Abstract
Background & Aims
Epigenetic alterations have been correlated with field cancerization in human patients, but evidence from experimental models that specific epigenetic changes can initiate cancer has been lacking. Although hormones have been associated with cancer risk, the mechanisms have not been determined. The peptide hormone, gastrin, exerts a suppressive effect on antral gastric carcinogenesis.
Methods
N-methyl-N-nitrosourea (MNU)–dependent gastric cancer was investigated in hypergastrinemic (INS-GAS), gastrin-deficient (GAS-/-), Tff1-deficient (Tff1+/-), and wild-type (WT) mice. Epigenetic alterations of the Trefoil Factor 1 (TFF1) tumor suppressor gene were evaluated in vitro and in vivo.
Results
Human intestinal-type gastric cancers in the antrum exhibited progressive TFF1 repression and promoter hypermethylation. Mice treated with MNU exhibited a field defect characterized by widespread Tff1 repression associated with histone H3 lysine 9 (H3K9) methylation and H3 deacetylation at the Tff1 promoter in epithelial cells. In MNU-induced advanced cancers, DNA methylation at the Tff1 promoter was observed. Tumor induction and Tff1 repression were increased in MNU-treated mice by Helicobacter infection. Hypergastrinemia suppressed MNU-dependent tumor initiation and progression in a manner that correlated with gene silencing and epigenetic alterations of Tff1. In contrast, homozygous gastrin-deficient and heterozygous Tff1-deficient mice showed enhanced MNU-dependent field defects and cancer initiation compared with WT mice. In gastric cancer cells, gastrin stimulation partially reversed the epigenetic silencing in the TFF1 promoter.
Conclusions
Antral gastric cancer initiation is associated with progressive epigenetic silencing of TFF1, which can be suppressed by the hormone gastrin.
Keywords: gastrin, gastric cancer, epigenetics
Introduction
While both genetic and epigenetic alterations have been characterized in diverse cancers, the critical early steps that result in cancer initiation remain poorly understood. Epigenetic alterations are often detected very early in tumorigenesis, in broad regions of histopathologically normal tissue prior to detection of the incipient tumor,1, 2 a concept referred to as a “field cancerization (defect)”. 3 Tumor suppressor genes can be silenced early through hypermethylation of CpG islands frequently found within gene promoter regions, a process that has frequently been associated with changes in histone modifications.1, 2 Growing evidence supports the concept that hormones can modulate cancer risk and regulate the epigenome.4 However, it remains uncertain whether epigenetic alterations can mediate the relationship between hormones and carcinogenesis. Further, the critical unanswered question is whether these alterations are sufficient to initiate cancer and are not just secondary events.
While Helicobacter pylori infection is the major risk factor for gastric cancer, it is often not sufficient on its own, and a diet of nitrate-rich foods, along with tobacco use, appear to be significant environmental inducers of gastric cancer.5 With the development of atrophy and hypochlorhydria during H. pylori associated carcinogenesis, dietary nitrates can be converted to endogenous N-nitroso compounds (NOCs) as a result of bacterial overgrowth.6 NOCs have been found to produce various tumors in animals, however, a causal association between exposure to NOCs and human cancer has not been established.7 Epidemiologically, increased endogenous formation of NOCs ia associated with non-cardia cancer risk in H. pylori infected patients.8 We have postulated that NOCs arising from bacterial overgrowth might be responsible for the conversion of metaplasia to dysplasia in human gastric cancer development.6 The synthetic NOC, N-Methyl-N-nitrosurea (MNU), has been used in experimental gastric carcinogenesis.9 While MNU is an alkylating agent that can potentially induce formation of DNA adducts and GC → TA transition mutations, only rare mutations have been observed in NOCs-induced gastric tumors of rodents.9 MNU is also known to modify amino acids in histone proteins, especially histone H3 lysine residues,10 leading to chromatin remodeling, although the epigenetic effects of NOCs have not been well studied.
Trefoil factor 1 (TFF1), a member of the trefoil factor family of peptides, is an antral stomach-specific tumor suppressor gene.11 Tff1-/- mice develop spontaneous antral and pyloric tumors, both adenomas and carcinomas.12 Another genetic model of antral gastric tumorigenesis, the gp130757F/F mouse, shows reduced Tff1 expression.13 A reduction in TFF1 gene expression has been observed in about 50% of human distal stomach cancers and promoter hypermethylation has also been reported.14, 15, 16 Interestingly, a well-defined positive transcriptional regulator of TFF1 is the peptide hormone, gastrin. 17
Gastrin, a peptide hormone secreted from antral gastrin-expressing (G) cells, was first characterized by its ability to stimulate acid secretion but has also been shown to stimulate proliferation of fundic epithelial cells.18, 19 Although the role of hypogastrinemia as a predisposing factor for antral gastric cancer in patients has not been studied, gastrin knockout (GAS-/-) mice develop spontaneous antral tumors under conventional housing conditions.20 While hypochlorhydria and bacterial overgrowth are thought to promote cancer in these mice,21 studies by our group and others suggest that tumorigenesis might be related to Tff1 repression in gastric antrum.17, 22
Here we show that progressive TFF1 epigenetic silencing is one of mechanisms during carcinogenesis in gastric antrum. Further, we demonstrate that the hormone, gastrin, inhibits TFF1 repression, and thus suppresses antral gastric carcinogenesis.
Materials and Methods
Human tissues and Mice
Human gastric mucosal tissues, which were collected from 4 pathological subgroups in gastric antrum; normal stomach, H. pylori-positive gastritis, intestinal-type cancer, and preneoplastic adjacent tissue showing intestinal metaplasia (IM) (Tables S1 and S2), were obtained (Melbourne Health Human Research Ethics Committee approval; Project 2004:176, Kanazawa University Ethics Committee for Human Genome Research approval 2008: 174) with the informed consent. The animal protocol was approved by the Columbia University Medical Center Institutional Animal Care and Use Committee. INS-GAS males (FVB background), gastrin-deficient, Tff1-deficient (B6), and wild-type (FVB and B6) mice were used in this study. Cell lines, chemical treatments, H. felis infection, tissue preparation, epithelial cell isolation, immunohistochemistry, qRT-PCR, methylation analysis, bisulfite modification, sequencing analysis, ChIP assay, and transfection are provided in Supplementary Materials and Methods. All the primers used are shown in Table S3.
Results
Progressive loss of TFF1 expression correlates with promoter methylation during intestinal-type carcinogenesis in human gastric antrum
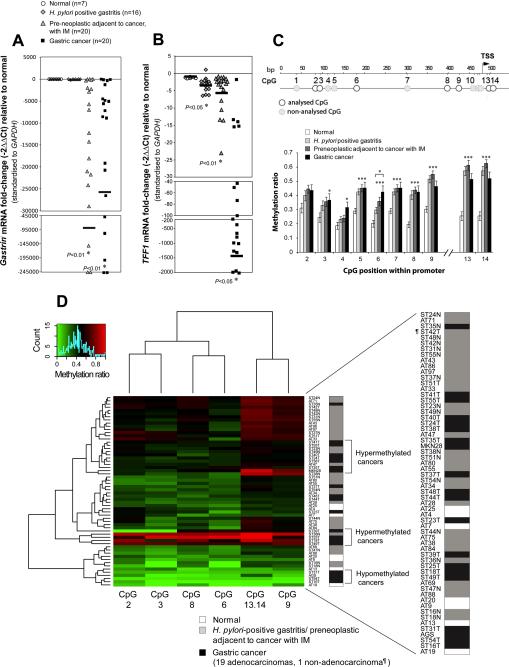
Gastrin mRNA expression was significantly decreased in preneoplastic mucosa with IM and intestinal-type cancers, but was not reduced in H. pylori-positive mucosa compared with normal mucosa (Figure 1A). TFF1 mRNA expression was significantly decreased in H. pylori-positive mucosa, preneoplastic mucosa, and cancers compared with normal mucosa (Figure 1B). CpG dinucleotides in the TFF1 proximal promoter were more frequently methylated in H. pylori-positive gastritis, preneoplastic mucosa, and cancers than in normal mucosa (Figure 1C). This shows that TFF1 promoter hypermethylation is associated with TFF1 repression at the earliest stages of carcinogenesis in gastric antrum. Therefore, although a modest suppression of TFF1 occurs independently of gastrin levels in H. pylori infection, there is a clear relationship between TFF1 epigenetic silencing and loss of gastrin in both IM and intestinal-type cancers in gastric antrum.
Figure 1.
Epigenetic silencing of TFF1 during intestinal-type carcinogenesis in human gastric antrum. qRT-PCR analysis of Gastrin (A) and TFF1 (B) mRNA levels from four indicated pathological groups. Y-axis shows mRNA fold-change relative to normal antral stomach. Black horizontal bars show mean fold-change. *P<0.05. (C) 500bp region of the TFF1 promoter analyzed for methylation. Position of the TFF1 transcription start site (TSS) is indicated. (C) TFF1 promoter methylation levels in human gastric tissues determined by EPITYPER analysis. *P<0.05. (D) Two-way hierarchical cluster analysis of methylation at TFF1 promoter CpGs (columns) in human gastric tissues (rows). CpG units in pair 13-14 are shown as combined averages (not resolved on the mass spectrum). Tissue pathology classification is shown on the right vertical axis. CpG methylation ratios 0 (green) to 1.0 (red; see color key). AGS and MKN28, gastric cancer cell lines
To extend these findings, we investigated whether samples from different pathological subgroups can be distinguished on the basis of TFF1 promoter methylation patterns by hierarchical clustering (Figure 1D). This analysis broadly partitioned the cancer samples (n=20) (Table S2) across three major groups, two with TFF1 hypermethylation containing the majority (50%, 10/20), and one (15%, 3/20) with TFF1 hypomethylation. A non-adenocarcinoma (choriocarcinoma) was not included in either group (Figure 1D). The division by methylation status was predictive for pathology but not for specific pathological subgroups. Interestingly, MKN28 gastric cancer cells were associated with the high methylation group, while AGS cells were associated with the low methylation group. Collectively, our results suggest that a subset of human gastric cancers in the antrum may arise by TFF1 epigenetic silencing under hypogastrinemic conditions.
Gastrin suppresses MNU-dependent antral gastric carcinogenesis
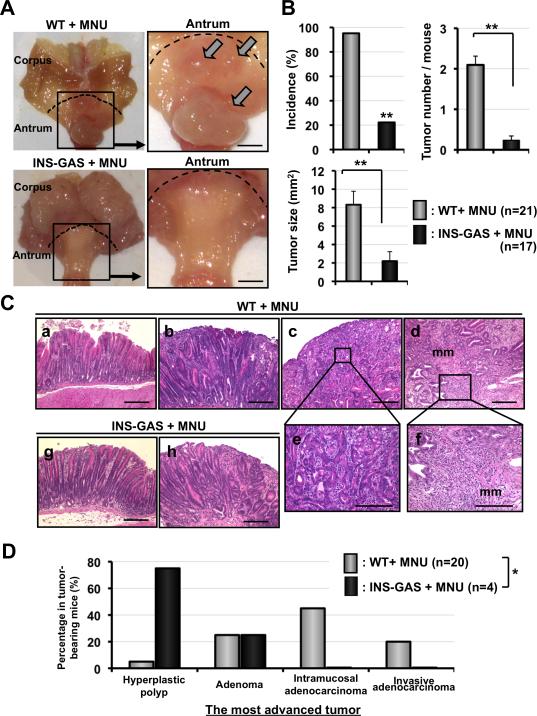
To investigate the role of TFF1 and gastrin in gastric carcinogenesis of the antrum, we administrated MNU (five 1-week courses) to WT and INS-GAS males, and followed the mice for up to 36 weeks (Figure S1). It is well established that INS-GAS mice spontaneously develop gastric tumors only in the corpus.23 Antral tumors were not detected in either cohort at 18 weeks. At 36 weeks, antral tumors were observed macroscopically in MNU-treated WT and INS-GAS mice, most showing a sessile and/or polypoid morphology (Figure 2A). In the corpus, there was no difference between INS-GAS and WT mice in the response to MNU; in contrast, in the antrum, the incidence, average tumor number, and tumor size were significantly greater MNU-treated WT mice compared to MNU-treated INS-GAS mice (Figure 2B). These results indicate that hypergastrinemia suppresses the initiation and promotion stage of MNU-induced antral tumors.
Figure 2.
Gastrin suppresses MNU-dependent gastric carcinogenesis. (A) Macroscopic photographs of the whole stomach (left) and antrum (right) in MNU-treated WT and INS-GAS mice at 36 weeks. Dotted lines define the border between the corpus and the antrum. Higher magnifications of boxed sections indicate the antrum. Arrows indicate macroscopic tumors. Bars, 2mm. (B) Incidence (percentage of tumor-bering mice/total examined mice in each group), tumor number, tumor size of gastric tumors in MNU-treated WT and INS-GAS mice. **P<0.01. (C) Histopathologic features of gastric tumors in MNU-treated WT and INS-GAS mice at 36 weeks. MNU-treated WT mice (a-f). a. Hyperplastic polyp b. Adenoma c. intramucosal adenocarcinoma d. Invasive adenocarcinoma e. and f. higher magnifications of boxed sections in (c) and (d). mm, muscularis mucosa. MNU-treated INS-GAS mice (g, h). g. Hyperplastic polyp h. Adenoma Bars, 100 µm (D) Histologic grade of the most advanced tumor in tumor-bearing MNU-treated WT and INS-GAS mice. *P<0.05.
Histopathlogical evaluation demonstrated significant decrease in the development of intramucosal and invasive adenocarcinomas in MNU-treated INS-GAS mice as compared with MNU-treated WT mice (Figure 2CD), consistent with a suppressive effect of gastrin on the progression to advanced antral gastric cancer. It is noteworthy that the MNU-treated WT mice often developed moderately and poorly differentiated adenocarcinomas, whereas MNU-treated INS-GAS mice did not. In contrast, MNU treatment of INS-GAS mice enhanced the development of Tff2-negative dysplastic glands during carcinogenesis in the corpus (Figure S2), while MNU treatment of WT mice showed no effect.
Hypergastrinemia suppresses MNU-dependent proliferation, apoptosis and progenitor cell expansion that correlates with inhibition of Tff1 repression
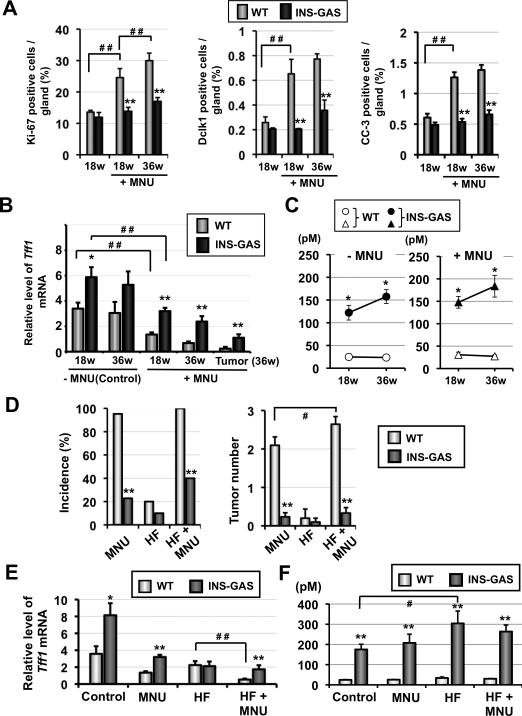
To clarify some of the inhibitory mechanisms of gastrin on gastric carcinogenesis, we assessed antral cell proliferative activity (Ki-67), apoptosis (cleaved caspase-3), and putative progenitor cell (doublecortin-like kinase 1, Dclk1) distribution in MNU-treated mice. Dclk1 was reported to be a transcript specifically upregulated in gut epithelial progenitors 24 and suggested to be a putative progenitor cell marker in the stomach.25 Although antral proliferation, apoptosis and Dclk1+ cell numbers increased over time in both set of mice, there was a significantly greater increase in all three parameters in MNU-treated WT mice compared with MNU-treated INS-GAS mice (Figure 3A and S3A). This suggests that suppression of antral proliferation, apoptosis, and progenitor cell expansion by gastrin is a likely mechanism for inhibition of antral carcinogenesis.
Figure 3.
Gastrin suppresses abnormal cell proliferation, progenitor cell expansion, and Tff1 repression during MNU-induced antral carcinogenesis, and H. felis infection promotes MNU-induced antral carcinogenesis with Tff1 repression. (A) Percentage of Ki-67+, Dclk1+, and cleaved caspase-3 (CC-3)+ cells in the antrum of WT and INS-GAS mice (n=10 for each group) at the indicated times. (B) Expression of Tff1 mRNA in tumor-free epithelial cells and tumors. *P < 0.05, **P < 0.01 for WT versus INS-GAS mice at the indicated times, # # P < 0.01 for comparisons indicated. (C) Plasma gastrin levels in untreated (-MNU) and MNU-treated (+MNU) WT and INS-GAS mice at the indicated times (n=5 for each group). *P < 0.05 for WT versus INS-GAS mice at the indicated times. (D) Incidence (percentage of tumor-bearing mice/total examined mice in each group) and average number of gastric tumors in MNU-treated and/or H. felis-infected WT and INS-GAS mice at 36 weeks. HF, H. felis **P < 0.01 for WT mice versus INS-GAS mice at the indicated times, # P < 0.05 for comparisons indicated. (E) Expression of Tff1 mRNA in the non-cancerous epithelium of MNU-treated and/or H. felis-infected WT and INS-GAS mice at 36 weeks. *P < 0.05, **P < 0.01 for WT versus INS-GAS mice at the indicated groups, # P < 0.05 for comparisons indicated. (F) Plasma gastrin levels in MNU-treated and/or H. felis-infected WT and INS-GAS mice at 36 weeks. **P < 0.01 for WT versus INS-GAS mice at the indicated groups, # P < 0.05 for comparisons indicated.
We next examined Tff1 mRNA expression in epithelial cells of gastric antrum during carcinogenesis. At 18 weeks following MNU exposure, antral Tff1 mRNA expression in WT mice was significantly lower than that of untreated controls and further reduced at 36 weeks. (Figure 3B). In addition, antral Tff1 mRNA expression was significantly lower in tumor tissues compared to adjacent non-neoplastic tissues at 36 weeks. In contrast, corpus Tff1 expression in surface pit cells and dysplastic glands by immunohistochemistry and qRT-PCR showed no change following MNU treatment in either WT or INS-GAS mice. (Figure S4). While plasma gastrin levels were higher in MNU-treated INS-GAS mice compared to MNU-treated WT mice (Figure 3C), both WT and INS-GAS mice showed a normal gastric pH at 18 weeks following MNU treatment, although the gastric pH increased at the time that the mice developed antral tumors (36 weeks) (Figure S3B), suggesting that gastrin effects were not mediated directly by alterations in acidity. Taken together, there is a correlation between Tff1 loss and progression to cancer, suggesting that progressive Tff1repression in the antrum represents a potential mechanism for MNU-induced carcinogenesis.
One day after a 1-week course of MNU treatment (Figure S5A), epithelial cell proliferation and Tff1 mRNA expression were increased in both WT and INS-GAS mice, but the proliferation normalized more rapidly in INS-GAS mice (Figure S5 BC). Tff1 mRNA expression fell rapidly following the 1-week MNU treatment, and was equivalent to normal at 2 and 4 weeks, while Tff1 mRNA levels in INS-GAS mice were higher than WT mice at 4 weeks. At 1-day and 4-weeks following the 1-week MNU treatment, neither plasma gastrin nor gastric pH was altered compared to that in untreated mice. (Figure S5DE). Short-term omeprazole treatment of WT mice produced a smaller increase in plasma gastrin, and thus an insignificant change in antral Tff1 mRNA expression (Figure S5F). Nevertheless, taken together, these data are consistent with the notion that gastrin-dependent induction of Tff1 may account for its inhibitory effect on tumorigenesis.
While the MNU mouse model of antral tumorigenesis reproduces some aspects of human antral gastric cancer, it lacks the infectious- and inflammatory- aspects of Helicobacter-driven neoplasia in humans. Histopathologic scoring confirmed that MNU alone was unable to induce a significant increase in tissue infiltration by neutrophils or mononuclear cells (Figure S6). No significant antral atrophy was observed in either WT or INS-GAS mice after MNU treatment.
Thus, to explore the impact of Helicobacter infection on Tff1 gene expression and antral carcinogenesis, we studied the combination of H. felis infection + MNU treatment in WT and INS-GAS mice. Eight- to ten-week old mice were inoculated with H. felis, followed two weeks later by administration of MNU in the drinking water (Figure S1). H. felis infection alone resulted in limited antral tumorigenesis (but no antral adenocarcinoma). H. felis infected WT mice developed adenomas in the antrum, while H. felis-infected INS-GAS mice only developed hyperplastic polyps (Figure S7). In contrast, H. felis infection + MNU-treatment of WT mice resulted in a significant increase in tumor number (Figure 3D). H. felis infection alone decreased Tff1 mRNA expression in the non-cancerous epithelium; however, the combination of H. felis infection + MNU resulted in a much greater reduction in antral Tff1 mRNA level (Figure 3E). In contrast, corpus Tff1 mRNA expression was not affected by H. felis infection and/or MNU treatment (Figure S4). H. felis infection increased the plasma gastrin levels in INS-GAS mice but not in WT mice (Figure 3F). Taken together, these results suggest that on its own, Helicobacter is a weak antral gastric carcinogen, and that the combined effects of H. felis infection and NOC exposure is most effective for suppressing Tff1 expression and inducing antral gastric carcinogenesis.
MNU induces epigenetic alterations in the Tff1 promoter
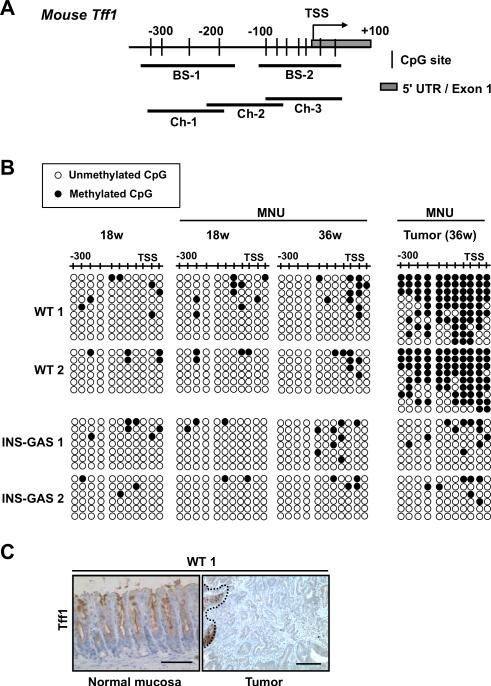
Gastric tissue-specific expression of TFF1 is regulated epigenetically by promoter methylation in mice.26 To determine if promoter methylation is a possible mechanism for Tff1 repression in MNU-induced gastric carcinogenesis, we examined isolated epithelial cells and adenocarcinomas by bisulfite sequencing (Figure 4A). Methylation at CpG sites in the Tff1 promoter increased in advanced tumors of MNU-treated WT mice but not INS-GAS mice (Figure 4B and Figure S8). Advanced adenocarcinomas showing increased promoter methylation were negative for Tff1 immunostaining, whereas disease-free epithelium with unaltered promoter methylation showed abundant Tff1 staining (Figure 4C). To exclude somatic mutation of Tff1 as a mechanism for tumor initiation by MNU, gastric tumors from MNU-treated WT and INS-GAS mice (n=3 each) were examined by gene sequencing. None were found in any of the tumors tested. These results indicate that Tff1 silencing, associated with promoter methylation in MNU-dependent tumorigenesis, is negatively regulated by gastrin.
Figure 4.
Methylation status at the Tff1 promoter during MNU-induced antral carcinogenesis. (A) A schematic of the promoter region of the mouse Tff1 gene. An arrow indicates the transcription start site (TSS). BS-1 and -2 (black bars) represent the examined PCR regions for bisulfite sequencing. Ch-1, -2, and -3 (black bars) represent the examined PCR regions for ChIP assays. (B) Methylation status of CpG sites by sequencing of bisulfite-modified DNA from tumor-free isolated epithelial cells and tumors in the antrum of WT and INS-GAS mice at the indicated times. (C) Representative Tff1 immunostaining for normal mucosa (left) and MNU-induced tumor (right) of the WT mouse indicated as WT1 in (B). A dotted line indicates the border between positive and negative lesion in the tumor. Bars, 100μm.
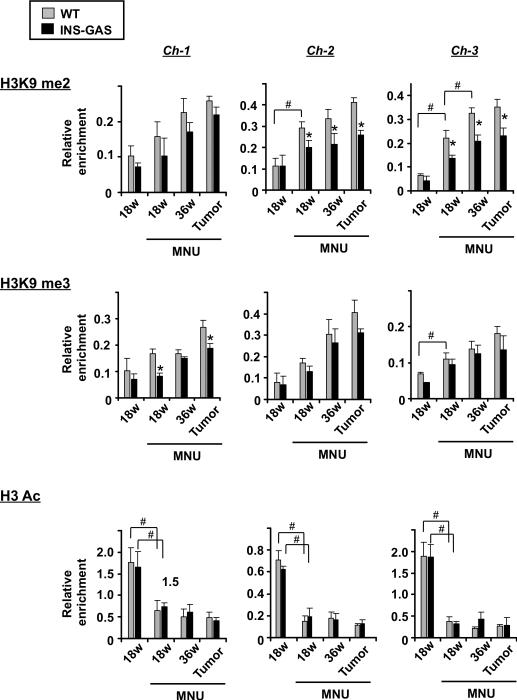
Paradoxically, 18 weeks post-MNU treatment, we observed decreased Tff1 mRNA expression (Figure 3B), without alteration to Tff1 promoter methylation in both WT and INS-GAS mice (Figure 4B). Recently, it has been reported that transcriptional repression associated with histone modifications may precede changes in DNA methylation and these modifications might be reversible.2 To determine if histone modifications might precede DNA methylation at the Tff1 promoter at the formative stages of silencing, we analyzed the repressive marks H3K9me2, H3K9me3, H3K27me3, and the activating mark, H3ac by chromatin immunoprecipitation (ChIP). Enrichment of H3K9me2 and H3K9me3 were detected in the antral epithelium at 18 weeks post-MNU treatment, and increased thereafter (Figure 5). H3K9me2 and H3K9me3 enrichment were less pronounced in MNU-treated INS-GAS mice compared to MNU-treated WT mice. H3K27me3 enrichment was not detected in either group (Figure S9). Active H3ac mark was decreased in epithelial cells and tumors of 18-week post MNU-treated WT and INS-GAS mice compared to untreated controls (Figure 5). Overall, these results indicate that increased H3K9 methylation correlates with progressive Tff1 silencing during MNU-induced antral carcinogenesis. Further, the suppressive effect of gastrin on MNU-induced carcinogenesis is closely associated with the level of H3K9 methylation at the Tff1 promoter.
Figure 5.
Histone modifications at the Tff1 promoter during MNU-induced antral carcinogenesis. ChIP analysis coupled with qPCR in the indicated amplicons (shown in Figure 4A) and antibodies recognizing repression-associated (H3K9me2 and me3) and activation-associated (H3ac) chromatin marks in tumor-free epithelial cells and tumors from WT and INS-GAS mice (n=3 per group). The relative enrichments (bound/input) encompassing the indicated regions are shown for each histone mark. *P < 0.05 for WT versus INS-GAS mice at the indicated times; # P < 0.05 for comparisons indicated.
Homozygous gastrin-deficient and heterozygous Tff1-deficient show increased susceptibility to cancer
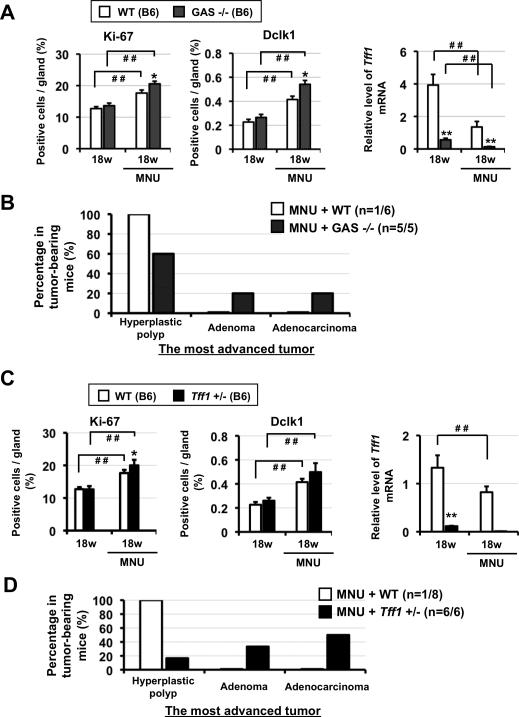
To confirm the role of gastrin in modulating susceptibility to MNU-induced carcinogenesis, we compared gastrin knockout (GAS-/-) mice to WT littermates in the MNU model (Figure S10A). While no significant differences were observed at baseline, both antral proliferation and progenitor cell number were significantly higher at 18 weeks following MNU treatment in GAS-/- mice compared to WT mice (Figure 6A and Figure S10B). Antral Tff1 mRNA expression was considerably lower at baseline in GAS-/- mice, decreased further at 18 weeks following MNU, and was significantly lower than that of MNU-treated WT mice (Figure 6A). Interestingly, we found that at 20 weeks, microadenocarcinomas developed in the antrum of MNU-treated GAS-/- mice (Figure S10C), while MNU-treated WT and untreated GAS-/- mice remained tumor free. Histopathlogical evaluation demonstrated an increase of progression of malignant tumors in MNU-treated GAS-/- mice compared with MNU-treated WT mice (Figure 6B). These results indicate that gastrin elicits a marked suppression of antral carcinogenesis by positively regulating Tff1 gene expression.
Figure 6.
Proliferation, progenitor cell expansion, and carcinogenesis in the antrum of MNU-treated GAS -/- and Tff1 +/- mice. (A) Percentage of Ki-67+ and Dclk1+ cells in the antrum of untreated and MNU-treated WT and GAS-/- mice (n=5 for each group) at the indicated times. Expression of Tff1 mRNA (left) in tumor-free epithelial cells. *P < 0.05, **P < 0.01 for WT versus GAS-/- mice at the indicated times; # # P < 0.01 for comparisons indicated. (B) Histological grade of the most advanced tumor in tumor-bearing MNU-treated WT and GAS-/- mice. (C) Percentage of Ki-67+ and Dclk1+ cells in the antrum of untreated and MNU-treated WT and Tff1+/- mice (n=5 for each group) at the indicated times. Expression of Tff1 mRNA (right) in tumor-free epithelial cells. *P < 0.05 for WT versus Tff1+/- mice at the indicated times; # # P < 0.01 for comparisons indicated. (D) Histological grade of the most advanced tumor in tumor-bearing MNU-treated Tff1+/+and Tff1+/- mice.
To address Tff1 transcriptional repression as a mechanism for MNU-driven antral tumorigenesis, we analyzed Tff1 mutant mice. Homozygous Tff1-/- mutants, which develop spontaneous antral adenomas and adenocarcinomas,12 showed at baseline increased proliferation and Dclk1+ cell number (Figure S11 AB). Heterozygous Tff1+/- mutants showed a reduced level of Tff1 mRNA compared to WT controls, but did not develop tumors (Figure 6C). By MNU exposure, both antral proliferation and progenitor cell number were significantly higher at 18 weeks in Tff1+/- mice compared to WT mice (Figure 6C and Figure S11C). Tff1 mRNA expression was almost lost in MNU-treated Tff1+/- mice at that time point. Interestingly, we found that at 18 weeks, microadenocarcinomas developed in the antrum of MNU-treated Tff1+/- mice (Figure S11D), while MNU-treated WT and untreated Tff1+/- mice remained tumor free. Finally, histopathlogical evaluation clearly demonstrated an increase of malignant tumors in MNU-treated Tff1+/- mice compared with MNU-treated WT mice (Figure 6D). These data indicate that reduced Tff1 gene expression markedly increases susceptibility to the initiation and progression in MNU-induced gastric carcinogenesis.
Gastrin stimulation changes epigenetic status at the TFF1 promoter in vitro
To investigate whether gastrin can directly modulate TFF1 epigenetic silencing in gastric cancer cells, first, we examined TFF1 mRNA expression and promoter methylation (Figure 7A) in four gastric cancer cell lines, AGS, AGS-E, MKN45, and MKN28. Significant TFF1 repression and promoter methylation were observed only in MKN28 cells (Figure S12AB). To confirm that TFF1 silencing in the TFF1-negative MKN28 cells can be indeed regulated by promoter methylation, MKN28 cells were treated with the DNA methyltransferase (DNMT) inhibitor, 5-aza-2'-deoxycytidine (5-AzadC) or the histone deacetylase inhibitor, trichostatin A (TSA). TFF1 expression was restored dose-dependently after five days of 5-AzadC treatment (Figure S12CD), while there was no significant effect of TSA (Figure S12E). These results indicate that TFF1 transcriptional repression requires promoter methylation in TFF1-silencing gastric cancer cells.
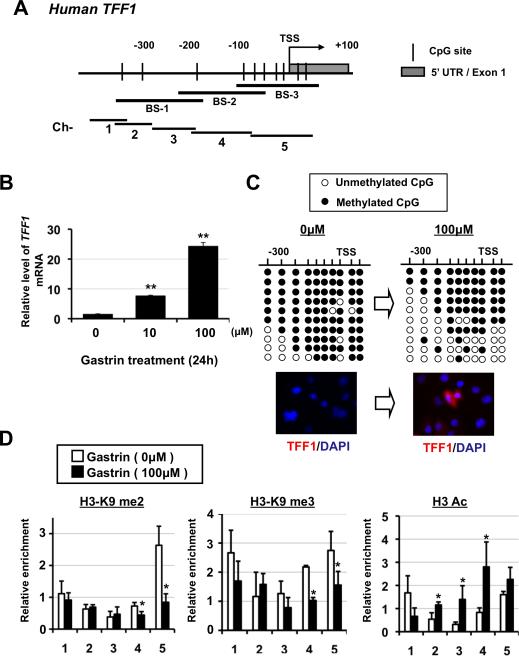
Figure 7.
Effect of gastrin stimulation on epigenetic alterations in TFF1-silencing gastric cancer cells. (A) A schematic of the human TFF1 gene promoter. An arrow indicates the transcription start site (TSS). BS-1, -2, and -3 (black bars) represents the examined PCR regions for bisulfite sequencing. Ch-1 to -5 (black bars) represents the examined PCR regions for ChIP assays. (B) Expression of TFF1 mRNA in MKN28E cells after gastrin treatment for 24 hours. **P < 0.01 for control (0μM) versus the indicated cells. (C) Methylation status (top) of CpG sites in untreated and gastrin-treated MKN28E cells. Representative images (bottom) showing TFF1(red) and DAPI(blue) immunofluorescence in untreated and gastrin-treated MKN28E cells. (D) ChIP analysis in the indicated amplicons (shown in Figure 7A) and antibodies recognizing histone marks in untreated and gastrin-treated MKN28E cells. The relative enrichments (bound/input) encompassing the indicated regions are shown for each histone mark. *P < 0.05 for untreated versus treated cells at the indicated regions.
The TFF1 transactivation by gastrin is induced both directly and indirectly through gastrin/CCKBR between neighboring cells.17 To examine the effect of gastrin on the epigenetic regulation of TFF1, MKN28 cells transfected transiently and stably with gastrin/CCKBR (stable clones, MKN28E) were treated with exogenous gastrin. Gastrin treatment of the MKN28E cells induced TFF1 mRNA expression in a dose-dependent manner, with a 25-fold increase observed with 100 micromolar concentration (Figure 7B). Gastrin treatment partially reversed methylation of the TFF1 promoter associated with TFF1 derepression (Figure 7C). Transiently transfected MKN28 cells also showed similar results (data not shown). These results suggest a mechanism by which gastrin-dependent restoration of TFF1 expression is mediated by changes in promoter methylation levels.
Finally, we analyzed histone modifications at the TFF1 promoter in response to gastrin treatment by using a ChIP assay. Following gastrin treatment, the active H3ac mark showed significant enrichment over the TFF1 promoter compared with untreated cells (Figure 7D). In addition, the repressive marks, H3K9me2 and me3, was significantly decreased, albeit in regions near the TSS at the proximal TFF1 promoter (Figure 7D). These results suggest that gastrin-dependent restoration of TFF1 expression may also be associated with the methylation level at histone lysine residues in the TFF1 proximal promoter.
Discussion
We demonstrate that one of the earliest changes that occur in the antral gland during gastric carcinogenesis in mice and humans is epigenetic repression of the tumor suppressor, TFF1. In mice, Tff1 promoter methylation and antral tumorigenesis was induced by NOC. Human studies suggest that TFF1 is frequently inactivated in human gastric cancer 14 and we show for the first time that hormonal inhibition of carcinogenesis is mediated by repression of the epigenetic changes that initiate cancer.
Epigenetic disruption might perturb the normal balance between undifferentiated progenitor cells and differentiated committed cells within a given anatomical compartment, either in number or in their capacity for normal differentiation, which provides a common mechanism for unifying neoplasia.1 Within the gastric epithelium, TFF1 has been suggested to regulate progenitor cells, since recombinant TFF1 inhibits G1-S transition and TFF1 depletion results in loss of epithelial cell maturation.27 WT mice treated in our study with MNU phenocopied the untreated Tff1-/- mice in the histological feature and tumor localization, with the development of antral tumors that was preceded by Tff1 epigenetic repression and an increase in proliferation and progenitor cell number. Methylation of histone H3K9 is a primary signal that is sufficient for initiating DNA methylation and gene silencing in vivo,28 and we showed in our MNU model substantial alterations in Tff1-associated H3K9 methylation in association with Tff1 promoter DNA methylation, which together generated an epigenetic field defect in the antrum. Thus, our results suggest that gastric progenitor cell expansion associated with epigenetic Tff1 repression might continue a first step that leading to the later onset of cancer development in the antrum.
In our studies of primary human tissues, we found that TFF1 was epigenetically repressed to a greater extent in H. pylori infected patients, more repressed in pre-neoplastic tissue and profoundly repressed in antral cancers. We found a similar pattern in our mouse models, with Tff1 repression by Helicobacter infection alone but more marked repression in the MNU-model following Helicobacter infection in the antrum. Since suppression of Tff1 appears to be necessary for initiation of antral tumors, this suggests that Helicobacter infection may not be sufficient, but instead requires the development of atrophy, achlorhydria and the production of NOCs to initiate tumorigenesis. Thus, the combination of MNU treatment following Helicobacter infection likely mimics best human antral carcinogenesis.
Interestingly, our MNU model of antral gastric cancer was phenocopied not only by Tff1-/- mice but also to a large extent by GAS-/- mice. GAS-/- mice have been reported in the past to develop spontaneous antral tumors, particularly in a mixed genetic background in conventional housing,20 and we have previously shown that gastrin is able to directly and indirectly induce TFF1 transcription 17 and that hypergastrinemia is able to inhibit the development of antral cancer in the setting of H. felis infection.22 Here, we show that untreated GAS-/- mice showed decreased Tff1 gene expression and consequently are much more susceptible to MNU-dependent antral carcinogenesis. In contrast, hypergastrinemic INS-GAS mice showed increased antral Tff1 gene expression and are extremely resistant to MNU-dependent gastric cancer. Gastrin/Cck2 receptors are present in the pyloric gland of the antrum (Figure S13), and thus could certainly mediate the effect. The relationship of hypergastrinemia to the development of human antral gastric cancer has not been specifically examined. However, moderate hypergastrinemia is typically seen in patients with duodenal ulcer disease,29 and interestingly duodenal ulcer patients have been shown to be protected against the development of gastric cancer.30 In addition, the protective effects of hypergastrinemia on antral carcinogenesis could explain the lack of promotion of proton pump inhibitors on gastric cancer rates, despite a previously reported synergy with H. pylori on the induction of corpus atrophy.31
Although previous studies have noted a relationship between epigenetic alterations and hormone-dependent cancers, such as breast and prostate cancers,4 we have shown for the first time that hormonal inhibition of cancer can be directly attributed to related epigenetic alterations of a tumor suppressor. One mode by which hormonal agents, such as estrogen and glucocorticoids, work is by modulating epigenetic events such as histone modifications and DNA methylation.4, 32 Indeed, we have demonstrated that gastrin may be able to reverse the epigenetic changes, with evidence for gastrin-dependent alterations of methylation at DNA and histone levels in gastric cancer cells, although the signaling pathways has not been defined. Recently, it has been reported that parathyroid hormone can induce active demethylation of target gene promoters by activating a protein kinase C (PKC)-dependent pathway. 32 Interestingly, we have found that the peptide hormone, gastrin, can regulate TFF1 transcriptional activity through a PKC-dependent pathway in gastric cancer cells. 17 In this respect, we can speculate that gastrin might induce demethylation of the TFF1 promoter by activating demethylases through a PKC-dependent pathway. In our study, hormonal-dependent epigenetic switching at DNA and histone levels reflects, at least in part, well demonstrated hormonal actions on gene regulation in case of gastric cancers.
In summary, our results show that the aberrant transcriptional silencing of TFF1 preceded its promoter methylation, and this may be triggered by chromatin remodeling associated with histone modifications, such as H3K9 methylation and H3 deacetylation in MNU-induced gastric carcinogenesis. These epigenetic alterations may contribute to the disruption of the cell proliferation and progenitor cell expansion, which are involved in the epigenetic field defect that leads to the later onset of cancer development in the antrum exposed to MNU (Figure S14). Further, the hormone, gastrin, is able to inhibit the initiation and progression of antral carcinogenesis through positive regulation of TFF1 and may have a potential as an epigenetic modifier. The findings may have an important implication for the prevention and treatment of cancers arising in gastric antrum.
Supplementary Material
Acknowledgement
We thank Justin DeGrazia and Ashley Whelan for their help with animal procedures.
Funding: This research was supported by grants from the National Institute of Health grants 5R01CA093405 and 5R01CA120979 (T.C.W.). H.T. was supported by the Uehara Memorial Foundation and the International College of Surgeons.
Abbreviations
- TFF
trefoil factor
- MNU
N-methyl-N-nitrosourea
- WT
wild type
- NOC
N-nitroso compound
- INS-GAS mouse
human amidated gastrin - overexpressing transgenic mouse
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- Dclk1
doublecortin-like kinase 1
- ChIP
chromatin immunoprecipitation
- BS
bisulfite sequence
- H3K9
histone H3 lysine 9
- me
methylation
- ac
acetylation
- H3K27me3
histone lysine 27 trimethylation
- 5-AzadC
5-aza-2'-deoxycytidine
- TSA
trichostatin A
- PKC
protein kinase C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors disclose no conflicts.
References
- 1.Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 2.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–16. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 3.Ushijima T. Epigenetic field for cancerization. J Biochem Mol Biol. 2007;40:142–50. doi: 10.5483/bmbrep.2007.40.2.142. [DOI] [PubMed] [Google Scholar]
- 4.Chiam K, Tilley WD, Butler LM, et al. The dynamic and static modification of the epigenome by hormones: a role in the developmental origin of hormone related cancers. Biochim Biophys Acta. 2009;1795:104–9. doi: 10.1016/j.bbcan.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 6.Fox JG, Wang TC. Helicobacter pylori--not a good bug after all! N Engl J Med. 2001;345:829–32. doi: 10.1056/NEJM200109133451111. [DOI] [PubMed] [Google Scholar]
- 7.Brambilla G, Martelli A. Genotoxic and carcinogenic risk to humans of drug-nitrite interaction products. Mutat Res. 2007;635:17–52. doi: 10.1016/j.mrrev.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Jakszyn P, Bingham S, Pera G, et al. Endogenous versus exogenous exposure to N-nitroso compounds and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study. Carcinogenesis. 2006;27:1497–501. doi: 10.1093/carcin/bgl019. [DOI] [PubMed] [Google Scholar]
- 9.Tsukamoto T, Mizoshita T, Tatematsu M. Animal models of stomach carcinogenesis. Toxicol Pathol. 2007;35:636–48. doi: 10.1080/01926230701420632. [DOI] [PubMed] [Google Scholar]
- 10.Boffa LC, Bolognesi C. Methylating agents: their target amino acids in nuclear proteins. Carcinogenesis. 1985;6:1399–401. doi: 10.1093/carcin/6.9.1399. [DOI] [PubMed] [Google Scholar]
- 11.Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–32. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- 12.Lefebvre O, Chenard MP, Masson R, et al. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science. 1996;274:259–62. doi: 10.1126/science.274.5285.259. [DOI] [PubMed] [Google Scholar]
- 13.Judd LM, Alderman BM, Howlett M, et al. Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology. 2004;126:196–207. doi: 10.1053/j.gastro.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 14.Beckler AD, Roche JK, Harper JC, et al. Decreased abundance of trefoil factor 1 transcript in the majority of gastric carcinomas. Cancer. 2003;98:2184–91. doi: 10.1002/cncr.11789. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto J, Yasui W, Tahara H, et al. DNA hypermethylation at the pS2 promoter region is associated with early stage of stomach carcinogenesis. Cancer Lett. 2000;149:125–34. doi: 10.1016/s0304-3835(99)00349-3. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho R, Kayademir T, Soares P, et al. Loss of heterozygosity and promoter methylation, but not mutation, may underlie loss of TFF1 in gastric carcinoma. Lab Invest. 2002;82:1319–26. doi: 10.1097/01.lab.0000029205.76632.a8. [DOI] [PubMed] [Google Scholar]
- 17.Khan ZE, Wang TC, Cui G, et al. Transcriptional regulation of the human trefoil factor, TFF1, by gastrin. Gastroenterology. 2003;125:510–21. doi: 10.1016/s0016-5085(03)00908-9. [DOI] [PubMed] [Google Scholar]
- 18.Johnson LR. New aspects of the trophic action of gastrointestinal hormones. Gastroenterology. 1977;72:788–92. [PubMed] [Google Scholar]
- 19.Wang TC, Koh TJ, Varro A, et al. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest. 1996;98:1918–29. doi: 10.1172/JCI118993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zavros Y, Eaton KA, Kang W, et al. Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene. 2005;24:2354–66. doi: 10.1038/sj.onc.1208407. [DOI] [PubMed] [Google Scholar]
- 21.Zavros Y, Rieder G, Ferguson A, et al. Genetic or chemical hypochlorhydria is associated with inflammation that modulates parietal and G-cell populations in mice. Gastroenterology. 2002;122:119–33. doi: 10.1053/gast.2002.30298. [DOI] [PubMed] [Google Scholar]
- 22.Takaishi S, Tu S, Dubeykovskaya ZA, et al. Gastrin is an essential cofactor for helicobacter-associated gastric corpus carcinogenesis in C57BL/6 mice. Am J Pathol. 2009;175:365–75. doi: 10.2353/ajpath.2009.081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang TC, Dangler CA, Chen D, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 24.Giannakis M, Stappenbeck TS, Mills JC, et al. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem. 2006;281:11292–300. doi: 10.1074/jbc.M512118200. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi M, Nagata H, Watanabe N, et al. Altered expression of a putative progenitor cell marker DCAMKL1 in the rat gastric mucosa in regeneration, metaplasia and dysplasia. BMC Gastroenterol. 2010;10:65. doi: 10.1186/1471-230X-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terada T, Sakagami R, Tabuchi Y, et al. Characterization of the mouse TFF1 (pS2) gene promoter region. Biol Pharm Bull. 2001;24:135–9. doi: 10.1248/bpb.24.135. [DOI] [PubMed] [Google Scholar]
- 27.Tomasetto C, Rio MC. Pleiotropic effects of Trefoil Factor 1 deficiency. Cell Mol Life Sci. 2005;62:2916–20. doi: 10.1007/s00018-005-5479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCabe MT, Brandes JC, Vertino PM. Cancer DNA methylation: molecular mechanisms and clinical implications. Clin Cancer Res. 2009;15:3927–37. doi: 10.1158/1078-0432.CCR-08-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson WL, Barnett CC, Evans DJ, Jr., et al. Acid secretion and serum gastrin in normal subjects and patients with duodenal ulcer: the role of Helicobacter pylori. Am J Gastroenterol. 1993;88:2038–43. [PubMed] [Google Scholar]
- 30.Hansson LE, Nyren O, Hsing AW, et al. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med. 1996;335:242–9. doi: 10.1056/NEJM199607253350404. [DOI] [PubMed] [Google Scholar]
- 31.Kuipers EJ, Sipponen P. Helicobacter pylori eradication for the prevention of gastric cancer. Helicobacter. 2006;11(Suppl 1):52–7. doi: 10.1111/j.1478-405X.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim MS, Kondo T, Takada I, et al. DNA demethylation in hormone-induced transcriptional derepression. Nature. 2009;461:1007–12. doi: 10.1038/nature08456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.