Abstract
SHP-1 has been implicated as a potential cancer therapeutic target by its negative regulation of immune cell activation and the activity of the SHP-1 inhibitor SSG that induced IFNγ+ cells for anti-tumor action. To develop more potent SHP-1-targeted anti-cancer agents, inhibitory leads were identified from a library of 34,000 drug-like compounds. Among the leads and active at low nM for recombinant SHP-1, tyrosine phosphatase inhibitor-1 (TPI-1) selectively increased SHP-1 phospho-substrates (pLck-pY394, pZap70 and pSlp76) in Jurkat T cells but had little effects on pERK1/2 or pLck-pY505 regulated by phosphatases SHP-2 or CD45, respectively. TPI-1 induced mouse splenic-IFNγ+ cells in vitro, ~58-fold more effective than SSG, and increased mouse splenic-pLck-pY394 and -IFNγ+ cells in vivo. TPI-1 also induced IFNγ+ cells in human peripheral blood in vitro. Significantly, TPI-1 inhibited (~83%, p <0.002) the growth of B16 melanoma tumors in mice at a tolerated oral dose in a T cell-dependent manner but had little effects on B16 cell growth in culture. TPI-1 also inhibited B16 tumor growth and prolonged tumor mice survival as a tolerated s.c. agent. TPI-1 analogs were identified with improved activities in IFNγ+ cell induction and in anti-tumor actions. In particular, analog TPI-1a4 as a tolerated oral agent completely inhibited the growth of K1735 melanoma tumors and was more effective than the parental lead against MC-26 colon cancer tumors in mice. These results designate TPI-1 and the analogs as novel SHP-1 inhibitors with anti-tumor activity likely via an immune mechanism, supporting SHP-1 as a novel target for cancer treatment.
Keywords: Melanoma, colon cancer, phosphatase inhibitor, SHP-1, IFNγ and SSG
INTRODUCTION
Activating immune cells for cancer treatment has been investigated intensively for decades with certain degrees of success and holds promise for increasing cancer cures (1). For instance, IL-2 is a potent immune cell activator capable of inducing complete responses durable for more than ten years in melanoma or renal cancer patients (2, 3). However, its application is restricted by high toxicity and the limitation of efficacy within a subpopulation of patients (4). With increased mechanistic understanding of immune cell signaling (5, 6), alternative approaches for immune cell activation could be exploited (7–9) and might lead to more efficacious and tolerated cancer treatments.
Targeting protein tyrosine phosphatases (PTPs) that negatively regulate immune cells with inhibitors is an attractive strategy. Like protein tyrosine kinases (PTKs) that have been targeted successfully with inhibitors for cancer treatment (10–12), PTPs are key regulators of intracellular signaling and potential targets for developing novel cancer therapeutics (13–17). Specifically, several PTPs have been identified as negative regulators of immune cells or cytokines and might be targeted to improve immunotherapy and cytokine therapy (14, 17–21). In addition, oncogenic PTPs play a causal role in oncogenesis (22–25) or metastasis (26) and could be targeted to block their pathogenic activities. Further, tumor suppressor PTPs have also been reported (27) and might be exploited through activating down-stream signaling molecules. However, it remains to be established that targeting PTPs could be an effective and safe anti-cancer strategy. Few PTP inhibitors with pre-clinical anti-tumor activity and clinical potential have been reported (13–17).
Anti-cancer potential of activating immune cells via targeting negative regulatory PTPs has been suggested by the anti-tumor actions of SHP-1 inhibitory sodium stibogluconate (SSG). SSG is an anti-leishmania drug with previously undefined mechanism of action (28). SSG selectively inhibited the PTP SHP-1 (29), which negatively controls the activation of immune cells (14, 21, 30) essential for anti-tumor immunity (1) and down regulates the signaling of anti-tumor cytokines (31–33). Consistent with targeting SHP-1, SSG had anti-renal tumor activity mediated via activating TH1 cells (IFNγ+ T cells) in mice when combined with IL-2 (34) and required IFNγ for anti-tumor action (35). SSG also synergized with IFNα in mouse models to eradicate melanoma tumors (36) and to inhibit the growth of prostate cancer tumors (37). This preclinical evidence provided a basis for moving SSG into clinical trials (NCT00311558, NCT00629200 & NCT00498979), one of which has reported evidence of augmenting anti-tumor immunity by SSG in cancer patients (38). These results together support the development of refined small molecule SHP-1 inhibitors as more effective cancer therapeutics.
In this work, TPI-1 and its analogs were identified as more effective than SSG in SHP-1 inhibition, immune cell activation and anti-tumor action. Our results provide important evidence supporting targeting SHP-1 to activate immune cells as an anti-cancer strategy and designate TPI-1 and the analogs as promising leads and a valuable platform for further investigation.
MATERIALS AND METHODS
Cells, cell culture and reagents
Recombinant protein of SHP-1 catalytic domain was described previously (39). Fluorescence substrate DIFMUP (6, 8-difluoro-4 methylumbelliferyl phosphate) was purchased (Molecular Probes). SSG, recombinant SHP-2 and MKP1 were reported previously (34, 36, 37). Human and mouse IFNγ ELISPOT Kit (R & D System), CD4+ Cell Intracellular IFNγ Detection Kit (BD Bioscience) and CD8+ Cell Intracellular IFNγ Detection Kit (BD Bioscience) were purchased from commercial sources. Jurkat human T cell line (40), B16 murine melanoma cell line (41) (ATCC), MC-26 murine colon cancer cell line (42) and K1735 murine melanoma cell line (43) (Institutional tumor core) were maintained in DMSO culture medium supplemented with 10% fetal calf serum (FCS). Antibodies against pLck-pY394 (Cell Signaling) (44), pLck-pY505 (Cell Signaling), pZap70 (pY319, BD Biosciences), pSlp76 (pY128, BD Biosciences) and pLat (pY226, BD Biosciences) were purchased from commercial sources. Pervanadate was prepared following an established protocol (45).
Evaluation of chemical compounds by PTP assays
A rapid SHP-1 PTP assay was developed for screening the compounds in a commercial library of 34,000 drug-like small chemicals (Chembridge, MA) and for evaluating lead compounds and analogs. Briefly, compounds were placed in 96-well plates (Falcon, 353072) and mixed with recombinant SHP-1 protein (0.1 µg/well) in 90 µl of HEPES buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.2 mM DTT and 0.1 mg/ml BSA). The plates were incubated at room temperature for 10 minutes prior to the addition of fluorescence substrate DIFMUP (40 µM stock in HEPES buffer, 10 µl/well) to initiate PTP reaction. Upon completion of PTP reaction at room temperature for 1hr in darkness, fluorescence signal of individual wells were recorded using a Victor2 Multilabel Counter (Victor, CA). They were compared to that of control SHP-1 PTP reaction (~ 10,000 units of fluorescence signal) in the absence of any compound (100%) for calculating relative SHP-1 inhibition induced by the compounds after subtracting the background signal (~ 500 units of fluorescence signal) of the substrate. The activities of lead compounds on recombinant SHP-2 or MKP1 were evaluated similarly. Analogs of lead compound TPI-1 from the library were identified via searching pubchem databases of ~ 1 million compounds based on structural similarities and purchased from commercial sources (Chembridge, MA). Their chemical features of druggability were extracted from pubchem databases.
Induction and detection of cellular protein tyrosine phosphorylation in Jurkat cells
Jurkat cells in culture medium (3 × 106 cells/ml) were treated with agents for designated times at room temperature. After brief centrifuging in a microfuge (4,000 rpm, 2 min), the cell pellet was lysed on ice for 30 min in 100 µl of cold lysis buffer (1% NP40, 50 mM Tris, pH 7.4, 150 mM NaCl, 20 mM NaF, 0.2 mM Na3VO4 and 1 mM Na3MO4) containing a cocktail of proteinase inhibitors (Sigma, 1 tablet/10 ml). The lysates were cleared by centrifuging (14,000 rpm, 10 min) in a microfuge at 4°C to remove insoluble parts, mixed with equal volume of 2 × SDS-PAGE sample buffer, boiled for 5 min and analyzed (~ 3 × 105 cells/well) by SDS-PAGE/Western blotting as described previously (46, 47). Relative intensities of phosphotyrosine bands were quantified through densitometry analysis.
Induction and quantification of mouse and human IFNγ+ cells
For induction of mouse primary IFNγ+ cells, splenocytes from female mice (129, 3–5-week old, Taconic Farms, Germantown, NY) were prepared as reported previously (34) following an established protocol approved by the Institutional Animal Care and Use Committee (IACUC) of the Cleveland Clinic. The splenocytes were cultured in RPMI 1640 medium supplemented with 10% FCS in the absence or presence of designated agents for 16 hrs in flat-bottom 96-well plates coated with a monoclonal antibody specific for mouse IFNγ (mouse IFNγ ELISPOT Kit, R & D System). The plates were then processed for in situ detection of IFNγ+ cells by ELISA following the manufacturer's procedure. Scanning and counting of IFNγ+ cells in the plates were accomplished using an automatic ELISPOT reader with Immunospot2 software (Cellular Technology Ltd).
For induction of human primary IFNγ+ cells, heparinized peripheral blood samples were obtained by vein-puncture from healthy volunteers following an established protocol approved by the Institutional Review Board (IRB) of Cleveland Clinic. To mimic in vivo drug-exposure, human peripheral blood samples were directly treated with different agents without pre-separation of white blood cells from other blood components. Blood samples (0.1 ml/sample) were mixed with the agents, incubated at 37°C for 4 hrs, diluted with 5 volumes of hypotonic solution (10 mM Tris, pH 7.4; 10 mM NaCl) to lyse RBC and centrifuged to pellet WBCs. The pellets were washed with hypotonic solution one time, re-suspended in RPMI 1640 medium (10% FCS) and used for ELISPOT assays (Human IFNγ ELISPOT Kit, R & D System) to quantify human IFNγ+ cells as outlined above.
Animals and animal studies
For in vivo induction of pLck-pY394 and IFNγ+ cells in mice, C57BL/6J mice (~8-week old, female, Taconic Farms, Germantown, NY) were treated with PBS or TPI-1 (~ 1 or 3 mg/kg, s.c.) for 4 days. Spleens were harvested one hour post-treatment on day 4 and processed into splenocytes, which were used for assessing pLck-pY394 levels by SDS-PAGE/Western blotting and for quantification of IFNγ+ cells by ELISPOT assays. Mice were also treated with TPI-1 (~10 mg/kg, daily, s. c., n = 2) to evaluate the toxicity of the compounds in vivo.
For assessing the anti-tumor activities of lead compounds, mice (6~8-week old, female, Taconic Farms, Germantown, NY) were inoculated (s.c.) at the flanks with tumor cells. Four days post-inoculation, the mice were treated with vehicle control or the lead compounds as indicated. Tumor volumes were measured during the study period and calculated using the formula for a prolate spheroid (V= 4/3 πa2b) (48). Student’s t test was used for assessing the significance of tumor volume differences among differential treatment groups. Mouse viability and body weights were also recorded during the study period. Internal organs of the mice were inspected visually upon their termination at the end of the experiment. All studies involving mice were approved by the Institutional Animal Care and Use Committee (IACUC) of the Cleveland Clinic.
RESULTS
Identification of SHP-1 inhibitor TPI-1 from a library of drug-like small chemicals
To identify novel SHP-1 inhibitors, compounds (~ 34,000) in a chemical library were screened for candidates capable of inhibiting recombinant SHP-1 in PTP assays. 29 compounds were identified (data not shown) and designated as leads.
Given their intended use for targeting SHP-1 in immune cells, the leads were evaluated for the capacity to inhibit cellular SHP-1 and thus increase SHP-1 substrate pLck-pY394 (49) in Jurkat T cells, in which the PTP and the substrate (49) were reported (50). Lead compound #5 (L5) was the most active among the leads and increased pLck-pY394 levels ~10-fold under the experimental conditions (Fig 1A). It was named as tyrosine phosphatase inhibitor-1 (TPI-1) (Fig 1B).
Fig 1.
TPI-1 inhibits recombinant and cellular SHP-1 and had little cytotoxicity in vitro and in mice. A) Jurkat cells in culture were treated with vehicle control or lead compounds #1 – 5 (10 µg/ml) for 10 min; total cell lysates (TCL) of the cells were prepared and analyzed by SDS-PAGE/Western blotting with antibodies as indicated. B) Chemical structures of lead compound TPI-1. C) Jurkat cells were cultured in the absence or presence of TPI-1 (A) for 6 days prior to quantification of cell growth by MTT assays. Data represent mean ± SD of triplicate samples. D) Viability of Balb/c mice treated with TPI-1 (~10 mg/kg, s. c., daily, 5d/wk) for two weeks. E) Relative activities of recombinant SHP-1, SHP-2 or MKP1 in the absence or presence of escalating doses of TPI-1 (mean ± SD of triplicates).
As an initial step to evaluate its potential for further development, TPI-1 was assessed for toxicity in vitro and in vivo. TPI-1 had little impact on Jurkat cell growth in vitro during 6 day co-culture (Fig 1C) and was tolerated by mice as a daily treatment at 10 mg/kg for two weeks (Fig 1D). Consistent with this indication of limited off-target effects for the lead, TPI-1 effectively in inhibited recombinant SHP-1 (IC50 ~ 0.01 µg/ml or 40 nM) but had limited impact on recombinant MKP1 (IC50 > 1 µg/ml) or SHP-2 (IC50 ~ 0.1 µg/ml) under the experimental conditions (Fig 1E). TPI-1 was therefore chosen for further evaluation.
TPI-1 selectively increases SHP-1 phospho-substrates in Jurkat T cells at low nM
To assess the potency and selectivity of TPI-1 for cellular SHP-1 in immune cells, the impacts of TPI-1 at a dose range on phospho-substrates of SHP-1, SHP-2 and CD45 in human Jurkat T cells were determined. Expression of SHP-1, SHP-2 and CD45 in Jurkat cells were reported previously (51, 52) and verified (data not shown).
TPI-1 was effective starting at 10 ng/ml in increasing SHP-1 phospho-substrates pLck-pY394 (49), pZap70 (53) and pSlp76 (54) in Jurkat cells (Fig 2A). pLAT, which functions down stream from pLck during T cell activation (55), was also elevated (Fig 2A). This TPI-1 action was selective since it failed to equally affect phospho-proteins pERK1/2 (56, 57) and pLck-pY505 (58) that are regulated by SHP-2 and CD45, respectively. pLck-pY505 is a substrate dephosphorylated by CD45 (58) and was not obviously affected by TPI-1 at the evaluated doses (Fig 2B). pERK1/2 are not directly dephosphorylated by SHP-2 but require active SHP-2 for their phosphorylation (56, 57). TPI-1 at lower doses (100 to 1 ng/ml) had little effects but reduced the levels of pERK1/2 at 1 µg/ml (Fig 2B). Therefore at its effective doses (10 – 100 ng/ml) for SHP-1 inhibition (Fig 2A), TPI-1 did not affect pLck-pY505 or pERK1/2, indicating a lack of inhibition of CD45 and SHP-2 (Fig 2B). It apparently inhibited SHP-2 only at a higher dose (1 µg/ml). In addition, TPI-1 did not markedly alter the profile of cellular phospho-tyrosine proteins in Jurkat cells except for the few with molecular weights corresponding to pLck-pY394, pZap70, pSlp76 and pLAT (Fig 2C). In contrast, induction of a comparable level of pLck-pY394 by the non-specific PTP inhibitor pervanadate (PV) (45) was accompanied by marked increases of multiple phosphotyrosine proteins in Jurkat cells (Fig 2D).
Fig 2.
TPI-1 selectively increases SHP-1 phospho-substrates in Jurkat cells at low nM levels. Jurkat cells were untreated or treated with TPI-1 (A–C) or non-specific phosphatase inhibitor PV (D) at various doses for 10 min. TCL of the cells were prepared and analyzed by SDS-PAGE/Western blotting using antibodies as indicated.
These results together provided evidence that TPI-1 selectively inhibited cellular SHP-1 in Jurkat T cells at low ng/ml concentrations.
TPI-1 increases IFNγ+ cells in mouse splenocytes and human peripheral blood in vitro
IFNγ is a TH1 cytokine expressed in activated anti-tumor immune cells (59, 60), in which SHP-1 is a key negative regulator (18). IFNγ+ cells were induced by SSG in its anti-Renca tumor action (34) and in human peripheral blood samples in vitro (35). As a further step to assess TPI-1, the capacity of the SHP-1 inhibitor to induce primary IFNγ+ cells in mouse splenocytes and human peripheral blood in vitro were evaluated in comparison with SSG.
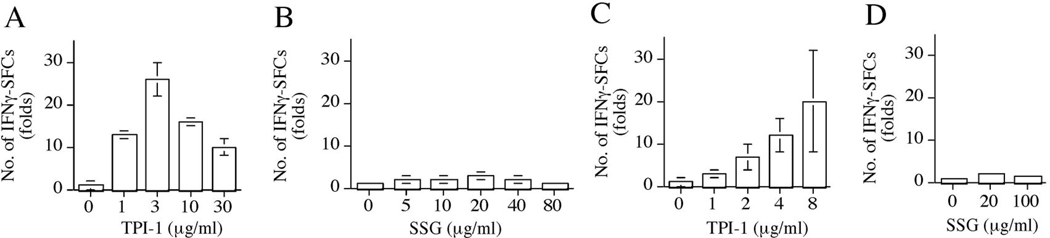
TPI-1 markedly induced IFNγ+ cells in mouse splenocytes (Fig 3A) and human peripheral blood (Fig 3C). IFNγ+ cells were increases in splenocytes treated with TPI-1 at 1 µg/ml (~14-fold), 3 µg/ml (~ 26-fold), 10 µg/ml (~17-fold) and 30 µg/ml (~10-fold) (Fig 3A). In contrast, SSG induced maximal increase ~ 3-fold at its optimal dose (20 µg/ml) (Fig 3B). IFNγ+ cells in human peripheral blood were also induced by TPI-1 (maximal 20-fold at 8 µg/ml) (Fig 3C), more effective than SSG (~ 2-fold at 20 µg/ml) (Fig 3D).
Fig 3.
TPI-1 induces IFNγ+ cells in mouse splenocytes and human peripheral blood in vitro. Relative numbers of IFNγ+ cells in mouse splenocytes (A and B) or human peripheral blood (C and D) cultured in the absence or presence of TPI-1 (A and C) or SSG (B and D) for 16 hrs as quantified by ELISPOT assays. Data present the mean ± SD of duplicate samples.
These results demonstrated that TPI-1 was a potent inducer of mouse and human primary IFNγ+ cells in vitro. When compared with SSG for maximal induction at a comparable dose, TPI-1 was more effective in inducing IFNγ+ cells in mouse splenocytes (~ 58-fold) and human peripheral blood (~ 20-fold).
TPI-1 increases mouse spleen pLck-pY394 and IFNγ+ cells in vivo
Given the TPI-1 capacity to induce phosphorylation of SHP-1 substrates and to induce IFNγ+ cells in vitro, we next determined whether TPI-1 possessed similar activities in vivo as well. Spleens from mice untreated or treated with TPI-1 were harvested for evaluation of pLck-pY394 levels and IFNγ+ cells in splenocytes.
Splenocyte pLck-pY394 was detectable in untreated mice (Fig 4A, lane 1) and was further increased (~ 3.3-fold, Fig 4B) in mice treated with TPI-1 at ~3 mg/kg of body weight (Fig 4A, lane 3). Spleen IFNγ+ cells were also increased approximately 3-fold (Fig 4C) in mice treated with a comparable dose of TPI-1. At a lower dose (1 mg/kg of body weight), TPI-1 had only a minor effect on pLck-pY394 (Fig 4A and B) under the experimental conditions. The effects of the low dose of TPI-1 on spleen IFNγ+ cells were not determined.
Fig 4.
TPI-1 increases spleen pLck-pY394 and IFNγ+ cells in mice. Mice were treated with vehicle control or TPI-1 (1 or 3 mg/kg, s.c., daily). A/B), Splenocytes from the mice were processed into total cell lysates (TCL) and analyzed by SDS-PAGE/Western blotting to quantify pLck-pY394 levels (A) for calculating induction by densitometry analysis (B). C, The splenocytes were also used in ELISPOT assays to quantify IFNγ+ cells (mean ± SD of duplicate samples).
Consistent with its in vitro activity, TPI-1 thus also increased pLck-pY394 and IFNγ+ cells in mice, demonstrating that the compound was effective in vivo as well. The reason for the lower levels of TPI-1-induced pLck–pY394and IFNγ+ cells in vivo (Fig 4) in comparison to those in vitro (Fig 2 and 3) have not been determined and could be resulted from TPI-1 clearance or metabolism in vivo.
TPI-1 inhibits the growth of B16 melanoma tumors in mice as a tolerated single agent
TPI-1 might have anti-cancer potential based on its induction of IFNγ+ cells critical in anti-tumor immunity. This potential was investigated by assessing its effects on B16 melanoma tumors in mice in comparison to IL-2 or SSG. The melanoma model (41) was chosen for optimal detection of TPI-1 anti-tumor effects via immunity in the absence of direct drug actions on cancer cells, since TPI-1 had little direct toxicity against B16 cells in culture at doses up to 10 µg/ml (Fig 5A). Like advanced human melanoma, B16 melanoma forms aggressive tumors in mice that are resistant to chemotherapeutics and poorly antigenic (61).
Fig 5.
TPI-1 inhibits B16 tumor growth as a tolerated single agent. A) Growth of B16 cells cultured with TPI-1 for 5 days (MTT, mean ± SD of triplicates). B) Tumor volumes in C57BL/B6 mice (n=5) bearing 4-day established B16 tumors (5 104 cells/inoculation) and treated with oral TPI-1 (3 mg/kg, daily, 5 d/wk). C) B16 tumor volumes in nude mice (n=5) treat as in B. D) Tumor volumes of C57BL/B6 mice (n=5) bearing 4-day established B16 tumors (105 cells/inoculation) and treated with TPI-1 (1 mg/kg, daily, 5 d/wk, s.c.). E) B16 tumor volumes in C57BL/B6 mice (n=5) bearing 4-day established tumors (5 × 104 cells/inoculation) and treated with SSG (12 mg, daily, 5 d/wk, s.c.). F) Body weights of the mice in B. G) Tumor volumes of C57BL/B6 mice (n = 5) bearing 4-day established tumors (105 cells/inoculation) and treated with vehicle control or TPI-1 (1 mg/kg, daily, 5d/wk, s.c.). H) Tumor volumes of C57BL/B6 mice (n=5) bearing 4-day established B16 tumors (105 cells/inoculation) and treated with vehicle control IL-2 (3 × 105 IU, bid, 5d/wk, ip). I) Viability of C57BL/B6 mice (n = 5) bearing 4-day established tumors (105 cells/inoculation) and treated with vehicle control or TPI-1 (1 mg/kg, daily, 5 d/wk, s.c.).
B16 tumors grew aggressively in control mice (Fig 5B), which had to be terminated by the third week due to large tumor burden and tumor ulceration. Growth of B16 tumors in mice treated with oral TPI-1 was slower and induced ~ 83% of growth inhibition of B16 tumors compared (p < 0.002) to that of the control (Fig 5B).
Supporting an immune mechanism of action, TPI-1 failed to inhibit B16 melanoma tumors in athymic nude mice with T-cell-deficiency under comparable experimental conditions (Fig 5C). To evaluate its effectiveness at a different dose and via an alternative route of delivery, TPI-1 (1 mg/kg, s.c.) also showed significant activity (p < 0.01) against B16 tumors in mice (Fig 5D). TPI-1 was tolerated: all the mice in the study survived to the end of study with comparable body weight (Fig 5F) and no obvious abnormalities in behaviors or gross anatomy (data not shown). Similar data were derived in a repeated experiment, in which the compound inhibited tumor growth (Fig 5G) and prolonged survival of melanoma tumor mice (Fig 5I). The optimal dose for TPI-1 has not been determined although oral TPI-1 at 30 mg/kg was tolerated by mice in a pilot experiment (data not shown).
B16 tumors were not responsive to an SSG treatment (Fig 5E), which was effective against several other types of tumors in mice (34, 36, 62). The growth of the tumors was inhibited (~ 61%) by high dose IL –2 (3 × 105 IU, bid) (Fig 5H), an FDA-approved front line treatment for melanoma and capable of inducing durable complete response (3). At a lower dose (1 × 105 IU, bid), IL-2 had little effect on B16 tumor growth (data not shown).
These results demonstrated an anti-B16 melanoma activity for TPI-1 that was more effective than SSG but comparable to IL-2 under the experimental conditions.
Identification of TPI-1 analogs with correlated activities in SHP-1 inhibition, IFNγ+ cell induction and anti-tumor action
Encouraged by the above data, ten chemical analogs (TPI-1a1-10) of TPI-1 were identified and evaluated for improved activities and for mechanistic insights.
Analogs TPI-1a1-5 effectively increased SHP-1 substrate pLck-pY394 in Jurkat cells in contrast to the inactive TPI-1a6-10 (Fig 6B, TPI-1a1-2 data not shown). Similar to their parental compound, the active TPI-1a1-5 were also found to have excellent druggability based on Lipinski’s ‘Rule of Five’ and Extensions (63) (Table 1). TPI-1a1-5 and the representative inactive TPI-1a10 (Fig 6A) were therefore further evaluated.
Fig 6.
TPI-1 analogs inhibit rSHP-1 in correlation with activity to increase SHP-1 phospho-substrate pLck-pY394 in Jurkat cells and induce IFNγ+ cells in mouse splenocytes. A) Chemical structures of TPI-1 analogs. B) pLck-pY394 levels in Jurkat cells treated with vehicle control, TPI-1 or selective analogs ( 10 µg/ml, 10 min) were quantified by Western blotting with antibodies as indicated. C) Activities of recombinant SHP-1 (rSHP-1) in the absence or presence of TPI-1 and selective analogs (10 µg/ml) as quantified by in vitro phosphatase assays (mean ± SD of triplicates). D) Numbers of IFNγ+ cells in mouse splenocytes cultured in the presence of vehicle control, TPI-1 or analogs for 16 hrs as quantified by ELISPOT assays (mean ± SD of duplicates).
Table 1.
Druggability of the lead compounds
| Rule of Five /Extensions(≤) |
TPI-1 | a1 | a2 | a3 | a4 | a5 | |
|---|---|---|---|---|---|---|---|
| M.W. | 500 | 253 | 253 | 219 | 234 | 228 | 214 |
| ClogP | 5 | 1.8 | 1.8 | 1.1 | 1.7 | 0.8 | 0.3 |
| H-bond donors | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| H-bondaccptors (N+O) | 10 | 2 | 2 | 2 | 2 | 3 | 3 |
| Rotatable bonds | 10 | 1 | 1 | 1 | 1 | 1 | 1 |
| Polar surface area | 140 | 34 | 34 | 34 | 34 | 43 | 43 |
| Sum of H-donors/acceptors | 12 | 2 | 2 | 2 | 2 | 3 | 3 |
The active analogs TPI-1a1-5 were all capable of inhibiting recombinant SHP-1 in vitro whereas TPI-1a10 was not active under comparable conditions (Fig 6C). The active analogs also induced IFNγ+ cells in mouse splenocytes in vitro and ~ 2–4 folds more effective than the parental TPI-1 at the dose range of 1 ng – 1,000 ng/ml (Fig 6D). TPI-1a10 failed to significantly induce IFNγ+ cells under comparable conditions at doses from 1 ng – 10 µg/ml (data not shown).
Two of the active analogs (TPI-1a2 and TPI-1a4) and the inactive analog L5a10 were assessed for activities in syngeneic mouse tumor models of B16 melanoma (41) or MC-26 colon cancer (42). TPI-1a2 and TPI-1a4 inhibited B16 tumor growth significantly (p < 0.01) (Fig 7A and B) whereas TPI-1a10 was not active (Fig 7C). TPI-1a4 as a single oral agent inhibited the growth (p = 0.04) of MC-26 tumors (Fig 7E), against which TPI-1 had modest but insignificant activity (p = 0.32) (Fig 7D). The compounds were tolerated as indicated by the viability of the treated mice (data not shown) and the comparable mouse body weights at the end of the experiment (Fig 7F).
Fig 7.
TPI-1 analogs TPI-1a2 and TPI-1a4 have anti-tumors in mice at tolerated oral doses. A/B) C57BL/6 mice bearing 4-day-established B16 tumors (s. c.) were treated with vehicle control TPI-1a2 (A) or TPI-1a4 (B) (3 mg/kg/daily, oral, 5d/week). Tumor volumes on day 22 were recorded (n = 5). C) Tumor volumes (n=5) in mice bearing 4-day established B16 tumors and treated with TPI-1a10 (3 mg/kg/day, oral, 5d/wk). D/E) Tumor volumes (n = 5) in Balb/c mice bearing 4-day established MC-26 tumors were treated with vehicle control, TPI-1 (3 mg/kg/daily, oral, 5 d/week) (D) or TPI-1a4 (3 mg/kg/daily, oral, 5d/week) (E). Data in D and E were from a single experiment and are presented in two panels for comparison. F) Body weights of the mice in D and E at the end of the experiment. G/H) C3H/HeJ mice (female, 8 wks, 5/group) bearing 4-day established K1735 tumors (s.c.) were treated with vehicle control or a4 (1 mg/kg, daily, oral, 5d/wk). Tumor volumes (G, n = 5) and mouse body weights (H, n = 5) were recorded. I) Growth of K1735 cells in the presence of vehicle control or a4 in culture for 3 days as quantified by MTT assays (mean ± SD of triplicates).
The anti-tumor activity of TPI-1a4 was further evaluated against the K1735 murine melanoma (43). Unlike the B16 melanoma from a spontaneous tumor (41), K1735 is similar to human melanoma in that it was UV-induced (43) and harbors the common N-Ras mutation (64). TPI-1a4 as a single oral agent completely inhibited the growth (p < 0.01) of 4-day established K1735 tumors in mice (Fig 7G). In contrast, K1735 tumors grew aggressively in the control mice (Fig 7G), which were moribund with large tumor burden by day 21. TPI-1a4 was tolerated by mice that maintained stead body weight (Fig 7H). It had little effect on K1735 cell growth in culture (Fig 7I).
Taken together, these data demonstrated correlated activities in SHP-1 inhibition, IFNγ+ cell induction and anti-tumor effects in mice for TPI-1 and its analogs, suggesting that the compounds target SHP-1 to induce IFNγ+ cells for anti-tumor action. In addition, the apparently improved activities of the analogs suggested feasibility of further refinement.
DISCUSSION
Based on our prior finding of SSG as an SHP-1-inhibitory compound (29) with anti-tumor activity that was mediated significantly via IFNγ+ cells (34, 35), we sought to identify novel and more potent SHP-1 inhibitors as potential anti-cancer agents.
Our current work demonstrated that TPI-1 was a potent and selective SHP-1 inhibitor effective at low nM levels (Fig 1 and 2). TPI-1 inhibited recombinant SHP-1 with IC50 at ~ 10 ng/ml (Fig 1) and increased SHP-1 phospho-substrates pLck-pY394, pZap70 and pSlp76 in Jurkat T cells starting at 10 ng/ml (Fig 2). Its selectivity was indicated by its reduced effectiveness for other PTPs in vitro and its limited impact on cellular phospho-proteins regulated by CD45 or SHP-2 (Fig 1 and 2). Interestingly, TPI-1 was effective on cellular SHP-2 only at 1 µg/ml (Fig 2) despite its lower IC50 (0.1 µg/ml) for recombinant SHP-2 (Fig 1). Assessment of cellular IC50s based on intracellular substrate phosphorylation has been common for characterizing PTK inhibitors (65, 66), including those approved for clinical use (67), and is believed more predictive for intracellular inhibition than data from recombinant enzymes. Indeed, several of our initial 29 lead compounds from the library failed to increase SHP-1 phospho-substrate in Jurkat T cells (Fig 1A), indicating their lack of activity for the cellular enzyme although they were inhibitory for the recombinant phosphatase.
Consistent with its capacity to inhibit SHP-1, TPI-1 was an effective inducer of IFNγ+ cells in mouse splenocytes and in human peripheral blood in vitro and ~ 10 – 20 × more effective than SSG (Fig 3). TPI-1 was also active in vivo and increased pLck-pY394 and IFNγ+ cells in mice (Fig 4). The induction of IFNγ+ cells by TPI-1 was not unexpected given its capacity to rapidly increase cellular pLck and pZap70 etc (Fig 2) that mediate activating signals in immune cells (6). Indeed, IFNγ+ cells could be induced rapidly by stimulation with anti-CD3 antibody that activates the Lck and Zap70 kinases (6, 68, 69). Thus, TPI-1-induced pLck and pZap70 might be sufficient as substitutions for extra-cellular stimuli in inducing IFNγ+ cells. Moreover, induction of IFNγ+ cells by SHP-1 inhibitors is also supported by the increases of IFNγ+ thymocyotes in SHP-1-deficient mice (70). Consistent with the functional role of Lck and Zap70 in T lymphocytes (6) that are major IFNγproducers (59), IFNγ+ cells were increased more in the CD3+ population (6.0 ×) than the CD3- cells (2.3 ×) in TPI-1-treated mouse splenocytes (our unpublished data). These observations provide a basis for future studies to determine the full impact of TPI-1 on differential immune cell types and to define its mechanism of action in inducing IFNγ+ cells.
Importantly, TPI-1 exhibited pre-clinical anti-melanoma tumor activity that was apparently via an immune mechanism and more effective than SSG. TPI-1 at a tolerated oral dose induced significant growth inhibition (~83%, p < 0.002) of B16 tumors (Fig 5B). This anti-melanoma activity was likely mediated by TPI-1-activated immune cells given that TPI-1 was capable of inducing IFNγ+ cells in mice (Fig 4) but lacked growth inhibitory activity against B16 melanoma cells in culture (Fig 5A). Such an immune mechanism of action was supported by the apparent requirement of T cells for TPI-1 anti-B16 tumor action (Fig 5D). TPI-1 also inhibited B16 tumors (Fig 5D) and prolonged survival of mice bearing B16 tumors when administered subcutaneously (Fig 5G).
The anti-B16 tumor activity of TPI-1 was comparable to those of IL-2 under the experimental conditions (Fig 5B/D/G/H). Although IL-2 induces durable complete responses in some patients, its clinical efficacy and application are limited by toxicity (2–4, 71, 72). Potential therapeutics derived from TPI-1 might be less toxic and more convenient as an oral agent. TPI-1 also represents an improvement over SSG that was inactive for B16 tumors (Fig 5E). Additional studies of mouse tumor models will help to define the mechanism of TPI-1 anti-melanoma action and to assess the optimal TPI-1 capacity to cure established tumors at higher doses or in combination with other agents. In this regard, it is worth noting that TPI-1 inhibitory activity against B16 tumors (Fig 6B) was achieved using a TPI-1 dose (~3 mg/kg) lower than its maximal tolerated dose (> 10 mg/kg) (Fig 2C).
At its effective doses, TPI-1 apparently acted predominantly against SHP-1 in immune cells with little or inconsequential interactions with other PTPs or cellular signaling molecules. This was indicated the observation that TPI-1 at effective SHP-1 inhibition doses (10 – 100 ng/ml) did not significantly affect pERK1/2 or pLck-pY505 that were regulated SHP-2 and CD45 PTPs, respectively (Fig 3C). It was further supported by the tolerance of immune cells and mice as a whole to the compound during its action to increase SHP-1 phospho-substrates, induce IFNγ+ cells and inhibit B16 tumor growth in mice. If other PTPs were also affected by TPI-1, it did not prevent TPI-1 from activating immune cells for anti-tumor action and might contribute to the effects due to functional overlaps with SHP-1. The potential value and clinical efficacy of a tolerated SHP-1 inhibitor with desired activity but targeting multiple molecules were indicated by the three FDA-approved kinase inhibitors (imatinib, sorafenib and sunitinib) that all target multiple kinases (10–12). Nevertheless, future studies will help to define the spectrum of PTPs affected by TPI-1 and to improve its specificity via chemical modifications.
TPI-1 analogs were identified with correlating and improved activities in SHP-1 inhibition, IFNγ+ cell induction and anti-tumor activity in mice, suggesting mechanistic insights and potential for further therapeutic development. Despite their close structural similarities, the active analogs (TPI-1a1-5) were effective in SHP-1 inhibition, IFNγ+ cell induction and anti-tumor activity while the inactive TPI-1a10 failed in each of the assays (Fig 6 and 7). These data provide additional and mechanistic evidence that the active analogs function via SHP-1 inhibition to activate immune cells for anti-tumor action. Interestingly, some of the analogs also showed improved activity in IFNγ+ cell induction (Fig 6) and anti-tumor action (Fig 7) comparing to the parental compound TPI-1. It suggests that the unique sub-structures in the more active analogs might be responsible for the improvement and could be further modified chemically for refinement. In addition, the conserved chemical structure among the active analogs might be of value as a platform for developing tolerated and orally available therapeutics that target differential phosphatases.
Of significance, TPI-1a4 was demonstrated to completely inhibit K1735 tumor growth as a single oral agent in mice under the experimental conditions (Fig 7). It suggests anti-cancer potential for this novel class of lead compounds and has other implications. The anti-tumor action was likely mediated via an immune mechanism since TPI-1a4 had little direct impact on K1735 cell growth in vitro (Fig 7). The putative mechanism might be distinct from that of IL-2 given that the cytokine had little activity for the melanoma in vivo as a single agent (73). Moreover, it was independent of TLR-4 since the receptor capable of mediating anti-tumor action (74) was defective in the host mice (C3H/HeJ) (75). The fact that K1735 melanoma was UV-induced and N-Ras mutated (64) also implicates the oncogenic signals and molecule as responding correlates for future investigation. In particular, optimal regimens for these small molecule inhibitors as single agents or in combinations with other cancer therapeutics need to be established for potential curative treatment, for assessing the immune specificity and duration of their anti-tumor action and for mechanistic analyses.
In summary, our work herein has identified a novel class of small molecule SHP-1 inhibitors that were more potent than SSG and had significant pre-clinical anti-tumor activities likely via activating immune cells. Their translational potential was suggested by tolerance in mice, oral availability, excellent druggability and capacity to activate human immune cells. Further evaluation of these leads is warranted and could lead to promising candidates for assessing therapeutic potential for cancers and other indications.
ACKKNOWLEDGEMENT
The authors thank Becky Haney for excellent technical assistance.
This work was supported by NIH grants CA096636 (TY), CA095020 (DJL), HL079441(ZJZ) and CA0890344 (EB).
Abbreviations
- SSG
Sodium stibogluconate
- IL-2
interleukin-2
- IFNγ
interferon-gamma
- PTK
protein tyrosine kinase
- PTP
protein tyrosine phosphatase
REFERECENS
- 1.Rosenberg SA. Shedding light on immunotherapy for cancer. N Engl J Med. 2004;350:1461–1463. doi: 10.1056/NEJMcibr045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA. Interleukin-2 and the development of immunotherapy for the treatment of patients with cancer. Cancer J Sci Am. 2000;2000:S2–S7. [PubMed] [Google Scholar]
- 3.Atkins MB. Cytokine-based therapy and biochemotherapy for advanced melanoma. Clin Cancer Res. 2006;12:2353s–2358s. doi: 10.1158/1078-0432.CCR-05-2503. [DOI] [PubMed] [Google Scholar]
- 4.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 5.Pentcheva-Hoang T, Corse E, Allison JP. Negative regulators of T-cell activation: potential targets for therapeutic intervention in cancer, autoimmune disease, and persistent infections. Immunol Rev. 2009;229:67–87. doi: 10.1111/j.1600-065X.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 6.Weiss A. TCR signal transduction: opening the black box. J Immunol. 2009;183:4821–4827. doi: 10.4049/jimmunol.0990083. [DOI] [PubMed] [Google Scholar]
- 7.Paulos CM, Kaiser A, Wrzesinski C, Hinrichs CS, Cassard L, Boni A, Muranski P, Sanchez-Perez L, Palmer DC, Yu Z, Antony PA, Gattinoni L, Rosenberg SA, Restifo NP. Toll-like receptors in tumor immunotherapy. Clin Cancer Res. 2007;13:5280–5289. doi: 10.1158/1078-0432.CCR-07-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarnaik AA, Weber JS. Recent advances using anti-CTLA-4 for the treatment of melanoma. Cancer J. 2009;15:169–173. doi: 10.1097/PPO.0b013e3181a7450f. [DOI] [PubMed] [Google Scholar]
- 10.Druker BJ. Molecularly targeted therapy: have the floodgates opened? Oncologist. 2004;9:357–360. doi: 10.1634/theoncologist.9-4-357. [DOI] [PubMed] [Google Scholar]
- 11.de Jonge MJ, Verweij J. Multiple targeted tyrosine kinase inhibition in the clinic: all for one or one for all? Eur J Cancer. 2006;42:1351–1356. doi: 10.1016/j.ejca.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, Ginsberg MS, Bacik J, Kim ST, Baum CM, Michaelson MD. Sunitinib in patients with metastatic renal cell carcinoma. Jama. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 13.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 14.Neel BG, Gu H, Pao L. The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 15.Zhang ZY. Protein tyrosine phosphatases: structure and function, substrate specificity, and inhibitor development. Annu Rev Pharmacol Toxicol. 2002;42:209–234. doi: 10.1146/annurev.pharmtox.42.083001.144616. [DOI] [PubMed] [Google Scholar]
- 16.Lazo JS, Wipf P. Small molecule regulation of phosphatase-dependent cell signaling pathways. Oncol Res. 2003;13:347–352. doi: 10.3727/096504003108748555. [DOI] [PubMed] [Google Scholar]
- 17.Yi T, Lindner DJ. The role and target potential of protein tyrosine phosphatases in cancer. Current Oncology Reports. 2008;10:114–121. doi: 10.1007/s11912-008-0019-6. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin Immunol. 1999;12:361–378. doi: 10.1006/smim.2000.0223. [DOI] [PubMed] [Google Scholar]
- 19.Joliat MJ, Shultz LD. The molecular bases of spontaneous immunological mutations in the mouse and their homologous human diseases. Clin Immunol. 2001;101:113–129. doi: 10.1006/clim.2001.5120. [DOI] [PubMed] [Google Scholar]
- 20.Long EO. Regulation of immune responses through inhibitory receptors. Annu Rev Immunol. 1999;17:875–904. doi: 10.1146/annurev.immunol.17.1.875. [DOI] [PubMed] [Google Scholar]
- 21.Dolton GM, Sathish JG, Matthews RJ. Protein tyrosine phosphatases as negative regulators of the immune response. Biochem Soc Trans. 2006;34:1041–1045. doi: 10.1042/BST0341041. [DOI] [PubMed] [Google Scholar]
- 22.Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H, van der Burgt I, Crosby AH, Ion A, Jeffery S, Kalidas K, Patton MA, Kucherlapati RS, Gelb BD. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 23.Tartaglia M, Kalidas K, Shaw A, Song X, Musat DL, van der Burgt I, Brunner HG, Bertola DR, Crosby A, Ion A, Kucherlapati RS, Jeffery S, Patton MA, Gelb BD. PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am J Hum Genet. 2002;70:1555–1563. doi: 10.1086/340847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tartaglia M, Gelb BD. Germ-line and somatic PTPN11 mutations in human disease. Eur J Med Genet. 2005;48:81–96. doi: 10.1016/j.ejmg.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Araki T, Mohi MG, Ismat FA, Bronson RT, Williams IR, Kutok JL, Yang W, Pao LI, Gilliland DG, Epstein JA, Neel BG. Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects of Ptpn11 mutation. Nat Med. 2004;10:849–857. doi: 10.1038/nm1084. [DOI] [PubMed] [Google Scholar]
- 26.Saha S, Bardelli A, Buckhaults P, Velculescu VE, Rago C, Croix BS, Romans KE, Choti MA, Lengauer C, Kinzler KW, Vogelstein B. A phosphatase associated with metastasis of colorectal cancer. Science. 2001;294:1343–1346. doi: 10.1126/science.1065817. [DOI] [PubMed] [Google Scholar]
- 27.Easty D, Gallagher W, Bennett DC. Protein tyrosine phosphatases, new targets for cancer therapy. Curr Cancer Drug Targets. 2006;6:519–532. doi: 10.2174/156800906778194603. [DOI] [PubMed] [Google Scholar]
- 28.Berman JD. Chemotherapy for leishmaniasis: biochemical mechanisms, clinical efficacy, and future strategies. Rev Infect Dis. 1988;10:560–586. doi: 10.1093/clinids/10.3.560. [DOI] [PubMed] [Google Scholar]
- 29.Pathak MK, Yi T. Sodium stibogluconate is a potent inhibitor of protein tyrosine phosphatases and augments cytokine responses in hemopoietic cell lines. J Immunol. 2001;167:3391–3397. doi: 10.4049/jimmunol.167.6.3391. [DOI] [PubMed] [Google Scholar]
- 30.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev. 2009;228:342–359. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.David M, Chen HE, Goelz S, Larner AC, Neel BG. Differential regulation of the alpha/beta interferon-stimulated Jak/Stat pathway by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol. 1995;15:7050–7058. doi: 10.1128/mcb.15.12.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiao H, Yang W, Berrada K, Tibrizi M, Shultz L, Yi T. Macrophages from motheaten and viable motheaten mutant mice show increased proliferative response to GM-CSF: detection of potential HCP substrates in GM-CSF signal transduction. Exp. Hematol. 1997;25:592–600. [PubMed] [Google Scholar]
- 33.Migone TS, Cacalano NA, Taylor N, Yi T, Waldmann TA, Johnston JA. Recruitment of SH2-containing protein tyrosine phosphatase SHP-1 to the interleukin 2 receptor; loss of SHP-1 expression in human T-lymphotropic virus type I-transformed T cells. Proc Natl Acad Sci U S A. 1998;95:3845–3850. doi: 10.1073/pnas.95.7.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan K, Zhou M, Pathak MK, Lindner DJ, Altuntas CZ, Tuohy VK, Borden EC, Yi T. Sodium stibogluconate interacts with IL-2 in anti-Renca tumor action via a T cell-dependent mechanism in connection with induction of tumor-infiltrating macrophages. J Immunol. 2005;175:7003–7008. doi: 10.4049/jimmunol.175.10.7003. [DOI] [PubMed] [Google Scholar]
- 35.Fan K, Borden EC, Yi T. IFNg is induced in human peripheral blood immune cells in vitro by SSG/IL-2 combination and mediates its anti-tumor activity in vivo. J Interferon Cytokine Res. 2009;29:451–460. doi: 10.1089/jir.2008.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yi T, Pathak MK, Lindner DJ, Ketterer ME, Farver C, Borden EC. Anticancer activity of sodium stibogluconate in synergy with IFNs. J Immunol. 2002;169:5978–5985. doi: 10.4049/jimmunol.169.10.5978. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Lindner DJ, Farver C, Borden EC, Yi T. Efficacy of SSG and SSG/IFNalpha2 against human prostate cancer xenograft tumors in mice: a role for direct growth inhibition in SSG anti-tumor action. Cancer Chemother Pharmacol. 2006 doi: 10.1007/s00280-006-0378-3. [DOI] [PubMed] [Google Scholar]
- 38.Naing A, Reuben J, Verschraegen C, Camacho L, Stephen S, Hong D, Wheler J, fu S, Martinez M, Akinsanmi L, Kurzrock R. Phase I dose-escalation study of sodium stibogluconate (SSG), a protein tyrosine phosphatase inhibitor, combined with interferon-alfa for patients with solid tumors. J Clin Oncol. 2008;26:3011. doi: 10.7150/jca.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang X, Meng W, Niu T, Zhao Z, Zhou GW. Expression, purification, and crystallization of the catalytic domain of protein tyrosine phosphatase SHP-1. J Struct Biol. 1997;120:201–203. doi: 10.1006/jsbi.1997.3927. [DOI] [PubMed] [Google Scholar]
- 40.Schneider U, Schwenk HU, Bornkamm G. Characterization of EBV-genome negative "null" and "T" cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer. 1977;19:621–626. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- 41.Fidler IJ. Selection of successive tumour lines for metastasis. Nat New Biol. 1973;242:148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- 42.Singh P, Walker JP, Townsend CM, Jr, Thompson JC. Role of gastrin and gastrin receptors on the growth of a transplantable mouse colon carcinoma (MC-26) in BALB/c mice. Cancer Res. 1986;46:1612–1616. [PubMed] [Google Scholar]
- 43.Kripke ML. Speculations on the role of ultraviolet radiation in the development of malignant melanoma. J Natl Cancer Inst. 1979;63:541–548. doi: 10.1093/jnci/63.3.541. [DOI] [PubMed] [Google Scholar]
- 44.Gerke C, Falkow S, Chien YH. The adaptor molecules LAT and SLP-76 are specifically targeted by Yersinia to inhibit T cell activation. J Exp Med. 2005;201:361–371. doi: 10.1084/jem.20041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haque SJ, Harbor P, Tabrizi M, Yi T, Williams BR. Protein-tyrosine phosphatase Shp-1 is a negative regulator of IL-4- and IL-13-dependent signal transduction. J Biol Chem. 1998;273:33893–33896. doi: 10.1074/jbc.273.51.33893. [DOI] [PubMed] [Google Scholar]
- 46.Yi T, Mui AL, Krystal G, Ihle JN. Hematopoietic cell phosphatase associates with the interleukin-3 (IL-3) receptor beta chain and down-regulates IL-3-induced tyrosine phosphorylation and mitogenesis. Molecular & Cellular Biology. 1993;13:7577–7586. doi: 10.1128/mcb.13.12.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yi T, Ihle JN. Association of hematopoietic cell phosphatase with c-Kit after stimulation with c-Kit ligand. Molecular & Cellular Biology. 1993;13:3350–3358. doi: 10.1128/mcb.13.6.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindner DJ, Borden EC, Kalvakolanu DV. Synergistic antitumor effects of a combination of interferons and retinoic acid on human tumor cells in vitro and in vivo. Clin Cancer Res. 1997;3:931–937. [PubMed] [Google Scholar]
- 49.Chiang GG, Sefton BM. Specific dephosphorylation of the Lck tyrosine protein kinase at Tyr-394 by the SHP-1 protein-tyrosine phosphatase. J Biol Chem. 2008;276:23173–23178. doi: 10.1074/jbc.M101219200. [DOI] [PubMed] [Google Scholar]
- 50.Mary F, Moon C, Venaille T, Thomas ML, Mary D, Bernard A. Modulation of TCR signaling by beta1 integrins: role of the tyrosine phosphatase SHP-1. Eur J Immunol. 1999;29:3887–3897. doi: 10.1002/(SICI)1521-4141(199912)29:12<3887::AID-IMMU3887>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 51.Musci MA, Beaves SL, Ross SE, Yi T, Koretzky GA. Surface expression of hemopoietic cell phosphatase fails to complement CD45 deficiency and inhibits TCR-mediated signal transduction in a Jurkat T cell clone. J Immunol. 1997;158:1565–1571. [PubMed] [Google Scholar]
- 52.Frearson JA, Alexander DR. The phosphotyrosine phosphatase SHP-2 participates in a multimeric signaling complex and regulates T cell receptor (TCR) coupling to the Ras/mitogen-activated protein kinase (MAPK) pathway in Jurkat T cells. J Exp Med. 1998;187:1417–1426. doi: 10.1084/jem.187.9.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plas DR, Johnson R, Pingel JT, Matthews RJ, Dalton M, Roy G, Chan AC, Thomas ML. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 1996;272:1173–1176. doi: 10.1126/science.272.5265.1173. [DOI] [PubMed] [Google Scholar]
- 54.Binstadt BA, Billadeau DD, Jevremovic D, Williams BL, Fang N, Yi T, Koretzky GA, Abraham RT, Leibson PJ. SLP-76 is a direct substrate of SHP-1 recruited to killer cell inhibitory receptors. J Biol Chem. 1998;273:27518–27523. doi: 10.1074/jbc.273.42.27518. [DOI] [PubMed] [Google Scholar]
- 55.Aguado E, Martinez-Florensa M, Aparicio P. Activation of T lymphocytes and the role of the adapter LAT. Transpl Immunol. 2006;17:23–26. doi: 10.1016/j.trim.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 56.Shi ZQ, Lu W, Feng GS. The Shp-2 tyrosine phosphatase has opposite effects in mediating the activation of extracellular signal-regulated and c-Jun NH2-terminal mitogen-activated protein kinases. J Biol Chem. 1998;273:4904–4908. doi: 10.1074/jbc.273.9.4904. [DOI] [PubMed] [Google Scholar]
- 57.Fragale A, Tartaglia M, Wu J, Gelb BD. Noonan syndrome-associated SHP2/PTPN11 mutants cause EGF-dependent prolonged GAB1 binding and sustained ERK2/MAPK1 activation. Hum Mutat. 2004;23:267–277. doi: 10.1002/humu.20005. [DOI] [PubMed] [Google Scholar]
- 58.Sieh M, Bolen JB, Weiss A. CD45 specifically modulates binding of Lck to a phosphopeptide encompassing the negative regulatory tyrosine of Lck. Embo J. 1993;12:315–321. doi: 10.1002/j.1460-2075.1993.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 60.Becker Y. Molecular immunological approaches to biotherapy of human cancers--a review, hypothesis and implications. Anticancer Res. 2006;26:1113–1134. [PubMed] [Google Scholar]
- 61.Beattie CW, Tissot R, Amoss M. Experimental models in human melanoma research: a logical perspective. Semin Oncol. 1988;15:500–511. [PubMed] [Google Scholar]
- 62.Li J, Lindner DJ, Farver C, Borden EC, Yi T. Efficacy of SSG and SSG/IFNalpha2 against human prostate cancer xenograft tumors in mice: a role for direct growth inhibition in SSG anti-tumor action. Cancer Chemother Pharmacol. 2007;60:341–350. doi: 10.1007/s00280-006-0378-3. [DOI] [PubMed] [Google Scholar]
- 63.Keller TH, Pichota A, Yin Z. A practical view of 'druggability'. Curr Opin Chem Biol. 2006;10:357–361. doi: 10.1016/j.cbpa.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 64.Melnikova VO, Bolshakov SV, Walker C, Ananthaswamy HN. Genomic alterations in spontaneous and carcinogen-induced murine melanoma cell lines. Oncogene. 2004;23:2347–2356. doi: 10.1038/sj.onc.1207405. [DOI] [PubMed] [Google Scholar]
- 65.Traxler PM, Furet P, Mett H, Buchdunger E, Meyer T, Lydon N. 4-(Phenylamino)pyrrolopyrimidines: potent and selective, ATP site directed inhibitors of the EGF-receptor protein tyrosine kinase. J Med Chem. 1996;39:2285–2292. doi: 10.1021/jm960118j. [DOI] [PubMed] [Google Scholar]
- 66.Murray LJ, Abrams TJ, Long KR, Ngai TJ, Olson LM, Hong W, Keast PK, Brassard JA, O'Farrell AM, Cherrington JM, Pryer NK. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis. 2003;20:757–766. doi: 10.1023/b:clin.0000006873.65590.68. [DOI] [PubMed] [Google Scholar]
- 67.Carlomagno F, Anaganti S, Guida T, Salvatore G, Troncone G, Wilhelm SM, Santoro M. BAY 43–9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006;98:326–334. doi: 10.1093/jnci/djj069. [DOI] [PubMed] [Google Scholar]
- 68.Favre N, Bordmann G, Rudin W. Comparison of cytokine measurements using ELISA, ELISPOT and semi-quantitative RT-PCR. J Immunol Methods. 1997;204:57–66. doi: 10.1016/s0022-1759(97)00033-1. [DOI] [PubMed] [Google Scholar]
- 69.Wang Q, Stanley J, Kudoh S, Myles J, Kolenko V, Yi T, Tubbs R, Bukowski R, Finke J. T cells infiltrating non-Hodgkin's B cell lymphomas show altered tyrosine phosphorylation pattern even though T cell receptor/CD3-associated kinases are present. J Immunol. 1995;155:1382–1392. [PubMed] [Google Scholar]
- 70.Yu WM, Wang S, Keegan AD, Williams MS, Qu CK. Abnormal Th1 cell differentiation and IFN-gamma production in T lymphocytes from motheaten viable mice mutant for Src homology 2 domain-containing protein tyrosine phosphatase-1. J Immunol. 2005;174:1013–1019. doi: 10.4049/jimmunol.174.2.1013. [DOI] [PubMed] [Google Scholar]
- 71.Atkins MB, Dutcher J, Weiss G, Margolin K, Clark J, Sosman J, Logan T, Aronson F, Mier J. Kidney cancer: the Cytokine Working Group experience (1986–2001): part I. IL-2-based clinical trials. Med Oncol. 2001;18:197–207. doi: 10.1385/MO:18:3:197. [DOI] [PubMed] [Google Scholar]
- 72.Bascon JU. Vascular leak syndrome: a troublesome side effect of immunotherapy, Immunopharmacology, 39/3 (1998) 255. Immunopharmacology. 1998;39:255–257. [PubMed] [Google Scholar]
- 73.Barnard AL, Farzaneh F, Gaken J, Darling D. Local versus systemic interleukin-2: tumor formation by wild-type and B7-1-positive murine melanoma cells. Cancer Gene Ther. 2000;7:207–214. doi: 10.1038/sj.cgt.7700087. [DOI] [PubMed] [Google Scholar]
- 74.Lee CH, Wu CL, Shiau AL. Toll-like receptor 4 mediates an antitumor host response induced by Salmonella choleraesuis. Clin Cancer Res. 2008;14:1905–1912. doi: 10.1158/1078-0432.CCR-07-2050. [DOI] [PubMed] [Google Scholar]
- 75.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]