Abstract
A highly plausible etiology for Gulf War Illness (GWI) is that the neural damage and cognitive deficits are associated with excessive exposure to cholinesterase-inhibiting cholinergic stimulants. Our previous SPECT study provided strong indication that cerebral blood flow (CBF) in veterans with GWI may be different from those of unaffected control veterans. The present study confirmed and extended previous findings that patients with GWI have abnormal response to an inhibitory cholinergic challenge, physostigmine infusion, when compared to age-gender-education matched control veterans. The MRI-based arterial spin labeling (ASL) and phase-contrast techniques have several key advantages over SPECT, including shorter experiment duration, complete non-invasiveness, and higher spatial and temporal resolutions, and therefore may provide a cost-effective biomarker for characterization of GWI.
Keywords: Arterial spin labeling MRI, Phase-contrast MRI, Physostigmine, Brain diseases, Cerebral blood flow, Gulf War Illness
1. Introduction
Gulf War Illness (GWI) refers to chronic multisymptom illness in veterans of the 1991 Gulf War (Binns et al., 2004, Golomb, 2008, Henderson et al., 2001). A highly plausible etiology for GWI is that the neural damage and cognitive deficits are associated with excessive exposure to cholinesterase-inhibiting cholinergic stimulants (Binns, Golomb, 2004, Golomb, 2008, Henderson, Barr, 2001). This hypothesis is supported by epidemiological findings that many case definitions are linked to cholinergic stimulants such as organophosphate pesticides, pyridostigmine bromide anti-nerve agent medications, and low-level sarin nerve gas in fallout from Coalition bombing of Iraqi ammunition storage sites (Golomb, 2008). Numerous animal experiments have further demonstrated chronic brain changes, including changes in behavior and brain function (Kassa et al., 2001), alteration of cholinergic receptors (Henderson et al., 2002), reduced parasympathetic nervous system function (Morris et al., 2007), and reduced glucocorticoid production (Henderson, Barr, 2002), from exposure to these chemicals either alone or in synergistic combinations.
In a previous single photon emission computed tomography (SPECT) study conducted by our group, a thoroughly studied group of chronically ill Gulf War veterans and unaffected control veterans were given the short-acting cholinesterase-inhibiting drug physostigmine and the effects of this cholinergic challenge on cerebral blood flow (CBF) were assessed (Haley et al., 2009). The results provided strong indication that Gulf war veterans with GWI have abnormal responses to physostigmine infusion, compared to control veterans. However, the SPECT experiment is lengthy, involves injection of radioactive tracer, and requires excessively several days of washout between consecutive experiments, making it unsuitable for large-scale studies or routinely screening.
Therefore, the purposes of the present study are two fold: 1) to use a different CBF technique to confirm the findings from the earlier SPECT study; 2) to establish a cost effective technique that does not require the use of radiotracers and can be routinely performed on clinical MRI systems. To minimize the confounding effect of different patient selection criteria, we recruited the same group of subjects that were studied in the original SPECT study.
2. Methods
2.1. Subjects
Veterans, selected from members of the U.S. Naval construction battalion surveyed in 1995–1996, were classified into one of three GWI syndromes or a healthy control group based on factor analysis of symptoms (Fukuda et al., 1998, Haley et al., 1997, Haley et al., 2001, Kang et al., 2002). The mean ages of veterans of Gulf War Syndrome complexes 1 (Syn 1, characterized by impaired cognition, N = 11), 2 (Syn 2, characterized by confusion-ataxia, N = 12) and 3 (Syn 3, characterized by central pain, N = 10), and healthy controls (NC, N = 14) were 51.4 ± 6.1, 60.9 ± 6.0, 57.3 ± 6.7, and 60.1 ± 6.3 years, respectively. All subjects were male members of the same U.S. Naval Reserve battalion with similar education levels. Subjects discontinued all medications that might affect cerebral blood flow or interact with physostigmine at least three half-lives before arriving for the ASL study and were hospitalized in the UT Southwestern Medical Center Clinical Translational Research Center (CTRC) for 7 days while undergoing a week-long suite of studies including the ASL exam. The recruitment of control and ill subjects were randomized during the study and the researchers were blinded to the diagnosis during the procedures. The protocol was approved by the Institutional Review Board (IRB) of University of Texas Southwestern Medical Center. Informed written consent was obtained before the enrollment of the subjects.
2.2. Physostigmine challenge
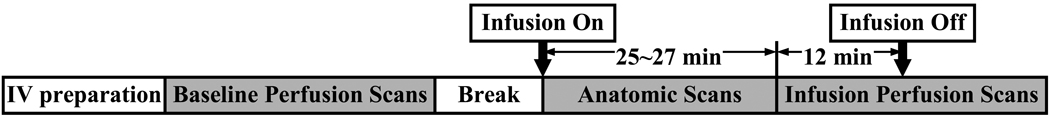
Each subject underwent two study sessions with one day gap in between, one session for CBF responses to drug infusion (challenge experiment) and the other session for CBF responses to saline infusion (control experiment). At each session subjects were prepared with an intravenous (IV) line for the infusion. The challenge experiment used the short-acting cholinesterase-inhibiting drug physostigmine, which is expected to stimulate the cholinergic system and decrease blood flow. Figure 1 illustrates the timing of the procedures in each session. Baseline perfusion scans without infusion were performed, followed by a brief break period outside the magnet. After the break, the subject was re-positioned and infusion of either saline or physostigmine was started. For physostigmine, the mean dose was approximately 0.6 mg. Since the maximal physostigmine effect is achieved by 25 minutes into the infusion and persists for at least 60 minutes more, we used the initial build-up time for anatomical scans. The perfusion scans were initiated about 25–27 minutes after the onset of infusion. During the imaging, subjects were awake with eyes closed. Before the IV infusion of physostigmine, a very low dose of glycopyrrolate (0.3 mg) was administrated IV to reduce peripheral side effects. Nearly all the MRI scans were performed in the early afternoon. In all subjects, session 1 used saline infusion and session 2 used physostigmine infusion. This order was chosen because some subjects may experience some nausea effects from physostigmine infusion and, if this happened in session 1, the stress level for session 2 might be affected. However, the participants were blinded to this order.
Figure 1.
Experimental procedures for the physostigmine challenge.
2.3. MRI methods
All experiments were performed on a 3 Tesla MRI system (Tim Trio, Siemens Medical Solutions, Erlangen, Germany). Standard configuration of body coil for transmission and 12-channel head coil for receiving was used. Two MRI sequences were used to assess cerebral blood flow (CBF): phase-contrast MRI and Arterial Spin Labeling (ASL) MRI covering the deep brain structures.
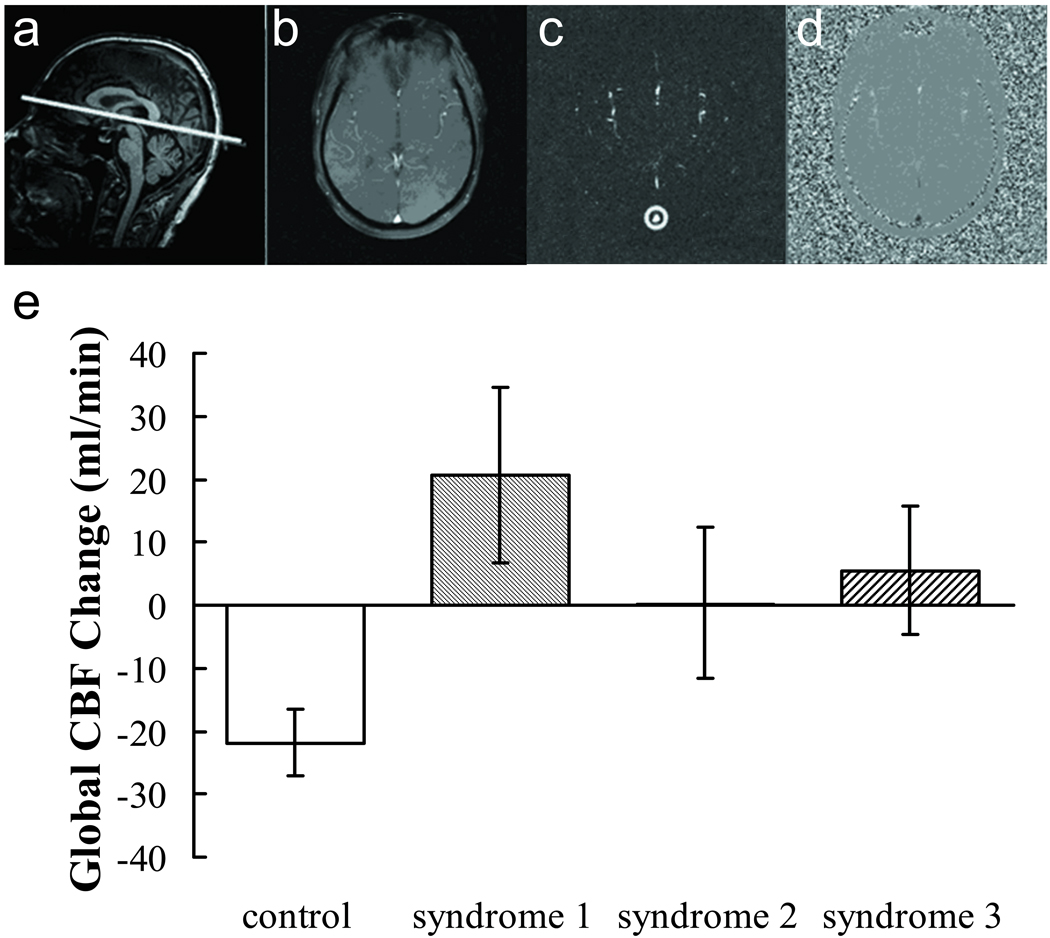
Phase-contrast MRI provides a quantitative assessment of global CBF. This technique utilizes the phase of an image to encode the velocity of moving spins. The pulse sequence is a typical gradient-echo except that a bipolar gradient is inserted before the data acquisition to encode the velocity information in the phase. Then the phase image is used to calculate the blood flow in major blood vessels. Although phase-contrast MRI does not provide regional CBF information, it is considered highly quantitative and is complementary to ASL which provides regional information but the CBF quantification is more complex. The following parameters were used: single slice, voxel size = 0.45×0.45×5 mm3, FOV = 230×230×5 mm3, maximum velocity encoding = 80 cm/s, scan duration 27 seconds. We chose to measure the flow in the sagittal sinus (Fig. 2a). Although sagittal sinus does not drain the blood flow of the entire brain, it provides a good estimation of global CBF changes with physostigmine infusion. A technological advantage of imaging the sagittal sinus is that the trajectory of sagittal sinus is relatively easy to identify, and thus can be reproducibly positioned without needing a venogram.
Figure 2.
Results of phase contrast MRI. (a) Illustration of the slice position in a representative subject. (b)–(d) show the raw image, magnitude image and phase image of the phase-contrast scan from the same subject, respectively. The manually drawn preliminary ROI (circle) is also displayed on the magnitude image (c). (e) Global CBF change due to cholinergic stimulation measured by phase-contrast MRI.
The ASL MRI was performed to cover the deep brain structures in the basal ganglia and thalamus. The following parameters were used: a modified flow sensitive alternating inversion recovery (FAIR) sequence with bolus control: bolus width = 800 ms; TR = 2.8 s, post bolus delay = 1200 ms, 10 mm gap on each side of the imaging slab, voxel size 3.48×3.48×3.48 mm3, 18 slices positioned axially, scan duration 6 minutes. Additionally, an M0 image was acquired using the same geometric parameters, with TR = 8 s and number of averages = 2.
The imaging slices in the two imaging sessions were reproducibly placed using Auto-Align, Siemens’ online co-registration and study planning tool. Due to subject-dependent variation in brain anatomy, translational adjustment was manually performed to ensure consistent slice position relative to selected anatomic landmarks across subjects, using MPRAGE T1-weighted high-resolution images for more accurate reference.
2.4. Data analysis
For phase-contrast MRI data, a preliminary ROI was drawn on the sagittal sinus based on the magnitude image (Fig. 2c). A signal intensity threshold was then applied to the magnitude image to obtain the final vessel mask. The threshold is set to be 5 times the background noise. This mask was applied to the phase image (velocity map) and the integration of the map (i.e. velocity × area) yielded cerebral blood flow, in units of mL/min. We have tested the inter-rater variability of this processing procedure and found that the results are highly consistent across raters.
The ASL MRI yielded 70 pairs of label and control images of deep brain structure. All 140 images were motion corrected with respect to the first image by using SPM. A custom MATLAB program was written to calculate absolute CBF (aCBF). Next, CBF maps were co-registered to their corresponding MPRAGE anatomical images, using SPM software. In order to conduct Region of Interest (ROI) analysis, FSL’s Integrated Registration and Segmentation Tool (FIRST) was used to extract deep brain structures in each subjects’ native space. FIRST uses mesh models trained with hand-segmented data to segment 7 subcortical brain structures: thalamus, caudate, putamen, pallidum, hippocampus, amygdala, nucleus accumbens. Another custom MATLAB program was written to create a mask for each structure and calculate the mean aCBF of each ROI. ROI analysis was also performed for cortical structures including frontal, temporal, occipital, cingulate, and insular cortices.
Statistical analysis was performed on the CBF values. Separate analysis was conducted for the global CBF values obtained from phase contrast MRI and the ASL-derived regional aCBF of the seven deep brain structures described above. A general linear mixed effects statistical model was used in which the CBF was the dependent variable and the subject group, session (saline vs physostigmine) and their interaction (group × session) were the independent variables. The within- and between-subject variance was modeled as random effects. The mixed model procedure in SAS (Cary, NC) was used for these analyses. Our primary interest was to determine whether or not the syndrome groups differed from controls at baseline (group effect) and whether or not syndrome groups differed from controls with respect to their response to physostigmine (group × session effect). For control of false positive error we employed a number of procedures in calculating individual contrast. First, we considered only planned effects/contrasts based on our a priori interest described above. In addition, we will consider a contrast only if a significant omnibus (F) test from the full mixed model was observed for the effect. Our primary tests are contrasts between each illness group and the control group. As secondary tests, the differences among the syndrome groups were also assessed. Finally, Bonferroni adjustments were performed for six comparisons within each ROI. A p value of 0.05 or less is considered statistically significant. A p value between 0.05 and 0.1 is considered having a trend of difference.
3. Results
3.1. Global CBF measured with phase-contrast MRI
Figure 2 shows the representative results of phase-contrast MRI. Figure 2a illustrates the position of the imaging slice on a sagittal image. Figure 2b–d show the raw image, magnitude image and phase image of the phase-contrast scan, respectively. The manually drawn preliminary ROI is also displayed on the magnitude image.
With physostigmine challenge, the control group showed a decrease of CBF by 21.8 ± 4.1 mL/min (P=0.004), consistent with the mechanism of physostigmine activating the inhibitory brain networks more than the excitatory networks. In contrast, the Syndromes 1, 2 and 3 GWI groups showed unchanged or slightly increased CBF upon physostigmine infusion (Fig. 2e). Comparing the control subjects to the GWI subjects (combining all syndrome groups), the CBF responses were significantly different (P=0.014). This may be because patients with GWI have lost considerable inhibitory cholinergic receptors and the overall effect of physostigmine tends towards excitatory. Mixed model analysis on the phystigmine effect showed that there was a trend of difference across groups (P=0.081). Comparing the individual Syndrome groups to the control group (NC), the cholinergic responses showed a trend of difference for Syndrome 1 (P=0.072), but no differences for Syndrome 2 and 3. No differences in CBF responses were observed among the Syndrome 1, 2 and 3 groups.
3.2. Regional CBF measured with ASL MRI
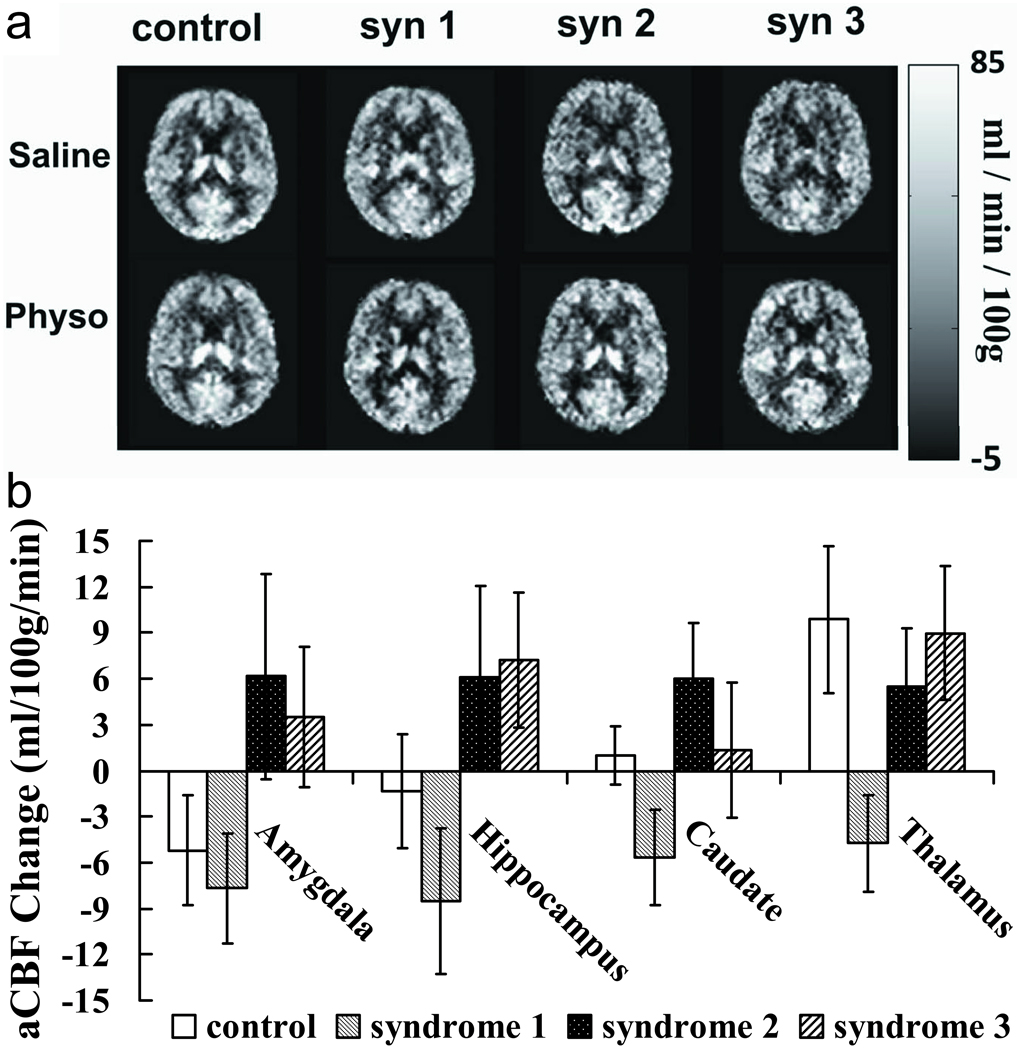
Next we investigated which brain regions contributed to the abnormal CBF responses in the GWI patients. Figure 3a shows CBF maps for each group and each infusion condition. Regional responses to physostigmine challenge for the four subject groups are plotted in Figure 3b. Among all the subcortical brain structure we analyzed, amygdala, hippocampus, caudate and thalamus showed significant group level differences in physostigmine effect according to the omnibus F-test of the mixed effect model (Table 1). The spatial pattern of the deficit is in good agreement with the previous SPECT finding (Haley, Spence, 2009). No differences were observed in physostigmine effect across groups in cortical gray matter.
Figure 3.
Regional CBF change due to cholinergic stimulation measured by standard resolution ASL MRI.
Table 1.
Summary of mixed model analysis of regional CBF changes due to cholinergic stimulation measured by standard resolution ASL MRI. P values in bold indicate significant differences (P<0.05). The other P values indicate a trend of difference (0.05–0.1).
| Comparison | P Values | ||||||
|---|---|---|---|---|---|---|---|
| Amygdala | Hippocampus | Caudate | Thalamus | Accumbens | Putamen | Pallidum | |
| Omnibus F test | 0.0073 | 0.0022 | 0.0019 | 0.0008 | NS | NS | NS |
| NC vs Syn 1 | NS | NS | NS | 0.0006 | NS | NS | NS |
| NC vs Syn 2 | 0.0557 | NS | NS | NS | NS | NS | NS |
| NC vs Syn 3 | NS | NS | NS | NS | NS | NS | NS |
| Syn 1 vs Syn 2 | 0.0179 | 0.006 | 0.0006 | 0.0505 | NS | NS | NS |
| Syn 1 vs Syn 3 | NS | 0.009 | NS | 0.0096 | NS | NS | NS |
| Syn 2 vs Syn 3 | NS | NS | NS | NS | NS | NS | NS |
As can be seen in Fig. 3b, Syndrome 2 and 3 groups showed reduced inhibitory effect in amygdala, hippocampus and caudate nucleus, compared to the control subjects. These findings in Syndrome 2 and 3 groups are also consistent with the phase contrast results, i.e. physostigmine decreased CBF in controls but increased CBF in patients. For the Syndrome 1 group, the CBF responses appear to be different from those observed using phase-contrast MRI. Specifically, the inhibitory effect of physostigmine is intact or even enhanced in Syndrome 1. The CBF responses for the syndrome 1 groups in deep brain structures are also significantly different from the responses for the Syndrome 2 and 3 groups (Table 1). No differences were observed between Syndrome 2 and 3 groups.
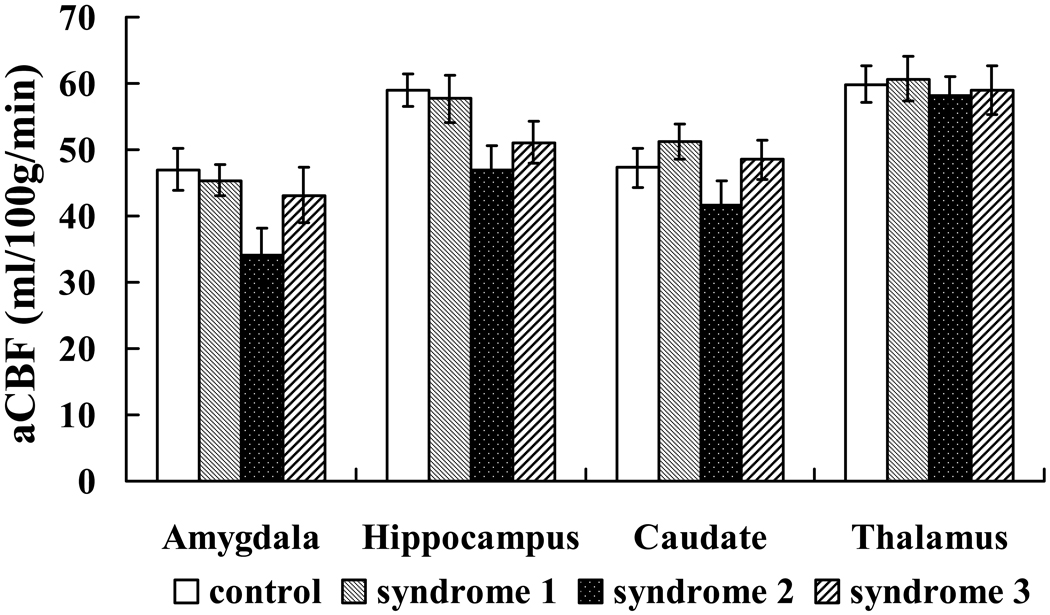
Comparison of baseline CBF (with saline infusion) across subject groups showed significant difference in amygdala (P=0.008) and a trend of difference in hippocampus (P=0.051). Comparing individual groups, it was found that Syndrome 2 patients had significantly lower CBF values in amygdala (P=0.006) compared to the control subjects and a trend of difference (P=0.072) in hippocampus (Figure 4).
Figure 4.
Comparison of baseline CBF across subject groups.
4. Discussion
The present study showed that patients with Gulf War Illness have abnormal responses to an inhibitory cholinergic challenge, physostigmine infusion, when compared to age-matched control veterans. The differential CBF responses to physostigmine challenge are similar to those found in the SPECT studies performed in 1997–1998 (Haley, Spence, 2009), indicating that the cholinergic deficits still persist a decade later. These findings, together with those reported in previous studies (Heaton et al., 2007, Menon et al., 2004, Meyerhoff et al., 2001, Proctor et al., 2006, Roland et al., 2000), indicate that there may be a nucleus of Gulf War veterans suffering from variants of a chronic encephalopathic syndrome related to different combinations of abnormal resting metabolism and cholinergic responsiveness of neurons in the brain, especially in deep brain structures.
Our findings further suggest that Syndrome 1 GWI may involve different neuropathologic mechanisms from those underlying the Syndromes 2 and 3. The Syndrome 1 group, which is characterized by “impaired cognition”, had statistically significant excessive decreases in CBF in deep brain structures compared to Syndrome 2 and 3 groups. Specifically, the inhibitory effect of physostigmine is intact or even enhanced in amygdala, hippocampus and caudate in Syndrome 1. These responses are different from those observed using phase-contrast MRI, in which the Syndrome 1 group showed an increase in global CBF with physostigmine challenge. The data suggest that cholinergic deficit in Syndrome 1 subjects may be located in other brain regions.
The current MRI study showed results similar to the previous SPECT study (Haley, Spence, 2009). The MRI-based technique has several key advantages compared to the SPECT technique. The SPECT method requires intravenous injection of the radiotracer technetium-99m hexamethylpropyleneamine oxime (99mTc-HMPAO) into the patients at the end of physostigmine infusion, and a ninety-minute washout period before the SPECT scans to allow the radiotracer to clear from blood and facial structures. In contrast, the MRI-based technique is completely non-invasive, non-radioactive, and more time efficient. Although the two infusion MRI scans in the present study were 2 days apart in order to be comparable with the earlier SPECT study (and to allow a comparison SPECT re-study, results not presented here), the flexibility of the MRI method permits conducting the two sessions, saline and physostigmine infusion, back-to-back in the same day. Such testings are currently being performed in our laboratory in a follow-up study of Gulf War veterans from a statistically representative samples.
5. Conclusion
The present study confirmed and extended previous findings that patients with Gulf War Illness have abnormal responses to an inhibitory cholinergic challenge, physostigmine infusion, when compared to control veterans. The findings are in general agreement with those observed in the SPECT studies performed in 1997–1998, indicating that the cholinergic deficits still persist a decade later. Previous reports were conducted using a radiotracer based SPECT technique and required a total more than 3 hours for the infusion and brain scan and three days for the physostigmine and control saline infusions. Our MRI-based technique has several key advantages including shorter experiment duration, complete non-invasiveness and higher spatial and temporal resolutions. This new technique may provide a cost-effective biomarker for characterization of Gulf War Illness.
Acknowledgements
This study was supported by IDIQ contract VA549-P-0027, awarded and administered by the Department of Veterans Affairs Medical Center, Dallas, TX, by DOD Grant Number DAMD 17-01-1-0741 from the U.S. Army Medical Research and Materiel Command, and by NIH (NCRR) Grant Number UL1RR024982. The content does not necessarily reflect the position or the policy of the Federal government or the sponsoring agencies, and no official endorsement should be inferred.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement
The authors declare that there are no conflicts of interest.
References
- Binns JH, Golomb BA, Graves JC, Haley RW, Knox ML, Meggs WJ, et al. Scientific Progress in Understanding Gulf War Veterans' Illnesses: Report and Recommendations. Washington: Illnesses RACoGWV, editor. 2004 Available at http://www1.va.gov/rac-gwvi/docs/ReportandRecommendations_2004.pdf.
- Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA. 1998;280:981–988. doi: 10.1001/jama.280.11.981. [DOI] [PubMed] [Google Scholar]
- Golomb BA. Acetylcholinesterase inhibitors and Gulf War illnesses. Proc Natl Acad Sci U S A. 2008;105:4295–4300. doi: 10.1073/pnas.0711986105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley RW, Kurt TL, Hom J. Is there a Gulf War Syndrome? Searching for syndromes by factor analysis of symptoms. JAMA. 1997;277:215–222. [PubMed] [Google Scholar]
- Haley RW, Luk GD, Petty F. Use of structural equation modeling to test the construct validity of a case definition of Gulf War syndrome: invariance over developmental and validation samples, service branches and publicity. Psychiatry Res. 2001;102:175–200. doi: 10.1016/s0165-1781(01)00241-4. [DOI] [PubMed] [Google Scholar]
- Haley RW, Spence JS, Carmack PS, Gunst RF, Schucany WR, Petty F, et al. Abnormal brain response to cholinergic challenge in chronic encephalopathy from the 1991 Gulf War. Psychiatry Res. 2009;171:207–220. doi: 10.1016/j.pscychresns.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Heaton KJ, Palumbo CL, Proctor SP, Killiany RJ, Yurgelun-Todd DA, White RF. Quantitative magnetic resonance brain imaging in US army veterans of the 1991 Gulf War potentially exposed to sarin and cyclosarin. Neurotoxicology. 2007;28:761–769. doi: 10.1016/j.neuro.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Henderson RF, Barr EB, Blackwell WB, Clark CR, Conn CA, Kalra R, et al. Response of rats to low levels of sarin. Toxicol Appl Pharmacol. 2002;184:67–76. [PubMed] [Google Scholar]
- Henderson RF, Barr EB, Blackwell WB, Clark CR, Conn CA, Kalra R, et al. Response of F344 rats to inhalation of subclinical levels of sarin: exploring potential causes of Gulf War illness. Toxicol Ind Health. 2001;17:294–297. doi: 10.1191/0748233701th105oa. [DOI] [PubMed] [Google Scholar]
- Kang HK, Mahan CM, Lee KY, Murphy FM, Simmens SJ, Young HA, et al. Evidence for a deployment-related Gulf War syndrome by factor analysis. Arch Environ Health. 2002;57:61–68. doi: 10.1080/00039890209602918. [DOI] [PubMed] [Google Scholar]
- Kassa J, Koupilova M, Herink J, Vachek J. The long-term influence of low-level sarin exposure on behavioral and neurophysiological functions in rats. Acta Medica (Hradec Kralove) 2001;44:21–27. [PubMed] [Google Scholar]
- Menon PM, Nasrallah HA, Reeves RR, Ali JA. Hippocampal dysfunction in Gulf War Syndrome. A proton MR spectroscopy study. Brain Res. 2004;1009:189–194. doi: 10.1016/j.brainres.2004.02.063. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Lindgren J, Hardin D, Griffis JM, Weiner MW. Metabolic abnormalities in the brain of subjects with Gulf War illness. Proc Intl Soc Mag Reson Med. 2001 [Google Scholar]
- Morris M, Key MP, Farah V. Sarin produces delayed cardiac and central autonomic changes. Exp Neurol. 2007;203:110–115. doi: 10.1016/j.expneurol.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Proctor SP, Heaton KJ, Heeren T, White RF. Effects of sarin and cyclosarin exposure during the 1991 Gulf War on neurobehavioral functioning in US army veterans. Neurotoxicology. 2006;27:931–939. doi: 10.1016/j.neuro.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Roland PS, Haley RW, Yellin W, Owens K, Shoup AG. Vestibular dysfunction in Gulf War syndrome. Otolaryngol Head Neck Surg. 2000;122:319–329. doi: 10.1067/mhn.2000.105783. [DOI] [PubMed] [Google Scholar]