Abstract
The final assembly of nonlytic envelope viruses requires the coordinated transport of either subviral particles or fully formed virions to the plasma membrane for release from the cell. Recent research has delved into the mechanisms viruses employ to hijack the host cell's cytoskeletal system for active transport to the site of final assembly and release. This review will look at recent findings that relate to the transport of virions to the cell periphery and out of the cell.
Keywords: Viral transport, Cytoskeleton, Microtubules, Cortical actin network, Kinesin, Myosin, Poxvirus, Herpesvirus, HIV, Paramyxovirus
Introduction
The spatial separation between the location in the cell where viruses replicate their genomes and the plasma membrane, where final assembly and exit from the cell occurs, can be vast. When one considers the small diffusion rate in the dense cytoplasm of large macromolecular complexes such as encapsidated genomes and nucleoprotein complexes, it should come as no surprise that viruses have figured out mechanisms of commandeering their host cell's transportation system for active and directional transport during egress. The ability to genetically tag viral proteins with fluorescent proteins (FP) has advanced both the study of viral entry and egress by allowing the real-time visualization of these chimeras in live cells. This review will summarize what is currently known about the transport of virions and subviral particles through the cytoplasm toward the plasma membrane during egress and will build on several excellent reviews on viral interactions with the cytoskeleton (Diefenbach et al., 2008, Greber and Way, 2006, Lyman and Enquist, 2009, Radtke et al., 2006).
Cytoskeletal transport
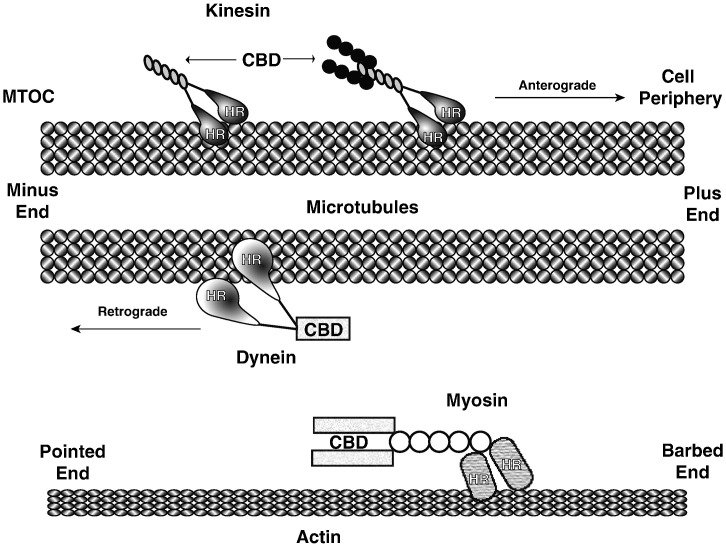
Almost all reviews on this topic (including this one) begin with an overview of the cellular transportation system along the cytoskeleton. The cytoskeleton has three major types of filaments, actin, microtubules and intermediate filaments. Although intermediate filaments can assemble into extensive networks in the cell and are involved in the sub-cellular positioning of lysosomes and mitochondria, there are no known motors that utilize them for transport (Toivola et al., 2010). The other two types of filaments, microtubules and actin, have specialized motor proteins that travel along them by converting chemical energy into mechanical work to transport cargo to various regions of the cell (Vale, 2003). Microtubules are polar filaments with a positive and a negative end. They are made up of protofilaments of α- and β-tubulin dimers. Filaments are nucleated at the microtubule-organizing center (MTOC), which is typically located next to the nucleus and represents the minus end of microtubules. From the MTOC, microtubules radiate out towards the periphery of the cell. The distal ends, or plus ends, terminate in the vicinity of the plasma membrane and are more dynamic than the negative end with a higher rate of microtubule growth and shrinkage by a process of either adding or removing α/β tubulin dimers. Two types of motors move along microtubules: kinesin and dynein (Fig. 1 ). Generally speaking kinesin motors move outward from the cell center toward the positive end of microtubules, although exceptions are known, and dynein moves toward the MTOC or negative end (Hirokawa, 1998, Hirokawa and Noda, 2008, Hirokawa et al., 2009). The kinesins are divided up into 15 families and mammals are thought to express over 45 individual motor proteins, called KIFs (Miki et al., 2001). This large number is thought to represent the diversity of cargo and their destinations within the cell. In their simplest form, kinesins consist of a globular head domain and a tail region (Akhmanova and Hammer, 2010, Skowronek et al., 2007). The head domain binds microtubules and hydrolyzes ATP to produce movement. Cargo binds through the tail region, which varies between the different types of motors and is selective for different types of cargo. The widely expressed KIF5B, conventional kinesin, is a heterotetramer made up of two heavy chains (KHC) and two light chains (KLC). Each heavy chain contains an N-terminal head domain followed by a stalk domain for dimerization. The two light chains also interact with the stalk region on the heavy chains. Cargo binds through a tetratricopeptide repeat domain on the light chain. Unbound kinesin is thought to be in an inactive state with the head and tail domains interacting (Akhmanova and Hammer, 2010). This interaction is disrupted by cargo binding thus activating the motor to commence transport.
Fig. 1.
Motors associated with cytoskeletal transport. Microtubules radiate out from their minus end at the MTOC to the periphery of the cell. Both kinesin and dynein travel along microtubules but in opposite directions. Myosin is the only known motor to travel along actin filaments. Motors bind filaments at their head region (HR) whereas their cargo binding domains (CBD) are located distal to the head region.
In contrast to the large family of kinesin, there is only one motor that transports cargo toward the minus end of microtubules (Kardon and Vale, 2009). Cytoplasmic dynein has both a head domain and a tail domain. Like kinesin, the head domain contains the microtubule binding site and hydrolyzes ATP for movement. The tail domain is involved in dimerization of dynein monomers. While the dynein dimer alone is sufficient for movement in vitro, in vivo, non-catalytic subunits are typically found associated with the dimer through the tail domain (Kardon and Vale, 2009). It is believed that these subunits regulate dynein function and cargo specificity.
Actin filaments are made up of a single protein, globular actin (G-actin). G-actin assembles into polar filaments (F-actin) with a barbed and pointed end (plus and minus ends respectively) (Carlsson, 2010, Olson and Nordheim, 2010). Unlike microtubules, actin filaments may branch and cross-link forming a mesh-like network. There is only one type of motor, the myosins, that moves along actin filaments. Myosins are divided into two classes termed conventional and unconventional (Redowicz, 2007). The conventional myosins form filaments and are typically found in muscle tissue. Unconventional myosins do not resemble the form found in muscle and can be found performing a large number of cellular functions, including intracellular trafficking. To date, only a few types of myosins are known to be involved in intracellular trafficking. Two of these, myosin V and myosin VI move toward the barbed and pointed ends, respectively (Ross et al., 2008). It should be noted, however, that with the exception of myosin VI, all known myosins move toward the barbed end. Myosin is a homodimer with the motor and actin binding functions contained within the head domain followed by a neck domain for dimerization and the cargo binding domain.
The majority of cargo, including viruses (McDonald et al., 2002, Moss and Ward, 2001, Smith et al., 2001, Suomalainen et al., 1999), is known to display movement in both directions (bidirectional) (Welte, 2004). This suggests that they are able to interact with both plus- and minus-end directed motors. While rapid switching between the two types of motors is possible, it is believed that both plus- and minus-end directed motors bind cargo simultaneously. In support of this notion, kinesin and dynein have been shown to be linked by the essential dynein adaptor dynactin (Berezuk and Schroer, 2007). Additionally, kinesin and myosin V interact and motors are thought to be transported together and function as a complex (Ali et al., 2008, DePina and Langford, 1999). If both types of motors are bound, it is unclear how one motor predominates to achieve overall movement in the proper direction (Welte, 2004).
Poxvirus egress
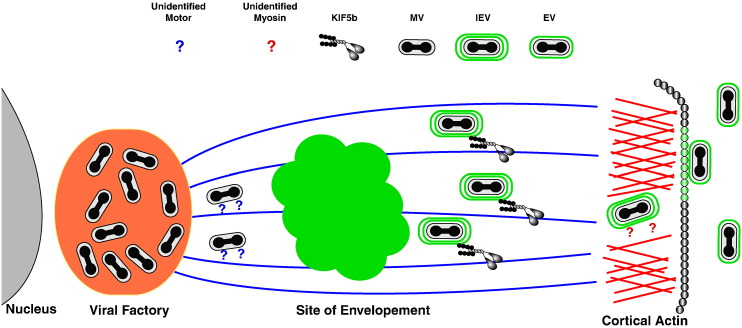
Poxviruses take full advantage of the cytoskeleton and its various motors for transport during egress. Vaccinia virus, the prototypical orthopoxvirus, produces two morphologically distinct forms of virions, both of which utilize intracellular transport. Viral replication occurs in the cytoplasm near the nucleus in a region termed the viral factory and results in the production of fully infectious particles termed mature virus (MV) (Moss, 2001) (Fig. 2 ). A subset of MV undergoes intracellular envelopment at the trans-Golgi network and endosomes to become intracellular enveloped virus (IEV) (Schmelz et al., 1994, Tooze et al., 1993). IEV are transported to the plasma membrane where they are released by fusion of their outermost membrane with the plasma membrane to become extracellular virions (EV) (Smith et al., 2002). Some EV remain attached to the cell and coordinate the polymerization of actin in the cytoplasm directly beneath them to form actin tails that facilitate cell-to-cell spread. Infectious EV production is required for plaque formation. The inhibition of either IEV formation or EV release leads to a complete abolishment of plaque formation (Ward, 2005b). The distance between the viral factory and the site of wrapping is sufficient to require active transport of MV. Indeed, the wrapping of MV was shown to be sensitive to the microtubule depolymerizing drug nocodazole (Sanderson et al., 2000). Furthermore, a recombinant vaccinia virus that expresses a capsid-YFP fusion and is incapable of making IEV revealed that MV movement approached speeds of 3 μm/s, consistent with microtubule transport (Ward, 2005b). The movement was bidirectional indicating that MV bind both dynein and kinesin, although it is still unclear which motor is responsible for transport toward the site of wrapping. It has been suggested that transport of MV is driven by kinesin (Schepis et al., 2007) and therefore toward the distal microtubule end. Predicting which motor is involved is confounded by the location of the viral factory, which typically forms in the perinuclear region (Carter et al., 2003, Mallardo et al., 2001) close to the MTOC. Alternatively, the transportation of cellular vesicles to the trans-Golgi network, the site of wrapping, is dynein driven (Hirokawa and Noda, 2008). While none of the vaccinia MV proteins are known to interact with motor proteins, the distantly related fowlpox virus protein 140 was recently reported to interact with the tetratricopeptide repeat of KLC using two photon-induced fluorescence resonance energy transfer fluorescence lifetime imaging microscopy (Jeshtadi et al., 2010). The vaccinia homolog H3 is found on MV and it will be of interest to determine if it is also able to interact with the tetratricopeptide repeat domain of KLC.
Fig. 2.
Poxvirus egress. Poxviruses such as vaccinia virus replicate in the cytoplasm producing infectious MV at the viral factory. Some MV are transported by an unidentified motor along microtubules to be enveloped at the TGN or endosomes to become IEV. IEV are transported to the cell periphery by KIF5b, where they engage an unknown myosin for active transport across the cortical actin network, fusion at the plasma membrane, and eventual release.
Once IEV is formed it is transported to the cell periphery for fusion with the plasma membrane and release. In accordance with IEV transport toward the plus end of microtubules and the plasma membrane, conventional kinesin, KIF5b, was shown to localize to IEV (Rietdorf et al., 2001). IEV have a different set of viral proteins exposed to the cytoplasm compared to MV (Smith et al., 2002) and two IEV specific proteins, A36 and F12, have been proposed to link kinesin to IEV. Deletion of either F12L or A36R causes a reduction in plaque size on cell monolayers but not a total abrogation (Parkinson and Smith, 1994, van Eijl et al., 2000), indicating that some EV are transported and released in the absence of either protein. Mutagenesis studies of A36 revealed two distinct functions (Ward and Moss, 2001, Ward et al., 2003). The first is the activation of the cell's actin polymerization complex for actin tail formation, which enhances cell-to-cell spread. Mutations in A36 that abrogate actin tail formation cause a reduction in plaque size, whereas deletion of the entire A36R gene results in a further reduction in plaque size. These results indicate that an additional function has been lost that is likely to be IEV transport. Visualization of IEV movement in infected cells in the absence of A36 shows an overall reduction in their transport along with a drop in their processivity (Herrero-Martinez et al., 2005, Ward et al., 2003). In addition, A36 was shown to interact with the tetratricopeptide repeat domain of KLC using the yeast two-hybrid system and GST-pulldowns (Ward and Moss, 2004). This interaction was recently confirmed in infected cells using two photon-induced fluorescence resonance energy transfer fluorescence lifetime imaging microscopy (Jeshtadi et al., 2010). A recent study reported an alignment of F12 with KLC. F12 has 12% amino acid identity (50% similarity) with KLC (Morgan et al., 2010) and it was suggested that F12 acts as a KLC mimic. While there were regions of the two proteins that showed alignment, F12 appears to be missing the heptad repeats of KLC that are involved in KHC binding. If F12 mimics the KLC function of binding cargo, it is unclear how it interacts with the heavy chains for transport. It was also reported that IEV made in the absence of F12 do not label with an antibody to kinesin, but a direct interaction between F12 and kinesin has yet to be reported.
IEV transport via kinesin and microtubules delivers them to the cell periphery. Vaccinia virus appears to have maximized intracellular transport by stimulating microtubule dynamics and increasing their targeting to the periphery (Arakawa et al., 2007b). This is accomplished by the F11 protein interacting with the Rho GTPase, RhoA and preventing its regulation of microtubule dynamics through mDia. The F11–RhoA interaction has the added benefit of thinning the cortical actin network (Arakawa et al., 2007a, Cordeiro et al., 2009). The cortical actin network makes a thick mesh just under the plasma membrane that give cells their rigidity and presents a physical barrier to cargo (both viruses and secretory vesicles) exiting the cell. The thinning of the actin in this region by down regulation of mDia would act to facilitate IEV transport through the cortex. It is thought that thinning alone would not be sufficient and that active transport by a myosin motor is needed. Indeed, imaging studies suggest that IEV undergo movement that is different than that seen by IEV outside of the actin cortex. Currently there are no reports of which myosin is involved although, myosin II has been suggested (Arakawa et al., 2007a).
Herpesvirus egress
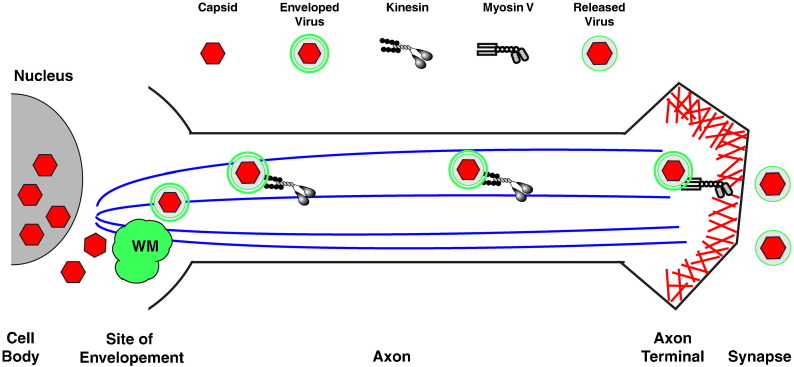
Herpesviruses also undergo a complex set of events for egress. Genome replication occurs and subsequent encapsidation occurs in the nucleus. Newly formed capsids traverse the nuclear envelope and are released into the cytoplasm where membrane acquisition occurs for intracellular envelopment and eventual release from the cell (Mettenleiter et al., 2009). Members of the alphaherpesviruses, such as pseudorabies virus and HSV1, are capable of replicating in neurons and need to travel the length of the axon for release (Lyman and Enquist, 2009) (Fig. 3 ). Fusion of GFP to the capsid protein UL35 of pseudorabies virus allowed the visualization of capsids as they traveled along dorsal root ganglia (Smith et al., 2001). The movement was bidirectional and approached speeds of ~ 2 μm/s, consistent with microtubule-based transport using kinesin. Since this finding, a debate has arisen as to what is actually transported in nerve cells or, to look at it another way, where does envelope acquisition occur? The controversy is whether capsids are enveloped immediately after exiting the nucleus and before transport down the axon or are capsids transported down the axon and then enveloped. Using a recombinant pseudorabies virus that expressed a capsid red fluorescent protein (RFP) chimera and a glycoprotein-GFP chimera, it was shown that approximately 85% of the RFP capsids that were undergoing anterograde transport had GFP associated with them, while the majority of the RFP only capsids were traveling retrograde (Antinone and Smith, 2006). Studies of HSV-1 failed to find colocalization of capsids and glycoprotein membranes during anterograde transport using both EM and immunofluorescence (Holland et al., 1999, Miranda-Saksena et al., 2000, Snyder et al., 2007, Snyder et al., 2006), suggesting a fundamental difference between these two alpha herpesviruses. In addition, a reconstituted in vitro transport system demonstrated that unenveloped capsids were capable of microtubule movement (Wolfstein et al., 2006). It was found that capsids that had their outer tegument proteins removed were still able to move along microtubules in an ATP dependent fashion. In contrast, capsids purified from the nucleus, which are devoid of tegument proteins, did not. In addition, it was shown that the cofactor dynactin, which interacts with both kinesin and dynein, was required for motility. This raised the possibility that they were visualizing both anterograde and/or retrograde transport in their system. Subsequently, this group showed that capsids that have not been treated to remove their inner tegument were capable of binding dynein, dynactin and kinesin-1 independently of other host proteins and that a single capsid could bind multiple motors simultaneously (Radtke et al., 2010). It seems logical that capsids would interact with both minus- and plus-end directed motors because they need to be transported to the nucleus for replication after entering the cell and upon exit from the nucleus they would require some form of transport, even if it is for a short distance, to reach wrapping membranes. Using an in vitro pull-down assay, the HSV-1 tegument protein US11 was found to bind the heavy chain of conventional kinesin (Diefenbach et al., 2002). This suggests a potential link for anterograde transport of capsids but its function during infection has not been explored. In a separate study using an in vitro trafficking system, Lee et al. showed that HSV-containing vesicles that cofractionated with the trans-Golgi network marker TG46 move in an ATP dependent manner on fluorescent microtubules (Lee et al., 2006). The movement in their assay was reduced by both dynein and kinesin specific inhibitors. While these assays establish that both capsids and enveloped virions are capable of bidirectional movement on microtubules, neither resolved the question of where envelope acquisition occurs for HSV-1 in neurons.
Fig. 3.
Alphaherpesvirus egress in neurons. Herpeseviruses replicate their genome in the nucleus. After nuclear encapsidation, capsids are released to the cytoplasm where they undergo envelopment at TGN or endosomes. Enveloped viruses engage kinesin for transport down the axon. At the axon terminal, virions engage myosin V for transport across the cortical actin network for fusion at the plasma membrane and release into the synapse.
Quite recently, papers have come out supporting the model that enveloped HSV-1 virions are transported down axons for release. Using a similar approach as they did previously for pseudorabies virus, Antinone et al. created a recombinant HSV-1 that expressed a capsid protein-RFP fusion and an envelope protein–GFP fusion (Antinone et al., 2010). Imaging of neurons infected with the recombinant showed that over 60% of the anterograde moving capsids were associated with GFP, indicating that enveloped virions are transported down the axon for egress. These results are supported by two studies that found enveloped particles in axons using electron microscopy (Huang et al., 2011, Negatsch et al., 2010). Notably, it was reported that HSV-1 produced significantly less virions compared to PRV, which suggests that this was the reason for the long-standing discrepancy. The transport of enveloped virions down the axon necessitates the recruitment of kinesin to the viral membrane. The HSV-2 membrane protein UL56 was found to interact with KIF1A in both a yeast two-hybrid assay and a GST pull-down assay (Koshizuka et al., 2005). It remains to be shown if the interaction has a function during herpesvirus infection.
Upon delivery of herpesviruses to the cell periphery, they must also cross the cortical actin network for access to the plasma membrane. A recent article reported that expression of a dominant negative form of the actin motor myosin V reduced herpesvirus release (Roberts and Baines, 2010). Myosin V is known to transport secretory vesicles across the cortical actin network, suggesting that crossing the cortical actin network is an active process and that myosin V is involved. The dominant negative form consists of the cargo binding and dimerization domains but has the actin binding head domain replaced with GFP. The dominant negative functions by binding cargo and preventing its interaction with normal myosin V and, therefore, transport. A protein involved in tethering HSV to the motor was not reported, but considering that the outer envelope is cellular in origin, the linking protein could be either viral or cellular.
Transport of HIV
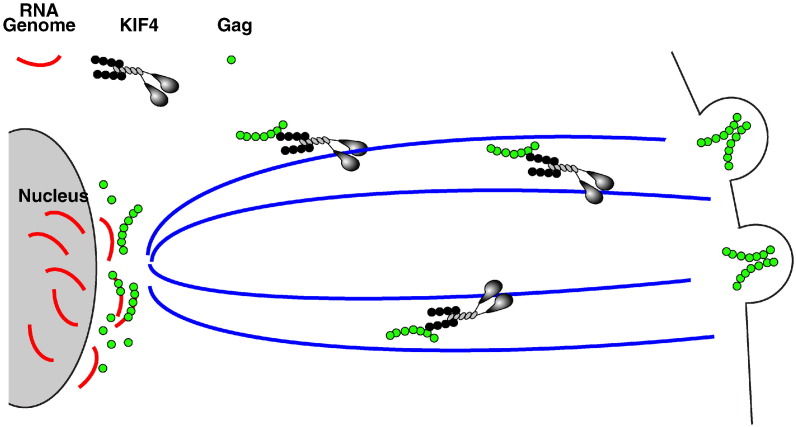
Much like viruses that assemble in the cytoplasm, viruses that assemble at the plasma membrane must transport their replicated genomes safely to the plasma membrane for assembly and release. A key step in the assembly of lentiviruses is the transport of genomic RNA to the plasma membrane for incorporation into virions (Fig. 4 ). Genomic RNA is produced in the nucleus and, at some point during assembly, needs to associate with the major viral structural protein Gag (Resh, 2004). Four domains and corresponding functions have been uncovered for Gag. Interaction with membranes occurs through the p17 matrix domain and requires myristalation. The p6 domain is involved in membrane pinch off and release. The p24 capsid domain and p7 nucleocapsid domains are involved in particle assembly and multimerization. In addition, the p7 domain interacts with specific packaging signals on the genome. Using a yeast two-hybrid assay and immunoprecipitation the p17 domain of Gag was shown to interact with the C-terminal tail of KIF4 (Kim et al., 1998, Tang et al., 1999). Subsequently, it was shown that knockdown of KIF4 led to an accumulation of Gag in the perinuclear region (Martinez et al., 2008). This raised the possibility that the genome and Gag were transported as a complex to the plasma membrane for assembly but it was unclear where in the cell the interaction first occurred. Using the ssRNA binding coat protein of the bacteriaphage MS2 fused to a fluorescent protein (MS2-XFP), Kemler et al. were able to visualize HIV and FIV RNA that was engineered to contain MS2 phage specific sequences (Kemler et al., 2010). In the absence of other viral proteins, the RNA/MS2-XFP complex appeared to localize to the nuclear envelope. The addition of Gag caused a redistribution of the RNA to the cytoplasm with a concentration at the plasma membrane. The redistribution was dependent on the RNA containing the packaging signal and a corresponding Gag protein. These results suggest that Gag binds newly replicated RNA genomes as they exit the nucleus. The Gag-RNA complexes interact with KIF4 in the perinuclear region and are transported to the plasma membrane for incorporation into budding virus.
Fig. 4.
HIV egress. RNA genomic copies are produced in the nucleus and transported to the cytoplasm where they interact with Gag at the nuclear envelope. The RNP complex engages KIF4 for microtubule transport to the plasma membrane where the RNP is incorporated into budding virions and released from the cell.
Transport of other ribonucleoprotein complexes
The Rab family of small GTPases is known for their role in regulating intracellular fusion events (Akhmanova and Hammer, 2010, Grosshans et al., 2006, Schimmoller et al., 1998, Stenmark, 2009). Over the past several years, they have been shown to interact with various motors and are now thought to recruit specific motors, through adaptor proteins, to specific organelles for vesicle transport. Rabs are capable of recruiting different types of motors to the same organelle. For example, Rab11 can recruit both myosin Vb and kinesin-2 to recycling endosomes through different adaptor proteins (Akhmanova and Hammer, 2010). Recently, it was shown that the ribonucleocapsid from Sendai virus (vRNP), a member of the Paramyxoviridae, trafficked with Rab11 (Chambers and Takimoto, 2010). This was accomplished by fusing the viral polymerase protein L, which is packaged into nascent vRNP, to GFP. In cells infected with a recombinant Sendai virus expressing the L-GFP chimera, fluorescent particles could be visualized moving through cells with speeds between 0.41 and 1.04 μm/s. This movement was sensitive to nocodazole indicating that it was microtubule based. In addition, the recycling endosome marker transferrin was imaged moving with vRNP. Taken together, these results suggest that the vRNP are transported through the cytoplasm along microtubules with vesicles from the recycling endosome. It also implies that Sendai vRNP is transported to the plasma membrane by kinesin-2.
The fusion of the coronavirus nucleocapsid protein to GFP permitted the visualization of its intracellular trafficking in cells cotransfected with plasmids that express M and E (Siu et al., 2008). Like Sendai virus, the N protein was found associated with vesicles in the cytoplasm. The vesicles moved and merged with each other. Although there was no analysis of the movement or use of nocodazole, the transport was sensitive to Brefeldin A, indicating that it may be interacting with secretory vesicles.
In a similar fashion, the phosphoprotein P of VSV, a subunit of the viral RNA polymerase was tagged with GFP (PeGFP) (Das et al., 2006). PeGFP was both stable and functional and incorporated into virions although at reduced levels compared to wild type P. The fusion permitted the visualization of RNP in live cells, which was reported to associate with mitochondria as they moved toward the periphery of the cell. The movement was sensitive to nocodazole, indicating that it was microtubule dependent and averaged 0.03 μm/s.
Summary and future directions
In order to exit the cell without lysis, viruses need to be efficiently transported to the plasma membrane for final assembly and egress. The microtubule network is emerging as the preferred choice for this transportation. This is not surprising, as it seems to be the most direct route to the plasma membrane. Of key interest is to determine which viral proteins mediate motor recruitment either directly or indirectly and how this recruitment is regulated. For viruses that undergo intracellular envelopment, proteins in the wrapping membrane that interact with motor proteins need to be regulated to ensure that they only interact after envelopment has occurred. Vaccinia virus has set up an interaction cascade that may be involved in just such a regulation (Ward, 2005a). Understanding how viruses regulate this interaction will not only give greater insight into viral processes but also help in understanding motor recruitment and activation by cellular membranes.
From the few examples known, there appears to be a trend of RNA viruses to transport their vRNP to the plasma membrane in conjunction with cellular membranes of the secretory system. Understanding this process for other RNA viruses will help determine if this is a common theme or if there are differences. Utilizing GFP fusions has proven to be a powerful tool for tracking vRNP egress. However, viruses that have strict genome packaging limits, such as orthomyxoviruses and arenaviruses, may not tolerate the addition of GFP to critical proteins. The use of packaging cell lines, in which some of the viral proteins are encoded by the cell (Martinez-Sobrido et al., 2010), could alleviate part of this restriction and allow FP fusions and subsequent trafficking studies of the capsids and vRNP in these viruses.
The cortical actin network presents a barrier that all viruses must cross for egress. Poxviruses and herpesviruses seem to have taken a proactive approach for transport through the network to the plasma membrane by thinning the network and engaging myosin. It seems likely that other viruses may employ a similar strategy. Exactly which myosin mediates transport across the network needs to be determined for each virus as different myosins are expressed in different cell types. As it seems likely that viruses carry both types (kinesin and myosin) with them as they travel through the cytoplasm, the molecular events that coordinate the switch from microtubule transport in the cytoplasm to actin transport at the cortical actin network will need to be worked out.
Acknowledgments
I thank members of my laboratory for helpful comments and discussions during the preparation of this review. Work in the author's laboratory is supported by National Institute of Allergy and Infectious Diseases grants AI067391 and AI081082 in addition to contract N01-AI-50020.
References
- Akhmanova A., Hammer J.A., III Linking molecular motors to membrane cargo. Curr. Opin. Cell Biol. 2010;22:479–487. doi: 10.1016/j.ceb.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M.Y., Lu H., Bookwalter C.S., Warshaw D.M., Trybus K.M. Myosin V and Kinesin act as tethers to enhance each others' processivity. Proc. Natl Acad. Sci. USA. 2008;105:4691–4696. doi: 10.1073/pnas.0711531105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinone S.E., Smith G.A. Two modes of herpesvirus trafficking in neurons: membrane acquisition directs motion. J. Virol. 2006;80:11235–11240. doi: 10.1128/JVI.01441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinone S.E., Zaichick S.V., Smith G.A. Resolving the assembly state of herpes simplex virus during axon transport by live-cell imaging. J. Virol. 2010;84:13019–13030. doi: 10.1128/JVI.01296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa Y., Cordeiro J.V., Schleich S., Newsome T.P., Way M. The release of vaccinia virus from infected cells requires RhoA-mDia modulation of cortical actin. Cell Host Microbe. 2007;1:227–240. doi: 10.1016/j.chom.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Arakawa Y., Cordeiro J.V., Way M. F11L-mediated inhibition of RhoA-mDia signaling stimulates microtubule dynamics during vaccinia virus infection. Cell Host Microbe. 2007;1:213–226. doi: 10.1016/j.chom.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Berezuk M.A., Schroer T.A. Dynactin enhances the processivity of kinesin-2. Traffic. 2007;8:124–129. doi: 10.1111/j.1600-0854.2006.00517.x. [DOI] [PubMed] [Google Scholar]
- Carlsson A.E. Actin dynamics: from nanoscale to microscale. Annu. Rev. Biophys. 2010;39:91–110. doi: 10.1146/annurev.biophys.093008.131207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter G.C., Rodger G., Murphy B.J., Law M., Krauss O., Hollinshead M., Smith G.L. Vaccinia virus cores are transported on microtubules. J. Gen. Virol. 2003;84:2443–2458. doi: 10.1099/vir.0.19271-0. [DOI] [PubMed] [Google Scholar]
- Chambers R., Takimoto T. Trafficking of Sendai virus nucleocapsids is mediated by intracellular vesicles. PLoS ONE. 2010;5:e10994. doi: 10.1371/journal.pone.0010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro J.V., Guerra S., Arakawa Y., Dodding M.P., Esteban M., Way M. F11-mediated inhibition of RhoA signalling enhances the spread of vaccinia virus in vitro and in vivo in an intranasal mouse model of infection. PLoS ONE. 2009;4:e8506. doi: 10.1371/journal.pone.0008506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.C., Nayak D., Zhou Y., Pattnaik A.K. Visualization of intracellular transport of vesicular stomatitis virus nucleocapsids in living cells. J. Virol. 2006;80:6368–6377. doi: 10.1128/JVI.00211-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePina A.S., Langford G.M. Vesicle transport: the role of actin filaments and myosin motors. Microsc. Res. Tech. 1999;47:93–106. doi: 10.1002/(SICI)1097-0029(19991015)47:2<93::AID-JEMT2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Diefenbach R.J., Miranda-Saksena M., Diefenbach E., Holland D.J., Boadle R.A., Armati P.J., Cunningham A.L. Herpes simplex virus tegument protein US11 interacts with conventional kinesin heavy chain. J. Virol. 2002;76:3282–3291. doi: 10.1128/JVI.76.7.3282-3291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach R.J., Miranda-Saksena M., Douglas M.W., Cunningham A.L. Transport and egress of herpes simplex virus in neurons. Rev. Med. Virol. 2008;18:35–51. doi: 10.1002/rmv.560. [DOI] [PubMed] [Google Scholar]
- Greber U.F., Way M. A superhighway to virus infection. Cell. 2006;124:741–754. doi: 10.1016/j.cell.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Grosshans B.L., Ortiz D., Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc. Natl Acad. Sci. USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Martinez E., Roberts K.L., Hollinshead M., Smith G.L. Vaccinia virus intracellular enveloped virions move to the cell periphery on microtubules in the absence of the A36R protein. J. Gen. Virol. 2005;86:2961–2968. doi: 10.1099/vir.0.81260-0. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Noda Y. Intracellular transport and kinesin superfamily proteins, KIFs: structure, function, and dynamics. Physiol. Rev. 2008;88:1089–1118. doi: 10.1152/physrev.00023.2007. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Noda Y., Tanaka Y., Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- Holland D.J., Miranda-Saksena M., Boadle R.A., Armati P., Cunningham A.L. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J. Virol. 1999;73:8503–8511. doi: 10.1128/jvi.73.10.8503-8511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Lazear H.M., Friedman H.M. Completely assembled virus particles detected by transmission electron microscopy in proximal and mid-axons of neurons infected with herpes simplex virus type 1, herpes simplex virus type 2 and pseudorabies virus. Virology. 2011;409:12–16. doi: 10.1016/j.virol.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeshtadi A., Burgos P., Stubbs C.D., Parker A.W., King L.A., Skinner M.A., Botchway S.W. Interaction of poxvirus intracellular mature virion proteins with the TPR domain of kinesin light chain in live infected cells revealed by two-photon-induced fluorescence resonance energy transfer fluorescence lifetime imaging microscopy. J. Virol. 2010;84:12886–12894. doi: 10.1128/JVI.01395-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon J.R., Vale R.D. Regulators of the cytoplasmic dynein motor. Nat. Rev. Mol. Cell Biol. 2009;10:854–865. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler I., Meehan A., Poeschla E.M. Live-cell coimaging of the genomic RNAs and Gag proteins of two lentiviruses. J. Virol. 2010;84:6352–6366. doi: 10.1128/JVI.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W., Tang Y., Okada Y., Torrey T.A., Chattopadhyay S.K., Pfleiderer M., Falkner F.G., Dorner F., Choi W., Hirokawa N., Morse H.C., III Binding of murine leukemia virus Gag polyproteins to KIF4, a microtubule-based motor protein. J. Virol. 1998;72:6898–6901. doi: 10.1128/jvi.72.8.6898-6901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshizuka T., Kawaguchi Y., Nishiyama Y. Herpes simplex virus type 2 membrane protein UL56 associates with the kinesin motor protein KIF1A. J. Gen. Virol. 2005;86:527–533. doi: 10.1099/vir.0.80633-0. [DOI] [PubMed] [Google Scholar]
- Lee G.E., Murray J.W., Wolkoff A.W., Wilson D.W. Reconstitution of herpes simplex virus microtubule-dependent trafficking in vitro. J. Virol. 2006;80:4264–4275. doi: 10.1128/JVI.80.9.4264-4275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman M.G., Enquist L.W. Herpesvirus interactions with the host cytoskeleton. J. Virol. 2009;83:2058–2066. doi: 10.1128/JVI.01718-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallardo M., Schleich S., Krijnse Locker J. Microtubule-dependent organization of vaccinia virus core-derived early mRNAs into distinct cytoplasmic structures. Mol. Biol. Cell. 2001;12:3875–3891. doi: 10.1091/mbc.12.12.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez N.W., Xue X., Berro R.G., Kreitzer G., Resh M.D. Kinesin KIF4 regulates intracellular trafficking and stability of the human immunodeficiency virus type 1 Gag polyprotein. J. Virol. 2008;82:9937–9950. doi: 10.1128/JVI.00819-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Sobrido L., Cadagan R., Steel J., Basler C.F., Palese P., Moran T.M., Garcia-Sastre A. Hemagglutinin-pseudotyped green fluorescent protein-expressing influenza viruses for the detection of influenza virus neutralizing antibodies. J. Virol. 2010;84:2157–2163. doi: 10.1128/JVI.01433-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D., Vodicka M.A., Lucero G., Svitkina T.M., Borisy G.G., Emerman M., Hope T.J. Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol. 2002;159:441–452. doi: 10.1083/jcb.200203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T.C., Klupp B.G., Granzow H. Herpesvirus assembly: an update. Virus Res. 2009;143:222–234. doi: 10.1016/j.virusres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Miki H., Setou M., Kaneshiro K., Hirokawa N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc. Natl Acad. Sci. USA. 2001;98:7004–7011. doi: 10.1073/pnas.111145398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Saksena M., Armati P., Boadle R.A., Holland D.J., Cunningham A.L. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 2000;74:1827–1839. doi: 10.1128/jvi.74.4.1827-1839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan G.W., Hollinshead M., Ferguson B.J., Murphy B.J., Carpentier D.C., Smith G.L. Vaccinia protein F12 has structural similarity to kinesin light chain and contains a motor binding motif required for virion export. PLoS Pathog. 2010;6:e1000785. doi: 10.1371/journal.ppat.1000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Poxviridae: The viruses and their replication. In: Fields B.N., Knipe D.M., Howley P.M., editors. Fields Virology. Fourth ed. Lippincott-Raven Publishers; Philadelphia: 2001. pp. 2849–2883. [Google Scholar]
- Moss B., Ward B.M. High-speed mass transit for poxviruses on microtubules. Nat. Cell Biol. 2001;3:E245–E246. doi: 10.1038/ncb1101-e245. [DOI] [PubMed] [Google Scholar]
- Negatsch A., Granzow H., Maresch C., Klupp B.G., Fuchs W., Teifke J.P., Mettenleiter T.C. Ultrastructural analysis of virion formation and intraaxonal transport of herpes simplex virus type 1 in primary rat neurons. J. Virol. 2010;84:13031–13035. doi: 10.1128/JVI.01784-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E.N., Nordheim A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat. Rev. Mol. Cell Biol. 2010;11:353–365. doi: 10.1038/nrm2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J.E., Smith G.L. Vaccinia virus gene A36R encodes a Mr 43-50 K protein on the surface of extacellular enveloped virus. Virology. 1994;204:376–390. doi: 10.1006/viro.1994.1542. [DOI] [PubMed] [Google Scholar]
- Radtke K., Dohner K., Sodeik B. Viral interactions with the cytoskeleton: a hitchhiker's guide to the cell. Cell. Microbiol. 2006;8:387–400. doi: 10.1111/j.1462-5822.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- Radtke K., Kieneke D., Wolfstein A., Michael K., Steffen W., Scholz T., Karger A., Sodeik B. Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures. PLoS Pathog. 2010;6:e1000991. doi: 10.1371/journal.ppat.1000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redowicz M.J. Unconventional myosins in muscle. Eur. J. Cell Biol. 2007;86:549–558. doi: 10.1016/j.ejcb.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Resh M.D. A myristoyl switch regulates membrane binding of HIV-1 Gag. Proc. Natl Acad. Sci. USA. 2004;101:417–418. doi: 10.1073/pnas.0308043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietdorf J., Ploubidou A., Reckmann I., Holmstrom A., Frischknecht F., Zettl M., Zimmermann T., Way M. Kinesin-dependent movement on microtubules precedes actin-based motility of vaccinia virus. Nat. Cell Biol. 2001;3:992–1000. doi: 10.1038/ncb1101-992. [DOI] [PubMed] [Google Scholar]
- Roberts K.L., Baines J.D. Myosin Va enhances secretion of herpes simplex virus 1 virions and cell surface expression of viral glycoproteins. J. Virol. 2010;84:9889–9896. doi: 10.1128/JVI.00732-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J.L., Ali M.Y., Warshaw D.M. Cargo transport: molecular motors navigate a complex cytoskeleton. Curr. Opin. Cell Biol. 2008;20:41–47. doi: 10.1016/j.ceb.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C.M., Hollinshead M., Smith G.L. The vaccinia virus A27L protein is needed for the microtubule- dependent transport of intracellular mature virus particles. J. Gen. Virol. 2000;81:47–58. doi: 10.1099/0022-1317-81-1-47. [DOI] [PubMed] [Google Scholar]
- Schepis A., Stauber T., Krijnse Locker J. Kinesin-1 plays multiple roles during the vaccinia virus life cycle. Cell. Microbiol. 2007;9:1960–1973. doi: 10.1111/j.1462-5822.2007.00927.x. [DOI] [PubMed] [Google Scholar]
- Schimmoller F., Simon I., Pfeffer S.R. Rab GTPases, directors of vesicle docking. J. Biol. Chem. 1998;273:22161–22164. doi: 10.1074/jbc.273.35.22161. [DOI] [PubMed] [Google Scholar]
- Schmelz M., Sodeik B., Ericsson M., Wolffe E.J., Shida H., Hiller G., Griffiths G. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J. Virol. 1994;68:130–147. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu Y.L., Teoh K.T., Lo J., Chan C.M., Kien F., Escriou N., Tsao S.W., Nicholls J.M., Altmeyer R., Peiris J.S., Bruzzone R., Nal B. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J. Virol. 2008;82:11318–11330. doi: 10.1128/JVI.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowronek K.J., Kocik E., Kasprzak A.A. Subunits interactions in kinesin motors. Eur. J. Cell Biol. 2007;86:559–568. doi: 10.1016/j.ejcb.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Smith G.A., Gross S.P., Enquist L.W. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc. Natl Acad. Sci. USA. 2001;98:3466–3470. doi: 10.1073/pnas.061029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G.L., Vanderplasschen A., Law M. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 2002;83:2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- Snyder A., Wisner T.W., Johnson D.C. Herpes simplex virus capsids are transported in neuronal axons without an envelope containing the viral glycoproteins. J. Virol. 2006;80:11165–11177. doi: 10.1128/JVI.01107-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A., Bruun B., Browne H.M., Johnson D.C. A herpes simplex virus gD-YFP fusion glycoprotein is transported separately from viral capsids in neuronal axons. J. Virol. 2007;81:8337–8340. doi: 10.1128/JVI.00520-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Suomalainen M., Nakano M.Y., Keller S., Boucke K., Stidwill R.P., Greber U.F. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J. Cell Biol. 1999;144:657–672. doi: 10.1083/jcb.144.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Winkler U., Freed E.O., Torrey T.A., Kim W., Li H., Goff S.P., Morse H.C., III Cellular motor protein KIF-4 associates with retroviral Gag. J. Virol. 1999;73:10508–10513. doi: 10.1128/jvi.73.12.10508-10513.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivola D.M., Strnad P., Habtezion A., Omary M.B. Intermediate filaments take the heat as stress proteins. Trends Cell Biol. 2010;20:79–91. doi: 10.1016/j.tcb.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Hollinshead M., Reis B., Radsak K., Kern H. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur. J. Cell Biol. 1993;60:163–178. [PubMed] [Google Scholar]
- Vale R.D. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- van Eijl H., Hollinshead M., Smith G.L. The vaccinia virus A36R protein is a type Ib membrane protein present on intracellular but not extracellular enveloped virus particles. Virology. 2000;271:26–36. doi: 10.1006/viro.2000.0260. [DOI] [PubMed] [Google Scholar]
- Ward B.M. The longest micron; transporting poxviruses out of the cell. Cell. Microbiol. 2005;7:1531–1538. doi: 10.1111/j.1462-5822.2005.00614.x. [DOI] [PubMed] [Google Scholar]
- Ward B.M. Visualization and characterization of the intracellular movement of vaccinia virus intracellular mature virions. J. Virol. 2005;79:4755–4763. doi: 10.1128/JVI.79.8.4755-4763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B.M., Moss B. Vaccinia virus intracellular movement is associated with microtubules and independent of actin tails. J. Virol. 2001;75:11651–11663. doi: 10.1128/JVI.75.23.11651-11663.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B.M., Moss B. Vaccinia virus A36R membrane protein provides a direct link between intracellular enveloped virions and the microtubule motor kinesin. J. Virol. 2004;78:2486–2493. doi: 10.1128/JVI.78.5.2486-2493.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B.M., Weisberg A.S., Moss B. Mapping and functional analysis of interaction sites within the cytoplasmic domains of the vaccinia virus A33R and A36R envelope proteins. J. Virol. 2003;77:4113–4126. doi: 10.1128/JVI.77.7.4113-4126.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte M.A. Bidirectional transport along microtubules. Curr. Biol. 2004;14:R525–R537. doi: 10.1016/j.cub.2004.06.045. [DOI] [PubMed] [Google Scholar]
- Wolfstein A., Nagel C.H., Radtke K., Dohner K., Allan V.J., Sodeik B. The inner tegument promotes herpes simplex virus capsid motility along microtubules in vitro. Traffic. 2006;7:227–237. doi: 10.1111/j.1600-0854.2005.00379.x. [DOI] [PubMed] [Google Scholar]