Abstract
The heart has complex mechanisms that facilitate the maintenance of an oxygen supply–demand balance necessary for its contractile function in response to physiological fluctuations in workload as well as in response to chronic stresses such as hypoxia, ischemia, and overload. Redox-sensitive signaling pathways are centrally involved in many of these homeostatic and stress-response mechanisms. Here, we review the main redox-regulated pathways that are involved in cardiac myocyte excitation–contraction coupling, differentiation, hypertrophy, and stress responses. We discuss specific sources of endogenously generated reactive oxygen species (e.g., mitochondria and NADPH oxidases of the Nox family), the particular pathways and processes that they affect, the role of modulators such as thioredoxin, and the specific molecular mechanisms that are involved—where this knowledge is available. A better understanding of this complex regulatory system may allow the development of more specific therapeutic strategies for heart diseases.
Abbreviations: AIF, apoptosis-inducing factor; ARC, apoptosis repressor with caspase recruitment domain; CamKII, calmodulin kinase II; CTGF, connective tissue growth factor; EB, embryoid body; ECC, excitation–contraction coupling; ER, endoplasmic reticulum; ES, embryonic stem; ETC, electron transport chain; G6PDH, glucose-6-phosphate dehydrogenase; GPCR, G-protein-coupled receptor; HDAC, histone deacetylase; Hif, hypoxia-inducible factor; MAO-A, monoamine oxidase-A; MI, myocardial infarction; MMP, matrix metalloproteinase; MPTP, mitochondrial permeability transition pore; mtDNA, mitochondrial DNA; NCX, Na/Ca exchanger; NOS, nitric oxide synthase; PHD, prolyl hydroxylase dioxygenase; PKA, protein kinase A; PKC, protein kinase C; PKG, protein kinase G; ROS, reactive oxygen species; RyR, ryanodine receptor; SERCA, sarcoplasmic reticulum calcium ATPase; SR, sarcoplasmic reticulum; Trx1, thioredoxin1; TNFα, tumor necrosis factor-α; VEGF, vascular endothelial growth factor
Keywords: Cardiac myocyte, Reactive oxygen species, Redox signaling, Hypertrophy, Heart failure, NADPH oxidase, Mitochondria, Free radicals
Introduction
The heart as a contractile pump has the highest O2 consumption among all body organs and can increase consumption eightfold or more under maximal workload conditions. Complex mechanisms have evolved to facilitate the maintenance of a balance between myocardial O2 supply and O2 consumption during physiological fluctuations in contractile function and workload, during growth and differentiation, and under pathological stresses such as hypoxia, ischemia, pressure, and volume overload. These mechanisms induce changes in cardiomyocyte structure and/or function through coordinated changes in gene and protein expression and/or the activities of various proteins. Redox mechanisms are centrally involved in the signaling pathways underlying many of these homeostatic mechanisms, both via the direct effects of O2 levels in the cardiomyocyte and through the effects of reactive oxygen species (ROS)1 (Fig. 1). From a clinical perspective, much attention has focused on the concept that oxidative stress may be a driver of cardiac disease progression (e.g., heart failure) but clinical interventions with antioxidants have had little or no impact on heart disease risk and progression. Among the reasons for the failure of such interventions, we may include the fact that an isolated focus on oxidative stress as detrimental is far too simplistic and neglects the wider role of ROS, redox balance (and perhaps O2 itself) in integrated cellular signaling and heart metabolism. Here, we review current knowledge on the involvement of ROS as well as O2 in modulating key signaling pathways and physiological and pathophysiological processes in cardiac myocytes. Although we focus on the cardiomyocyte, functional communication and cross talk among the various cardiac cell types (i.e., cardiomyocytes, fibroblasts, endothelial cells, vessels) are also critical for normal heart function and responses to stress [1,2].
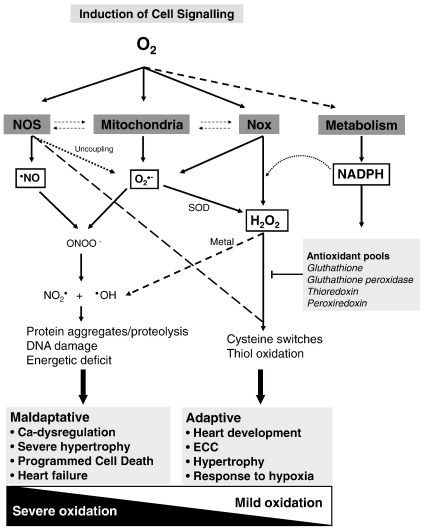
Fig. 1.
Main ROS sources and effects in cardiac myocytes. A diverse range of signals can initiate redox signaling, e.g., GPCR agonists, growth factors, and stresses such as hypoxia. These may result in the induction of and/or changes in ROS generation by sources that include NO synthases (NOS), NADPH oxidases (Nox), and mitochondria. Emerging data support cross talk among the ROS-generating systems. The main species that are generated in the first instance are NO, O2•−, or H2O2, with recent data suggesting that Nox4 may generate predominantly H2O2 rather than O2•−. O2-dependent metabolism also influences redox signaling significantly, notably through changes in the generation of NADPH, which is critical for the maintenance of major antioxidant pools. ROS often act at a molecular level by mediating protein posttranslational modifications within diverse signaling pathways. In general, low-level production of ROS may be involved in potentially adaptive processes—such as adaptation to hypoxia and modulation of excitation–contraction coupling (ECC). On the other hand, the generation of higher levels of ROS and/or more potent oxidants such as •OH may result in significant pathology as a result of macromolecular damage as well as detrimental signaling.
General considerations on redox signaling in cardiomyocytes
The propagation of electrons through coupled transfer partners is a key aspect of basic O2-dependent eukaryotic cell processes such as oxidative phosphorylation, protein folding in the endoplasmic reticulum, the enzymatic activities of many oxygenases, and the generation of reductive power (e.g., NADP+/NADPH; FAD/FADH2) during metabolism. In redox signaling involving O2 or O2-derived reactive species (ROS), the transmission and amplification of signals generally involves posttranslational redox protein modifications. ROS in cardiomyocytes are generated by various sources among which the most important are mitochondria, NADPH oxidases of the Nox family, and nitric oxide synthases (NOSs) (Fig. 1). The quantity of ROS generation by these various sources may increase severalfold under different cellular contexts (e.g., disease) or upon specific stimulation (e.g., for certain NADPH oxidases). Both one-electron oxidants (e.g., the free radicals O2•− and NO) and two-electron oxidants (e.g., the nonradicals H2O2 and ONOO−) are generated, the effects of which are influenced not only by their sites and level of production but also by cell compartment-specific antioxidant pools and by interplay between one- and two-electron species to form more powerful oxidants such as •OH [3,4]. The physiological and pathological roles of •NO generated in cardiac myocytes have been covered in recent reviews [5–7] and are not considered in detail in this article. It should be noted, however, that the inactivation of NO by O2•− may lead to modulation of signaling pathways both as a consequence of ONOO− generation and because of the decrease in NO bioavailability [8].
Even low concentrations of moieties such as •OH (subnanomolar) may cause severe oxidation of macromolecules and organelles and lead to maladaptive cardiac dysfunction, whereas less oxidative ROS (such as O2•−, NO, and H2O2) are commonly involved in cellular signaling that may have an impact on both adaptive and maladaptive cardiac responses [9] (Fig. 1). The concept that oxidative stress resulting from an imbalance between increased ROS generation and inadequate endogenous antioxidant pools contributes to heart failure is well established and this may contribute to the activation of maladaptive signaling cascades, e.g., those leading to impaired calcium (Ca) handling—a fundamental feature of most forms of advanced heart disease. Much less attention has been paid to the idea of cell compartment-specific or “localized” redox signaling, which may have quite distinct and more restricted effects. Moreover, an imbalance between ROS and antioxidants in the opposite direction, i.e., leading to so-called reductive stress, has recently also been suggested to be detrimental in certain cardiac conditions [10]. Such varying effects on cell signaling can be considered within an overall scheme in which O2 and local compartment-specific generation of ROS modulate signaling pathways that can potentially drive adaptive or maladaptive stress responses. Ultimately, the balance between such pathways determines whether the heart adapts or fails in different pathological settings (e.g., cardiac overload or ischemia).
Posttranslational redox modifications to myocardial proteins (such as cysteine and methionine thiol oxidation, proline and arginine hydroxylation, and tyrosine nitration) may affect the conformation, stability, and activity of diverse receptors, ion transporters (pumps/exchangers/channels), kinases, phosphatases, caspases, translocators (GTPases), transcription factors, and structural/contractile proteins. Perhaps the most susceptible redox targets of “signaling” ROS such as H2O2 are protein cysteine thiols, the oxidation of which may result in reversible intra- or intermolecular disulfide formation or other thiol modifications such as nitrosylation and glutathiolation [4,11]. The activities of proteins such as the phosphatases PTP1B and PTEN, caspase-3, and STAT3 are well known to be modulated by such thiol redox switching between reduced and oxidized states [12]. In the heart, examples of redox-modulated protein activities that may be particularly important for cardiomyocyte function include protein kinase A (PKA) [13], protein kinase G (PKG) [14], the ryanodine receptor (RyR) [15], the small G protein Ras [16], class II histone deacetylases (HDACs) [17], and the metabolic enzyme glyceraldehyde-3-phosphate dehydrogenase [18,19]. The balance between oxidized and reduced forms of such signaling proteins is influenced by both local ROS generation and reductants such as glutathione that can reduce a wide range of oxidized proteins. A more specific modulator of redox switching of key signaling proteins in the heart is the protein thioredoxin 1 (Trx1), which catalyzes the reduction of cysteine disulfides and nitrosothiols in selected proteins (e.g., Ras, apoptosis signal regulating kinase-1 (ASK1), and class II HDACs during cardiac hypertrophy) after specific protein–protein interactions [20,21]. Indeed, transgenic mice overexpressing Trx1 specifically in the heart had reduced cardiac hypertrophy after overload stress compared to wild-type mice [22]. Recent proteomic analyses from tissues of mice with cardiac-specific overexpression of Trx1 revealed increased levels of proteins associated with the creatine–phosphocreatine shuttle, the mitochondrial permeability transition pore (MPTP) complex, and the contractile apparatus, suggesting that Trx1 may be involved in the coordination of a wide array of cellular functions for maintaining proper cardiac energy dynamics and facilitating contractile function [23].
Irreversible acute protein oxidation may also modulate signaling, secondary to the proteasomal degradation of the oxidized protein. The best example of this is probably the proline and arginine hydroxylation of the transcription factor hypoxia-inducible factor 1α (Hif1α; see later). More commonly, severe irreversible protein oxidation correlates with the formation of insoluble protease-resistant protein aggregates [24]. Indeed, both aged and failing hearts show increased levels of irreversibly oxidized protein amino acids, e.g., 3-nitrotyrosine [25]. The correlation between protein oxidation and aggregate formation is, however, complex. Increased reductive power was also found to promote the accumulation of protein aggregates in hearts expressing a mutant αB-crystallin, a chaperone that stabilizes cytoskeletal desmin filaments [10]. These results suggest that a fine balance between redox state and metabolism is more important than oxidative stress per se and that an imbalance in either the oxidative or the reductive direction could be detrimental.
Role of heart metabolism
The heart in metazoan organisms exemplifies the major importance of oxygen metabolism for efficient high-energy processes (in particular, contractile function) on the one hand, and the use of oxygen and ROS for modulating complex intracellular signaling cascades on the other. The intracellular organelle content and distribution play a key role in the metabolism and integrated signaling network of cardiomyocytes [26,27]. Mitochondria occupy 30% of cardiomyocyte volume and are organized in ordered rows regularly spaced between myofilaments, closely related to the sarcoplasmic reticulum (SR), and also clustered around the nucleus. Such an organization facilitates the functional interplay between mitochondrial ATP production, SR-regulated Ca homeostasis and myofilament-dependent contraction [28–30]. This architecture also favors efficient high energy output through the matching of local ATP generation and consumption.
In contrast to organs such as brain, which depend mainly on glucose metabolism, the adult mammalian heart normally uses lipids as the major fuel, and mitochondria supply over 90% of the total ATP through β-oxidation of plasma fatty acids [31]. During hypoxia, under ischemia or settings of increased cardiac workload, there is a substantial increase in glycolytic ATP generation, which may be cardioprotective during ischemia–reperfusion by ensuring an adequate ATP supply for membrane and SR ion pumps [31,32]. Such a metabolic shift is accompanied by changes in levels and activities of numerous proteins involved in glucose metabolism, among which a major increase in the expression and activity of glucose-6-phosphate dehydrogenase (G6PDH; the rate-limiting enzyme in the pentose phosphate pathway) contributes to the generation of NADPH. Recent studies indicate that a metabolic shift to glycolysis has important implications for redox status in the heart. NADPH is critical for the maintenance of antioxidant defense through the regeneration of reduced pools of glutathione, and G6PDH activity was shown to be of major importance for the maintenance of redox status, Ca homeostasis, and contractile function in cardiomyocytes subjected to oxidative stress [33]. Similarly, the hearts of mice lacking G6PDH have reduced glutathione (GSH) levels and demonstrate impaired contractile function after ischemia–reperfusion [34]. On the other hand, it has been suggested that increased NADPH levels may fuel NADPH oxidase-derived ROS generation in failing hearts, and this could be partially reversed by a G6PDH inhibitor, 6-aminonicotinamide [35]. In this regard, the relative importance of NADPH levels for antioxidant balance secondary to the regeneration of glutathione and thioredoxin versus possible promotion of NADPH-dependent ROS generation (e.g., via NADPH oxidases) remains to be established. The intracellular locations of the various types of NADPH-dependent processes and their integration with cell signaling pathways may also be important. Adding further complexity, an increased activity of the G6PDH–NADPH pathway has also been shown to contribute to detrimental “reductive stress” associated with increased levels of GSH in a transgenic mouse model of cardiomyopathy caused by a mutation in αB-crystallin [10]. In this case, the use of a G6PDH blocker could partially rescue such detrimental effects [10]. Therefore, it may be that an appropriate G6PDH/NADPH/GSH balance is more important than absolute levels of glutathione.
Sources of ROS in cardiomyocytes
Mitochondrial electron transport chain-derived ROS
Mitochondria have long been recognized as one of the most important sources of cellular ROS, which can increase severalfold depending upon the context [36,37]. ROS are thought to be generated mainly at complexes I and III of the electron transport chain (ETC) through “leakage” during respiration [36,37]. It is important to remember that mitochondrial ROS levels are influenced not only by the ROS generation rate but also by ROS-scavenging systems. In this regard, there is close linkage and cross talk between the redox couples involved in substrate oxidation and the ETC (i.e., NADH/NAD+) and those involved in antioxidant defense through NADPH-regenerating reactions that maintain reduced pools of glutathione, glutaredoxin, and thioredoxin, i.e., NADPH/NADP+ [38]. The precise relationship between electron transport chain flux, ROS generation, and the mitochondrial membrane potential and the mechanisms underlying increases in mitochondrial ROS remain controversial [38]. It has been suggested that in settings in which the mitochondrial redox potential is significantly reduced (e.g., hypoxia), low electron flow is accompanied by increased ROS generation. On the other hand, ROS levels may also be higher in settings in which the mitochondrial redox potential is highly oxidized, e.g., during increased workload in failing hearts. In this case, the increase in ROS is related more to a depletion of the antioxidant capacity as a result of decreased NADPH levels than an increase in ROS production per se [38]. Recently, it was demonstrated that the latter mechanism may be modulated by changes in ionic homeostasis in failing hearts. Kohlhaas et al. [39] found that an elevated intracellular [Na+], which leads to a reduction in mitochondrial Ca, caused a decrease in NADPH levels during increased workload and a consequent increase in ROS levels. The reduction in NADPH was attributed to a decreased activity of Ca-dependent Krebs-cycle dehydrogenases and presumably led to reduced regeneration of antioxidant pools.
Mitochondrial ROS generation may have beneficial or detrimental effects. For example, ROS generation during cardiac ischemic preconditioning may be beneficial, as discussed later in this article. In contrast, increased mitochondrial ROS generation in failing hearts [40] may induce damage to mitochondrial DNA (mtDNA), e.g., after myocardial infarction (MI) [41] or in the hypertrophied heart [42]. mtDNA is thought to be more susceptible to oxidative damage than nuclear DNA because of the proximity of ROS generation and the lack of protective histone. Mitochondrial ROS generation during ischemia–reperfusion can also trigger the MPT and lead to a burst of further ROS release—a phenomenon termed mitochondrial ROS-induced ROS release [43]. This is not only capable of depolarizing individual mitochondria but also can propagate among long chains of mitochondria and perhaps cells, thus amplifying cell injury [43,44]. Interestingly, recent studies have suggested that a transient opening of the MPTP in quiescent unstressed cells can functionally couple to ETC-dependent ROS production and lead to so-called “superoxide flashes” [45]. Although such flashes were especially evident during reoxygenation after anoxia in cardiomyocytes, the authors also suggested that basally produced superoxide flashes could potentially be involved in modulating local signaling [45].
Significant evidence for a pathological role of increased mitochondrial ROS in heart disease comes from studies in gene-modified mice in which mitochondrial antioxidant levels have been perturbed. Mice with deletion of mitochondrial thioredoxin reductase 2 are embryonically lethal because of impaired hematopoiesis and impaired cardiac function [46], whereas mice with complete deletion of mitochondrial Mn superoxide dismutase develop severe fatal dilated cardiomyopathy [47]. In contrast, transgenic mice overexpressing mitochondrial peroxiredoxin III [48] or glutathione peroxidase [49] demonstrate a significant attenuation of adverse left ventricular remodeling post-MI. Similarly, mice with a mitochondrial-targeted overexpression of catalase demonstrate a prolonged life span with improved cardiac function [50] and an attenuation of cardiac aging [51].
NADPH oxidase-derived ROS
The Nox family NADPH oxidases generate ROS (O2•− and H2O2) through electron transfer from NADPH to molecular O2. Each of the seven oxidase family members is based on a distinct catalytic subunit (i.e., Nox1–5 and Duox1 and 2) and has differing requirements for additional protein subunits [52–57]. The prototypic member of the Nox family, Nox2 oxidase (aka gp91phox oxidase) is best known for its role in neutrophil phagocytosis and the fact that genetic defects in the enzyme result in chronic granulomatous disease, a condition in which affected children suffer from recurrent severe fungal and bacterial infections due to defective phagocyte function [52,53]. However, Nox2 is also expressed in many other cell types and Nox family oxidases as a whole are very widely expressed in a tissue-specific manner. Detailed reviews on the structure, function, and roles of these fascinating enzymes have been published recently [52–55]. Apart from the roles of some of the Nox's (e.g., phagocyte Nox2 and epithelial Duox's) in host defense, a large number of studies have demonstrated that these enzymes generate ROS that are involved in redox signaling [9,55–57]. In many cases, Nox-dependent redox signaling involves the regulated and spatially restricted production of low levels of ROS in the vicinity of target proteins, and such effects have been implicated in signaling pathways that regulate processes such as cell differentiation, proliferation, survival, senescence, and migration [9,55–57].
The two Nox isoforms for which there are good data on expression and functional effects in cardiac myocytes are Nox2 [58–61] and Nox4 [62–65] (Fig. 2). Each of these isoforms exists as a heterodimer with a lower molecular weight p22phox subunit and is predicted to be membrane-bound but there are several major differences between the isoforms. Nox2 is normally quiescent and is acutely activated by stimuli such as G-protein-coupled receptor (GPCR) agonists (e.g., angiotensin II, endothelin-1), growth factors, and cytokines in a tightly regulated process in which cytosolic subunits (p47phox, p67phox, p40phox, and Rac1) associate with the Nox2–p22phox heterodimer to initiate enzyme activity [52–54]. In contrast, Nox4 does not have a requirement for additional regulatory subunits, has constitutive low-level activity, and seems to be regulated largely by changes in abundance [52–55]. Therefore, Nox4 may be regarded as an inducible isoform. Additionally, recent independent studies from several groups suggest that, in contrast to Nox2, Nox4 may generate predominantly H2O2 rather than O2•− [65–69], although it should be noted that some papers have reported O2•− generation [70], possibly related to experimental conditions and methodological issues. Finally, it is clear that the intracellular locations of the two isoforms in cardiomyocytes are distinct. There is a broad consensus that activated Nox2 is found predominantly on the plasma membrane, whereas Nox4 is found intracellularly [9,55–57,60,66–68]. Several groups, including our own, have found Nox4 to be in an endoplasmic reticulum (ER)-related perinuclear location [65] but others reported that it may also be present in the mitochondria [64].
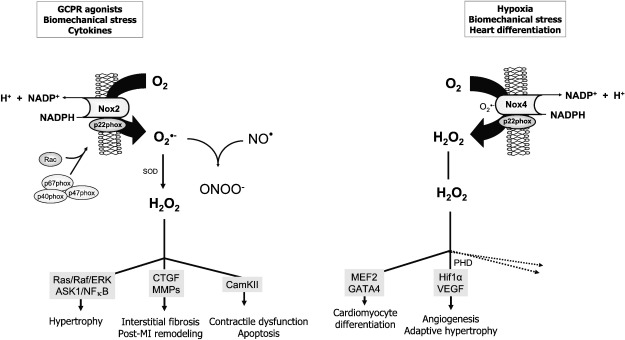
Fig. 2.
Regulation and downstream effects of Nox2 and Nox4 in cardiac myocytes. Nox2 is normally quiescent and is activated through the binding of cytosolic regulatory components to generate O2•−, which may react with NO or be converted to H2O2. Nox4 on the other hand is constitutively active but its expression level is increased by stresses such as hypoxia or during development. Recent studies suggest that Nox4 generates predominantly H2O2. The downstream effects of the two Nox isoforms are divergent, with Nox2 generally driving detrimental effects, whereas Nox4 may facilitate adaptive processes such as angiogenesis. Abbreviations as in the text.
Nox2 may be activated by several different cardiac stresses and an increasing body of data suggests important pathophysiological roles for this isoform in cardiac disease [9]. Previous work from our laboratory and others has shown that Nox2 contributes to the development of angiotensin II-induced left ventricular hypertrophy; to contractile dysfunction, interstitial fibrosis, and apoptosis in the pressure-overloaded or remodeling heart; and to ischemic preconditioning [9,59,62,71]. Although the detailed mechanisms responsible for these effects still remain to be defined, it is clear that Nox-derived ROS modulate several signaling pathways in cardiomyocytes, as are discussed further later (Fig. 2). Much less has been known about the role of Nox4 in the heart until recently, apart from it being suggested to be involved in cardiomyogenesis [63,72]. An emerging literature from studies in other tissues suggests that Nox4 expression increases during stresses such as hypoxia [73], ER stress [74,75], and mitochondrial dysfunction [76,77], and we have recently found that myocardial Nox4 expression also increases during hypoxia, myocardial ischemia, or in vivo pressure overload [62,65,71]. Furthermore, our studies demonstrate an unanticipated protective role of this isoform during the cardiac response to chronic pressure overload [65], as discussed further later (Fig. 2).
Uncoupled NO synthases
Cardiomyocytes constitutively express both neuronal and endothelial NOS isoforms (nNOS and eNOS, respectively), whereas inducible NOS (iNOS) may be expressed in various pathological situations. NO generated by nNOS and eNOS has important physiological effects on myocardial contractile function [5,6]. Although we do not discuss NO-mediated signaling in detail in this review, it should be noted that NO-dependent nitrosylation of cysteine residues in proteins may modulate signal transduction cascades in a manner analogous to the effects of cysteine oxidation [78]. NOSs, however, can also become “uncoupled” and generate O2•− in settings in which there is a deficiency of the essential NOS cofactor tetrahydrobiopterin (BH4) [79]. Because BH4 is degraded by ROS, including those derived from Nox's [80], ROS production by uncoupled NOSs can act as an amplifying mechanism for both the deleterious and the signaling effects of ROS. The concomitant production of NO and O2•− is generally associated with nitroxidative stress and protein nitration via ONOO− or heme peroxidase-dependent nitration [3,25]. 3-Nitrotyrosine provides a useful marker for NO-associated stress in various diseases [81] and increased levels are found in failing hearts, notably in proteins such as myofibrillar creatine kinase, α-actinin, and sarcoplasmic reticulum Ca2+ ATPase [25].
Other sources of ROS
Xanthine oxidoreductase exists in two forms, xanthine dehydrogenase and xanthine oxidase (XO), which are interconvertible [82]. Only XO generates ROS, producing more O2•− or H2O2 depending on the environmental conditions, e.g., substantially more H2O2 than O2•− during hypoxia [83]. Upregulation of XO is reported in experimental models of heart failure and in human end-stage failing myocardium and its inhibition is reported to enhance mechanical efficiency and improve cardiac remodeling [84,85]. Interestingly, the activity of XO is also reported to be linked to that of nNOS such that XO-derived ROS production is enhanced when nNOS is deficient [85,86].
Apart from the ETC, there may be other sources of ROS in mitochondria, for example, enzyme systems such as 2-oxoglutarate, pyruvate dehydrogenase, and flavoprotein acyl-CoA dehydrogenase [36,87]. Recently, mitochondrial monoamine oxidase-A (MAO-A) enzymes were reported to be important ROS sources through the catabolism of prohypertrophic neurotransmitters such as norepinephrine and serotonin, resulting in the generation of H2O2. In mice with pressure overload-induced heart failure, either an MAO-A inhibitor, clorgyline, or a dominant-negative MAO-A reduced oxidative stress, inhibited contractile dysfunction, and inhibited matrix metalloproteinase and caspase-3 activation [88].
Cardiomyocyte differentiation and proliferation
Adult cardiomyocytes are terminally differentiated cells with absent or extremely limited proliferative capacity and therefore differentiation and proliferation are processes that are pertinent mainly to the developing heart. The molecular mechanisms that underlie early cardiomyocyte differentiation have largely been studied in vitro, often in cultured embryonic stem (ES) cell systems. ES cells can be induced to differentiate in vitro into embryoid bodies (EBs) comprising many different cell types, including cardiomyocytes, which can be seen to “beat” in culture. An increase in the proportion of beating cardiomyocytes in such cultures is commonly taken as a measure of increased cardiotypic differentiation but it should be noted that an increase in proliferative capacity of a cardiac progenitor resulting in increased numbers of cardiomyocytes would have a similar effect.
The overall cellular and compartment-specific redox balance is a critical regulator of differentiation and proliferation in many cell types [89,90], including cardiomyocytes [91–93]. Both mechanical strain and electrical stimulation, which increase the levels of intracellular ROS, also increase the proportion of beating cardiomyocytes within an EB population [72,94,95]. By contrast, agents that scavenge or reduce ROS levels (e.g., Trolox, pyrrolidine dithiocarbamate, catalase, and N-acetylcysteine) impair cardiomyocyte formation in EBs [63,94]. Intriguingly, however, the incubation of ES cells with H2O2 either enhances or impairs the cardiomyogenic program, dependent upon the concentration and timing during differentiation—suggesting both a dose-dependency and a cellular competency in the response to ROS [63,92,96].
It has been suggested that an important source of the ROS involved in cardiomyogenesis is Nox4 NADPH oxidase, which is abundantly expressed in ES cells. Li et al. [63] showed that small interfering RNA (siRNA)-mediated downregulation of Nox4 reduced the potential of ES cells to express cardiac-specific markers and form beating EBs, an effect that was reversed by a pulse of low-dose exogenous H2O2. The activation of phosphatidylinositol 3-kinase seems to be required upstream of ROS generation [91,97] and the activation of PPARα has also been implicated [98]. Nox2 is also expressed in ES cells but the overexpression of a dominant-negative Rac mutant that inhibits Nox2 activation did not alter the ability to form beating EBs [96], suggesting that Nox2- and Nox4-derived ROS are functionally distinct during early cardiomyogenesis. In Xenopus laevis embryos, the mitochondrial respiratory chain was found to play an essential role in heart formation through Ca-dependent activation of the transcription factor NFAT [99]. In that study, embryos in which either of two subunits of the respiratory chain, GRIM-19 or NDUFS3, was knocked down showed severe defects in heart formation that could be rescued by a constitutively active form of mouse NFATc4. Although the signaling pathway between mitochondria and NFAT was not established in detail, other studies have shown that mitochondrial respiration and aerobic energy production are dispensable during very early cardiomyocyte differentiation [100], suggesting that other mitochondrial respiratory chain-dependent mechanisms such as ROS production may be involved.
The molecular mechanisms involved in redox regulation of cardiomyocyte differentiation remain poorly understood and deserve further study. In cardiotrophin-1-treated EBs, a ROS-dependent activation of Jak-2, STAT3, and ERK1/2 and the nuclear translocation of NF-κB were reported [97]. Also, siRNA-mediated downregulation of Nox4 inhibited the activation of p38MAPK in ES cells during cardiotypic differentiation [63]. Moreover, the specific inhibition of p38MAPK signaling resulted in reduced cardiomyogenesis and abnormal myofibrillogenesis of cardiac-committed cells together with an impaired nuclear translocation of the cardiac-restricted transcription factor MEF2C. In addition to changes in ROS production, alterations in antioxidant balance may also be involved in regulating cardiomyocyte differentiation and proliferation. For example, in adult cardiac stem cells, the redox effector protein-1 seems to regulate ROS-dependent increases in cardiac differentiation markers [93].
Excitation–contraction coupling
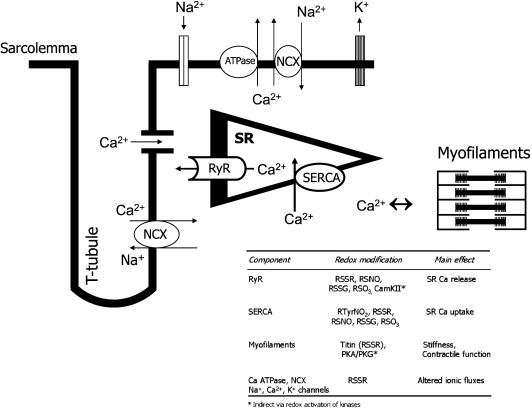
The process of excitation–contraction coupling (ECC) is essential for cardiomyocyte contractile activity and becomes dysregulated in cardiac conditions such as heart failure [101]. During ECC, depolarization of the sarcolemmal membrane results in the opening of voltage-gated L-type Ca channels and subsequent Ca-induced Ca release from the SR through the opening of RyR Ca-release channels (Fig. 3). The binding of Ca to troponin C initiates actomyosin cross-bridge cycling and contraction. During relaxation, Ca is actively taken back into the SR via the SR Ca ATPase (SERCA). In addition, other sarcolemmal ion pumps and exchangers (e.g., the Na/Ca exchanger) and ion fluxes between the cytoplasm and the mitochondria also contribute to Ca homeostasis. The control of SR Ca release and uptake is regulated by a specialized molecular machinery comprising the RyR and SERCA as well as several accessory proteins, including phospholamban, FKBP12.6, calsequestrin, triadin, and junctin. Appropriate regulation of Ca cycling is essential for normal contractile function and is a major site of potential pathologic failure, contributing not only to contractile dysfunction but also to arrhythmia, energetic dysfunction, and mitochondrial ROS production [101–104]. Redox signaling involving the oxidative modification of target proteins can affect several components of the cardiomyocyte ECC machinery [105–107] (Fig. 3).
Fig. 3.
Components of the excitation–contraction coupling machinery in cardiomyocytes and the main redox-sensitive targets. The table indicates the main redox-sensitive targets and types of protein modification, i.e., RSSR, disulfide bond; RSNO, thiol nitrosylation; RSSG, thiol glutathiolation; RSO3, sulfonic acid; PTyrNO2, tyrosine nitration. In the case of redox-activated protein kinases, the modification is protein phosphorylation.
Redox modulation of RyR
The cardiac RyR (RyR2) has around 90 cysteine residues per subunit of which approximately 20 are in a reduced state and potential targets for various redox modifications, including S-nitrosylation, S-glutathionylation, and disulfide crosslinking [15]. Many in vitro studies and experiments in synthetic lipid bilayers have convincingly demonstrated that moieties such as NO, H2O2, nitroxyl (HNO), and hydroxyl radicals induce redox-dependent alterations in RyR activity [106–109]. The functional consequences of such modification are likely to depend upon the nature, concentration, and exposure time of the oxidizing agent; the type of chemical modification; and the specific cysteine residues that are affected. In skeletal muscle RyR (RyR1), it has been shown that although some cysteines are nonspecific targets for various redox agents, others are exclusively S-nitrosylated (Cys1040, Cys1303) or S-glutathionylated (Cys1591, Cys3193) [110]. In general, the RyR2 open probability and therefore SR Ca release is increased by oxidizing conditions, whereas reducing reagents such as dithiothreitol reverse these effects [106,110,111]. However, prolonged exposure to high doses of oxidizing agents may cause irreversible and detrimental RyR activation [111,112].
Although redox modification of RyR activity is well established, its physiological relevance and role in relation to endogenously generated oxidants remains incompletely understood. Significant evidence suggests that RyR2 may be modulated by NO derived from both eNOS (under conditions of myocardial stretch) and nNOS [106,113]. Indeed, nNOS coimmunoprecipitates with RyR2 in the heart [114] and eNOS, which is normally found in sarcolemmal caveolae, can translocate to the SR in failing hearts [115,116]. The effects of NO may depend upon the level of PKA activation [113]. It has not yet been definitively established, however, whether these effects of NO involve redox modification of RyR2. Additional redox-sensitive modulation may arise from the inactivation of NO by endogenously generated O2•−. For example, nNOS is reported to colocalize with xanthine oxidase in the heart so that interaction between xanthine oxidase-derived ROS and nNOS-derived NO may be relevant [86,117]. In addition, cardiac SR is reported to have NADH oxidase activity, which influences SR Ca release, although the nature of the oxidase was not established in that study [118]. Hidalgo and colleagues reported the presence of a Nox2 oxidase (which utilizes NADPH rather than NADH) in cardiac and skeletal muscle SR and found a stimulation of RyR Ca release via S-glutathionylation [119,120], in the former case in response to tachycardia. The functional effect of a transient increase in RyR open probability in the physiological setting has been suggested to be an augmentation of contractile force by increasing Ca transient amplitude, but it is also argued that isolated transient RyR2 activation will have no sustained effect on the Ca transient or contraction, at least in isolated cells [121]. Recently, the positive inotropic effects of nitroxyl donors (or HNO, the one-electron reduction product of NO) in the form of Angeli's salt were reported to involve redox-dependent enhancement of both RyR Ca release and SR Ca uptake [122]—exemplifying the concept that effects additional to RyR Ca release may be required to induce changes in contractile function.
Growing evidence suggests that redox modification of RyR2 may contribute to abnormal Ca handling in disease states. RyR2 dysfunction with an increase in diastolic Ca leak from the SR may reduce SR Ca content and the Ca transient and thereby contribute to a reduced contractile force in the failing heart as well as an increased likelihood of arrhythmia [123,124]. Proposed mechanisms underlying such abnormal RyR2 behavior include a hyperphosphorylation of RyR2 by PKA [123] or calmodulin kinase II (CamKII) [125], or redox modification of RyR2 [126,127]. Interestingly, myocardial CamKII activation has been found to be enhanced by ROS independent of Ca (discussed further under myocyte apoptosis) [128], raising the possibility that multiple redox mechanisms could contribute to RyR2 dysfunction. Recently, it was also suggested that diastolic Ca leak and impaired contraction in cardiomyocytes from rats with spontaneous hypertensive heart failure was attributable not only to increased RyR2 oxidation by xanthine oxidase-derived O2•− but also to a reduced RyR2 nitrosylation due to less bioavailable NO [129]. Several studies in experimental models of heart failure have provided support for a redox-dependent mechanism for RyR2 dysregulation, including canine tachypacing models [130,131], sudden cardiac death models [132], and a model of muscular dystrophy [133].
Redox modulation of SERCA
It has been known for many years that SERCA may be posttranslationally modified by cysteine oxidation or tyrosine nitration, and several of the specific residues that can be oxidatively modified have been mapped by mass spectrometry studies in noncardiomyocyte systems [134–137]. These include nitration of Tyr294 and Tyr295 [136], S-glutathiolation of Cys669 and Cys674 [135], and the oxidation of several other cysteine residues [137]. It is suggested that low levels of ROS may reversibly increase SERCA activity, whereas higher levels may cause inactivation as a result of irreversible oxidative modifications [134,137]. In skeletal muscle, the age-dependent decrease in SERCA activity was suggested to be related to such oxidation of cysteine residues [137]. In vascular smooth muscle, SERCA2b (the dominant isoform in this tissue) has been shown to be redox-activated via a peroxynitrite-mediated reversible S-glutathiolation at Cys674, resulting in a reduced intracellular Ca level and vasorelaxation [135]. This mechanism was suggested to play an important role in NO-dependent cyclic GMP-independent vasorelaxation. Furthermore, the irreversible oxidation of this residue rendered SERCA unresponsive to NO-dependent regulation. In platelets, there is evidence for the modification of SERCA activity by H2O2 [138].
Although the above studies strongly support redox regulation of SERCA as a biologically important mechanism, information on the redox regulation of the major SERCA isoform expressed in cardiac myocytes—i.e., SERCA2a—and the functional consequences of such modification is still quite limited. Tocchetti et al. [122] reported that the positive inotropic effects of HNO involved an increase in SR Ca uptake, and Lancel et al. [139] subsequently demonstrated that the mechanism of this effect is an HNO-mediated reversible S-glutathiolation of Cys674. The same authors have recently reported that contractile dysfunction in transgenic mice overexpressing Gαq subunits (an established model of heart failure that involves increased GPCR signaling) is attributable, at least in part, to a ROS-dependent increase in SERCA2a oxidation [140]. Irreversible oxidative modifications comprising sulfonylation at Cys674 and nitration at Tyr294/295 together with a reduction in maximal SERCA activity were identified, which contributed to marked abnormalities of myocyte Ca transients and contractile function. Crossbreeding the Gαq transgenic mice with animals that had a cardiomyocyte-specific overexpression of catalase reduced both Cys674 sulfonylation and Tyr294/295 nitration, restored SERCA activity, and improved myocyte Ca transients and contraction—suggesting that H2O2 was involved in these effects. The reduced cardiomyocyte Ca transients in the setting of H2O2-induced oxidative stress may involve a decrease in SERCA activity as well as an increase in Na/Ca exchanger activity, which together lead to a reduced SR Ca content [140]. Oxidation of cardiomyocyte SERCA2a associated with a reduction in Ca uptake was also reported in a rodent model of obesity associated with contractile dysfunction [141]. In that study, the authors found similar effects when cardiomyocytes were treated with angiotensin II and proposed that activation of Nox2 may be involved. In the setting of myocardial ischemia–reperfusion, it has been suggested that activation of the polyol pathway (comprising aldose reductase and sorbitol dehydrogenase) contributes to irreversible SERCA2a and RyR oxidation as a result of increased peroxynitrite formation [142]. The treatment of isolated rat hearts subjected to ischemia–reperfusion with polyol pathway inhibitors reduced SERCA Cys674–SO3H (sulfonic) formation and ameliorated postischemic contractile dysfunction. A similar mechanism of contractile dysfunction was also suggested in the setting of hyperglycemia [143].
Redox modulation of myofilament proteins
Direct inhibitory effects of ROS on myofilament function have been recognized for many years although the precise mechanisms that are involved remain unclear [144,145]. Potential sources of such ROS include xanthine oxidase [146,147]. Recently, the passive stiffness of cardiac muscle, which depends to a significant extent on the giant elastic protein titin, was shown to be redox-modulated through disulfide bridge formation in the cardiac-specific N2-B-unique extensible segment [148]. Elevated oxidation increased titin-based stiffness in isolated human heart myofibrils, an effect that could be reversed by thioredoxin.
In addition to direct mechanisms, ROS may indirectly modulate myofilament function through effects on key protein kinases that induce posttranslational phosphorylation of various myofilament proteins such as troponin I and myosin-binding protein C. Type 1 PKA is centrally involved in the positive inotropic actions of β-adrenergic stimulation through effects on the L-type Ca channel, SERCA2a, troponin I, and myosin-binding protein C. Brennan et al. [13] found that ROS-induced formation of an interprotein disulfide bond between its two regulatory RI subunits leads to a subcellular translocation and activation of the kinase. The activated PKA localizes to myofilaments through interaction with the α-myosin heavy chain (which serves as a PKA anchor protein) and phosphorylates troponin I and myosin-binding protein C, leading to an increase in contractile shortening that is independent of β-adrenergic stimulation and cAMP elevation. PKG also modulates contractile function through the phosphorylation of troponin I [149] and it has been found that oxidants activate PKG1α through the formation of an interprotein disulfide linking its two subunits [14]. The latter mechanism was found to contribute to NO-independent vasorelaxation [14] but whether redox activation of PKG1α has physiological effects in cardiac myocytes remains to be established. Redox-dependent protein kinase C (PKC) activation was reported to increase the phosphorylation of troponin I and myosin-binding protein C in cardiac myocytes and thereby to increase the Ca sensitivity of force production [150].
Other ECC targets
Redox modifications may also affect L-type Ca channels, the plasmalemmal Ca ATPase, the Na/Ca exchanger, and other ion channels [106,151]. The pore-forming α1C subunit of the L-type Ca channel contains > 10 cysteine residues, and a modulation of channel activity by thiol oxidizing agents is reported [151]. In isolated cardiomyocytes, endothelin-1 reportedly increases the open probability of L-type Ca channels through a mechanism that involves Nox2 oxidase activation [152]. 20-Hydroxyeicosatetraenoic acid, an eicosanoid that is increased during ischemia–reperfusion, also activated L-type Ca current via a Nox2/PKC-dependent mechanism [153]. The Na/Ca exchanger comprises nine transmembrane domains and intramolecular disulfide bonds between cysteine residues in different domains are thought be functionally important [154]. Na/Ca exchanger activity is reported to be significantly increased by ROS [155–157] but the source of these ROS remains unclear. Angiotensin II-induced NADPH oxidase activation is reported to reduce membrane K+ currents in streptozotocin-induced diabetes in rats [158] and swelling-activated Cl− current in rabbit ventricular myocytes [159]. Nox2-derived ROS may also modulate the expression of ion channels, for example, an increase in the L-type Ca channel α1C subunits [160], a decrease in Scn5a Na+ channel mRNA abundance [161], and the destabilization of cardiac Kv4.3 channel mRNA [162].
Myocardial hypoxia and ischemia–reperfusion
A reduction in oxygen supply (hypoxia) critically affects heart function and is a major driver of pathophysiology in various acute and chronic disease settings, e.g., ischemic heart disease, MI, ischemia–reperfusion, and pressure overload hypertrophy. Prolonged hypoxia or ischemia beyond a critical threshold results in cell death by necrosis and/or apoptosis due to the lack of O2, disruption of Ca and pH homeostasis, mitochondrial injury, increased ROS generation, and oxidative DNA damage. Reperfusion (or reoxygenation) is essential for cardiomyocyte survival but is itself associated with significant cell damage that is mediated at least in part via ROS-dependent mechanisms [163]. As discussed earlier, mitochondrial ROS generation and the opening of the MPTP are thought to be causally involved in acute reperfusion-associated damage [164].
Adaptation to hypoxia
Cardiac myocytes are generally relatively resistant to moderate hypoxia [165]. This is largely related to hypoxia-induced activation of a broad range of adaptive pathways, a process in which the redox-sensitive transcription factor Hif plays a central role [166–169]. Hif1-dependent transcription requires the dimerization of Hif1α (or Hif2α) with Hif1β (also called aryl hydrocarbon receptor nuclear translocator) and the recruitment of coactivators such as CBP/p300. The primary regulator of Hif1 transcriptional activity is the level of Hif1 or 2α, which is very low under normoxic conditions but becomes elevated during hypoxia or in response to certain other stimuli—notably those that generate ROS. In the presence of oxygen, prolyl hydroxylase dioxygenase family enzymes (PHD1–3) hydroxylate Hif-α at conserved proline residues (i.e., Pro402 and Pro564) in a process that requires nonheme Fe(II), ascorbate, and 2-oxoglutarate [168,170]. Hydroxylated Hif-α binds to the von Hippel-Lindau (vHL)–E3 ligase and is targeted for rapid proteasomal degradation. PHD activity is substantially inhibited during hypoxia, leading to increased Hif-α levels and an increase in the transcription of a multitude of Hif-regulated genes—including genes involved in regulating O2 delivery and consumption, glycolysis, glucose metabolism, mitochondrial function, cell survival, antioxidant defense, angiogenesis, and many others. During sustained chronic hypoxia, PHD levels themselves are also upregulated and act as a brake on sustained Hif activation, which is thought to be detrimental [171].
In addition to the direct effects of a reduction in O2 levels, an increase in ROS also results in Hif activation through other mechanisms that increase Hif1α levels. The transcription of both Hif1α and Hif2α is redox-sensitive [166,167], whereas Hif1α translation may also be subject to redox regulation via changes in the activity of mTOR [172]. In addition, PHD activity is inhibited by ROS, potentially through oxidation of the nonheme iron and/or reduction in ascorbate levels [173]. It is worth noting that NO also has similar effects on Hif1α [174]. During hypoxia, both mitochondrial ROS [175,176] and those derived from NADPH oxidases—in particular, Nox4 [65,177]—may contribute to Hif activation. Nox4 levels increase in response to hypoxia in fibroblasts [73] and cardiomyocytes [65], and we have recently reported that a major mechanism underlying cardiomyocyte Hif activation during hypoxia is a Nox4-dependent increase in Hif1α levels secondary to reduced Hif1α hydroxylation at both Pro402 and Pro564 residues [65].
The activation of Hif-regulated genes is likely to contribute to protection during and/or after ischemia through several mechanisms such as an increase in glycolytic capacity, enhanced antioxidant defense, activation of cell survival pathways, and an increase in angiogenesis. Indeed, increased levels of Hif1α and Hif-regulated genes such as heme oxygenase-1 (HO-1), iNOS, and VEGF are found in the peri-infarct zone after MI [178,179]. Mice with a cardiomyocyte-specific deletion of Hif1α demonstrate a reduction in vascularization, contractility, and high-energy phosphate content that is attributable to the altered expression of genes involved in angiogenesis, calcium handling, and glucose metabolism, indicating a major role for Hif1α in coordinating energy availability and utilization in the heart [180]. Transgenic overexpression of HIF1α in the myocardium of mice reduces infarct size and improves cardiac function 4 weeks after MI in association with evidence of increased capillary density in the peri-infarct region [181]. Similarly, the siRNA-mediated silencing of PHD expression in murine hearts in vivo was found to increase HIF1α protein stabilization and to decrease infarct size and cardiac dysfunction after ischemia–reperfusion [182]. These effects seemed to involve Hif1-induced increases in iNOS (which has been shown to be cardioprotective in several studies) because they were lost in iNOS−/− mice [183]. Other studies have implicated increases in HO-1 as a downstream mediator of Hif1-dependent reduction in cardiac ischemia–reperfusion injury [184]. Hif1 activation may also have favorable effects in nonischemic cardiac pathology, such as pressure-overload hypertrophy, which is discussed in a later section. Although the data described above indicate that short-term Hif1 activation is protective after MI, it should be noted that several studies suggest that chronic sustained activation of the Hif1 pathway in ischemic myocardium may be maladaptive. For example, mice with a cardiac-specific deletion of vHL [185], a genetic deletion of PHD2/3 [186,187], or overexpression of an O2-insensitive form of Hif1α [188] all develop severe cardiomyopathy.
Ischemic preconditioning
Transient episodes of myocardial ischemia are protective against cell injury and death caused by subsequent prolonged ischemia, a phenomenon known as ischemic preconditioning [189,190]. In addition to ischemia per se, such cardioprotection can be evoked by a wide variety of stimuli, notably many GPCR agonists. The pathways underlying this potent phenomenon still remain incompletely understood but inhibition of the MPT is considered an important terminal mechanism. Many studies have suggested that redox signaling during the initial preconditioning period may be involved in the eventual cardioprotective effect, at least for some stimuli that evoke preconditioning [191–194]. These signaling ROS can be derived from mitochondria (related to slight mitochondrial swelling and an increase in respiration) [193] or from Nox2 NADPH oxidase [195,196] and are thought to act through the redox activation of PKC, although it is possible that other kinases involved in preconditioning could also be redox-activated. Several studies have suggested that the signaling pathways activated by diverse preconditioning stimuli converge on glycogen synthase kinase-3β (GSK-3β), the phosphorylation and inactivation of which inhibits the MPTP, although the precise molecular target(s) of GSK-3β remains to be established [193,197]. However, some studies have argued against a central role for GSK-3β inhibition in preconditioning (e.g., [198–200]).
Preconditioning triggers not only induce immediate protection against ischemia–reperfusion injury but also provide delayed protection 24 hours or more after the initial trigger—termed delayed or late preconditioning. Several Hif-dependent genes are implicated in late ischemic preconditioning, for example, iNOS [201], cyclooxygenase-2 [202], and HO-1 [203], suggesting that redox signaling may be involved. The protective effect may, in part, involve an upregulation of antioxidants such as HO-1 and other proteins [203].
Cardiac hypertrophy
Chronic increases in cardiac workload result in heart hypertrophy, a situation in which there is a significant increase in cardiomyocyte size that contributes to increased chamber mass and wall thickness. In “physiological” hypertrophy, e.g., with chronic endurance exercise or pregnancy, the increase in size is an adaptation to the increased workload and is associated with the maintenance of normal contractile function and the absence of long-term ill effects. In contrast, persistent “pathological” hypertrophy, e.g., with chronic hypertension or after MI, is associated with a progressive energetic deficit, contractile dysfunction, and eventual heart failure. As well as changes at the cardiomyocyte level, pathological hypertrophy is characterized by significant rarefaction of myocardial capillaries and major changes in the extracellular matrix that lead to fibrosis and ventricular dilatation. Specific signaling pathways are thought to drive adaptive versus maladaptive programs of gene and protein expression that contribute to these abnormalities and the balance between these may determine whether the heart fails [204–206]. Abnormalities in myocyte ECC and tissue hypoxia (both discussed earlier) and the occurrence of myocyte apoptosis (discussed later) are important contributors to the development of heart failure. In this section, we focus on ROS-modulated pathways that promote cardiomyocyte hypertrophy as well as those that are protective in this setting (Fig. 2).
Hypertrophic pathways modulated by endogenous ROS
Numerous experimental studies have reported that exogenous ROS can modulate many signaling pathways known to be involved in cardiomyocyte hypertrophy, such as the activation of ERK1/2, JNK, p38MAPK, Akt, PKCs, and NF-κB [207]. Here we focus on studies that have addressed the involvement of endogenous ROS in hypertrophic signaling pathways, identified the sources of such ROS, and/or provided information on molecular mechanisms involved in such redox signaling.
Cardiomyocyte hypertrophy induced by GPCR agonists such as angiotensin II, α-adrenoceptor agonists, and endothelin-1 has been shown to involve endogenous ROS generation and the activation of ERK1/2 and NF-κB [208–210]. In the case of α-adrenoceptor stimulation, it was demonstrated that the Trx1-sensitive oxidation of specific cysteine thiols on the small G protein Ras is a key molecular mechanism that induces activation of the Ras–Raf–MEK1/2–ERK1/2 signaling pathway leading to cardiomyocyte hypertrophy [211]. Another molecular mechanism that has been elegantly dissected is the Trx1-sensitive oxidation of class II HDACs [17], which are master negative regulators of cardiac hypertrophy that inhibit the transcription of MEF2-dependent genes [204,206]. It was found that Trx1 forms a multiprotein complex with HDAC4 and a heat shock protein, DnaJb5, in cardiomyocytes. During α-adrenoceptor-induced cardiomyocyte hypertrophy, specific cysteine residues in HDAC4 (Cys667, Cys669) and DnaJb5 (Cys274, Cys276) were oxidized to form intramolecular disulfide bonds, leading to the phosphorylation-independent nuclear export of HDAC4 and the induction of hypertrophy [17].
Redox-dependent activation of the MAPKKK ASK1 has been implicated upstream of NF-κB activation in GPCR-induced hypertrophy [210]. Tumor necrosis factor-α (TNFα)-induced cardiomyocyte hypertrophy was also reported to involve ROS-dependent activation of NF-κB [212]. These investigators subsequently showed that Ca-dependent activation of Pyk leads to Rac1 activation [213], which is itself upstream of ASK1 and NF-κB activation [214], raising the possibility that cardiomyocyte Nox2 (which requires Rac1 for its activation) may the ROS source. Indeed, Nox2 has been confirmed to be involved in cultured cardiomyocyte hypertrophy induced by angiotensin II [215,216] and endothelin-1 [217], and Hingtgen et al. [216] provided evidence that Akt activation may also be involved. Further strong evidence for an involvement of Nox2 in angiotensin II-induced cardiac hypertrophy comes from in vivo studies in gene-modified mice. We found that hypertrophy induced by chronic angiotensin II infusion was significantly inhibited in Nox2-null mice independent of changes in blood pressure, in conjunction with a failure to increase myocardial NADPH oxidase activity [59]. Satoh et al. [218] reported that angiotensin II-induced in vivo cardiac hypertrophy was similarly inhibited in mice with a cardiomyocyte-specific deletion of Rac1 in conjunction with a reduction in Nox2 activation. Angiotensin II-induced in vivo cardiac hypertrophy was also inhibited in ASK1-null mice [219].
Redox signaling is also implicated in pressure-overload-induced cardiac hypertrophy [220]. Mechanical strain may be at least one prohypertrophic stimulus during pressure overload and it has been shown that mechanical stress-induced cardiomyocyte hypertrophy may involve Rac1–ROS-dependent pathways that activate ERK1/2 [221] and p38MAPK [222], consistent with the involvement of Nox2. ROS-dependent S-glutathiolation of Ras at Cys118 and consequent activation of the Raf–MEK–ERK pathway were involved in mechanical strain-induced hypertrophy [223], similar to findings in GPCR-induced hypertrophy [211]. The expression and activation of Nox2 do in fact increase during chronic pressure overload [58,224,225], and increases in Nox2 expression and activity have also been demonstrated in myocardial samples from failing human hearts [60,226,227]. Mice with a cardiomyocyte-specific deletion of Rac1 were protected against pressure-overload hypertrophy [218] and Nox2-null mice developed less contractile dysfunction than wild-type littermates when subjected to chronic pressure overload [228], supporting a prohypertrophic role of Nox2. However, the extent of hypertrophy in Nox2-null mice was similar to that in wild-type littermates [62,229,230], suggesting that although Nox2 can exert prohypertrophic effects during chronic pressure overload other pathways may compensate in its absence.
Redox-sensitive protective pathways activated during cardiac hypertrophy
We have recently identified a potent Nox4-dependent protective pathway that is activated during chronic pressure overload [65]. Earlier studies had shown that although the level of Nox4 is low in the healthy adult heart, its expression increases significantly during pressure overload or after myocardial ischemia [62,71]. It was therefore speculated that Nox4 might compensate for Nox2 in the setting of pressure overload hypertrophy. As discussed under Sources of ROS in cardiomyocytes, however, the two Nox isoforms exhibit significant differences in regulation, activation, subcellular location, and nature of ROS generation. Nox4, unlike Nox2, does not require agonist stimulation and is regulated mainly by its expression level [66,67]. It is found in an intracellular perinuclear location [65] in contrast to activated Nox2, which is located at the cardiomyocyte sarcolemmal membrane [60]. Furthermore, Nox4 seems to generate predominantly H2O2 rather than O2•−, in contrast to Nox2 [65–69]. To investigate the role of Nox4 during pressure overload, we generated mouse models with a deletion of Nox4 or a cardiomyocyte-targeted increase in Nox4 expression. Neither model developed significant changes in cardiac hypertrophy or function in the absence of stress, and there were no changes in myocardial Nox2 levels [65]. In response to chronic pressure overload, however, Nox4-null mice developed significantly greater cardiac hypertrophy and contractile dysfunction than control animals, whereas Nox4-transgenic mice exhibited protection against pressure-overload-induced hypertrophy and failure. Investigation of the mechanisms underlying the protective effect of Nox4 revealed that it facilitated the preservation of myocardial capillary density during pressure overload by augmenting stress-induced cardiomyocyte Hif1 activation and the release of VEGF, resulting in increased paracrine angiogenic activity [65]. The main mechanism by which Nox4 regulated cardiomyocyte Hif1 activation was through inhibition of Hif prolyl hydroxylase activity and a subsequent increase in Hif1α protein levels. These data extend the findings from recent studies showing that the extent of myocardial capillarization is a key determinant of functional cardiac compensation during chronic pressure overload [231,232], with Hif1 being a major driver of this process [233]. An insufficient increase in capillary number relative to the increase in cardiomyocyte size during hypertrophy may promote pathological remodeling of the heart with increased hypertrophy, fibrosis, cardiac dilatation, and contractile failure. Whether other Nox4-dependent antihypertrophic pathways are also involved in its beneficial effects requires further investigation. The above data on Nox4 taken together with previous studies on Nox2 indicate that the two isoforms contrast markedly in their effects during pressure-overload-induced cardiac hypertrophy, with Nox2 being detrimental and Nox4 beneficial (Fig. 2). It should be noted that a recent study using different mouse models of Nox4 deletion and overexpression suggested that Nox4 contributed to detrimental O2•− production, which enhanced heart failure in response to pressure overload, although no effects on cardiac hypertrophy were found in that study and Hif1 activation was not studied [70]. The reasons for this discrepancy remain to be elucidated but the finding of substantial Nox4-dependent O2•− production in this study is at odds with recent data from several independent laboratories that Nox4 generates predominantly H2O2 rather than O2•− [65–69,234,235]. It should also be noted that persistent Hif1 activation in the setting of pathological cardiac hypertrophy could be detrimental by promoting maladaptive metabolic changes in glycolytic and lipid anabolic pathways [236].
Myocyte apoptosis
Cardiomyocyte apoptosis is recognized to be important in both ischemia–reperfusion injury and the transition from compensated cardiac hypertrophy to heart failure, at least in part through redox-dependent mechanisms [237,238]. The signaling pathways that regulate apoptosis in cardiomyocytes have been reviewed in detail [237,238] In brief, apoptosis may be activated by extrinsic or intrinsic pathways. The extrinsic pathway is stimulated by factors such as TNFα that cause the formation of a death-inducing signaling complex, which leads to the activation of a cascade of caspases and eventual caspase-3-mediated proteolysis of intracellular proteins and oligonucleosomal DNA cleavage. The intrinsic apoptotic pathway uses mitochondria to evoke cell death through the opening of the MPTP or the permeabilization of the outer membrane, resulting in the release of cytochrome c and other proteins (e.g., apoptosis-inducing factor, AIF) into the cytoplasm. Cytochrome c facilitates the formation of an apoptosome complex with apoptotic protease activating factor-1, dATP, and caspase-9 and leads to caspase-3 activation. Bcl-2 family proteins are key regulators of mitochondrial membrane integrity in apoptosis and comprise proapoptotic (e.g., Bax, Bak) and antiapoptotic proteins (e.g., Bcl-2, Bcl-xL) that are transcriptionally and posttranslationally regulated. BH3-only proteins are additional death effectors that can activate proapoptotic or inactivate antiapoptotic Bcl-2 family members.
The importance of oxidative stress in cardiomyocyte apoptosis is illustrated by the finding that the overexpression of various antioxidant proteins, including catalase [221], glutathione peroxidase 1 [49], metallothionein [239], mitochondrial glutaredoxin-2 [240], and peroxiredoxin II [241], reduces apoptosis and improves contractile dysfunction after ischemia–reperfusion injury in mice in vivo. Oxidative stress activates the intrinsic apoptotic pathway in cardiomyocytes (e.g., during ischemia–reperfusion) through multiple mechanisms, such as the induction of MPTP opening, DNA damage-induced activation of the p53 transcription factor, translocation of Bax and Bad to the mitochondria, and caspase activation [237,238,242]. An alternative mode of oxidative stress-induced activation of the intrinsic apoptotic pathway may involve induction of the ER stress response, leading to caspase-12 activation and/or Ca-dependent opening of the MPTP [238]. During oxidative stress, p53 induces the E3 ubiquitin ligase, MDM2, which promotes the ubiquitination and proteasomal degradation of a key antiapoptotic regulator, ARC (apoptosis repressor with caspase recruitment domain), which is known to interact with Bax and inhibit apoptosis involving the mitochondrial pathway [243]. This seems to be an important pathogenic mechanism based on the findings that overexpression of ARC inhibits oxidant-induced apoptosis in isolated myocytes [244] and that ARC knockout mice develop larger infarcts after ischemia–reperfusion and more rapidly develop pressure-overload-induced heart failure [245]. However, it should be noted that MDM2 may have complex effects because it also promotes the degradation of p53 itself, which may be cardioprotective [246]. AIF also provides protection against oxidative stress-induced apoptosis in cardiomyocytes, and AIF-deficient mice show increased apoptosis and faster progression to heart failure when subjected to chronic pressure overload [247].
GPCR signaling induced by catecholamines, angiotensin II, prostaglandin F2α, or endothelin-1, which induces cardiomyocyte hypertrophy, may also induce apoptosis at higher intensities of GPCR activation. One mechanism that has been quite well characterized is a Gαq-mediated PKC-dependent transcriptional upregulation of the Bcl-2 family member Nix, which activates the mitochondrial death pathway [248]. PKC activation is potentially redox-sensitive but whether this is involved in the upregulation of Nix remains to be established. Cardiomyocyte apoptosis induced by 5-HT was shown to involve MAO-A-dependent ROS generation, which led to upregulation of Bax and downregulation of Bcl-2 and subsequent mitochondrial release of cytochrome c [249]. Other pathways linking GPCR activation and the induction of apoptosis may involve the activation of kinases such as ASK1, JNK, and p38MAPK [250]. In adult rat cardiomyocytes, β-adrenoceptor-induced apoptosis was shown to involve ROS-dependent activation of JNK, which in turn activated the mitochondrial death pathway and cytochrome c release [251]. ASK1 may be redox-activated upstream of p38MAPK and JNK to induce cardiomyocyte apoptosis, and ASK1 activity and ASK1-dependent apoptosis are inhibited by Trx1 and Trx2 [252].
A novel redox mechanism involved in angiotensin II-induced cardiomyocyte apoptosis has recently been identified as the Ca-independent activation of CamKII as a result of the oxidation of methionine residues 281 and 282 in the regulatory domain of the kinase [128]. In vivo oxidative activation of CamKII in the hearts of mice infused with angiotensin II was accompanied by increased apoptosis, which was inhibited in mice overexpressing an inhibitory peptide against CamKII (AC3-I). The authors also provided evidence that the ROS responsible for CamKII activation and apoptotic death via the mitochondrial pathway were derived from Nox2 oxidase activation (Fig. 2), because the effects were abolished in mice lacking p47phox (which is essential for Nox2 activity) [128]. The methionine oxidation of CamKII is reversible by methionine sulfoxide reductase A (MsrA) and mice deficient in MsrA were found to exhibit exaggerated CamKII oxidation, cardiomyocyte apoptosis, cardiac dysfunction, and mortality after myocardial infarction [128]. Interestingly, however, isoproterenol induced activation of CamKII, and induction of apoptosis did not involve ROS. Palomeque et al. [253] also reported a similar angiotensin II-induced ROS-dependent cardiomyocyte apoptotic pathway involving CamKII activation and found that p38MAPK activation was downstream of CamKII in the proapoptotic pathway.
It should be noted that recent studies in various noncardiomyocyte settings have reported that Nox4-derived ROS may inhibit apoptosis, e.g., in pulmonary fibroblasts [73], human glioma cells [254], pancreatic carcinoma cells [255–257], and hepatocytes [258]. The mechanisms involved in Nox4-dependent antiapoptotic effects may include the activation of kinases such as Akt [257] and Jak-2 [259]. Whether such ROS-dependent antiapoptotic effects are relevant in cardiomyocytes remains to be established.
Conclusions
In this article, we have reviewed the main redox-regulated physiological and pathological pathways that are involved in cardiac myocyte ECC, growth, differentiation, hypertrophy, survival, and responses to stresses such as hypoxia, ischemia, and chronically increased workload. The sources of the endogenously generated ROS that are involved in modulating these various pathways are gradually being identified and it is increasingly evident that in many cases, regulatory pathways are source-specific. A number of molecular mechanisms through which ROS modulate specific pathways in cardiomyocytes have been defined (e.g., the redox activation of RyR, PKA, and Ras, or the posttranslational modification of HDAC4) but many others remain to be elucidated. In addition to such direct posttranslational protein modifications mediated by ROS, redox protein recycling systems such as Trx1 and glutaredoxin and the metabolic processes that influence redox status (e.g., through the regeneration of antioxidant pools) are of major importance. The latter are especially sensitive to the cardiomyocyte O2 supply, and the interrelationship between the direct effects of O2 levels and ROS-dependent regulation is also a key aspect of redox signaling in the cardiomyocyte. The data reviewed in this article clearly show that redox signaling is integral to the maintenance of cardiomyocyte homeostasis and is centrally involved in the integrated cellular signaling machinery that underlies cardiac responses to stress. Future work in the field needs to better address several key aspects that include (a) cardiomyocyte compartment-specific redox regulation and signaling in various settings, (b) mechanisms that regulate and integrate intercompartmental signaling, (c) the integration of specific ROS generation with antioxidant balance and metabolic processes (which may be especially important in myocytes), (d) the detrimental and/or protective roles of specific ROS generators, and (e) the identification of key molecular mechanisms that could be therapeutically targeted. A better understanding of this complex regulatory system may allow the development of more specific therapeutic strategies for heart diseases.
Acknowledgments
The authors’ research is supported by the British Heart Foundation (RG/08/011/25922; CH/99001; RE/08/003), a Leducq Foundation Transatlantic Network of Excellence award, EU FP6 Grant LSHM-CT-2005-018833 (EUGeneHeart), and a National Institute for Health Research comprehensive Biomedical Research Centre award to Guy's & St Thomas’ NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust.
References
- 1.Brutsaert D.L. Cardiac endothelial–myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol. Rev. 2003;83:59–115. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
- 2.Kakkar R., Lee R.T. Intramyocardial fibroblast myocyte communication. Circ. Res. 2010;106:47–57. doi: 10.1161/CIRCRESAHA.109.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augusto O., Bonini M.G., Amanso A.M., Linares E., Santos C.C., De Menezes S.L. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radic. Biol. Med. 2002;32:841–859. doi: 10.1016/s0891-5849(02)00786-4. [DOI] [PubMed] [Google Scholar]
- 4.Winterbourn C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 5.Massion P.B., Balligand J.L. Relevance of nitric oxide for myocardial remodeling. Curr. Heart Fail. Rep. 2007;4:18–25. doi: 10.1007/s11897-007-0021-6. [DOI] [PubMed] [Google Scholar]
- 6.Seddon M., Shah A.M., Casadei B. Cardiomyocytes as effectors of nitric oxide signalling. Cardiovasc. Res. 2007;75:315–326. doi: 10.1016/j.cardiores.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 7.Kass D.A., Takimoto E., Nagayama T., Champion H.C. Phosphodiesterase regulation of nitric oxide signaling. Cardiovasc. Res. 2007;75:303–314. doi: 10.1016/j.cardiores.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–324. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cave A.C., Brewer A.C., Narayanapanicker A., Ray R., Grieve D.J., Walker S., Shah A.M. NADPH oxidases in cardiovascular health and disease. Antioxid. Redox Signaling. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 10.Rajasekaran N.S., Connell P., Christians E.S., Yan L.J., Taylor R.P., Orosz A., Zhang X.Q., Stevenson T.J., Peshock R.M., Leopold J.A., Barry W.H., Loscalzo J., Odelberg S.J., Benjamin I.J. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130:427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton P. Protein thiol oxidation in health and disease: techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radic. Biol. Med. 2006;40:1889–1899. doi: 10.1016/j.freeradbiomed.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 12.Forman H.J., Maiorino M., Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan J.P., Bardswell S.C., Burgoyne J.R., Fuller W., Schröder E., Wait R., Begum S., Kentish J.C., Eaton P. Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J. Biol. Chem. 2006;281:21827–21836. doi: 10.1074/jbc.M603952200. [DOI] [PubMed] [Google Scholar]
- 14.Burgoyne J.R., Madhani M., Cuello F., Charles R.L., Brennan J.P., Schröder E., Browning D.D., Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 15.Xu L., Eu J.P., Meissner G., Stamler J.S. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 16.Kuster G.M., Siwik D.A., Pimentel D.R., Colucci W.S. Role of reversible, thioredoxin-sensitive oxidative protein modifications in cardiac myocytes. Antioxid. Redox Signaling. 2006;8:2153–2159. doi: 10.1089/ars.2006.8.2153. [DOI] [PubMed] [Google Scholar]
- 17.Ago T., Liu T., Zhai P., Chen W., Li H., Molkentin J.D., Vatner S.F., Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 18.Eaton P., Byers H.L., Leeds N., Ward M.A., Shattock M.J. Detection, quantitation, purification, and identification of cardiac proteins S-thiolated during ischemia and reperfusion. J. Biol. Chem. 2002;277:9806–98011. doi: 10.1074/jbc.M111454200. [DOI] [PubMed] [Google Scholar]
- 19.Eaton P., Wright N., Hearse D.J., Shattock M.J. Glyceraldehyde phosphate dehydrogenase oxidation during cardiac ischemia and reperfusion. J. Mol. Cell. Cardiol. 2002;34:1549–1560. doi: 10.1006/jmcc.2002.2108. [DOI] [PubMed] [Google Scholar]
- 20.Berndt C., Lillig C.H., Holmgren A. Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. Am. J. Physiol. 2007;292:H1227–H1236. doi: 10.1152/ajpheart.01162.2006. [DOI] [PubMed] [Google Scholar]
- 21.Oka S., Ago T., Kitazono T., Zablocki D., Sadoshima J. The role of redox modulation of class II histone deacetylases in mediating pathological cardiac hypertrophy. J. Mol. Med. 2009;87:785–791. doi: 10.1007/s00109-009-0471-2. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M., Yang G., Hong C., Liu J., Holle E., Yu X., Wagner T., Vatner S.F., Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J. Clin. Invest. 2003;112:1395–1406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu C., Wu C., Liu T., Ago T., Zhai P., Sadoshima J., Li H. Elucidation of thioredoxin target protein networks in mouse. Mol. Cell. Proteomics. 2009;8:1674–1687. doi: 10.1074/mcp.M800580-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiffin R., Bandyopadhyay U., Cuervo A.M. Oxidative stress and autophagy. Antioxid. Redox Signaling. 2006;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- 25.Peluffo G., Radi R. Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc. Res. 2007;75:291–302. doi: 10.1016/j.cardiores.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Weiss J.N., Yang L., Qu Z. Systems biology approaches to metabolic and cardiovascular disorders: network perspectives of cardiovascular metabolism. J. Lipid Res. 2006;47:2355–2366. doi: 10.1194/jlr.R600023-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi T., Martone M.E., Yu Z., Thor A., Doi M., Holst M.J., Ellisman M.H., Hoshijima M. Three-dimensional electron microscopy reveals new details of membrane systems for Ca2+ signaling in the heart. J. Cell Sci. 2009;122:1005–1013. doi: 10.1242/jcs.028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pizzo P., Pozzan T. Mitochondria–endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol. 2007;17:511–517. doi: 10.1016/j.tcb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M., Tuft R.A., Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 30.Kornmann B., Currie E., Collins S.R., Schuldiner M., Nunnari J., Weissman J.S., Walter P. An ER–mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opie L.H., Sack M.N. Metabolic plasticity and the promotion of cardiac protection in ischemia and ischemic preconditioning. J. Mol. Cell. Cardiol. 2002;34:1077–1089. doi: 10.1006/jmcc.2002.2066. [DOI] [PubMed] [Google Scholar]
- 32.Bolukoglu H., Goodwin G.W., Guthrie P.H., Carmical S.G., Chen T.M., Taegtmeyer H. Metabolic fate of glucose in reversible low-flow ischemia of the isolated working rat heart. Am. J. Physiol. 1996;270:H817–H826. doi: 10.1152/ajpheart.1996.270.3.H817. [DOI] [PubMed] [Google Scholar]
- 33.Jain M., Brenner D.A., Cui L., Lim C.C., Wang B., Pimentel D.R., Koh S., Sawyer D.B., Leopold J.A., Handy D.E., Loscalzo J., Apstein C.S., Liao R. Glucose-6-phosphate dehydrogenase modulates cytosolic redox status and contractile phenotype in adult cardiomyocytes. Circ. Res. 2003;93:e9–e16. doi: 10.1161/01.RES.0000083489.83704.76. [DOI] [PubMed] [Google Scholar]
- 34.Jain M., Cui L., Brenner D.A., Wang B., Handy D.E., Leopold J.A., Loscalzo J., Apstein C.S., Liao R. Increased myocardial dysfunction after ischemia–reperfusion in mice lacking glucose-6-phosphate dehydrogenase. Circulation. 2004;109:898–903. doi: 10.1161/01.CIR.0000112605.43318.CA. [DOI] [PubMed] [Google Scholar]
- 35.Gupte S.A., Levine R.J., Gupte R.S., Young M.E., Lionetti V., Labinskyy V., Floyd B.C., Ojaimi C., Bellomo M., Wolin M.S., Recchia F.A. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J. Mol. Cell. Cardiol. 2006;41:340–349. doi: 10.1016/j.yjmcc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Kowaltowski A.J., de Souza-Pinto N.C., Castilho R.F., Vercesi A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009;47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aon M.A., Cortassa S., O'Rourke B. Redox-optimized ROS balance: a unifying hypothesis. Biochim. Biophys. Acta. 2010;1797:865–877. doi: 10.1016/j.bbabio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kohlhaas M., Liu T., Knopp A., Zeller T., Ong M.F., Böhm M., O'Rourke B., Maack C. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 2010;121:1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ide T., Tsutsui H., Kinugawa S., Utsumi H., Kang D., Hattori N., Uchida K., Arimura K., Egashira K., Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ. Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 41.Ide T., Tsutsui H., Hayashidani S., Kang D., Suematsu N., Nakamura K., Utsumi H., Hamasaki N., Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ. Res. 2001;88:529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- 42.Marín-García J., Goldenthal M.J., Moe G.W. Abnormal cardiac and skeletal muscle mitochondrial function in pacing-induced cardiac failure. Cardiovasc. Res. 2001;52:103–110. doi: 10.1016/s0008-6363(01)00368-6. [DOI] [PubMed] [Google Scholar]
- 43.Zorov D.B., Filburn C.R., Klotz L.O., Zweier J.L., Sollott S.J. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aon M.A., Cortassa S., Marbán E., O'Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 45.Wang W., Fang H., Groom L., Cheng A., Zhang W., Liu J., Wang X., Li K., Han P., Zheng M., Yin J., Wang W., Mattson M.P., Kao J.P., Lakatta E.G., Sheu S.S., Ouyang K., Chen J., Dirksen R.T., Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conrad M., Jakupoglu C., Moreno S.G., Lippl S., Banjac A., Schneider M., Beck H., Hatzopoulos A.K., Just U., Sinowatz F., Schmahl W., Chien K.R., Wurst W., Bornkamm G.W., Brielmeier M. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol. Cell. Biol. 2004;24:9414–9423. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y., Huang T.T., Carlson E.J., Melov S., Ursell P.C., Olson J.L., Noble L.J., Yoshimura M.P., Berger C., Chan P.H., Wallace D.C., Epstein C.J. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 48.Matsushima S., Ide T., Yamato M., Matsusaka H., Hattori F., Ikeuchi M., Kubota T., Sunagawa K., Hasegawa Y., Kurihara T., Oikawa S., Kinugawa S., Tsutsui H. Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2006;113:1779–1786. doi: 10.1161/CIRCULATIONAHA.105.582239. [DOI] [PubMed] [Google Scholar]
- 49.Shiomi T., Tsutsui H., Matsusaka H., Murakami K., Hayashidani S., Ikeuchi M., Wen J., Kubota T., Utsumi H., Takeshita A. Overexpression of glutathione peroxidase prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2004;109:544–549. doi: 10.1161/01.CIR.0000109701.77059.E9. [DOI] [PubMed] [Google Scholar]
- 50.Schriner S.E., Linford N.J., Martin G.M., Treuting P., Ogburn C.E., Emond M., Coskun P.E., Ladiges W., Wolf N., Van Remmen H., Wallace D.C., Rabinovitch P.S. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 51.Dai D.F., Santana L.F., Vermulst M., Tomazela D.M., Emond M.J., MacCoss M.J., Gollahon K., Martin G.M., Loeb L.A., Ladiges W.C., Rabinovitch P.S. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lambeth J.D.N.O.X. enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 53.van der Vliet A. NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radic. Biol. Med. 2008;44:938–955. doi: 10.1016/j.freeradbiomed.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 55.Brandes R.P., Weissmann N., Schröder K. NADPH oxidases in cardiovascular disease. Free Radic. Biol. Med. 2010;49:687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 56.Brown D.I., Griendling K.K. Nox proteins in signal transduction. Free Radic. Biol. Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid. Redox Signaling. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J.M., Gall N.P., Grieve D.J., Chen M., Shah A.M. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. 2002;40:477–484. doi: 10.1161/01.hyp.0000032031.30374.32. [DOI] [PubMed] [Google Scholar]
- 59.Bendall J.K., Cave A.C., Heymes C., Gall N., Shah A.M. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 60.Heymes C., Bendall J.K., Ratajczak P., Cave A.C., Samuel J.L., Hasenfuss G., Shah A.M. Increased myocardial NADPH oxidase activity in human heart failure. J. Am. Coll. Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 61.Hingtgen S.D., Tian X., Yang J., Dunlay S.M., Peek A.S., Wu Y., Sharma R.V., Engelhardt J.F., Davisson R.L. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol. Genomics. 2006;26:180–191. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- 62.Byrne J.A., Grieve D.J., Bendall J.K., Li J.M., Gove C., Lambeth J.D., Cave A.C., Shah A.M. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ. Res. 2003;93:802–805. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- 63.Li J., Stouffs M., Serrander L., Banfi B., Bettiol E., Charnay Y., Steger K., Krause K.H., Jaconi M.E. The NADPH oxidase NOX4 drives cardiac differentiation: role in regulating cardiac transcription factors and MAP kinase activation. Mol. Biol. Cell. 2006;17:3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ago T., Kuroda J., Pain J., Fu C., Li H., Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang M., Brewer A.C., Schröder K., Santos C.X., Grieve D.J., Wang M., Anilkumar N., Yu B., Dong X., Walker S.J., Brandes R.P., Shah A.M. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc. Natl Acad. Sci. USA. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martyn K.D., Frederick L.M., von Loehneysen K., Dinauer M.C., Knaus U.G. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell. Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 67.Serrander L., Cartier L., Bedard K., Banfi B., Lardy B., Plastre O., Sienkiewicz A., Fórró L., Schlegel W., Krause K.H. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dikalov S.I., Dikalova A.E., Bikineyeva A.T., Schmidt H.H., Harrison D.G., Griendling K.K. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic. Biol. Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nisimoto Y., Jackson H.M., Ogawa H., Kawahara T., Lambeth J.D. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry. 2010;49:2433–2442. doi: 10.1021/bi9022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuroda J., Ago T., Matsushima S., Zhai P., Schneider M.D., Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl Acad. Sci. USA. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Looi Y.H., Grieve D.J., Siva A., Walker S.J., Anilkumar N., Cave A.C., Marber M., Monaghan M.J., Shah A.M. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51:319–325. doi: 10.1161/HYPERTENSIONAHA.107.101980. [DOI] [PubMed] [Google Scholar]
- 72.Schmelter M., Ateghang B., Helmig S., Wartenberg M., Sauer H. Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. FASEB J. 2006;20:1182–1184. doi: 10.1096/fj.05-4723fje. [DOI] [PubMed] [Google Scholar]
- 73.Li S., Tabar S.S., Malec V., Eul B.G., Klepetko W., Weissmann N., Grimminger F., Seeger W., Rose F., Hänze J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid. Redox Signaling. 2008;10:1687–1698. doi: 10.1089/ars.2008.2035. [DOI] [PubMed] [Google Scholar]
- 74.Santos C.X., Tanaka L.Y., Wosniak J., Laurindo F.R. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid. Redox Signaling. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 75.Pedruzzi E., Guichard C., Ollivier V., Driss F., Fay M., Prunet C., Marie J.C., Pouzet C., Samadi M., Elbim C., O'dowd Y., Bens M., Vandewalle A., Gougerot-Pocidalo M.A. Lizard, G; Ogier-Denis, E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell. Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wosniak J., Jr., Santos C.X., Kowaltowski A.J., Laurindo F.R. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid. Redox Signaling. 2009;11:1265–1278. doi: 10.1089/ars.2009.2392. [DOI] [PubMed] [Google Scholar]
- 77.Govindarajan B., Sligh J.E., Vincent B.J., Li M., Canter J.A., Nickoloff B.J., Rodenburg R.J., Smeitink J.A., Oberley L., Zhang Y., Slingerland J., Arnold R.S., Lambeth J.D., Cohen C., Hilenski L., Griendling K., Martínez-Diez M., Cuezva J.M., Arbiser J.L. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J. Clin. Invest. 2007;117:719–729. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lima B., Forrester M.T., Hess D.T., Stamler J.S. S-nitrosylation in cardiovascular signaling. Circ. Res. 2010;106:633–646. doi: 10.1161/CIRCRESAHA.109.207381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vásquez-Vivar J. Tetrahydrobiopterin, superoxide, and vascular dysfunction. Free Radic. Biol. Med. 2009;47:1108–1119. doi: 10.1016/j.freeradbiomed.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Landmesser U., Dikalov S., Price S.R., McCann L., Fukai T., Holland S.M., Mitch W.E., Harrison D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beckmann J.S., Ye Y.Z., Anderson P.G., Chen J., Accavitti M.A., Tarpey M.M., White C.R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol. Chem. Hoppe Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 82.Nishino T., Okamoto K., Eger B.T., Pai E.F., Nishino T. Mammalian xanthine oxidoreductase—mechanism of transition from xanthine dehydrogenase to xanthine oxidase. FEBS J. 2008;275:3278–3289. doi: 10.1111/j.1742-4658.2008.06489.x. [DOI] [PubMed] [Google Scholar]
- 83.Kelley E.E., Khoo N.K., Hundley N.J., Malik U.Z., Freeman B.A., Tarpey M.M. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic. Biol. Med. 2010;48:493–498. doi: 10.1016/j.freeradbiomed.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Minhas K.M., Saraiva R.M., Schuleri K.H., Lehrke S., Zheng M., Saliaris A.P., Berry C.E., Barouch L.A., Vandegaer K.M., Li D., Hare J.M. Xanthine oxidoreductase inhibition causes reverse remodeling in rats with dilated cardiomyopathy. Circ. Res. 2006;98:271–279. doi: 10.1161/01.RES.0000200181.59551.71. [DOI] [PubMed] [Google Scholar]
- 85.Saavedra W.F., Paolocci N., St John M.E., Skaf M.W., Stewart G.C., Xie J.S., Harrison R.W., Zeichner J., Mudrick D., Marbán E., Kass D.A., Hare J.M. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ. Res. 2002;90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- 86.Kinugawa S., Huang H., Wang Z., Kaminski P.M., Wolin M.S., Hintze T.H. A defect of neuronal nitric oxide synthase increases xanthine oxidase-derived superoxide anion and attenuates the control of myocardial oxygen consumption by nitric oxide derived from endothelial nitric oxide synthase. Circ. Res. 2005;96:355–362. doi: 10.1161/01.RES.0000155331.09458.A7. [DOI] [PubMed] [Google Scholar]
- 87.St-Pierre J., Buckingham J.A., Roebuck S.J., Brand M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 88.Kaludercic N., Takimoto E., Nagayama T., Feng N., Lai E.W., Bedja D., Chen K., Gabrielson K.L., Blakely R.D., Shih J.C., Pacak K., Kass D.A., Di Lisa F., Paolocci N. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ. Res. 2010;106:193–202. doi: 10.1161/CIRCRESAHA.109.198366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Go Y.M., Jones D.P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yanes O., Clark J., Wong D.M., Patti G.J., Sánchez-Ruiz A., Benton H.P., Trauger S.A., Desponts C., Ding S., Siuzdak G. Metabolic oxidation regulates embryonic stem cell differentiation. Nat. Chem. Biol. 2010;6:411–417. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sauer H., Rahimi G., Hescheler J., Wartenberg M. Role of reactive oxygen species and phosphatidylinositol 3-kinase in cardiomyocyte differentiation of embryonic stem cells. FEBS Lett. 2000;476:218–223. doi: 10.1016/s0014-5793(00)01747-6. [DOI] [PubMed] [Google Scholar]
- 92.Buggisch M., Ateghang B., Ruhe C., Strobel C., Lange S., Wartenberg M., Sauer H. Stimulation of ES-cell-derived cardiomyogenesis and neonatal cardiac cell proliferation by reactive oxygen species and NADPH oxidase. J. Cell Sci. 2007;120:885–894. doi: 10.1242/jcs.03386. [DOI] [PubMed] [Google Scholar]
- 93.Gurusamy N., Mukherjee S., Lekli I., Bearzi C., Bardelli S., Das D.K. Inhibition of ref-1 stimulates the production of reactive oxygen species and induces differentiation in adult cardiac stem cells. Antioxid. Redox Signaling. 2009;11:589–600. doi: 10.1089/ars.2008.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sauer H., Rahimi G., Hescheler J., Wartenberg M. Effects of electrical fields on cardiomyocyte differentiation of embryonic stem cells. J. Cell. Biochem. 1999;75:710–723. doi: 10.1002/(sici)1097-4644(19991215)75:4<710::aid-jcb16>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 95.Serena E., Figallo E., Tandon N., Cannizzaro C., Gerecht S., Elvassore N., Vunjak-Novakovic G. Electrical stimulation of human embryonic stem cells: cardiac differentiation and the generation of reactive oxygen species. Exp. Cell Res. 2009;315:3611–3619. doi: 10.1016/j.yexcr.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pucéat M., Travo P., Quinn M.T., Fort P. A dual role of the GTPase Rac in cardiac differentiation of stem cells. Mol. Biol. Cell. 2003;14:2781–2792. doi: 10.1091/mbc.E02-09-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sauer H., Neukirchen W., Rahimi G., Grünheck F., Hescheler J., Wartenberg M. Involvement of reactive oxygen species in cardiotrophin-1-induced proliferation of cardiomyocytes differentiated from murine embryonic stem cells. Exp. Cell Res. 2004;294:313–324. doi: 10.1016/j.yexcr.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 98.Sharifpanah F., Wartenberg M., Hannig M., Piper H.M., Sauer H. Peroxisome proliferator-activated receptor alpha agonists enhance cardiomyogenesis of mouse ES cells by utilization of a reactive oxygen species-dependent mechanism. Stem Cells. 2008;26:64–71. doi: 10.1634/stemcells.2007-0532. [DOI] [PubMed] [Google Scholar]
- 99.Chen Y., Yuen W.H., Fu J., Huang G., Melendez A.J., Ibrahim F.B., Lu H., Cao X. The mitochondrial respiratory chain controls intracellular calcium signaling and NFAT activity essential for heart formation in Xenopus laevis. Mol. Cell. Biol. 2007;27:6420–6432. doi: 10.1128/MCB.01946-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spitkovsky D., Sasse P., Kolossov E., Böttinger C., Fleischmann B.K., Hescheler J., Wiesner R.J. Activity of complex III of the mitochondrial electron transport chain is essential for early heart muscle cell differentiation. FASEB J. 2004;18:1300–1302. doi: 10.1096/fj.03-0520fje. [DOI] [PubMed] [Google Scholar]
- 101.Bers D.M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 102.Del Monte F., Hajjar R.J. Intracellular devastation in heart failure. Heart Fail. Rev. 2008;13:151–162. doi: 10.1007/s10741-007-9071-9. [DOI] [PubMed] [Google Scholar]
- 103.Molkentin J.D. Dichotomy of Ca2+ in the heart: contraction versus intracellular signaling. J. Clin. Invest. 2006;116:623–626. doi: 10.1172/JCI27824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aon M.A., Cortassa S., O'Rourke B. Mitochondrial oscillations in physiology and pathophysiology. Adv. Exp. Med. Biol. 2008;641:98–117. doi: 10.1007/978-0-387-09794-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morad M., Suzuki Y.J. Redox regulation of cardiac muscle calcium signaling. Antioxid. Redox Signaling. 2000;2:65–71. doi: 10.1089/ars.2000.2.1-65. [DOI] [PubMed] [Google Scholar]
- 106.Zima A.V., Blatter L.A. Redox regulation of cardiac calcium channels and transporters. Cardiovasc. Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 107.Hidalgo C., Donoso P. Cross-talk between calcium and redox signalling: from molecular mechanisms to health implications. Antioxid. Redox Signaling. 2008;10:1275–1312. doi: 10.1089/ars.2007.1886. [DOI] [PubMed] [Google Scholar]
- 108.Gonzalez D.R., Treuer A., Sun Q.A., Stamler J.S., Hare J.M. S-nitrosylation of cardiac ion channels. J. Cardiovasc. Pharmacol. 2009;54:188–195. doi: 10.1097/FJC.0b013e3181b72c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aracena-Parks P., Goonasekera S.A., Gilman C.P., Dirksen R.T., Hidalgo C., Hamilton S.L. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J. Biol. Chem. 2006;281:40354–40368. doi: 10.1074/jbc.M600876200. [DOI] [PubMed] [Google Scholar]
- 110.Abramson J.J., Salama G. Sulfhydryl oxidation and Ca2+ release from sarcoplasmic reticulum. Mol. Cell. Biochem. 1998;82:81–84. doi: 10.1007/BF00242520. [DOI] [PubMed] [Google Scholar]
- 111.Eager K.R., Roden L.D., Dulhunty A.F. Actions of sulfhydryl reagents on single ryanodine receptor Ca2+-release channels from sheep myocardium. Am. J. Physiol. 1997;272:C1908–C1918. doi: 10.1152/ajpcell.1997.272.6.C1908. [DOI] [PubMed] [Google Scholar]
- 112.Marengo J.J., Hidalgo C., Bull R. Sulfhydryl oxidation modifies the calcium dependence of ryanodine-sensitive calcium channels of excitable cells. Biophys. J. 1998;74:1263–1277. doi: 10.1016/S0006-3495(98)77840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lim G., Venetucci L., Eisner D.A., Casadei B. Does nitric oxide modulate cardiac ryanodine receptor function? Implications for excitation–contraction coupling. Cardiovasc. Res. 2008;77:256–264. doi: 10.1093/cvr/cvm012. [DOI] [PubMed] [Google Scholar]
- 114.Barouch L.A., Harrison R.W., Skaf M.W., Rosas G.O., Cappola T.P., Kobeissi Z.A., Hobai I.A., Lemmon C.A., Burnett A.L., O'Rourke B., Rodriguez E.R., Huang P.L., Lima J.A., Berkowitz D.E., Hare J.M. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 115.Bendall J.K., Damy T., Ratajczak P., Loyer X., Monceau V., Marty I., Milliez P., Robidel E., Marotte F., Samuel J.L., Heymes C. Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in beta-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation. 2004;110:2368–2375. doi: 10.1161/01.CIR.0000145160.04084.AC. [DOI] [PubMed] [Google Scholar]
- 116.Damy T., Ratajczak P., Shah A.M., Camors E., Marty I., Hasenfuss G., Marotte F., Samuel J.L., Heymes C. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet. 2004;363:1365–1367. doi: 10.1016/S0140-6736(04)16048-0. [DOI] [PubMed] [Google Scholar]
- 117.Khan S.A., Lee K., Minhas K.M., Gonzalez D.R., Raju S.V., Tejani A.D., Li D., Berkowitz D.E., Hare J.M. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation–contraction coupling. Proc. Natl Acad. Sci. USA. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cherednichenko G., Zima A.V., Feng W., Schaefer S., Blatter L.A., Pessah I.N. NADH oxidase activity of rat cardiac sarcoplasmic reticulum regulates calcium-induced calcium release. Circ. Res. 2004;94:478–486. doi: 10.1161/01.RES.0000115554.65513.7C. [DOI] [PubMed] [Google Scholar]
- 119.Sánchez G., Pedrozo Z., Domenech R.J., Hidalgo C., Donoso P. Tachycardia increases NADPH oxidase activity and RyR2 S-glutathionylation in ventricular muscle. J. Mol. Cell. Cardiol. 2005;39:982–991. doi: 10.1016/j.yjmcc.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 120.Hidalgo C., Sánchez G., Barrientos G., Aracena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S-glutathionylation. J. Biol. Chem. 2006;281:26473–26482. doi: 10.1074/jbc.M600451200. [DOI] [PubMed] [Google Scholar]
- 121.Trafford A.W., Díaz M.E., Sibbring G.C., Eisner D.A. Modulation of CICR has no maintained effect on systolic Ca2+: simultaneous measurements of sarcoplasmic reticulum and sarcolemmal Ca2+ fluxes in rat ventricular myocytes. J. Physiol. 2000;522:259–270. doi: 10.1111/j.1469-7793.2000.t01-2-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tocchetti C.G., Wang W., Froehlich J.P., Huke S., Aon M.A., Wilson G.M., Di Benedetto G., O'Rourke B., Gao W.D., Wink D.A., Toscano J.P., Zaccolo M., Bers D.M., Valdivia H.H., Cheng H., Kass D.A., Paolocci N. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ. Res. 2007;100:96–104. doi: 10.1161/01.RES.0000253904.53601.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marx S.O., Reiken S., Hisamatsu Y., Jayaraman T., Burkhoff D., Rosemblit N., Marks A.R. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 124.Kubalova Z., Terentyev D., Viatchenko-Karpinski S., Nishijima Y., Györke I., Terentyeva R., da Cuñha D.N., Sridhar A., Feldman D.S., Hamlin R.L., Carnes C.A., Györke S. Abnormal intrastore calcium signaling in chronic heart failure. Proc. Natl Acad. Sci. USA. 2005;102:14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anderson M.E. Calmodulin kinase signaling in heart: an intriguing candidate target for therapy of myocardial dysfunction and arrhythmias. Pharmacol. Ther. 2005;106:39–55. doi: 10.1016/j.pharmthera.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 126.Zissimopoulos S., Lai F.A. Redox regulation of the ryanodine receptor/calcium release channel. Biochem. Soc. Trans. 2006;34:919–921. doi: 10.1042/BST0340919. [DOI] [PubMed] [Google Scholar]
- 127.Györke S., Carnes C. Dysregulated sarcoplasmic reticulum calcium release: potential pharmacological target in cardiac disease. Pharmacol. Ther. 2008;119:340–354. doi: 10.1016/j.pharmthera.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Erickson J.R., Joiner M.L., Guan X., Kutschke W., Yang J., Oddis C.V., Bartlett R.K., Lowe J.S., O'Donnell S.E., Aykin-Burns N., Zimmerman M.C., Zimmerman K., Ham A.J., Weiss R.M., Spitz D.R., Shea M.A., Colbran R.J., Mohler P.J., Anderson M.E. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gonzalez D.R., Treuer A.V., Castellanos J., Dulce R.A., Hare J.M. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J. Biol. Chem. 2010;285:28938–28945. doi: 10.1074/jbc.M110.154948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mochizuki M., Yano M., Oda T., Tateishi H., Kobayashi S., Yamamoto T., Ikeda Y., Ohkusa T., Ikemoto N., Matsuzaki M. Scavenging free radicals by low-dose carvedilol prevents redox-dependent Ca2+ leak via stabilization of ryanodine receptor in heart failure. J. Am. Coll. Cardiol. 2007;49:1722–1732. doi: 10.1016/j.jacc.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 131.Terentyev D., Györke I., Belevych A.E., Terentyeva R., Sridhar A., Nishijima Y., de Blanco E.C., Khanna S., Sen C.K., Cardounel A.J., Carnes C.A., Györke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ. Res. 2008;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Belevych A.E., Terentyev D., Viatchenko-Karpinski S., Terentyeva R., Sridhar A., Nishijima Y., Wilson L.D., Cardounel A.J., Laurita K.R., Carnes C.A., Billman G.E., Gyorke S. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc. Res. 2009;84:387–395. doi: 10.1093/cvr/cvp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ullrich N.D., Fanchaouy M., Gusev K., Shirokova N., Niggli E. Hypersensitivity of excitation–contraction coupling in dystrophic cardiomyocytes. Am. J. Physiol. 2009;297:H1992–H2003. doi: 10.1152/ajpheart.00602.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schöneich C., Sharov V.S. Mass spectrometry of protein modifications by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2006;41:1507–1520. doi: 10.1016/j.freeradbiomed.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 135.Adachi T., Weisbrod R.M., Pimentel D.R., Ying J., Sharov V.S., Schöneich C., Cohen R.A. S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 136.Xu S., Ying J., Jiang B., Guo W., Adachi T., Sharov V., Lazar H., Menzoian J., Knyushko T.V., Bigelow D., Schöneich C., Cohen R.A. Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging. Am. J. Physiol. 2006;290:H2220–H2227. doi: 10.1152/ajpheart.01293.2005. [DOI] [PubMed] [Google Scholar]
- 137.Sharov V.S., Dremina E.S., Galeva N.A., Williams T.D., Schöneich C. Quantitative mapping of oxidation-sensitive cysteine residues in SERCA in vivo and in vitro by HPLC-electrospray-tandem MS: selective protein oxidation during biological aging. Biochem. J. 2006;394:605–615. doi: 10.1042/BJ20051214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Redondo P.C., Salido G.M., Rosado J.A., Pariente J.A. Effect of hydrogen peroxide on Ca2+ mobilisation in human platelets through sulphydryl oxidation dependent and independent mechanisms. Biochem. Pharmacol. 2004;67:491–502. doi: 10.1016/j.bcp.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 139.Lancel S., Zhang J., Evangelista A., Trucillo M.P., Tong X., Siwik D.A., Cohen R.A., Colucci W.S. Nitroxyl activates SERCA in cardiac myocytes via glutathiolation of cysteine 674. Circ. Res. 2009;104:720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kuster G.M., Lancel S., Zhang J., Communal C., Trucillo M.P., Lim C.C., Pfister O., Weinberg E.O., Cohen R.A., Liao R., Siwik D.A., Colucci W.S. Redox-mediated reciprocal regulation of SERCA and Na+–Ca2+ exchanger contributes to sarcoplasmic reticulum Ca2+ depletion in cardiac myocytes. Free Radic. Biol. Med. 2010;48:1182–1187. doi: 10.1016/j.freeradbiomed.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li S.Y., Yang X., Ceylan-Isik A.F., Du M., Sreejayan N., Ren J. Cardiac contractile dysfunction in Lep/Lep obesity is accompanied by NADPH oxidase activation, oxidative modification of sarco(endo)plasmic reticulum Ca2+-ATPase and myosin heavy chain isozyme switch. Diabetologia. 2006;49:1434–1446. doi: 10.1007/s00125-006-0229-0. [DOI] [PubMed] [Google Scholar]
- 142.Tang W.H., Kravtsov G.M., Sauert M., Tong X.Y., Hou X.Y., Wong T.M., Chung S.K. Man Chung, S. S. Polyol pathway impairs the function of SERCA and RyR in ischemic–reperfused rat hearts by increasing oxidative modifications of these proteins. J. Mol. Cell. Cardiol. 2010;49:58–69. doi: 10.1016/j.yjmcc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tang W.H., Cheng W.T., Kravtsov G.M., Tong X.Y., Hou X.Y., Chung S.K., Chung S.S. Cardiac contractile dysfunction during acute hyperglycemia due to impairment of SERCA by polyol pathway-mediated oxidative stress. Am. J. Physiol. 2010;299:C643–C653. doi: 10.1152/ajpcell.00137.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shattock M.J. Myocardial stunning: do we know the mechanism? Basic Res. Cardiol. 1997;92(Suppl. 2):18–22. doi: 10.1007/BF00797199. [DOI] [PubMed] [Google Scholar]
- 145.Hertelendi Z., Tóth A., Borbély A., Galajda Z., van der Velden J., Stienen G.J., Edes I., Papp Z. Oxidation of myofilament protein sulfhydryl groups reduces the contractile force and its Ca2+ sensitivity in human cardiomyocytes. Antioxid. Redox Signaling. 2008;10:1175–1184. doi: 10.1089/ars.2007.2014. [DOI] [PubMed] [Google Scholar]
- 146.Duncan J.G., Ravi R., Stull L.B., Murphy A.M. Chronic xanthine oxidase inhibition prevents myofibrillar protein oxidation and preserves cardiac function in a transgenic mouse model of cardiomyopathy. Am. J. Physiol. 2005;289:H1512–H1518. doi: 10.1152/ajpheart.00168.2005. [DOI] [PubMed] [Google Scholar]
- 147.Tziomalos K., Hare J.M. Role of xanthine oxidoreductase in cardiac nitroso-redox imbalance. Front. Biosci. 2009;14:237–262. doi: 10.2741/3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Grützner A., Garcia-Manyes S., Kötter S., Badilla C.L., Fernandez J.M., Linke W.A. Modulation of titin-based stiffness by disulfide bonding in the cardiac titin N2-B unique sequence. Biophys. J. 2009;97:825–834. doi: 10.1016/j.bpj.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Layland J., Li J.M., Shah A.M. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J. Physiol. 2002;540:457–467. doi: 10.1113/jphysiol.2001.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Avner B.S., Hinken A.C., Yuan C., Solaro R.J. H2O2 alters rat cardiac sarcomere function and protein phosphorylation through redox signaling. Am. J. Physiol. 2010;299:H723–H730. doi: 10.1152/ajpheart.00050.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hool L.C., Corry B. Redox control of calcium channels: from mechanisms to therapeutic opportunities. Antioxid. Redox Signaling. 2007;9:409–435. doi: 10.1089/ars.2006.1446. [DOI] [PubMed] [Google Scholar]
- 152.Zeng Q., Zhou Q., Yao F., O'Rourke S.T., Sun C. Endothelin-1 regulates cardiac L-type calcium channels via NAD(P)H oxidase-derived superoxide. J. Pharmacol. Exp. Ther. 2008;326:732–738. doi: 10.1124/jpet.108.140301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zeng Q., Han Y., Bao Y., Li W., Li X., Shen X., Wang X., Yao F., O'Rourke S.T., Sun C. 20-HETE increases NADPH oxidase-derived ROS production and stimulates the L-type Ca2+ channel via a PKC-dependent mechanism in cardiomyocytes. Am. J. Physiol. 2010;299:H1109–H1117. doi: 10.1152/ajpheart.00067.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nicoll D.A., Ottolia M., Lu L., Lu Y., Philipson K.D. A new topological model of the cardiac sarcolemmal Na+–Ca2+ exchanger. J. Biol. Chem. 1999;274:910–917. doi: 10.1074/jbc.274.2.910. [DOI] [PubMed] [Google Scholar]
- 155.Reeves J.P., Bailey C.A., Hale C.C. Redox modification of sodium–calcium exchange activity in cardiac sarcolemmal vesicles. J. Biol. Chem. 1986;261:4948–4955. [PubMed] [Google Scholar]
- 156.Goldhaber J.I. Free radicals enhance Na+/Ca2+ exchange in ventricular myocytes. Am. J. Physiol. 1996;271:H823–H833. doi: 10.1152/ajpheart.1996.271.3.H823. [DOI] [PubMed] [Google Scholar]
- 157.Zeitz O., Maass A.E., Van Nguyen P., Hensmann G., Kögler H., Möller K., Hasenfuss G., Janssen P.M. Hydroxyl radical-induced acute diastolic dysfunction is due to calcium overload via reverse-mode Na+–Ca2+ exchange. Circ. Res. 2002;90:988–995. doi: 10.1161/01.res.0000018625.25212.1e. [DOI] [PubMed] [Google Scholar]
- 158.Shimoni Y., Hunt D., Chuang M., Chen K.Y., Kargacin G., Severson D.L. Modulation of potassium currents by angiotensin and oxidative stress in cardiac cells from the diabetic rat. J. Physiol. 2005;567:177–190. doi: 10.1113/jphysiol.2005.090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ren Z., Raucci F.J., Jr., Browe D.M., Baumgarten C.M. Regulation of swelling-activated Cl− current by angiotensin II signalling and NADPH oxidase in rabbit ventricle. Cardiovasc. Res. 2008;77:73–80. doi: 10.1093/cvr/cvm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tsai C.T., Wang D.L., Chen W.P., Hwang J.J., Hsieh C.S., Hsu K.L., Tseng C.D., Lai L.P., Tseng Y.Z., Chiang F.T., Lin J.L. Angiotensin II increases expression of alpha1C subunit of L-type calcium channel through a reactive oxygen species and cAMP response element-binding protein-dependent pathway in HL-1 myocytes. Circ. Res. 2007;100:1476–1485. doi: 10.1161/01.RES.0000268497.93085.e1. [DOI] [PubMed] [Google Scholar]
- 161.Shang L.L., Sanyal S., Pfahnl A.E., Jiao Z., Allen J., Liu H., Dudley S.C., Jr. NF-kappaB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am. J. Physiol. 2008;294:C372–C379. doi: 10.1152/ajpcell.00186.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou C., Ziegler C., Birder L.A., Stewart A.F., Levitan E.S. Angiotensin II and stretch activate NADPH oxidase to destabilize cardiac Kv4.3 channel mRNA. Circ. Res. 2006;98:1040–1047. doi: 10.1161/01.RES.0000218989.52072.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zweier J.L., Talukder M.A. The role of oxidants and free radicals in reperfusion injury. Cardiovasc. Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 164.Halestrap A.P., Clarke S.J., Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim. Biophys. Acta. 2007;1767:1007–1031. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Malhotra R., Brosius F.C. Glucose uptake and glycolysis reduce hypoxia-induced apoptosis in cultured neonatal rat cardiac myocytes. J. Biol. Chem. 1999;274:12567–12575. doi: 10.1074/jbc.274.18.12567. [DOI] [PubMed] [Google Scholar]
- 166.Rey S., Semenza G.L. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc. Res. 2010;86:236–242. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Loor G., Schumacker P.T. Role of hypoxia-inducible factor in cell survival during myocardial ischemia–reperfusion. Cell Death Differ. 2008;15:686–690. doi: 10.1038/cdd.2008.13. [DOI] [PubMed] [Google Scholar]
- 168.Schofield C.J., Ratcliffe P.J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 169.Aragonés J., Fraisl P., Baes M., Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009;9:11–22. doi: 10.1016/j.cmet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 170.Mole D.R. Iron homeostasis and its interaction with prolyl hydroxylases. Antioxid. Redox Signaling. 2010;12:445–458. doi: 10.1089/ars.2009.2790. [DOI] [PubMed] [Google Scholar]
- 171.Ginouvès A., Ilc K., Macías N., Pouysségur J., Berra E. PHDs overactivation during chronic hypoxia "desensitizes" HIFalpha and protects cells from necrosis. Proc. Natl Acad. Sci. USA. 2008;105:4745–4750. doi: 10.1073/pnas.0705680105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yuan G., Nanduri J., Khan S., Semenza G.L., Prabhakar N.R. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. Cell Physiol. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gerald D., Berra E., Frapart Y.M., Chan D.A., Giaccia A.J., Mansuy D., Pouysségur J., Yaniv M., Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 174.Berchner-Pfannschmidt U., Yamac H., Trinidad B., Fandrey J. Nitric oxide modulates oxygen sensing by hypoxia-inducible factor 1-dependent induction of prolyl hydroxylase2. J. Biol. Chem. 2007;282:1788–1796. doi: 10.1074/jbc.M607065200. [DOI] [PubMed] [Google Scholar]
- 175.Guzy R.D., Schumacker P.T. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp. Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 176.Chandel N.S., Maltepe E., Goldwasser E., Mathieu C.E., Simon M.C., Schumacker P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl Acad. Sci. USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Diebold I., Petry A., Hess J., Görlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol. Biol. Cell. 2010;21:2087–2096. doi: 10.1091/mbc.E09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jürgensen J.S., Rosenberger C., Wiesener M.S., Warnecke C., Hörstrup J.H., Gräfe M., Philipp S., Griethe W., Maxwell P.H., Frei U., Bachmann S., Willenbrock R., Eckardt K.U. Persistent induction of HIF-1alpha and -2alpha in cardiomyocytes and stromal cells of ischemic myocardium. FASEB J. 2004;18:1415–1417. doi: 10.1096/fj.04-1605fje. [DOI] [PubMed] [Google Scholar]
- 179.Lee S.H., Wolf P.L., Escudero R., Deutsch R., Jamieson S.W., Thistlethwaite P.A. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N. Engl. J. Med. 2000;342:626–633. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- 180.Huang Y., Hickey R.P., Yeh J.L., Liu D., Dadak A., Young L.H., Johnson R.S., Giordano F.J. Cardiac myocyte-specific HIF-1alpha deletion alters vascularization, energy availability, calcium flux, and contractility in the normoxic heart. FASEB J. 2004;18:1138–1140. doi: 10.1096/fj.04-1510fje. [DOI] [PubMed] [Google Scholar]
- 181.Kido M., Du L., Sullivan C.C., Li X., Deutsch R., Jamieson S.W., Thistlethwaite P.A. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J. Am. Coll. Cardiol. 2005;46:2116–2124. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 182.Natarajan R., Salloum F.N., Fisher B.J., Kukreja R.C., Fowler A.A. Hypoxia inducible factor 1 activation by prolyl 4-hydroxylase-2 gene silencing attenuates myocardial ischemia reperfusion injury. Circ. Res. 2006;98:133–140. doi: 10.1161/01.RES.0000197816.63513.27. [DOI] [PubMed] [Google Scholar]
- 183.Jones S.P., Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J. Mol. Cell. Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 184.Pachori A.S., Melo L.G., Hart M.L., Noiseux N., Zhang L., Morello F., Solomon S.D., Stahl G.L., Pratt R.E., Dzau V.J. Hypoxia-regulated therapeutic gene as a preemptive treatment strategy against ischemia/reperfusion tissue injury. Proc. Natl Acad. Sci. USA. 2004;101:12282–12287. doi: 10.1073/pnas.0404616101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lei L., Mason S., Liu D., Huang Y., Marks C., Hickey R., Jovin I.S., Pypaert M., Johnson R.S., Giordano F.J. Hypoxia-inducible factor-dependent degeneration, failure, and malignant transformation of the heart in the absence of the von Hippel-Lindau protein. Mol. Cell. Biol. 2008;28:3790–3803. doi: 10.1128/MCB.01580-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Minamishima Y.A., Moslehi J., Padera R.F., Bronson R.T., Liao R., Kaelin W.G., Jr. A feedback loop involving the Phd3 prolyl hydroxylase tunes the mammalian hypoxic response in vivo. Mol. Cell. Biol. 2009;29:5729–5741. doi: 10.1128/MCB.00331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Moslehi J., Minamishima Y.A., Shi J., Neuberg D., Charytan D.M., Padera R.F., Signoretti S., Liao R., Kaelin W.G., Jr. Loss of hypoxia-inducible factor prolyl hydroxylase activity in cardiomyocytes phenocopies ischemic cardiomyopathy. Circulation. 2010;122:1004–1016. doi: 10.1161/CIRCULATIONAHA.109.922427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bekeredjian R., Walton C.B., MacCannell K.A., Ecker J., Kruse F., Outten J.T., Sutcliffe D., Gerard R.D., Bruick R.K., Shohet R.V. Conditional HIF-1alpha expression produces a reversible cardiomyopathy. PLoS ONE. 2010;5:e11693. doi: 10.1371/journal.pone.0011693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Downey J.M., Davis A.M., Cohen M.V. Signaling pathways in ischemic preconditioning. Heart Fail. Rev. 2007;12:181–188. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 190.Granfeldt A., Lefer D.J., Vinten-Johansen J. Protective ischaemia in patients: preconditioning and postconditioning. Cardiovasc. Res. 2009;83:234–246. doi: 10.1093/cvr/cvp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Forbes R.A., Steenbergen C., Murphy E. Diazoxide-induced cardioprotection requires signaling through a redox-sensitive mechanism. Circ. Res. 2001;88:802–809. doi: 10.1161/hh0801.089342. [DOI] [PubMed] [Google Scholar]
- 192.Pain T., Yang X.M., Critz S.D., Yue Y., Nakano A., Liu G.S., Heusch G., Cohen M.V., Downey J.M. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ. Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 193.Juhaszova M., Zorov D.B., Kim S.H., Pepe S., Fu Q., Fishbein K.W., Ziman B.D., Wang S., Ytrehus K., Antos C.L., Olson E.N., Sollott S.J. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Robin E., Guzy R.D., Loor G., Iwase H., Waypa G.B., Marks J.D., Hoek T.L., Schumacker P.T. Oxidant stress during simulated ischemia primes cardiomyocytes for cell death during reperfusion. J. Biol. Chem. 2007;282:19133–19143. doi: 10.1074/jbc.M701917200. [DOI] [PubMed] [Google Scholar]
- 195.Bell R.M., Cave A.C., Johar S., Hearse D.J., Shah A.M., Shattock M.J. Pivotal role of NOX-2-containing NADPH oxidase in early ischemic preconditioning. FASEB J. 2005;14:2037–2039. doi: 10.1096/fj.04-2774fje. [DOI] [PubMed] [Google Scholar]
- 196.Kimura S., Zhang G.X., Nishiyama A., Shokoji T., Yao L., Fan Y.Y., Rahman M., Suzuki T., Maeta H., Abe Y. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension. 2005;45:860–866. doi: 10.1161/01.HYP.0000163462.98381.7f. [DOI] [PubMed] [Google Scholar]
- 197.Tong H., Imahashi K., Steenbergen C., Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase-dependent pathway is cardioprotective. Circ. Res. 2007;90:377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 198.Clarke S.J., Khaliulin I., Das M., Parker J.E., Heesom K.J., Halestrap A.P. Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circ. Res. 2008;102:1082–1090. doi: 10.1161/CIRCRESAHA.107.167072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Skyschally A., van Caster P., Boengler K., Gres P., Musiolik J., Schilawa D., Schulz R., Heusch G. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ. Res. 2009;104:15–18. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- 200.Nishino Y., Webb I.G., Davidson S.M., Ahmed A.I., Clark J.E., Jacquet S., Shah A.M., Miura T., Yellon D.M., Avkiran M., Marber M.S. Glycogen synthase kinase-3 inactivation is not required for ischemic preconditioning or postconditioning in the mouse. Circ. Res. 2008;103:307–314. doi: 10.1161/CIRCRESAHA.107.169953. [DOI] [PubMed] [Google Scholar]
- 201.Guo Y., Jones W.K., Xuan Y.T., Tang X.L., Bao W., Wu W.J., Han H., Laubach V.E., Ping P., Yang Z., Qiu Y., Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc. Natl Acad. Sci. USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang Y., Kodani E., Wang J., Zhang S.X., Takano H., Tang X.L., Bolli R. Cardioprotection during the final stage of the late phase of ischemic preconditioning is mediated by neuronal NO synthase in concert with cyclooxygenase2. Circ. Res. 2004;95:84–91. doi: 10.1161/01.RES.0000133679.38825.a6. [DOI] [PubMed] [Google Scholar]
- 203.Yoshida T., Maulik N., Ho Y.S., Alam J., Das D.K. H(mox-1) constitutes an adaptive response to effect antioxidant cardioprotection: a study with transgenic mice heterozygous for targeted disruption of the heme oxygenase-1 gene. Circulation. 2001;103:1695–1701. doi: 10.1161/01.cir.103.12.1695. [DOI] [PubMed] [Google Scholar]
- 204.Heineke J., Molkentin J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 205.Catalucci D., Latronico M.V., Ellingsen O., Condorelli G. Physiological myocardial hypertrophy: how and why? Front. Biosci. 2008;13:312–324. doi: 10.2741/2681. [DOI] [PubMed] [Google Scholar]
- 206.Hill J.A., Olson E.N. Cardiac plasticity. N. Engl. J. Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 207.Sugden P.H., Clerk A. Oxidative stress and growth-regulating intracellular signaling pathways in cardiac myocytes. Antioxid. Redox Signaling. 2006;8:2111–2124. doi: 10.1089/ars.2006.8.2111. [DOI] [PubMed] [Google Scholar]
- 208.Xiao L., Pimentel D.R., Wang J., Singh K., Colucci W.S., Sawyer D.B. Role of reactive oxygen species and NAD(P)H oxidase in alpha(1)-adrenoceptor signaling in adult rat cardiac myocytes. Am. J. Physiol. 2002;282:C926–C934. doi: 10.1152/ajpcell.00254.2001. [DOI] [PubMed] [Google Scholar]
- 209.Xiao L., Pimental D.R., Amin J.K., Singh K., Sawyer D.B., Colucci W.S. MEK1/2-ERK1/2 mediates alpha1-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J. Mol. Cell. Cardiol. 2001;33:779–787. doi: 10.1006/jmcc.2001.1348. [DOI] [PubMed] [Google Scholar]
- 210.Hirotani S., Otsu K., Nishida K., Higuchi Y., Morita T., Nakayama H., Yamaguchi O., Mano T., Matsumura Y., Ueno H., Tada M., Hori M. Involvement of nuclear factor-kappaB and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyocyte hypertrophy. Circulation. 2002;105:509–515. doi: 10.1161/hc0402.102863. [DOI] [PubMed] [Google Scholar]
- 211.Kuster G.M., Pimentel D.R., Adachi T., Ido Y., Brenner D.A., Cohen R.A., Liao R., Siwik D.A., Colucci W.S. Alpha-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes is mediated via thioredoxin-1-sensitive oxidative modification of thiols on Ras. Circulation. 2005;111:1192–1198. doi: 10.1161/01.CIR.0000157148.59308.F5. [DOI] [PubMed] [Google Scholar]
- 212.Higuchi Y., Otsu K., Nishida K., Hirotani S., Nakayama H., Yamaguchi O., Matsumura Y., Ueno H., Tada M., Hori M. Involvement of reactive oxygen species-mediated NF-kappa B activation in TNF-alpha-induced cardiomyocyte hypertrophy. J. Mol. Cell. Cardiol. 2002;34:233–240. doi: 10.1006/jmcc.2001.1505. [DOI] [PubMed] [Google Scholar]
- 213.Hirotani S., Higuchi Y., Nishida K., Nakayama H., Yamaguchi O., Hikoso S., Takeda T., Kashiwase K., Watanabe T., Asahi M., Taniike M., Tsujimoto I., Matsumura Y., Sasaki T., Hori M., Otsu K. Ca2+-sensitive tyrosine kinase Pyk2/CAK beta-dependent signaling is essential for G-protein-coupled receptor agonist-induced hypertrophy. J. Mol. Cell. Cardiol. 2004;36:799–807. doi: 10.1016/j.yjmcc.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 214.Higuchi Y., Otsu K., Nishida K., Hirotani S., Nakayama H., Yamaguchi O., Hikoso S., Kashiwase K., Takeda T., Watanabe T., Mano T., Matsumura Y., Ueno H., Hori M. The small GTP-binding protein Rac1 induces cardiac myocyte hypertrophy through the activation of apoptosis signal-regulating kinase 1 and nuclear factor-kappa B. J. Biol. Chem. 2003;278:20770–20777. doi: 10.1074/jbc.M213203200. [DOI] [PubMed] [Google Scholar]
- 215.Nakagami H., Takemoto M., Liao J.K. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J. Mol. Cell. Cardiol. 2003;35:851–859. doi: 10.1016/s0022-2828(03)00145-7. [DOI] [PubMed] [Google Scholar]
- 216.Hingtgen S.D., Tian X., Yang J., Dunlay S.M., Peek A.S., Wu Y., Sharma R.V., Engelhardt J.F., Davisson R.L. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol. Genomics. 2006;26:180–191. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- 217.Tanaka K., Honda M., Takabatake T. Redox regulation of MAPK pathways and cardiac hypertrophy in adult rat cardiac myocyte. J. Am. Coll. Cardiol. 2001;37:676–685. doi: 10.1016/s0735-1097(00)01123-2. [DOI] [PubMed] [Google Scholar]
- 218.Satoh M., Ogita H., Takeshita K., Mukai Y., Kwiatkowski D.J., Liao J.K. Requirement of Rac1 in the development of cardiac hypertrophy. Proc. Natl Acad. Sci. USA. 2006;103:7432–7437. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Izumiya Y., Kim S., Izumi Y., Yoshida K., Yoshiyama M., Matsuzawa A., Ichijo H., Iwao H. Apoptosis signal-regulating kinase 1 plays a pivotal role in angiotensin II-induced cardiac hypertrophy and remodeling. Circ. Res. 2003;93:874–883. doi: 10.1161/01.RES.0000100665.67510.F5. [DOI] [PubMed] [Google Scholar]
- 220.Date M.O., Morita T., Yamashita N., Nishida K., Yamaguchi O., Higuchi Y., Hirotani S., Matsumura Y., Hori M., Tada M., Otsu K. The antioxidant N-2-mercaptopropionyl glycine attenuates left ventricular hypertrophy in in vivo murine pressure-overload model. J. Am. Coll. Cardiol. 2002;39:907–912. doi: 10.1016/s0735-1097(01)01826-5. [DOI] [PubMed] [Google Scholar]
- 221.Pimentel D.R., Amin J.K., Xiao L., Miller T., Viereck J., Oliver-Krasinski J., Baliga R., Wang J., Siwik D.A., Singh K., Pagano P., Colucci W.S., Sawyer D.B. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ. Res. 2001;89:453–460. doi: 10.1161/hh1701.096615. [DOI] [PubMed] [Google Scholar]
- 222.Aikawa R., Nagai T., Tanaka M., Zou Y., Ishihara T., Takano H., Hasegawa H., Akazawa H., Mizukami M., Nagai R., Komuro I. Reactive oxygen species in mechanical stress-induced cardiac hypertrophy. Biochem. Biophys. Res. Commun. 2001;289:901–907. doi: 10.1006/bbrc.2001.6068. [DOI] [PubMed] [Google Scholar]
- 223.Pimentel D.R., Adachi T., Ido Y., Heibeck T., Jiang B., Lee Y., Melendez J.A., Cohen R.A., Colucci W.S. Strain-stimulated hypertrophy in cardiac myocytes is mediated by reactive oxygen species-dependent Ras S-glutathiolation. J. Mol. Cell. Cardiol. 2006;41:613–622. doi: 10.1016/j.yjmcc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 224.Nadruz W., Jr., Lagosta V.J., Moreno H., Jr., Coelho O.R., Franchini K.G. Simvastatin prevents load-induced protein tyrosine nitration in overloaded hearts. Hypertension. 2004;43:1060–1066. doi: 10.1161/01.HYP.0000124252.43470.2c. [DOI] [PubMed] [Google Scholar]
- 225.Li H.L., Huang Y., Zhang C.N., Liu G., Wei Y.S., Wang A.B., Liu Y.Q., Hui R.T., Wei C., Williams G.M., Liu D.P., Liang C.C. Epigallocathechin-3 gallate inhibits cardiac hypertrophy through blocking reactive oxidative species-dependent and -independent signal pathways. Free Radic. Biol. Med. 2006;40:1756–1775. doi: 10.1016/j.freeradbiomed.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 226.Maack C., Böhm M., Vlaskin L., Dabew E., Lorenz K., Schäfers H.J., Lohse M.J., Engelhardt S. Partial agonist activity of bucindolol is dependent on the activation state of the human beta1-adrenergic receptor. Circulation. 2003;108:348–353. doi: 10.1161/01.CIR.0000080325.94345.8B. [DOI] [PubMed] [Google Scholar]
- 227.Nediani C., Borchi E., Giordano C., Baruzzo S., Ponziani V., Sebastiani M., Nassi P., Mugelli A. d'Amati, G.; Cerbai, E. NADPH oxidase-dependent redox signaling in human heart failure: relationship between the left and right ventricle. J. Mol. Cell. Cardiol. 2007;42:826–834. doi: 10.1016/j.yjmcc.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 228.Grieve D.J., Byrne J.A., Siva A., Layland J., Johar S., Cave A.C., Shah A.M. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J. Am. Coll. Cardiol. 2006;47:817–826. doi: 10.1016/j.jacc.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 229.Johar S., Cave A.C., Narayanapanicker A., Grieve D.J., Shah A.M. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006;20:1546–1548. doi: 10.1096/fj.05-4642fje. [DOI] [PubMed] [Google Scholar]
- 230.Maytin M., Siwik D.A., Ito M., Xiao L., Sawyer D.B., Liao R., Colucci W.S. Pressure overload-induced myocardial hypertrophy in mice does not require gp91phox. Circulation. 2004;109:1168–1171. doi: 10.1161/01.CIR.0000117229.60628.2F. [DOI] [PubMed] [Google Scholar]
- 231.Shiojima I., Sato K., Izumiya Y., Schiekofer S., Ito M., Liao R., Colucci W.S., Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J. Clin. Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Heineke J., Auger-Messier M., Xu J., Oka T., Sargent M.A., York A., Klevitsky R., Vaikunth S., Duncan S.A., Aronow B.J., Robbins J., Crombleholme T.M., Molkentin J.D. Cardiomyocyte GATA4 functions as a stress-responsive regulator of angiogenesis in the murine heart. J. Clin. Invest. 2007;117:3198–3210. doi: 10.1172/JCI32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sano M., Minamino T., Toko H., Miyauchi H., Orimo M., Qin Y., Akazawa H., Tateno K., Kayama Y., Harada M., Shimizu I., Asahara T., Hamada H., Tomita S., Molkentin J.D., Zou Y., Komuro I. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 234.Helmcke I., Heumüller S., Tikkanen R., Schröder K., Brandes R.P. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid. Redox Signaling. 2009;11:1279–1287. doi: 10.1089/ars.2008.2383. [DOI] [PubMed] [Google Scholar]
- 235.von Löhneysen K., Noack D., Wood M.R., Friedman J.S., Knaus U.G. Structural insights into Nox4 and Nox2: motifs involved in function and cellular localization. Mol. Cell. Biol. 2010;30:961–975. doi: 10.1128/MCB.01393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Krishnan J., Suter M., Windak R., Krebs T., Felley A., Montessuit C., Tokarska-Schlattner M., Aasum E., Bogdanova A., Perriard E., Perriard J.C., Larsen T., Pedrazzini T., Krek W. Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab. 2009;9:512–524. doi: 10.1016/j.cmet.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 237.van Empel V.P., Bertrand A.T., Hofstra L., Crijns H.J., Doevendans P.A., De Windt L.J. Myocyte apoptosis in heart failure. Cardiovasc. Res. 2005;67:21–29. doi: 10.1016/j.cardiores.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 238.Foo R.S., Mani K., Kitsis R.N. Death begets failure in the heart. J. Clin. Invest. 2005;115:565–571. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kang Y.J., Li Y., Sun X., Sun X. Antiapoptotic effect and inhibition of ischemia/reperfusion-induced myocardial injury in metallothionein-overexpressing transgenic mice. Am. J. Pathol. 2003;163:1579–1586. doi: 10.1016/S0002-9440(10)63514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nagy N., Malik G., Tosaki A., Ho Y.S., Maulik N., Das D.K. Overexpression of glutaredoxin-2 reduces myocardial cell death by preventing both apoptosis and necrosis. J. Mol. Cell. Cardiol. 2008;44:252–260. doi: 10.1016/j.yjmcc.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 241.Zhao W., Fan G.C., Zhang Z.G., Bandyopadhyay A., Zhou X., Kranias E.G. Protection of peroxiredoxin II on oxidative stress-induced cardiomyocyte death and apoptosis. Basic Res. Cardiol. 2009;104:377–389. doi: 10.1007/s00395-008-0764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.von Harsdorf R., Li P.F., Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation. 1999;99:2934–2941. doi: 10.1161/01.cir.99.22.2934. [DOI] [PubMed] [Google Scholar]
- 243.Foo R.S., Chan L.K., Kitsis R.N., Bennett M.R. Ubiquitination and degradation of the anti-apoptotic protein ARC by MDM2. J. Biol. Chem. 2007;282:5529–5535. doi: 10.1074/jbc.M609046200. [DOI] [PubMed] [Google Scholar]
- 244.Neuss M., Monticone R., Lundberg M.S., Chesley A.T., Fleck E., Crow M.T. The apoptotic regulatory protein ARC (apoptosis repressor with caspase recruitment domain) prevents oxidant stress-mediated cell death by preserving mitochondrial function. J. Biol. Chem. 2001;276:33915–33922. doi: 10.1074/jbc.M104080200. [DOI] [PubMed] [Google Scholar]
- 245.Donath S., Li P., Willenbockel C., Al-Saadi N., Gross V., Willnow T., Bader M., Martin U., Bauersachs J., Wollert K.C., Dietz R., von Harsdorf R. Apoptosis repressor with caspase recruitment domain is required for cardioprotection in response to biomechanical and ischemic stress. Circulation. 2006;113:1203–1212. doi: 10.1161/CIRCULATIONAHA.105.576785. [DOI] [PubMed] [Google Scholar]
- 246.Toth A., Nickson P., Qin L.L., Erhardt P. Differential regulation of cardiomyocyte survival and hypertrophy by MDM2, an E3 ubiquitin ligase. J. Biol. Chem. 2006;281:3679–3689. doi: 10.1074/jbc.M509630200. [DOI] [PubMed] [Google Scholar]
- 247.van Empel V.P., Bertrand A.T., van der Nagel R., Kostin S., Doevendans P.A., Crijns H.J., de Wit E., Sluiter W., Ackerman S.L., De Windt L.I. Downregulation of apoptosis-inducing factor in harlequin mutant mice sensitizes the myocardium to oxidative stress-related cell death and pressure overload-induced decompensation. Circ. Res. 2005;96:e92–e101. doi: 10.1161/01.RES.0000172081.30327.28. [DOI] [PubMed] [Google Scholar]
- 248.Yussman M.G., Toyokawa T., Odley A., Lynch R.A., Wu G., Colbert M.C., Aronow B.J., Lorenz J.N., Dorn G.W. Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat. Med. 2002;8:725–730. doi: 10.1038/nm719. [DOI] [PubMed] [Google Scholar]
- 249.Bianchi P., Kunduzova O., Masini E., Cambon C., Bani D., Raimondi L., Seguelas M.H., Nistri S., Colucci W., Leducq N., Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005;112:3297–3305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- 250.Matsuzawa A., Ichijo H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid. Redox Signaling. 2005;7:472–481. doi: 10.1089/ars.2005.7.472. [DOI] [PubMed] [Google Scholar]
- 251.Remondino A., Kwon S.H., Communal C., Pimentel D.R., Sawyer D.B., Singh K., Colucci W.S. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ. Res. 2003;92:136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- 252.Nishida K., Otsu K. The role of apoptosis signal-regulating kinase 1 in cardiomyocyte apoptosis. Antioxid. Redox Signaling. 2006;8:1729–1736. doi: 10.1089/ars.2006.8.1729. [DOI] [PubMed] [Google Scholar]
- 253.Palomeque J., Rueda O.V., Sapia L., Valverde C.A., Salas M., Petroff M.V., Mattiazzi A. Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species. Circ. Res. 2009;105:1204–1212. doi: 10.1161/CIRCRESAHA.109.204172. [DOI] [PubMed] [Google Scholar]
- 254.Shono T., Yokoyama N., Uesaka T., Kuroda J., Takeya R., Yamasaki T., Amano T., Mizoguchi M., Suzuki S.O., Niiro H., Miyamoto K., Akashi K., Iwaki T., Sumimoto H., Sasaki T. Enhanced expression of NADPH oxidase Nox4 in human gliomas and its roles in cell proliferation and survival. Int. J. Cancer. 2008;123:787–792. doi: 10.1002/ijc.23569. [DOI] [PubMed] [Google Scholar]
- 255.Vaquero E.C., Edderkaoui M., Pandol S.J., Gukovsky I., Gukovskaya A.S. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J. Biol. Chem. 2004;279:34643–34654. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- 256.Edderkaoui M., Hong P., Vaquero E.C., Lee J.K., Fischer L., Friess H., Buchler M.W., Lerch M.M., Pandol S.J., Gukovskaya A.S. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am. J. Physiol. 2005;289:G1137–G1147. doi: 10.1152/ajpgi.00197.2005. [DOI] [PubMed] [Google Scholar]
- 257.Mochizuki T., Furuta S., Mitsushita J., Shang W.H., Ito M., Yokoo Y., Yamaura M., Ishizone S., Nakayama J., Konagai A., Hirose K., Kiyosawa K., Kamata T. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25:3699–3707. doi: 10.1038/sj.onc.1209406. [DOI] [PubMed] [Google Scholar]
- 258.Murillo M.M., Carmona-Cuenca I., Del Castillo G., Ortiz C., Roncero C., Sánchez A., Fernández M., Fabregat I. Activation of NADPH oxidase by transforming growth factor-beta in hepatocytes mediates up-regulation of epidermal growth factor receptor ligands through a nuclear factor-kappaB-dependent mechanism. Biochem. J. 2007;405:251–259. doi: 10.1042/BJ20061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lee J.K., Edderkaoui M., Truong P., Ohno I., Jang K.T., Berti A., Pandol S.J., Gukovskaya A.S. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133:1637–1648. doi: 10.1053/j.gastro.2007.08.022. [DOI] [PubMed] [Google Scholar]