Abstract
The rapid advancement in basic biology knowledge, especially in the stem cell field, has created new opportunities to develop biomaterials capable of orchestrating the behavior of transplanted and host cells. Based on our current understanding of cellular differentiation, a conceptual framework for the use of materials to program cells in situ is presented, namely a domino versus a switchboard model, to highlight the use of single versus multiple cues in a controlled manner to modulate biological processes. Further, specific design principles of material systems to present soluble and insoluble cues that are capable of recruiting, programming and deploying host cells for various applications are presented. The evolution of biomaterials from simple inert substances used to fill defects, to the recent development of sophisticated material systems capable of programming cells in situ is providing a platform to translate our understanding of basic biological mechanisms to clinical care.
Keywords: Biomaterials, scaffolds, stem cells, translational medicine, tissue engineering
Introduction
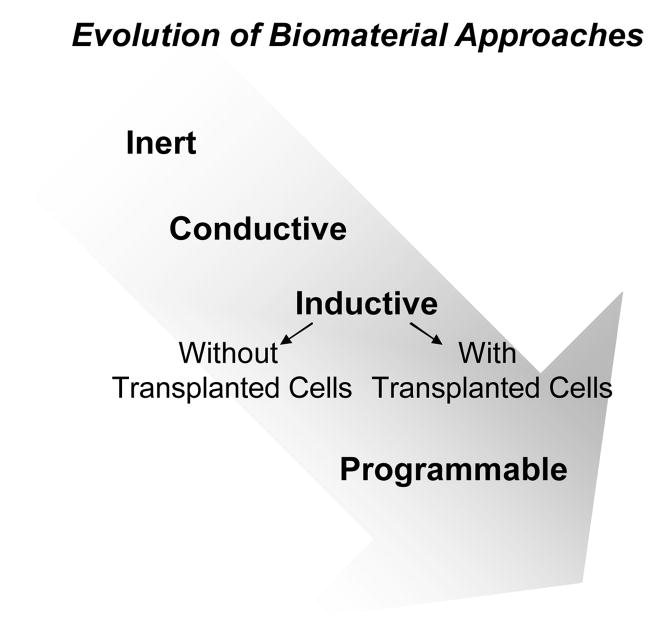
The mention of materials in dentistry usually conjures up images of artificial substitutes to fill tooth defects in restorative or prosthodontic procedures. The first generation of these materials were designed to be as inert as possible so as to illicit a minimal host tissue reaction. However, the popular use of implants has brought the role of ‘bio’ materials in dental surgical sciences to the forefront with their implicit biological interactions as a key frontier at the interface of material engineering and biological sciences. Based on their ability to modulate biological processes, modern biomaterials are typically defined as either conductive or inductive (Figure 1). Conductive materials are meant to provide a barrier or scaffolding function and physical support to aid host driven regeneration. A good example in periodontics is the use of polytetrafluroethane (PTFE) membranes for Guided Tissue Regeneration (GTR), or application of macroporous alginate or collagen sponges for treating cranial defects (Parrish et al., 2009, von Lindern et al., 2002). Inductive materials, in contrast, are aimed at inducing host cells to undergo specific biological responses that can lead to regeneration. These materials usually incorporate controlled delivery systems for biological agents like growth factors, cDNA or pharmaceuticals to control cell responses. The use of biomaterials as carriers for transplanted cells intended to enhance surgical restoration or re-engineering of craniofacial defects resulting from trauma or congenital defects is under intense investigation. However, limitations of current cell therapies include the need to harvest autologous bone or soft tissues, limited cell yield and donor site morbidity (Mooney & Vandenburgh, 2008).
Figure 1.
Cartoon showing evolution of biomaterial approaches from initially biologically noninteractive (inert), to a permissive (conductive), and then to actively promoting regeneration (inductive) with bioactive agents and/or exogenously transplanted cells. Present materials systems are now capable of programming host or transplanted cells.
As the functions of the biological interface and inductive properties of these materials became more evident, the importance of promoting a defined tissue-interaction with the materials has become increasing clear. This usually involves either controlled release of soluble biomolecules (delivery of soluble cues) and/or tailoring the material biophysical-biomechanical properties (insoluble cues) to initiate cellular responses (Langer & Vacanti, 1993, Discher et al., 2009, Huebsch & Mooney, 2009). Examples of these inductive approaches in dentistry include the use of growth factors and gene delivery of factors like bone morphogenetic protein (BMP) 2 and 7 to improve osseous healing (King & Cochran, 2002, Jung et al., 2003, Taba et al., 2005), platelet derived growth factor (PDGF) and fibroblast growth factor (FGF) seeded scaffolds and implants (Murakami et al., 1999, Kitamura et al., 2008) and the use of enamel matrix protein to promote periodontal regeneration (Bosshardt, 2008, Esposito et al., 2009). Major limitations of these approaches include cost, an inability to precisely control factor efficacy as tissue fluid dilutes-diffuses the agents from the delivery site, and safety issues due to poor control over systemic distribution. Materials capable of responding to in vivo or external signals (‘smart’ responsive materials) have also gained much interest for use as need-based, sense and respond systems (Furth et al., 2007, Huebsch & Mooney, 2009).
There is considerable interest in utilizing either lineage restricted cells or cells with increasing stemness as therapeutic agents. The former approach is aimed at repopulating a target niche population such as osteoblasts in a calvarial defect, myoblasts in a muscle repair or endothelial precursors to aid therapeutic vasculogenesis (Hill E et al.2006, Kaigler et al., 2006, Silva E et al 2008). The latter use of cells with increasing stemness has the power of providing multi-lineage cellular subsets that would be required in complex in vivo biological scenarios like regeneration of organ systems. Both approaches at the moment have significant limitations in terms of survival of transplanted cells, host integration, immune rejection and the risk of transformation of transplanted stem cells. Cell transplantation approaches also have other significant practical limitations including technical (cost, processing, storage and quality control), medical and ethical issues (Mooney & Vandenburgh, 2008).
The development of materials to direct biological process has spanned two distinct forums. First, these materials are being used to create in vitro 3D tissue equivalents to aid investigational studies or perform screening. Second, materials specifically developed for in vivo therapeutic approaches are designed to specifically interact and modulate transplanted cells and host biological responses. Recent exciting work in vitro with biomaterials has adapted combinatorial properties to simulate or mimic the in vivo conditions of the tissues being studied such as spleen and liver (Khetani & Bhatia, 2008, Yung et al., 2009) but this will not be addressed in this review. In contrast to the current cell transplantation approaches, biomaterial strategies under development aim to build off recent insights into mechanistic underpinnings of stem cell biology and immunology, with the goal to use materials alone to mediate recruitment, programming and trafficking of host cells, obviating the need for transplanting cells (Figure 2) (Mooney & Vandenburgh, 2008). Further, this concept of programming either host or transplanted cells in situ has gained ground with the advent of nanoscale designed and injectable systems that provide flexibility in terms of biological control and ease of handling for a broad range of applications. This review highlights the changing framework of our current understanding of basic biological mechanisms that is guiding the design of materials to program host cells, and provides recent examples of various applications. We hope to highlight the promise of biomaterials in translational medicine as tools capable of applying basic science advances to clinical care.
Figure 2.
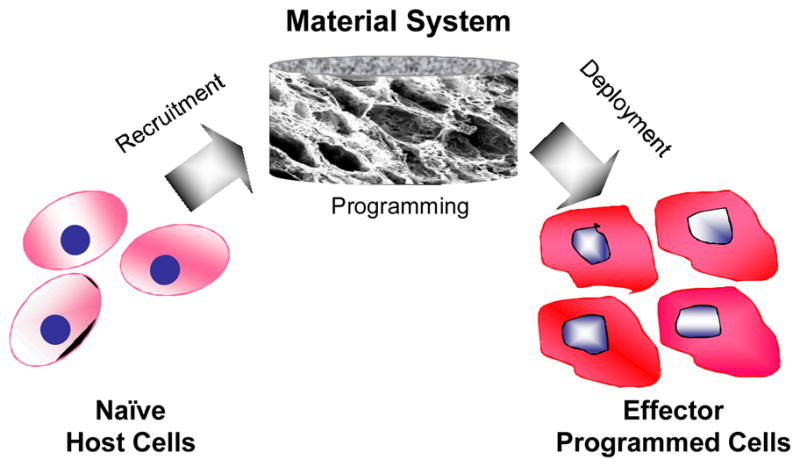
Programming cells in vivo with materials only involving first, recruiting naive host cells, then programming material-resident cells and finally, deploying programmed cells to drive biological processes, all without a need for ex vivo cell manipulations.
Overview of Cellular Differentiation
Information incorporated into material systems are intended to modulate biological responses, including Initiation (regulating cell cycle phase of target cell population), Proliferation (controlled increase in specific lineage subsets), tissue-organ Patterning (including apoptosis), Trafficking (chemotactic cell migration and release) and Maturation (morpho-differentiation and functional activation). In any given application, a combination of these effects is usually desired for final therapeutic outcomes. For example, proliferation may be coupled with maturation, or morpho-differentiation coupled to patterning of target cell populations. A key issue in the design of programmable materials is our understanding of the regulatory pathways, as this typically limits our ability to precisely modulate the biological processes.
The question of pivotal cues
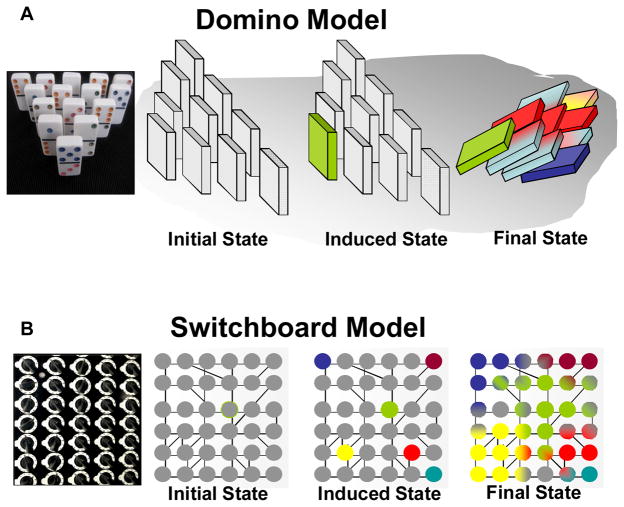
A critical question in directing cellular behavior is whether single or multiple pivotal cues are necessary and/or sufficient to be included in the design of material approaches (Figure 2) to appropriately program cells in situ. Based on our current understanding of cellular differentiation, two conceptual models for material based programming to direct biological behavior can be summarized as either a Domino model or the Switchboard model (Figure 3). Both models rely on the basic premise that the differentiated state of a cell is defined by its transcriptome and subsequent proteome which, in turn, defines the form and function of the cells, the tissues, and the organs they form. Both mechanisms rely on local triggering events that cause a global change in the genotypic landscape. The basic difference between the two is that the domino model is based on a single initiation step, genetic or epigenetic, that can trigger global changes, while the switchboard model relies on multiple, independent, spatially distinct but temporally concurrent events to initiate a cascade of effector biological pathways. It is important to emphasize here that to achieve an ultimate biological outcome, even the domino model results in multiple sequential processes but, in contrast to the switchboard model, these would be temporally consecutive (Figure 3). Spatial and quantitative aspects of these cues in various biological contexts are being intensely investigated but remain to be fully elucidated.
Figure 3.
The two broad conceptual frameworks underlying material based programming approaches for directing differentiation, (A) Domino Model with photograph of dominos (left), the scheme (right) showing the baseline (initial) state of a cell that can be induced with a single event (green colored domino) that in turn starts of a cascade of events (different colors) that can modulate cellular response and behavior, (B) Switchboard model with photograph (left) and scheme (right) depicting the initiation of multiple primary events (various colors) simultaneously that in turn bring about a complex change (partial/overlapping domains are represented by bi-colored gradients) in cellular response and behavior.
Where are the target sites? Intrinsic versus Extrinsic cell regulators
A key question for material design is what types and kind of regulation it should provide? Material systems can deliver regulated cues in either a local or global (soluble cue via systemic circulation) format and it becomes increasingly apparent that its sphere of influence must arise along its spatial scale of biological interactions. The centricity of genetic versus epigenetic determination of a cell’s genotype, and hence phenotype, has long been debated. The classical somatic cell nuclear transfer experiments, the unraveling of the complete genome and ability to clone whole organisms are clear evidence that the nuclear genetic information are key regulatory elements in the ultimate phenotype and functionality of cellular function (Gurdon, 1964, Campbell et al., 1996, Venter et al., 2001, Lander et al., 2001). But it is also clear that a higher level of integrative function lies in the regulatory framework controlling gene expression as demonstrated the global long range interactions of the genome defining spatial correlation of active versus inactive chromatin structure and hence its gene expression as well as global transcriptional modifiers of the whole genome such as the Polycomb and Tri-thorax group of proteins (Schwartz & Pirrotta, 2007, Schuettengruber et al., 2007, Lieberman-Aiden et al., 2009, Misteli & Soutoglou, 2009). These recent discoveries have opened new vistas in our understanding and, potentially our ability to regulate gene expression via delivery of these intrinsic cues via material systems.
The major motivation for designing biomaterials to deliver extrinsic regulatory cues arose from the discovery of growth factors, morphogen cues and extracellular molecules that bind cellular receptors to enable a cell to sense and respond in an integrative manner via complex cell signaling networks (Sporn & Roberts, 1986).. As a single event, stochastic or deterministic, can potentially tilt the physiological state into a pathological state or, vice versa, correct a diseased state (Aldridge et al., 2006), regulation of normal homeostatic mechanisms via active cell programming will hinge on precise and effective use of extrinsic and intrinsic regulators. The interplay between extrinsic and intrinsic growth factors in terms of receptor expression or transcriptional state of the cell demonstrate opportunities to manipulate these regulatory networks, both within and outside the cell, across various length scales. Other possibilities include the incorporation of biophysical and biochemical elements in material systems to manipulate non-equilibrium, steady state behavior of the targeted biological system. Thus, modulation of these key intrinsic and/or extrinsic regulatory elements provide a logical target site for material based cues to direct biological processes at the cell, tissue and organismal level.
Programming versus Re-Programming
Another important question in biomaterial design is whether it should aim to program or reprogram cells. Most data supporting theories of programming comes from our understanding of cellular differentiation, especially during embryonic development. Carefully done studies in a variety of organisms have traced the genome wide expression of lineage specific factors, most elaborately with the hematopoetic cells (Orkin & Zon, 2008, Slack, 2009). Recent advances in the inducible stem cell field, with its demonstration that either a single or a select combination of transcription factors can induce stemness supports the promise of reprogramming cells via both the Domino and Switchboard models of differentiation (Takahashi & Yamanaka, 2006, Meissner et al., 2007, Kim et al., 2009). While these studies definitively establish reversibility of a cell fate by re-programming to a stem-like totipotent state, it is important to acknowledge that this would rarely be a significant therapeutic endpoint application, as the resultant undifferentiated cell population would be devoid of function and could form a neoplastic tumor. Reprogramming would be simply the first step of a subsequent differentiation program to generate therapeutically useful cells. The knowledge that one can directly switch lineages of one differentiated tissue type into another in a controlled manner termed cellular or lineage reprogramming, trans-differentiation or trans-determination (Hadorn, 1968, Brambrink et al., 2006, Zhou et al., 2008, Slack, 2009) will probably have significant implications for material based programming in the future.
Implications for Programmable Material Design
The current premise of material design is that incorporation of extrinsic signals, such as growth factors or cytokines can drive and maintain a differentiated state of a cell, or intrinsic factors, such as transcription factors or regulatory RNA molecules can manipulate complex cellular regulatory networks and thus, program cells. Delivery of small molecules that can act at various cellular compartments, extrinsically or intrinsically, to achieve programming is also at an early stage of investigation. Material approaches may exploit inducible cellular states as an initial step to recruit host cells, reprogram these cells to perform specialized functions and promote integration in the host (Figure 2). As per the domino model, materials could provide the key, decisive cue in a controlled spatial and temporal distribution that would dictate the biological responses and eventual therapeutic outcomes (Figure 3). However, a single factor may often not be sufficient in a more complex in vivo scenario. The switchboard model which utilizes multiple cues may provide the most efficacious route in many situations (Figure 3). The material systems described below exploit these models to recruit and/or program host and transplanted cells to generate desirable outcomes.
Design Principles for Material Systems for Directed Differentiation
This review focuses on two aspects of materials design, the regulation of soluble cues via regulated release or uptake, and presentation of insoluble cues to provide loco-regional physical and mechanical niches to program and direct cells in vivo (Figure 2 and 3).
Soluble Cues - regulated presentation of biomolecules
A variety of soluble signaling biomolecules including proteins such as growth factors and cytokines, nucleic acids encoding a gene of interest or regulatory transcriptional factors, or pathway specific small molecules can direct cellular responses. Although the use of controlled delivery of protein factors has been the most explored application of polymeric systems to date, major challenges include low payload, and risk of denaturation and loss of activity during fabrication. To address these issues, many investigators have been exploring the presentation of nucleic acids that can be taken up by the host cells following release from polymeric systems, to effect the expression of the downstream effector proteins in a context dependent manner. A third more recent approach is the use of pathway specific small molecules. These approaches are individually discussed below.
Delivery of growth factor and morphogens
The proof of principle that various growth factors can induce a biological response has been demonstrated by directly injecting dissolved factors into a tissue (Driever et al., 1990). However, transient and uncontrolled tissue exposure with this delivery approach limits biological response and has spurred the development of polymeric delivery systems. Material systems providing sustained and localized delivery have led to increased efficacy and therapeutic utility of molecules such as BMP (2, 4 or 7) and TGF-β (1, 2 or 3) for osseous healing, bFGF, EGF, PDGF-AA, AB or BB and IGF-1 for soft tissue healing, especially in periodontal defects, VEGF, HGF, PDGF in vasculogenesis, and IGF, HGF in muscle repair, among others (Alsberg et al., 2001b, Mao et al., 2006). Materials used in these contexts have varied from natural polymeric systems like alginate and collagen, ceramics such as hydroxyapatite and tricalcium phosphates, to synthetic materials ranging from polylactide-co-glycolide, poly(methyl methacrylate), poly(dioxanoneco-glycolide) or polyethylene oxide/poly butylene copolymers.
Key issues with delivering these cues include the concentration expected to be efficacious in the biological context, sustained temporal presentation of the factor at these concentrations, and the spatial distribution due to zoning effects (Chen et al., 2007). The precise amount of the growth factor required for each targeted cell lineage is also critical, as it is well known that the growth factors have a wide range of physiological roles in a context and concentration dependent manner. For example, the use of TGF-β1 in concentrations ranging from 200–900ng/ml were most effective in aiding osteochondral repair, while concentrations above 900ng/ml resulted in adverse effects such as osteophyte formation, synovitis and cartilage erosions (Mierisch et al., 2002). Similarly, optimal GM-CSF concentrations resulted in successful recruitment and proliferation of dendritic cells into polymeric system but higher concentrations (>100ng/ml) inhibited lymph node migration, and expression of key surface receptors (CCL19, CCR7 and MHCII) essential for their function (Ali et al., 2009b).
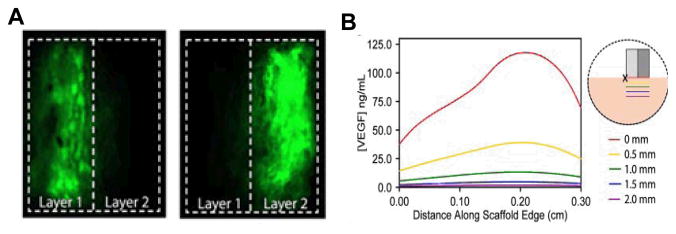
A single ‘burst’ release of a growth factor may be appropriate, as per the domino model, to kick start a biological response, assuming the resident cells are responsiveness and capable of executing the desired function (Figure 3). If the agent needs to be maintained at sustained levels for longer durations, a sustained release profile is more appropriate. Both of these release kinetics are routinely achieved with material systems by associating the factors with the carrier via covalent interactions, physical adsorption and/or entrapment (Richardson et al., 2001). As an ideal material will allow its own replacement with regenerating host tissue, regulating the material degradation rate provides a tool to both tailor availability of the factor (via disintegration of polymer material over time) and impact the volume of regenerating tissues. The complete loss of material and, hence the soluble cue would thus conveniently terminate its action, preventing undesirable sequelae of the factor. The spatial location and release kinetics defines the resulting temporal concentrations and spatial distributions resulting in gradient fields (Figure 4) (Chen et al., 2007).
Figure 4.
Distribution of material deployed growth factor to develop morphogen fields, (A) PLG Scaffolds with spatially compartmentalized VEGF were fabricated and loaded protein remains confined to each layer, dotted lines indicates separation between layers, (B) Predicted VEGF distribution in ischemic hindlimbs with scaffolds designed to create a VEGF gradient extending away from the femoral artery/vein (X) into muscle tissue (pink), guiding new capillaries to orient down ischemic limb. VEGF concentrations in a layered scaffold were modeled and solved in COMSOL, and steady-state VEGF concentrations, predicted to be reached after 30 min, are shown. Images adapted with permission from FASEB Journal (Chen et al., 2007).
The use of a single cue might be most effective in certain scenarios, such as normal healing of bone or soft tissue, but in more complex cases where there is such significant loss of tissue that the resident host milieu cannot correct easily, or in the context of underlying pathological states such as in diabetic wounds, a combination of factors acting in a synergistic manner may be more efficacious, as per the switch board model (Figure 3). The factors could be administered simultaneously if the target populations are distinct and no significant cross-talk is expected, such that each factor may act on distinct, non-redundant components of the targeted process. Recently, this approach was successfully used in muscle regeneration where VEGF was used to recruit endothelial and build new vessels, while IGF was used to stimulate skeletal muscle regeneration (Figure 5) (Borselli et al., 2009). Alternatively, distinct factors may be presented in a phased manner to move along different stages of the same process. For example, delivery of VEGF, a potent pro-angiogenic growth factor, can aid therapeutic revascularization in a limb ischemia model (Silva et al., 2008). In this case, VEGF acted to recruit host cells via sprouting and neoangiogenesis from pre-existing vessels. While these findings were significant versus baseline controls, an addition of slower released PDGF led to subsequent recruitment of smooth muscle cells to the newly formed vessels, aiding their maturation and increased perfusion to even higher levels (Figure 6) (Richardson et al., 2001, Sun et al., 2010). Similar combinatorial approaches with multiple growth factors have been successfully demonstrated in angiogenesis with FGF-PDGF (Cao et al., 2003), and osseous repair with BMP4-VEGF (Huang et al., 2005a) and TGFβ-IGF (Kandziora et al., 2002). Although the provision of single or multiple soluble cues may initiate desirable sequelae of events, they must be carefully tailored to be self-limiting and appropriately compartmentalized to prevent adverse events. Careful mechanistic studies in appropriate in vitro models will be critical in aiding future design of material systems and guiding their effective use in vivo. Although unexplored to date, an interesting concept common in developmental processes such as axial patterning (Struhl et al., 1992) involves the spatially distinct availability of two agonists, such as complimentary growth factors, or one agonist and one antagonist, such as pathway specific inhibitors, to produce precise and spatially restricted gradient boundaries to aid regeneration.
Figure 5.
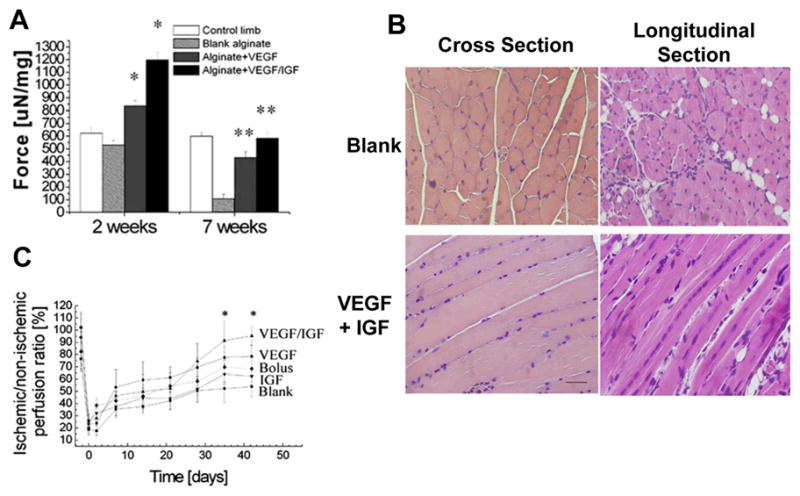
Promoting skeletal muscle regeneration in a hind limb ischemia model with dual delivery of VEGF and IGF-1 (A) Functional muscle regeneration, measured as tetanic force of the anterior tibialis muscles of mice at 2 and 7 weeks after induction of ischemia, and no treatment (blank alginate), or treatment with VEGF alone and combination of VGEF and IGF-1 (VEGF+IGF-1), normalized to each muscle’s weight to obtain weight-corrected specific force. Mean values are presented with SD, p<0.05. Control condition of undamaged muscle (control limb) is also shown, (B) Representative cross-section and longitudinal tissue sections from tibialis ischemic muscles treated with alginate gel only (blank) or alginate gels delivering VEGF and IGF1 at postoperative week 2, stained with H&E, scale bars 50 μm (C) Blood perfusion measured by Laser Doppler perfusion imaging (LDPI) of C57 mice hindlimbs treated with blank alginate gel, alginate gel delivering VEGF or IGF-1 alone or VEGF and IGF1 together and bolus delivery of VEGF and IGF1 in PBS, *p < 0.05 vs. blank alginate gel and bolus; mean values are presented with SD. Images adapted from the original article in Proceedings of the National Academy of Sciences, USA (Borselli et al., 2009).
Figure 6.
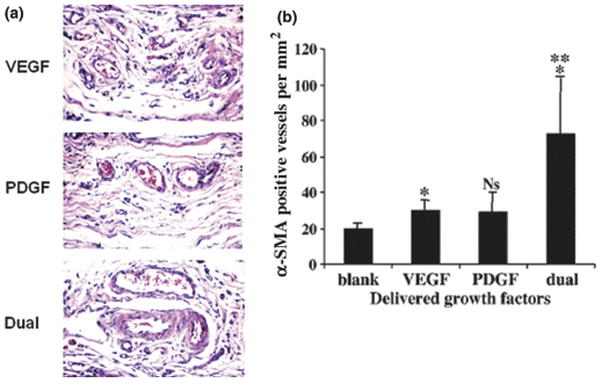
Sequential growth factor delivery from polymer demonstrating their synergistic efficacy in promoting vessel maturation in non-obese diabetic mice subjected to femoral artery ligation (A) Scaffolds incorporating VEGF alone, PDGF alone, and both VEGF/PDGF were implanted in ischemic hind limb. Compared to scaffolds rapidly releasing VEGF (Top panel) or slower release of PDGF (middle panel), the scaffold releasing both VEGF and PDGF (bottom panel) demonstrated increased vascular density, with mature vessels (magnification 400x) (B) Quantitation of α smooth muscle actin staining of tissue sections demonstrating mature, well formed vessels with dual delivery (magnification was 1000x). Images adapted from the original article in Nature Biotechnology (Richardson et al., 2001).
Nucleic acids
Genetic information encoded in nucleic acid sequences are key drivers of cellular form and function. Although extraneous nucleic acids released by material systems and taken up by target cells are not expected to be subjected to the same tightly regulated expression as endogenous genetic elements, significant control can be exerted with a variety of approaches and provide a need-based sustained expression of the factor. To specifically facilitate uptake of nucleic acids by target cells, viral or non-viral vectors are routinely used. The use of viral gene transfer from a material depot has the advantage of sustained, high expression of target gene due to the high transfection efficiencies of viruses (Shin et al., 2010). However, viral vector delivery carries the risk of non-selective cell transduction, immunogenicity and random permanent integration. Non-viral modes of gene transfer include the use of polycationic liposomes such as polyethylenimine, polylysine or polyaminobutyl glycolic acid to condense plasmid DNA that can then be incorporated into polymeric systems like PLGA or collagen scaffolds for sustained delivery. This approach has been used to incorporate BMP-4 or parathyroid hormone cDNA into collagen sponges to induce bone formation in critical defects in rats and beagle dogs (Fang et al., 1996, Bonadio et al., 1999). A similar approach using VEGF 165 cDNA has been shown to improve bone formation in a mouse femoral fracture healing model (Keeney et al., 2010). A potential limitation with nucleic based approaches is that some proteins that undergo complex post-translational processing for their complete biological activity may not be best suited for this approach. For example TGF-β is secreted as a latent complex, and is activated by various physico-chemical or biological means. Thus, gene delivery of a full length TGF-β could result in increased protein expression, but the resultant protein will not be physiologically viable if it remains in its latent form (Munger et al., 1999).
The use of transcription factors as master regulators of gene expression have been demonstrated in the inducible stem cell field and material based approaches may enable one to exploit these factors in vivo. Ongoing work has already utilized simple nucleic acid sequences for specific applications such as the use of cytosine-phosphate-guanisine (CpG) oligonucelotide to activate immune surveillance mechanisms. A recent combinatorial strategy described the use of GM-CSF and CpG-ODN to recruit and proliferate dendritic cells which were then sensitized to melanoma tumor lysates to activate potent anti-tumor responses (Figure 7) (Ali et al., 2009a). These material systems could be modified for other tumor types as well as infectious diseases and immune diseases where one desires to program or reprogram the immune system.
Figure 7.
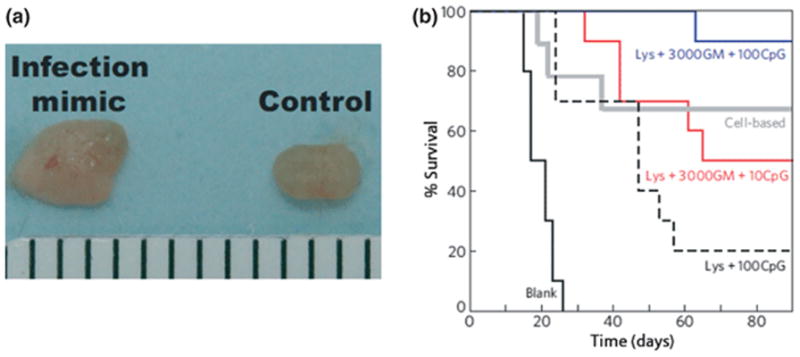
Materials to recruit, program and deploy anti-tumor dendritic APCs in treating melanomas (A) Inguinal lymph nodes from normal mice (control, right) and after 10 days implantation of matrices incorporating 10 μg CpG, 3000ng GM-CSF (infection mimic, left), demonstrating significant dispersion of programmed immune cells to lymph node (note enlarged lymph node with infection mimic) (B) Comparison of survival time in C57BL/6J mice subcutaneously implanted with scaffolds and challenged 14days later with a subcutaneous injection of 105 B16-F10 melanoma cells. The PLG scaffolds implanted were empty (black), Lysate and 100 μgCpG (broken line), lysate, 3000ng GM-CSFC and 10μg CpG (red) and lysate, 3000ng GM-CSF, 100μg CpG (blue). Animals were also immunized with a cell-based vaccine (grey) that consisted of radiated B16 cells over-expressing GM-CSF as has been described previously (Okano et al., 2005). Images adapted from the original article in Nature Materials (Ali et al., 2009b).
Small molecules
The use of material-delivered small molecules for programming is extremely attractive, as in addition to easy handling and synthesis, they can act either in an intrinsic or extrinsic manner to modulate biological processes across various cellular compartments (Cao et al., 2003, Ichida et al., 2009, Firestone & Chen, 2010). While a small molecule can directly modulate a target site, acting in a domino manner, another approach is to modulate different processes or levels of the same process akin to the switchboard analogy. One example of this approach is the presentation of a small molecule, gamma secretase inhibitor, which enhances VEGFR2 levels in endothelial cells, making them more responsive to VEGF that is presented concomitantly and enhancing VEGF-driven angiogenesis (Cao et al., 2009). Given the huge strides in development of targeted chemical biology approaches, these small molecule approaches are likely to be useful agents for material based delivery systems.
Insoluble cues - controlling adhesion and mechanics to modulate local cellular behavior
Most cells in the body require adhesion to the extracellular matrix for survival and function. As host cells are recruited in the programming approaches, the materials can provide specific adhesion cues analogous to ECM to direct cell organization and regulate gene expression. Scaffolding materials are often modeled on connective tissue and basement membrane components that support epithelial stratification, maturation and function. A wide range of natural polymers are used to mimic the ECM niche (Table 1) (Sohier et al., 2008). These materials can provide requisite physical support and allow natural tissue patterning and morpho-differentiation and this has been specifically demonstrated in regenerating pulp tissue (Bohl et al., 1998, El-Backly et al., 2008). However, there are concerns of mechanical integrity, immune rejection and batch-to-batch variations. Another major limitation with these naturally derived polymeric systems is that complex cell-matrix interactions are not easily definable, making some of the biological responses unpredictable.
Table 1.
Representative polymeric systems
| Natural Polymer Systems | Synthetic Polymer Systems |
|---|---|
| Collagens | Poly(lactic) acid [PLA], |
| Fibrin | Poly(glycolic) acid [PGA], |
| Matrigel | Poly(lactic-co-glycolic) acid [PLGA] |
| Alginate | Poly(ethylene glycol)-diacrylates [PEG-DA] |
| Chitosan | Poly(ε-caprolactone) [PCL], |
| Hyaluronate | Poly(ethylene glycol) terephtalate [PEGT], |
| Silk | Poly (butylene) terepthalate [PBT], |
| Polyhydroxyalkanoates | Polyphospho-esters [PPEs], Polyphosphazenes [PPAs], Polyanhydrides [PAs], Polyortho-esters [POEs], Poly(propylene fumarate)-diacrylates [PPF-DA] |
A variety of synthetic polymeric systems (Table 1) have also been developed to address limitation of naturally derived materials, including controlled manufacturing at large scales and providing a precisely tailored cellular niche. Polymeric materials with suitably designed chemistry (Table 2) can be used to not only define the cell-matrix interaction, but also aid in directing cellular responses by acting as depots to develop spatially restricted morphogen fields. Many techniques (Table 3) are routinely used to fabricate polymeric systems with a variety of geometries and architecture and precise control over pore size, shape and connectivity (Sohier et al., 2008). Spatially precise fabrication techniques have more recently been developed to provide better control of pore scaffold architecture, internal and external pore connectivity (Sohier et al., 2008). The conventional and newer techniques often involve the use of heat and solvents that can result in denaturation and loss of incorporated protein activity. One approach to overcome this limitation is by the use of two phase systems in which the polymeric fabrication step is decoupled from the biological incorporation step. Two common techniques used are addition of microspheres encapsulating proteins to a pre-formed scaffold or adsorption of the protein onto the polymer scaffold post-fabrication (Bouhadir et al., 2001, Kong et al., 2004). In addition, processing techniques that utilize non harmful solvents (such as CO2, H20) allow biologically active molecules to be incorporated without diminishing their activity (Mooney et al., 1996, Silva & Mooney, 2007).
Table 2.
Design principles for programmable material design
| Material Properties | Utility |
|---|---|
| Biocompatible | Minimal inflammatory and immune rejection |
| Controlled pore architecture | Control over cell trafficking, morpho-differentiation and spatial organization, host integration |
| Encapsulate or binding of soluble signaling molecules | Spatiotemporal controlled delivery |
| Cell adhesive | Cell attachment and promotion of interface dependent cellular behavior |
| Controlled biodegradation | Replacement by host tissue, avoid chronic host responses |
| Dynamic properties | Responsive to local environment or external stimuli |
| Gelability, injectable or micro/nanoparticulate form | Ease of delivery and minimize trauma |
Table 3.
Material Processing Techniques
| Techniques |
|---|
Conventional architectures/geometry
|
| More precise Architectural control |
Fused diffusion molding
|
Solid free form techniques
|
Synthetic polymers largely lack intrinsic cell adhesiveness requiring modification to enable control over cell interactions. Full length proteins can be combined with synthetic polymer scaffolds, but smaller peptide fragments are preferred as they can be readily synthesized and covalently coupled to the polymers to provide a highly defined cell-material interface. Routinely used peptides include those containing the Arginine-Glycine-Aspartic acid (RGD), sequence found in many ECM proteins, various fibronectin fragments (eg; FNIII) and laminin derived adhesion motifs (eg; IKAV). These motifs have been successfully grafted onto a wide range of natural and synthetic materials including PEO (Hu et al., 2008), fibrin (Schense & Hubbell, 1999), chitosan (Kawamata et al., 2002), dextran (Massia & Stark, 2001), PLA (Quirk et al., 2001), PLG (Eid et al., 2001) and alginate (Alsberg et al., 2002). It should be noted that small adhesion peptides may have lower bioactivity than full length ECM proteins due to the absence of complementary domains (Cutler & Garcia, 2003).
The specific adhesive ligand and dimensionality (2D versus 3D presentation) will define the receptors used by cells to adhere to the material, and the peptide density, nanoscale distribution and mechanical properties of the substrate have profound effects on proliferation, migration and differentiation of interacting cells (Koo et al., 2002, Mann & West, 2002, Tosatti et al., 2004, Kong et al., 2005, Schuler et al., 2006, Comisar et al., 2007). Interestingly, given the right context, cells can also use the adhesive motifs to rearrange the macrostructure of the material, creating a dynamic feedback to modulate fate decisions (Kong et al., 2005, Comisar et al., 2007, Huebsch et al., 2010). The mechanical properties of the material presenting the peptides also influence cell fate decisions (Engler et al., 2006, Huebsch et al., 2010) providing another variable to modulate tissue regeneration. The precise cellular mechanisms underlying the effects of adhesion ligand presentation are being intensely investigated and are speculated to result, at least in part, from modulation of adhesion contacts and from clustering of cellular receptor that in turn modulate the cytoskeleton and intrinsic cell signaling pathways (Chen et al., 1997, Geiger et al., 2001, Comisar et al., 2006, Discher et al., 2009). Interestingly, as there is better understanding of the biological basis of these material’s biophysical interactions at the nanoscale, the material composition itself can be designed to serve as an extrinsic cue to trigger specific signal transduction pathways, as demonstrated by direct induction of focal adhesion kinase signaling in a recent report (Poondra et al., 2009). In vivo, mechanically tailored material systems, both natural and synthetic, have demonstrated efficacy in aiding regeneration of cardiac muscle (van Amerongen et al., 2006, Valarmathi et al., 2010) and PDL (Moioli et al., 2008), as well as modulating scar tissue formation (Agrawal et al., 2010).
Future Directions
Classical tissue engineering approaches have resulted in significant progress towards the goal of regenerating craniofacial tissues including bone, cartilage, oral mucosa, skin, pulp, dentin, periodontium, cranial sutures, and temporomandibular joints (Alsberg et al., 2001b, Taba et al., 2005, Mao et al., 2006). While these approaches have used either conductive or inductive approaches along with cell transplantation to achieve their goals, the concept of using materials to program cells in situ is leading to a paradigm shift in our understanding and use of materials in biomedical applications. The increasing understanding of biological mechanisms underlying stem cell biology is enabling the major steps of first recruiting, then programming and finally dispersing cells to be exploited as three interlinked modules (Figure 2). The ability to exploit individual modules, such as the recruitment of specific cell subpopulations useful in therapeutic angiogenesis, skeletal muscle, peripheral nerve, dental pulp and bone regeneration (Richardson et al., 2001, Huang et al., 2005a, Kaigler et al., 2005, Hill et al., 2006, Borselli et al., 2009, Kim et al., 2010) can provide significant utility in isolation from other modules. However, systems capable of integrating these modular functions are the ultimate goal and would enable one to bypass ex vivo cell manipulations completely (Ali et al., 2009a). Future materials are expected to progress from single step inductive processes, as illustrated by the domino differentiation model, to more sophisticated multi-step regulation of complex processes as per the switchboard analogy (Figure 3). The use of soluble cues such as aptamers, ribozymes, small regulatory RNAs (si and miRNA) and pathway specific small molecules, material hydrophobicity and electrical, magnetic or photonic fields that could affect cell polarity and morpho-differentiation are all exciting future avenues for material based programming systems.
Acknowledgments
We would like to Nathaniel Huebsch, David Nusinow and Will Yuen and for reading and providing helpful comments on the paper. The research in the authors’ laboratory is funded by NIH, DOD and NSF; PRA is a recipient of a Harvard Presidential Scholarship.
Footnotes
Competing Interests: The authors declare they have no competing interests.
References
- Agrawal V, Johnson SA, Reing J, Zhang L, Tottey S, Wang G, Hirschi KK, Braunhut S, Gudas LJ, Badylak SF. Epimorphic regeneration approach to tissue replacement in adult mammals. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3351–5. doi: 10.1073/pnas.0905851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge BB, Burke JM, Lauffenburger DA, Sorger PK. Physicochemical modelling of cell signalling pathways. Nature cell biology. 2006;8:1195–203. doi: 10.1038/ncb1497. [DOI] [PubMed] [Google Scholar]
- Ali OA, Emerich D, Dranoff G, Mooney DJ. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Science translational medicine. 2009a;1:8ra19. doi: 10.1126/scitranslmed.3000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nature materials. 2009b;8:151–8. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell-interactive alginate hydrogels for bone tissue engineering. Journal of dental research. 2001a;80:2025–9. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- Alsberg E, Anderson KW, Albeiruti A, Rowley JA, Mooney DJ. Engineering growing tissues. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12025–30. doi: 10.1073/pnas.192291499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsberg E, Hill EE, Mooney DJ. Craniofacial tissue engineering. Crit Rev Oral Biol Med. 2001b;12:64–75. doi: 10.1177/10454411010120010501. [DOI] [PubMed] [Google Scholar]
- Bohl KS, Shon J, Rutherford B, Mooney DJ. Role of synthetic extracellular matrix in development of engineered dental pulp. Journal of biomaterials science. 1998;9:749–64. doi: 10.1163/156856298x00127. [DOI] [PubMed] [Google Scholar]
- Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nature medicine. 1999;5:753–9. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proceedings of the National Academy of Sciences of the United States of America. 2009;107:3287–92. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshardt DD. Biological mediators and periodontal regeneration: a review of enamel matrix proteins at the cellular and molecular levels. Journal of clinical periodontology. 2008;35:87–105. doi: 10.1111/j.1600-051X.2008.01264.x. [DOI] [PubMed] [Google Scholar]
- Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnology progress. 2001;17:945–50. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- Brambrink T, Hochedlinger K, Bell G, Jaenisch R. ES cells derived from cloned and fertilized blastocysts are transcriptionally and functionally indistinguishable. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:933–8. doi: 10.1073/pnas.0510485103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–6. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- Cao L, Arany PR, Wang WS, Mooney DJ. Promoting angiogenesis via manipulation of VEGF responsiveness with Notch signaling. Biomaterials. 2009;30:4085–93. doi: 10.1016/j.biomaterials.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post MJ, Wahlberg E, Leboulch P, Cao Y. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nature medicine. 2003;9:604–13. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science (New York, NY. 1997;276:1425–8. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharmaceutical research. 2007;24:258–64. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- Comisar WA, Hsiong SX, Kong HJ, Mooney DJ, Linderman JJ. Multi-scale modeling to predict ligand presentation within RGD nanopatterned hydrogels. Biomaterials. 2006;27:2322–9. doi: 10.1016/j.biomaterials.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Comisar WA, Kazmers NH, Mooney DJ, Linderman JJ. Engineering RGD nanopatterned hydrogels to control preosteoblast behavior: a combined computational and experimental approach. Biomaterials. 2007;28:4409–17. doi: 10.1016/j.biomaterials.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Cutler SM, Garcia AJ. Engineering cell adhesive surfaces that direct integrin alpha5beta1 binding using a recombinant fragment of fibronectin. Biomaterials. 2003;24:1759–70. doi: 10.1016/s0142-9612(02)00570-7. [DOI] [PubMed] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science (New York, NY. 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W, Siegel V, Nusslein-Volhard C. Autonomous determination of anterior structures in the early Drosophila embryo by the bicoid morphogen. Development. 1990;109:811–20. doi: 10.1242/dev.109.4.811. [DOI] [PubMed] [Google Scholar]
- Eid K, Chen K, Griffith L, Glowacki J. Effect of RGD coating on osteocompatibility of PLGA-polymerdisks in rat tibial wound. J Biomed Mat Res. 2001;57:224–31. doi: 10.1002/1097-4636(200111)57:2<224::aid-jbm1162>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- El-Backly RM, Massoud AG, El-Badry AM, Sherif RA, Marei MK. Regeneration of dentine/pulp-like tissue using a dental pulp stem cell/poly(lactic-co-glycolic) acid scaffold construct in New Zealand white rabbits. Aust Endod J. 2008;34:52–67. doi: 10.1111/j.1747-4477.2008.00139.x. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Esposito M, Grusovin MG, Papanikolaou N, Coulthard P, Worthington HV. Enamel matrix derivative (Emdogain(R)) for periodontal tissue regeneration in intrabony defects. Cochrane database of systematic reviews (Online) 2009:CD003875. doi: 10.1002/14651858.CD003875.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Zhu YY, Smiley E, Bonadio J, Rouleau JP, Goldstein SA, McCauley LK, Davidson BL, Roessler BJ. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5753–8. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone AJ, Chen JK. Controlling destiny through chemistry: small-molecule regulators of cell fate. ACS chemical biology. 2010;5:15–34. doi: 10.1021/cb900249y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth ME, Atala A, Van Dyke ME. Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials. 2007;28:5068–73. doi: 10.1016/j.biomaterials.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nature reviews. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Gurdon JB. The transplantation of living cell nuclei. Advances in morphogenesis. 1964;4:1–43. doi: 10.1016/b978-1-4831-9951-1.50004-8. [DOI] [PubMed] [Google Scholar]
- Hadorn E. Transdetermination in cells. Scientific American. 1968;219:110-4. doi: 10.1038/scientificamerican1168-110. passim. [DOI] [PubMed] [Google Scholar]
- Hill E, Boontheekul T, Mooney DJ. Designing scaffolds to enhance transplanted myoblast survival and migration. Tissue Eng. 2006;12:1295–304. doi: 10.1089/ten.2006.12.1295. [DOI] [PubMed] [Google Scholar]
- Hu Z, Luo F, Pan Y, Hou C, Ren L, Chen J, Wang J, Zhang Y. Arg-Gly-Asp (RGD) peptide conjugated poly(lactic acid)-poly(ethylene oxide) micelle for targeted drug delivery. Journal of biomedical materials research. 2008;85:797–807. doi: 10.1002/jbm.a.31615. [DOI] [PubMed] [Google Scholar]
- Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005a;20:848–57. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- Huang YC, Simmons C, Kaigler D, Rice KG, Mooney DJ. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene therapy. 2005b;12:418–26. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nature materials. 2010;9:518–26. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebsch N, Mooney DJ. Inspiration and application in the evolution of biomaterials. Nature. 2009;462:426–32. doi: 10.1038/nature08601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida JK, Blanchard J, Lam K, Son EY, Chung JE, Egli D, Loh KM, Carter AC, Di Giorgio FP, Koszka K, Huangfu D, Akutsu H, Liu DR, Rubin LL, Eggan K. A small-molecule inhibitor of TGF-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell stem cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Glauser R, Scharer P, Hammerle CH, Sailer HF, Weber FE. Effect of rhBMP-2 on guided bone regeneration in humans. Clinical oral implants research. 2003;14:556–68. doi: 10.1034/j.1600-0501.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- Kaigler D, Krebsbach PH, Wang Z, West ER, Horger K, Mooney DJ. Transplanted endothelial cells enhance orthotopic bone regeneration. Journal of dental research. 2006;85:633–7. doi: 10.1177/154405910608500710. [DOI] [PubMed] [Google Scholar]
- Kaigler D, Krebsbach PH, West ER, Horger K, Huang YC, Mooney DJ. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. Faseb J. 2005;19:665–7. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- Kandziora F, Schmidmaier G, Schollmeier G, Bail H, Pflugmacher R, Gorke T, Wagner M, Raschke M, Mittlmeier T, Haas NP. IGF-I and TGF-beta1 application by a poly-(D,L-lactide)-coated cage promotes intervertebral bone matrix formation in the sheep cervical spine. Spine. 2002;27:1710–23. doi: 10.1097/00007632-200208150-00006. [DOI] [PubMed] [Google Scholar]
- Kawamata Y, Nagayama Y, Nakao K, Mizuguchi H, Hayakawa T, Sato T, Ishii N. Receptor independent augmentation of adenovirus-mediated gene transfer with chitosan in vitro. Biomaterials. 2002;23:4573–9. doi: 10.1016/s0142-9612(02)00203-x. [DOI] [PubMed] [Google Scholar]
- Keeney M, van den Beucken JJ, van der Kraan PM, Jansen JA, Pandit A. The ability of a collagen/calcium phosphate scaffold to act as its own vector for gene delivery and to promote bone formation via transfection with VEGF(165) Biomaterials. 2010;31:2893–902. doi: 10.1016/j.biomaterials.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nature biotechnology. 2008;26:120–6. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- Kim JB, Sebastiano V, Wu G, Arauzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, Meyer J, Hubner K, Bernemann C, Ortmeier C, Zenke M, Fleischmann BK, Zaehres H, Scholer HR. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–9. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Kim K, Lee CH, Kim BK, Mao JJ. Anatomically Shaped Tooth and Periodontal Regeneration by Cell Homing. Journal of dental research. 2010 doi: 10.1177/0022034510370803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GN, Cochran DL. Factors that modulate the effects of bone morphogenetic protein induced periodontal regeneration: a critical review. Journal of periodontology. 2002;73:925–36. doi: 10.1902/jop.2002.73.8.925. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Nakashima K, Kowashi Y, Fujii T, Shimauchi H, Sasano T, Furuuchi T, Fukuda M, Noguchi T, Shibutani T, Iwayama Y, Takashiba S, Kurihara H, Ninomiya M, Kido J, Nagata T, Hamachi T, Maeda K, Hara Y, Izumi Y, Hirofuji T, Imai E, Omae M, Watanuki M, Murakami S. Periodontal tissue regeneration using fibroblast growth factor-2: randomized controlled phase II clinical trial. PloS one. 2008;3:e2611. doi: 10.1371/journal.pone.0002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong HJ, Kaigler D, Kim K, Mooney DJ. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules. 2004;5:1720–7. doi: 10.1021/bm049879r. [DOI] [PubMed] [Google Scholar]
- Kong HJ, Polte TR, Alsberg E, Mooney DJ. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4300–5. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo LY, Irvine DJ, Mayes AM, Lauffenburger DA, Griffith LG. Co-regulation of cell adhesion by nanoscale RGD organization and mechanical stimulus. Journal of cell science. 2002;115:1423–33. doi: 10.1242/jcs.115.7.1423. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Langer R, Vacanti JP. Tissue engineering. Science (New York, NY. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, Sandstrom R, Bernstein B, Bender MA, Groudine M, Gnirke A, Stamatoyannopoulos J, Mirny LA, Lander ES, Dekker J. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science (New York, NY. 2009;326:289–93. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann BK, West JL. Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J Biomed Mater Res. 2002;60:86–93. doi: 10.1002/jbm.10042. [DOI] [PubMed] [Google Scholar]
- Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, Shi S. Craniofacial tissue engineering by stem cells. Journal of dental research. 2006;85:966–79. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massia SP, Stark J. Immobilized RGD peptides on surface-grafted dextran promote biospecific cell attachment. J Biomed Mater Res. 2001;56:390–9. doi: 10.1002/1097-4636(20010905)56:3<390::aid-jbm1108>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nature biotechnology. 2007;25:1177–81. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- Mierisch CM, Cohen SB, Jordan LC, Robertson PG, Balian G, Diduch DR. Transforming growth factor-beta in calcium alginate beads for the treatment of articular cartilage defects in the rabbit. Arthroscopy. 2002;18:892–900. doi: 10.1053/jars.2002.36117. [DOI] [PubMed] [Google Scholar]
- Misteli T, Soutoglou E. The emerging role of nuclear architecture in DNA repair and genome maintenance. Nature reviews. 2009;10:243–54. doi: 10.1038/nrm2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moioli EK, Clark PA, Chen M, Dennis JE, Erickson HP, Gerson SL, Mao JJ. Synergistic actions of hematopoietic and mesenchymal stem/progenitor cells in vascularizing bioengineered tissues. PloS one. 2008;3:e3922. doi: 10.1371/journal.pone.0003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney DJ, Baldwin DF, Suh NP, Vacanti JP, Langer R. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17:1417–22. doi: 10.1016/0142-9612(96)87284-x. [DOI] [PubMed] [Google Scholar]
- Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell stem cell. 2008;2:205–13. doi: 10.1016/j.stem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha V beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–28. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Murakami S, Takayama S, Ikezawa K, Shimabukuro Y, Kitamura M, Nozaki T, Terashima A, Asano T, Okada H. Regeneration of periodontal tissues by basic fibroblast growth factor. Journal of periodontal research. 1999;34:425–30. doi: 10.1111/j.1600-0765.1999.tb02277.x. [DOI] [PubMed] [Google Scholar]
- Okano F, Merad M, Furumoto K, Engleman EG. In vivo manipulation of dendritic cells overcomes tolerance to unmodified tumor-associated self antigens and induces potent antitumor immunity. J Immunol. 2005;174:2645–52. doi: 10.4049/jimmunol.174.5.2645. [DOI] [PubMed] [Google Scholar]
- Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–44. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish LC, Miyamoto T, Fong N, Mattson JS, Cerutis DR. Non-bioabsorbable vs. bioabsorbable membrane: assessment of their clinical efficacy in guided tissue regeneration technique. A systematic review. Journal of oral science. 2009;51:383–400. doi: 10.2334/josnusd.51.383. [DOI] [PubMed] [Google Scholar]
- Poondra RR, Kumar NN, Bijian K, Prakesch M, Campagna-Slater V, Reayi A, Reddy PT, Choudhry A, Barnes ML, Leek DM, Daroszewska M, Lougheed C, Xu B, Schapira M, Alaoui-Jamali MA, Arya P. Discovery of Indoline-Based, Natural-Product-like Compounds as Probes of Focal Adhesion Kinase Signaling Pathways. Journal of combinatorial chemistry. 2009 doi: 10.1021/cc8001525. [DOI] [PubMed] [Google Scholar]
- Quirk RA, Chan WC, Davies MC, Tendler SJ, Shakesheff KM. Poly(L-lysine)-GRGDS as a biomimetic surface modifier for poly(lactic acid) Biomaterials. 2001;22:865–72. doi: 10.1016/s0142-9612(00)00250-7. [DOI] [PubMed] [Google Scholar]
- Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nature biotechnology. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- Schense JC, Hubbell JA. Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa. Bioconjugate chemistry. 1999;10:75–81. doi: 10.1021/bc9800769. [DOI] [PubMed] [Google Scholar]
- Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–45. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Schuler M, Owen GR, Hamilton DW, de Wild M, Textor M, Brunette DM, Tosatti SG. Biomimetic modification of titanium dental implant model surfaces using the RGDSP-peptide sequence: a cell morphology study. Biomaterials. 2006;27:4003–15. doi: 10.1016/j.biomaterials.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schwartz YB, Pirrotta V. Polycomb silencing mechanisms and the management of genomic programmes. Nature reviews. 2007;8:9–22. doi: 10.1038/nrg1981. [DOI] [PubMed] [Google Scholar]
- Shin S, Salvay DM, Shea LD. Lentivirus delivery by adsorption to tissue engineering scaffolds. Journal of biomedical materials research. 2010;93:1252–9. doi: 10.1002/jbm.a.32619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva EA, Kim ES, Kong HJ, Mooney DJ. Material-based deployment enhances efficacy of endothelial progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14347–52. doi: 10.1073/pnas.0803873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5:590–8. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- Slack JM. Metaplasia and somatic cell reprogramming. The Journal of pathology. 2009;217:161–8. doi: 10.1002/path.2442. [DOI] [PubMed] [Google Scholar]
- Sohier J, Moroni L, van Blitterswijk C, de Groot K, Bezemer JM. Critical factors in the design of growth factor releasing scaffolds for cartilage tissue engineering. Expert opinion on drug delivery. 2008;5:543–66. doi: 10.1517/17425247.5.5.543. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Roberts AB. Peptide growth factors and inflammation, tissue repair, and cancer. The Journal of clinical investigation. 1986;78:329–32. doi: 10.1172/JCI112580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Johnston P, Lawrence PA. Control of Drosophila body pattern by the hunchback morphogen gradient. Cell. 1992;69:237–49. doi: 10.1016/0092-8674(92)90405-2. [DOI] [PubMed] [Google Scholar]
- Sun Q, Silva EA, Wang A, Fritton JC, Mooney DJ, Schaffler MB, Grossman PM, Rajagopalan S. Sustained release of multiple growth factors from injectable polymeric system as a novel therapeutic approach towards angiogenesis. Pharmaceutical research. 2010;27:264–71. doi: 10.1007/s11095-009-0014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taba M, Jr, Jin Q, Sugai JV, Giannobile WV. Current concepts in periodontal bioengineering. Orthodontics & craniofacial research. 2005;8:292–302. doi: 10.1111/j.1601-6343.2005.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tosatti S, Schwartz Z, Campbell C, Cochran DL, VandeVondele S, Hubbell JA, Denzer A, Simpson J, Wieland M, Lohmann CH, Textor M, Boyan BD. RGD-containing peptide GCRGYGRGDSPG reduces enhancement of osteoblast differentiation by poly(L-lysine)-graft-poly(ethylene glycol)-coated titanium surfaces. Journal of biomedical materials research. 2004;68:458–72. doi: 10.1002/jbm.a.20082. [DOI] [PubMed] [Google Scholar]
- Valarmathi MT, Goodwin RL, Fuseler JW, Davis JM, Yost MJ, Potts JD. A 3-D cardiac muscle construct for exploring adult marrow stem cell based myocardial regeneration. Biomaterials. 2010;31:3185–200. doi: 10.1016/j.biomaterials.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen MJ, Harmsen MC, Petersen AH, Kors G, van Luyn MJ. The enzymatic degradation of scaffolds and their replacement by vascularized extracellular matrix in the murine myocardium. Biomaterials. 2006;27:2247–57. doi: 10.1016/j.biomaterials.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, et al. The sequence of the human genome. Science (New York, NY. 2001;291:1304–51. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- von Lindern JJ, Niederhagen B, Appel T, Berge S. Treatment of soft tissue defects with exposed bone in the head and face region with alginates and hydrocolloid dressings. J Oral Maxillofac Surg. 2002;60:1126–30. doi: 10.1053/joms.2002.34979. [DOI] [PubMed] [Google Scholar]
- Yung CW, Fiering J, Mueller AJ, Ingber DE. Micromagnetic-microfluidic blood cleansing device. Lab on a chip. 2009;9:1171–7. doi: 10.1039/b816986a. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32. 21. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]