Abstract
Cryoprotectant (CPA) cytotoxicity constitutes a challenge in developing cryopreservation protocols, specifically in vitrification where high CPA concentrations are necessary to achieve the ice-free, vitreous state. Few cytotoxicity studies have investigated vitrification-relevant concentrations of CPAs, and the benefits and disadvantages of cocktail solutions and of incorporating non-permeating solutes have not been fully evaluated. In this study, we address these issues by determining the cytotoxicity kinetics for dimethylsulfoxide (Me2SO) and 1,2-propanediol (PD) on alginate-encapsulated βTC-tet mouse insulinomas for a range of concentrations and temperatures. Cytotoxicity kinetics were also determined for two cocktails, DPS (3M Me2SO + 3M PD + 0.5M sucrose) and PEG400 (1M Me2SO + 5M PD + 0.34 M poly(ethylene)glycol with M.W. of 400). PD was found to be more cytotoxic than Me2SO at higher concentrations and temperatures. This was reflected in PEG400 being more cytotoxic at room temperature than PEG400 at 4°C or DPS at either temperature. Addition of non-permeating solutes increased the cytotoxicity of cocktails. Furthermore, results indicate that CPA cytotoxicity may not be additive and that combining CPAs may increase cytotoxicity synergistically. Finally, when comparing cytotoxic effects towards encapsulated HepG2 and βTC-tet cells, and towards βTC-tet cells in capsules and in monolayers, CPAs appear more cytotoxic towards cells with higher metabolic activity. The incorporation of these results in the rational design of CPA addition/removal processes in vitrification is discussed.
Introduction
Within the field of Cryobiology, much work has been done to determine the cytotoxic effects of the cryoprotectant agents (CPAs) necessary for preservation. The cytotoxicity of CPAs has been shown to increase with time, temperature and concentration [1]. Cytotoxicity is especially critical for vitrification, which requires much higher concentrations of CPAs. Recently, vitrification has been touted by some to be the most promising method of preservation for tissues [2] as well as tissue-engineered constructs [3–4] due to the need to minimize or eliminate ice formation during preservation. Very few of the cytotoxicity studies available achieve the high concentrations of cryoprotectants necessary for successful vitrification [1, 5]. To improve the vitrification process, several investigators have chosen to use cocktail solutions combining CPAs to achieve the necessary concentrations. These cocktails have gained widespread use for two reasons. The combination of different permeating and non-permeating CPAs has been shown to decrease the total concentration necessary to achieve successful vitrification [6–7]. Also, the addition of non-permeating CPAs may improve the viability and function of the cells or tissues that are preserved [8–10]. Some studies have focused on determining the predictability of vitrification [11] and vitrification solution toxicity [2, 12]. However, few of these studies have directly compared the cocktail solutions to their individual CPA components to determine if cytotoxicity may be additive or have synergistic effects to either reduce or increase cytotoxicity. Most that have investigated this have focused on the addition of additives that do not contribute to the overall glass-forming ability of the solution, such as amides [13–14].
Additional questions that remain on the use of CPAs in cryopreservation include variations on CPA cytotoxicity towards different cell types or even the same cell in different types of culture. Evidence for these differences can be seen in a review of studies which range from the preservation of embryos [10, 15] to tissues [1, 16] or cells [5, 17]. To our knowledge, although cytotoxicity studies have been carried out for many of these, no studies have investigated differences in cytotoxicity towards cells cultured in monolayers and cells in tissue constructs or a tissue itself.
In this study, we address these critical issues concerning the cytotoxicity of CPAs. Alginate-encapsulated mouse insulinoma βTC-tet cells were chosen for the majority of the experiments due to their use as a pancreatic substitute [18–19]. Cytotoxicity measurements were performed in a systematic way so as to investigate the effects of temperature, concentration and exposure time. Initial studies focused on single-component CPAs applied at concentrations of 2M to 6M in order to be relevant for both conventional freezing and vitrification. The cytotoxicity of cocktail CPAs with and without non-permeating solutes was compared to single-component CPAs. To address the effects of culturing method on cytotoxicity, the cytotoxic effects of CPAs towards βTC-tet cells in monolayers and in capsules were evaluated and compared. Lastly, variations of CPA cytotoxicity towards different cell types were studied by comparing CPA effects on encapsulated HepG2 cells and βTC-tet insulinomas. Conclusions regarding fundamental issues of CPA cytotoxicity and the use of such systematic studies in designing optimized cryopreservation protocols are discussed.
Materials and Methods
Cell Culture
Mouse insulinoma βTC-tet cells were obtained from Dr. Efrat, Albert Einstein College of Medicine, Bronx, NY (Fleischer 1998). Monolayer cultures were initiated from frozen stocks and propagated in T-flasks in complete growth medium consisting of Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (Gemini Bioproducts, West Sacramento, CA), 1% L-glutamine (Mediatech, Inc., Manassas, VA) and 1% penicillin/streptomycin (Mediatech, Inc.). Monolayer human liver carcinoma HepG2 cells (American Type Culture Collection, Manassas, VA) were cultured in DMEM (Cellgro by Mediatech, Inc.) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin as above. Cells were incubated in a humidified incubator at 37°C and 5% CO2 and were split at a ratio of 1:5 (βTC-tet) or 1:10 (HepG2) when 90% confluent. Experiments were performed with βTC-tet cells of passage 38–44 and with HepG2 cells of passage 18–23.
For monolayer studies, βTC-tet cells were plated at a density of 500,000 cells/well in 24-well cell-culture treated plates (Corning Inc., Corning, NY). The cells were cultured as above for two days before cytotoxicity studies were performed.
Alginate Encapsulation
Encapsulation of βTC-tet and HepG2 cells was carried out using previously published protocols [20], briefly as follows. Cells were detached from monolayer cultures by trypsinization (0.25% Trypsin-EDTA, Mediatech Cellgro) and suspended at a density of 3.0×107 cells/ml of 2% sodium alginate (Pronova Ultra Pure LVM alginate NovaMatrix of FMC BioPolymer AS, Norway). An electrostatic droplet generator (Nisco Engineering AG, Zurich, Switzerland) was used to generate droplets which fell into a well-stirred 1.1% CaCl2 bath, forming beads of gel containing entrapped cells. Complete growth medium was used to wash and store the beads. Beads were cultured overnight in a non-tissue culture treated T-flask on a rocker plate in a 37°C and 5% CO2, humidified incubator. For cytotoxicity studies, beads were transferred to a 100 µm cell strainer (Becton-Dickinson, Franklin Lakes, NJ) and exposed to CPA solutions in a non-treated 6-well plates (Corning). Beads were agitated throughout CPA addition and removal except for final addition step where they were agitated for 4 minutes regardless of incubation time.
CPA Solutions
Solutions for cytotoxicity studies were prepared using a concentrated and modified version of the EuroCollins carrier solution containing 174.76 g/L dextrose, 5.6 g/L KCl, 4.2 g/L NaHCO3 and 8.2 g/L NaCl. This concentrated EuroCollins solution was diluted in the final solution volume at a ratio of 1:5. The cocktail solutions were DP6 (3M Me2SO + 3M PD), DPS (3M Me2SO + 3M PD + 0.5M Sucrose), PEG400 (1M Me2SO + 5M PD + 0.34M polyethylene glycol with M.W. 400) and 5/1 (1M Me2SO + 5M PD). All chemicals were purchased from Sigma-Aldrich except sucrose and NaHCO3 (Fisher). DP6 was compared to the complete solution, DPS, and 5/1 compared to the complete solution, PEG400, in order to investigate the effect of the non-permeating solutes. CPAs were added in a step-wise fashion. Protocols for addition and removal were designed using a previously established model [21]. Cells were incubated in the final solution for different times to determine the kinetics of cytotoxicity. Incubation times for other addition and removal steps remained constant for all solutions. The addition/removal protocols for cocktails and high concentration single-component CPA solutions are shown in Table 1. All addition steps were carried out at the indicated temperature (4°C or room temperature) and all removal steps were carried out at room temperature. Room temperature was 25°C ± 1°C, while 4°C was achieved by keeping solutions in an ice/water bath.
Table 1.
Addition and Removal Protocols for 6M PD, 6M Me2SO, DPS, PEG400, DP6 and 5/1. Sucrose is denoted as S. Lower concentration single component CPAs were added and removed in the same manner: 2M was added in one step (A2) and removed in one step (R4) and 4M was added in two steps (A2 and A3) and removed in two steps (R3 and R4) for corresponding CPA.
| Solution | 6M PD | 6M Me2SO | DPS | PEG400 | DP6 | 5/1 | Time (min) |
|---|---|---|---|---|---|---|---|
| (M) | PD/S | Me2SO/S | Me2SO/PD/S | Me2SO/PD/PEG | Me2SO/PD/S | Me2SO/PD/S | |
| A1 | -- | -- | -- | 0.25/1/0 | -- | -- | 4 |
| A2 | 2/0 | 2/0 | 1/1/0.15 | 0.5/2/0.1 | 1/1/0 | 0.33/1.67/0 | 4 |
| A3 | 4/0 | 4/0 | 2/2/0.3 | 0.75/3.5/0.2 | 2/2/0 | 0.67/3.33/0 | 4 |
| A4 | 6/0 | 6/0 | 3/3/0.5 | 1/5/0.3384 | 3/3/0 | 1/5/0 | 15* |
| R1 | -- | -- | 2.25/2.25/0.3 | 0.75/4/0.2 | -- | -- | 2 |
| R2 | 4/0.5 | 4/0.6 | 1.5/1.5/0.2 | 0.5/2/0.2 | 2/2/0.5 | 0.67/3.33/0.5 | 2 |
| R3 | 2/0.25 | 2/0.35 | 0.75/0.75/0.1 | 0.25/1/0 | 1/1/0.25 | 0.33/1.67/0.3 | 2 |
| R4 | 0/0 | 0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 4 |
Incubation in final addition step was changed to determine cytotoxicity kinetics.
Metabolic Activity and Viability
To determine the metabolic activity of cells, 100 µL alginate beads or a cell monolayer were incubated with a solution of alamarBlue™ consisting of 100 µL alamarBlue™ and 1 mL complete growth medium in a 12 well plate for 3 hours (encapsulated βTC-tet cells), 4 hours (monolayer βTC-tet cells) or 1.5 hours (encapsulated HepG2 cells) in a 37°C and 5% CO2, humidified incubator. Incubation times for alamarBlue™ were varied due to different cell number (encapsulated βTC-tet cells vs. monolayer βTC-tet cells) or metabolic activity of the cells (βTC-tet cells vs. HepG2 cells). With the selected incubation times, all assays fell within range wherein fluorescence was proportional to metabolic activity. A control consisting of 100 µL of alamarBlue™ and 1 mL medium was incubated for the same length of time. After this incubation, 100 µL supernatant from each well was transferred to a black 96 well plate and the fluorescence read using a SPECTRAMAX Gemini Fluorescent plate reader (Molecular Devices, Sunnyvale, CA) using an excitation wavelength of 544 nm and an emission wavelength of 590 nm. The data obtained from treated encapsulated cells using this assay were normalized to the data from untreated beads. For monolayers, the untreated control was subjected to the same number of washes as the test groups but was washed with complete growth medium. This was done to account for any detachment due simply to washing.
Cell viability was assessed using the dye exclusion stain Trypan Blue (0.4% Sigma). Beads were dissolved using 2% sodium citrate (Fisher). The resulting cell suspension was added to Trypan Blue in a 1:1 ratio and stained cells with compromised membranes and non-stained cells were counted. Trypan blue-linked viability, henceforth referred to simply as viability, was normalized to the data from untreated beads.
Statistics
Although regression analysis is often used when considering time as a variable, the time points investigated were discrete and the data were collected from independent test groups. Therefore, statistical calculations were performed using a one-way ANOVA and Tukey’s test to determine differences due to exposure time, temperature and concentration of CPA. For the comparison of single-component CPAs to cocktails, test groups were only compared to other test groups at the same temperature and exposure time. For comparisons of βTC-tet vs. HepG2 cells and of encapsulated cells vs. monolayers, statistical differences were evaluated only between cell type or culture method keeping all other variables the same. A p value of less than 0.05 was considered to indicate statistical difference.
Results and Discussion
CPA cytotoxicity is of particular concern in vitrification, which, to achieve the vitreous state, requires much higher CPA concentrations than conventional freezing. The chemical identity of the CPA used, the exposure time, temperature and concentration all play a role in the survival of cells during addition and removal procedures. Single-component CPAs and CPA cocktails were investigated to determine the role that these parameters play in the overall cytotoxicity of the solution. These studies were carried out with encapsulated βTC-tet cells. The same system was used to address the question of additivity of cytotoxicity in cocktail solutions relative to single-component CPAs. To determine the effect of the mode of cell culture on CPA cytotoxicity, experiments were carried out with βTC-tet cells cultured in monolayers and in capsules. Finally, cytotoxicity studies were performed with encapsulated βTC-tet cells and encapsulated HepG2 cells to investigate the extent to which cytotoxic effects vary between cell types. Results are described and discussed below.
Single-component CPAs
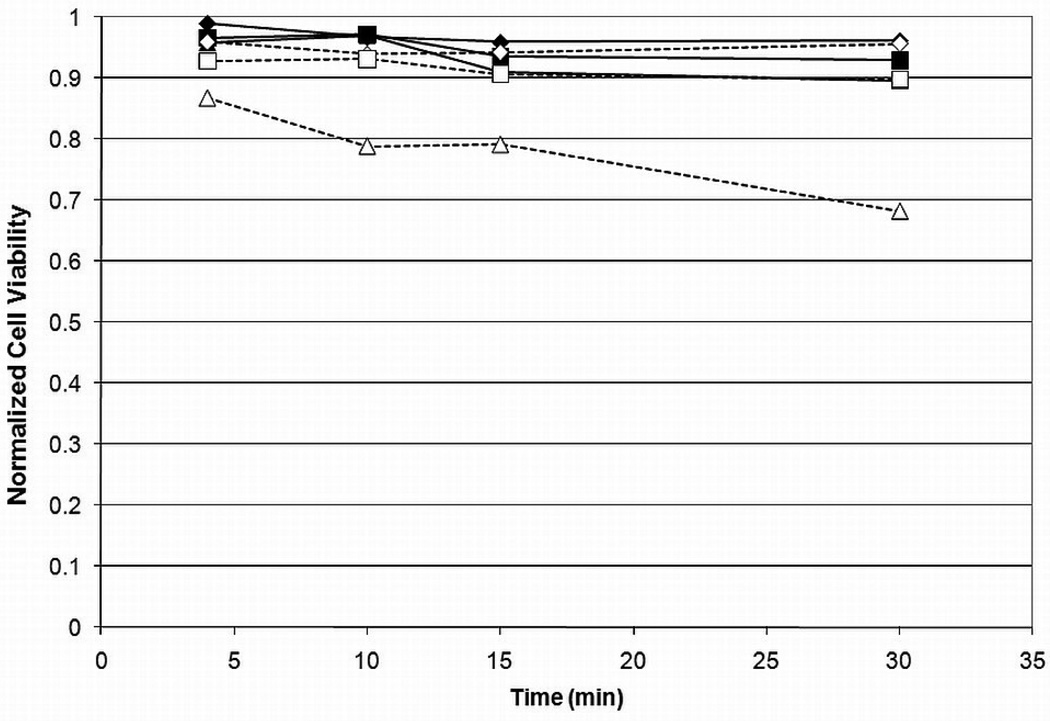
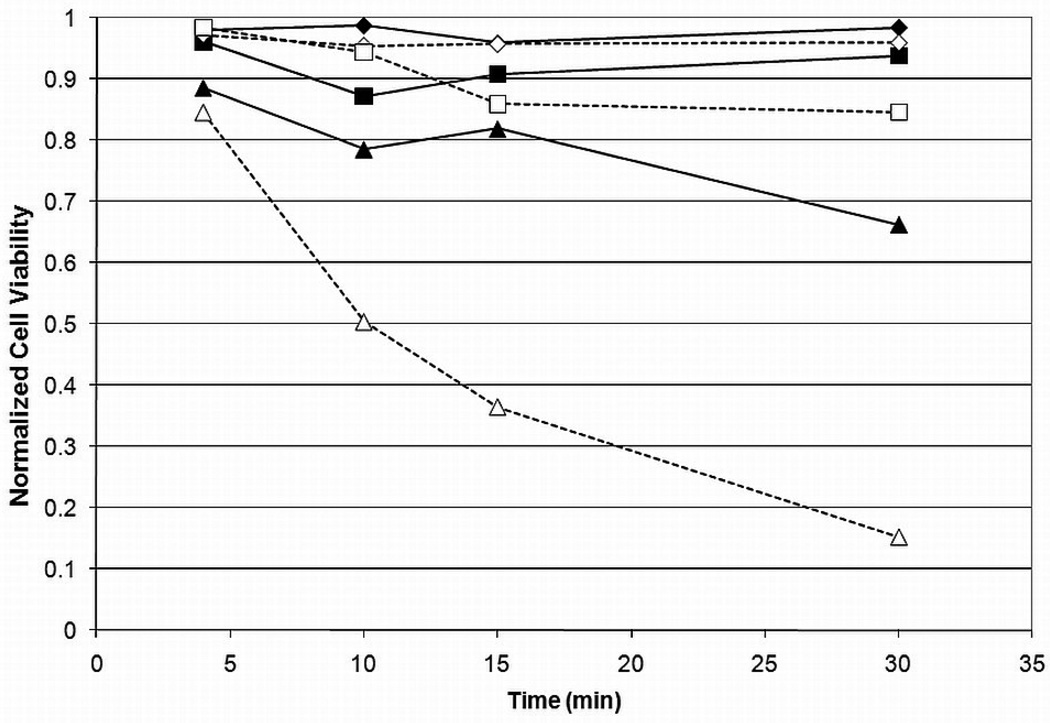
Results on the viability of encapsulated βTC-tet cells exposed to Me2SO or PD for various times, at different concentrations, and at two temperatures, are shown in Figure 1 and Figure 2. Pairwise comparisons were done for all groups and the results are shown in Table 2. As expected, cell viability generally decreased with an increase in time, temperature or concentration for either CPA. Viability decreased only slightly when cells were exposed to Me2SO with the only significant decrease occurring at room temperature as concentration was increased from 2M to 6M with an incubation time of 30 minutes. The relatively high viabilities remaining after incubation in 6M Me2SO at shorter incubation times also indicate that cell death due to osmotic excursions has been minimized. PD resulted in lower cell viabilities than Me2SO at higher concentrations and temperature. Although cell viability did not decrease significantly over a change in temperature or time for incubation in 2M or 4M PD, incubation in 6M PD caused a significantly higher loss of viability, which increased with time and temperature. This is an indication that temperature control is much more important for PD than for Me2SO. These results corroborate several previous studies which have shown that PD is more cytotoxic [17, 22]. It has been shown that temperature control during CPA addition and removal is critical for maintaining cell viability during vitrification [23–24]. However, the importance of temperature control may vary with the identity of CPAs, alone or in cocktails. To our knowledge, these data are the first to show that PD cytotoxicity is more dependent on temperature than that of Me2SO.
Figure 1.
Cell viability vs. time for Me2SO for 2M ( ), 4M (
), 4M ( ) and 6M (
) and 6M ( ) at 4°C or room temperature (empty) for encapsulated βTC-tet cells. Cell viabilitydetermined by Trypan Blue exclusion assay, normalized to untreated control. Error bars not shown for clarity. Statistical significance shown in Table 2, n=3.
) at 4°C or room temperature (empty) for encapsulated βTC-tet cells. Cell viabilitydetermined by Trypan Blue exclusion assay, normalized to untreated control. Error bars not shown for clarity. Statistical significance shown in Table 2, n=3.
Figure 2.
Cell viability vs. time for PD for 2M ( ), 4M (
), 4M ( ) and 6M (
) and 6M ( ) at 4°C or room temperature (empty) for encapsulated βTC-tet cells. Cell viability determined by Trypan Blue exclusion assay, normalized to untreated control. Error bars not shown for clarity. Statistical significance shown in Table 2, n=3.
) at 4°C or room temperature (empty) for encapsulated βTC-tet cells. Cell viability determined by Trypan Blue exclusion assay, normalized to untreated control. Error bars not shown for clarity. Statistical significance shown in Table 2, n=3.
Table 2.
Pairwise Comparisons of Single-Component Cytotoxicity Kinetics. p<0.05 for corresponding letters (e.g. p<0.05 when comparing 2M and 6M Me2SO at 30 minutes at room temperature, letter A)
| Trypan blue- linked viability |
Incubation at 4°C | Incubation at Room Temperature | ||||||
|---|---|---|---|---|---|---|---|---|
| 4 min | 10 min | 15 min | 30 min | 4 min | 10 min | 15 min | 30 min | |
| 2M Me2SO | - | - | - | - | - | - | - | A |
| 4M Me2SO | - | - | - | - | - | - | - | - |
| 6M Me2SO | - | - | - | - | - | - | - | A |
| 2M PD | - | - | - | S | L | J | G | D,E |
| 4M PD | - | - | - | T | M | K | H | D,F |
| 6M PD | U | V | B | C,S,T,U | L,M,N,O,P | V,J,K,N,Q | B,G,H,O,R | C,E,F,P,Q,R |
The metabolically active cell number measured by alamarBlue™ exhibited the same trends although it was consistently lower than viability and appeared to be more susceptible to the CPA cytotoxicity as concentration, time or temperature increased (results not shown). There are several possible reasons for this difference, the most likely being that exposure to CPAs lowers the metabolism of cells before compromising the integrity of cell membranes. Indeed, studies with human hepatocytes [25] and other cell types [26] indicate that cryopreservation reduces cellular metabolism, although it is unclear if this occurred because of the CPA exposure, the cooling/warming process, or both, as these studies investigated the aggregate effect. Another possibility for the difference in the assays is that exposure to the CPAs may activate apoptotic pathways, as demonstrated in porcine embryos [27]. This may become evident in metabolic activity assays before causing a loss in membrane integrity. Additionally, the processes of vitrification [28] and conventional freezing [29–30] have both been shown to activate apoptosis, although again these studies evaluated the aggregate CPA addition/removal and cooling/warming effects. In our studies, direct apoptosis measurements were not performed. In several of the experiments, encapsulated cells subjected to CPA addition/removal were cultured for another day and the viability measured. This indirect measure indicated that no significant apoptosis occurred (data not shown). We chose to present trypan blue-linked viability, rather than total metabolic activity, for the majority of the figures, as this is a more direct measure of cellular necrosis. Additionally, a depression in cellular metabolism may be reversible while the loss of membrane integrity necessary for Trypan blue to enter the cell is not.
CPA Cocktails
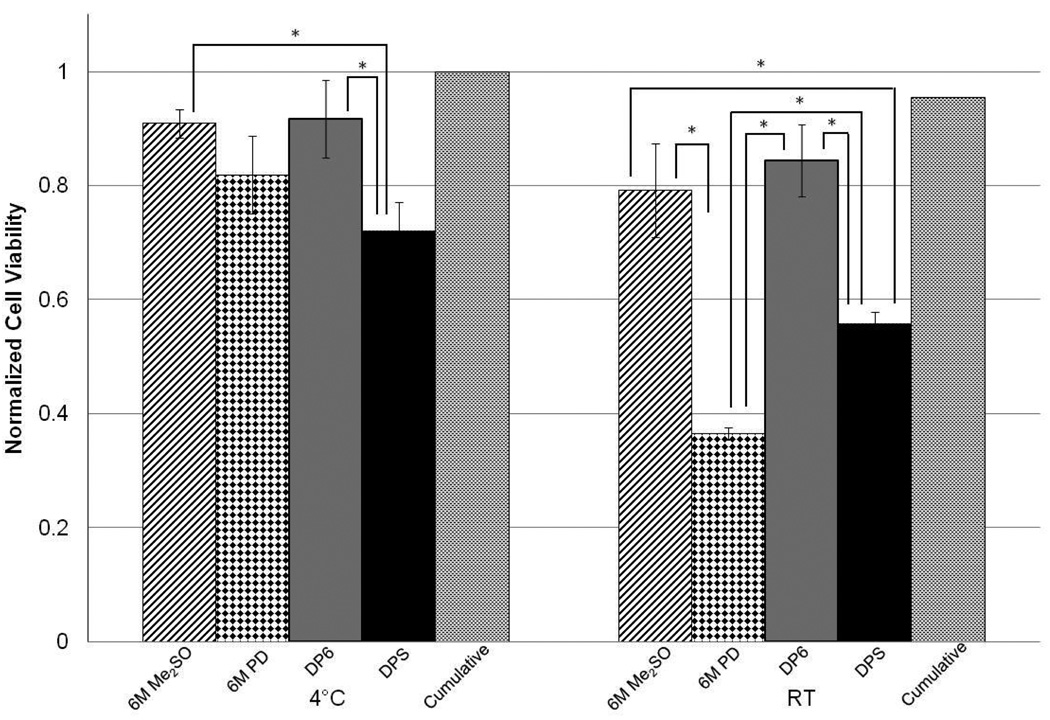
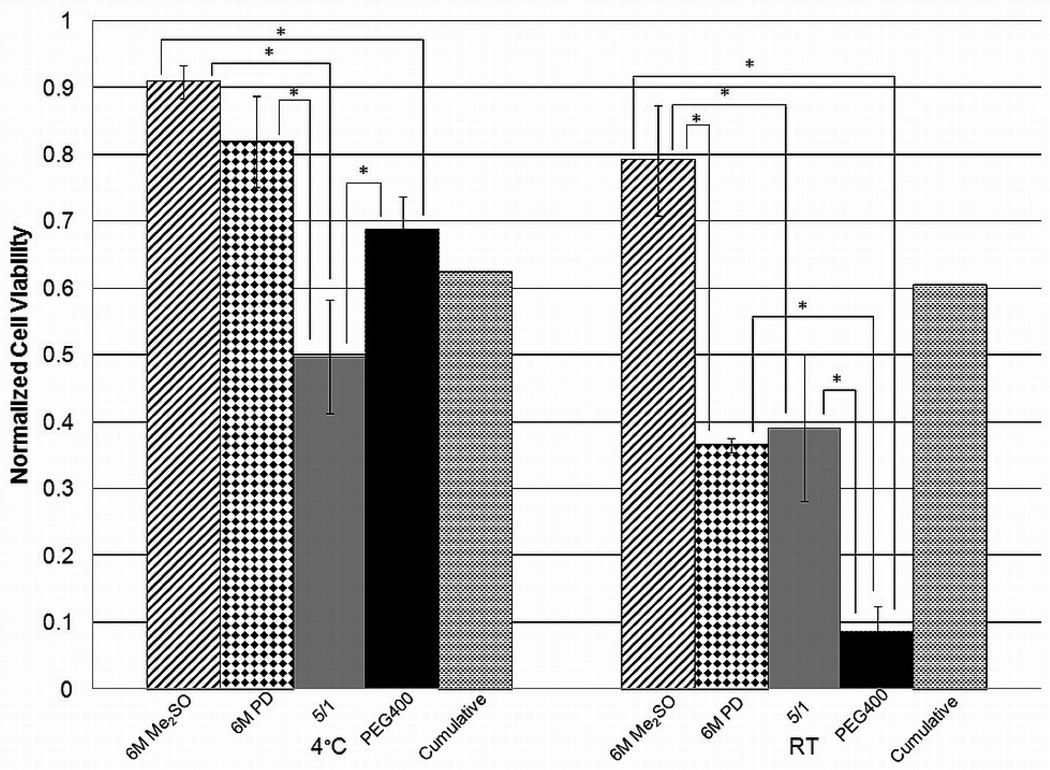
The toxicity kinetics for DPS and PEG400 were studied and compared (Figure 3). The toxicity of PEG400 was comparable to DPS at 4°C; however, PEG400 exhibited a much higher increase in toxicity as temperature was increased. In this case, statistical comparisons were made between PEG400 at room temperature and DPS at both temperatures as well as PEG400 at 4°C. Encapsulated cells exposed to PEG400 at room temperature had a statistically lower viability at each time point relative to PEG400 at 4°C or DPS at either temperature. These results indicate that temperature control is much more important for PEG400 than for DPS; they are also compatible with the single-component cytotoxicity results, as the concentration of PD is higher in PEG400 than DPS, resulting in the viability being more temperature-dependent for the PEG400 cocktail. On the other hand, the loss of cell viability caused by CPA cocktails was higher than the sum of cell viability loss caused by the pure CPAs at the concentrations found in the cocktail, with the latter obtained or estimated from Figures 1 and 2. Therefore, further experiments were carried out to directly compare the cytotoxicity of the single components to those of the cocktails without the non-permeating CPAs (DP6 and 5/1) and finally, to those of the full cocktails (DPS and PEG400). These studies were performed at a single incubation time (15 minutes) for the individual permeating CPAs as well as the cocktails.
Figure 3.
Cell viability vs. time for PEG400( ) and DPS (
) and DPS ( ) at 4°C or room temperature (empty) for encapsulated βTC-tet cells. Cell viability determined by Trypan Blue exclusion assay, normalized to untreated control. Error bars indicate standard deviations, n=3. #, † indicate p<0.05 when comparing groups. Additionally, p<0.05 when comparing PEG400 at RT to all other groups at each time point (not indicated for clarity).
) at 4°C or room temperature (empty) for encapsulated βTC-tet cells. Cell viability determined by Trypan Blue exclusion assay, normalized to untreated control. Error bars indicate standard deviations, n=3. #, † indicate p<0.05 when comparing groups. Additionally, p<0.05 when comparing PEG400 at RT to all other groups at each time point (not indicated for clarity).
Results from these studies are shown in Figure 4 and Figure 5. These figures also show the “cumulative viability” values calculated as shown below using average viabilities at the corresponding temperature and concentration of each CPA in the cocktail (e.g., 3M PD and 3M Me2SO viabilities used to determine “cumulative viability” for DP6). These values have no error bars and statistical significance is not indicated.
Figure 4.
Cell viability for 6M Me2SO, 6M PD, DP6 and DPS compared to “cumulative viability” for an incubation period of 15 minutes for encapsulated βTC-tet cells. Cell viability determined by Trypan Blue exclusion assay, normalized to untreated control. Error bars indicate standard deviations, no error bars for cumulative viability, n=3. * indicates p<0.05
Figure 5.
Cell viability for 6M Me2SO, 6M PD, 5/1 and PEG400 compared to “cumulative viability” for an incubation period of 15 minutes for encapsulated βTC-tet cells. Cell viability determined by Trypan Blue exclusion assay, normalized to untreated control. Error bars indicate standard deviations, no error bars for cumulative viability, n=3. * indicates p<0.05
It should also be noted that, in our hands, 6M PD, 6M Me2SO, 5/1 and DP6 are not vitrifiable (as evidenced by macroscopic observation) and in order to consistently achieve vitrification, non-permeating solutes need to be added. Addition of non-permeating solutes may allow for a reduction in the concentration of permeating CPA necessary to achieve vitrification [6, 10] and may inhibit ice formation during vitrification [31]. Currently, non-permeating CPAs are considered to have little effect on or be beneficial for the overall solution cytotoxicity [7, 32–33] and may stabilize cell membranes [34]. However, those results were obtained with low concentrations of CPAs which may not be significantly toxic to cells. Our results show that the viabilities of cells exposed to complete cocktails were lower than those of cells exposed to incomplete cocktails, consisting only of the permeating CPAs, and the latter were closer to the cumulative viabilities. Although the introduction of a non-permeating solute may improve the consistency of solution vitrification, it resulted in an increase in cytotoxicity, the only exception being 5/1 relative to PEG400 at 4°C (Figure 5). A possible reason for this is that the addition of non-permeating CPAs results in an increase in the intracellular concentrations of permeating CPAs, as also predicted by membrane permeability models [35]. Overall, it is important that the possibility of significantly increasing cytotoxicity be considered when adding non-permeating CPAs to solutions of permeating CPAs.
Some interesting comments can also be made in comparing single-component cytotoxicity to that of cocktails. The less cytotoxic CPA, Me2SO at 6M concentration, resulted in comparable or higher viabilities relative to cocktail solutions at either temperature. This was also the case for 6M PD at 4°C. However, at room temperature 6M PD performed worse than DP6 and DPS and was comparable to 5/1, as expected given that 5/1 is mostly PD. However, addition of the non-permeating PEG in forming PEG400 significantly increased the cytotoxicity of the latter relative to 6M PD and 5/1. These results suggest that the presence of PD at a high concentration in a cocktail affects the temperature dependence significantly.
Notably, PEG400 and 5/1 performed differently at the two temperatures, with PEG400 being less cytotoxic than 5/1 at 4°C but more cytotoxic at room temperature. The increase in cytotoxicity at room temperature is likely due to the decreased intracellular water content as discussed above. The difference at 4°C might be due to the protective effect of non-permeating solutes cited in several publications [7, 32–34]. These results therefore suggest that the protective effect of non-permeating CPAs is present at low temperatures, but it is outweighed by the negative osmotic effects at higher temperatures.
As seen in Figure 4 and Figure 5, the cumulative viabilities appear higher than those obtained with the incomplete cocktails. This suggests that CPA cocktail cytotoxicity may not be additive and that combining CPAs may have a synergistic effect, causing increased cytotoxicity.
Comparison of culturing method
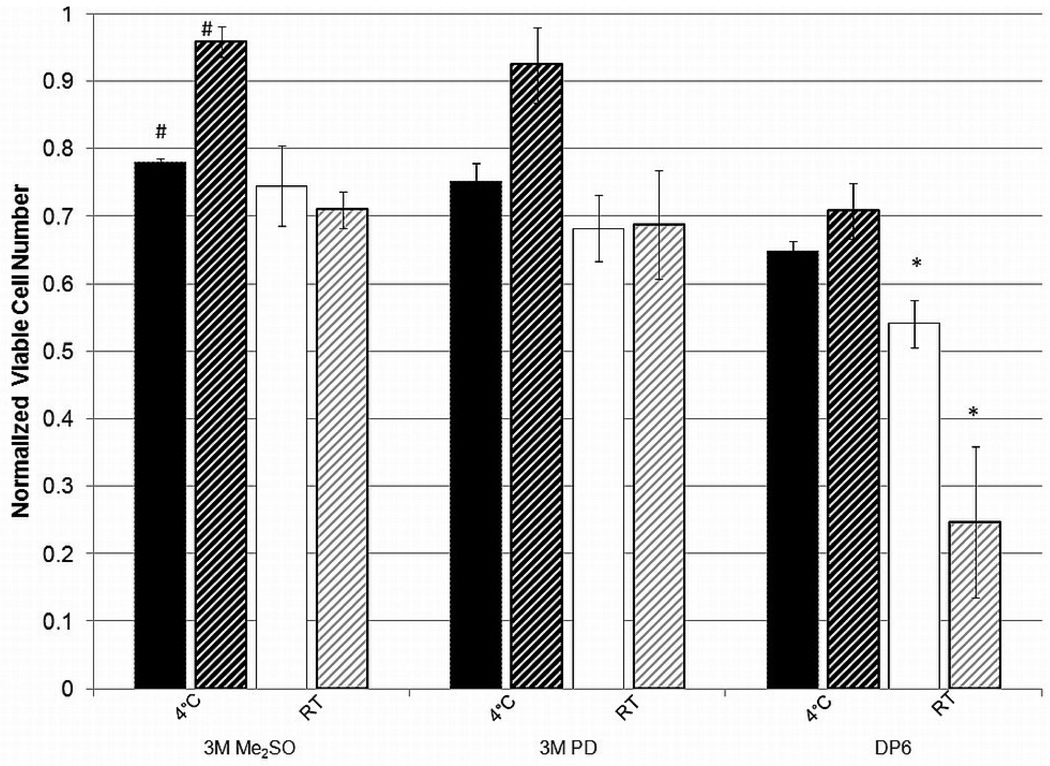
Figure 6 shows the normalized number of metabolically active cells, measured by alamarBlue™ for βTC-tet cells in suspension within an alginate matrix and in monolayers exposed to different CPAs at 4°C and room temperature for 15 min. It appears that the cells in a monolayer fare better than encapsulated cells at low temperatures and concentrations while they experience more cytotoxicity at higher CPA concentrations (DP6) and higher temperature. There are several differences between the two systems that may explain these results. The most obvious difference is that there is a short period of time necessary for diffusion of the CPAs towards the center of the alginate bead. Previous studies [36] investigated the diffusion of different CPAs into and out of alginate beads at room temperature. Those results, also temperature-corrected for diffusion at 4°C, indicate that equilibration is essentially reached within two to three minutes at either temperature, which is negligible given the exposure time of 15 minutes. Hence, diffusional resistance is an unlikely cause of the observed difference. Additionally, the cytotoxicity experienced by the encapsulated cells is not consistently lower due to a shorter exposure time. Another difference between the two systems is the culture itself. βTC-tet cells encapsulated in alginate are suspended whereas those in a monolayer are adhered to the tissue culture surface. This difference is the most likely cause for the results as studies investigating the metabolism of a similar cell line, βTC3 murine insulinomas, have shown that the metabolism of monolayers is significantly higher than encapsulated cells [37]. This increase in metabolism would cause an increase in the cytotoxic effects at room temperature and higher concentrations. At the lower temperature, the effects of higher metabolism are tempered as metabolism is significantly depressed [38]. These effects would also be less noticeable at lower concentrations where the CPAs are less cytotoxic. The reason for the differences at lower temperatures is less clear. It might be that the results showing that monolayers experience less cytotoxicity when exposed to a lower concentration of CPAs at a lower temperature is actually a reflection of the control group that was used for normalization. The control monolayer group was subjected to the maximum number of washes, corresponding to the number of washes used in the addition and removal for DP6, albeit with supplemented DMEM. These extra washes may have caused some detachment of living cells which then resulted in artificially high normalized viability. This difference would not be seen at room temperature where the metabolism, and possibly attachment, is higher. These data indicate that monolayer cytotoxicity studies may not be directly applicable to the same cells in suspension, seeded in a construct, or present in a natural tissue.
Figure 6.
Viable cell number for 3M Me2SO, 3M PD and DP6 for monolayer βTC-tet cells (stripes) compared to encapsulated βTC-tet cells (solids) for an incubation period of 15 minutes. Viable cell number determined by alamarBlue™, normalized to untreated control. Error bars indicate standard deviations, n=3. *, # indicates p<0.05.
Comparison of cell type
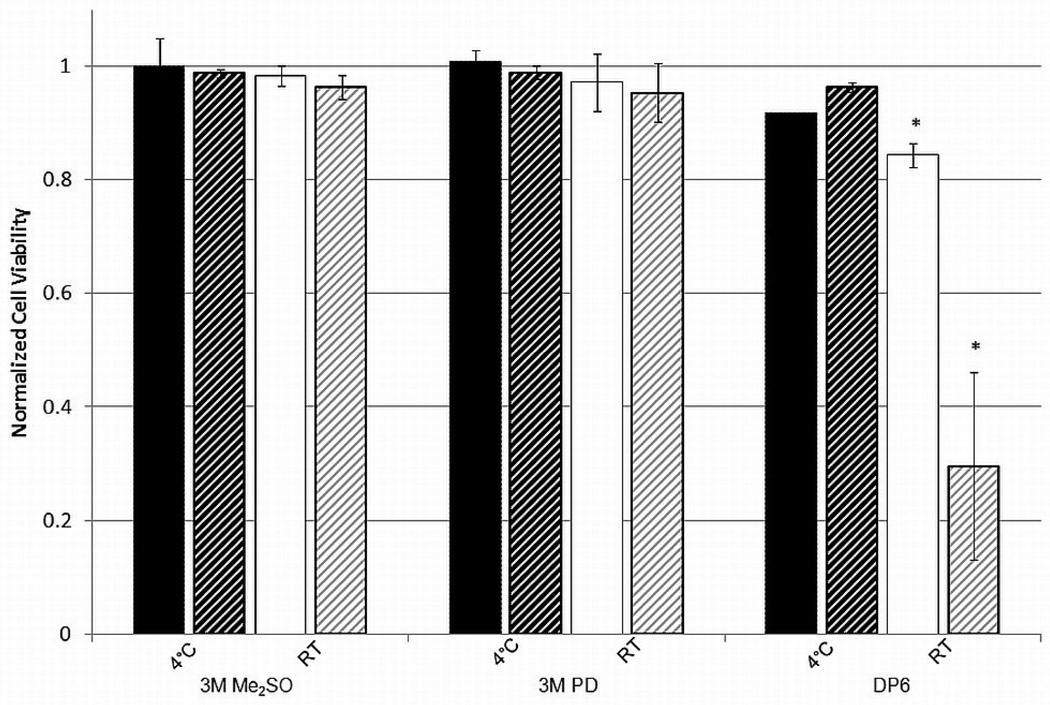
The cytotoxic effects of Me2SO, PD and DP6 on encapsulated βTC-tet and HepG2 cells were determined for an incubation period of 15 minutes at 4°C and room temperature. Results on the normalized cell viability measured by Trypan Blue are shown in Figure 7. The cytotoxicity of the single-components is very similar between the two cell types. However, the cocktail solution DP6 had a much higher cytotoxicity towards HepG2 than βTC-tet cells at room temperature. This is likely due to the difference in metabolism and the rate of cell growth between the two cell lines. βTC-tet cells encapsulated in 2% LVM alginate double in approximately two weeks [19] while HepG2s encapsulated in 1% LVM alginate triple in ten days [39]. Despite the small differences in alginate concentration, it is expected that the HepG2 cells are more metabolically active than βTC-tet cells. This is similar to what was observed when comparing monolayers and encapsulated cells and indicates that cytotoxicity likely increases as the metabolism of the cells is higher.
Figure 7.
Cell viability for 3M Me2SO, 3M PD and DP6 for encapsulated βTC-tet cells (solids) compared to encapsulated HepG2 cells (stripes) for an incubation period of 15 minutes. Cell viability determined by Trypan Blue exclusion assay, normalized to untreated control. Error bars indicate standard deviations, n=3. * indicates p<0.05
Summary
Our studies determined the cytotoxicity kinetics on encapsulated βTC-tet cells of two commonly used CPAs, Me2SO and PD, at different concentrations and two temperatures. Studies were extended to investigate the additivity of CPA cytotoxicity in cocktail solutions. Results generally agree with the literature but allow insight into the importance of temperature control and the use of CPA cocktails. Although it may be beneficial to add non-permeating solutes to cocktails to improve their ability to vitrify, this addition may not be as innocuous as previously thought. The importance of temperature control varies between CPAs and CPA cocktails and is more critical for cells with higher metabolism, either due to cell type or culturing method.
Acknowledgements
The authors wish to thank Candice Castellino for technical assistance and Dr. Ying C. Song for useful discussions and input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Wang X, et al. Cryopreservation of tissue-engineered dermal replacement in Me2SO: Toxicity study and effects of concentration and cooling rates on cell viability. Cryobiology. 2007;55(1):60–65. doi: 10.1016/j.cryobiol.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Fahy GM, Wowk B, Wu J. Cryopreservation of complex systems: The missing link in the regenerative medicine supply chain (vol 9, pg 279, 2006) Rejuvenation Research. 2006;9(4):509–509. doi: 10.1089/rej.2006.9.279. [DOI] [PubMed] [Google Scholar]
- 3.Kuleshova LL, Gouk SS, Hutmacher DW. Vitrification as a prospect for cryopreservation of tissue-engineered constructs. Biomaterials. 2007;28(9):1585–1596. doi: 10.1016/j.biomaterials.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 4.Yin HY, et al. Vitreous cryopreservation of tissue engineered bone composed of bone marrow mesenchymal stem cells and partially demineralized bone matrix. Cryobiology. 2009;59(2):180–187. doi: 10.1016/j.cryobiol.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Wusteman MC, et al. Vitrification media: toxicity, permeability, and dielectric properties. Cryobiology. 2002;44(1):24–37. doi: 10.1016/S0011-2240(02)00002-0. [DOI] [PubMed] [Google Scholar]
- 6.Sutton RL. Critical Cooling Rates for Aqueous Cryoprotectants in the Presence of Sugars and Polysaccharides. Cryobiology. 1992;29(5):585–598. doi: 10.1016/0011-2240(92)90063-8. [DOI] [PubMed] [Google Scholar]
- 7.Petrenko YA, Jones DRE, Petrenko AY. Cryopreservation of human fetal liver hematopoietic stem/progenitor cells using sucrose as an additive to the cryoprotective medium. Cryobiology. 2008;57(3):195–200. doi: 10.1016/j.cryobiol.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Beattie GM, et al. Trehalose: A cryoprotectant that enhances recovery and preserves function of human pancreatic islets after long-term storage. Diabetes. 1997;46(3):519–523. doi: 10.2337/diab.46.3.519. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, et al. Effects of different cryoprotectants on the viability and biological characteristics of porcine preadipocyte. Cryobiology. 2006;53(2):240–247. doi: 10.1016/j.cryobiol.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Kuleshova LL, et al. Sugars exert a major influence on the vitrification properties of ethylene glycol based solutions and have low toxicity to embryos and oocytes. Cryobiology. 1999;38(2):119–130. doi: 10.1006/cryo.1999.2153. [DOI] [PubMed] [Google Scholar]
- 11.Shaw JM, et al. Vitrification properties of solutions of ethylene glycol in saline containing PVP, Ficoll, or dextran. Cryobiology. 1997;35(3):219–229. doi: 10.1006/cryo.1997.2043. [DOI] [PubMed] [Google Scholar]
- 12.Fahy GM, et al. Improved vitrification solutions based on the predictability of vitrification solution toxicity. Cryobiology. 2004;48(1):22–35. doi: 10.1016/j.cryobiol.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Fahy GM. Cryoprotectant toxicity neutralization. Cryobiology. 2010;60(3):S45–S53. doi: 10.1016/j.cryobiol.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Fahy GM, Levy DI, Ali SE. Some Emerging Principles Underlying the Physical-Properties, Biological Actions, and Utility of Vitrification Solutions. Cryobiology. 1987;24(3):196–213. doi: 10.1016/0011-2240(87)90023-x. [DOI] [PubMed] [Google Scholar]
- 15.Mukaida T, et al. Vitrification of human embryos based on the assessment of suitable conditions for 8-cell mouse embryos. Human Reproduction. 1998;13(10):2874–2879. doi: 10.1093/humrep/13.10.2874. [DOI] [PubMed] [Google Scholar]
- 16.Elmoazzen H, et al. Dimethyl sulfoxide toxicity kinetics in intact articular cartilage. Cell Tissue Banking. 2007;8:125–133. doi: 10.1007/s10561-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 17.Wusteman MC, et al. Vitrification of rabbit tissues with propylene glycol and trehalose. Cryobiology. 2008;56(1):62–71. doi: 10.1016/j.cryobiol.2007.10.177. [DOI] [PubMed] [Google Scholar]
- 18.Gross JD, et al. Monitoring of dissolved oxygen and cellular bioenergetics within a pancreatic substitute. Biotechnology and Bioengineering. 2007;98(1):261–270. doi: 10.1002/bit.21421. [DOI] [PubMed] [Google Scholar]
- 19.Simpson NE, et al. Effects of growth regulation on conditionally-transformed alginate-entrapped insulin secreting cell lines in vitro. Biomaterials. 2005;26(22):4633–4641. doi: 10.1016/j.biomaterials.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 20.Stabler C, et al. The effects of alginate composition on encapsulated beta TC3 cells. Biomaterials. 2001;22(11):1301–1310. doi: 10.1016/s0142-9612(00)00282-9. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee IN, Song YC, Sambanis A. Cryoprotectant delivery and removal from murine insulinomas at vitrification-relevant concentrations. Cryobiology. 2007;55(1):10–18. doi: 10.1016/j.cryobiol.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai S, Rawson DM, Zhang T. Studies on Cryoprotectant Toxicity to Early Stage Zebrafish (Danio Rerio) Ovarian Follicles. Cryoletters. 2008;29(6):477–483. [PubMed] [Google Scholar]
- 23.Wang LH, et al. Further work on the cryopreservation of articular cartilage with particular reference to the liquidus tracking (LT) method. Cryobiology. 2007;55(2):138–147. doi: 10.1016/j.cryobiol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Pegg DE, Wang LH, Vaughan D. Cryopreservation of articular cartilage. Part 3: The liquidus-tracking method. Cryobiology. 2006;52(3):360–368. doi: 10.1016/j.cryobiol.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Stephenne X, et al. Cryopreservation of human hepatocytes alters the mitochondrial respiratory chain complex I. Cell Transplantation. 2007;16(4):409–419. doi: 10.3727/000000007783464821. [DOI] [PubMed] [Google Scholar]
- 26.He SY, Woods C. Effects of dimethyl sulfoxide and glycine on cryopreservation induced damage of plasma membranes and mitochondria to striped bass (Morone saxatilis) sperm. Cryobiology. 2004;48(3):254–262. doi: 10.1016/j.cryobiol.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Rajaei F, et al. Analysis of DNA fragmentation of porcine embryos exposed to cryoprotectants. Reproduction in Domestic Animals. 2005;40(5):429–432. doi: 10.1111/j.1439-0531.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- 28.Rahimi G, et al. Apoptosis in Human Ovarian Tissue after Conventional Freezing or Vitrification and Xenotransplantation. Cryoletters. 2009;30(4):300–309. [PubMed] [Google Scholar]
- 29.Heng BC, et al. Loss of viability during freeze-thaw of intact and adherent human embryonic stem cells with conventional slow-cooling protocols is predominantly due to apoptosis rather than cellular necrosis. Journal of Biomedical Science. 2006;13(3):433–445. doi: 10.1007/s11373-005-9051-9. [DOI] [PubMed] [Google Scholar]
- 30.Baust JM, Van Buskirk R, Baust JG. Cell viability improves following inhibition of cryopreservation-induced apoptosis. In Vitro Cellular & Developmental Biology-Animal. 2000;36(4):262–270. doi: 10.1290/1071-2690(2000)036<0262:cvifio>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Wang HY, et al. Inhibition of nucleation and growth of ice by poly(vinyl alcohol) in vitrification solution. Cryobiology. 2009;59(1):83–89. doi: 10.1016/j.cryobiol.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Katenz E, et al. Cryopreservation of primary human hepatocytes: The benefit of trehalose as an additional cryoprotective agent. Liver Transplantation. 2007;13(1):38–45. doi: 10.1002/lt.20921. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues JP, et al. Evaluation of trehalose and sucrose as cryoprotectants for hematopoietic stem cells of umbilical cord blood. Cryobiology. 2008;56(2):144–151. doi: 10.1016/j.cryobiol.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Rudolph AS, Crowe JH. Membrane Stabilization during Freezing - the Role of 2 Natural Cryoprotectants, Trehalose and Proline. Cryobiology. 1985;22(4):367–377. doi: 10.1016/0011-2240(85)90184-1. [DOI] [PubMed] [Google Scholar]
- 35.Kleinhans FW. Membrane permeability modeling: Kedem-Katchalsky vs a two-parameter formalism. Cryobiology. 1998;37(4):271–289. doi: 10.1006/cryo.1998.2135. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee IN. School of Chemical & Biomolecular Engineering. Atlanta, GA: Georgia Institute of Technology; 2008. A Rational Design Approach for the Cryopreservation of Natural and Engineered Tissues. [Google Scholar]
- 37.Simpson NE, et al. Biochemical consequences of alginate encapsulation: A NMR study of insulin-secreting cells. Biomaterials. 2006;27(12):2577–2586. doi: 10.1016/j.biomaterials.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 38.Belzer FO. Principles of Organ Preservation. Transplantation Proceedings. 1988;20(1):925–927. [PubMed] [Google Scholar]
- 39.Chin K, et al. Hydrogel-perfluorocarbon composite scaffold promotes oxygen transport to immobilized cells. Biotechnology Progress. 2008;24(2):358–366. doi: 10.1021/bp070160f. [DOI] [PubMed] [Google Scholar]