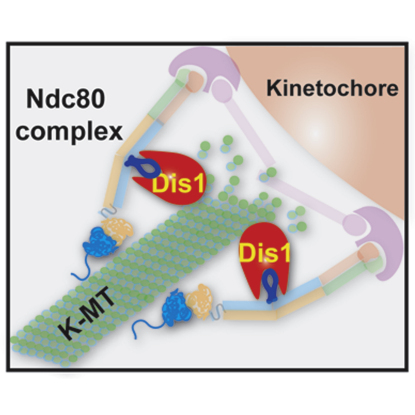
Summary
The Ndc80 complex, a conserved outer kinetochore complex, comprising four components (Ndc80/Hec1, Nuf2, Spc24, and Spc25), constitutes one of the core microtubule-binding sites within the kinetochore [1–3]. Despite this knowledge, molecular mechanisms by which this complex contributes to establishment of correct bipolar attachment of the kinetochore to the spindle microtubule remain largely elusive [1, 2, 4, 5]. Here we show that the conserved internal loop [6, 7] of fission yeast Ndc80 directly binds the Dis1/TOG microtubule-associated protein [8–10], thereby coupling spindle microtubule dynamics with kinetochore capture. Ndc80 loop mutant proteins fail to recruit Dis1 to kinetochores, imposing unstable attachment and frequent spindle collapse. In these mutants, mitotic progression is halted attributable to spindle assembly checkpoint activation, and chromosomes remain in the vicinity of the spindle poles without congression. dis1 deletion precisely phenocopies the loop mutants. Intriguingly, forced targeting of Dis1 to the Ndc80 complex rescues loop mutant's defects. We propose that Ndc80 comprises two microtubule-interacting interfaces: the N-terminal region directly binds the microtubule lattice, while the internal loop interacts with the plus end of microtubules via Dis1/TOG. Therefore, our results provide a crucial insight into how the Ndc80 complex establishes stable bipolar attachment to the spindle microtubule.
Graphical Abstract
Highlights
► Ndc80-loop mutant is defective in spindle-kinetochore attachment ► Ndc80 internal loop directly interacts with Dis1/TOG ► Dis1 dissociates from the kinetochore in Ndc80-loop mutants ► Tethering Dis1 to the kinetochore rescues Ndc80-loop mutant defects
Results and Discussion
We undertook the isolation of temperature-sensitive (ts) ndc80 mutants by using error-prone PCR from fission yeast that are specifically defective in spindle microtubule dynamics and consequently obtained one mutant (ndc80-21, see Supplemental Experimental Procedures and Figure S1A available online). Like other mitotic mutants, ndc80-21 exhibited hypersensitivity to a benomyl-derivative tubulin drug, thiabendazole (Figure S1A). Synchronous culture analysis showed that the ndc80-21 mutant lost viability during the first mitosis with missegregating chromosomes (Figure S1B).
Live imaging uncovered two characteristic features of ndc80-21: unstable spindle microtubule morphology and abnormal kinetochore movement. Unlike wild-type cells (Figure 1A, top three panels), the ndc80-21 mutant (bottom three panels) exhibited very weak intensities of spindle microtubules, in which blobs around the two poles were observed with faint interpolar microtubules (white arrowheads in 2 min time-point in the top right corner, see Movie S1 and Movie S2). Consistent with this, the distance of the two poles was often shortened, which we call spindle collapse (18 out of 23 cells observed).
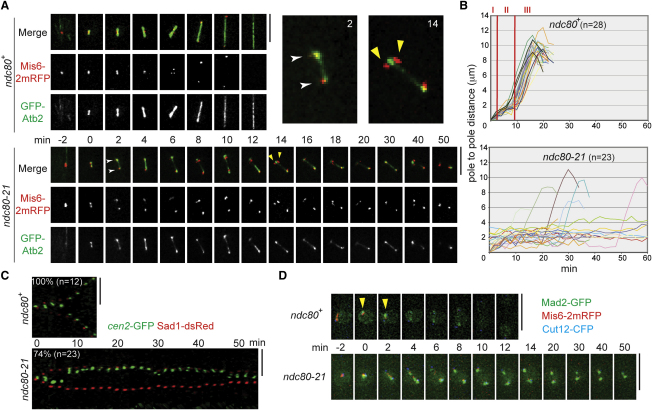
Figure 1.
The ndc80-21 Mutant Is Defective in Spindle-Kinetochore Attachment with Activation of the Spindle Assembly Checkpoint
(A) Prolonged mitotic delay with unstable spindle microtubules. Time-lapse fluorescence montages of spindle microtubules (GFP-Atb2, α2-tubulin, green) and kinetochores (Mis6-2mRFP, kinetochore component Mis6 tagged with two copies of monomeric RFP, red) are shown (wild-type, top, and ndc80-21, bottom, cultured at 36°C). Merged images of two time points (2 min and 14 min) were enlarged in the top right corner. See Movie S1 (wild-type) and Movie S2 (ndc80-21).
(B) Profiles of mitotic progression. Changes of the inter-SPB distance are plotted against time in wild-type (top) or ndc80-21 (lower). Phase I is a period of initial spindle elongation (I), while phase II represents the duration in which spindle length is constant (II) and phase III corresponds to anaphase B (III) [11].
(C) Time-lapse analysis of sister centromere behavior. Wild-type (top) or ndc80-21 (bottom) cells containing cen2-GFP (green) and Sad1-dsRed (red, an SPB marker) cultured at 36°C were recorded (2 min intervals) and converted to kymograph. See Movie S3 (wild-type) and Movie S4 (ndc80-21).
(D) Mad2 localization at kinetochores. Wild-type (top) or ndc80-21 (bottom) cells containing Mad2-GFP (green), Mis6-2mRFP (kinetochore, red), and Cut12-CFP (SPB) was shifted to 36°C for 40 min and mitotic images were recorded (see Supplemental Experimental Procedures). Arrowheads show transient kinetochore localization (∼2 min) of Mad2 in wild-type. See Figures S1C and S1D for additional data.
Scale bars represent 5 μm.
In terms of kinetochore movement, kinetochore markers located in close proximity to the two separated spindle poles (Figure 1A). Closer inspection of individual time frames, however, showed that kinetochores were not always associated with the poles, but instead they often detached from the poles and even from microtubules (see the top right corner, yellow arrowheads). We envision that the ndc80-21 mutation enforced destabilization of spindle microtubules, by which kinetochores attach to spindle microtubules only weakly and therefore remain localizing closely to the spindle poles.
Live imaging of individual cells also showed the prolonged mitotic phase, corresponding to the period between prometaphase and anaphase A (phase II) [11] (Figures 1A and 1B), which was ascribable to activation of the spindle assembly checkpoint (SAC) [12] (see below). In fact, observation of individual sister chromatids (cen2-GFP) [11, 13] showed that in the ndc80-21 mutant, two cen2-GFP signals remained attached to the same pole (Figure 1C, the bottom row, 17 out of 23 samples) instead of splitting into the two poles (wild-type, top). Nonetheless, a pair of cen2-GFP sometimes segregated apart and two dots were distinguishable within a short distance (e.g., see time points after 30 min, see Movie S3 and Movie S4), but these two cen2-GFP signals were still situated in close vicinity of one side of the spindle poles. This indicated that each of the sister kinetochores was likely to be pulled from the opposite poles, although stable attachment was never established.
Given the prolonged mitotic arrest in ndc80-21, we examined SAC activation by observing Mad2 localization. As shown in Figure 1D (top row), whereas Mad2 localized to the kinetochore only transiently during early mitosis in wild-type (∼2–3 min, arrowheads), in ndc80-21, Mad2 foci colocalized with kinetochore markers for more than 50 min in close proximity to the SPB (spindle pole body) (Figure 1D, bottom row). This suggested that kinetochores in ndc80-21 lack tension, resulting in alternating the physical configurations between attached and detached state, by which Mad2 is recruited to these kinetochores and the SAC is activated. Further analysis (in Figures S1C and S1D) indicated that mitotic arrest in ndc80-21 is dependent on SAC components such as Mad2 and a tension sensor Aurora B kinase [12, 14–16].
We next determined the mutation sites in ndc80-21. Three mutations were identified (T307A, E379G, and L405P). E379 and L405 locate in the vicinity of and within the internal loop, respectively, of hitherto unknown function [1, 6, 7, 17]. This loop, consisting of ∼70 amino acid resides, is sandwiched by adjacent coiled coil regions (Figure 2A; Figures S2A and S2B), which are responsible for forming a heterotetramer with Nuf2, Spc24, and Spc25 [18, 19]. In order to identify the critical mutation(s) that are responsible for temperature sensitivity, ndc80 genes carrying individual single or double mutations were introduced into ndc80-21. It turned out that Ndc80-L405P failed to complement this mutant (Figures 2B). When combined with E379G, which is silent on its own, this double mutant recapitulated the robust ts phenotype of ndc80-21. It is of note that amino acid residues around L405 show relatively high homology among different species, though L405 itself is not conserved (Figure S2B).
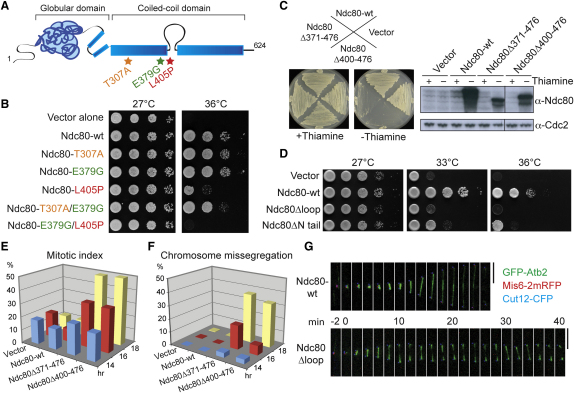
Figure 2.
The Internal Loop Is Essential for Ndc80's Role in Spindle Microtubule Stabilization
(A) A schematic view of Ndc80 with three mutation sites (asterisks) in ndc80-21. See Figures S2A and S2B for more information.
(B) Identification of the mutation site(s) responsible of temperature sensitivity. The ndc80 gene containing indicated mutation(s) were introduced into an ndc80-21 strain as episomal plasmids (Table S2). 10-fold serial dilution (5 × 104 cells in the first spot) was applied on rich media, followed by incubation at 27°C or 36°C for 3 days.
(C) Dominant-negative effects of overproduced loop-less Ndc80. Cells carrying indicated plasmids were streaked on minimal plates with (left plate, repressed) or without (right plate, derepressed) thiamine. For immunoblotting, 30 μg protein extracts were run on the gel. The Ndc80 protein was detected with Ndc80 antibody (Supplemental Experimental Procedures).
(D) Nonfunctionality of loop-less Ndc80. Plasmids producing wild-type, loop-less (Ndc80Δ400-476, Ndc80Δloop), or N-terminally truncated (Ndc80Δ2-94, Ndc80ΔN) Ndc80 were introduced into an ndc80-21 strain.
(E and F) Defective mitosis in cells overproducing loop-less Ndc80. Cells used in (C) were grown in minimal liquid media in the absence of thiamine, and the percentage of mitotic cells (E, microtubule morphology and the number of SPBs) and patterns of chromosome segregation (F, kinetochores and DAPI) were observed (n > 200).
(G) Mitotic arrest accompanied with unstable spindle microtubules. Live image analysis was performed in cells overproducing Ndc80ΔN (16 hr in the absence of thiamine, bottom row). Scale bars represent 5 μm.
Identification of L405P as a mutation causing unstable kinetochore-spindle attachment suggested that the loop is directly or indirectly involved in spindle microtubule dynamics. To scrutinize this notion, we created two types of Ndc80 deletion constructs that lack the internal loop. One construct (Ndc80Δ400-476) lacked only the loop region, whereas in the other construct (Ndc80Δ371-476), an additional 30 amino acids were also deleted. These mutants were then episomally introduced into wild-type cells under the thiamine-repressible nmt1 promoter. It was found that both constructs, but not wild-type Ndc80, were lethal in wild-type cells upon overexpression (−Thiamine, Figure 2C). Thus, loop-less Ndc80 mutant proteins acted in a dominant-negative manner. In line with this, these two mutants neither complemented ndc80-21 (Figure 2D) nor did they rescue ndc80-deleted strains (data not shown), indicating that the loop is essential for Ndc80 function. Like ndc80-21 (Figure S1A), these loop-less mutants retained kinetochore-localizing activities (Figure S2C). We, therefore, envisage that overall structural integrities of the Ndc80 complex in Ndc80-loop mutants are not impaired, if not the same as wild-type.
It is noteworthy that another Ndc80 mutant that lacks the N-terminal 94 amino acid extension, which is believed to be required for microtubule-binding activity, in particular binding to the microtubule lattice [2, 4, 20, 21], was capable of rescuing ndc80-21, albeit partially (Figure 2D, Ndc80ΔN tail). This illuminated a functional differentiation between this N-terminal extension and the internal loop. Phenotypic analysis of overproducing loop-less Ndc80Δ371-476 or Ndc80Δ400-476 indicated that these cells exhibited a high mitotic index (∼50%, Figure 2E) with missegregating chromosomes (Figure 2F). Importantly, spindle microtubules of these overproducing cells displayed very fragile morphologies, two blobs around the opposite SPBs, to which kinetochores were associated in close proximity (Figure 2G). These defects were virtually phenocopies of those observed in ndc80-21 cells (Figure 1A). Taken together, our data show that the internal loop of Ndc80 plays a critical role in establishment of proper spindle-kinetochore attachment.
Having seen the striking instability of spindle microtubules in Ndc80-loop mutants, we wondered whether some microtubule-binding proteins might compensate for spindle defects when overproduced. Among six such proteins tested, only one protein, Dis1 (a homolog of TOG/XMAP215) [8, 9], partially rescued temperature sensitivity of the ndc80-21 mutant (Figure 3A; Figure S3A). As Dis1/TOG family members are not only microtubule-associated proteins but also microtubule polymerases [22], suppression by overproduced Dis1 appeared coherent. Intriguingly in accordance with this, Dis1 is reportedly an M-phase-specific kinetochore protein [23] (see below).
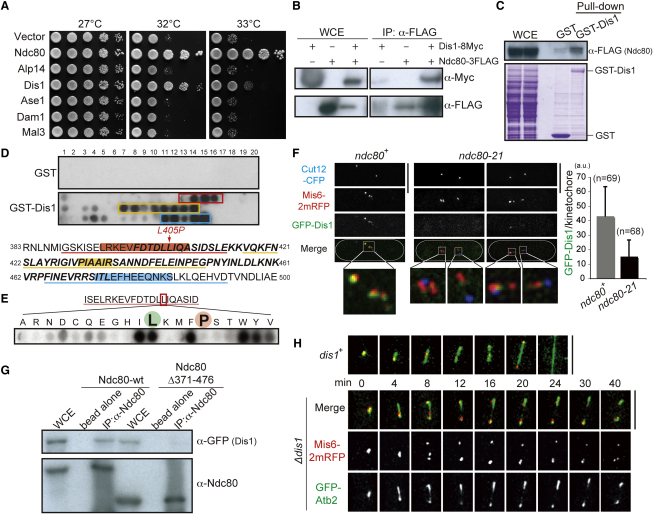
Figure 3.
Dis1/TOG Microtubule-Associated Protein Is a Factor Responsible for Ndc80 Loop-Dependent Kinetochore Microtubule Stabilization
(A) Rescue of ndc80-21 by multicopy plasmids containing dis1+. See Figure S3A.
(B) Interaction between Dis1 and Ndc80. Protein extracts (1.5 mg) were prepared from indicated cells, followed by immunoprecipitation (see Supplemental Experimental Procedures). WCE (whole cell extract), 50 μg.
(C) Pull-down of Ndc80 by GST-Dis1. Bacterial GST-Dis1 bound on GSH beads was passed through fission yeast cell extracts (2.7 mg) containing Ndc80-3FLAG. Eluted fractions were immunoblotted with FLAG antibody (top) or stained with coomassie blue (bottom). WCE, 50 μg.
(D) Peptide array assay. Peptides that showed positive interactions with Dis1 are boxed or underlined (395–408, red; 431–436, yellow; and 473–484, blue). Amino acid residues inside the loop (Figure S2B) are shown in bold and italics.
(E) Binding assay between GST-Dis1 and mutated peptides. Leucine (wild-type) and proline (Ndc80-21) are marked with green and red, respectively. See Figure S3B for comprehensive data.
(F) Delocalization of Dis1 from kinetochores. Cells were cultured at 36°C for 2 hr and mitotic cells were pictured. Quantification is shown on the right corner. Error bars represent SD (standard deviation).
(G) Reduced binding of loop-less Ndc80 to Dis1. Protein extracts (1 mg) were prepared from indicated cells and immunoprecipitation was performed (Supplemental Experimental Procedures). WCE, 30 μg.
(H) Unstable mitotic spindles in the dis1 deletion. Cells were grown at 20°C for 4 hr and time-lapse imaging was performed.
Scale bars represent 5 μm.
Suppression by multicopy plasmids raised a trivial possibility that the rescue is attributed to nonspecific microtubule-stabilizing activities of overproduced Dis1 independent of Ndc80 loop function. However, the internal loop is predicted to form a β sheet structure, implying that this domain might represent a protein-protein interaction motif [6, 7]. To address this point, we examined whether Ndc80 and Dis1 physically interact. Coimmunoprecipitation experiments showed that it is the case (Figure 3B). Furthermore, bacterially produced GST-Dis1 proteins pulled down the Ndc80 protein from fission yeast cell extracts (Figure 3C). In order to examine whether Dis1 directly binds the Ndc80 loop, we analyzed the interaction via a peptide array screen (Supplemental Experimental Procedures). A membrane was spotted with 20-residue peptides covering the loop sequence with a 2-residue start increment per spot. This array was probed with GST-Dis1 or GST alone. As shown in Figure 3D (top two filters), this assay identified three main sites of interaction in the loop (exact amino acid sequences were colored in the bottom). Intriguingly L405, which is mutated in ndc80-21, was found in one of the binding peptides (red). Next, a series of mutant peptides that contain 20 different amino acids at the position 405, including leucine (wild-type Ndc80) and proline (ndc80-21), were synthesized and binding assay was performed. As expected, a peptide containing proline at 405 did not bind GST-Dis1, and moreover mutated peptides that retained binding capability were those containing conservative hydrophobic residues (I, F, W, Y, and V; Figure 3E; Figure S3B). These results indicated that the Ndc80 loop interacts directly with Dis1.
To explore Dis1's roles at the kinetochore in more detail, we performed the following three experiments. First, we examined kinetochore localization of Dis1 in ndc80-21. As shown in Figure 3F, GFP-Dis1 signals largely disappeared from the kinetochore. Second, interaction between loop-less Ndc80 and Dis1 was assessed. Immunoprecipitation in cells expressing mutant loop-less Ndc80 (Ndc80Δ371-476) and Dis1 showed that amount of the Dis1 protein coprecipitating with Ndc80 was reduced (Figure 3G). Third, we examined spindle microtubule morphologies of dis1-deleted mutants by live imaging (note that dis1-deletion cells are cold sensitive for growth) [24]. At the low temperature (20°C), we found that spindle microtubules were extremely unstable with prolonged mitotic arrest (Figure 3H and see Figure S3 for further information and discussion), literally indistinguishable from those observed in ndc80-21 mutants and cells overproducing loop-less Ndc80 (Figures 1A and 2G, respectively). Collectively, the major defect of Ndc80 loop-less mutants lies in unstable spindle-kinetochore attachment and microtubule instability ascribable to Dis1 delocalization from the kinetochore.
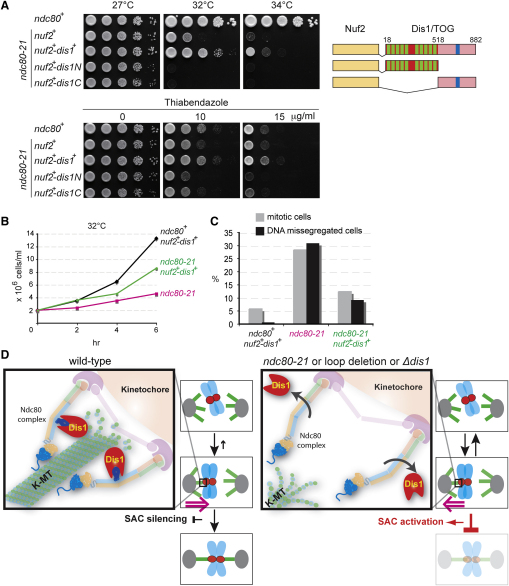
Results so far point toward the idea that Dis1 is a critical microtubule/kinetochore factor that determines proper spindle-kinetochore attachment in a manner dependent upon the Ndc80 loop. This scenario poses a crucial prediction, that is, tethering of Dis1 to the Ndc80 complex would rescue the ndc80-21 mutant at the physiological level of Dis1 without the need for overexpression. In order to test this possibility, we constructed a strain containing the nuf2+-dis1+ fusion gene (expressed under the endogenous nuf2+ promoter) and crossed it with ndc80-21. Remarkably this Ndc80 complex-targeted Dis1 (Nuf2-Dis1) suppressed temperature sensitivity and thiabendazole hypersensitivity, although not completely (Figure 4A). Liquid culture analysis indicated that ndc80-21 containing Nuf2-Dis1 indeed divided faster than ndc80-21 (Figure 4B) and contained less mitotic cells or cells displaying missegregating chromosomes (Figure 4C). For this suppression to occur, full-length Dis1 is necessary, because either N- or C-terminally truncated Dis1 fused to Nuf2 failed to complement ndc80-21 (Figure 4A, Nuf2-Dis1N and Nuf2-Dis1C). We also made fusion construct with Mis12, a kinetochore component interacting with the Ndc80 complex but located proximal to the inner kinetochore side [1, 2]. Interestingly, in this case Mis12-Dis1 did not rescue ndc80-21 (Figure S4B), suggesting that the closer geometrical proximity is required for suppression of ndc80-21 by targeting Dis1. Taken together our results establish a central role for Dis1 and Ndc80 in kinetochore-spindle attachment. Dis1 binds Ndc80, most likely via the internal loop of Ndc80, which allows a physical connection between the plus end of the spindle microtubule and the outer kinetochore, thereby ensuring stable bipolar attachment.
Figure 4.
Tethering of Dis1 to the Ndc80 Complex Rescues ndc80-21 Mutants and a Model
(A) Rescue of ndc80-21 by targeting Dis1 to the Ndc80 complex. Full-length, N-terminal, or C-terminal Dis1 was fused to Nuf2 and produced in the ndc80-21 mutant. The N-terminal part of Dis1 contains two TOG repeats, each consisting of five HEAT repeats (green boxes), and the C-terminal domain contains central coiled coil domain (blue boxes) [8].
(B and C) Liquid culture analysis of ndc80-21 containing Nuf2-Dis1. Indicated strains were grown in rich liquid media and incubated at 32°C. At each time point, cell number (B) and percentages of mitotic cells and cells displaying chromosome missegregation (stained with DAPI and Calcofluor) were counted (C, n > 100). See Figure S4A for additional data.
(D) A model. Ndc80 contains two independent microtubule-interacting domains. The N-terminal domain (shown in blue oval with extension) binds directly the microtubule lattice (green, K-MT, kinetochore microtubule) [2, 4, 20, 21], while the internal loop (dark blue) interacts with Dis1/TOG (red) and indirectly binds the plus end of microtubules (left). Dis1 ensures stability of kinetochore microtubules, thereby promoting proper spatial attachment between the kinetochore and the spindle microtubule. In the absence of loop function (ndc80-21 or loop-less Ndc80 mutants) or dis1 deletion (right), the Ndc80 complex is incapable of establishing robust attachment. Under these conditions, spindle microtubules are unstable and the spindle assembly checkpoint is activated.
The molecular functions for the Ndc80 loop remain largely unknown, though several hypotheses have been proposed [1, 7, 17], including fine-tuning for microtubule capture and a device as a part of the tension-sensing machinery. Here we have shown that the loop is indispensable in vivo and that the loop's essential role lies in its binding to Dis1/TOG. Our results together with previous studies lead us to a model in which Dis1 plays a key role in connecting the Ndc80 complex to the spindle microtubule (Figure 4D). We propose that Ndc80 constitutes two functional microtubule-binding domains, the N-terminal domain and the internal loop, each of which is essential and acts coordinately to establish proper kinetochore-microtubule attachment. Previous work has shown that the N-terminal region is required for interaction of the Ndc80 complex to the microtubule lattice [3, 20, 21, 25, 26], perhaps a mode of the initial interaction between the kinetochore and the spindle microtubule in yeasts and vertebrates [27]. We have shown in this study that the internal loop interacts with microtubules indirectly via Dis1/TOG. Importantly Dis1/TOG localizes to the kinetochore and the plus end of the microtubule and promotes microtubule assembly with microtubule polymerase activities [22]. Ndc80-Dis1/TOG interaction thus not only helps secure a physical association between the kinetochore and the spindle microtubule but also could alter microtubule dynamics at the kinetochore site (Figure 4D). Thus, our finding may account for, at least in part, a long-standing question as to how kinetochores regulate microtubule dynamics [28, 29] and furthermore supports the recent report showing that tension applied to kinetochore particles in vitro stabilizes an interaction with microtubules, which favors polymerization [30].
In budding yeast, Tanaka and colleagues have found that the Dam1 complex and the Ndc80 loop interact, albeit probably not directly, which is a key step to establish end-on attachment in this yeast ([31], this issue). Although kinetochore localization of Stu2, the budding yeast Dis1/TOG homolog, requires Spc24 [32], whether or not Stu2 plays any roles in Ndc80-Dam1 complex-dependent end-on attachment remains to be established [31]. We addressed whether fission yeast Dam1 [33, 34] is involved in Ndc80 loop-dependent spindle-kinetochore attachment. As far as we could tell, Dam1, unlike Dis1, did not bind Ndc80, nor did it play a direct role in this process (see Figures S4C–S4F for data and further discussions). It is, therefore, possible that fission yeast and budding yeast have divergently evolved, by which these two yeasts utilize different microtubule/kinetochore-associated factors in the process of Ndc80-loop-dependent bipolar attachment. Further work including analyses in higher systems is necessary to address evolutionary conservation of roles for Ndc80 and Dis1/TOG in microtubule dynamics and spindle-kinetochore attachment.
Experimental Procedures
Yeast Genetics, Strains, and General Methodologies
Strains used in this study are listed in Table S1. Standard methods for yeast genetics and molecular biology were used [35, 36]. Plasmids used in this study are provided in Table S2.
Acknowledgments
We thank Fred Chang, Usula Fleig, Silke Hauf, Yasushi Hiraoka, Jonathan Millar, Kayoko Tanaka and Yoshinori Watanabe, Ayumu Yamamoto, and Mitsuhiro Yanagida for the gift of reagents used in this study and Martin Singleton's laboratory (Macromolecular Structure and Function Laboratory, Cancer Research UK, London Research Institute) for help. We are grateful to Nicola O'Reilly and the Peptide Synthesis unit for preparations of the peptide arrays. We thank Tomoyuki Tanaka for informing us of his results on budding yeast Ndc80 and Dam1 prior to publication. We are grateful to Martin Singleton for critical reading of the manuscript and stimulating discussions. This work was supported by Cancer Research UK (T.T.).
Published online: January 20, 2011
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, two tables, and four movies and can be found with this article online at doi:10.1016/j.cub.2010.12.048.
Supplemental Information
Fluorescent proteins used are Msi6-2mRFP (kinetochore) and GFP-Atb2 (microtubule). Anaphase B starts within 10 min (see Figure 1A for time scale). Movie corresponds to Figure 1A, top three rows.
Fluorescent proteins used are Msi6-2mRFP (kinetochore) and GFP-Atb2 (microtubule). Note a prolonged mitotic stage (>50 min) with unstable spindle microtubules. Movie corresponds to Figure 1A, bottom three rows.
Centromere on chromosome II are visualized with GFP (cen2-GFP) and SPBs are marked with Sad1-dsRed. cen2-GFP splits into two at around 8 min (see Figure 1B for time scale). Movie corresponds to Figure 1B, top.
Centromere on chromosome II is visualized with GFP (cen2-GFP) and SPBs are marked with Sad1-dsRed. Note that a pair of cen2-GFP signals locates in close proximity to one of the SPBs (top). Movie corresponds to Figure 1B, bottom.
References
- 1.Wan X., O'Quinn R.P., Pierce H.L., Joglekar A.P., Gall W.E., DeLuca J.G., Carroll C.W., Liu S.T., Yen T.J., McEwen B.F. Protein architecture of the human kinetochore microtubule attachment site. Cell. 2009;137:672–684. doi: 10.1016/j.cell.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheeseman I.M., Chappie J.S., Wilson-Kubalek E.M., Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 3.Wei R.R., Al-Bassam J., Harrison S.C. The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat. Struct. Mol. Biol. 2007;14:54–59. doi: 10.1038/nsmb1186. [DOI] [PubMed] [Google Scholar]
- 4.Powers A.F., Franck A.D., Gestaut D.R., Cooper J., Gracyzk B., Wei R.R., Wordeman L., Davis T.N., Asbury C.L. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell. 2009;136:865–875. doi: 10.1016/j.cell.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEwen B.F., Dong Y. Contrasting models for kinetochore microtubule attachment in mammalian cells. Cell. Mol. Life Sci. 2010;67:2163–2172. doi: 10.1007/s00018-010-0322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciferri C., Pasqualato S., Screpanti E., Varetti G., Santaguida S., Dos Reis G., Maiolica A., Polka J., De Luca J.G., De Wulf P. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell. 2008;133:427–439. doi: 10.1016/j.cell.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H.W., Long S., Ciferri C., Westermann S., Drubin D., Barnes G., Nogales E. Architecture and flexibility of the yeast Ndc80 kinetochore complex. J. Mol. Biol. 2008;383:894–903. doi: 10.1016/j.jmb.2008.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohkura H., Garcia M.A., Toda T. Dis1/TOG universal microtubule adaptors-one MAP for all? J. Cell Sci. 2001;114:3805–3812. doi: 10.1242/jcs.114.21.3805. [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita K., Habermann B., Hyman A.A. XMAP215: A key component of the dynamic microtubule cytoskeleton. Trends Cell Biol. 2002;12:267–273. doi: 10.1016/s0962-8924(02)02295-x. [DOI] [PubMed] [Google Scholar]
- 10.Howard J., Hyman A.A. Microtubule polymerases and depolymerases. Curr. Opin. Cell Biol. 2007;19:31–35. doi: 10.1016/j.ceb.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Nabeshima K., Nakagawa T., Straight A.F., Murray A., Chikashige Y., Yamashita Y.M., Hiraoka Y., Yanagida M. Dynamics of centromeres during metaphase-anaphase transition in fission yeast: Dis1 is implicated in force balance in metaphase bipolar spindle. Mol. Biol. Cell. 1998;9:3211–3225. doi: 10.1091/mbc.9.11.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musacchio A., Salmon E.D. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 13.Hauf S., Biswas A., Langegger M., Kawashima S.A., Tsukahara T., Watanabe Y. Aurora controls sister kinetochore mono-orientation and homolog bi-orientation in meiosis-I. EMBO J. 2007;26:4475–4486. doi: 10.1038/sj.emboj.7601880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawashima S.A., Tsukahara T., Langegger M., Hauf S., Kitajima T.S., Watanabe Y. Shugoshin enables tension-generating attachment of kinetochores by loading Aurora to centromeres. Genes Dev. 2007;21:420–435. doi: 10.1101/gad.1497307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen J., Hagan I.M. S. pombe aurora kinase/survivin is required for chromosome condensation and the spindle checkpoint attachment response. Curr. Biol. 2003;13:590–597. doi: 10.1016/s0960-9822(03)00205-7. [DOI] [PubMed] [Google Scholar]
- 16.Vanoosthuyse V., Hardwick K.G. A novel protein phosphatase 1-dependent spindle checkpoint silencing mechanism. Curr. Biol. 2009;19:1176–1181. doi: 10.1016/j.cub.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joglekar A.P., Bloom K., Salmon E.D. In vivo protein architecture of the ekaryotic kinetochore with nanometer scale accuracy. Curr. Biol. 2009;19:694–699. doi: 10.1016/j.cub.2009.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciferri C., De Luca J., Monzani S., Ferrari K.J., Ristic D., Wyman C., Stark H., Kilmartin J., Salmon E.D., Musacchio A. Architecture of the human Ndc80-Hec1 complex, a critical constituent of the outer kinetochore. J. Biol. Chem. 2005;280:29088–29095. doi: 10.1074/jbc.M504070200. [DOI] [PubMed] [Google Scholar]
- 19.Wei R.R., Sorger P.K., Harrison S.C. Molecular organization of the Ndc80 complex, an essential kinetochore component. Proc. Natl. Acad. Sci. USA. 2005;102:5363–5367. doi: 10.1073/pnas.0501168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guimaraes G.J., Dong Y., McEwen B.F., Deluca J.G. Kinetochore-microtubule attachment relies on the disordered N-terminal tail domain of Hec1. Curr. Biol. 2008;18:1778–1784. doi: 10.1016/j.cub.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller S.A., Johnson M.L., Stukenberg P.T. Kinetochore attachments require an interaction between unstructured tails on microtubules and Ndc80Hec1. Curr. Biol. 2008;18:1785–1791. doi: 10.1016/j.cub.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouhard G.J., Stear J.H., Noetzel T.L., Al-Bassam J., Kinoshita K., Harrison S.C., Howard J., Hyman A.A. XMAP215 is a processive microtubule polymerase. Cell. 2008;132:79–88. doi: 10.1016/j.cell.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakaseko Y., Goshima G., Morishita J., Yanagida M. M phase-specific kinetochore proteins in fission yeast microtubule-associating Dis1 and Mtc1 display rapid separation and segregation during anaphase. Curr. Biol. 2001;11:537–549. doi: 10.1016/s0960-9822(01)00155-5. [DOI] [PubMed] [Google Scholar]
- 24.Ohkura H., Adachi Y., Kinoshita N., Niwa O., Toda T., Yanagida M. Cold-sensitive and caffeine supersensitive mutants of the Schizosaccharomyces pombe dis genes implicated in sister chromatid separation during mitosis. EMBO J. 1988;7:1465–1473. doi: 10.1002/j.1460-2075.1988.tb02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alushin G.M., Ramey V.H., Pasqualato S., Ball D.A., Grigorieff N., Musacchio A., Nogales E. The Ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature. 2010;467:805–810. doi: 10.1038/nature09423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deluca J.G., Gall W.E., Ciferri C., Cimini D., Musacchio A., Salmon E.D. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka K., Mukae N., Dewar H., van Breugel M., James E.K., Prescott A.R., Antony C., Tanaka T.U. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature. 2005;434:987–994. doi: 10.1038/nature03483. [DOI] [PubMed] [Google Scholar]
- 28.Mitchison T., Evans L., Schulze E., Kirschner M. Sites of microtubule assembly and disassembly in the mitotic spindle. Cell. 1986;45:515–527. doi: 10.1016/0092-8674(86)90283-7. [DOI] [PubMed] [Google Scholar]
- 29.Salmon E.D., Goode D., Maugel T.K., Bonar D.B. Pressure-induced depolymerization of spindle microtubules. III. Differential stability in HeLa cells. J. Cell Biol. 1976;69:443–454. doi: 10.1083/jcb.69.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akiyoshi B., Sarangapani K.K., Powers A.F., Nelson C.R., Reichow S.L., Arellano-Santoyo H., Gonen T., Ranish J.A., Asbury C.L., Biggins S. Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature. 2010;468:576–579. doi: 10.1038/nature09594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maure J.-F., Komoto S., Oku Y., Mino A., Pasqualato S., Natsume K., Clayton L., Musacchio A., Tanaka T.U. The Ndc80 loop region facilitates formation of kinetochore attachment to the dynamic microtubule plus end. Curr. Biol. 2011;21:207–213. doi: 10.1016/j.cub.2010.12.050. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma L., McQueen J., Cuschieri L., Vogel J., Measday V. Spc24 and Stu2 promote spindle integrity when DNA replication is stalled. Mol. Biol. Cell. 2007;18:2805–2816. doi: 10.1091/mbc.E06-09-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez-Perez I., Renwick S.J., Crawley K., Karig I., Buck V., Meadows J.C., Franco-Sanchez A., Fleig U., Toda T., Millar J.B. The DASH complex and Klp5/Klp6 kinesin coordinate bipolar chromosome attachment in fission yeast. EMBO J. 2005;24:2931–2943. doi: 10.1038/sj.emboj.7600761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X., McLeod I., Anderson S., Yates J.R., He X. Molecular analysis of kinetochore architecture in fission yeast. EMBO J. 2005;24:2919–2930. doi: 10.1038/sj.emboj.7600762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreno S., Klar A., Nurse P. Molecular genetic analyses of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:773–782. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 36.Bähler J., Wu J., Longtine M.S., Shah N.G., McKenzie A., III, Steever A.B., Wach A., Philippsen P., Pringle J.R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluorescent proteins used are Msi6-2mRFP (kinetochore) and GFP-Atb2 (microtubule). Anaphase B starts within 10 min (see Figure 1A for time scale). Movie corresponds to Figure 1A, top three rows.
Fluorescent proteins used are Msi6-2mRFP (kinetochore) and GFP-Atb2 (microtubule). Note a prolonged mitotic stage (>50 min) with unstable spindle microtubules. Movie corresponds to Figure 1A, bottom three rows.
Centromere on chromosome II are visualized with GFP (cen2-GFP) and SPBs are marked with Sad1-dsRed. cen2-GFP splits into two at around 8 min (see Figure 1B for time scale). Movie corresponds to Figure 1B, top.
Centromere on chromosome II is visualized with GFP (cen2-GFP) and SPBs are marked with Sad1-dsRed. Note that a pair of cen2-GFP signals locates in close proximity to one of the SPBs (top). Movie corresponds to Figure 1B, bottom.