Abstract
Schizotypal personality disorder (SPD) individuals and borderline personality disorder (BPD) individuals have been reported to show neuropsychological impairments and abnormalities in brain structure. However, relationships between neuropsychological function and brain structure in these groups are not well understood. This study compared visual-spatial working memory (SWM) and its associations with dorsolateral prefrontal cortex (DLPFC) and ventrolateral prefrontal cortex (VLPFC) gray matter volume in 18 unmedicated SPD patients with no BPD traits, 18 unmedicated BPD patients with no SPD traits, and 16 healthy controls (HC). Results showed impaired SWM in SPD but not BPD, compared with HC. Moreover, among the HC group, but not SPD patients, better SWM performance was associated with larger VLPFC (BA44/45) gray matter volume (Fisher's Z p-values<0.05). Findings suggest spatial working memory impairments may be a core neuropsychological deficit specific to SPD patients and highlight the role of VLPFC subcomponents in normal and dysfunctional memory performance.
Keywords: working memory, borderline personality disorder, schizotypal personality disorder, dorsolateral prefrontal cortex, ventrolateral prefrontal cortex, MRI
Schizotypal personality disorder (SPD) is characterized by asocial tendencies, difficulties with language, paranoia, odd behavior, and magical thinking. Borderline Personality Disorder (BPD), on the other hand, is characterized by affective instability and impulsive behavior and was first included in DSM-III [1,2]. Many of the earlier SPD studies included symptoms related to conceptions of BPD, but diagnostic overlap was somewhat modified when the criterion of paranoid ideation under stress in BPD was introduced in DSM-III-R [3,4]. Nonetheless, co-morbidity of SPD and BPD is not uncommon [5], which may be due to overlapping areas of impairment [6] or to similar diagnostic nomenclature. Given the potentially overlapping and diverging neurobiological and phenotypic aspects of SPD and BPD, as well as the inherent complexity of both, this study worked to clarify commonalities and distinctions among SPD, BPD and HC individuals on one specific type of neuropsychological functioning, visual-spatial working memory, and the dorsolateral prefrontal cortex (DLPFC) and ventrolateral prefrontal cortex (VLPFC) morphometry correlates.
Previous studies reviewing neuropsychological impairments in individuals with SPD consistently document disruptions in working memory [7-12]. Neuropsychological investigations of individuals with BPD also reveal deficits in non-verbal domains of functioning [13-17], including visual-spatial working memory [18]. Nonetheless, although a meta-analysis of neuropsychological findings in BPD by Ruocco [16] revealed that BPD deficits seem to be primarily lateralized to the right hemisphere [19], it also included studies that failed to detect any neuropsychological difficulties in BPD. Ruocco [16] concluded that further examination of brain-behavior relationships is required to better characterize and elucidate neurocognitive functioning in BPD and personality disorders in general. In the current study, our aim was to investigate one aspect of this brain-behavior relationship, namely volume of DLPFC and VLPFC regions subserving visual-spatial working memory function in SPD and BPD—two personality disorder groups shown to exhibit cognitive deficits and frontal lobe dysfunction [20,21]—as compared with HC. Thus, our goal was to investigate the association between spatial working memory functioning and volume of frontal brain regions thought to subserve working memory function.
Given this aim to explore the potential association between nonverbal deficits and prefrontal cortex morphometric correlates, it is important to note previous studies implicating the DLPFC Brodmann Areas (BA9/10/46) and VLPFC Brodmann Areas (BA44/45) in working memory processes [22-26]. In addition to this work, functional neuroimaging studies examining SPD and BPD patients provide additional support for the idea that abnormalities in DLPFC and VLPFC may underlie neuropsychological impairments [27-31]. Evidence also exists for DLPFC and VLPFC volumetric abnormalities in BPD and SPD [32-35]. Further, research supports the notion that smaller prefrontal regions in SPD are associated with executive function deficits in a healthy control group with schizotypal personality features [36] and reduced volume of BA10 is related to increased impulsiveness in BPD [32].
To date, there is little work comparing neurocognitive function across different personality disorder groups and its relationship with prefrontal cortex volume. The current study sought to determine whether performance on measures of visual-spatial working memory differs for both patient groups, as compared with HC, and/or if deficits are specific to one personality disorder, and characterize the relationship between visual-spatial working memory and DLPFC/VLPFC volume. As such, this study works to advance our limited understanding of the phenotypic and endophenotypic aspects among and within SPD and BPD patients, respectively. We hypothesized that both groups would evidence visual-spatial working memory deficits but, given more consistent reporting of poor performance in SPD [10,31] patients, we predicted more impairment in this group. The second goal of this study was to explore the volumetric correlates of visual-spatial working memory function in these groups. Because both DLPFC and VLPFC have been shown to play a role in working memory in HC, and both regions have been shown to be dysfunctional in SPD and BPD patients, we hypothesized that, among HC, larger prefrontal cortex volume would be associated with better performance on measures of visual-spatial working memory, but these relationships would not be evidenced in SPD nor BPD patients.
All diagnoses were made through interviews by a psychologist using the Structured Clinical Interview for DSM-IV Axis I disorders [SCID-I; 37] and the Structured Interview for DSM-IV Personality Disorders [SIDP; 38] followed by a consensus meeting. Diagnostic methods have been reported previously [39]. Patients with a history of schizophrenia, a psychotic disorder, bipolar (type I) affective disorder, substance abuse within 6 months of study entry or current (in the last 6 months) major depressive disorder (MDD) were excluded. All patients were unmedicated at the time of the study (>2 weeks prior to both testing sessions). Healthy volunteers diagnosed with an Axis I or II psychiatric illness or an Axis I diagnosis in a first-degree relative were excluded from this study. Exclusion criteria for all participants included: severe medical illness, neurological illness, head injury, past substance dependence, as well as substance abuse in the past 6 months, positive urine toxicology test on scan day, and females with a positive pregnancy test on scan day. Written informed consent approved by the Mount Sinai Institutional Review Board was provided by all participants.
A total of 52 adults participated in the study. We excluded one HC who was > 2 SD from the healthy control mean on SWM performance. There were two additional HC participants who approached 2 SD on SWM so we conducted all analyses with and without these participants, and the results were essentially the same and significant either way. Therefore, we present analyses with these two HC subjects included in order to be more conservative. We studied 18 SPD patients with no BPD traits (11 M/7 F; mean age = 35.33±11.0); 18 BPD patients with no SPD traits (7 M/11 F; mean age = 33.50±9.52); and 16 HCs (9 M/7 F; mean age = 30.63±8.11). Participants in the three groups did not differ on age, sex, years of education (SPD mean = 14.94±3.32; BPD mean = 14.0±2.50; HC mean = 15.63±3.28), or Wechsler Abbreviated Scale of Intelligence Full Scale [WASI FSIQ; 40] (SPD mean = 104.76 ±9.91; BPD mean = 107.07±17.62; HC mean = 108.19±18.13), all p's > 0.11.
SWM performance errors were significantly correlated with WASI FSIQ (r = -0.51, p <0.05), years of education (r = -0.29, p < 0.05), and age (r = 0.46, p < 0.05) across all participants regardless of group. Therefore, we used two covariates: age and years of education, instead of WASI FSIQ, because we had data for all participants on this variable, whereas 5 of the 52 participants were missing WASI data. See Table 1 for clinical symptom severity ratings and self-report ratings. To calculate the level of clinical symptom severity, we added up the individual symptom ratings for each DSM-IV diagnostic criterion. Ratings were on a 4-point scale (0=absent, 0.5=somewhat present, 1.0=definitely present/prototypic, 2.0=severe, pervasive).
Table 1. Clinical Interview and Self-Report Ratings in Healthy Control, Borderline Personality Disorder, Schizotypal Personality Disorder Groups.
| Healthy Controls | Borderline PD Patients | Schizotypal PD Patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | ||||
| Symptom Severity* | 16 | --- | --- | 18 | 7.53 | 0.98 | 18 | 7.14 | 1.22 | |||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | ||||
| State-Trait Anxiety Inventory for Adults-Trait (STAI-T) | 13 | 30.46a | 7.47 | 18 | 51.22a | 11.75 | 17 | 41.53a | 11.69 | |||
| Past MDD** | n | % | n | % | n | % | ||||||
| --- | --- | 10b | 56% | 4b | 22% | |||||||
Symptom severity = total of individual symptom ratings for each DSM-IV diagnostic criterion.
MDD = past major depressive disorder (MDD) was defined as prior episode occurring > two months from time of fMRI scan.
Means in same row that share subscripts differ at p<0.05. Given these group differences, we correlated STAI-Trait [65] as well as Past MDD with SWM performance, and they were not significant. Thus, STAI-T and Past MDD were not used as covariates.
Gray matter volume [39] in the Brodmann areas and diffusion tensor imaging (DTI) [41] has been reported previously for this sample. Neuropsychological measures were not a component of the study initially and thus were administered only to a subset of the original sample published previously [39].
All participants completed the Spatial Working Memory (SWM) subtest of the Cambridge Neuropsychological Test Automated Battery [CANTAB; 42], a standardized computer battery, as part of the full CANTAB battery. Findings from the remaining tests of the battery are reported elsewhere [43,44]. The CANTAB has demonstrated adequate reliability [45,46].
Spatial Working Memory (SWM)
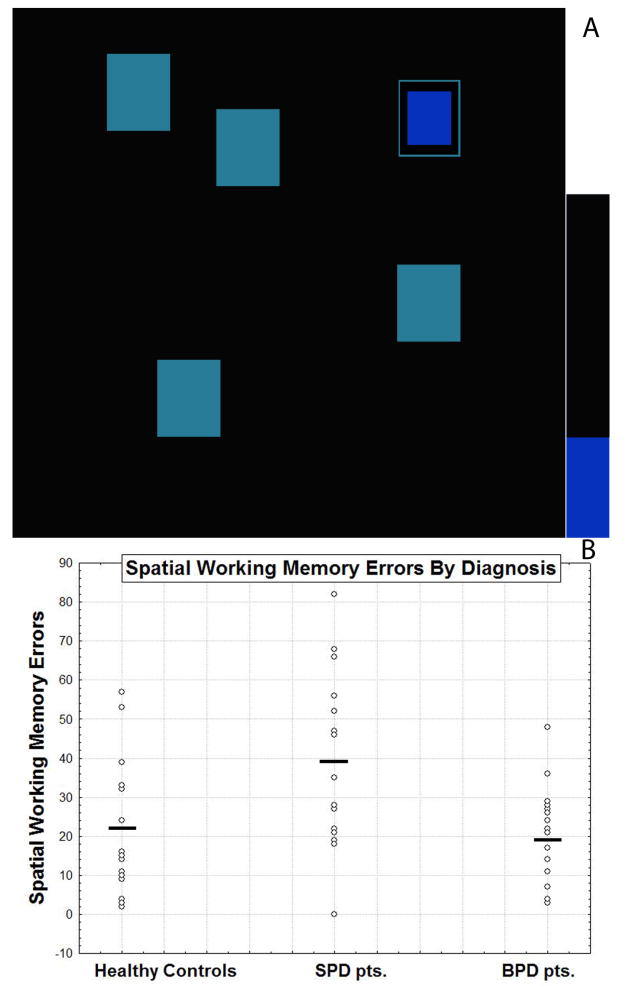
See Figure 1A for details on the test. There are two outcome measures: a ‘Between Search Error’ involving the number of errors made (i.e. touching boxes that are empty or revisiting boxes that have already been checked) and a ‘Strategy Score’ which consists of how often a predetermined search sequence was employed by beginning with the same box during each trial (a low score indicates a more effective use of this strategy).
Figure 1. Spatial working memory task and performance in healthy controls, schizotypal personality disorder patients and borderline personality disorder patients.
A: An example of the visual-spatial working memory task is shown. In the task, blue tokens are “hidden” behind colored squares on the screen and the participant is asked to locate the tokens using the process of elimination until they have found enough tokens to fill up an empty column on the side of the screen. Only one token is hidden on each trial and a token is never hidden more than once in the same location. B: Scatterplot for performance scores on the Spatial Working Memory Task are shown for healthy control, SPD, and BPD participants. The horizontal bar represents the unadjusted means for each group and are as follows: (SPD: 38.67±25.14; BPD: 19.33±12.04; HC: 22.13±17.24). Note that two participants had a score of 32 in the HC group; two participants had a score of 0, two had a score of 27, two had a score of 82 in the SPD group; and two participants had a score of 17, three had a score of 7 in the BPD group. Adjusted means (adjusted for age and years of education) are as follows: (SPD: 37.16±16.32; BPD: 17.47±16.37; HC: 25.93±16.52). There was a significant Group × Error score interaction, F(2,47) = 6.54, p = 0.003 and SPD participants performed significantly worse than the BPD participants (p = 0.0008, Fisher's LSD test) and healthy controls (p = 0.005, Fisher's LSD test).
As described in Goldstein et al. [39], imaging in this sample was conducted on a head-dedicated Siemens Allegra 3-T scanner. T1-weighted MP-RAGE (Magnetization Prepared Rapid Gradient Echo) scans were acquired with the following parameters: 208 slices with slice thickness=0.82 mm, matrix size=256×256×208, FOV=21 cm, TR=2500 ms, TE=4.38 ms, T1=1100ms, and an 8° flip angle FLASH acquisition.
Volumes for the correlational analyses were obtained for gray matter within each individual BA in the DLPFC and VLPFC using a semi-automated parcellation technique described in detail elsewhere [28,47,48] and based upon a digital version of a histologically-based atlas [49].
FSL-FAST was used to segment the images into matter type (gray, white, CSF). Validation of the segmentation method is reported elsewhere [48,50]. Volume measures were expressed in mm3 and obtained by computing relative size as the ratio of (area of ROI)/(volume of brain)×1000. The intraclass correlation coefficients for gray and white matter components for two tracers were 0.98 and 0.99. To address the current study's primary hypotheses, we examined gray matter volume in BAs within the DLPFC (BA9/10/46) and VLPFC (BA44/45) which were developed on a theoretical and anatomical basis.
Neurocognitive data were analyzed using one-way analysis of covariance (ANCOVA). Follow-up Fisher's Least Significant Difference (LSD) tests were conducted to determine the nature of significant main effects and interactions with group. Pearson r product-moment correlations were used to examine the association between specific BA gray matter volumes and performance on the neurocognitive measures. To minimize the number of tests, we ran correlations in diagnostic groups where we found significant between-group differences from HC on the SWM task. A Bonferroni correction was applied for multiple comparisons (alpha level of p = 0.0025 to account for the number of comparisons conducted), and a Fisher's Z test was used to assess between-group differences in correlation coefficients. All significance tests were two-tailed. Correlation coefficients that did not survive Bonferroni correction but were significantly different between groups are mentioned.
Neurocognitive Measures
A one-way ANCOVA was performed on the Spatial Working Memory (SWM) test and, controlling for age and years of education, results revealed a significant main effect of group, F(2,47) = 6.54, p = 0.003. As hypothesized, SPD patients showed more impaired performance (more SWM errors) than BPD patients (p < 0.01) and HC (p < 0.01) (see Figure 1B). BPD patients and HC did not differ, p = 0.62. Results from analyses without age and education covariates also revealed a significant main effect of group, F(2,49) = 5.38, p < 0.01). SPD patients had significantly more SWM errors than HC (p < 0.05) and BPD patients (p < 0.01). The three groups did not differ in strategy use whether controlling for age and education or not, F's < 1. The ‘Strategy Use’ Score is based on how frequently a searching sequence was initiated from the same box during a trial. Thus, a low strategy score is indicative of searching systematically and using a more extensive strategy whereas a high score represents poor use of this strategy.
Neurocognitive Correlates
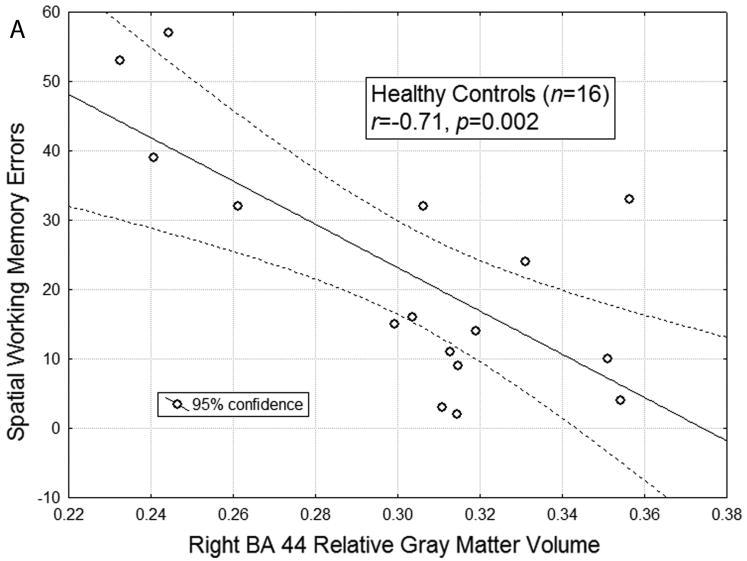
Given our finding of normal-SPD differences in SWM, Pearson's correlations were calculated for these groups to examine the association between DLPFC/VLPFC volume and task performance. All of the following correlational analyses controlled for age and education. Among the healthy control participants but not the SPD patients, fewer errors on the SWM test was associated with larger gray matter volume: right BA44 (HC: r = -0.89, p < 0.005, Figure 2A; SPD: r = 0.04, p = ns; HC vs SPD, Fisher's Z test = -3.86, p < 0.01) and right BA45 (HC: r = -0.68, p < 0.01; SPD: r = -0.02, p = ns; HC vs SPD, Fisher's Z test = -2.14 p < 0.05). The association between right BA44 and SWM in HCs survived the Bonferroni correction of 0.0025, but the relation between BA45 and SWM in HCs did not.
Figure 2. Individual differences in right BA 44 volume and spatial working memory errors.
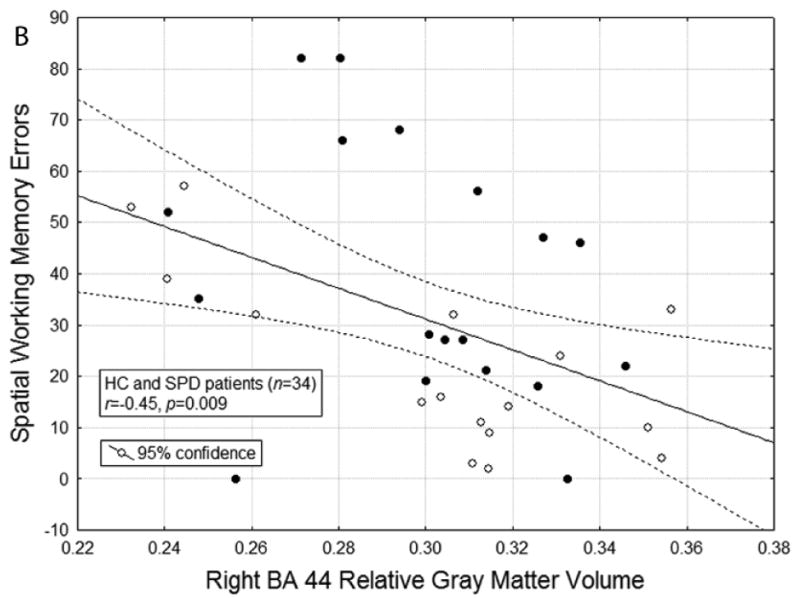
Figure A: The scatterplot for the correlation between relative right BA 44 gray matter volume and spatial working memory errors in healthy controls is shown (r(16) = -0.71, p = 0.002). When controlling for age and education, the correlation between relative right BA 44 gray matter volume and spatial working memory errors is r(12) = -0.89, p = 0.0001. The r for healthy controls (n = 16) was significantly different from the r = 0.04 for SPD patients (n = 18), p<0.01. B: The scatterplot for the correlation between relative right BA 44 gray matter volume and spatial working memory errors in the entire sample of HC and SPD participants is shown (r(34) = -0.45, p = 0.007). Solid black circles represent SPD participants and white circles represent HC participants. When controlling for age and education, the correlation remains significant (r(30) = -0.45, p = 0.009).
In order to see if there was utility in PFC volumes predicting SWM performance, we also calculated the correlation coefficient for the entire sample of HC and SPD participants, given that they showed significant differences in SWM performance. The correlation coefficient was significant conventionally (but did not survive Bonferroni correction of 0.0025) and, as expected, greater right BA44 volume was associated with better SWM performance (see Figure 2B; r = -0.45, p = 0.009).
Correlations between SWM performance, DLPFC/VLPFC gray matter volume and clinical symptomatology (as assessed by interview and self report) for SPD patients did not reach significance.
The first novel finding of this study is that patients with SPD demonstrate visual-spatial working memory impairment relative to BPD patients and HC. These results add to previous work by suggesting that SWM abilities may help differentiate SPD patients from BPD. SWM may thus represent a core neuropsychological deficit in SPD [8] and potentially serve as an important biomarker for differentiating between personality disorders. The second major finding is that, consistent with our hypothesis, larger volume of right BA44 was associated with better task performance (fewer errors) in HC but not in the SPD group alone, the diagnostic group that showed significantly impaired performance. However, the association between larger right BA 44 volume and better SWM was observed across the HC-SPD spectrum.
Our finding of visual-spatial working memory impairment in SPD patients is consistent with prior work [7,8,11] as well as studies involving schizophrenia patients [51,52] thought to evidence similar genetic, neurobiological and neuropsychological abnormalities as those with SPD [53]. Although some previous studies report intact visual-spatial working memory in individuals with BPD [16,54-56], a few studies have reported BPD deficits in this domain [18]. However, much of this work included BPD patients who met criteria for other Axis II disorders, including SPD, thus rendering it difficult to establish a clear neurocognitive profile for BPD. The present study addressed these comorbidity issues by including BPD patients without SPD traits (i.e. no more than three SPD diagnostic criteria with two items rated as 1.0 (definitely present) and one item rated as 0.5 (somewhat present)) and SPD patients without BPD traits. Taken together, our findings indicate SPD but not BPD patients exhibit SWM deficits and underscore the diagnostic specificity of this deficit.
SPD patients demonstrated a specific type of spatial working memory deficit in this study, involving a high number of erroneous returns to previously searched locations. On the ‘Strategy Use’ score, however, SPD patients performed within normal limits. These findings suggest that SPD patients can adopt a spatial-search strategy, but it does not aid in the accuracy of their performance. Thus, while caution is necessary in drawing conclusions from these results, it is possible to argue that SPD erroneous re-visits to boxes where they previously had found tokens may be better attributed to difficulties in executive functioning, such as perseverative behavior and/or temporary storage and/or manipulation of spatial and/or visual information deficits [57], rather than impairments in strategy use. Consistent with this idea, Raine and colleagues [36] reported that individuals with high scores on schizotypal personality measures demonstrated more perseverative errors on an executive functioning task. Further research is needed to understand executive functioning difficulties in SPD patients and potential ways in which cognitive interventions, e.g., cognitive remediation and/or training in strategy-use abilities might improve function.
The present study's visual-spatial working memory task and its measures of errors and strategy use have been shown to be primarily sensitive to frontal-lobe functioning [58]. Additionally, Petrides and colleagues [59,60] have proposed that working memory processes can be mapped onto two distinct cytoarchitectonic regions—VLPFC, where information is maintained in working memory, and DLPFC, where information is monitored and manipulated in working memory [61]. Consistent with the above-mentioned research, as well as animal and human work demonstrating dorsolateral and ventral prefrontal cortex involvement in spatial working memory [23,62], this study showed that larger right BA44 volume was associated with better spatial working memory performance in HC. This same relationship was not observed in the SPD group alone. Given these findings, it is important to note our most recent paper reporting brain volume in this same sample [39] did not detect SPD-HC group differences in VLPFC (i.e. BA44) volume. Although some prior work suggests VLPFC volumetric abnormalities in SPD individuals [35], our failure to find group differences in this region is not entirely surprising given our previous work [34] and other research reporting no SPD-HC prefrontal volume differences, suggesting these regions may be relatively preserved in SPD individuals [53]. However, in the absence of group differences in prefrontal volume, the current study's correlational results across the HC-SPD spectrum suggest there is an association between volume and neurocognitive performance. Our data provide support for the new NIMH Research Domain Criteria (RDoC) strategic plan which calls for studies to classify psychopathology based on dimensions of “observable behavior and neurobiological measures.”
Although this study did not include fMRI to determine whether SPD patients demonstrate dysfunctional prefrontal cortex activation during spatial working memory processing, our previous imaging work implicates disrupted right BA44 and BA45 function in SPD patients during visual-spatial working memory tasks [31]. Koenigsberg and colleagues [31] demonstrated that HC showed increased activation in right BA44/45/47, bilateral premotor areas (BA6), as well as additional parietal regions during a visual-spatial working memory task. SPD patients, on the other hand, showed reduced activation during the maintenance period of a visual-spatial working memory task in left BA44/45/47/10, left intraparietal cortex, and left posterior inferior gyrus. Importantly, our prior finding of an SPD-related deficit in right BA44/45 activation [31] is consistent with our current finding of a lack of association between volume of BA44 and SWM performance in SPD. One potential explanation for our results may be differential recruitment of the neuronal network subserving this function in SPD patients. For example, research in HC provides evidence of VLPFC (i.e. BA44/45) connections with posterior sensory association areas [59,60] and reports VLPFC's role in the process of maintaining visual information in working memory [63]. It is also significant that our results were localized to the right hemisphere, consistent with research linking hemispheric lateralization with type of task, (i.e. spatial with right, verbal with left) [59,60].
Our study has several limitations. First, given the constraints of the task, we were not able to offer greater specificity regarding the exact component of spatial working memory that is impaired in SPD patients. For example, our prior study [31] reported that during the encoding and maintenance period of a SWM task, HC showed increased activation in right BA44/45/47, among other prefrontal and parietal regions. In contrast, SPD patients showed decreased activation in left BA44/45/47 and other regions during this same period of maintaining the spatial locations in memory. Taken together with these findings and evidence of BA44/45 involvement in maintenance functions in HC [63], we can only speculate that SPD patients experience difficulty during the maintenance period in particular but cannot offer evidence to support this idea. Future research needs to investigate potential SWM deficits in greater detail and ideally include a spectrum sample examining HC, SPD, and schizophrenia patients. Also, despite the present study's significant results, the findings need to be replicated in a larger sample, and it would be beneficial to include a group of schizophrenia patients to examine the full schizophrenia spectrum. Lastly, research suggests that stress, which is thought to play an important role in the etiology and course of personality disorders, may impair prefrontal cortex working memory performance in humans and animals [65]. Thus, further research examining the effect of stress on prefrontal cortex functioning in BPD and SPD patient groups would help better characterize SWM abnormalities in these disorders.
The present study's findings highlight visual-spatial working memory deficits in SPD patients and, given the complexity of personality disorder groups and their many overlapping and diverging aspects, suggest that SWM may be a potential biomarker for differentiating among/between them. Also, given our finding of a relation between prefrontal cortex volume and SWM performance, these results suggest the need to further investigate functional prefrontal correlates of different working memory tests and how they may or may not contribute to cognitive, behavioral and emotional disturbances that may underpin SPD and BPD traits, respectively. Use of the SWM test in personality disorder studies examining change with treatment may help, and baseline predictors of treatment response may be fruitful.
Acknowledgments
This work was supported by NIMH grant R01MH073911 to Dr. Hazlett. Other support came from Grant Number MO1-RR00071 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and the Mental Illness Research, Education and Clinical Center, VISN 3 Veterans Health Administration. We thank Dr. Monte Buchsbaum who kindly allowed use of in-house MIPS software.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gunderson JG, Singer MT. Defining borderline patients: An overview. American Journal of Psychiatry. 1975;132:1–10. doi: 10.1176/ajp.132.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Kernberg O. The structural diagnosis of borderline personality organization. In: Hartocollis P, editor. Borderline Personality Disorders: the Concept, the Syndrome, the Patient. New York: International Universities Press, Inc.; 1977. pp. 87–121. [Google Scholar]
- 3.Livesley WJ. The DSM-IV personality disorders. New York: The Guilford Press, Inc.; 1995. [Google Scholar]
- 4.Spitzer RL, Endicott J, Gibbon M. Crossing the border into borderline personality and borderline schizophrenia. Archives of General Psychiatry. 1979;36:17–24. doi: 10.1001/archpsyc.1979.01780010023001. [DOI] [PubMed] [Google Scholar]
- 5.Zanarini MC, Frankenburg FR, Dubo ED, Sickel AE, Trikha A, Levin A, et al. Axis II comorbidity of borderline personality disorder. Comprehensive Psychiatry. 1998;39:296–302. doi: 10.1016/s0010-440x(98)90038-4. [DOI] [PubMed] [Google Scholar]
- 6.McGlashan TH, Grilo CM, Skodol AE, Gunderson JG, Shea MT, Morey LC, et al. The collaborative longitudinal personality disorders study: baseline Axis I/II and II/II diagnostic cooccurrence. Acta Psychiatrica Scandinavica. 2000;102:256–64. doi: 10.1034/j.1600-0447.2000.102004256.x. [DOI] [PubMed] [Google Scholar]
- 7.Farmer CM, O'Donnell BF, Niznikiewicz MA, Voglmaier MM, McCarley RW, Shenton ME. Visual perception and working memory in schizotypal personality disorder. American Journal of Psychiatry. 2000;157:781–8. doi: 10.1176/appi.ajp.157.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roitman SE, Mitropoulou V, Keefe RS, Silverman JM, Serby M, Harvey PD, et al. Visuospatial working memory in schizotypal personality disorder patients. Schizophrenia Research. 2000;41:447–55. doi: 10.1016/s0920-9964(99)00085-7. [DOI] [PubMed] [Google Scholar]
- 9.McClure MM, Barch DM, Flory JD, Harvey PD, Siever L. Context Processing in Schizotypal Personality Disorder: Evidence of Specificity of Impairment to the Schizophrenia Spectrum. Journal of Abnormal Psychology. 2008;117:342–54. doi: 10.1037/0021-843X.117.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitropoulou V, Harvey PD, Zegarelli G, New AS, Silverman JM, Siever LJ. Neuropsychological performance in schizotypal personality disorder: importance of working memory. American Journal of Psychiatry. 2005;162:1896–903. doi: 10.1176/appi.ajp.162.10.1896. [DOI] [PubMed] [Google Scholar]
- 11.Park S, McTigue K. Working memory and the syndromes of schizotypal personality. Schizophrenia Research. 1997;26:213–20. doi: 10.1016/s0920-9964(97)00051-0. [DOI] [PubMed] [Google Scholar]
- 12.Voglmaier MM, Seidman LJ, Niznikiewicz MA, Dickey CC, Shenton ME, McClarley RW. Verbal and nonverbal neuropsychological test performance in subjects with schizotypal personality disorder. American Journal of Psychiatry. 2000;157:787–93. doi: 10.1176/appi.ajp.157.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beblo T, Saavedra AS, Mensebach C, Lange W, Markowitsch HJ, Rau H, et al. Deficits in visual functions and neuropsychological inconsistency in borderline personality disorder. Psychiatry Research. 2006;145:127–35. doi: 10.1016/j.psychres.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Dell'Osso B, Berlin HA, Serati M, Altamura AC. Neuropsychobiological aspects, comorbidity patterns and dimensional models in borderline personality disorder. Neuropsychobiology. 2010;61:169–79. doi: 10.1159/000297734. [DOI] [PubMed] [Google Scholar]
- 15.Dinn WM, Harris CL, Aycicegi A, Greene PB, Kirkley SM, Reilly C. Neurocognitive function in borderline personality disorder. Progress in Neuropsychopharmacology and Biological Psychiatry. 2004;28:329–41. doi: 10.1016/j.pnpbp.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Ruocco AC. The neuropsychology of borderline personality disorder. Psychiatry Research. 2005;137:191–202. doi: 10.1016/j.psychres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Seres I, Unoka Z, Bódi N, Aspán N, Kéri S. The neuropsychology of borderline personality disorder: relationship with clinical dimensions and comparison with other personality disorders. Journal of Personality Disorders. 2009;23:555–62. doi: 10.1521/pedi.2009.23.6.555. [DOI] [PubMed] [Google Scholar]
- 18.LeGris J, van Reekum R. The neuropsychological correlates of borderline personality disorder and suicidal behavior. The Canadian Journal of Psychiatry. 2006;51:131–42. doi: 10.1177/070674370605100303. [DOI] [PubMed] [Google Scholar]
- 19.Stein DJ, Hollander E, Cohen L, Frenkel M, Saoud JB, DeCaria C, et al. Neuropsychiatric impairment in impulsive personality disorders. Psychiatry Research. 1993;48:257–66. doi: 10.1016/0165-1781(93)90076-s. [DOI] [PubMed] [Google Scholar]
- 20.McCloskey MS, Phan KL, Coccaro EF. Neuroimaging and personality disorders. Current Psychiatry Reports. 2005;7:65–72. doi: 10.1007/s11920-005-0027-2. [DOI] [PubMed] [Google Scholar]
- 21.New AS, Goodman M, Triebwasser J, Siever LJ. Recent advances in the biological study of personality disorders. Psychiatric Clinics of North America. 2008;31:441–61. doi: 10.1016/j.psc.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Arnsten AF, Goldman-Rakic PS. Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–6. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- 23.Goldman-Rakic PS. Circuitry of primate prefrontal cortex and the regulation of behaviour by representation memory. In: Plum F, Mountcastle V, editors. Handbook of Physiology: The Nervous System, Higher Functions of the Brain. Maryland: American Physiological Society; 1987. pp. 373–417. [Google Scholar]
- 24.Hazlett EA, Buchsbaum MS, Jeu LA, Nenadic I, Fleischman MB, Shihabuddin L, et al. Hypofrontality in unmedicated schizophrenia patients studied with PET during performance of a serial verbal learning task. Schizophrenia Research. 2000;43:33–46. doi: 10.1016/s0920-9964(99)00178-4. [DOI] [PubMed] [Google Scholar]
- 25.Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychonomic Bulletin & Review. 2002;9:637–71. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- 26.Petrides M, Milner B. Deficits on subject ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20:249–62. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- 27.Buchsbaum MS, Trestman RL, Hazlett E, Siegel BV, Jr, Schaefer CH, Luu-Hsia C, et al. Regional cerebral blood flow during the Wisconsin Card Sort Test in schizotypal personality disorder. Schizophrenia Research. 1997;27:21–8. doi: 10.1016/S0920-9964(97)00081-9. [DOI] [PubMed] [Google Scholar]
- 28.Buchsbaum MS, Nenadic I, Hazlett EA, Spiegel-Cohen J, Fleischman MB, Akhavan A, et al. Differential metabolic rates in prefrontal and temporal Brodmann areas in schizophrenia and schizotypal personality disorder. Schizophrenia Research. 2002;54:141–50. doi: 10.1016/s0920-9964(01)00361-9. [DOI] [PubMed] [Google Scholar]
- 29.Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. A positron emission tomography study of memories of childhood abuse in borderline personality disorder. Biological Psychiatry. 2004;55:759–65. doi: 10.1016/j.biopsych.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Völlm B, Richardson P, Stirling J, Elliott R, Dolan M, Chaudhry I, et al. Neurobiological substrates of antisocial and borderline personality disorder: preliminary results of a functional fMRI study. Criminal Behaviour and Mental Health. 2006;14:39–54. doi: 10.1002/cbm.559. [DOI] [PubMed] [Google Scholar]
- 31.Koenigsberg HW, Buchsbaum MS, Buchsbaum BR, Schneiderman JS, Tang CY, New A, et al. Functional MRI of visuospatial working memory in schizotypal personality disorder: a region-of-interest analysis. Psychological Medicine. 2005;35:1019–30. doi: 10.1017/s0033291705004393. [DOI] [PubMed] [Google Scholar]
- 32.Hazlett EA, New AS, Newmark R, Haznedar MM, Lo JN, Speiser LJ, et al. Reduced anterior and posterior cingulate gray matter in borderline personality disorder. Biological Psychiatry. 2005;58:614–23. doi: 10.1016/j.biopsych.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 33.Völlm BA, Zhao L, Richardson P, Clark L, Deakin JF, Williams S, et al. A voxel-based morphometric MRI study in men with borderline personality disorder: preliminary findings. Criminal Behaviour and Mental Health. 2009;19:64–72. doi: 10.1002/cbm.716. [DOI] [PubMed] [Google Scholar]
- 34.Hazlett EA, Buchsbaum MS, Haznedar MM, Newmark R, Goldstein KE, Zelmanova Y, et al. Cortical gray and white matter volume in unmedicated schizotypal and schizophrenia patients. Schizophrenia Research. 2008;101:111–23. doi: 10.1016/j.schres.2007.12.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawasaki Y, Suzuki M, Nohara S, Hagino H, Takahashi T, Matsui M, et al. Structural brain differences in patients with schizophrenia and schizotypal disorder demonstrated by voxel-based morphometry. European Archives of Psychiatry and Clinical Neuroscience. 2004;254:406–14. doi: 10.1007/s00406-004-0522-1. [DOI] [PubMed] [Google Scholar]
- 36.Raine A, Sheard S, Reynolds GP, Lencz T. Prefrontal structural and functional deficits associated with individual differences in schizotypal personality. Schizophrenia Research. 1992;7:237–47. doi: 10.1016/0920-9964(92)90018-z. [DOI] [PubMed] [Google Scholar]
- 37.First MB, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for Axis I Disorders-Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 1996. [Google Scholar]
- 38.Pfohl B, Blum N, Zimmerman M. Structured Clinical Interview for DSM-IV Personality (SIDP-IV) Washington, D.C.: American Psychiatric Press; 1997. [Google Scholar]
- 39.Goldstein KE, Hazlett EA, New AS, Haznedar MM, Newmark RE, Zelmanova Y, et al. Smaller superior temporal gyrus volume specificity in schizotypal personality disorder. Schizophrenia Research. 2009;112:14–23. doi: 10.1016/j.schres.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Psychological Corp . Wechsler Abbreviated Scale of Intelligence. Texas: Author; 1999. [Google Scholar]
- 41.Hazlett EA, Collazo T, Zelmanova Y, Entis JJ, Byne W, Chu KW, et al. Volume, anisotropy, and fiber-tract couting in the anterior limb of the internal capsule in schizotypal and borderline personality disorder. doi: 10.1016/j.schres.2012.08.022. Unpublished results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luciana M, Nelson CA. Assessment of neuropsychological function through use of the Cambridge Neuropsychological Testing Automated Battery: performance in 4- to 12-year-old children. Developmental Neuropsychology. 2002;22:595–624. doi: 10.1207/S15326942DN2203_3. [DOI] [PubMed] [Google Scholar]
- 43.Berlin HA, Hamilton HK, Goldstein KE, Rogers K, Siever LJ, New AS, et al. Neurocognition and temperament in borderline and schizotypal personality disorder. Paper presented at the meeting of the Society for Biological Psychiatry; New Orleans, LA. 2010. [Google Scholar]
- 44.Berlin HA, Hamilton H, Rogers K, Goldstein KE, Hazlett EA, Siever LJ, et al. Neurocognition and temperament in borderline and schizotypal personality disorder Unpublished results. [Google Scholar]
- 45.Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, McInnes L, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. Journal of International Neuropsychological Society. 1998;4:474–90. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- 46.Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–81. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 47.Hazlett EA, Buchsbaum MS, Haznedar MM, Singer MB, Schnur DB, Jimenez EA, et al. Prefrontal cortex glucose metabolism and startle eyeblink modification abnormalities in unmedicated schizophrenia patients. Psychophysiology. 1998;35:186–98. [PubMed] [Google Scholar]
- 48.Mitelman SA, Buchsbaum MS, Brickman AM, Shihabuddin L. Cortical intercorrelations of frontal area volumes in schizophrenia. Neuroimage. 2005;27:753–70. doi: 10.1016/j.neuroimage.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 49.Perry R, Oakley A, Perry E. Coronal brain map and dissection guide: Localization of Brodman areas in coronal sections. 1991 [Google Scholar]
- 50.Mitelman S, Shihabuddin L, Brickman A, Hazlett E, Buchsbaum MS. MRI assessment of gray and white matter distribution in Brodmann areas of the cortex in patients with schizophrenia with good and poor outcomes. American Journal of Psychiatry. 2003;160:2154–68. doi: 10.1176/appi.ajp.160.12.2154. [DOI] [PubMed] [Google Scholar]
- 51.Carter C, Robertson L, Nordahl T, Chaderjian M, Kraft L, O'Shora-Celaya L. Spatial working memory deficits and their relationship to negative symptoms in unmedicated schizophrenia patients. Biological Psychiatry. 1996;40:930–2. doi: 10.1016/S0006-3223(96)00350-2. [DOI] [PubMed] [Google Scholar]
- 52.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Archives of General Psychiatry. 1992;49:975–82. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 53.Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. American Journal of Psychiatry. 2004;161:398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- 54.Bazanis E, Rogers RD, Dowson JH, Taylor P, Meux C, Staley C, et al. Neurocognitive deficits in decision-making and planning of patients with DSM-III-R borderline personality disorder. Psychological Medicine. 2002;32:1395–405. doi: 10.1017/s0033291702006657. [DOI] [PubMed] [Google Scholar]
- 55.Berlin HA, Rolls ET, Iversen SD. Borderline personality disorder, impulsivity, and the orbitofrontal cortex. American Journal of Psychiatry. 2005;162:2360–73. doi: 10.1176/appi.ajp.162.12.2360. [DOI] [PubMed] [Google Scholar]
- 56.Kunert HJ, Druecke HW, Sass H, Herpertz SC. Frontal lobe dysfunctions in borderline personality disorder? Neuropsychological Findings. Journal of Personality Disorders. 2003;17:497–509. doi: 10.1521/pedi.17.6.497.25354. [DOI] [PubMed] [Google Scholar]
- 57.Baddeley AD, Hitch GJ. Working memory. In: Bower G, editor. The psychology of learning and motivation: Advances in research and theory. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- 58.Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–34. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- 59.Petrides M. Frontal lobes and working memory: evidence from investigations of the effects of cortical excisions in nonhuman primates. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Vol. 9. Amsterdam: Elsevier; 1994a. pp. 59–82. [Google Scholar]
- 60.Petrides M. Functional organization of the human frontal cortex for mneumonic processing. Evidence from neuroimaging studies Annals of the New York Academy of Sciences. 1995;769:85–96. doi: 10.1111/j.1749-6632.1995.tb38133.x. [DOI] [PubMed] [Google Scholar]
- 61.Smith EE, Jonides J. Storage and executive processes in the frontal lobe. Science. 1999;283:1657–61. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 62.D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Research, Cognitive Brain Research. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 63.McLaughlin NCR, Wiebe D, Fulwiler C, Gansler DA. Differential contributions of lateral prefrontal cortex regions to visual memory processes. Brain Imaging and Behavior. 2009;3:202–11. [Google Scholar]
- 64.Arnsten AF. Stress impairs prefrontal cortical function in rats and monkeys: role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Progress in Brain Research. 2000;126:183–92. doi: 10.1016/S0079-6123(00)26014-7. [DOI] [PubMed] [Google Scholar]
- 65.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. California: Consulting Psychologists Press; 1970. [Google Scholar]