AK, Blazquez AB, Zhang S, Mayer L, Sampson HA, Berin MC. CD4 T cells activated in the mesenteric lymph node mediate gastrointestinal food allergy in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1234–43. doi: 10.1152/ajpgi.00323.2007. [DOI] [PubMed] [Google Scholar]
- 46.Proust B, Astier C, Jacquenet S, Ogier V, Magueur E, Roitel O, et al. A single oral sensitization to peanut without adjuvant leads to anaphylaxis in mice. Int Arch Allergy Immunol. 2008;146:212–8. doi: 10.1159/000115889. [DOI] [PubMed] [Google Scholar]
- 47.Khodoun M, Strait R, Orekov T, Hogan S, Karasuyama H, Herbert DR, et al. Peanuts can contribute to anaphylactic shock by activating complement. J Allergy Clin Immunol. 2009;123:342–51. doi: 10.1016/j.jaci.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Godfraind C, Louahed J, Faulkner H, Vink A, Warnier G, Grencis R, et al. Intraepithelial infiltration by mast cells with both connective tissue-type and mucosal-type characteristics in gut, trachea, and kidneys of IL-9 transgenic mice. J Immunol. 1998;160:3989–96. [PubMed] [Google Scholar]
- 50.Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–83. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 51.Matsuzawa S, Sakashita K, Kinoshita T, Ito S, Yamashita T, Koike K. IL-9 enhances the growth of human mast cell progenitors under stimulation with stem cell factor. J Immunol. 2003;170:3461–7. doi: 10.4049/jimmunol.170.7.3461. [DOI] [PubMed] [Google Scholar]
- 52.Jones TG, Hallgren J, Humbles A, Burwell T, Finkelman FD, Alcaide P, et al. Antigen-induced increases in pulmonary mast cell progenitor numbers depend on IL-9 and CD1d-restricted NKT cells. J Immunol. 2009;183:5251–60. doi: 10.4049/jimmunol.0901471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faulkner H, Humphreys N, Renauld JC, Van Snick J, Grencis R. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur J Immunol. 1997;27:2536–40. doi: 10.1002/eji.1830271011. [DOI] [PubMed] [Google Scholar]
- 54.Temann UA, Ray P, Flavell RA. Pulmonary overexpression of IL-9 induces Th2 cytokine expression, leading to immune pathology. J Clin Invest. 2002;109:29–39. doi: 10.1172/JCI13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strait RT, Morris SC, Smiley K, Urban JF, Jr, Finkelman FD. IL-4 exacerbates anaphylaxis. J Immunol. 2003;170:3835–42. doi: 10.4049/jimmunol.170.7.3835. [DOI] [PubMed] [Google Scholar]