Abstract
The prevalence of central nervous system (CNS) neurologic dysfunction associated with human immunodeficiency virus (HIV) infection continues to increase, despite the use of antiretroviral therapy. Previous work has focused on the deleterious effects of HIV on mature neurons and on development of neuroprotective strategies, which have consistently failed to show a meaningful clinical benefit. It is now well established that new neurons are continuously generated in discrete regions in the adult mammalian brain, and accumulating evidence supports important roles for these neurons in specific cognitive functions. In a transgenic mouse model of HIV neurologic disease with glial expression of the HIV envelope protein gp120, we demonstrate a significant reduction in proliferation of hippocampal neural progenitors in the dentate gyrus of adult animals, resulting in a dramatic decrease in the number of newborn neurons in the adult brain. We identify amplifying neural progenitor cells (ANPs) as the first class of progenitors affected by gp120, and we also demonstrate that newly generated neurons exhibit aberrant dendritic development. Furthermore, voluntary exercise and treatment with a selective serotonin reuptake inhibitor increase the ANP population and rescue the observed deficits in gp120 transgenic mice. Thus, during HIV infection, the envelope protein gp120 may potently inhibit adult hippocampal neurogenesis, and neurorestorative approaches may be effective in ameliorating these effects. Our study has significant implications for the development of novel therapeutic approaches for HIV-infected individuals with neurologic dysfunction and may be applicable to other neurodegenerative diseases in which hippocampal neurogenesis is impaired.
Keywords: adult neurogenesis, dendritic development, neural progenitor, gp120, exercise, SSRI
INTRODUCTION
Soon after the discovery of the human immunodeficiency virus (HIV) it was realized that the virus may cause a dementing illnesss (Navia et al., 1986a) and that it may enter the brain early in the course of infection. With the availability of combination antiretroviral therapies, the severity of neurocognitive impairment has become milder but the prevalence continues to increase (Sacktor, 2002). In recent years closer attention has been paid to the asymptomatic and milder forms of neurologic impairment. In a recent study, the prevalence of HIV associated neurocognitive disorders (HAND) in aviremic patients was 84% among patients with cognitive complaints and 64% among non-complainers (Simioni et al., 2009). While it is widely accepted that neuroprotective strategies are needed to treat HAND, to date this approach has shown little or no effect (McArthur et al., 2005), in part because the underlying substrate mediating neurologic dysfunction remains poorly understood. While much attention has previously focused on frontostriatal dysfunction in HIV infection (Chang et al., 2001; Ernst et al., 2002), the hippocampus is increasingly recognized as an important target of HIV-mediated neurotoxicity. The presence of atrophy, high viral loads, neuronal cell loss, and functional MRI abnormalities in the hippocampus of HIV-infected patients suggests that the hippocampus may be a target of HAND (Archibald et al., 2004; Castelo et al., 2006; Sa et al., 2004; Wiley et al., 1998).
Within the dentate gyrus of the adult hippocampus, new granule cells are continuously generated throughout life in all mammals studied, including humans (Johnson et al., 2009; Lledo et al., 2006; Ming and Song, 2005a; Zhao et al., 2008). Neurogenesis involves a coordinated series of steps including proliferation, survival and differentiation of neural progenitor cells (NPC) and maturation of newly generated neurons (Duan et al., 2008; Johnson et al., 2009; Lledo et al., 2006; Zhao et al., 2008). While the exact functions of adult neurogenesis are still elusive, rapidly accumulating evidence from behavioral analyses supports a contribution of new neurons in the adult brain to learning, memory and cognition (Kempermann et al., 2004; Kitabatake et al., 2007). At the cellular level, adult-generated dentate granule cells exhibit a high degree of synaptic plasticity within a critical period (Ge et al., 2007), and are preferentially incorporated over pre-existing granule cells into spatial memory networks (Kee et al., 2007). In addition, pathogenic processes that decrease neurogenesis interfere with hippocampal-dependent memory formation (Haughey et al., 2002; Monje et al., 2003). How adult hippocampal neurogenesis may be affected by HIV infection is largely unknown (Venkatesan et al., 2007). An autopsy study demonstrated fewer proliferating NPCs in the dentate gyrus of patients with HAND (Krathwohl and Kaiser, 2004). In another study, injection of HIV-infected monocyte derived macrophages into the brains of adult mice led to a decrease in hippocampal neurogenesis (Poluektova et al., 2005). More recently, the HIV envelope protein gp120, an important mediator of HAND (Mattson et al., 2005), was found to decrease adult hippocampal neural precursor cell proliferation via activation of the p38 mitogen-activated protein kinase (MAPK) cascade with subsequent arrest at the G1 phase of the cell cycle (Okamoto et al., 2007). Importantly, gp120 immunoreactivity has been demonstrated throughout the brains of individuals infected with HIV, including the hippocampus (Jones et al., 2000).
Here, we focus on characterizing and rescuing defective adult hippocampal neurogenesis in a well-established transgenic mouse model system of HIV neurologic disease, in which HIV gp120 is expressed within glial cells in the brain (Toggas et al., 1994). We first demonstrate that there is a significant defect of adult hippocampal neurogenesis in gp120-transgenic mice, resulting from a reduction of progenitor cell proliferation. Proliferation of amplifying neural progenitor cells (ANPs) is decreased, while quiescent neural progenitor cells (QNPs) are not affected by gp120 expression. In addition, dendritic development of newly generated adult hippocampal neurons is abnormal in gp120 transgenic mice, implying cell-stage specific effects of gp120. We next determined whether the observed defects in neurogenesis in gp120 transgenic mice could be rescued by methods that increase proliferating ANPs. Both voluntary exercise and treatment with selective serotonin receptor inhibitors (SSRIs) have been shown to increase ANPs in some, but not all, disease models (van Praag et al., 1999; van Praag et al., 1999; Malberg et al., 2000; Santarelli et al., 2003; Encinas et al., 2006). We therefore sought to determine whether the deficits in adult hippocampal neurogenesis observed in gp120 transgenic mice could be reversed by these physiological and pharmacological measures.
MATERIALS AND METHODS
Transgenic mice
Eight to nine week old gp120 transgenic mice (B6×SJL strain) in which the expression of gp120 is driven by a glial fibrillary acidic protein (GFAP) promoter (Toggas et al., 1994) and littermate wildtype (wt) controls were used. To confirm gp120 expression in transgenic animals, Western blot of hippocampal lysates was performed with gp120 antibody (Cat # 1101; 1:200; Immunodiagnostics, Woburn, MA). The same blot was re-probed with antibodies against GAPDH as a loading control (Cat# G8795; 1:10,000, Sigma). Blots were developed using the ECL Plus™ kit per manufacturer's instructions (Amersham Biosciences, Piscataway, NJ). A minimum of four animals per group were used for each experimental condition. To assess cell proliferation, bromodeoxyuridine (BrdU; 200 mg/kg) was injected intraperitoneally (i.p.), and animals were euthanized 2 hours later. For studies of cell fate determination and cell survival, BrdU (50 mg/kg) was injected i.p. daily for seven days, followed by euthanization at 14 or 28 days after the initial BrdU injection. In separate experiments to assess the effects of exercise or paroxetine on cell fate determination, BrdU (50 mg/kg) was injected i.p. four times at 2 hour intervals, then animals were euthanized 20 days after the BrdU injection.
Retroviral labeling of newly generated neurons
Engineered self-inactivating murine oncoretroviruses were used to express green fluorescent protein (GFP) specifically in proliferating cells and their progeny (Duan et al., 2007; van Praag et al., 2002). For dendritic analysis, three-dimensional reconstruction of entire dendritic processes of each neuron was made from Z series stacks of confocal images. The two-dimensional projection images were traced with NIH ImageJ. GFP+ dentate granule cells with largely intact dendritic trees were analyzed for total dendritic length and branch number as described (Ge et al., 2006). Sholl analysis for dendritic complexity was carried out by counting the number of dendrites that cross a series of concentric circles from the soma as previously described (Ge et al., 2006). Mice were analyzed at either one or two weeks following retroviral labeling. Data shown are from a minimum of 10 individual GFP+ neurons from at least four animals in each group.
Immunohistochemistry, microscopy, and quantification
Animals were transcardially perfused with saline followed by 4% (w/v) paraformaldehyde (PFA). After postfixing in PFA overnight, brains were immersed in a 30% (v/v) sucrose solution. On the following day, brains were cut in 40 μm thick coronal sections on a sliding microtome. Every sixth brain section spanning the entire hippocampal dentate gyrus was washed in Tris-Buffered Saline (TBS) prior to incubation in blocking solution (TBS with 0.5% (v/v) Triton-X and 2.5% (v/v) donkey serum). Primary antibodies were diluted in blocking solution as follows: anti-bromodeoxyuridine (BrdU) (Cat#OBT0030; 1:1000; Accurate, Westbury, NY, USA), anti-NeuN (Cat# MAB377B; 1:250, Chemicon, Temecula, CA, USA), anti-doublecortin (DCX; Cat# sc-8066;1:250; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-GFAP (Cat# Z0334; 1:2,000; Dako, Carpinteria, CA, USA), anti-Sox2 (Cat#AB5608; 1:500; Chemicon, Temecula, CA), anti-Tbr2 (Cat#ab23345; 1:500; Abcam, Cambridge, MA, USA) or anti-gp120 (cat#1101; 1:200; ImmunoDiagnostics, Woburn, MA, USA) and incubated with slices overnight at 4°C. For detection of BrdU, slices were treated with 1M HCl at 37°C for 30 min prior to application of the primary antibody. After washing with TBS, slices were incubated with the appropriate fluorescent conjugated secondary antisera (1:250, Jackson ImmunoResearch, West Grove, PA, USA), followed by washing and counterstaining with 4’,6-diamidino-2-phenylindole (DAPI) to label all nuclei. Stained slices were dried, mounted on slides, and viewed with a Zeiss confocal laser microscope. Z-stacks (2 μm thick, spanning the entire 40 mm thickness of each slice) were constructed for each image. All immunopositive cells within the subgranular zone and granule cell layer of every sixth slice spanning the hippocampal dentate gyrus were counted by a modified stereological method as previously described (Lie et al., 2005). Double- and triple-immunolabeling was confirmed for each cell by orthogonal analysis using LSM 5 Image Browser. Positively labeled cells were expressed as cells per volume of the granule cell layer, as assessed by staining of the nuclei with DAPI.
Voluntary exercise
For voluntary running experiments, mice were housed in a cage containing a running wheel. The voluntary wheel system consists of an 11.5-cm-diameter wheel with a 5.0-cm-wide running surface (model 61390, Petsmart, Phoenix, AZ, USA) equipped with a digital magnetic wireless counter (model CC-MC100W, CatEye, Boulder, CO, USA) that is activated by wheel rotation. During the running period, running duration and distance were recorded daily for each cage. Two to three mice were housed per cage in all experiments. Running distance per mouse was calculated by dividing total distance by number of mice in each cage. For proliferation experiments, mice were allowed access to running wheels for 10 days. For experiments in which BrdU+NeuN+ newly generated neurons were assessed, mice were allowed access to running wheels for 21 days. For analysis of the effects of exercise on initial dendritic development, exercise was initiated 3 days after retroviral labeling, and continued until 2 weeks post injection.
Paroxetine treatment
Chronic administration of paroxetine (Tocris Cookson, Ellisville, MO, USA) was accomplished by subcutaneous implantation of an osmotic mini-pump (model 2004, Alzet, Palo Alto, CA, USA) according to manufacturer's instructions. Pumps containing either paroxetine or saline were activated prior to implantation by overnight incubation at 37°C as per manufacturer's instructions. Pumps were placed subdermally in the interscapular region of mice under deep anaesthesia, and a pump flow rate of 0.25 μl/h allowed for delivery of paroxetine at a dose of 10 mg/kg per day for four weeks. Upon completion of experiments, pumps were removed and residual volume was determined, to ensure that the appropriate amount of drug had indeed been delivered to each mouse.
Human neural progenitor cell (NPC) culture
Human NPCs were cultured from human fetal brain specimens of 7-8 weeks gestation in accordance with NIH guidelines and following approval by the Institutional Review Board at the Johns Hopkins University. The tissues were then dissociated after removing meninges and blood vessels. After centrifugation at 1000 rpm for 10 min, cells were resuspended in DMEM/F12 media [containing 8 mM glucose, 1× N2 supplement, 1% antibiotics, 0.1% albumin (Sigma), human fibroblast growth factor-beta (hFGFb; 20 ng/mL) and human epithelial growth factor (hEGF) (20 ng/mL)] and plated on poly-D-lysine (Sigma) coated T 25 cm2 tissue culture flasks. When cell cultures reached 60% confluence, they were treated with 0.0125% trypsin (Sigma) and replated at a density of 2×104 cells/ml on coverslips in 24 well plates. Media was replaced every other day. NPC cultures were ready for experiments 4-5 days after replating and >98% of the cells expressed the neural stem cell marker nestin while <1% of the cells expressed GFAP (a marker for astrocytes) or beta-III tubulin (a neuronal cell marker) as determined by immunocytochemistry. Cells were exposed to HIV gp120 IIIB (Immunodiagnostics), and heat inactivation was accomplished by heating the protein at 90°C for 10 minutes. Percentage of proliferating NPCs (BrdU+nestin+ cells/nestin+ cells) was assessed by incubating the cells with BrdU labeling reagent (1:100, ZYMED, San Francisco, CA) for 4 hours followed by fixation and immunostaining for BrdU and nestin. To assess for cell death, cells were subjected to a Live/Dead Viability assay (Invitrogen Molecular Probes, Cat # L-3224) as per manufacturer's protocol and immediately visualized under 20x magnification. Alternatively, cells were stained with trypan blue for 5 min, washed with PBS, pH 7.4 (PBS), and immediately visualized under 20× magnification. Quantification of cells was performed in four predetermined fields per well, and each experiment was done in triplicate wells.
RESULTS
HIV-1 gp120 impairs adult hippocampal neurogenesis by inhibiting proliferation of NPCs
We first determined whether gp120 was expressed in the hippocampus of gp120-transgenic mice. Western blot analysis of hippocampi of transgenic animals showed the HIV gp120 protein band of approximate molecular mass 120 kDa, which was absent from littermate control wt hippocampal lysates (Fig. 1A). Immunohistochemical analysis demonstrated expression of gp120 in stellate-shaped GFAP+ astrocytes throughout the dentate gyrus as well as in subgranular zone GFAP+ cells that adopted a radial glial morphology. As expected, all cells exhibiting gp120 immunoreactivity were GFAP+ (Fig 1B,C).
Figure 1. Characterization of gp120 expression in transgenic mice.
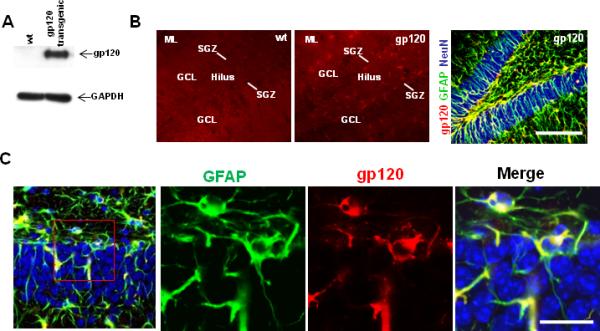
A. Western blot of hippocampal lysates from wt and transgenic mice demonstrates gp120 expression in gp120 transgenic but not in wt. B. Immunohistochemistry of hippocampal sections with gp120 antibody demonstrates gp120 expression (red) in hippocampal molecular layer (ML), hilus, granule cell layer (GCL), and subgranular zone (SGZ) in gp120 mice but not in wt. Triple-labeling immunohistochemistry of gp120 mouse hippocampal sections (red, gp120; green, GFAP; blue, NeuN) demonstrates extensive colocalization of gp120 and GFAP (yellow), particularly in the hilus of gp120 transgenic mice, in close proximity to the neurogenic region of the hippocampal dentate gyrus. Scale bar 100 um. C. High powered view (40x) of hippocampal dentate gyrus in gp120 transgenic mice demonstrates that each cell that expresses gp120 also expresses GFAP.
We next determined whether gp120 expression affects the proliferation of adult hippocampal NPCs in vivo. Adult mice were administered a single dose of BrdU to label proliferating cells, and euthanized 2 hours later. Quantitative analysis showed a 40% reduction of BrdU+ cells in the dentate gyrus of gp120 transgenic mice as compared to their littermate wt mice (Fig. 2A,B), suggesting that expression of gp120 inhibits proliferation of adult hippocampal NPCs. The observed decrease in proliferation in gp120 transgenic mice was comparable to that seen in a recent study (Okamoto et al., 2007), in which many of the BrdU+ cells were also found to express the marker PSA-NCAM, suggesting that the cells were neuronal, rather than glial, precursor cells. To confirm that the observed reduction in proliferation of adult hippocampal NPCs in gp120-transgenic mice results in a decrease in newly generated neuronal cells, gp120-transgenic and littermate wt mice were injected with BrdU for 7 days, and animals were analyzed at four weeks after the first BrdU injection. We used immunocytochemical markers to examine the fate of BrdU+ cells, using NeuN for mature neurons, doublecortin (DCX) for immature neurons, and glial fibrillary acidic protein (GFAP) for stellate-shaped astrocytes (Fig. 2C). Triple-label immunohistochemistry and confocal analysis (Fig. S1) showed a 45% and 55% reduction in the number of newly generated mature neurons (BrdU+NeuN+) and immature neurons (BrdU+DCX+NeuN-) respectively in the dentate gyrus of gp120 mice as compared to littermate wt mice (Fig. 2D). In contrast, no significant differences in cell fate specification of hippocampal NPCs were observed. The percentages of BrdU+ cells that acquired phenotypes of NeuN+ mature neurons, DCX+NeuN- immature neurons,or GFAP+ astrocytes were similar between wt and gp120 mice (Fig. 2E). Thus, HIV gp120 reduces generation of new neurons in the adult hippocampus, but does not appear to affect cell fate specification of adult hippocampal NPCs.
Figure 2. gp120 mice exhibit impairment of adult hippocampal neurogenesis.

A, B. Representative images (A) and quantification (B) of proliferating (BrdU+, green) hippocampal cells in the neurogenic region of wt and gp120 transgenic mice. Tissue is counterstained with DAPI (blue). SGZ, subgranular zone. GCL, granule cell layer. Values represent mean + SEM; n=5 per group; * p<0.01 Student's t test . Scale bar 100 um. C. Representative images of cells triple labeled with BrdU, DCX, and NeuN to identify newly generated neurons (BrdU+NeuN+) and neuroblasts (BrdU+DCX+NeuN-). Scale bar 100 um. D. Quantification of data in C. * p<0.05 E. Quantification of percentages of newly generated cells that differentiate into mature neurons (NeuN+), immature neurons (DCX+/NeuN-), and astrocytes (GFAP+) reveals no significant differences in cell fate specification between wt and gp120 transgenic mice (corresponding p-values are > 0.05 as assessed by ANOVA with Bonferroni post-test. F. Survival of newborn neurons assessed by injection of BrdU for 7 days and analysis at 2 and 4 weeks after the initial injection. Left, BrdU+ cells decrease at a similar rate in both wt and gp120 transgenic animals (p>0.05). Right, BrdU+NeuN+ newborn neurons decrease at a similar rate between 2 weeks and 4 weeks in both wt and gp120 transgenic animals (p>0.05). p-values are calculated from 2-way ANOVA comparisons to detect two-factor interactions (genotype × time).
To determine whether gp120 also regulates the survival of newborn neurons in the adult hippocampus, another group of mice was labeled with BrdU for 7 days followed by euthanization at 2 and 4 weeks after the first BrdU injection. Quantitative comparison was made between the number of labeled cells at 2 and 4 weeks after initial BrdU injection. As shown in Fig 2F, the total number of BrdU+ cells decreased over time in both wt and gp120 transgenic mice, reflecting loss of newly generated cells. However, the rates of change in total BrdU+ newborn cells and BrdU+NeuN+ newborn neurons were not significantly different between the 2 and 4 week time-points, suggesting that gp120 does not impair survival of newly generated cells during this period of time (Tashiro et al., 2006). In addition, TUNEL analysis showed only rare apoptotic cells in the hippocampal dentate gyrus of either wt or gp120 transgenic mice. Taken together, these results indicate that the reduction in adult hippocampal neurogenesis in gp120-transgenic mice results mainly from decreased proliferation rather than impairment of either neuronal fate specification or survival of newly generated neurons.
HIV-1 gp120 expression decreases proliferation of ANPs
To further characterize the subtypes of NPCs affected in gp120-transgenic mice, we examined the expression of Sox2 and GFAP in the subgranular zone (SGZ) of the hippocampus. In the SGZ, Sox2 expression occurs in both quiescent neural progenitor cells (QNPs) and in amplifying neural progenitor cells (ANPs), while GFAP is expressed in QNPs. Although astrocytes can also co-express Sox2 and GFAP, these cells exhibit a stellate morphology that allows for ready differentiation from QNPs (Komitova and Eriksson, 2004; Segi-Nishida et al., 2008). Mice were euthanized two hours after BrdU injection, and immunohistochemical labeling and morphologic criteria were used to unambiguously identify ANPs and QNPs (Fig 3A). We observed a 50% decrease in BrdU+Sox2+GFAP- cells (proliferating ANPs) in gp120 mice compared to wt, while numbers of BrdU+Sox2+GFAP+ cells (proliferating QNPs) were similar between the two groups (Fig 3B,C). The decrease in ANP proliferation was accompanied by a 60% reduction in total numbers of ANPs in gp120 transgenic animals as compared to wt, while total QNPs were similar between the two groups (Fig 3D,E). These results demonstrate that gp120 expression preferentially impairs proliferation of ANPs, while sparing the QNP population.
Figure 3. Proliferating ANPs are reduced in gp120 transgenic mice.

Mice were analyzed 2 hours after BrdU injection. A. Triple labeling with antibodies to GFAP, Sox2, and BrdU allows unambiguous identification of QNPs (GFAP+Sox2+; arrows) and ANPs (GFAP-Sox2+; arrowhead). In gp120 mice, proliferating ANPs (B) and total ANPs (D) are reduced compared to wt, while numbers of proliferating and total QNPs (C,E) are similar between the two groups.
Exercise rescues adult hippocampal neurogenesis in gp120 transgenic mice
Exercise enhances adult hippocampal neurogenesis by increasing proliferation of ANPs in wt rodents, but effects in disease models have been variable (Kohl et al., 2007; Kronenberg et al., 2003; Luo et al., 2007; Naylor et al., 2008; Redila et al., 2006; van Praag et al., 1999). Since we observed a decrease in proliferating ANPs in gp120 transgenic mice, we sought to determine whether voluntary exercise can rescue neurogenesis defects in gp120 transgenic mice. Over the course of ten days, both wt and gp120 transgenic mice housed in cages with running wheels averaged running approximately 4-5 km/day, consistent with previous reports of voluntary exercise in mice. We found that running increased NPC proliferation in hippocampi of wt mice by 56%, similar to previous reports (van Praag et al., 1999). Importantly, the same ten day period of voluntary running was sufficient to completely rescue the deficit in NPC proliferation in the dentate gyrus of gp120 mice, increasing NPC proliferation by over 100% to reach levels similar to wt animals (Fig 4A). In addition, we found that ANP proliferation increased significantly in gp120 transgenic mice upon running, and this was also accompanied by an increase in total numbers of ANPs (Fig 4B,C). To further confirm that ANPs were rescued by exercise, we analyzed expression of Tbr2, a marker expressed in ANPs in the adult hippocampus (Hodge et al., 2008). Total numbers of Tbr2 cells were markedly decreased in gp120 transgenic animals compared to wt, concordant with our observations of Sox2+GFAP- ANPs. Upon exercise, both BrdU+Tbr2+ and total Tbr2+ cells increased markedly in gp120 transgenic mice, again consistent with a rescue of proliferating ANPs (Fig 4D,E). To determine whether this rescue of ANPs resulted in the formation of new dentate granule neurons, we allowed mice access to running wheels for a total of three weeks. Voluntary running resulted in a marked increase in the number of newly generated hippocampal neurons formed in gp120 transgenic mice, to levels in excess of that observed in wt non-running mice (Fig 4F). We also observed a small effect on differentiation following exercise; running resulted in a slightly higher percentage of BrdU+ cells that acquired a neuronal phenotype as compared to non-runners (89% vs. 78%; data not shown), consistent with previous reports that exercise regulates survival and differentiation of newly generated neurons in addition to effects on NPC proliferation (van Praag et al., 1999). Of note, total numbers of BrdU+/NeuN+ cells in this experiment and in succeeding experiments examining neurogenesis following paroxetine treatment (Fig 6C) are lower than in Fig 2, because a different injection paradigm was used. Here, mice were given a total of four doses of BrdU (50 mg/kg) every two hours on a single day, to ensure that BrdU+ cells were labeled over a short time period. Overall, these experiments demonstrate that voluntary exercise rescues neurogenesis defects in gp120 transgenic mice by increasing the proliferation of ANPs, leading to increased generation of newly born neurons.
Figure 4. Exercise rescues ANP proliferation and neurogenesis in gp120 transgenic mice.

A-E, mice were allowed free access to a running wheel for 10 days, followed by BrdU injection (200 mg/kg) and analysis two hours later. A. Voluntary running increases proliferating NPCs in both wt and gp120 transgenic mice. B,C. Exercise increases proliferating and total ANPs in gp120 mice to levels comparable to wt mice. D,E. Proliferating and total Tbr2+ cells are decreased in gp120 mice compared to wt, and are increased to levels similar to wt after exercise. * p<0.05, ANOVA. F, Mice received four injections of BrdU (50 mg/kg every 2 hrs) and were allowed access to a running wheel for 21 days. Exercise markedly increases the generation of newly born neurons in gp120 mice. * p<0.05, Student's t test.
Figure 6. Paroxetine rescues adult hippocampal neurogenesis in gp120 transgenic mice.
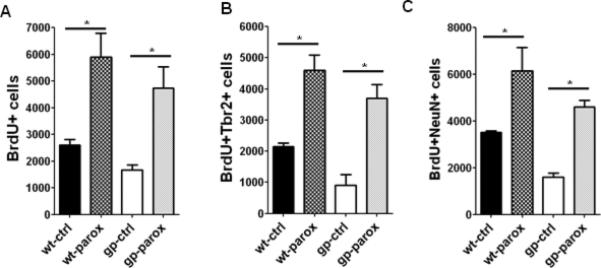
A. Mice implanted with either saline- or paroxetine-containing pumps for 28 days were analyzed 2 hours after BrdU injection. Paroxetine administration increases NPC proliferation in gp120 transgenic mice B. BrdU+Tbr2+ cells, representing proliferating ANPs, were quantified in wt and gp120 mice. C. Mice were administered daily injections of BrdU (50 mg/kg) for seven days, beginning 1 week after pump implantation, and analyzed 28 days after the first BrdU injection. BrdU+NeuN+ newly generated neurons are increased in gp120 mice upon paroxetine administration *p<0.05, Student's t test.
Aberrant initial dendritic development of newborn neurons in gp120 transgenic mice is rescued by exercise
Since functional neurogenesis requires proper development of newly generated neurons, we also determined whether gp120 impairs initial dendritic development of newly generated adult hippocampal neurons. We employed retrovirus-mediated GFP labeling (Ge et al., 2006; van Praag et al., 2002) to mark a population of GFP+ newly born dentate granule neurons whose “birthdates” are within several days of each other (Ge et al., 2006). Surprisingly, reconstructions of the dendritic arborizations of individual GFP+ neurons by confocal microscopy demonstrated markedly elaborated dendritic trees in gp120 transgenic mice as compared to wt mice at 2 weeks post injection (wpi) of retrovirus (Fig 5A). The difference in dendritic length was observed as early as 1 wpi (Fig 5B; mean wt length 201 ± 20 μm, mean gp120 length 317 ± 27 μm) and increased at 2 wpi (Fig 5C; mean wt length 373 ± 24 μm; mean gp120 length 660 ± 33 μm). Dendritic branch number was also increased in gp120 transgenic mice at both 1 wpi and 2 wpi (Figs 5B,C). Furthermore, Sholl analysis demonstrated greater dendritic complexity in neurons of gp120 transgenic mice as compared to wt. Thus, initial dendritic development appears to be aberrantly accelerated in newly generated adult hippocampal neurons in gp120 transgenic mice. We next asked whether the aberrant dendritic development observed in gp120 transgenic mice could also be reversed by voluntary exercise. Mice were provided access to running wheels following retroviral injection, and analyzed at 2 wpi. Dendritic lengths were similar in exercised gp120 transgenic mice as compared to exercised wt littermates (mean gp120 runner length 381 ± 36 μm; mean wt runner length 426 ± 25 μm), as was dendritic branch number and complexity (Fig 5D). Indeed, dendritic length, branch number, and complexity were also similar in exercised gp120 transgenic mice as compared to non-running wt mice (compare Fig 5C,D). Thus, voluntary exercise normalized the parameters of initial dendritic development in gp120 transgenic mice.
Figure 5. gp120 mice exhibit aberrant initial dendritic development of newly generated adult hippocampal neurons.
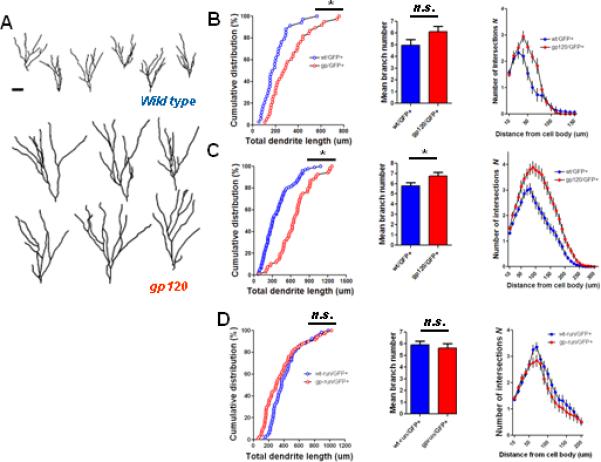
A. Retroviral labeling of newly generated neurons in dentate gyrus, followed by 3D confocal reconstruction of GFP+ dentate granule cells at 2 wpi. Shown are samples of 2D projection trajectories of 3D confocal reconstruction of dendrites of GFP+ neurons. Scale bar 20um. B and C, Summaries of dendrite properties at 1 wpi (B) and 2 wpi (C). Shown are cumulative distribution plots of dendrite length, graph of dendrite branch number, and Sholl analysis of dendritic complexity. Overall, gp120 transgenic mice demonstrate increased dendritic lengths, branch number, and complexity at 1 wpi and 2 wpi . D. Voluntary exercise results in normalization of parameters of initial dendritic development in gp120 mice at 2wpi (* p<0.05 Kolomogorov-Smirnov test for dendrite distribution; *p<0.05 Student's t test for dendritic length and branch number).
Rescue of adult hippocampal neurogenesis in gp120 transgenic mice by paroxetine
We next sought to determine whether SSRIs, which exert effects specifically upon the ANP population (Encinas et al., 2006), also led to restoration of the defects in ANP proliferation and neurogenesis in gp120 transgenic mice. As expected, subcutaneous administration of paroxetine for thirty days led to an increase in hippocampal NPC proliferation in wt mice (Fig 6A). Importantly, in gp120 transgenic mice, paroxetine administration also increased hippocampal NPC proliferation by over 100% (Fig 6A). Likewise, BrdU+Tbr2+ cells were increased by a similar extent, demonstrating that proliferating ANPs were rescued by paroxetine (Fig 6B). We next asked whether rescue of the ANP population resulted in rescue of the generation of new neurons in gp120 transgenic mice. Indeed, we found that paroxetine administration in gp120 transgenic animals led to a marked increase in BrdU+ NeuN+ cells in the dentate gyrus (Fig 6C), to levels comparable to wt mice. Thus, the SSRI paroxetine rescues both proliferating NPCs and generation of new neurons in gp120 transgenic mice.
Extracellular gp120 reduces human NPC proliferation
We next sought to determine whether gp120 directly impairs neurogenesis of human NPCs. Dissociated human NPC cultures were exposed to varying concentrations of gp120, and BrdU incorporation was assessed. Addition of extracellular gp120 decreases human NPC proliferation in a dose-dependent manner (Fig 7A), at concentrations similar to that which decreased rodent NPC proliferation (Okamoto et al., 2007). Viability of human NPCs was not affected by gp120 (Fig 7B). Thus, we demonstrate that gp120 can directly impair proliferation of human NPCs.
Figure 7. Extracellular HIV-1 gp120 decreases human NPC proliferation in vitro.
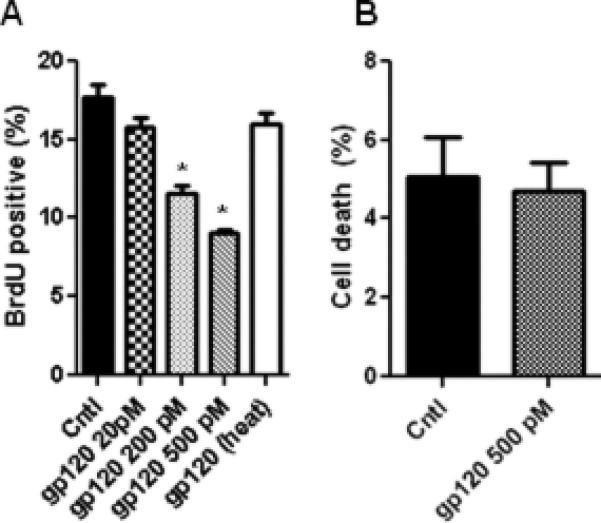
A. Recombinant gp120 IIIB was added to human NPC cultures for 48 hrs, followed by BrdU labeling for 4 hrs. heat, heat-inactivated. B. Live/Dead assay reveals no increase in NPC death upon addition of gp120 at the highest concentration used. * p<0.05 compared to control, ANOVA with Bonferroni.
DISCUSSION
Using a transgenic rodent model to study the effects of HIV infection in vivo, we provide evidence that the HIV envelope protein gp120 negatively regulates adult hippocampal neurogenesis, and we identify strategies to rescue the observed defects in neurogenesis. Mechanistically, the defect in NPC proliferation in gp120-transgenic animals is due to a reduction in proliferating ANPs. Initial dendritic development of newly generated neurons is also impaired, but NPC differentiation and survival are largely unaffected. These findings suggest that ANPs and newly generated neurons are particularly vulnerable to gp120, reflecting cell stage specificity in response to this viral protein. Importantly, the defects in neurogenesis in gp120 transgenic mice can be rescued during adulthood, by either voluntary exercise or by treatment with the SSRI paroxetine.
A key reservoir for HIV infection is the central nervous system, where HIV can infect infiltrating macrophages, microglia, and astrocytes through interactions between the viral envelope protein gp120 and cell surface receptors including CD4, CXCR4, and CCR5 (Dunfee et al., 2006). Macrophage (M)-tropic strains of HIV are thought to gain entry into the CNS by infecting cells through the coreceptor CCR5, while in later phases of infection a switch to T cell tropic strains recognizing the CXCR4 coreceptor can occur (Dunfee et al., 2006). Both M- and T-tropic viruses are cytotoxic, although they may have distinct pathogenic effects (Bachis et al., 2010). In the current antiretroviral era where CCR5 antagonists are increasingly being used, it is likely that there may be a selection for T-tropic strains. Hence, it may be critical to better understand the role of these viral strains in the neuropathogenesis of HIV infection. Although the mechanisms of neurotoxicity caused by HIV infection remain poorly understood, gp120 has emerged as an important mediator of the pathogenesis of HIV-associated neurodegeneration and cognitive dysfunction (Kaul et al., 2005). Gp120 can be secreted from a wide variety of cell types (Churchill et al., 1996; Paul et al., 1993) including astrocytic cells (Venkatesan, unpublished observations). When released from infected glial cells, the protein can directly cause apoptosis in mature neurons and can activate glial cells to secrete substances toxic to mature neurons in vitro (Mattson et al., 2005; Nath and Geiger, 1998). The importance of the pathogenic effects of gp120 in HIV-associated neurodegeneration is underscored by findings in the gp120-transgenic mouse model, in which the the gp120 IIIB protein, derived from a T-tropic virus, is expressed within glial cells in the brain. The spectrum of neuronal and glial changes in gp120-transgenic mice closely resembles that seen in the brains of HIV-infected patients (Toggas et al., 1994). In addition, gp120-transgenic mice develop several age-related behavioral deficits, including impairment of open field activity and spatial reference memory, that are thought to reflect neuromotor and cognitive deficits in patients with HAND (D'Hooge et al., 1999).
In addition to deleterious effects on mature neurons, gp120 can also impair function of developing neurons in the adult brain. We demonstrate a marked decrease in proliferating ANPs in gp120 transgenic mice with sparing of the QNP population, thus identifying ANPs as the major class of NPCs to be affected by gp120 expression. In the transgenic mice used in this study, expression of gp120 is restricted to GFAP+ cells, including astrocytes and QNPs, and was not detected in ANPs. Despite gp120 expression within QNPs, the numbers of proliferating QNPs as well as the total pool of QNPs was unchanged compared to wt animals. Therefore, the observed reduction in proliferating and total ANPs does not result from changes in the total QNP pool. Rather, it is likely that impairment of ANP proliferation in the transgenic mice results from gp120 release from either neighboring astrocytes or QNPs. Studies of rodent NPCs have identified several potential mechanisms by which gp120 may decrease NPC proliferation (Venkatesan et al., 2007). In vitro, gp120 IIIB interferes with the mitogenic effects of the stromal derived factor-1/CXCR4 interaction on NPCs, resulting in decreased proliferation (Tran et al., 2005). Such a mechanism may be particularly relevant in vivo, since in the adult hippocampus SDF-1 is expressed in the granule cell layer in close proximity to subgranular zone NPCs expressing CXCR4 (Berger et al., 2007; Tran et al., 2007). Additionally, gp120 IIIB may act by increasing the release of cytokines such as TNF-α (Buriani et al., 1999) from glial cells, or by decreasing levels of growth factors such as brain-derived neurotrophic factor, thereby affecting neighboring NPC proliferation (Iosif et al., 2006; Nosheny et al., 2004). Interestingly, recent evidence also suggests that HIV can infect human NPCs, thereby allowing for intracellular expression of gp120 within progenitor cells and potential impairment of neurogenesis (Schwartz et al., 2007). Gp120 may also regulate intracellular proteins through direct interactions with surfaced-expressed molecules on NPCs. Extracellular gp120 IIIB stimulates the p38 MAPK cascade in NPCs, thereby initiating withdrawal from the cell cycle and causing arrest in G1 phase, with concomitantly decreased proliferation (Okamoto et al., 2007). In addition, studies in neuronal cells have demonstrated that gp120 can regulate expression and function of a number of proteins including p53, Apaf-1, Rb, and E2F-1, in a CXCR4-dependent manner (Khan et al., 2003; Khan et al., 2005). Such proteins affect important aspects of cell cycle progression, survival, and differentiation, and may play a role in the gp120-mediated effects on NPC proliferation and dendritic development observed here. . Notably, we have found that addition of extracellular gp120 to human NPCs in vitro directly results in decreased proliferation, consistent with previous reports utilizing rodent hippocampal NPCs (Okamoto et al., 2007; Tran et al., 2005) and further supporting the relevance of gp120-induced alterations of neurogenesis in human disease. Thus, it is likely that at least some of the observed effects of gp120 on proliferation in vivo are accounted for by direct, cell autonomous effects of gp120 on NPCs.
Given the accumulating evidence that impairment of adult hippocampal neurogenesis may contribute to a decline in normal cognitive function (Zhao et al., 2008), an important question is whether HIV-mediated defects in neurogenesis can be rescued. In addition to HIV infection, a growing number of other psychiatric and neurologic conditions are characterized by impairments in adult hippocampal neurogenesis (Eisch et al., 2008; Ming and Song, 2005a).Interestingly, restoration of impaired neurogenesis has been demonstrated in some, but not all, of these pathological conditions. For example, voluntary running, which increases adult hippocampal NPC proliferation and neurogenesis in wild type rodents (Ming and Song, 2005a; van Praag et al., 1999), rescues neurogenesis following prenatal ethanol exposure, cranial irradiation in young mice, and ischemia in adult mice, but does not rescue hippocampal neurogenesis in mouse models of Huntington's disease or in a model of Alzheimer's disease (Kohl et al., 2007; Luo et al., 2007; Naylor et al., 2008; Redila et al., 2006; Wolf et al., 2006). Similarly, SSRIs enhance adult hippocampal neurogenesis in wt rodents (Encinas et al., 2006; Malberg et al., 2000), but effects in pathological models have been variable. Fluoxetine and sertraline, respectively, rescue adult hippocampal neurogenesis in a mouse model of Down's syndrome and the R6/2 Huntington's disease mouse model (Clark et al., 2006; Peng et al., 2008). However, fluoxetine did not rescue adult hippocampal neurogenesis after ischemia (Choi et al., 2007). Such differences in whether adult neurogenesis can be rescued likely arise both from methodological differences between studies and from disease-specific differences in mechanisms of impaired neurogenesis. Since we identified ANPs as the first class of NPCs impaired in gp120 transgenic mice, we sought to employ measures that restored this class of cells. We found that a relatively short duration (ten days) of voluntary running resulted in dramatic rescue of NPC proliferation in gp120 transgenic mice, such that numbers of proliferating ANPs were similar to that seen in normal wt mice. Continued exercise over the course of three weeks led to even more striking increases in numbers of newly generated neurons in gp120 transgenic mice. Such a profound increase in the generation of new neurons is consistent with previous observations that exercise can influence several phases of neurogenesis, including proliferation of ANPs, neuronal differentiation, and survival of newly generated neurons in wt mice (van Praag et al., 1999).
Since running can affect several phases of neurogenesis, we also sought to determine whether SSRIs, which exert effects specifically upon the ANP population (Encinas et al., 2006), also led to restoration of neurogenesis in gp120 transgenic mice. We found that the SSRI paroxetine rescues overall NPC proliferation, proliferation of ANPs, and neurogenesis in gp120 transgenic mice. The magnitude of increased neurogenesis was less than that observed after exercise, consistent with a more restricted action of SSRIs as compared to exercise on NPCs. Along with recent evidence that SSRIs possess anti-retroviral activity and may restore natural killer cell cytolytic activity in the setting of HIV infection (Evans et al., 2008; Letendre et al., 2007), our results suggest that SSRIs can target multiple viral and cellular pathways that may serve to limit HAND. Overall, we demonstrate that targeting the restoration of ANPs can dramatically rescue the neurogenesis defect in a mouse model of HIV neurologic disease.
Since functional neurogenesis requires proper maturation, in addition to formation, of newly generated neurons (Ming and Song, 2005b), we also determined whether gp120 affects initial dendritic maturation of newly generated adult hippocampal neurons. We found that dendritic trees from newly generated neurons in gp120 transgenic mice were longer and more complex than those arising from wt mice. Interestingly, enhanced dendritic outgrowth has also been noted in another pathological setting, following pilocarpine-induced seizures in mice (Overstreet-Wadiche et al., 2006). In these mice, increased dendritic outgrowth led to accelerated functional integration, thereby potentially contributing to epileptogenicity. Aberrant dendritic outgrowth was also noted in a rat model of seizures, in which kainic acid-induced status epilepticus led to extension of hilar basal dendrites in newly generated neurons (Jessberger et al., 2007). Since we found that voluntary exercise resulted in a marked increase in newly generated neurons in gp120 transgenic mice, an important question was whether these newly generated neurons also exhibited aberrant dendritic outgrowth. Strikingly, we found that voluntary exercise resulted in normalization of initial dendritic length, branch number, and complexity in newborn neurons in gp120 transgenic mice compared to wt mice. Consistent with a previous report, initial dendritic maturation of newly generated neurons in wt mice was not significantly affected by exercise (Zhao et al., 2006). Although the cellular and molecular mechanisms by which dendritic arborization is altered in gp120 mice and rescued upon exercise remain to be elucidated, we demonstrate that voluntary running rescues both the formation and initial dendritic development of dentate granule neurons in gp120 transgenic mice.
The observation that hippocampal neurogenesis is impaired in vivo by HIV gp120 has several important implications. Given the emerging evidence suggesting a role for continuous hippocampal neurogenesis in cognitive functioning, a gp120-induced reduction in newly generated neurons may result in impaired maintenance of hippocampal-dependent learning and memory (Jessberger et al., 2009; Kee et al., 2007; Leuner et al., 2006). Additionally, the inability to form new brain cells may impair repair in the setting of HIV-induced neurodegeneration, thereby potentially contributing to the brain atrophy seen in patients with HIV infection (Ge et al., 2003). Most importantly, however, it may in part explain why clinical trials with neuroprotective therapies have consistently failed in patients with HAND (Turchan et al., 2003). In addition to countering the deleterious effects of HIV on mature neuronal cells, therapeutic strategies may need to be directed at increasing and optimizing neurogenesis in this patient population. Both physiological measures such as exercise and pharmacological measures such as the use of SSRIs may be beneficial in restoring hippocampal neurogenesis and maintaining neurocognitive function in HIV-infected individuals.
Supplementary Material
ACKNOWLEDGEMENTS
We thank L. Mucke for kindly providing gp120 transgenic mice and P. Uapinyoying, L. Rajbhandari, L-h. Liu and K. Sailor for technical assistance. This work was supported by NIH (K08DA022946) and Howard Hughes Medical Institute to A.V., NIH (R01NS039253, P30 MH075673, R01DA024593) to A.N., and NIH (AG024984, NS047344) and NARSAD to H.S., NIH (NS048271) to G.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archibald SL, et al. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol. 2004;61:369–76. doi: 10.1001/archneur.61.3.369. [DOI] [PubMed] [Google Scholar]
- Bachis A, et al. M-tropic HIV envelope protein gp120 exhibits a different neuropathological profile than T-tropic gp120 in rat striatum. Eur J Neurosci. 2010 doi: 10.1111/j.1460-9568.2010.07325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger O, et al. Expression of SDF-1 and CXCR4 during reorganization of the postnatal dentate gyrus. Dev Neurosci. 2007;29:48–58. doi: 10.1159/000096210. [DOI] [PubMed] [Google Scholar]
- Buriani A, et al. Human immunodeficiency virus type 1 envelope glycoprotein gp120 induces tumor necrosis factor-alpha in astrocytes. J NeuroAIDS. 1999;2:1–13. doi: 10.1300/J128v02n02_01. [DOI] [PubMed] [Google Scholar]
- Castelo JM, et al. Altered hippocampal-prefrontal activation in HIV patients during episodic memory encoding. Neurology. 2006;66:1688–1695. doi: 10.1212/01.wnl.0000218305.09183.70. [DOI] [PubMed] [Google Scholar]
- Chang L, et al. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57:1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Choi YS, et al. Fluoxetine does not affect the ischemia-induced increase of neurogenesis in the adult rat dentate gyrus. Arch Pharm Res. 2007;30:641–5. doi: 10.1007/BF02977660. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, et al. The rev-responsive element negatively regulates human immunodeficiency virus type 1 env mRNA expression in primate cells. J Virol. 1996;70:5786–90. doi: 10.1128/jvi.70.9.5786-5790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S, et al. Fluoxetine rescues deficient neurogenesis in hippocampus of the Ts65Dn mouse model for Down syndrome. Exp Neurol. 2006;200:256–61. doi: 10.1016/j.expneurol.2006.02.005. [DOI] [PubMed] [Google Scholar]
- D'Hooge R, et al. Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gp120. Eur J Neurosci. 1999;11:4398–402. doi: 10.1046/j.1460-9568.1999.00857.x. [DOI] [PubMed] [Google Scholar]
- Duan X, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–58. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, et al. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18:108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunfee R, et al. Mechanisms of HIV-1 neurotropism. Curr HIV Res. 2006;4:267–78. doi: 10.2174/157016206777709500. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, et al. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28:11785–91. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, et al. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, et al. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59:1343–1349. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- Evans DL, et al. Selective serotonin reuptake inhibitor and substance P antagonist enhancement of natural killer cell innate immunity in human immunodeficiency virus/acquired immunodeficiency syndrome. Biol Psychiatry. 2008;63:899–905. doi: 10.1016/j.biopsych.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, et al. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–66. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, et al. Whole brain imaging of HIV-infected patients: quantitative analysis of magnetization transfer ratio histogram and fractional brain volume. AJNR Am J Neuroradiol. 2003;24:82–7. [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, et al. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- Hodge RD, et al. Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J Neurosci. 2008;28:3707–17. doi: 10.1523/JNEUROSCI.4280-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosif RE, et al. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–12. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–54. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, et al. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci. 2007;27:9400–7. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, et al. Cell-intrinsic signals that regulate adult neurogenesis in vivo: insights from inducible approaches. BMB Rep. 2009;42:245–59. doi: 10.5483/bmbrep.2009.42.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, et al. Immunolocalization of HIV envelope gp120 in HIV encephalitis with dementia. Aids. 2000;14:2709–13. doi: 10.1097/00002030-200012010-00010. [DOI] [PubMed] [Google Scholar]
- Kaul M, et al. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–92. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kee N, et al. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G, et al. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–91. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Khan MZ, et al. The chemokine receptor CXCR4 regulates cell-cycle proteins in neurons. J Neurovirol. 2003;9:300–14. doi: 10.1080/13550280390201010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MZ, et al. Regulation of neuronal P53 activity by CXCR 4. Mol Cell Neurosci. 2005;30:58–66. doi: 10.1016/j.mcn.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitabatake Y, et al. Adult neurogenesis and hippocampal memory function: new cells, more plasticity, new memories? Neurosurg Clin N Am. 2007;18:105–13. x. doi: 10.1016/j.nec.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl Z, et al. Physical activity fails to rescue hippocampal neurogenesis deficits in the R6/2 mouse model of Huntington's disease. Brain Res. 2007;1155:24–33. doi: 10.1016/j.brainres.2007.04.039. [DOI] [PubMed] [Google Scholar]
- Komitova M, Eriksson PS. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci Lett. 2004;369:24–7. doi: 10.1016/j.neulet.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. HIV-1 promotes quiescence in human neural progenitor cells. J Infect Dis. 2004;15:216–226. doi: 10.1086/422008. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, et al. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–63. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Letendre SL, et al. The role of cohort studies in drug development: clinical evidence of antiviral activity of serotonin reuptake inhibitors and HMG-CoA reductase inhibitors in the central nervous system. J Neuroimmune Pharmacol. 2007;2:120–7. doi: 10.1007/s11481-006-9054-y. [DOI] [PubMed] [Google Scholar]
- Leuner B, et al. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lledo PM, et al. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–93. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Luo CX, et al. Voluntary exercise-induced neurogenesis in the postischemic dentate gyrus is associated with spatial memory recovery from stroke. J Neurosci Res. 2007;85:1637–46. doi: 10.1002/jnr.21317. [DOI] [PubMed] [Google Scholar]
- Malberg JE, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, et al. Cell death in HIV dementia. Cell Death Differ. 2005;12(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- McArthur JC, et al. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–55. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005a;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005b;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Monje ML, et al. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Nath A, Geiger J. Neurobiological aspects of human immunodeficiency virus infection: neurotoxic mechanisms. Prog Neurobiol. 1998;54:19–33. doi: 10.1016/s0301-0082(97)00053-1. [DOI] [PubMed] [Google Scholar]
- Navia BA, et al. The AIDS dementia complex: I. Clinical features. Annals of Neurology. 1986a;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- Naylor AS, et al. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0711128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosheny RL, et al. Human immunodeficiency virus type 1 glycoprotein gp120 reduces the levels of brain-derived neurotrophic factor in vivo: potential implication for neuronal cell death. Eur J Neurosci. 2004;20:2857–64. doi: 10.1111/j.1460-9568.2004.03764.x. [DOI] [PubMed] [Google Scholar]
- Okamoto S, et al. HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell. 2007;1:230–6. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, et al. Seizures accelerate functional integration of adult-generated granule cells. J Neurosci. 2006;26:4095–4103. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul NL, et al. Expression of HIV-1 envelope glycoproteins by Semliki Forest virus vectors. AIDS Res Hum Retroviruses. 1993;9:963–70. doi: 10.1089/aid.1993.9.963. [DOI] [PubMed] [Google Scholar]
- Peng Q, et al. The antidepressant sertraline improves the phenotype, promotes neurogenesis and increases BDNF levels in the R6/2 Huntington's disease mouse model. Exp Neurol. 2008;210:154–63. doi: 10.1016/j.expneurol.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poluektova L, et al. Macrophage-induced inflammation affects hippocampal plasticity and neuronal development in a murine model of HIV-1 encephalitis. Glia. 2005;52:344–53. doi: 10.1002/glia.20253. [DOI] [PubMed] [Google Scholar]
- Redila VA, et al. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16:305–11. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- Sa MJ, et al. Dendritic changes in the hippocampal formation of AIDS patients: a quantitative Golgi study. Acta Neuropathol (Berl) 2004;107:97–110. doi: 10.1007/s00401-003-0781-3. [DOI] [PubMed] [Google Scholar]
- Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8(Suppl 2):115–21. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- Schwartz L, et al. Evidence of human immunodeficiency virus type 1 infection of nestin-positive neural progenitors in archival pediatric brain tissue. J Neurovirol. 2007;13:274–83. doi: 10.1080/13550280701344975. [DOI] [PubMed] [Google Scholar]
- Segi-Nishida E, et al. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc Natl Acad Sci U S A. 2008;105:11352–7. doi: 10.1073/pnas.0710858105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simioni S, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. Aids. 2009 doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Tashiro A, et al. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–33. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- Toggas SM, et al. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Tran PB, et al. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–33. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, et al. The HIV-1 coat protein gp120 regulates CXCR4-mediated signaling in neural progenitor cells. J Neuroimmunol. 2005;160:68–76. doi: 10.1016/j.jneuroim.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan J, et al. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003;60:307–314. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- van Praag H, et al. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–4. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan A, et al. Adult hippocampal neurogenesis: regulation by HIV and drugs of abuse. Cell Mol Life Sci. 2007;64:2120–32. doi: 10.1007/s00018-007-7063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CA, et al. Distribution of brain HIV load in AIDS. Brain Pathol. 1998;8:277–284. doi: 10.1111/j.1750-3639.1998.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SA, et al. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer's disease. Biol Psychiatry. 2006;60:1314–23. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Zhao C, et al. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–60. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao C, et al. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


