Abstract
Self-assembled monolayers (SAMs) can decorate surfaces with `smart´ functional units possessing reversible stimulus–response behavior for optical, thermal, magnetic or redox-chemical stimuli. An independent performance of individual functional groups in such a film is desirable, which can be, in particular, ensured by fairly large lateral separations between tailgroups in the SAM. Adsorbate molecules with multiple attachment points are very promising in this context owing to their large surface footprint, which covers a surface area exceeding the lateral dimensions of the functional groups. To address these design constraints, novel tridentate long-chain tripodal thioether ligands with central adamantine units and a redox-active ferrocenyl tailgroup, 1-[4-(ferrocenylethynyl)phenyl]-3,5,7-tri[(4-n-octylsulfanyl)phenyl]adamantine (T8) and 1-[4-(ferrocenylethynyl)phenyl]-3,5,7-tri[(4-n-dodecylsulfanyl)phenyl]adamantine (T12), were synthesized and used as tripodal adsorbate molecules for the fabrication of redox-active ferrocenyl-terminated SAMs on Au(111). These SAMs were characterized by X-ray photoelectron spectroscopy, near edge X-ray absorption fine structure spectroscopy and sum frequency generation spectroscopy. The data suggest that T8 and T12 form almost contamination-free, well-aligned and fairly densely-packed SAMs on Au(111) with laterally separated ferrocenyl units. The SAMs show a homogeneous binding chemistry, an important requirement for high fidelity SAMs. SFG results indicate lateral interactions between neighboring molecules via the long-chain binding units.
Introduction
Self-assembled monolayers (SAMs)1–4 fabricated from adsorbate molecules which contain functional tail groups are of great current interest, in particular, since they can exhibit `smart´ properties based on a reversible stimulus–response behaviour.5 The archetypal and most commonly used adsorbate system in this regard is that of thiols (or closely related suitable sulfur-containing compounds like disulfides or thioacetates) on gold, giving rise to thiolate-type SAMs.3 Different tail groups which can respond, for example, to optical,6–21 thermal,22–24 magnetic 25 or redox-chemical stimuli 26–34 have been utilized in this context.
An important aspect is the fact that, owing to their proximity in the SAM, tail groups can interact with one another to an extent that their stimulus–response characteristics differ from those of the respective parent compounds in solution. For example, the (E)–(Z) photoisomerisation of azobenzene-based adsorbate species is often severely hindered or even completely blocked in a densely packed SAM, which can be ascribed to a lack of free space necessary for the change of the molecular conformation associated with the isomerization process. However, by judicious choice of molecular arrangement, cooperative behavior can be realized. For example, a molecular domino effect was reported for the (E)–(Z) photoisomerization of a tailor-made azobenzene-based SAM.35 In the case of SAMs fabricated from adsorbate species with terminal redox-active ferrocenyl (Fc) groups on silicon substrates, fast lateral charge hopping upon local electrochemical oxidation was observed, giving rise to a conducting ferrocene-based monolayer on the nonconducting substrate.27
In many cases, an essentially independent behavior of individual tail groups (similar to that observed in isotropic solution) is desirable. This can be ensured by fairly large lateral separations between tailgroups in the SAM, which can be achieved in particular by the use of large-footprint headgroups which cover a surface area exceeding the lateral dimensions of the tailgroups.36 In this context, adsorbate molecules with multiple attachment points have been widely used. Since a plane is defined by three points, a particularly straightforward approach is based on C3 symmetric tripodal adsorbate molecules with three identical anchor moieties attached to a central tetrahedral branching unit, which is also connected to the tail group. The branching unit may be a single atom like C or Si. It may also be based on a polyatomic unit, which exhibits molecular Td symmetry. Due to synthetic reasons, the most popular unit in this regard is adamantane, which allows particularly easy access to C3 symmetric scaffolds.37 The synthesis of adamantane-based tripodal adsorbate species with sulfur-containing anchor groups for chemisorption on gold was pioneered by Keana and coworkers.38–40 First examples of thiolate-type SAMs fabricated from tripodal adamantane-based thiols and thioacetates on gold were recently characterised by scanning tunneling microscopy (STM) 41–43 and by sum frequency generation (SFG) vibrational spectroscopy, respectively.12 As an alternative to these systems, adamantane-based tripodal thioether ligands can be considered.
Note that we 44, 45 and others 46–50 have investigated the use of thioether units instead of thiols for multipoint attachment of adsorbate species on gold. Thioethers are assumed to be more mobile on the surface, which may be important for lateral diffusion during self-assembly, to avoid the formation of incomplete monolayers (also referred to as the “car parking problem”). Further, thioethers are chemically more robust and easier to handle than thiols. On a gold surface, however, complications may still arise from unwanted cleavage of C–S bonds at the thioether side chain, that has been an issue of some debate, since the occurrence and extent of this process can depend on the subtleties of film preparation.51 We recently investigated the chemisorption of the ferrocene-based thioethers [Fe{C5H4(SMe)}2] and [Fe{C5H3(SMe)2}2] and found pronounced C–S bond cleavage on gold in the case of the former, dipodal, derivative, whereas no such reaction was evident for the latter, tetrapodal, species.52 Our studies of SAMs fabricated from tripodal thioethers of the type Ph-(p-C6H4)n-Si(CH2SMe)3 (n = 0, 1) also indicate that –SMe units are prone to C–S bond cleavage.44, 45 A uniform binding chemistry is an important prerequisite for the formation of high fidelity SAMs.
A study of SAMs fabricated from ferrocenyl-functionalised long-chain thioethers of the type Fc-C(O)-(CH2)m-S-(CH2)n-Fc on gold revealed that these compounds chemisorb without the cleavage of C–S bond at the thioether side chain. This gives rise to SAMs of thioether molecules in a disordered, mobile, liquid-like state on the substrate.53 Although the first examples of ferrocenyl-functionalised tripodal thioethers, viz. [Fc-B(CH2SMe)3]54 and Fc-p-C6H4-C(CH2SMe)3,55 date back approximately one decade, such compounds have not been utilized so far for the fabrication of SAMs on gold. A somewhat related study was performed by Li et al., who investigated the formation of gold colloids using the tripodal Fc-C(O)NH-C[(CH2)11-S-C10H21]3 as a stabilizing ligand.56
In the present study we synthesized long-chain tripodal thioether ligands (Figure 1), which are adamantane-based large-footprint adsorbate species containing a redox-active ferrocenyl tailgroup, and used them for the preparation of SAMs on Au(111) substrates. These SAMs were characterized using x-ray photoelectron spectroscopy (XPS), near-edge x-ray absorption fine structure (NEXAFS) spectroscopy and SFG spectroscopy.
Figure 1.
Scheme of the tripodal ligands T8 and T12 used in this study.
2. Experimental methods
2.1. Chemical synthesis
2.1.1. General
Synthetic work was routinely performed under an atmosphere of dry nitrogen by using standard Schlenk techniques or a conventional glovebox. Solvents were appropriately dried and purified. Ethinylferrocene57 and 1,3,5,7-tetra(4-iodophenyl)adamantane58 were prepared according to published procedures. All other chemicals were procured from standard commercial sources and used as received. NMR spectra were recorded with a Varian Unity INOVA 500 spectrometer operating at 500.13 MHz for 1H. MALDI mass spectra were obtained with a BiFlex IV instrument (Bruker Daltonics, Bremen, Germany; DCTB (2-[(2E)-3-(4-tert-butylphenyl)-2-methylprop-2-enylidene]malononitrile) matrix, 337 nm N2 laser, 3 ns pulse width). High-resolution MALDI mass spectra were obtained with an Ultraflex instrument (Bruker Daltonics, Bremen, Germany) under the same conditions. Mass calibration was performed immediately prior to the measurement with a polystyrene standard (silver adduct).
2.1.2. Preparation of 1-[4-(ferrocenylethynyl)phenyl]-3,5,7-tri(4-iodophenyl)adamantane (1)
i-Pr2NH (10 mL), [PdCl2(PPh3)2] (211 mg, 0.3 mmol) and CuI (57 mg, 0.3 mmol) were added to a solution of 1,3,5,7-tetra(4-iodophenyl)adamantane (2.70 g, 2.86 mmol) in THF (100 mL). A solution of ethinylferrocene (660 mg, 3.1 mmol) in THF (100 mL) was added dropwise with stirring over the course of 1.5 h. Stirring was continued for 24 h. Dichloromethane (100 mL) was added, followed by 2 N hydrochloric acid (100 mL). The organic layer was separated off and washed neutral with brine (3 × 100 mL). The washings were combined and extracted with dichloromethane (3 × 100 mL). The combined organic layers were dried with MgSO4. Volatile components were removed in vacuo. The remaining solid was purified by column chromatography (silica gel, n-hexane/chloroform 4/1), affording the product as an orange, microcrystalline solid (Rf = 0.20). Yield 705 mg (24%).
1H NMR (CDCl3): δ = 2.07 (s, 6 H, CH2), 2.09 (s, 6 H, CH2), 4.24 (s, 7 H, Cp and C5H4), 4.50 (s, 2 H, C5H4), 7.20 (“d”, apparent J = 8.8 Hz, 6 H, p-C6H4-I), 7.38 (“d”, apparent J = 9.0 H, 2 H, p-C6H4-C≡CFc), 7.49 (“d”, apparent J = 9.0 H, 2 H, p-C6H4-C≡CFc), 7.67 (“d”, apparent J = 8.8 Hz, 6 H, p-C6H4-I). 13C{1H} NMR (CDCl3): δ = 39.1, 46.6, 68.7, 70.0, 71.3, 81.8, 91.7, 102.5, 121.3, 126.9, 137.6, 138.3, 148.4 (not all signals due to quaternary C atoms could be detected). MS (MALDI): m/z (%) 1026 (100) [M]+, correct isotope pattern.
2.1.3. Preparation of 1-[4-(ferrocenylethynyl)phenyl]-3,5,7-tri[(4-n-octylsulfanyl)phenyl]adamantine (T8)
n-Octane thiol (111 mg, 0.76 mmol) and KOt-Bu (85 mg, 0.76 mmol) were added to a stirred solution of 1 (236 mg, 0.23 mmol) in DME (5 mL) placed in a thick-walled `Rotaflo´ ampoule. The catalyst solution obtained by dissolving Pd(OAc)2 (8 mg, 0.04 mmol) and Josiphos SL-J009-1 (22 mg,0.04 mmol) in DME (4 mL) was added dropwise. The mixture was heated to 110 °C for 96 h and was subsequently allowed to cool to room temperature. Dichloromethane (20 mL) and was added, followed by 2 N hydrochloric acid (20 mL). The organic layer was separated off and washed neutral with brine (3 × 20 mL). The washings were combined and extracted with dichloromethane (3 × 20 mL). The combined organic layers were dried with MgSO4. Volatile components were removed in vacuo. The remaining solid was purified by column chromatography (silica gel, n-hexane/chloroform 4/1), affording T8 as a very viscous orange oil (Rf = 0.15). To remove potential traces of unreacted thiol, the product was dissolved in dichloromethane (10 mL) and filtered through a pad of silver powder mixed with silica gel. Yield 224 mg (90%).
1H NMR (CDCl3): δ = 0.87 (t, J = 7.5 H, 9 H, Me), 1.26 (br. m, 24 H, CH2), 1.41 (m, 6 H, CH2), 1.63 (m, 6 H, CH2), 2.11 (m, 12 H, CH2), 2.90 (t, J = 7.5 Hz, 6 H, SCH2), 4.25 (s, 7 H, Cp and C5H4),), 4.51 (s, 2 H, C5H4), 7.31 (“d”, apparent J = 8.5 Hz, 6 H, p-C6H4-S), 7.38 (“d”, apparent J = 8.5 Hz, 6 H, p-C6H4-S), 7.41 (“d”, apparent J = 8.0 H, 2 H, p-C6H4-C≡CFc), 7.46 (“d”, apparent J = 8.0 H, 2 H, p-C6H4-C≡CFc). 13C{ 1H} NMR (CDCl3): δ = 14.1, 22.7, 28.8, 29.3, 29.7, 31.9, 33.8, 39.0, 47.0, 68.0, 70.0, 72.1, 88.1, 92.0, 118.5, 125.0, 125.6, 129.1, 131.4, 134.6, 146.6, 146.8 (not all signals due to quaternary C atoms could be detected). HRMS (MALDI): m/z 1080.5410, calc. for [C70H88FeS3]+ 1080.5398.
2.1.4. Preparation of 1-[4-(ferrocenylethynyl)phenyl]-3,5,7-tri[(4-n-dodecylsulfanyl)phenyl]adamantane (T12)
This compound was obtained by a procedure essentially identical to that described for T8, utilising n-dodecane thiol (154 mg, 0.76 mmol). Yield 259 mg (90%).
1H NMR (CDCl3): δ = 0.88 (t, J = 7.5 H, 9 H, Me), 1.25 (br. m, 48 H), 1.41 (m, 6 H, CH2), 1.63 (m, 6 H, CH2), 2.11 (m, 12 H, CH2), 2.90 (t, J = 7.5 Hz, 6 H, SCH2), 4.28 (s, 7 H, Cp and C5H4),), 4.55 (s, 2 H, C5H4), 7.31 (“d”, apparent J = 9.0 Hz, 6 H, p-C6H4-S), 7.38 (“d”, apparent J = 9.0 Hz, 6 H, p-C6H4-S), 7.41 (“d”, apparent J = 8.5 H, 2 H, p-C6H4-C≡CFc), 7.45 (“d”, apparent J = 8.5 H, 2 H, p-C6H4-C≡CFc). 13C{1H} NMR (CDCl3): δ = 14.1, 22.7, 28.8, 29.0, 29.3, 29.7, 30.0, 31.9, 33.8, 39.0, 47.0, 68.1, 70.0, 72.0, 88.1, 92.0, 118.5, 125.0, 125.5, 129.1, 131.4, 134.6, 146.6, 146.8 (not all signals due to quaternary C atoms could be detected). HRMS (MALDI): m/z 1248.7282, calc. for [C82H112FeS3]+ 1248.7275.
2.2 Film preparation
The gold substrates for the SAM fabrication were prepared by thermal evaporation of 200 nm gold (99.99% purity) onto polished single-crystal silicon (111) wafers (Silicon Sense) primed with a 5 nm titanium layer for adhesion promotion. The resulting films were polycrystalline with a grain size of 20–50 nm and predominantly had a (111) orientation.59 The films were formed by immersion of freshly prepared gold substrates in 10 µM solutions of T8 and T12, respectively, in ethanol (pre-dissolved in small amounts of toluene) at room temperature overnight. After immersion, the samples were carefully rinsed with copious amounts of ethanol, blown dry with nitrogen, and then kept in plastic or glass containers filled with nitrogen until they were characterized.
In addition to the T8 and T12 films, we have also prepared SAMs of dodecanethiol, C12H25SH (DoDT). The preparation procedure was the same as for the T8 and T12 films. The DoDT concentration was 1 mM with no pre-dissolving in toluene. The DoDT SAMs were used as a reference.
2.3 X-ray photoelectron spectroscopy
The T8 and T12 films were characterized by XPS. The measurements were carried out on a Kratos AXIS Ultra DLD instrument (Kratos, Manchester, England) in the hybrid mode using a monochromatic AlKα X-ray source (hν=1486.6 eV) and normal emission geometry. The binding energy (BE) scale was calibrated to the Au 4f7/2 emission of the underlying gold substrate at 84.0 eV. The energy resolution was better than 400 meV. All XP spectra were fitted by symmetric Voigt functions and Shirley-type background. To fit the S 2p3/2,1/2 doublets, we used a branching ratio of 2 and a spin-orbit splitting (verified by fit) of 1.18 eV.60 The fits were carried out self-consistently, i.e., the same parameters were used for identical spectral regions. The reported composition data represent an average over 6 spots on two different samples.
2.4 NEXAFS
NEXAFS measurements were performed at the HE-SGM beamline of the synchrotron storage ring BESSY II in Berlin, Germany. Spectra at the carbon K-edge and iron L-edge were collected with a retardation voltage of −150 V and −450 V, respectively. Linearly polarized light with a polarization factor of ~0.82 was used. The energy resolution was approximately 0.4 eV, and the incidence angle of the X-ray light was varied from 90° to 20° in 10–20° steps. Raw NEXAFS spectra were normalized to the incident photon flux by division through a spectrum of a clean, freshly sputtered gold sample. The photon energy scale was referenced to the most intense π* resonance of freshly cleaved highly oriented pyrolytic graphite at 285.38 eV.61 Further, the carbon K-edge spectra were reduced to the standard form by subtracting linear pre-edge background and normalizing to the unity edge jump determined by a horizontal plateau 40–50 eV above the absorption edge. The Fe L-edge spectra were not normalized to the edge jump.
2.5 SFG Setup and Data Acquisition
The SFG vibrational spectra were acquired on an EKSPLA instrument (EKSPLA, Vilnius, Lithuania) by overlapping `visible and tunable IR laser pulses (25 ps) in time and space at incidence angles of 60° and 54°, respectively. Details of the instrumentation setup are published elsewhere.62 Briefly, the visible beam with a wavelength of 532 nm was delivered by an EKSPLA Nd:YAG laser operating at 50 Hz, which was also used to pump an EKSPLA optical parametric generation/amplification and difference frequency unit based on barium borate and AgGaS2 crystals to generate tunable IR laser radiation from 1000–4000 cm−1. The bandwidth was 1 cm−1 for the visible pump pulses and 1 cm−1 for the IR laser radiation. Both beams were unfocussed and had a diameter of approximately 2 mm at the sample. The energy for both beams was 160 µJ per pulse. The spectra were collected with 400 shots per data point in 2 cm−1 increments. All spectra were recorded in the ppp (sum, visible, and infrared) polarization combination and were normalized by a reference SFG signal generated in a ZnS crystal. SFG is a coherent non-linear optical process where spectrally tunable infrared and fixed visible laser pulses are overlapped in time and space at an interface and generate photons at the sum of the pump beam frequencies.
The intensity of the generated SF light ISF is given by:63, 64
| (1) |
here, Ivis and .IIR are the infrared and visible pump beam intensities, respectively, and denotes the effective second-order nonlinear susceptibility of the interface which can be written as:65
| (2) |
Here, is the second order nonlinear susceptibility of the non-resonant background, Aν is the strength of the νth vibrational mode, ϕν denotes the phase of the respective mode and ωIR refers to the frequency of the incident IR field. ων and Γν are the resonance position and width of the respective modes, respectively. Fitting eq 2 to the spectral data allows us to determine Aν, ων and Γν. In the fits, the Lorentzian line widths were set to 2 cm−1 and ℘⎨ was allowed to vary since the two contributions to the total line width could not be separated within the accuracy of the measurements. Phase values are reported as relative values to the non-resonant gold background and identical phases were assumed for all methyl and methylene related stretch vibrations.
3. Results and discussion
3.1. Chemical synthesis
The synthesis of the T8 and T12 compounds is outlined in Fig. 1. A Sonogashira coupling reaction was performed with ethinylferrocene and 1,3,5,7-tetra(4-iodophenyl)adamantane, which can be prepared in multigram quantities by a two-step procedure starting from commercially available 1-bromoadamantane. The starting materials were used in a 1:1 ratio. Not unexpectedly, this procedure resulted in the formation of a statistical mixture of coupling products and unreacted starting material.66 The desired mono-functionalised compound 1 was isolated in 24% yield by column chromatography. It was subsequently reacted with n-octane thiol and n-dodecane thiol, respectively, using Hartwig/Buchwald crosscoupling conditions.67–69 70 The coupling product was obtained in 90% yield in each case after chromatographic workup.
3.2 Analysis of binding chemistry and SAM composition
3.2.1 X-ray photoelectron spectroscopy (XPS)
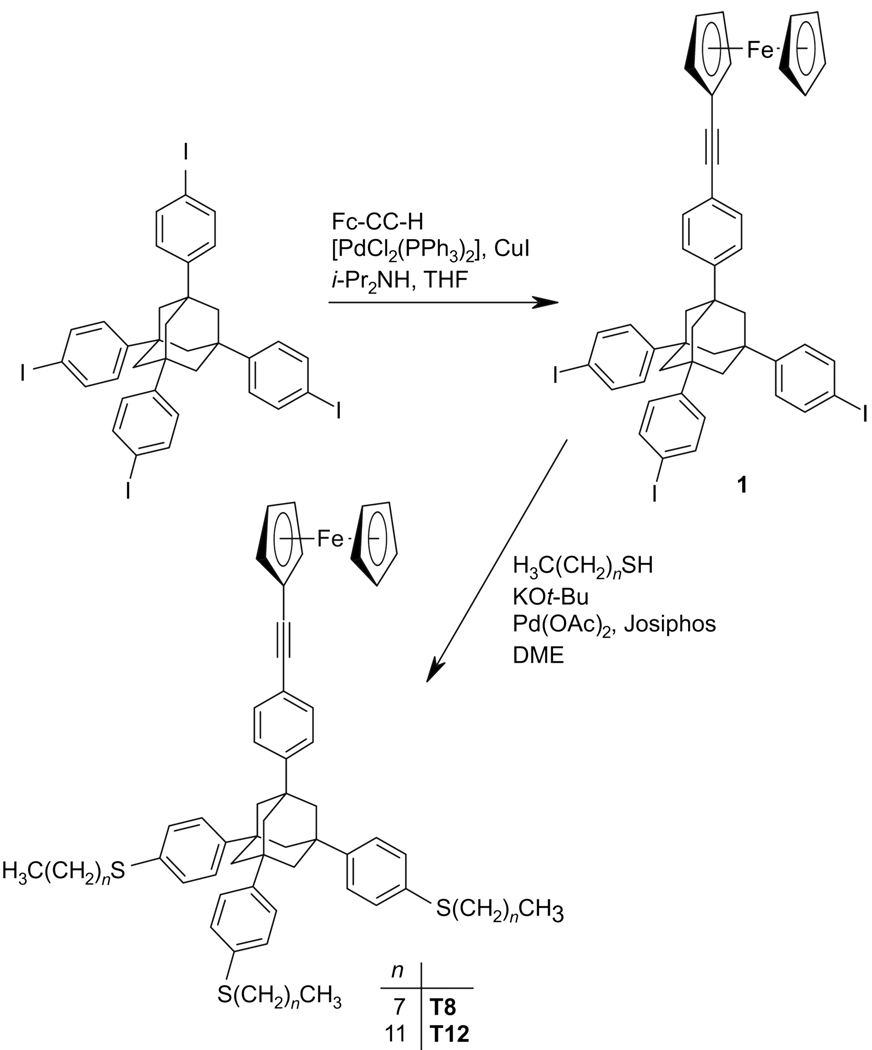
The chemical composition of the T8 and T12 films was studied by XPS. Normalized C 1s spectra of the target SAMs are presented in Figure 2. The spectra exhibit a single emission near 284.9 eV related to the merged signal of the aromatic phenylene and cyclopentadienyl rings and the aliphatic chains; a single peak is typical of the SAMs with complex hydrocarbon backbone.71 No peaks related to possible oxygen-containing contamination or to the oxidation of the molecular backbone are observed. Since oxygen-containing contamination is always observed on the surface of even freshly prepared Au substrate, its absence in the target SAMs implies that the self-cleaning process, typical of the formation of densely packed SAMs, occurred. This can only happen upon binding of the adsorbate molecules to the substrate.
Figure 2.
Normalized C 1s XPS spectra (open circles) of the T8 and T12 SAMs on Au(111). The respective fits (solid lines) and a background (dotted line) are also shown.
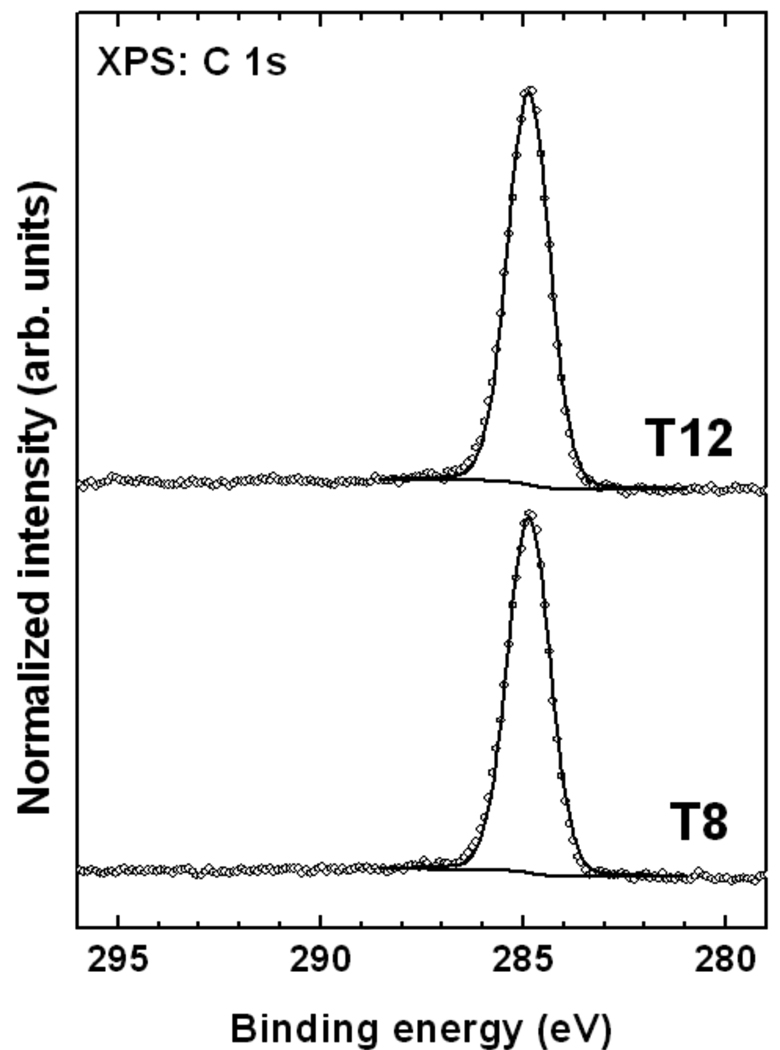
The S 2p XP spectra of the T8 and T12 SAMs are presented in Figure 3. The spectra are dominated by a pronounced S 2p3/2,1/2 doublet near 163.3 eV (S 2p3/2) accompanied by a weaker doublet at ca. 162.0 eV (S 2p3/2). The latter doublet is commonly assigned to a thiolate species, i.e., sulfur atoms strongly bound to the gold substrate after the cleavage of S–H, S–S, or C–S bonds (at the side chain) in the case of thiol, disulfide, and thioether, respectively.72 No traces of oxidized sulfur species (higher BEs) are observed. A doublet at 163.3 eV is commonly associated with weakly bound sulfur, unbound sulfur, or a disulfide moiety.72–74 In our case we assign this feature to thioethers with weak coordination-type binding to the substrate. Contributions from unbound thioether species cannot be completely excluded, but large amounts of unbound sulfur are unlikely in view of the self-cleaning upon the SAM formation (see above) and the location of the thioether groups close to the gold surface, which is inferred from the strong attenuation of the sulfur signal by the carbon overlayer (see discussion of the composition data below). This is consistent with previous reports that show that thioethers can adsorb intact on gold.51, 73, 75, 76 However, cleavage of one of the thioether C–S bonds can also occur for a part of the adsorbates,51 resulting in a thiolate-like bonding to the substrate. In the present case, in view of the low intensity of the 162.0 eV signal, C–S bond cleavage seems to occur only to a very limited extent (≈10–15%).
Figure 3.
Normalized S 2p XPS spectra (open circles) of the T8 and T12 SAMs on Au(111). The decomposition of these spectra into individual contributions (solid lines) and a background (dotted line) is also shown.
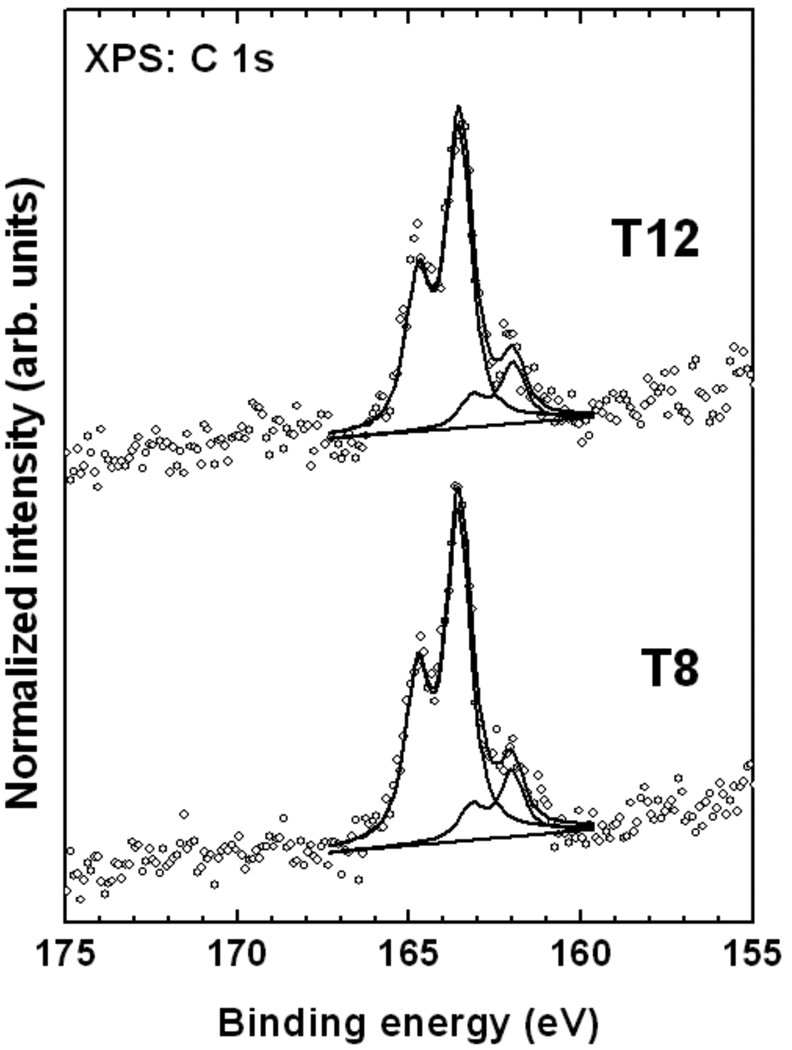
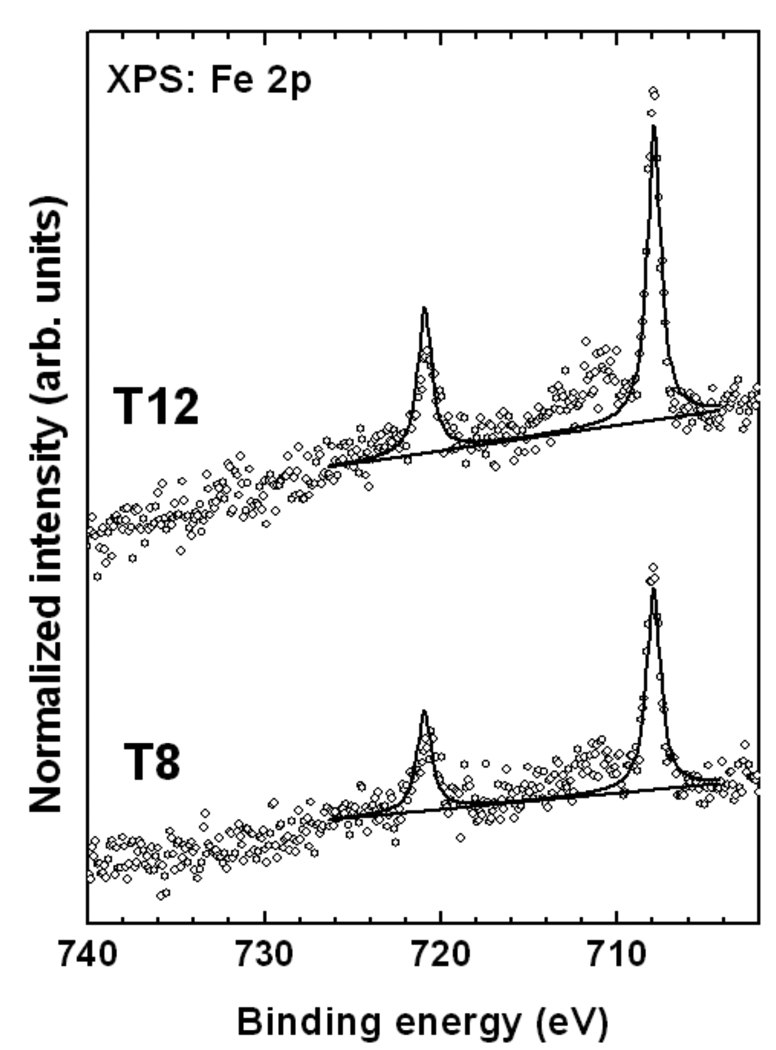
The XPS Fe 2p spectra of the T8 and T12 SAMs in Figure 4 is dominated by the characteristic Fe 2p3/2,1/2 doublet at a BE of about 708.0 eV (2p3/2), related to the intact ferrocenyl groups. There are only small amounts (<15%) of additional Fe species associated with the partial oxidation and decomposition of the organometallic moieties visible at a higher BE (~712 eV). The vast majority of the ferrocenyl units in the T8 and T12 films remain intact after adsorption.
Figure 4.
Normalized Fe 2p XPS spectra (open circles) of the T8 and the T12 SAMs on Au(111). A fit of the data (solid lines) and a background (dotted line) are also shown.
XPS can also provide quantitative information about the elemental composition of surfaces and, omitting the substrate contribution, enables the SAM composition to be compared with the precursor molecule’s stoichiometry. As shown in Table 1, the T8 and T12 SAMs show all the expected elements. The small amount of oxygen (<5%) detected could be due to a minor portion of oxidized ferrocenyl units (see the high resolution data), adventitious oxygen not replaced from the Au substrate during self-assembly, or residual solvent molecules. The XPS-derived carbon concentration is somewhat higher while the XPS-derived iron concentration is somewhat lower and the XPS-derived sulfur concentration is significantly lower than theoretically predicted. In view of the proposed SAM-like arrangement of the target molecules, the low intensity of the S 2p signal can be explained by the attenuation of the sulfur signal, which is a well-established phenomenon for densely packed SAMs on gold, since the sulfur species are usually located at the SAM-substrate interface. The extent of signal attenuation for the T12 SAM (ca. 62% of theoretical value) is comparable to the effect observed for dodecanthiol SAMs on gold (ca. 45% of theoretical value), which are known to exhibit only marginal (if any) amounts of unbound sulfur. This supports the assumption that the majority of the thioethers are close to the substrate and, thus, most likely coordinated to it. In view of such a molecular geometry, it can be assumed that the packing of the T8 and T12 molecules in the respective SAMs is determined by the size of the adamantine-thioether foot, providing the necessary spatial separation for the ferrocenyl units.
Table 1.
Elemental composition for the T8 and T12 SAMs
| SAM | Ca (atomic %) | Oa (atomic %) | Fea (atomic %) | Sa (atomic %) | Thickness (Å) |
|---|---|---|---|---|---|
| T8 experimental | 91.6 (1.1) | 4.9 (1.2) | 0.8 (0.2) | 2.6 (0.4) | 14.2 |
| T8 theoretical | 94.5 | 0 | 1.4 | 4.1 | ~20.0 |
| T12 experimental | 92.3 (0.9) | 4.8 (0.7) | 1.0 (0.2) | 2.2 (0.4) | 15.1 |
| T12 theoretical | 95.3 | 0 | 1.2 | 3.5 | ~20 |
The C, O, Fe and S atomic percents were renormalized to 100 atomic % to show the composition of the SAMs without the substrate gold signal. Standard deviations of the XPS compositions are shown in parentheses.
Attenuation effects are also expected for the Fe species, but to a much lower degree than for sulfur because in a tridentate binding geometry the ferrocenyl unit is expected to be close to the SAM-ambient interface. In such an ordered configuration, the signal gets slightly attenuated by the cyclopentadienyl rings. Indeed, the observed values, even though somewhat smaller, are close to the predicted Fe percentages for both films, thus indicating indirectly a significant degree of orientational order in the target films. Interestingly, for T12 the Fe percentage is closer to the predicted value than for T8 indicating indirectly a better molecular alignment in the former SAM. In the T8 SAM, some of the Fe sites are probably imbedded deeper into the film due to partial disorder, leading to an additional attenuation of the Fe signal.
In addition to the above analysis of the C 1s spectra, the effective thickness of the SAMs was calculated within the standard theoretical framework, using reported attenuation lengths.77, 78 The obtained values of the effective thickness are 14.2 Å and 15.1 Å for T8 and T12, respectively. These obtained thickness values are considerably lower than the theoretical estimates of ~20 Å, based on the sum of the respective molecular lengths and the substrate-S bond length (~1.8 Å) for the tridentate binding configurations and assuming an upright orientation of the molecules in the SAMs. This can be explained by the fact that the overall density of the tripod ligand films, even in the case of the dense packing of the adamantine-thioether anchors, should be significantly lower than that in the densely packed alkanethiol films used as a reference. In the former case, the packing is governed by the large footprints; in the latter – by the molecular chains. Thus, the lower (as compared to the molecular length) values of the effective thickness in the case of the T8 and T12 films are a manifestation of the molecular structure and organization in the films rather than the true thickness values. Significantly, these values are in agreement with the proposed adsorption model, within of which the molecules are oriented mostly upright in a tridentate binding geometry. For T8, the derived thickness value was slightly lower than for T12, reflecting the shorter alkyl chains and a lower overall film density, not necessarily a thinner film.
3.2.2 Near-edge X-ray absorption fine structure (NEXAFS) spectroscopy
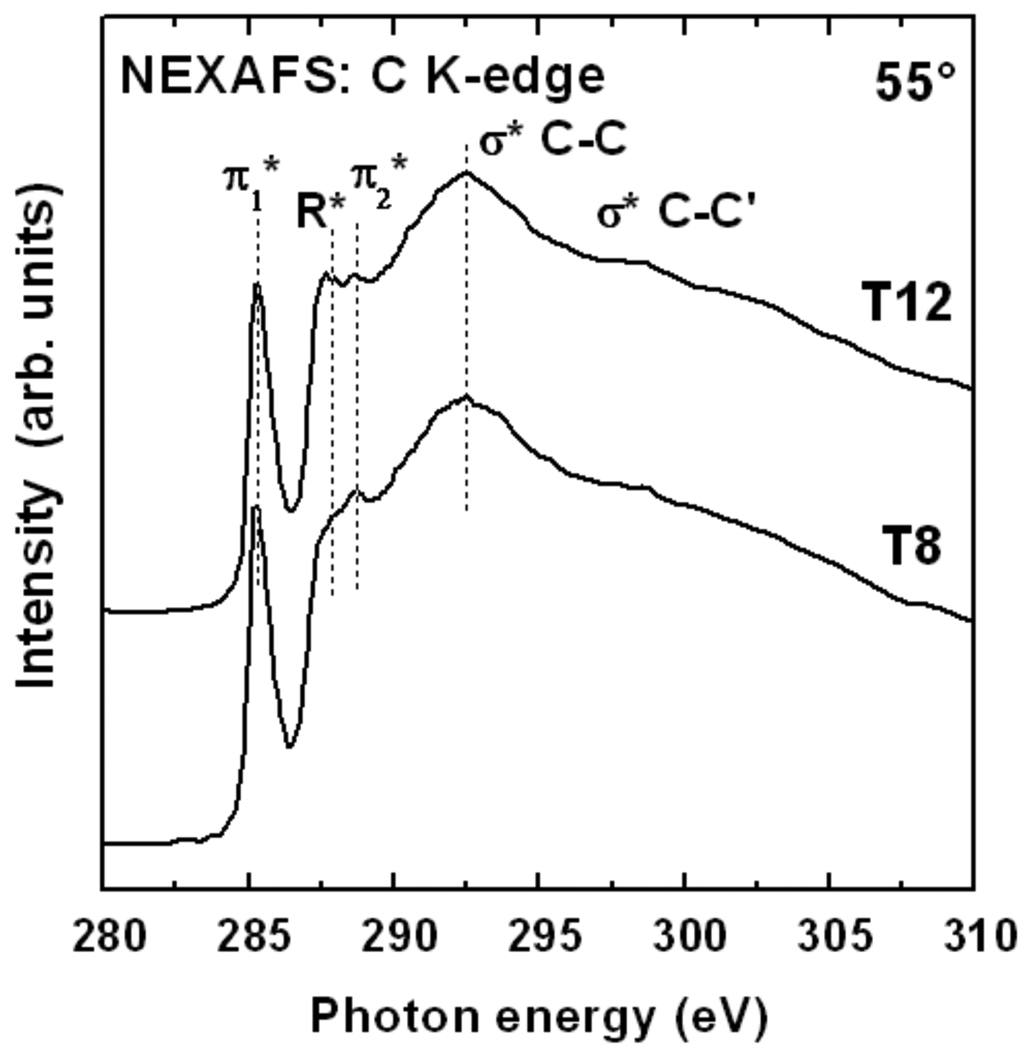
C K-edge NEXAFS spectra of T8 and T12 on gold are presented in Figure 5. These spectra were acquired at 55°, the so-called magic angle of X-ray incidence at which the spectra are independent of orientational effects.79 The spectra are dominated by the intense pre-edge π* resonance near 285.5 eV related to the phenylene and cyclopentandienyl rings. Other spectral features include a weak π2* resonance from phenylene at ~288.7 eV, a R*/σ*(C–H) resonance from the aliphatic part of the molecules and several broad σ*(C–C) features at higher photon energies. These spectral assignments were made based on results from with refs 52 and 80,81,82. The spectra of the T8 and T12 films are very similar. The most significant difference is the noticeably higher R*/σ*(C–H) resonance intensity for T12, which is expected from the longer alkyl chains of the T12 SAMs. The spectra exhibit no detectable contamination peaks, in particular no CO peaks that especially strong when contamination is present.83
Figure 5.
C K-edge NEXAFS spectra of the T8 and T12 SAMs on Au(111) acquired at an X-ray incidence angle of 55°.
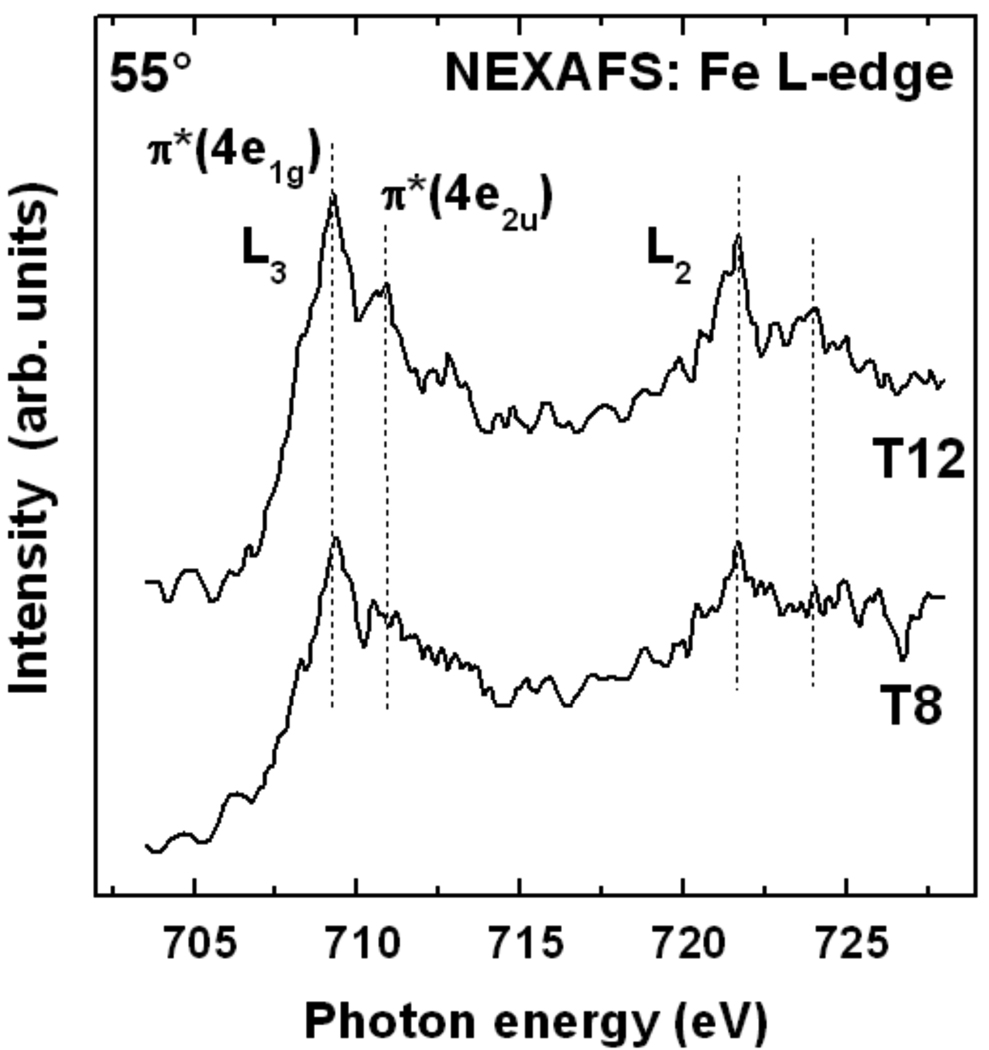
The NEXAFS spectra at the C K-edge are complemented by the Fe L-edge data. As shown in Figure 6, the spectra of both T8 and T12 films exhibit the characteristic π* resonances related to transitions into the 4e1g and 3e2u ferrocene-type orbitals at the L2 and L3-edges84, 85. The intensity for the T12 SAM is somewhat higher in agreement with the Fe 2p XPS data. Since the edge jump in the Fe NEXAFS spectra was not normalized to unity, the intensities are proportional to the Fe density. The results thus indicate a higher ferrocenyl density for T12 as compared to T8. The second possible explanation for the difference, namely a better film alignment, leading to less attenuation of photoelectrons due to ferrocenyl units getting covered by different SAM components, is less applicable in the given case as compared to XPS due to a smaller attenuation of the NEXAFS partial electron yield (PEY) signal at the retardation voltage of −450 V used here as compared with the elastic photoelectrons in XPS.86 The photon energy position of the L3 π*(4e1g) resonance is near 709.2 eV which is significantly higher than the position observed for solid ferrocene (~0.7 eV) 84, 85 or densely packed ferrocenyl-terminated SAMs.82 Such a blue-shift of photon energies has been reported by Shaporenko et al. 82 for specially designed SAMs with low ferrocenyl densities and was attributed to isolated ferrocenyl groups in the film. This indicates that the ferrocene units are also well separated from each other in the T8 and T12 SAMs.
Figure 6.
Fe L-edge NEXAFS spectra of the T8 and T12 SAMs on Au(111) acquired at an X-ray incidence angle of 55°.
Angle-dependent NEXAFS spectra, which can provide an insight into the average orientation of adsorbed species, were also acquired for the tripod ligand films and showed weak dichroism for both the aromatic and the aliphatic parts of the molecules (data not shown). However, in view of the intrinsically broad distribution of ring orientations in the precursor molecules, it is impossible to gain insight into the film alignment. The weak dischroism in the aliphatic part leads to the conclusion that the degree of orientational order in the films was relatively low or that the chains are strongly inclined. Based on our SFG analysis of the SAMs (v.i.), it is likely that both factors are involved.
3.3 Analysis of SAM alignment using sum frequency generation (SFG)
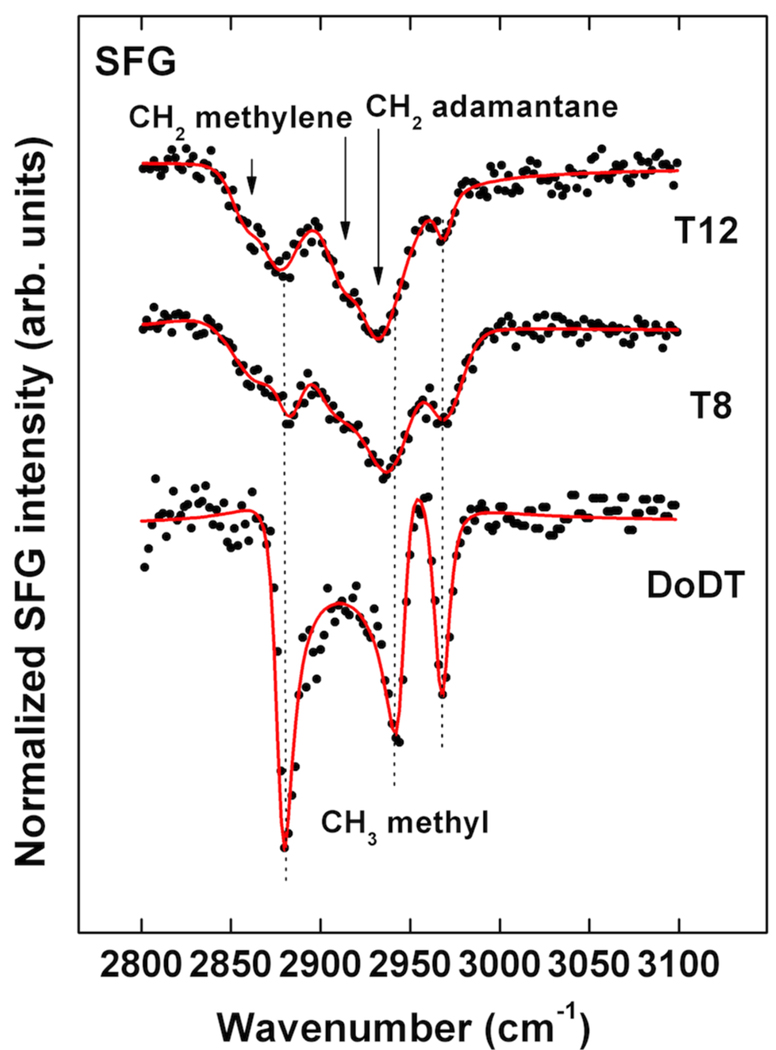
SFG spectra of the T8 and T12 SAMs and a reference DoDT SAM along with the corresponding fits are shown in Figure 7. The spectrum of DoDT SAM exhibits the expected resonances for terminal methyl units at 2969 cm−1 (r−), 2936 cm−1 (r−FR), and 2882 cm−1 (r+). These three modes are typical of well-ordered and densely packed alkane thiol SAMs on gold.87 As expected for DoDT, there are no CH2 related stretching modes visible in the spectrum, indicating that the alkyl chains are predominantly in an all-trans conformation.87 The T8 and T12 SAMs show much weaker intensity for the methyl modes, but the peaks related to the thioether alkyl chains are clearly visible. The low intensity of the signal is likely due to two effects, the lower surface density of alkyl chains in the tripod films compared to a pure alkane thiol SAM and a somewhat lower degree of orientational order among the chains. Symmetric and asymmetric methylene modes near 2860 cm−1 and 2915 cm−1, respectively, indicate also a considerable amount of gauche defects in the chains. The CH2/CH3 SFG peak amplitude ratio can be used as a semi-quantitative indicator for the amount of gauche defects in SAMs. The ratio, taken for the symmetric modes, is significantly higher for T8 (1.1) compared to T12 (0.5), showing that the chains have fewer defects for the letter SAM. This is additionally supported by Fourier transform infrared reflection absorption spectroscopy of the films. The symmetric and asymmetric methylene modes are observed near 2854 cm−1 and 2925 cm−1, respectively, which is a clear indication for a low chain order (data not shown).88 A strong resonance observed near 2930 cm−1 can either be assigned to alkyl chain CH2 Fermi resonance and asymmetric CH2 modes shifted by a liquid-like environment or to CH2 stretches related to the adamantane unit.89 Broad resonances of methylene are typical for trans-gauche defects in alkane thiols, but with SFG they are usually observed near 2915 cm−1 for the antisymmetric methylene stretching mode (d−),87, 90 while methylene Fermi resonances of completely disordered methylenes are rarely observed in disordered alkane thiol films. This makes an assignment of the 2930 cm−1 mode to the adamantane unit more likely here. There are no peaks visible related to the aromatic moieties. This could be explained by a lower degree of order for these units or by canceling of the SFG signal due to symmetry effects in the rings.64, 91
Figure 7.
SFG spectrum of T8 and T12 SAMs on Au(111) along with a spectrum of DoDT SAM taken as reference (bottom curve). Solid lines are best fits based on equation 1.
In summary, the SFG data is consistent with fairly well aligned alkyl chains in the SAMs with a considerable degree of gauche defects. Since the SFG peaks appear as dips instead of peaks in the spectra we can conclude, based on the phases of the SAM SFG signals relative to the non-resonant SFG background, that the chains are pointing away from the gold surface.87, 92, 93
4. Summary and discussion
According to the XPS, NEXAFS and SFG data, the long-chain tripodal thioether ligands with an adamantane-based large surface footprint and terminal redox-active ferrocenyl tailgroup T8 and T 12 form almost contamination-free, well-aligned and fairly densely-packed SAMs on Au(111). The SAMs show a homogeneous binding chemistry with only marginal extent of C–S bond cleavage at the thioether side chain, which is an important requirement for high fidelity thioether-based SAMs.44, 45, 52 The calculated thickness based on the XPS data is in agreement with a monolayer coverage. The upright orientation and extend of alignment observed for alkyl chains indicates that lateral interactions between neighboring molecules via the long-chain binding units plays an important role for the surface assembly. The tilt angles, in particular for the T12 films, are close to those observed for well-ordered alkane thiol SAMs on gold. T8, which has shorter alkyl chains, shows a lower packing density and film order than T12. The fact that chain length plays an important role for the film order indicates that self-assembly occurs via lateral van der Waals interactions of adjacent side chains, similar to alkane thiols on gold. The orientation of the ferrocenyl layer could not be assessed by SFG and NEXAFS, likely because of the inherently broad orientational distribution of aromatic units in the precursor molecules.
However, the XPS and NEXAFS Fe L-edge data clearly show that the ferrocenyl units are mostly non-oxidized and well separated in the film. In the case of monodentate binding units, such a separation has otherwise only been achieved in mixed monolayers.82
The results obtained here suggest that the long-chain thioethers can overcome steric effects of bulky tail groups and be a viable route for the assembly of tripodal molecules on coinage metal substrates.
Acknowledgements
M. Z. thanks M. Grunze for the support of this work, C. Wöll and A. Nefedov for technical cooperation at BESSY II, and the BESSY II staff for the assistance during the NEXAFS experiments. This work has been supported by German BMBF (05KS4VHA/4) and DFG (ZH 63/9-3). T. W. and D. G. C. thank NIH grant EB-002027 for support. T. W. thanks the German Research Foundation (DFG) for a research fellowship.
References
- 1.Schreiber F. Prog. Surf. Sci. 2000;65:151. [Google Scholar]
- 2.Schreiber F. J. Phys.: Condens. Matter. 2004;16:R881. [Google Scholar]
- 3.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Chem. Rev. 2005;105:1103. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 4.Onclin S, Ravoo BJ, Reinhoudt DN. Angew. Chem. Int. Ed. 2005;44:6282. doi: 10.1002/anie.200500633. [DOI] [PubMed] [Google Scholar]
- 5.Bunker BC. Material Science and Engineering R. 2008;62:157. [Google Scholar]
- 6.Weiss PS. Acc. Chem. Res. 2008;41:1772. doi: 10.1021/ar8001443. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Chen Q, Wan L-J. Phys. Chem. Chem. Phys. 2008;10:6467. doi: 10.1039/b810304f. [DOI] [PubMed] [Google Scholar]
- 8.Browne WR, Feringa BL. Annu. Rev. Phys. Chem. 2009;60:407. doi: 10.1146/annurev.physchem.040808.090423. [DOI] [PubMed] [Google Scholar]
- 9.Katsonis N, Lubomska M, Pollard MM, Feringa BL, Rudolf P. Prog. Surf. Sci. 2007;82:407. [Google Scholar]
- 10.Sekkat Z, Knoll W. In: Photoreactive Organic Thin Films. Sekkat Z, Knoll W, editors. San Diego, CA: Academic Press; 2002. [Google Scholar]
- 11.Siemeling U, Bruhn C, Bretthauer F, Borg N, Träger F, Vogel F, Azzam W, Badin M, Strunskus T, Wöll C. Dalton Trans. 2009:8593. doi: 10.1039/b905025f. [DOI] [PubMed] [Google Scholar]
- 12.Wagner S, Leyssner F, Kördel C, Zarwell S, Schmidt R, Weinelt M, Rück-Braun K, Wolf M, Tegeder P. Phys. Chem. Chem. Phys. 2009;11:6242. doi: 10.1039/b823330f. [DOI] [PubMed] [Google Scholar]
- 13.Suda M, Kameyama N, Ikegami A, Einaga Y. J. Am. Chem. Soc. 2009;131:865. doi: 10.1021/ja808231c. [DOI] [PubMed] [Google Scholar]
- 14.Weidner T, Bretthauer F, Ballav N, Motschmann H, Orendi H, Bruhn C, Siemeling U, Zharnikov M. Langmuir. 2008;24:11691. doi: 10.1021/la802454w. [DOI] [PubMed] [Google Scholar]
- 15.Mativetsky JM, Pace G, Elbing M, Rampi MA, Mayor M, Samorí P. J. Am. Chem. Soc. 2008;130:9192. doi: 10.1021/ja8018093. [DOI] [PubMed] [Google Scholar]
- 16.Elbing M, Blaszczyk A, von Haenisch C, Mayor M, Ferri V, Grave C, Rampi MA, Pace G, Samori P, Shaporenko A, Zharnikov M. Adv. Funct. Mater. 2008;18:2972. [Google Scholar]
- 17.Zhang X, Wen Y, Li Y, Li G, Du S, Guo H, Yang L, Ljiang L, Gao H, Song Y. Angew. Chem. Int. Ed. 2008;47:3407. [Google Scholar]
- 18.Ferri V, Elbing M, Pace G, Dickey MD, Zharnikov M, Samori P, Mayor M, Rampi MA. Angew. Chem. Int. Ed. 2008;47:3407. doi: 10.1002/anie.200705339. [DOI] [PubMed] [Google Scholar]
- 19.Kumar AS, Ye T, Takami T, Yu B-C, Flatt AK, Tour JM, Weiss PS. Nano Lett. 2008;8:1644. doi: 10.1021/nl080323+. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich P, Michalik F, Schmidt R, Gahl C, Mao G, Breusing M, Raschke MB, Priewisch B, Elsässer T, Mendelsohn R, Weinelt M, Rück-Braun K. Appl. Phys. A. 2008;93:285. [Google Scholar]
- 21.Ah Qune LFN, Akiyama H, Nagahiro T, Tamada K, Wee ATS. Appl. Phys. Lett. 2008;93:083109. [Google Scholar]
- 22.Demirel G, Rzaev Z, Patir S, Pişkin E. J. Nanosci. Nanotechnol. 2009;9:1865. doi: 10.1166/jnn.2009.388. [DOI] [PubMed] [Google Scholar]
- 23.Ernst O, Lieske A, Holländer A, Lankenau A, Duschl C. Langmuir. 2008;24:10259. doi: 10.1021/la801026y. [DOI] [PubMed] [Google Scholar]
- 24.Zareie HM, Boyer C, Bulmus V, Nateghi E, Davis TP. ACS Nano. 2008;2:757. doi: 10.1021/nn800076h. [DOI] [PubMed] [Google Scholar]
- 25.Mas-Torrent M, Crivillers N, Mugnaini V, Ratera I, Rovira C, Veciana J. J. Mater. Chem. 2009;19:1691. [Google Scholar]
- 26.Norman LL, Badia A. J. Am. Chem. Soc. 2009;131:2328. doi: 10.1021/ja808400s. [DOI] [PubMed] [Google Scholar]
- 27.Hauquier F, Ghilane J, Fabre B, Hapiot P. J. Am. Chem. Soc. 2008;130:2748. doi: 10.1021/ja711147f. [DOI] [PubMed] [Google Scholar]
- 28.Müller-Meskamp L, Lüssem B, Karthäuser S, Prikhodovski S, Homberger M, Simon U, Waser R. Phys. Status Solidi A. 2006;203:1448. [Google Scholar]
- 29.Li QMG, Gowda S, Surthi S, Zhao Q, Yu L, Lindsey JS, Bocian DF, Misra V. Adv. Mater. 2004;16:133. [Google Scholar]
- 30.Luk YY, Abbott NL. Science. 2003;301:623. doi: 10.1126/science.1084527. [DOI] [PubMed] [Google Scholar]
- 31.Smalley JFF, Harry O, Chidsey, Christopher ED, Linford, Matthew R, Creager, Stephen E, Ferraris, John P, Chalfant Keli, Zawodzinsk Thomas, Feldberg, Stephen W, Newton, Marshall D. J. Am. Chem. Soc. 2004;125:2004. doi: 10.1021/ja028458j. [DOI] [PubMed] [Google Scholar]
- 32.Mirkin CA, Xu F, Zhu J. Adv. Mater. 1997;9:167. [Google Scholar]
- 33.Kondo T, Horiuchi S, Yagi I, Ye S, Uosaki K. J. Am. Chem. Soc. 1999;121:391. [Google Scholar]
- 34.Gryko DT, Zhao F, Yasseri AA, Roth KM, Bocian DF, Kuhr WG, Lindsey JS. J. Org. Chem. 2000;65:7356. doi: 10.1021/jo0004862. [DOI] [PubMed] [Google Scholar]
- 35.Pace G, Ferri V, Grave C, Elbing M, von Hanisch C, Zharnikov M, Mayor M, Rampi MA, Samori P. Proc. Natl. Acad. Sci. U. S. A. 2007;104:9937. doi: 10.1073/pnas.0703748104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wöll C. Angew. Chem. Int. Ed. 2009;48:8406. doi: 10.1002/anie.200902974. [DOI] [PubMed] [Google Scholar]
- 37.Pannier N, Maison W. Eur. J. Org. Chem. 2008:1278. doi: 10.1021/jo702310g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q, Jin C, Petukhov PA, Rukavishnikov AV, Zaikova TO, Phadke A, LaMunyon DH, Lee MD, Keana JFW. J. Org. Chem. 2004;69:1010. doi: 10.1021/jo035640+. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Rukavishnikov AV, Petukhov PA, Zaikova TO, Keana JFW. Org. Lett. 2002;4:3631. doi: 10.1021/ol026495+. [DOI] [PubMed] [Google Scholar]
- 40.Rukavishnikov AV, Phadke A, Lee MD, LaMunyon DH, Pethukov PA, Keana JFW. Tetrahedron Lett. 1999;40:6353. [Google Scholar]
- 41.Katano S, Kim Y, Kitagawa T, Kawai M. Jpn. J. Appl. Phys. 2008;47:6156. [Google Scholar]
- 42.Katano S, Kim Y, Matsubara H, Kitagawa T, Kawai M. J. Am. Chem. Soc. 2007;129:2511. doi: 10.1021/ja065893v. [DOI] [PubMed] [Google Scholar]
- 43.Kitagawa T, Idomoto Y, Matsubara H, Hobara D, Kakiuchi T, Okazaki T, Komatsu K. J. Org. Chem. 2006;71:1362. doi: 10.1021/jo051863j. [DOI] [PubMed] [Google Scholar]
- 44.Weidner T, Ballav N, Siemeling U, Troegel D, Walter T, Tacke R, Castner DG, Zharnikov M. Journal of Physical Chemistry C. 2009;113:19603. doi: 10.1021/jp906367t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weidner T, Krämer A, Bruhn C, Zharnikov M, Shaporenko A, Siemeling U, Träger F. Dalton Trans. 2006:2767. doi: 10.1039/b515727g. [DOI] [PubMed] [Google Scholar]
- 46.Kittredge KW, Minton MA, Fox MA, Whitesell JK. Helv. Chim. Acta. 2002;85:788. [Google Scholar]
- 47.Schönherr H, Vansco GJ, Huisman B-H, van Veggel FCJM, Reinhoudt DN. Langmuir. 1999;15:5541. [Google Scholar]
- 48.Huisman B-H, Rudkevich DM, van Veggel FCJM, Reinhoud DN. J. Am. Chem. Soc. 1996;118:3523. [Google Scholar]
- 49.Huisman B-H, Thoden van Velzen EU, van Veggel FCJM, Engbersen JFJ, Reinhoudt DN. Tetrahedron Lett. 1995;36:3273. [Google Scholar]
- 50.Thoden van Velzen EU, Van Veggel FCJM, Engbersen JFJ, Reinhoudt DN. J. Am. Chem. Soc. 1995;116:3597. [Google Scholar]
- 51.Takiguchi H, Sato K, Ishida T, Abe K, Yase K, Tamada K. Langmuir. 2000;16:1703. [Google Scholar]
- 52.Weidner T, Ballav N, Zharnikov M, Priebe A, Long NJ, Maurer J, Winter R, Rothenberger A, Fenske D, Rother D, Bruhn C, Fink H, Siemeling U. Chem Eur. J. 2008;14:4346. doi: 10.1002/chem.200701936. [DOI] [PubMed] [Google Scholar]
- 53.Leavy MC, Bhattacharyya S, Cleland WE, Jr, Hussey CL. Langmuir. 1999;16:1703. [Google Scholar]
- 54.Schebler PJ, Riordan CG, Liable-Sands L, Rheingold AL. Inorg. Chim. Acta. 1998;270:543. [Google Scholar]
- 55.Hu J, Mattern DL. J. Org. Chem. 2000;65:2277. doi: 10.1021/jo9909812. [DOI] [PubMed] [Google Scholar]
- 56.Li X-M, de Jong MR, Inoue K, Shinkai S, Huskens J, Reinhoudt DN. J. Mater. Chem. 2001;11:1919. [Google Scholar]
- 57.Polin J, Schottenberger H. Org. Synth. 1996;73:262. [Google Scholar]
- 58.Reichert VR, Mathias LJ. Macromolecules. 1994;27:7015. [Google Scholar]
- 59.Heister K, Zharnikov M, Grunze M, Johansson LSO. J. Phys. Chem. B. 2001;105:4058. [Google Scholar]
- 60.Moulder JF, Stickle WF, Sobol PE, Bomben KD. Handbook of X-ray Photoelectron Spectroscopy. Eden Prairie: Perkin-Elmer Corp.; 1992. [Google Scholar]
- 61.Batson PE. Phys. Rev. B. 1993;48:2608. doi: 10.1103/physrevb.48.2608. [DOI] [PubMed] [Google Scholar]
- 62.Baio JE, Weidner T, Brison J, Graham DJ, Gamble LJ, Castner DG. J. Electron. Spectrosc. Relat. Phenom. 2009;172:2. doi: 10.1016/j.elspec.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boyd RW. Nonlinear Optics. 1 ed. London: Academic Press; 1992. [Google Scholar]
- 64.Shen YR. The Principles of Nonlinear Optics. 1 ed. New York: John Wiley & Sons; 1984. [Google Scholar]
- 65.Bain CD, Davies PB, Ong TH, Ward RN, Brown MA. Langmuir. 1991;7:1563. [Google Scholar]
- 66.Wei Q, Galoppini E. Tetrahedron. 2004;60:8497. [Google Scholar]
- 67.Murata SL, Buchwald SL. Tetrahedron. 2004;60:7782. [Google Scholar]
- 68.Fernández-Rodríguez MA, Shen Q, Hartwig JF. Chem. Eur. J. 2006;12:7782. doi: 10.1002/chem.200600949. [DOI] [PubMed] [Google Scholar]
- 69.Fernández-Rodríguez MA, Hartwig JF. J. Org. Chem. 2009;74:1663. doi: 10.1021/jo802594d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartwig JF. Acc. Chem. Res. 2008;41:1534. doi: 10.1021/ar800098p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zharnikov M. J. Electron. Spectrosc. Relat. Phenom. 2010;178–179:380. [Google Scholar]
- 72.Castner DG, Hinds K, Grainger DW. Langmuir. 1996;12:5083. [Google Scholar]
- 73.Zhong C-J, Brush RC, Anderegg J, Porter MD. Langmuir. 1999;15:518. [Google Scholar]
- 74.Heister K, Zharnikov M, Grunze M, Johansson LSO, Ulman A. Langmuir. 2000;17:8. [Google Scholar]
- 75.Trevor JL, Lykke KR, Pellin MJ, Hanley L. Langmuir. 1998;14:1664. [Google Scholar]
- 76.Beulen MWJ, Huisman B-H, van der Heijden PA, van Veggel FCJM, Simons MG, Biemond EMEF, de Lange PJ, Reinhoudt DN. Langmuir. 1996;12:6170. [Google Scholar]
- 77.Thome J, Himmelhaus M, Zharnikov M, Grunze M. Langmuir. 1998;14:7435. [Google Scholar]
- 78.Lamont CLA, Wilkes J. Langmuir. 1999;15:2037. [Google Scholar]
- 79.Stöhr J. NEXAFS Spectroscopy. Vol. 25. Berlin: Springer-Verlag; 1992. [Google Scholar]
- 80.Frey S, Stadler V, Heister K, Eck W, Zharnikov M, Grunze M, Zeysling B, Terfort A. Langmuir. 2001;17:2408. [Google Scholar]
- 81.Zharnikov M, Grunze M. J. Phys.: Condens. Matter. 2001;13:11333. [Google Scholar]
- 82.Shaporenko A, Rossler K, Lang H, Zharnikov M. J. Phys. Chem. B. 2006;110:24621. doi: 10.1021/jp064943f. [DOI] [PubMed] [Google Scholar]
- 83.Shaporenko A, Adlkofer K, Johansson LSO, Tanaka M, Zharnikov M. Langmuir. 2003;19:4992. [Google Scholar]
- 84.Ruehl EHAP. J. Am. Chem. Soc. 1989;111:5069. [Google Scholar]
- 85.Rühl E, Heinzel C, Baumgartel H, Hitchcock AP. Chem. Phys. 1993;169:243. [Google Scholar]
- 86.Zharnikov M, Frey S, Heister K, Grunze M. J. Electron. Spectrosc. Relat. Phenom. 2002;124:15. [Google Scholar]
- 87.Himmelhaus M, Eisert F, Buck M, Grunze M. J. Phys. Chem. B. 2000;104:576. [Google Scholar]
- 88.Porter MD, Bright TB, Allara DL, Chidsey CED. J. Am. Chem. Soc. 1987;109:3559. [Google Scholar]
- 89.Bellamy LD. The infra-red spectra of complex molecules. 3rd ed. Thetford, Norfolk: Lowe & Brydone; 1975. [Google Scholar]
- 90.Yang CSC, Richter LJ, Stephenson JC, Briggman KA. Langmuir. 2002;18:7549. [Google Scholar]
- 91.Varsanyi G. Assignments for Vibrational Spectra of Seven Hundred Benzene Derivatives. Vol. 1.1. London: Adam Hilger; 1974. 1.2, 2. [Google Scholar]
- 92.Ward RN, Davies PB, Bain CD. J. Phys. Chem. 1993;97:7141. [Google Scholar]
- 93.Weidner T, Apte J, Gamble LJ, Castner DG. Langmuir. 2010;26:3433. doi: 10.1021/la903267x. [DOI] [PMC free article] [PubMed] [Google Scholar]