Abstract
Nerve growth factor has been proposed to mediate many structural and chemical changes in bladder sensory neurons after injury or inflammation. We have examined the expression of receptors for the glial cell line-derived neurotrophic factor (GDNF) family within sensory terminals located in the sacral spinal cord and in bladder-projecting sacral dorsal root ganglion neurons of adult female Sprague-Dawley rats. Nerve fibers immunolabelled for GFRα1 (GDNF receptor), GFRα2 (neurturin receptor) or GFRα3 (artemin receptor) showed distinct distribution patterns in the spinal cord, suggesting separate populations of sensory fibers with different functions: GFRα1-labeled fibers were in outer lamina II, the lateral-collateral pathway and associated with autonomic interneurons and preganglionic neurons; GFRα2-labeled fibers were only in inner lamina II; GFRα3-labeled fibers were in lamina I, the lateral-collateral pathway and areas surrounding dorsal groups of preganglionic neurons and associated interneurons. Immunofluorescence studies of retrogradely-labelled bladder- projecting neurons in sacral dorsal root ganglia showed that ~25% expressed GFRα1- or GFRα3-immunoreactivity, the preferred receptors for GDNF and artemin, respectively. Following cyclophosphamide-induced bladder inflammation, fluorescence intensity of GFRα1-positive fibers increased within the dorsal horn, but there was no change in the GFRα2- or GFRα3-positive fibers. These studies have shown that GDNF and artemin may target bladder sensory neurons and potentially mediate plasticity of sacral visceral afferent neurons following inflammation. Our results have also revealed three distinct subpopulations of sensory fibers within the sacral spinal cord, which have not been identified previously using other markers.
Keywords: visceral pain, GDNF, nociceptor, micturition reflex, inflammation
INTRODUCTION
The sensory innervation of the urinary bladder has a number of functions. During bladder filling, the stretch stimulus activates sensory neurons in sacral dorsal root ganglia (DRG) to inform spinal and supraspinal centers that may then initiate a micturition reflex (voiding) (de Groat and Yoshimura, 2001; Holstege and Mouton, 2003; Holstege 2005). Stretch also activates sensory neurons indirectly, by stimulating ATP release from the urothelium to excite sensory terminals lying in the sub-urothelial tissue (Vlaskovska et al., 2001). Some bladder sensory neurons have a nociceptive function, particularly under inflammatory conditions (Björling et al., 2003; Nazif et al., 2007). Many studies have shown that, using animal models for clinical conditions of bladder dysfunction after spinal cord injury or inflammation, bladder sensory neurons undergo structural and chemical changes that may underlie a change in gain of the micturition reflex (e.g. leading to bladder overactivity) or bladder pain (Yoshimura and de Groat, 1997; de Groat et al., 1998; Yoshimura and de Groat, 1999). For example, following spinal cord injury, peptidergic sacral DRG neurons sprout and increase their peptide expression (Zvarova et al., 2004, 2005; Zinck et al., 2007). Plasticity of sacral primary afferent pathways also occurs after bladder inflammation, such as following cyclophosphamide (CYP) treatment, where cyclophosphamide is metabolised in the kidneys to form acrolein, which then inflames the lower urinary tract (Cox, 1979; Maggi et al., 1993). This treatment initiates many changes in the chemistry of sacral bladder-projecting DRG neurons and an increased terminal density in the sacral spinal cord, both of which are believed to contribute to an increased excitability and signaling of pain (Vizzard et al., 1996; Vizzard, 2001; Qiao and Vizzard, 2002; Hu et al., 2003).
It is well established that nerve growth factor (NGF) has powerful effects on axon growth and sensitisation of nociceptor neurons (Pezet and McMahon, 2006). NGF has also been strongly implicated in the mechanisms underlying the structural and chemical plasticity in adult bladder sacral DRG neurons (Steers et al., 1996; de Groat et al., 1997; Chuang et al., 2001; Yoshimura et al., 2006). Levels of neurotrophic factors within the bladder wall increase under conditions where these bladder sensory neurons undergo growth and increased sensitivity, such as CYP treatment, diabetes and spinal cord injury (Tuttle et al., 1994; Lowe et al., 1997; Clemow et al., 1998; Steinbacher and Nadelhaft, 1998) and intrathecal administration of NGF mimics these changes (Yoshimura et al., 2006). Together this information strongly suggests that NGF is at least partly responsible for altered ion channel expression leading to increased excitability in peptidergic sacral sensory neurons, sprouting of their terminals within the sacral spinal cord, and increased expression of neuropeptides under these conditions.
NGF-sensitive sensory neurons typically express the tyrosine kinase receptor, trkA, and the co-receptor, p75 (Kaplan and Miller, 2000). Many neurons expressing this combination of proteins synthesise one or more neuropeptides, most commonly calcitonin gene-related peptide (CGRP); conversely, most CGRP-positive sensory neurons express receptors for NGF (Averill et al., 1995; Bennett et al., 1996). To date, most studies investigating the plasticity of bladder sensory neurons have focused on this NGF-sensitive, peptidergic population. Despite the likely importance of NGF in the plasticity of bladder sensory neurons, various reports estimate that ~20–40% of bladder sensory neurons do not synthesise CGRP or other neuropeptides (Su et al., 1986; Keast and de Groat, 1992; Bennett et al., 1996; Vizzard, 2001; Bennett et al., 2003). Many of these neurons have been identified by their ability to bind the plant lectin isolectin B4 (IB4) (Averill et al., 1995; Bennett et al., 1996). Most IB4-positive, peptide-negative neurons do not respond to NGF and many instead express receptors for one or more members of the glial cell line-derived neurotrophic factor (GDNF) family (Bennett et al., 1998; Wanigasekara and Keast, 2006). A recent report showed that application of IB4-saporin to the sacral spinal cord, which destroyed most of the IB4-binding sensory terminals, reduced irritant-evoked bladder overactivity but did not alter normal micturition reflexes (Nishiguchi et al., 2004). Moreover, GDNF levels increase in the bladder wall following CYP treatment (Vizzard, 2000b) and GDNF levels are increased in the urine of interstitial cystitis patients (Okragly et al., 1999). Therefore, we hypothesised that one or more members of the GDNF family may influence the structure and activity of non-peptidergic bladder sensory neurons. We focused on GDNF, neurturin and artemin, which have many potent actions on the nervous system (Bespalov and Saarma, 2007), but which have not yet been explored in the context of bladder sensory function. We did not include the fourth member of the GDNF family, persephin, in our study, because there is relatively little information currently available strongly supporting a major role for endogenous persephin (or its receptor, GFRα4) in the nervous system or in nociceptive modulation.
In this study, we have utilised antibodies against three receptors (GFRα1, GFRα2 and GFRα3), which are the preferred receptors for three members of the GDNF family – GDNF, neurturin and artemin, respectively – to determine their expression within pelvic visceral afferent fibers within the sacral spinal cord of adult female rats. This revealed that each antibody labels populations of sensory fibers within the sacral spinal cord that have unique targets and may have distinct functions in control of pelvic visceral function. We have also shown that these receptors are expressed by ~25% of the bladder afferent neurons and examined the effect of CYP treatment on expression of these receptors within the sacral spinal cord. Together, our results have revealed new markers of functionally distinct populations of sensory fibers within the sacral spinal cord, and provided evidence for the GDNF family ligands being new potential targets for therapies to modify activity of bladder sensory nerves.
METHODS
Animal treatments
All procedures were approved by the University of Sydney and Royal North Shore Hospital ethics committees, as required by the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (National Health and Medical Research Council of Australia). Twenty adult female Sprague-Dawley rats (6–8 weeks old) were used for these experiments. These comprised: 3 naïve rats (immunohistochemistry only), 10 rats used for the cyclophosphamide (CYP) study (6 treated with CYP, 4 controls treated with saline) and 7 rats used for application of retrograde tracer to the urinary bladder.
To administer CYP, animals were briefly anesthetised with isoflurane then injected with CYP in sterile 0.9% sodium chloride (75 mg/kg, i.p), every three days (Qiao and Vizzard, 2002). On day 10, animals were heavily anesthetised with sodium pentobarbitone (80 mg/kg i. p) and perfused intra-cardially with freshly made 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4), prior to removal of spinal cord tissue. Spinal cord tissue was also removed from naïve rats perfused with fixative. Spinal tissue was post-fixed overnight and then washed in 0.1M phosphate-buffered saline (PBS) and stored in PBS containing 0.1% sodium azide until sectioning.
To identify bladder-projecting sensory neurons in DRG, the fluorescent retrograde tracer dye, FluoroGold (FG; 4% in sterile water; Fluorochrome, Englewood, CO) or the equivalent compound, hydroxystilbamidine (2% in sterile water; Invitrogen, Mt Waverley, Victoria, Australia) was microinjected into 6–8 sites of the urinary bladder wall (bladder base) using an insulin syringe fitted with a 30g needle (total volume ~10 μl), as described previously (Bennett et al., 2003). One to two weeks later, animals were heavily anesthetised and perfused with fixative as described above. DRG (L6, S1) were removed and post-fixed overnight in the same fixative, then washed and stored as described above.
Antibody characterization
Immunostaining for neuronal nitric oxide synthase (NOS) was performed in spinal cord sections to compare the distribution of GFR immunoreactivity with the location of preganglionic neurons in the sacral preganglionic nucleus, the majority of which express this enzyme (Burnett et al., 1995; Vizzard et al. 1995). Because NOS also labels many primary afferent terminals in lamina II of the sacral dorsal horn (Vizzard et al., 1995, 1996), NOS immunostaining also provided a reference point for GFR immunoreactivity in this region. The NOS antiserum was purchased from Zymed (now Invitrogen, Cat. No. 61-7000; 1:500) and was raised in rabbit against the N-terminal 195 amino acids of rat neuronal NOS protein. This antibody reacts with 160kDa neuronal NOS, shows no cross-reactivity with eNOS or iNOS and identifies a single ~160kD band on Western blots of rat and mouse brain tissue lysates (manufacturer’s information).
GFR antisera were all purchased from R&D Systems (Minneapolis, MN) and raised in goat (GFRα1, Cat. No. AF560, 1:200; GFRα2, Cat. No. AF429, 1:500; GFRα3, Cat. No. AF2645, 1:300). These antisera have been affinity purified using a column of specific recombinant protein; GFRα1- and GFRα2-antibody specificities have also been demonstrated using Western blotting, showing a single band (manufacturer’s technical information). The GFRα3 antibody recognised the recombinant GFRα3 protein in Western blotting (manufacturer’s information) and its specificity has been documented as detailed below. Western blotting of GFRs can be problematical and lead to multiple bands, due to variable dimerization, glycosylation states and ligand binding (Jing et al., 1996). Furthermore, GFRα1 and GFRα2 have very close molecular weights, so Western blotting alone is not ideal for identifying non-specificity. In the present study, the completely distinctive patterns of staining within the spinal cord and DRG using these antibodies (see Results) demonstrated that they do not show significant cross-reactivity with an inappropriate GFR.
GFRα1 antiserum was raised against recombinant rat GFRα1 extracellular domain (see Accession Number U59486). The immunogen consisted of amino acid residues Ala19-Leu445 of the rat GFRα1 extracellular domain fused to Fc with a linker sequence CSIEGRMD; the Fc was then cleaved, leaving Ala19-Leu445-CSIEGR. This antibody is specific for GFRα1: it blocks binding of GDNF to immobilized GFRα1/Fc chimera in a functional ELISA assay; in ELISA or western blotting this antibody shows no cross-reactivity with ret or GFRα3 and 10% cross-reactivity with GFRα2 (manufacturer’s technical information). The same antibody showed a band of ~55 kDa in extracts of cultured sympathetic ganglion neurons (Pierchala et al., 2006). It produced a similar pattern of immunoreactivity in the sacral dorsal horn as shown previously in more rostral segments (Widenfalk et al., 2001); in this previous study, using the same antibody as our study, the GFRα1 immunostaining present in rat dorsal root ganglion and kidney closely matched the pattern of GFRα1 mRNA as determined by in situ hybridisation. In addition, following spinal cord injury, there was a parallel increase in GFRα1 immunoreactivity and GFRα1 mRNA levels in spinal cord. GFRα1-IR distribution in DRG of our current study closely matched the results of previous in situ hybridisation studies on rat DRG (Kashiba et al., 2003), which showed neurons of similar frequency, distribution and size as we observed with immunofluorescence. In rat striatum, immunostaining with this antibody closely matches results from in situ hybridisation (Cho et al., 2004). Furthermore, this antibody immunostains many DRG neurons in wild type but not GFRα1 knockout mice (Rakowicz et al., 2002).
GFRα2 antiserum was raised against recombinant mouse GFRα2 extracellular domain (Accession Number NM008115). The immunogen consisted of amino acid residues Thr160-Ser441 of the GFRα2 extracellular domain fused to Fc with a linker sequence DIEGRMD; the Fc was then cleaved, leaving Thr160-Ser441-CSIEGR. As well as the control experiments described above, this antibody has been previously shown to produce labeling in pelvic ganglia and brain of wild type but not GFRα2 knockout mice (Voikar et al., 2004; Wanigasekara et al., 2004). This antiserum shows specific binding and functional reactivity in ELISA and western blotting assays, where it shows a single band; it also shows no cross-reactivity with GFRα3 (manufacturer’s technical information) and a similar pattern of staining in dorsal horn of rat spinal cord as another antibody (goat anti-GFRα2, Cat. No. GT1004, Lot 4900084, Neuromics, Edina, MN).
GFRα3 antiserum was raised against purified extracellular domain of the recombinant mouse GFRα3 protein (amino acid sequence 17-386; see Accession number O35118) linked to an Fc sequence as described above. The antibody was affinity purified, using methods identical to those described above for GFRα1 and GFRα2 antisera. According to the manufacturer’s technical information, in direct ELISA this antibody shows less than 2% cross-reactivity with GFRα2 and GFRα4 and in Western blot reacts with recombinant GFRα3, showing a 70 kDa band under reducing conditions (for the Fc chimera protein, comprising extracellular domain and Fc), and a 43 kDa band (for the extracellular domain alone). In our hands, this antibody shows identical patterns of localisation in DRG as described previously using the same antibody (Malin et al., 2006) and the same as described previously using another antibody, raised against residues 108–120 of murine GFRα3 and shown to be specific to GFRα3 using Western blotting and ELISA (Orozco et al., 2001). The pattern of staining in our study mimics the results of in situ hybridisation for GFRα3 mRNA in rat DRG (Bennett et al., 2000, 2006). Using this antibody, we have also found that ~50% of the GFRα3 neurons in adult rat sacral DRG express CGRP (data not shown), comparable to the coexpression shown with substance P described previously, using in situ hybridisation for GFRα3 mRNA (Bennett et al., 2006).
Immunohistochemistry
Tissues were cryoprotected overnight in phosphate-buffered saline (PBS) containing 30% sucrose and embedded in an inert mounting medium (OCT Tissue-Tek, Sakura, Torrance, CA, USA) prior to sectioning. Spinal cord was sectioned transversely (40μm) using a freezing microtome and sections processed free floating as described previously (Kalous et al., 2007), using the antisera and dilutions as listed above. Sections were distributed sequentially into three groups, to stain for each GFR simultaneously with NOS. GFR-immunoreactivity (IR) was visualised with fluorophore-conjugated species-specific secondary antibody (Cy3 conjugate of donkey anti-goat, 1:1000; Jackson Immunoresearch Laboratories, West Grove, PA), whereas NOS-IR was visualised with a fluorescein isothiocyanate conjugate of donkey anti-rabbit (1:100, Jackson Immunoresearch Laboratories). Briefly, sections were incubated in primary antisera for 48 hours, then secondary antisera for 4 hours before coverslipping with 0.5 M bicarbonate-buffered glycerol (pH 8.6). DRGs were cryosectioned (14 μm) and 10-15 sections at least 60 μm apart collected onto 1% gelatinised slides. Sections were air-dried, washed with PBS, blocked and permeabilised for 1 hr with PBS containing 10% horse serum (JRH Biosciences, Brooklyn, Vic, Australia) and 0.1% Triton X-100 (Sigma) in PBS. This was followed by incubation with GFR antibodies overnight and Cy3-conjugated anti-goat secondary antibody for 2–3 hours, both at room temperature. Slides were mounted with 0.5 M bicarbonate-buffered glycerol (pH 8.6) before coverslipping.
Image analysis and figure production
Sections were viewed under an Olympus BX51 fluorescence microscope. Images (8-bit monochrome) were captured using an RT Spot camera (Diagnostic Instruments, Sterling Heights, MI, USA) and digitised using Image Pro Plus software (Media Cybernetics, Silver Spring, MD, USA). For figure production, images were colorised digitally, where necessary making minor adjustments to contrast and brightness to best represent the immunostaining viewed under the microscope, using Adobe Photoshop (Version 8).
To assess GFR expression in bladder-projecting DRG neurons, nucleated profiles of FG-labelled neurons were classified as GFR-positive or -negative and expressed as a proportion of all FG-labelled profiles. At least 80 FG-positive neurons were assessed in each animal for each GFR. Data from L6 and S1 ganglia were pooled. To compare GFR expression in spinal cord, three regions were assessed (Fig. 1): lateral and medial regions of superficial laminae of the dorsal horn, and the central spinal cord dorsal to the central canal (dorsal gray commissure). In each of these areas, exposure times were kept constant and the intensity of immunofluorescence was quantified (raw grey level values of pixels) in a defined area and expressed as mean pixel intensity for that region (see Fig. 1). For each animal, at least 6 randomly selected sections were analysed, with control and CYP groups stained in the same run. Control and CYP values were expressed as mean ± SE and compared with an unpaired t-test. Because of the variable size and shape of the sacral preganglionic nucleus (SPN) between sections, the immunoreactivity in this region was not quantified.
Figure 1.
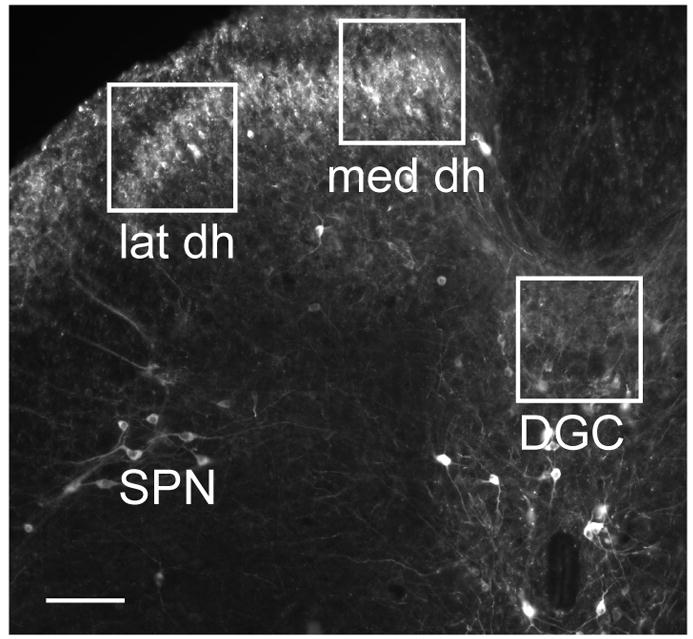
Regions of spinal cord chosen for densitometric quantitation of GFR-immunoreactivity. Cryosection of sacral spinal cord immunostained for nitric oxide synthase (NOS) showing size and position of regions chosen for densitometric analysis of GFR-immunoreactivity, using images taken under the 10x objective. Boxes were 250 pixels x 250 pixels and were aligned with the medial and lateral margins of the superficial dorsal horn (abbreviated med dh and lat dh, respectively). A similar area was also analysed in the dorsal gray commissure (DGC). The location of the sacral preganglionic nucleus (SPN) is also indicated. Calibration represents 100 μm.
RESULTS
GFR-immunoreactive fibers showed distinct patterns of distribution within sacral spinal cord
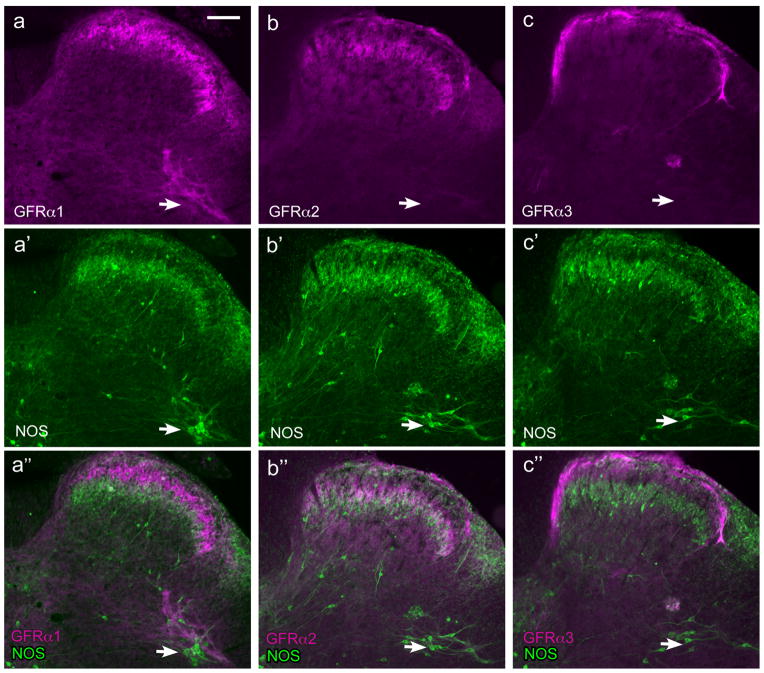
GFRα1-, GFRα2- and GFRα3-immunoreactive (IR) fibers each had a distinct distribution within the sacral spinal cord. This is demonstrated in Figure 2(a’’-c’’), where double-labelling with antisera against NOS was used to demonstrate the location of sacral preganglionic neurons and to provide a reference point for dorsal horn laminae (Zhang et al., 1993; Herdegen et al., 1994; Burnett et al., 1995; Vizzard et al., 1995, 1996). The distributions of NOS and each type of GFR were consistent between the 3 naïve animals and 6 controls for the CYP experiments. As described previously, NOS-IR neurons were located in the dorsal horn, near the central canal and in the dorsal gray commissure, and in the sacral preganglionic nucleus. Numerous varicose fibers were located in the dorsal horn, especially in the superficial laminae and being most concentrated in laminae II (inner) and lamina III. Many varicose fibers were also located in the dorsal gray commissure and lateral spinal nucleus. We did not observe NOS-IR fibers in the lateral collateral pathway, agreeing with a previous report that these only become evident after visceral nerve injury (Vizzard et al., 1995).
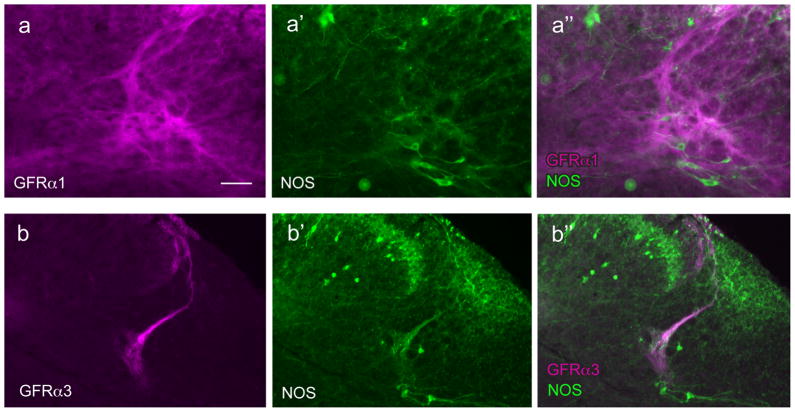
Figure 2.
Distribution of fibers showing GFRα1-, GFRα2- and GFRα3-immunoreactivity in sacral spinal cord. Each vertical set of three micrographs shows immunoreactivity (IR) for a GFR (a–c), nitric oxide synthase (NOS) to indicate dorsal horn lamination and preganglionic neurons (arrows) (a’–c’) and merged image (a’’–c’’). Images are oriented with dorsal surface at the top and medial cord on the left. a, GFRα1-IR fibers in the dorsal horn, and immediately dorsal to preganglionic neurons. b, GFRα2-IR fibers in the dorsal horn but not associated with preganglionic neurons. c, GFRα3-IR fibers in the dorsal horn and a small region dorsal to preganglionic neurons. Calibration bar represents 100 μm.
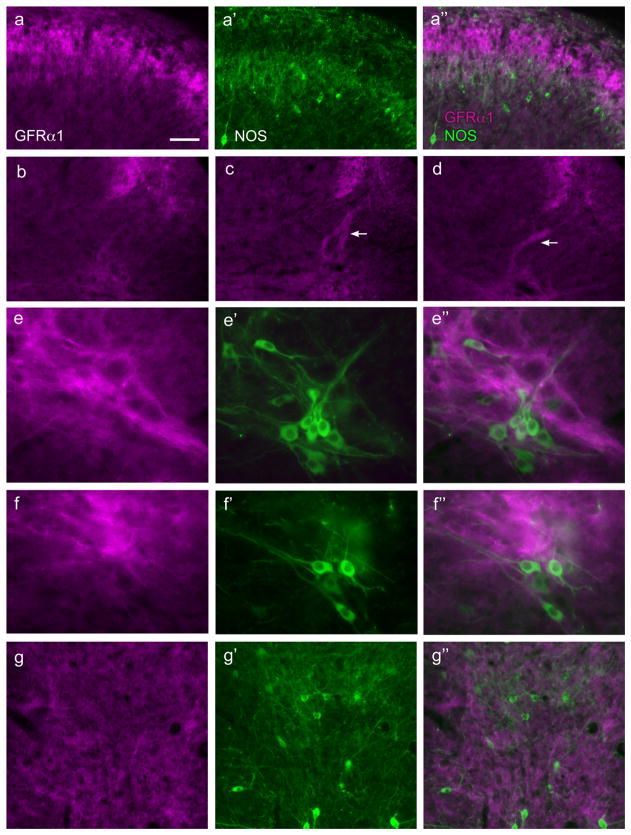
GFRα1-IR fibers were found in laminae II (outer) in the dorsal horn, where they formed a dense band (Figs. 2a, 3a). No GFRα1-IR somata were identified in this region. A moderate number of fibers were present in the lateral spinal nucleus (Fig. 2a). In occasional sections, a dense tract of GFRα1-IR fibers was seen in the lateral-collateral pathway, travelling along the lateral edge of the dorsal horn grey matter (Fig. 3b–d). These fibers largely terminated in a region slightly dorsal to the sacral preganglionic nucleus, although some GFRα1-IR fibers were also closely associated with NOS-IR preganglionic neurons (Fig. 3e,f). In some sections weakly stained GFRα1-IR somata were identified in this region, all of which were NOS-positive. Numerous fine GFRα1-IR fibers were also present in the dorsal gray commissure (Fig. 3g), where autonomic interneurons and dendrites of preganglionic neurons are located. GFRα1-IR motoneurons were commonly observed in the ventral horn (not shown), as previously reported (Trupp et al., 1997; Widenfalk et al., 1997).
Figure 3.
Distribution of GFRα1-immunoreactive fibers in the sacral spinal cord. Horizontal setS of three micrographs labelled a, e, f and g show immunoreactivity (IR) for GFRα1 (a, e, f, g), nitric oxide synthase (NOS; a’, e’, f’, g’), and a merged image (a’’, e’’, f’’, g’’). Images are oriented with dorsal surface at the top (all images) and medial cord on the left (a–f); g is located in the midline, dorsal to the central canal. a, fibers in superficial dorsal horn show minimal coexpression between GFRα1-IR and NOS. b–d, examples of variable prevalence of GFRα1-IR fibers in the lateral-collateral pathway between sections, with some sections showing no fibers (b) and others showing dense projections (c,d as indicated by arrows); panels b–d show identical regions of spinal cord. e,f, numerous fine GFRα1-IR fibers in the region dorsal to NOS-positive preganglionic neurons and also closely associated with a minority of preganglionic neurons. g, many fine GFRα1-IR fibers in the dorsal gray commissure; many NOS-positive neurons are located in this area but GFRα1-IR fibers do not appear to be preferentially localised with NOS-positive neurons. Calibration bar represents 25 μm (e,f), 40 μm (a,g) or 60 μm (b–d).
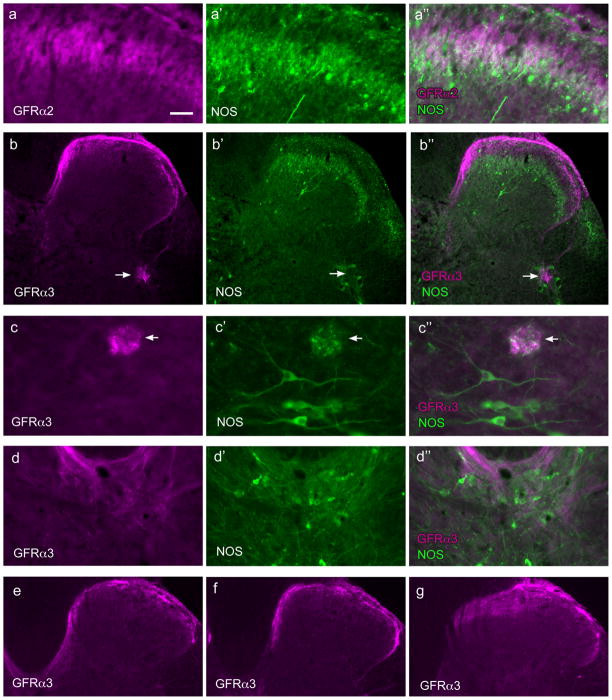
GFRα2-IR fibers were found in laminae II (inner) in the dorsal horn, where they formed a dense band (Fig. 2b, 4a). Although double staining for GFRα1 and GFRα2 could not be performed because both antibodies were raised in the same host species, comparison of each with NOS-IR fiber location in the dorsal horn clearly showed that GFRα1-IR fibers were located more superficially than GFRα2-IR fibers. No GFRα2-IR fibers were located in the lateral-collateral pathway or associated with the sacral preganglionic nucleus, or the region dorsal to the sacral preganglionic nucleus (putative interneurons). No GFRα2-IR fibers were found in the dorsal gray commissure. A moderate number of weakly stained GFRα2-IR fibers were found in the lateral spinal nucleus. No GFRα2-IR somata were identified in any region, including the ventral horn.
Figure 4.
Distribution of GFRα2- and GFRα3-immunoreactive fibers in the sacral spinal cord. Each of the top four horizontal sets of three micrographs shows immunoreactivity (IR) for a GFR (a–d), nitric oxide synthase (NOS, a’–d’), and a merged image (a’’–d’’). Images are oriented with dorsal surface at the top (all images) and medial cord on the left (a–c,e); d is located in the midline, dorsal to the central canal. a, GFRα2-IR fibers co-located in a similar region of lamina II as NOS-IR fibers. b, GFRα3-IR fibers in the dorsal horn extend in a medial band almost to the midline where they branched off into the dorsal gray commissure, while laterally some GFRα3-IR fibers were found in the lateral-collateral pathway, terminating in a small region within the more dorsal preganglionic neurons (arrow). c shows example of a small patch of GFRα3-IR fibers (arrows) lying dorsal to the region containing preganglionic neurons; this region also contains some NOS-IR fibers. d, dorsal gray commissure contains some GFRα3-IR fibers, especially in the most dorsal region. Panels e, f and g show progressive changes in the distribution of GFRα3-IR fibers moving more rostrally in the spinal cord; e shows caudal L6 where the medially located fibers travelling towards the midline; f shows rostral L6 where these fibers extend less medially and g shows L5 where there are no medially projecting GFRα3-IR fibers; note that in L5 GFRα3-IR fibers extend well into lamina II of the medial dorsal horn. Calibration bar represents 20 μm (c), 30 μm (a), 60 μm (d) or 100 μm (b, e–g).
GFRα3-IR fibers were found primarily in lamina I with a few fibers in lamina II (outer) (Fig. 2c, 4b). GFRα3-IR fibers were also found in the lateral collateral pathway (Fig. 4b,f) but in transverse sections appeared to supply a smaller terminal field, compared with GFRα1-IR fibers. Some GFRα3-IR fibers appeared to terminate in the most dorsal aspect of the region proposed to contain autonomic interneurons (i.e. dorsal to the sacral preganglionic nucleus), as shown in Fig. 2c, but in other sections the GFRα3-IR fibers terminated in small areas surrounding the preganglionic neurons (Fig. 4b). No GFRα3-IR fibers were present in the lateral spinal nucleus. In contrast to GFRα1- and GFRα2-IR fibers, GFRα3-IR fibers in the dorsal horn formed a dense band that extended medially, in some sections comprising an almost continuous band of fibers in lamina I (Fig. 2c, 4b,e,f). In many sections, fibers could be seen to exit this band to supply the dorsal gray commissure (Fig. 4b,d). When sections of spinal cord were examined in rostral L6 (Fig. 4f), this medial band became discontinuous and was absent in L5 (Fig. 4g). The emergence of this medial band of fibers extending across the spinal cord in more caudal levels correlated closely with the appearance of NOS-positive preganglionic neurons, consistent with a role of the GFRα3-IR fibers in visceral function. No GFRα3-IR somata were identified in any region, including the ventral horn.
Cyclophosphamide (CYP) treatment had selective actions on GFRα1-IR in sacral spinal cord
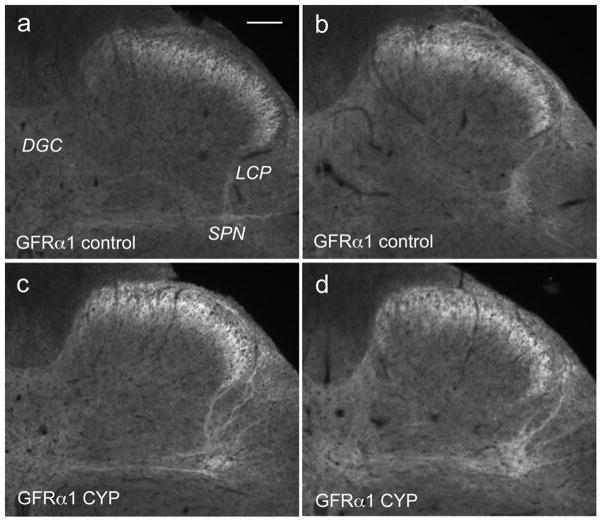
Quantitation of GFR-IR in sections of sacral spinal cord from CYP-treated animals (n=4 rats) immunostained in the same staining runs as sections from control animals (n=6 rats) showed a selective effect of the treatment. GFRα1-IR fibers were generally brighter in spinal cord sections from CYP animals (Fig. 5c,d) compared with controls (Fig. 5a,b), and the lateral-collateral pathway appeared brighter and more densely innervated (Fig. 5c, d, 6a). This increased intensity could be due to sprouting of fibers or increased staining intensity of existing fibers. The prevalence of GFRα1-IR in the lateral collateral pathway tract did not change significantly between control and CYP-treated animals (51 ± 6% control sections showed this tract c.f. 75 ± 13% CYP sections, P=0.11). In addition, in tissues from CYP animals both the sacral preganglionic nucleus and region immediately dorsal to it containing autonomic interneurons appeared to be more heavily innervated with bright GFRα1-IR fibers, which seemed to extend to a larger area compared with controls (Fig. 5c,d, 6a).
Figure 5.
Effects of cyclophosphamide (CYP) treatment on GFRα1-immunoreactivity in sacral spinal cord. GFRα1-immunoreactivity(IR) is shown in sacral spinal cord from control (a,b) and CYP (c,d) treated animals. All images were taken in the same session and using the same camera settings and have not been processed in any way other than cropping. Images from CYP-treated animals show brighter fibers in the superficial dorsal horn, in the lateral collateral pathway (LCP) dorsal to the sacral preganglionic nucleus (SPN), and in the dorsal gray commissure (DGC). Calibration bar represents 100μm.
Figure 6.
GFR-immunoreactivity associated with the sacral preganglionic nucleus in CYP-treated rats. Images are oriented with dorsal surface at the top (all images) and medial cord on the left. Each horizontal set of three micrographs shows a GFR (a,b), NOS (a’,b’) or a merged image (a’’,b’’). a, Extensive distribution of GFRα1-IR fibers immediately dorsal to the SPN, demonstrated by the location of NOS-IR somata. b, GFRα3-IR fibres formed a dense bundle in the lateral-collateral pathway and appeared to terminate dorsal to the SPN, indicated by the location of NOS-IR somata. Calibration bar represents 60 μm (a) and 100 μm (b).
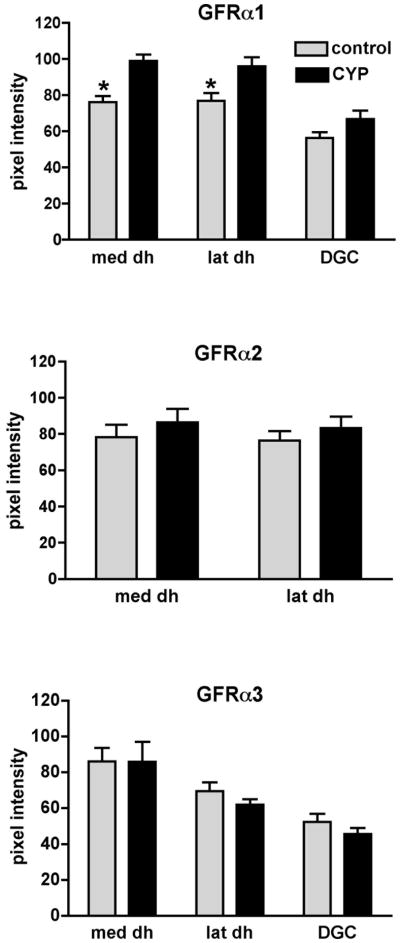
Because of the nature of the immunostaining, where many areas contained both distinct fibers and bright amorphous immunofluorescence, we were unable to perform morphometry to determine the area supplied by immunoreactive nerve fibers, i.e. it was not possible to confidently determine a threshold grey level. Instead, the raw fluorescence intensity of GFRα1-IR was quantified in three regions (see Methods), i.e. dorsal horn (medial and lateral) and dorsal gray commissure (Fig. 7). These analyses showed that after CYP there was a statistically significant increase in fluorescence intensity of GFRα1-IR in the medial and lateral dorsal horn and the possibility of a small increase in the dorsal gray commissure, although the latter was not a statistically significant effect (Fig. 7).
Figure 7.
Densitometric analysis of GFR-immunoreactivity in sacral spinal cord of control and cyclophosphamide (CYP)-treated animals. Histograms show mean ± SE of raw pixel intensity measurements taken from 6–10 randomly selected sections for each group and immunostain. CYP treatment caused a significant increase in GFRα1-IR pixel intensity in both medial and lateral dorsal dorsal horn but had no significant effect on the dorsal gray commissure, although there appeared to be a small increase (medial, P=0.002, lateral, P= 0.02, DGC, P=0.08, unpaired t test; n=6 control, n=4 CYP). CYP treatment had no effect on GFRα2- or GFRα3-IR pixel intensity (n=4 for both control and CYP groups). med dh, medial dorsal horn; lat dh, lateral dorsal horn; DGC, dorsal grey commissure.
Similar analyses were performed on sections stained in parallel for GFRα2-IR and GFRα3-IR, although no analyses were performed on the dorsal gray commissure for GFRα2-IR because immunoreactive fibers were absent from this region in control and CYP-treated animals. No effect of CYP treatment could be detected for GFRα2-IR fibers in the dorsal horn or for GFRα3-IR fibers in the dorsal horn or dorsal gray commissure (Fig. 7). While GFRα3-IR fibers in the lateral-collateral pathway were not observed more commonly after CYP treatment (63 ± 4% control sections c.f. 71 ± 3% CYP sections, P=0.2), GFRα3-IR fibers appeared to supply a larger terminal field in the region containing autonomic interneurons in sections from CYP animals compared with controls (Fig 6b).
Some sacral bladder sensory neurons showed GFR-immunoreactivity
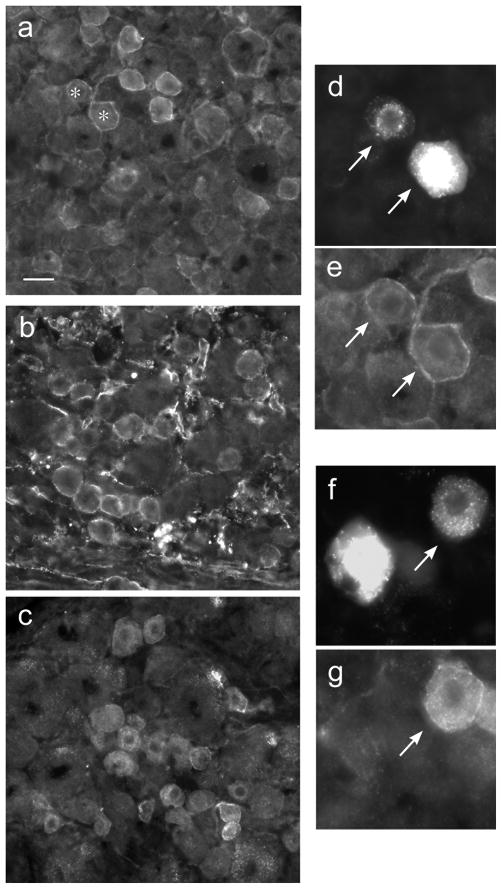
Similar patterns and intensity of GFR staining were seen in DRG from 3 naïve rats and 7 control (i.e. saline-treated) rats. In each animal, many neuronal somata were immunolabelled for GFRα1, GFRα2 and GFRα3 (Fig. 8a–c). Each type of immunoreactivity showed both cytoplasmic and plasma membrane distribution. Many axons surrounding groups of somata and within the associated roots were also stained for each GFR. FG-labelled neurons were identified in L6 and S1 DRG as previously described (Keast and de Groat, 1992). Assessment of FG-labelled neurons showed that 15.4 ± 1.5% (n=7 animals) were immunolabelled for GFRα1 (Fig. 8d,e) and 8.4 ± 1.8% (n=7 animals) were immunolabelled for GFRα3 (Fig 8f,g). GFRα2-immunoreactive FG neurons were rarely found (0.9 ± 0.6%; n=6 animals).
Figure 8.
Distribution of GFR – immunoreactivity in sacral dorsal root ganglia. a, Numerous GFRα1-immunoreactive (IR) neurons, showing weak to moderate cytoplasmic labelling but pronounced plasma membrane labelling. b, Many small- and medium-sized GFRα2-IR neurons, showing moderate cytoplasmic labelling and pronounced plasma membrane labelling. c, Many small- and medium-sized GFRα3-IR neurons, showing bright cytoplasmic labelling and pronounced plasma membrane labelling. d shows two bladder-projecting neurons retrogradely labelled with FluoroGold (arrows), one of which is much brighter than the other; both of these FluoroGold neurons show GFRα1-IR, as shown in panel e (note that these two neurons are present in the larger field shown at lower magnification in a, as indicated with asterisks). f shows another pair of FluoroGold-labeled bladder-projecting neurons but in this case only the neuron on the right (arrow) shows GFRα3-IR (g). Calibration represents 15 μm (d–g) and 30 μm (a–c).
DISCUSSION
In our study of adult female rats, immunolabelling for GFRα1 (GDNF receptor), GFRα2 (neurturin receptor) and GFRα3 (artemin receptor) revealed distinct distribution patterns in the sacral spinal cord, suggesting separate populations of sensory fibers with different functions. Being able to separately identify different populations of sensory neuron projections to the sacral cord, we now have available new tools to track structural changes within the visceral sensory system, such as during development and after injury or other manipulations.
These three populations have not been revealed previously with other markers, primarily because most emphasis to date has been placed on peptidergic sensory nerves, as typically identified by immunoreactivity for CGRP. In the sacral spinal cord, CGRP labels terminals in laminae I and II, as well as terminals in the regions containing autonomic preganglionic neurons and interneurons (Chung et al., 1993; Vizzard, 2001), whereas each population of GFR-IR fibers innervates a distinct subgroup of sacral afferent neurons. Based on the distinctive patterns of immunostaining in the sacral spinal cord, the most obvious interpretation is that the three different GFRs are synthesised by separate populations of neurons, even though an in situ hybridisation study performed in more rostral levels suggests a high degree of coexpression of GFRα2 -and GFRα3-IR (Kashiba et al., 2003). The present study showed that in the sacral cord GFRα3-IR fibers are located in lamina I whereas GFRα2-IR fibers are located in lamina II. This suggests GFRα3-IR fibers innervate projection neurons in nociceptive pathways (in lamina I). In contrast, GFRα2-IR fibers are not found in lamina I so must innervate nociceptive interneurons. This disagreement with the previous in situ hybridisation study may indicate that there are significant differences in GFR coexpression patterns at different spinal levels.
GFRα1- and GFRα2-IR were generally exhibited by peptide-negative, IB4-positive neurons (Kalous et al., 2007) and in the lumbosacral cord showed distinct locations. As well as supplying different parts of lamina II (as also shown in thoracic cord; Kalous et al., 2007), suggesting innervation of different populations of nociceptive interneurons, GFRα1-IR fibers (and to a lesser extent GFRα3-IR fibers) were closely associated with autonomic preganglionic neurons and interneurons. GFRα2-IR fibers were absent from these two latter locations. Furthermore, both GFRα1- and GFRα3-IR fibers were present in the lateral collateral pathway of Lissauer’s tract, often referred to simply as the lateral collateral pathway (LCP). GFRα2-IR fibers were absent from this tract. The LCP comprises the central projection of pelvic visceral afferent fibers, and enters at lamina I of the dorsal horn, extending to the regions containing autonomic interneurons and preganglionic neurons (Morgan et al., 1981). The presence of GFR-positive fibers in this tract suggests a role in mediation or modulation of reflex behaviours within the viscera, at least for the GFRα1- and GFRα3-IR populations. Only one population of GFR-IR fibers (GFRα3) was found in the medial collateral pathway, that may innervate a functionally distinct population of interneurons located dorsal to the central canal (Morgan et al., 1981). In DRG of other spinal levels, GFRα3 has recently been reported to partially coexist with substance P (Bennett et al., 2006), which in sacral DRG is in turn strongly co-expressed with CGRP (Keast and de Groat, 1992; Bennett et al., 2003). The GFRα3-IR fiber distribution we observed in the sacral dorsal horn was quite distinct from that of CGRP-IR fibers (Chung et al., 1993; Vizzard 2001). This is consistent with only a proportion of CGRP fibers co-expressing GFRα3, and provides further evidence for a unique function of these neurons or involvement in distinct ascending pathways.
The effects of GDNF family ligands on sacral DRG neurons have been poorly investigated. However, various studies in DRG neurons from other spinal levels have demonstrated potent pro-nociceptive effects (Stucky et al., 2002; Gardell et al., 2003; Vellani et al., 2004; Albers et al., 2006; Bespalov and Saarma, 2007). For example, in skin nociceptors the GDNF family ligands potentiate TRPV1 signalling with doses 10-100x less than required for NGF (Malin et al., 2006). In normal healthy adult rats, GDNF and neurturin are synthesised by a number of pelvic organs including the bladder (Widenfalk et al., 1997; Golden et al., 1998; Laurikainen et al., 2000; Vizzard, 2000b; Widenfalk et al., 2000; Kawakami et al., 2002, 2003), so it is feasible that these growth factors are involved in maintenance of structural and physiological properties of GFR-expressing bladder neurons or other pelvic visceral sensory neurons expressing GFRs. The synthesis of artemin by pelvic organs has not been investigated. After inflammation or spinal cord injury, levels of GDNF within the bladder increase (Vizzard, 2000b), providing a potential mechanism to stimulate structural remodelling or increased excitability of pelvic visceral sensory neurons. Moreover, spinal cord injury triggers an increase of GDNF within the spinal cord (Satake et al., 2000; Nakamura and Bregman, 2001; Widenfalk et al., 2001; Nakashima et al., 2004), especially close to the injury site, so this could be another mechanism involved with altered pelvic visceral sensory function. It is not known if neurturin and artemin levels are also increased at this time.
Many structural and chemical changes occur in bladder sensory neurons after injury or inflammation, leading to changes in reflex behaviour (e.g. decreased threshold for activation), and increased urgency and pain. Much of the focus on possible mechanisms underlying these changes has been on NGF, but the results of our current study raise the possibility that one or more members of the GDNF family may also involved in plasticity of pelvic visceral circuits, including those related to bladder control. This is also supported by experiments where IB4-positive sensory neurons innervating the bladder (most or all of which will express GFRs) were destroyed following intrathecal administration of IB4-saporin, after which capsaicin- or ATP-evoked bladder hyperreactivity was reduced, with no effect on normal voiding (Nishiguchi et al., 2004). Immunofluorescence studies of retrogradely-labelled bladder-projecting neurons showed that ~25% showed immunoreactivity for GFRα1 or GFRα3, the preferred receptors for GDNF and artemin, respectively. Further, bladder inflammation induced by CYP caused an up-regulation of GFRα1-IR in the dorsal horn and possibly the regions associated with autonomic interneurons and preganglionic neurons. We do not know if the increased intensity of GFRα1-IR fluorescence indicates an increase in GFRα1 synthesis or transport along the central process of sacral dorsal root ganglion neurons. However, our result is comparable to previous observations on NGF-sensitive, peptidergic bladder sensory neurons, which show an up-regulation of neurotrophin receptors, trkA and trkB, after CYP-induced cystitis (Qiao and Vizzard, 2002). There was a much smaller effect on GFRα3 and no effect on GFRα2. This strongly correlates with the absence of GFRα2-IR in sacral bladder afferent neurons. These data indicate that GDNF and artemin may target bladder sensory neurons and potentially mediate plasticity of sacral visceral afferent neurons following inflammation. This is supported by the report of increased levels of GDNF within the bladder following CYP treatment (Birder and de Groat, 1992; Birder et al., 1999; Vizzard, 2000b). Neurturin and artemin have not yet been measured under these conditions. Following bladder inflammation, a larger number of neurons express the immediate early gene product, fos, following a period of elicited micturition reflexes (Vizzard, 2000a). Our results raise the possibility that some of the fos-positive neurons will lie in the peptide-negative afferent pathway.
In the context of visceral reflexes, it is not known whether peptide-positive and -negative neurons activate different central pathways, however studies on somatic nociceptive pathways in mice indicate that these pathways are functionally quite distinct (Stucky and Lewin, 1999) and activate quite different supraspinal structures (Braz et al., 2002). However, because most of the GFR-IR neurons will bind IB4 (Averill et al., 1995; Bennett et al., 1996), and loss of IB4-positive sensory fibers in the sacral spinal cord attenuates ATP- and capsaicin-evoked bladder hyperactivity without affecting normal voiding (Nishiguchi et al., 2004), we propose that many of the GFRα1- and GFRα3-IR bladder sensory nerves are C fibers that mediate nociceptive responses. That is, only a minority may be the source of Aδ fibers required for initiating the micturition reflex by activation of the pontine micturition center. However, GFR-IR was observed in fibers closely associated with sacral preganglionic neurons and nearby interneurons, suggesting that these populations of sensory fibers are able to play some role in modulation of the micturition reflex or other pelvic visceral reflexes, and that the relevant neurotrophic factors may influence these synapses. It is also possible that some of the GFRα1- and GFRα3-IR fibers apparently terminating in this region are components of the spinal reflex circuit that is present in the neonatal period and re-activated following spinal cord injury (de Groat et al., 1998).
Our results showed a broader distribution of sensory terminals in the sacral spinal cord than previously revealed by application of horseradish peroxidase to the bladder wall (Steers et al., 1991) or the pelvic nerve (Nadelhaft and Booth, 1984), both of which revealed only a thin band of terminals in lamina 1, as well as fibers in the lateral collateral pathway and adjacent to the sacral preganglionic nucleus. Our results and many previous studies showing extensive distribution of substance P and CGRP fibers in the dorsal horn of the rat sacral spinal cord (e.g. Chung et al., 1993; Vizzard 2001), lead us to propose that previous tract tracing methods have significantly underestimated the central terminal field of sacral dorsal root ganglion neurons. While tract tracing methods are valuable, they cannot guarantee complete labelling of all axons in a given projection, and do not distinguish between different chemical or functional classes of axons within a projection.
GFRα1-IR and GFRα3-IR are found in sensory terminals that innervate sacral autonomic interneurons and preganglionic neurons. The effects of GDNF family ligands on autonomic reflex circuits have not been examined, although based on their effects on nociceptors, one might predict that exposure to centrally- or peripherally-derived GDNF, neurturin or artemin may increase the gain of this circuit. In the thoracic cord, GDNF is a pro-survival factor for axotomised preganglionic neurons, the effect being mediated byGFRα1 (Schober et al., 1999). This may also be the case for sacral preganglionic neurons, given the modest immunostaining for GFRα1 by some of these neurons. GDNF has a similar role in many somatic motor neurons (Bohn, 204).
Our study provided evidence for GDNF and artemin having a role in sacral bladder afferent pathways, however these neurotrophic factors and neurturin are probably also involved in modulation of sacral sensory neurons innervating other pelvic organs. Retrograde tracing methodology is unable to guarantee labelling of all neurons projecting in a given tract, and in our study only small volumes of tracer dye were injected into the bladder wall to minimise dye leakage and spurious labelling from other nearby pelvic organs. Therefore, we are likely to have labelled only a minority of bladder-projecting afferent neurons and so cannot estimate how many GFR-positive neurons innervate the bladder. Nevertheless, the prevalence of sacral DRG neurons expressing each GFR and the absence of GFRα2 from bladder-projecting neurons strongly suggests that other pelvic visceral afferent neurons express one or more of these receptors. Therefore, the GDNF family of neurotrophic factors may influence the activity of many functional classes pelvic visceral afferent neurons.
Conclusions
This study has revealed new markers of functionally distinct populations of sensory fibres within the sacral spinal cord. Our results also provide evidence for the GDNF family ligands, especially GDNF and artemin, targeting sacral bladder sensory neurons, and that neurons responding to these ligands may be affected by cyclophosphamide-induced bladder inflammation. Together these observations raise the possibility that the GDNF family ligands may be new potential targets for therapies to modify activity of pelvic visceral afferent nerves, including some of those that innervate the bladder.
Acknowledgments
This study was supported by the National Institutes of Health (DK069351-03 to JK), National Health and Medical Research Council of Australia (Senior Research Fellowship 358709 to JK), and the Spinal Research Foundation (grant-in-aid to JK).
LITERATURE CITED
- Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. Glial cell-line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. J Neurosci. 2006;26:2981–2990. doi: 10.1523/JNEUROSCI.4863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7:1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DLH, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Letts. 1996;206:33–36. doi: 10.1016/0304-3940(96)12418-6. [DOI] [PubMed] [Google Scholar]
- Bennett DLH, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DLH, Boucher TJ, Armanini MP, Poulsen KT, Michael GJ, Priestley JV, Phillips HS, McMahon SB, Shelton DL. The glial cell line-derived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury. J Neurosci. 2000;20:427–437. doi: 10.1523/JNEUROSCI.20-01-00427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL, Boucher TJ, Michael GJ, Popat RJ, Malcangio M, Averill SA, Poulsen KT, Priestley JV, Shelton DL, McMahon SB. Artemin has potent neurotrophic actions on injured C-fibres. J Peripher Nerv Syst. 2006;11:330–345. doi: 10.1111/j.1529-8027.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Bennett HL, Gustafsson JA, Keast JR. Estrogen receptor expression in lumbosacral dorsal root ganglion cells innervating the female rat urinary bladder. Auton Neurosci. 2003;105:90–100. doi: 10.1016/S1566-0702(03)00044-4. [DOI] [PubMed] [Google Scholar]
- Bespalov MM, Saarma M. GDNF family receptor complexes are emerging drug targets. Trends Pharmacol Sci. 2007;28:68–74. doi: 10.1016/j.tips.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Birder LA, de Groat WC. Increased c-fos expression in spinal neurons after irritation of the lower urinary tract in the rat. J Neurosci. 1992;12:4878–4889. doi: 10.1523/JNEUROSCI.12-12-04878.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Roppolo JR, Erickson VL, de Groat WC. Increased c-fos expression in spinal lumbosacral projection neurons and preganglionic neurons after irritation of the lower urinary tract in the rat. Brain Res. 1999;834:55–65. doi: 10.1016/s0006-8993(99)01546-2. [DOI] [PubMed] [Google Scholar]
- Björling DE, Beckman M, Saban R. Neurogenic inflammation of the bladder. Adv Exp Med Biol. 2003;539 (Pt B):551–583. doi: 10.1007/978-1-4419-8889-8_37. [DOI] [PubMed] [Google Scholar]
- Bohn MC. Motoneurons crave glial cell line-derived neurotrophic factor. Exp Neurol. 2004;190:263–275. doi: 10.1016/j.expneurol.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Braz JM, Rico B, Basbaum AI. Transneuronal tracing of diverse CNS circuits by Cre-mediated induction of wheat germ agglutinin in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:15148–15153. doi: 10.1073/pnas.222546999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett AL, Saito S, Maguire MP, Yamaguchi H, Chang TSK, Hanley DF. 5. Localization of nitric oxide synthase in spinal nuclei innervating pelvic ganglia. J Urol. 1999;153:212–217. doi: 10.1097/00005392-199501000-00079. [DOI] [PubMed] [Google Scholar]
- Cho JW, Yarygina O, Oo TF, Kholodilov NG, Burke RE. Glial cell line-derived neurotrophic factor GFRα1 is expressed in the rat striatum during postnatal development. Mol Brain Res. 2004;127:96–104. doi: 10.1016/j.molbrainres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Chuang YC, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol. 2001;165:975–979. [PubMed] [Google Scholar]
- Chung K, Lee WT, Park MJ. Spinal projections of pelvic visceral afferents of the rat: a calcitonin gene-related peptide (CGRP) immunohistochemical study. J Comp Neurol. 1993;337:63–69. doi: 10.1002/cne.903370104. [DOI] [PubMed] [Google Scholar]
- Clemow DB, Steers WD, McCarty R, Tuttle JB. Altered regulation of bladder nerve growth factor and neurally mediated hyperactive voiding. Am J Physiol. 1998;275 :R1279–1286. doi: 10.1152/ajpregu.1998.275.4.R1279. [DOI] [PubMed] [Google Scholar]
- Cox PJ. Cyclophosphamide cystitis-–identification of acrolein as the causative agent. Biochem Pharmacol. 1979;28:2045–2049. doi: 10.1016/0006-2952(79)90222-3. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Kruse MN, Vizzard MA, Cheng CL, Araki I, Yoshimura N. Modification of urinary bladder function after spinal cord injury. Adv Neurol. 1997;72:347–364. [PubMed] [Google Scholar]
- de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR. Developmental and injury induced plasticity in the micturition reflex pathway. Behav Brain Res. 1998;92:127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Pharmacology of the lower urinary tract. Annu Rev Pharmacol Toxicol. 2001;41:691–721. doi: 10.1146/annurev.pharmtox.41.1.691. [DOI] [PubMed] [Google Scholar]
- Gardell LR, Wang R, Ehrenfels C, Ossipov MH, Rossomando AJ, Miller S, Buckley C, Cai AK, Tse A, Foley SF, Gong B, Walus L, Carmillo P, Worley D, Huang C, Engber T, Pepinsky B, Cate RL, Vanderah TW, Lai J, Sah DW, Porreca F. Multiple actions of systemic artemin in experimental neuropathy. Nat Med. 2003;9:1383–1389. doi: 10.1038/nm944. [DOI] [PubMed] [Google Scholar]
- Golden JP, Baloh RH, Kotzbauer PT, Lampe PA, Osborne PA, Milbrandt J, Johnson EM. Expression of neurturin, GDNF, and their receptors in the adult mouse CNS. J Comp Neurol. 1998;398:139–150. doi: 10.1002/(sici)1096-9861(19980817)398:1<139::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Rudiger S, Mayer B, Bravo R, Zimmermann M. Expression of nitric oxide synthase and colocalisation with Jun, Fos and Krox transcription factors in spinal cord neurons following noxious stimulation of the rat hindpaw. Brain Res Mol Brain Res. 1994;22:245–258. doi: 10.1016/0169-328x(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Holstege G. Micturition and the soul. J Comp Neurol. 2005;493:15–20. doi: 10.1002/cne.20785. [DOI] [PubMed] [Google Scholar]
- Holstege G, Mouton LJ. Central nervous system control of micturition. Int Rev Neurobiol. 2003;56:123–145. doi: 10.1016/s0074-7742(03)56004-4. [DOI] [PubMed] [Google Scholar]
- Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;284:R574–585. doi: 10.1152/ajpregu.00465.2002. [DOI] [PubMed] [Google Scholar]
- Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tami R, Antonio L, Hu Z, Cupples R, Louis J-C, Hu S, Altrock BW, Fox GM. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-α, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- Kalous A, Osborne PB, Keast JR. Acute and chronic changes in dorsal horn innervation by primary afferents and descending supraspinal pathways after spinal cord injury. J Comp Neurol. 2007;504:238–253. doi: 10.1002/cne.21412. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Uchida Y, Senba E. Distribution and colocalization of NGF and GDNF family ligand receptor mRNAs in dorsal root and nodose ganglion neurons of adult rats. Mol Brain Res. 2003;110:52–62. doi: 10.1016/s0169-328x(02)00584-3. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Wakabayashi Y, Aimi U, Isono T, Okada Y. Developmental expression of glial cell-line derived neurotrophic factor, neurturin, and their receptor mRNA in the rat urinary bladder. Neurourol Urodyn. 2003;22:83–88. doi: 10.1002/nau.10074. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Wakabayashi Y, Isono T, Aimi Y, Okada Y. Expression of neurotrophin messenger RNAs during rat urinary bladder development. Neurosci Lett. 2002;329:77–80. doi: 10.1016/s0304-3940(02)00598-0. [DOI] [PubMed] [Google Scholar]
- Keast JR, de Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- Laurikainen A, Hiltunen JO, Thomas-Crussells J, Vanhatalo S, Arumäe U, Airaksinen MS, Klinge E, Saarma M. Neurturin is a neurotrophic factor for penile parasympathetic neurons in adult rat. J Neurobiol. 2000;43:198–205. doi: 10.1002/(sici)1097-4695(200005)43:2<198::aid-neu9>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol. 1997;79:572–577. doi: 10.1046/j.1464-410x.1997.00097.x. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Lecci A, Santicioli P, Del Bianco E, Giuliani S. Cyclophosphamide-induced cystitis in rats: involvement of capsaicin-sensitive primary afferents. Agents Actions. 1993;38(C28–30) doi: 10.1007/BF01991127. [DOI] [PubMed] [Google Scholar]
- Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26:8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C, Nadelhaft I, de Groat WC. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol. 1981;201:415–440. doi: 10.1002/cne.902010308. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J Comp Neurol. 1984;226:238–245. doi: 10.1002/cne.902260207. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Bregman BS. Differences in neurotrophic factor gene expression profiles between neonate and adult rat spinal cord after injury. Exp Neurol. 2001;169:407–415. doi: 10.1006/exnr.2001.7670. [DOI] [PubMed] [Google Scholar]
- Nakashima S, Matsuyama Y, Yu Y, Kiuchi K, Ishiguro N. Suppression of GDNF production by MPSS treatment following spinal cord injury in the rat. Neuroreport. 2004;15:2337–2340. doi: 10.1097/00001756-200410250-00007. [DOI] [PubMed] [Google Scholar]
- Nazif O, Teichman JMH, Gebhart GF. Neural upregulation in interstitial cystitis. Urology. 2007;69 (Suppl 4A):24–33. doi: 10.1016/j.urology.2006.08.1108. [DOI] [PubMed] [Google Scholar]
- Nishiguchi J, Sasaki K, Seki S, Chancellor MB, Erickson KA, de Groat WC, Kumon H, Yoshimura N. Effect of isolectin B4-conjugated saporin, a targeting cytotoxin, on bladder overactivity induced by bladder irritation. Eur J Neurosci. 2004;20:474–482. doi: 10.1111/j.1460-9568.2004.03508.x. [DOI] [PubMed] [Google Scholar]
- Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol. 1999;161:438–441. [PubMed] [Google Scholar]
- Orozco OE, Walus L, Sah DWY, Pepinsky RB, Sanicola M. GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci. 2001;13:2177–2182. doi: 10.1046/j.0953-816x.2001.01596.x. [DOI] [PubMed] [Google Scholar]
- Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- Pierchala BA, Milbrandt J, Johnson EM., Jr Glial cell line-derived neurotrophic factor-dependent recruitment of ret into lipid rafts enhances signalling by partitioning ret from proteosome-dependent degradation. J Neurosci. 2006;26:2777–2787. doi: 10.1523/JNEUROSCI.3420-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao LY, Vizzard MA. Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways. J Comp Neurol. 2002;454:200–211. doi: 10.1002/cne.10447. [DOI] [PubMed] [Google Scholar]
- Rakowicz WP, Staples CS, Milbrandt J, Brunstrom JE, Johnson EM., Jr Glial cell line-derived neurotrophic factor promotes the survival of early postnatal spinal motor neurons in the lateral and medial motor columns in slice culture. J Neurosci. 2002;22:3953–3962. doi: 10.1523/JNEUROSCI.22-10-03953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake K, Matsuyama Y, Kamiya M, Kawakami H, Iwata H, Adachi K, Kiuchi K. Up-regulation of glial cell line-derived neurotrophic factor (GDNF) following traumatic spinal cord injury. Neuroreport. 2000;17:3877–3881. doi: 10.1097/00001756-200011270-00054. [DOI] [PubMed] [Google Scholar]
- Schober A, Hertel R, Arumae U, Farkas L, Jaszai J, Krieglstein K, Saarma M, Unsicker K. Glial cell line-derived neurotrophic factor rescues target-deprived sympathetic spinal cord neurons but requires transforming growth factor-b as cofactor in vivo. J Neurosci. 1999;19:2008–2015. doi: 10.1523/JNEUROSCI.19-06-02008.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steers WD, Ciambotti J, Etzel B, Erdman S, de Groat WC. Alterations in afferent pathways from the urinary bladder of the rat in response to partial urethral obstruction. J Comp Neurol. 1991;310:401–410. doi: 10.1002/cne.903100309. [DOI] [PubMed] [Google Scholar]
- Steers WD, Creedon DJ, Tuttle JB. Immunity to nerve growth factor prevents afferent plasticity following urinary bladder hypertrophy. J Urol. 1996;155:379–385. [PubMed] [Google Scholar]
- Steinbacher BC, Nadelhaft I. Increased levels of nerve growth factor in the urinary bladder and hypertrophy of dorsal root ganglion neurons in the diabetic rat. Brain Res. 1998;782:255–260. doi: 10.1016/s0006-8993(97)01287-0. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Lewin GR. Isolectin B4-positive and -negative nociceptors are functionally distinct. J Neurobiol. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky CL, Rossi J, Airaksinen MS, Lewin GR. GFRα2/neurturin signalling regulates noxious heat transduction in isolectin B4-binding mouse sensory neurons. J Physiol. 2002;545:43–50. doi: 10.1113/jphysiol.2002.027656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HC, Wharton J, Polak JM, Mulderry PK, Ghatei MA, Gibson SJ, Terenghi G, Morrison JF, Ballesta J, Bloom SR. Calcitonin gene-related peptide immunoreactivity in afferent neurons supplying the urinary tract: combined retrograde tracing and immunohistochemistry. Neuroscience. 1986;18:727–747. doi: 10.1016/0306-4522(86)90066-7. [DOI] [PubMed] [Google Scholar]
- Trupp M, Belluardo N, Funakoshi H, Ibanez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle JB, Steers WD, Albo M, Nataluk E. Neural input regulates tissue NGF and growth of the adult rat urinary bladder. J Autonom Nerv Syst. 1994;49:147–158. doi: 10.1016/0165-1838(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Vellani V, Zachrissen O, McNaughton PA. Functional bradykinin B1 receptors are expressed in nociceptive neurones and are upregulated by the neurotrophin GDNF. J Physiol. 2004;560:391–401. doi: 10.1113/jphysiol.2004.067462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzard MA. Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol. 2000a;278:R1027–1039. doi: 10.1152/ajpregu.2000.278.4.R1027. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000b;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat. 2001;21:125–138. doi: 10.1016/s0891-0618(00)00115-0. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Erdman SL, de Groat WC. Increased expression of neuronal nitric oxide synthase (NOS) in visceral neurons after nerve injury. J Neurosci. 1995;15:4033–4045. doi: 10.1523/JNEUROSCI.15-05-04033.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzard MA, Erdman SL, de Groat WC. Increased expression of neuronal nitric oxide synthase in bladder afferent pathways following chronic bladder irritation. J Comp Neurol. 1996;370:191–202. doi: 10.1002/(SICI)1096-9861(19960624)370:2<191::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voikar V, Rossi J, Rauvala H, Airaksinen MS. Impaired behavioral flexibility and memory in mice lacking GDNF family receptor alpha2. Eur J Neurosci. 2004;20:308–312. doi: 10.1111/j.1460-9568.2004.03475.x. [DOI] [PubMed] [Google Scholar]
- Wanigasekara Y, Keast JR. Nerve growth factor, glial cell line-derived neurotrophic factor and neurturin prevent semaphorin 3A–mediated growth cone collapse in adult sensory neurons. Neuroscience. 2006;142:369–379. doi: 10.1016/j.neuroscience.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Nosrat C, Tomac A, Westphal H, Hoffer B, Olson L. Neurturin and glial cell line-derived neurotrophic factor receptor- β (GDNFR-β), novel proteins related to GDNF and GDNFR-α with specific cellular patterns of expression suggesting roles in the developing and adult nervous system and in peripheral organs. J Neurosci. 1997;17:8506–8519. doi: 10.1523/JNEUROSCI.17-21-08506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widenfalk J, Parvinen M, Lindqvist E, Olson L. Neurturin, RET, GFRα1 and GFRα2, but not GFRα3, mRNA are expressed in mice gonads. Cell Tissue Res. 2000;299:409–415. doi: 10.1007/s004419900068. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Lundstromer K, Jubran M, Brene S, Olsen L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J Neurosci. 2001;21:3457–3475. doi: 10.1523/JNEUROSCI.21-10-03457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Plasticity of Na+ channels in afferent neurones innervating rat urinary bladder following spinal cord injury. J Physiol. 1997;503:269–276. doi: 10.1111/j.1469-7793.1997.269bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006;26:10847–10855. doi: 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Verge V, Wiesenfeld-Hallin Z, Ju G, Bredt D, Synder SH, Hokfelt T. Nitric oxide synthase-like immunoreactivity in lumbar dorsal root ganglia and spinal cord of rat and monkey and effect of peripheral axotomy. J Comp Neurol. 1993;335:563–575. doi: 10.1002/cne.903350408. [DOI] [PubMed] [Google Scholar]
- Zinck ND, Rafuse VF, Downie JW. Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Exp Neurol. 2007;204:777–790. doi: 10.1016/j.expneurol.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Zvarova K, Murray E, Vizzard MA. Changes in galanin immunoreactivity in rat lumbosacral spinal cord and dorsal root ganglia after spinal cord injury. J Comp Neurol. 2004;475:590–603. doi: 10.1002/cne.20195. [DOI] [PubMed] [Google Scholar]
- Zvarova K, Dunleavy JD, Vizzard MA. Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp Neurol. 2005;192:46–59. doi: 10.1016/j.expneurol.2004.10.017. [DOI] [PubMed] [Google Scholar]