Abstract
Subpopulations of Kenyon cells, the intrinsic neurons of the insect mushroom bodies, are typically sequentially generated by dedicated neuroblasts that begin proliferating during embryogenesis. When present, Class III Kenyon cells are thought to be the first born population of neurons by virtue of the location of their cell somata, farthest from the position of the mushroom body neuroblasts. In the adult tobacco hornworm moth Manduca sexta, the axons of Class III Kenyon cells form a separate Y tract and dorsal and ventral lobelet; surprisingly, these distinctive structures are absent from the larval Manduca mushroom bodies. BrdU labeling and immunohistochemical staining reveal that Class III Kenyon cells are in fact born in the mid-larval through adult stages. The peripheral position of their cell bodies is due to their genesis from two previously undescribed protocerebral neuroblasts distinct from the mushroom body neuroblasts that generate the other Kenyon cell types. These findings challenge the notion that all Kenyon cells are produced solely by the mushroom body neuroblasts, and may explain why Class III Kenyon cells are found sporadically across the insects, suggesting that when present, they may arise through de novo recruitment of neuroblasts outside of the mushroom bodies. In addition, lifelong neurogenesis by both the Class III neuroblasts and the mushroom body neuroblasts was observed, raising the possibility that adult neurogenesis may play a role in mushroom body function in Manduca.
Introduction
The mushroom bodies of the insect brain play a role in a multitude of higher functions including sensory integration, attention-like processes and olfactory processing and olfactory learning (Li et al., 1999; Perez-Orive et al., 2002; Blum et al., 2009; van Swinderen and Brembs, 2010). The mushroom bodies are composed of morphologically and functionally distinct subpopulations of intrinsic neurons called Kenyon cells (Farris, 2005b). Kenyon cell dendrites form the mushroom body calyces, which receive sensory input that in most insects arises predominantly from first-order olfactory centers, the antennal lobes. Kenyon cell axons form a thick tract into the protocerebrum called the pedunculus, then bifurcate to form the medial and vertical lobes.
Kenyon cell subpopulations are produced sequentially during development, and their cell bodies and axonal processes are organized by birthdate in the adult mushroom bodies (Farris et al., 1999; Farris and Strausfeld, 2001; Kurusu et al., 2002; Farris and Sinakevitch, 2003). In all insect species studied to date, Kenyon cells appear to be generated by dedicated mushroom body neuroblasts located near the center of the calyx (Ito et al., 1997), and their somata are passively pushed outwards by continuing rounds of neurogenesis so that the earliest born cells are located at the periphery of the Kenyon cell body cluster, farthest from the position of the neuroblasts (Farris et al., 1999; Farris and Strausfeld, 2001; Malaterre et al., 2002). Studies in the fruit fly Drosophila melanogaster have shown that the mushroom body neuroblasts, which begin neurogenesis in the embryo and are continually mitotically active through the mid-pupal stage, are the sole source of Kenyon cells (Ito and Hotta, 1992; Ito et al., 1997).
In some insects, the mushroom bodies contain a distinct population of mushroom body intrinsic neurons, the Class III Kenyon cells, that often receive input from gustatory centers in the deutocerebrum, tritocerebrum and subesophageal ganglion (Weiss, 1981; Frambach and Schürmann, 2004; Farris, 2008c). Class III Kenyon cells are further typified by a unique morphology in which their axons form separate “lobelets” outside of the medial and vertical lobes, and may also form a separate accessory calyx with their dendrites (Weiss, 1981; Farris and Strausfeld, 2003; Farris, 2008c). Despite these unique features, Class III Kenyon cells are considered true mushroom body intrinsic neurons, largely because they all possess tiny, cytoplasm-poor cell bodies and share a high affinity for the anti-DC0 polyclonal antibody (Farris and Strausfeld, 2003; Farris et al., 2004; Farris, 2005a; Sjöholm et al., 2006; Farris, 2008a; Farris, 2008c; Fukushima and Kanzaki, 2009). The anti-DC0 antibody recognizes the catalytic subunit of Drosophila protein kinase A, which like other components of the cAMP signaling pathway is highly expressed in Kenyon cells and is important for the learning and memory functions of the mushroom bodies (Skoulakis et al., 1993). Class III Kenyon cell bodies are located at the periphery of the Kenyon cell soma clusters in the dorso-posterior protocerebrum; they are thus presumed to be generated by the mushroom body neuroblasts at the earliest stage of mushroom body development, and their cell bodies pushed to the farthest edges of the soma clusters by adulthood (Farris and Strausfeld, 2003). Aside from this inference, however, little is known about the origin of Class III Kenyon cells and the development of the unique structures formed by their axons and dendrites.
The tobacco hornworm moth Manduca sexta is an important model system for studies of the development and function of the olfactory system and olfaction-driven behavior (Rössler et al., 1999; Daly et al., 2004; Ito et al., 2009; Lei at al., 2009). Most attention has focused on the antennal lobe, and very little is known about the structure and development of the mushroom bodies in this species despite their being a major target of antennal lobe output. From neuroarchitectural and developmental standpoints, Manduca mushroom bodies are also of interest because Class III Kenyon cells have been observed in their mushroom bodies and those of other moths belonging to the order Lepidoptera, where their axons form a morphologically unique Y tract and lobelets (Pearson, 1971; Sjöholm et al., 2005; Fukushima and Kanzaki, 2009; Huetteroth et al., 2010). The present study describes the morphology and development of the Manduca mushroom bodies, with particular emphasis on the developmental origin of Class III Kenyon cells and the morphogenesis of their unique neuropils.
Methods
1.1 Insects
All moths were reared under standard rearing conditions (Bell and Joachim, 1976). Larvae were staged according to head capsule width. At the onset of wandering (W0) in the fifth larval instar, larvae were placed in individual pupation chambers and collected as needed throughout the four day wandering stage and the 18 day pupal stage. At pupal stage 17, individuals were placed into brown paper bags and kept in an incubator (Percival Scientific I66VLC8, Perry, IA) in a reversed 16/8 light/dark cycle at 25° C and 75% relative humidity.
1.2 Anti-DC0 and phalloidin labeling
Anti-DC0 is a polyclonal antibody derived against the catalytic subunit of protein kinase A in Drosophila melanogaster (Skoulakis et al., 1993). This antibody has a high affinity for mature Kenyon cell populations across a wide range of insect species (Farris and Strausfeld, 2003; Farris et al., 2004; Farris, 2005a; Farris, 2008a; Farris, 2008c). Fluorophore-conjugated phalloidin labels filamentous actin enriched in extending axons and dendrites of newborn Kenyon cells (Farris et al., 2004).
Prior to brain dissection, insects were chilled on ice until immobile. Brains were dissected in O’Shea-Adams saline (O’Shea and Adams, 1981) and fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS, pH 7.2) overnight at 4° C. Fixed brains were washed 3 × 10 min in PBS, embedded in 8% agarose and vibratome sectioned at 80 μm. Sections were transferred to a 24-well culture dish and washed 3 × 10 min in PBST (PBS with 0.1% Triton X-100), then blocked in 10% normal goat serum (NGS) in PBST for at least an hour at room temperature on an orbital shaker. The blocking solution was then removed and replaced with a primary antibody solution consisting of 1% NGS in PBST and the anti-DC0 primary antibody (a generous gift of Dr. Daniel Kalderon) at a 1:1000 concentration. Tissue was incubated in the primary antibody overnight at room temperature.
The following day, tissue was washed 6 × 20 min in PBST and then placed in a secondary antibody solution of 1% NGS in PBST with Texas Red-conjugated goat anti-rabbit secondary antibody (Jackson Immunochemicals, West Grove, PA) added at a 1:200 concentration. Alexa 488-conjugated phalloidin (Invitrogen (Molecular Probes), Eugene, OR) was also added to the solution at a 1:500 concentration, and tissue was incubated overnight on the orbital shaker at room temperature. The next day, tissue was washed 6 × 20 min in PBST and cleared in 60% glycerol in PBS for 30 min and 80% glycerol in PBS for 1h. Sections were coverslipped under 80% glycerol in PBS and viewed using an Olympus Fluoview 1000 confocal microscope. Image stacks were captured on the confocal and saved as AVI files, opened in NIH Image and broken into TIFF files of individual optical sections. Projections of portions of the stack were made in Adobe Photoshop CS4 (Adobe Systems Incorporated, USA) using the “lighten” function, and color balance, contrast and brightness adjusted as needed.
1.3 BrdU Labeling
For labeling of neuroblasts and their immediate progeny in late larvae, pupae and adult moths, 25 mg/ml 5′-bromo-2-deoxyuridine (BrdU) in O’Shea-Adams saline was loaded into a 1cc tuberculin syringe and injections made using a 31ga needle into cold-anesthetized insects. For first and second instar larvae that were too small for injections, the insects were chilled until immobile and a droplet of BrdU solution placed on their mouthparts. The insects ingested the solution as they revived from the cold anesthesia. All BrdU-treated insects were allowed to revive at room temperature for 4-24 hours, after which they were again chilled on ice and their brains dissected, fixed, embedded and sectioned as described previously.
For birthdating of Kenyon cell populations, BrdU treatment was performed as described above. Insects were then allowed to continue development until the late pupal stage and then processed for BrdU immunostaining.
Prior to BrdU immunostaining, fixed and sectioned tissue was washed 3 × 10 min in PBST, then incubated in 2N HCl for 40 minutes at room temperature to denature the DNA and allow access to the anti-BrdU antibody. After acid treatment, tissue was washed 3 × 5 minutes in PBST and placed in 10% NGS blocking solution for one hour. Mouse anti-BrdU (Becton-Dickinson, Franklin Lakes, NJ) was added at a 1:500 concentration to a 1% solution of NGS in PBST, and the tissue was incubated in this solution overnight at room temperature. Tissue was also double labeled with the anti-DC0 antibody as described above.
The next day, the primary antibody solution was removed and the tissue washed 6 × 20 minutes prior to incubation overnight in Alexa 488-conjugated goat anti-mouse secondary antibody (Invitrogen (Molecular Probes), Eugene, OR) added at a 1:200 concentration to a solution of 1% NGS in PBST. Texas Red conjugated goat anti-rabbit secondary for visualization of the anti-DC0 antibody was also added at this time. The following day, the tissue was washed, cleared, coverslipped and viewed as described above.
1.4 Fluorescent dextran tracing
Live larvae or pharate adults were cold anesthetized and the brains removed rapidly in cold O’Shea-Adams saline. Pharate adults were used because brains removed from eclosed adults typically did not remain alive long enough to allow complete filling of the targeted neurons (R.J. Bayline, personal communication). To apply the fluorescent dextrans, the tip of a pulled glass electrode was broken against a glass slide and then coated in a concentrated solution of Texas Red- or fluorescein-conjugated 3000 MW dextran (Molecular Probes, Inc. (Invitrogen), Eugene, OR) that had been dried at room temperature to a gel-like consistency. The dextran-coated electrode was applied to the subesophageal ganglion by hand to label outputs from centers receiving gustatory input from the proboscis (Kent and Hildebrand, 1987; Kvello et al., 2006), to the optic lobes, or to the antennal lobes. Brains were then incubated in O’Shea-Adams saline for 2-4h on a rapidly rotating orbital shaker, after which they were fixed in 4% paraformaldehyde in PBS, embedded in agarose, sectioned and cleared, and viewed on the confocal microscope.
Results
1.1 Morphology of the adult Manduca mushroom bodies and inputs to the calyces
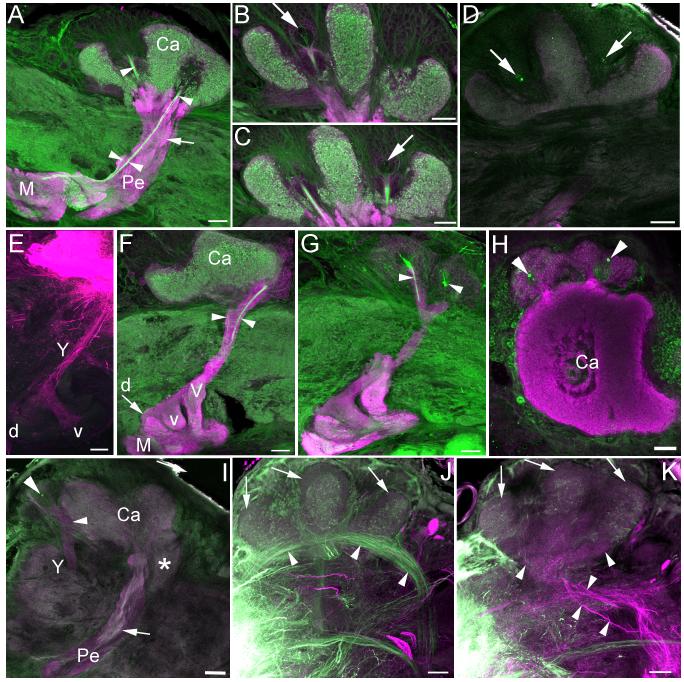
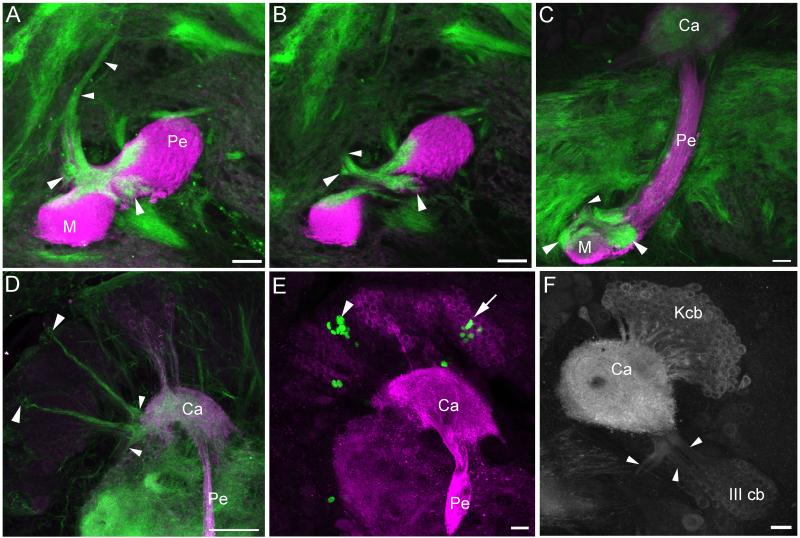
Anti-DC0 labeling showed the mushroom bodies of adult Manduca sexta to be much like those observed in other lepidopteran moths (Pearson, 1971; Sjöholm et al., 2005; Fukushima and Kanzaki, 2009). Two fused, invaginated calyces in the dorsoposterior protocerebrum are surrounded and filled by Kenyon cell bodies (Figures 1a-d). Ventral to the calyces the pedunculus projects anteroventrally into the protocerebrum and gives rise to subdivided vertical and medial lobes (corresponding to the segregated axons of γ, α’/β’, and α/β Kenyon cells as observed in other moths; Figure 1a; Sjöholm et al., 2005; Fukushima and Kanzaki, 2009). Phalloidin labeling revealed a thin ingrowth core arising from a small number of phalloidin-labeled Kenyon cell bodies at the center of each calyx, fusing in the pedunculus and bifurcating into the vertical and medial lobes (Figures 1a-c). This suggests that Kenyon cells are still growing into the mushroom bodies in the adult, as phalloidin heavily labels the outgrowing axons of immature neurons (Farris and Sinakevitch, 2003; Farris et al., 2004). Phalloidin also labeled the cell membrane of a large neuroblast-like cell at the center of each calyx (Figures 1b-c). 24h BrdU labeling confirmed the presence of a single mitotically active neuroblast in the center of each calyx, which gave rise to one or two labeled cells in this time period (Figure 1d). Neurogenesis as revealed by BrdU incorporation and phalloidin labeling of outgrowing Kenyon cell processes persisted to day 12 of adult life, effectively constituting lifelong mushroom body neurogenesis for this insect.
Figure 1. Mushroom bodies of adult Manduca sexta.
A-C. Anti-DC0 (magenta) and phalloidin (green) labeling of the mushroom body in one hemisphere of the protocerebrum in a twelve day old adult. Phalloidin labels an ingrowth core (arrowheads) made up of the axons of newborn Kenyon cells, which are generated by one neuroblast at the center of each calyx (large arrows). Small arrow in A indicates dense neuropil island in the pedunculus that forms in the pupal stage and is innervated by protocerebral neurons (Figure 6). D. Anti-DC0 (magenta) and anti-BrdU labeling (green) confirms mitotic activity of the mushroom body neuroblasts (arrows) in a six day old adult. E. Fluorescent dextran labeling of the Y-tract (Y), dorsal (d) and ventral (v) lobelets in the pharate adult. F-G. Anti-DC0 (magenta) and phalloidin (green) labeling of outgrowing Class III Kenyon cell axons (arrowheads) in the Y tract, emerging from two clusters of Class III Kenyon cell somata (G) in a twelve day old adult. H-I. Anti-DC0 (magenta) and anti-BrdU (green) labeling confirms mitotic activity of two proliferating neuroblasts amidst the Class III Kenyon cell soma (arrowheads), outside of the mushroom body calyces (H = pharate adult, I = six day old adult). Small arrow in I indicates neuropil island in the pedunculus. J-K. Fluorescent dextran labeling of inputs to the calyces from the antennal lobes (green) and the gustatory center of the subesophageal ganglion (magenta) in a pharate adult. Inner antennocerebral tract (iACT) projection neurons from the antennal lobe innervate most of the calyx neuropil (J, arrowheads) while tritocerebral tract (TT) projection neurons from the subesophageal ganglion innervate a ventroposterior putative accessory calyx neuropil (K, arrowheads). A dorsal compartment of the calyces characterized by small, diffuse microglomeruli was not innervated by neurons from either source (J-K, arrows). Ca- calyx, M- medial lobe, Pe- pedunculus, V- vertical lobe, *- accessory calyx. Scale bars = 50μm for all panels.
Two clusters of Class III Kenyon cell bodies are positioned anterolateral to the calyces; their processes form two branches, one of which enters the base of the lateral calyx and the other of which forms the Y tract (Figures 1e-g, i). The Y tract projects anterior to the pedunculus and bifurcates to form dorsal and ventral lobelets atop the medial lobe. Lobelet morphology was much like that observed in Spodoptera (Sjöholm et al., 2005); Manduca Class III Kenyon cells did not form a medial lobelet as has been observed in Bombyx (Fukushima and Kanzaki, 2009). Phalloidin also labeled two bundles of axons in the Y tract (Figures 1e-g), suggesting that some Class III cells were still extending axons and were thus immature in the adult. This finding was unexpected as the peripheral location of Class III Kenyon cell bodies relative to the position of neuroblasts in the calyx would suggest that they are among the earliest born mushroom body intrinsic neurons, and thus their development should be complete long before adult eclosion. Instead, both phalloidin and BrdU labeling revealed the presence of two proliferating neuroblasts amidst the Class III Kenyon cell somata (Figures 1h-i). This finding suggests that not only are Class III Kenyon cells in Manduca not an early born population of Kenyon cells, but that they are produced by two neuroblasts lying outside the calyces, distinct from the mushroom body neuroblasts located at each calyx center. These findings were confirmed by a detailed reconstruction of Manduca mushroom body development during the larval and pupal stages (see following sections).
Phalloidin and anti-DC0 double labeling combined with fluorescent dextran fills in the adult revealed three calycal subcompartments, two of which could be correlated with zones of afferent input. The majority of the calyx neuropil received afferent input from the antennal lobe via the inner antennocerebral tract (Figure 1i). A separate ventroposterior neuropil contained diffuse microglomeruli and received input from the gustatory center of the subesophageal ganglion (Figure 1j; Jørgensen et al., 2006), suggesting that this neuropil is an accessory calyx, perhaps composed of Class III Kenyon cell dendrites. The dorsal lips of the calyces were defined by their minute microglomeruli and exclusion by projection neuron inputs from the inner antennocerebral tract (Figure 1a, i). Neither this nor any other calyx subcompartment received input from the optic lobes (data not shown); we were unable to identify the source of input to this dorsal compartment. In Spodoptera, a similar dorsomedial calyx compartment with unidentified afferent input has also been observed (Sjöholm et al., 2005).
1.2 Morphology of the early larval mushroom bodies
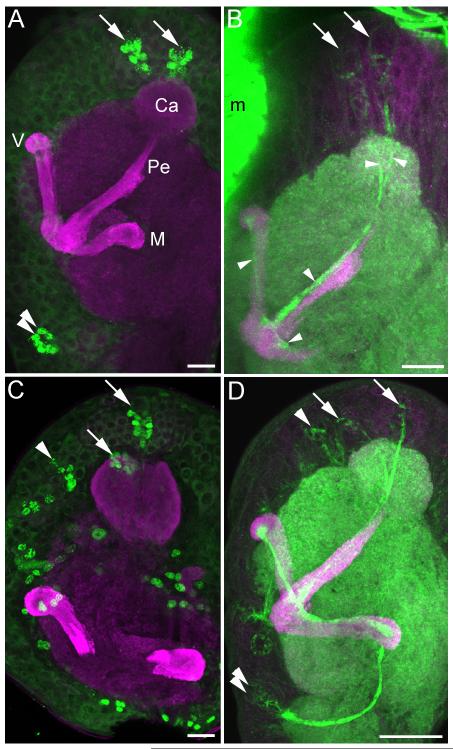
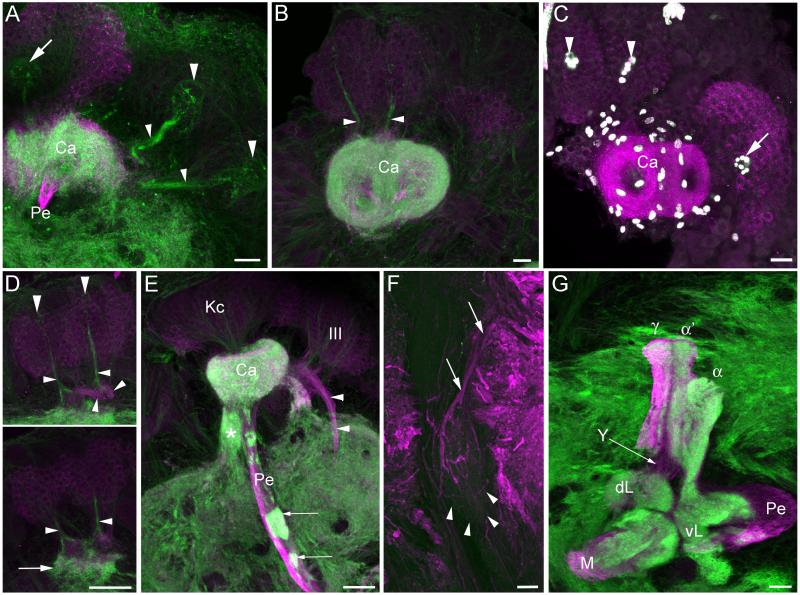
The mushroom bodies of Manduca sexta larvae were notably simpler in structure than those of the adult. Anti-DC0/phalloidin double labeling in all larval instars revealed a pedunculus and unsubdivided medial and vertical lobes containing an ingrowth core of outgrowing Kenyon cell axons with high affinity for phalloidin (Figures 2-4). The calyces of each mushroom body were fused into a single spherical neuropil, penetrated by two phalloidin-labeled tracts each arising from a cluster of newborn Kenyon cells generated by a single neuroblast (arrows, Figures 2b, d). As in the adult, the cell membranes of neuroblasts and their most recent progeny were prominently labeled with phalloidin (compare phalloidin labeling in Figures 2b, d with BrdU labeling of neuroblast progeny in Figures 2a, c); thus, we used phalloidin labeling as well as anti-BrdU labeling to localize neuroblasts and their recent, immature progeny throughout development.
Figure 2. Mushroom body development and neurogenesis in first and second instar larvae.
Anti-DC0 (magenta, all panels) staining shows simple mushroom bodies in the Manduca sexta larva, composed of a single calyx (Ca), medial lobe (M), vertical lobe (V) and pedunculus (Pe). A. BrdU labeling (green) in the first instar reveals neurogenesis by only three neuroblasts per hemisphere, the two mushroom body neuroblasts (arrows) and one neuroblast in the deutocerebrum (double arrows). B. Phalloidin labeling (green) is concentrated in the membranes of proliferating neuroblasts, newborn Kenyon cells beneath each mushroom body neuroblast (arrows) and their axons extending through the calyces and into the pedunculus and lobes (arrowheads). C. BrdU labeling (green) in the second instar larva reveals additional neurogenesis throughout the brain, including the mushroom body neuroblasts (arrows) and a protocerebral neuroblast anterior to the calyx (arrowhead). Tracing this latter neuroblast through larval development suggests that it contributes to the Class III Kenyon cell pool (see Figure 3). D. Phalloidin labeling (green) reveals newborn Kenyon cells produced by the mushroom body neuroblasts (arrows) and likely Class III Kenyon cells produced by the anterior protocerebral neuroblast (arrowhead). Axons of newborn neurons generated by the deutocerebral neuroblast are also labeled with phalloidin (double arrowheads). m- muscle, Scale bars = 20 μm for all panels.
Figure 4. Mushroom body development and innervation of the calyces in feeding fifth instar larvae.
A, B. Phalloidin labeling (green) of newborn Class III Kenyon cells growing into the Y tract and calyx, generated by one (A) or two (B) anterior protocerebral neuroblasts (large arrowhead, axons in Y tract indicated by small arrowheads). The mushroom body neuroblasts continue to contribute axons to the main mushroom body neuropil via the ingrowth core (arrows). C. Fluorescent dextran labeling of the larval antennal lobe reveals complete innervation of the mushroom body calyx by antennal lobe projection neurons in the inner antennocerebral tract (black arrowheads). D. Dextran labeling of the subesophageal ganglion reveals ascending projection neurons with blebby processes (inset) that arborize in the lateral protocerebrum and anterior to the mushroom body calyx. E. Double labeling with phalloidin (green) shows the anterior protocerebral neuroblasts and their newborn Class III Kenyon cell progeny extending processes anterior to the calyx in the region of dextran labeled axons from the subesophageal gangion (magenta) and into the Y tract (arrowheads). Ca- calyx, M- medial lobe, Pe- pedunculus, V- vertical lobe. Scale bars = 20 μm for all panels.
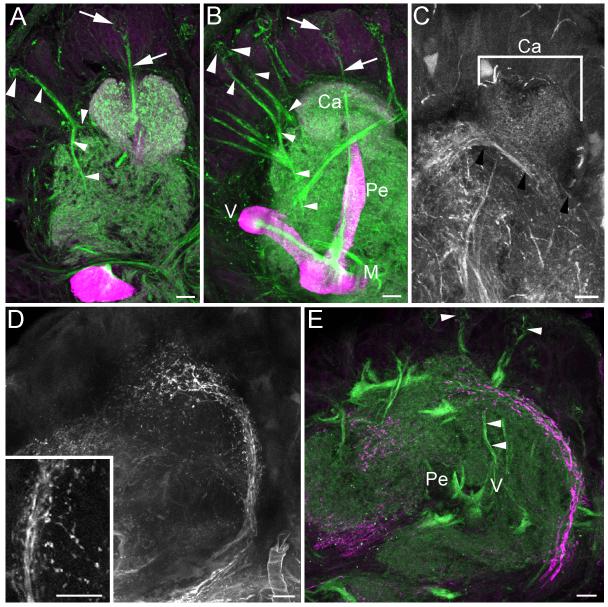
Notably, the larval mushroom bodies appeared to completely lack anti-DC0 labeling of a Y tract and lobelets (Figures 2-4). Only at the third instar did phalloidin labeling reveal a thin tract of outgrowing axons in the anterior protocerebrum with a morphology matching that of the adult Y tract (small arrowheads in Figure 3a, compare with sagittal view of adult Y tract in Figure 3c). Like the axons of Class III Kenyon cells making up the adult Y tract, the phalloidin-labeled axons in the third instar larva branched to send one collateral towards the calyx and one into the protocerebrum. In the fourth instar larva, the developing Y tract had extended deeper into the protocerebrum, nearly reaching the vertical lobe (Figure 3b).
Figure 3. Mushroom body development and neurogenesis in third and fourth instar larvae.
A, B. Phalloidin labeling (green) of the initial outgrowth of Class III Kenyon cell axons into the Y tract (small arrowheads) in the third instar larva (A) and fourth instar larva (B). Newborn Class III Kenyon cells are positioned beneath a neuroblast in the anterior protocerebrum, outside of the mushroom bodies (large arrowheads). The Y tract of a pharate adult is shown for comparison (C). D, E. Anti-BrdU labeling (green) of newborn Class III Kenyon cells around the anterior protocerebral neuroblast (arrowheads). F. Anti-DC0 staining of the fourth instar larval lobes reveals no Y tract, suggesting that Class III Kenyon cells forming the tract are immature and not yet expressing the DC0 antigen. Magenta staining in A, B, D and E and single channel staining in C and F is anti-DC0 labeling. Ca- calyx, m- muscle, M- medial lobe, Pe- pedunculus, V- vertical lobe. Scale bars = 20 μm for A, B, D, E; 50 μm for C, F.
As observed in the adult, outgrowing Class III Kenyon cell axons in the larva did not originate from newborn neurons adjacent to the mushroom body neuroblasts dorsal to the calyces, but rather originated from a cluster of newborn neurons positioned directly beneath a large anterior protocerebral neuroblast (Figure 3a, b, d, e; large arrowhead); a neuroblast surrounded by progeny was observed in a similar location in the second instar larva (Figures 2c, d), but no axon projections into the calyces and protocerebrum were visible at this developmental stage (Figure 2d). This neuroblast was not visible in the first instar larva and was perhaps quiescent like the majority of brain neuroblasts at this developmental stage (Truman and Bate, 1988; compare number of neuroblasts and the outgrowing axons of their progeny in first through fourth instar larvae, Figures 2 and 3).
Anti-DC0 labeling of the fourth instar larval mushroom bodies showed them to have the same relatively simple structure as observed in earlier instars, although they were larger due to the addition of Kenyon cells since that time (Figure 3f). Notably, the Y tract was still not labeled with anti-DC0 at this stage, indicating that no were no mature neurons in the tract, and that phalloidin labeling in earlier instars captured the first stages of Class III Kenyon cell development. Class III Kenyon cells are thus not the earliest-born neurons in the Manduca mushroom bodies as predicted by the peripheral position of their cell bodies in the adult. Rather, their production begins in the second larval instar (Figures 2c, d), and process outgrowth begins in the third instar. Most surprisingly, Class III Kenyon cells originate from a protocerebral neuroblast extrinsic to the mushroom bodies (hereafter referred to as a Class III neuroblast), not from the two mushroom body neuroblasts. This finding challenges the canonical view that all intrinsic neurons of the mushroom bodies are produced by the mushroom body neuroblasts.
1.3 Morphology of the late larval mushroom bodies
Phalloidin labeling in the feeding fifth instar larva revealed continued outgrowth of Class III Kenyon cells into the Y tract (Figures 4a, b). At this developmental stage some preparations showed that a second neuroblast had been recruited to produce Class III neurons, whereas only one protocerebral neuroblast was observed in this position in earlier instars. Two neuroblasts contributed Class III progeny for the remainder of postembryonic development.
To identify sources of afferent input to the larval mushroom bodies, fluorescent dextrans were used to label outputs from the larval antennal lobes and subesophageal ganglia, which receive olfactory and gustatory input from the head appendages, respectively (Kent and Hildebrand, 1987). Dextran labeling of antennal lobe_projection neurons indicates that these neurons project to and innervate the entire larval calyx (Figure 4c), while projection neurons from the subesophageal ganglion arborize anterior to the calyces (Figures 4d, e). Two tracts of phalloidin-labeled Class III Kenyon cell axons arising from the two Class III neuroblasts were observed projecting to this region (arrowheads, Figure 4e). The tract of gustatory projection neurons contained blebby processes reminiscent of growth cones (arrows Figures 4d), suggesting that gustatory inputs to newborn Class III Kenyon cells were just forming at this developmental stage. This finding suggests that Manduca Class III Kenyon cells, like those in other insects, may receive input from gustatory projection neurons; in Manduca these connections are newly generated in the late larva.
1.4 Morphology of the wandering larval and early pupal mushroom bodies
Formation of the lobelets by Class III Kenyon cell axons was first observed in the wandering (late 5th instar) larva (Figures 5a-c). The lobelets were diffuse and labeled heavily with phalloidin throughout the four day long wandering stage (W0-W3); the adult dorsal and ventral lobelet morphologies were evident by W2-W3 (Figure 5c).
Figure 5. Mushroom body development and neurogenesis in wandering fifth instar larvae and early pupae.
A-C. Phalloidin labeling (green) shows the formation of the lobelet neuropils (large arrowheads) by outgrowing Class III Kenyon cell axons in the Y tract (small arrowheads) of the W1 (wandering fifth instar, A, B) and W3 (C) larva. D. Phalloidin labeling of newborn Class III Kenyon cells generated by two anterior protocerebral neuroblasts (large arrowheads), sending processes into the calyx (Ca) and Y tract (small arrowheads) in the W3 larva. E. BrdU labeling (green) of newborn Kenyon cells produced by a mushroom body neuroblast dorsal to the calyx (arrow) and an anterior protocerebral neuroblast (arrowhead) in the P1 pupa. F. Anti-DC0 labeling of Kenyon cell bodies (Kcb) and Class III Kenyon cell bodies (III cb), the latter of which project into the calyx and the Y tract (arrowheads). Anti-DC0 labeling is magenta in panels A-E. M- medial lobe, Pe- pedunculus. Scale bars = 20 μm for all panels.
Neurogenesis by both the mushroom body neuroblasts and the Class III neuroblasts continued throughout the wandering stage. Two groups of newborn Class III Kenyon cells, each arising from a single neuroblast located at the periphery of the anterodorsal protocerebrum, produced long phalloidin-labeled neurites that branched into the calyx and Y tract (Figure 5d-f).
Metamorphosis of the calyx was first evident at W2-W3, as microglomerular structure was lost and the calyx took on a uniform “mushy” appearance (Figure 5c-f). Large-scale reorganization of Kenyon cell processes in the lobes was not observed. The unsubdivided larval lobes are likely composed of γ Kenyon cells, as these neurons are observed to be produced throughout the larval stage in holometabolous insects (Farris and Sinakevitch, 2003). The γ Kenyon cells underwent some modification in shape through metamorphosis in Manduca, such that the medial lobe lengthened towards the midline and the vertical lobe lengthened and curved anteriorly at its tip (data not shown). The processes of α’/β’, and α/β Kenyon cells were added to the lobes during the pupal stage (Huetteroth et al., 2010; Figure 6f).
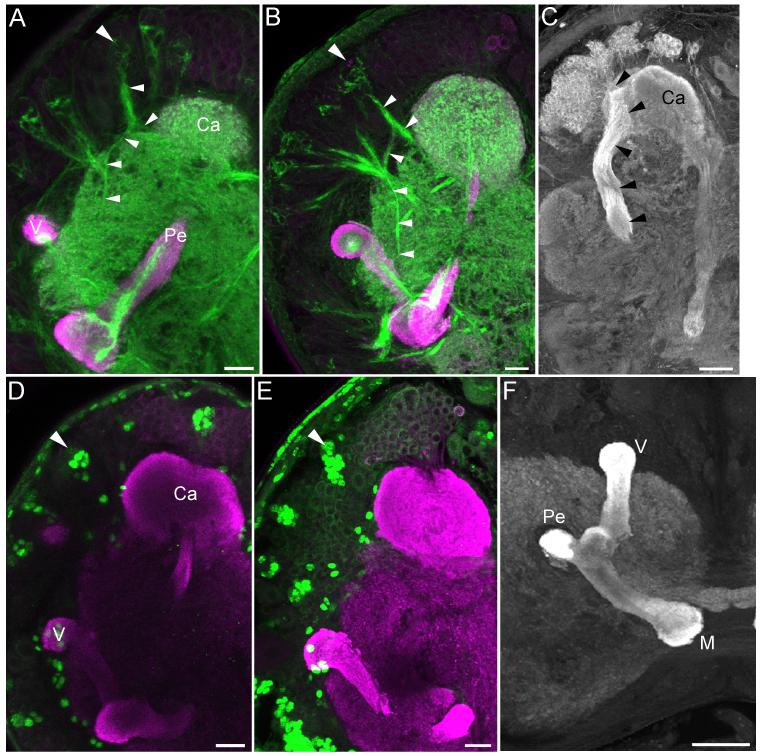
Figure 6. Mushroom body development and neurogenesis in pupae.
A, B, D. Phalloidin labeling (green) of anterior protocerebral (Class III) neuroblasts and their progeny in sagittal section (A, large arrowheads, P7 pupa), horizontal section (B, P6 pupa) and frontal section (D, both panels P7 pupa). Small arrowheads in all panels indicate processes of newborn Class III Kenyon cell forming the Y tract; outgrowth of Class III Kenyon cell dendrites anterior to the calyx is indicated by small arrow in the bottom panel of D. One of the mushroom body neuroblasts is visible dorsal to the calyx in panel A (large arrow). C. BrdU labeling (white) of progeny of the two Class III neuroblasts (arrowheads) and one of the mushroom body neuroblasts (arrow) in the P7 pupa. Other labeled cells around the calyx neuropil are likely to be glial cells. E. Sagittal section of a P8 pupa shows Kenyon cells projecting into the main calyx (Ca) and pedunculus (Pe) neuropils (Kc) and Class III Kenyon cells (III) projecting into the Y tract (arrowheads). A diffuse posterior neuropil, the putative accessory calyx, first becomes visible at this stage (indicated by *). Heavy phalloidin labeling (green) of blobs in the pedunculus (small arrows) likely represent ingrowing innervation by extrinsic neurons. F. Fluorescent dextran fill of the dorsal protocerebrum in the pharate adult showing innervation of a dense neuropil island in the pedunculus. G. All components of the adult lobes are visible in the P7 pupa including the dorsal and ventral lobelets (dL and vL) provided by Class III Kenyon cells in the Y tract (Y) and γ, α’/β’ (α’) and α/β (α) Kenyon cells in the vertical lobe. Anti-DC0 staining is magenta in all panels. M- medial lobe. Scale bars = 20 μm for all panels.
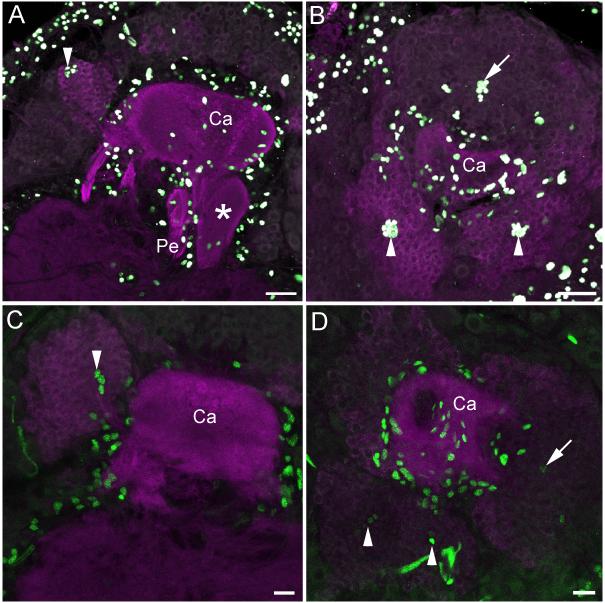
1.5 Morphology of the late pupal mushroom bodies
BrdU and phalloidin labeling showed continued neurogenesis by both the mushroom body neuroblasts and Class III neuroblasts and the incorporation of their progeny into the mushroom body calyces and lobes through the duration of the pupal stage (Figures 6a-d, 7). Long-term BrdU treatments (pulse of BrdU at pupal stage P9, immunostaining at P15) confirmed that cells produced by the Class III neuroblasts (Figures 7a-b) were incorporated into the anterolateral clusters of Class III Kenyon cell bodies (Figures 7c-d). BrdU labeling experiments performed during the pupal stage also revealed a great deal of gliogenesis around the mushroom bodies and other brain neuropils (Figures 6c, 7).
Figure 7. Neurogenesis by mushroom body neuroblasts and Class III neuroblasts in late pupae.
A, B. Sagittal (A) and horizontal (B) sections of P13 pupae labeled with BrdU (white) showing incorporation into progeny of Class III neuroblasts (arrowheads) and mushroom body neuroblasts (arrows). Additional cells labeling around the mushroom body neuropil are likely to be glial cells. C, D. BrdU labeling (green) of P9 pupae followed by immunostaining at P15 reveals labeled Class III Kenyon cell bodies (arrowheads) and other Kenyon cells derived from the mushroom body neuroblasts (arrows). Glial labeling around the mushroom body neuropil is also evident. Anti-DC0 labeling is magenta in all panels. Ca- calyx, Pe- pedunculus. Scale bars = 20 μm for all panels.
Phalloidin labeling also revealed dense blobs of staining in the pedunculus of the mid-to late pupa (Figure 6e). These blobs appear to correspond to dense neuropil “islands” in the otherwise fibrous pedunculus of the adult (Figures 1a, i). Dextran fills to the protocerebrum of pharate adults labeled large processes innervating these islands (Figure 6f), suggesting that like some other insects (honey bee and cockroach, for example; Strausfeld and Li, 1999; Strausfeld 2002), the mushroom bodies of Manduca may receive afferent input to the pedunculus.
Also of interest during pupal development was the formation of the ventro-posterior calyx neuropil beginning around P4, and increasing in size throughout the pupal stage (Figures 1i, 6e, 7a). The position of this neuropil is reminiscent of accessory calyces formed by Class III Kenyon cells in other insects; like other accessory calyces, this structure receives gustatory input in the Manduca adult (Figure 1k). Formation of a distinct accessory calyx neuropil by Class III Kenyon cells has not been reported in any of the other Lepidoptera studied to date, including the closely related sphingid Sphinx ligustri; in these species, Class III dendrites are incorporated within the main calyx neuropil along with the dendrites of the other types of Kenyon cells (Pearson, 1971; Sjöholm et al., 2005; Fukushima and Kanzaki, 2009). We were unable to confirm that Manduca Class III Kenyon cell dendrites make up this accessory calyx-like structure; however, its morphology and source of afferent input suggest that this may be the case.
Discussion
1.1 Commonalities of mushroom body architecture and development in holometabolous insects
Insect mushroom bodies share an architectural and developmental groundplan, and those of Manduca sexta are no exception. The majority of Kenyon cells making up the adult Manduca mushroom bodies are generated by two single neuroblasts in each hemisphere of the brain, forming two Kenyon cell clusters that provide dendrites to two partially fused calyces. Most insects studied to date show a similar pattern of dual neuroblasts/neuroblast clusters, dual Kenyon cell soma groups and dual calyces (the latter of which range in morphology from fully fused to fully separated; Farris, 2005b). Some exceptions do exist, perhaps most notably in the brachyceran flies (Diptera) in which three Kenyon cell groups are widespread (Panov, 2009a), and neuroblast numbers range from four to twenty (Gundersen and Larsen, 1978; Ito and Hotta, 1992; Panov, 2009b). However, given how widespread this developmental pattern of two progenitors: two Kenyon cell soma groups: two calyces is across the insects (including taxa in the primitively wingless Apterygota and the majority of taxa in the Neoptera), it is likely that this represents the ancestral mushroom body configuration.
The mushroom body lobes of Manduca sexta consist of γ, α’/β’, and α/β subdivisions made up of the bifurcated axons of these Kenyon cell populations. These subdivisions are added sequentially during development via an ingrowth core consisting of the axons of newborn Kenyon cells arising from the two mushroom body neuroblasts. Taken together with previous studies, this also appears to represent a groundplan for insect mushroom body lobe organization and development, with subdivision into γ, α’/β’, and α/β lobes in particular appearing to be conserved amongst holometabolous species (Crittenden et al., 1998; Strausfeld et al., 2003; Sjöholm et al., 2006; Zhao et al., 2008; Huetteroth et al., 2010). Exceptions exist here as well, and are also likely to be derived relative to the ancestral groundplan, such as the single γ lobe characteristic of the fruit fly Drosophila melanogaster (Strausfeld et al., 2003), the fused and laminated lobes of the Hymenoptera (Ehmer and Hoy, 2000; Strausfeld, 2002), and the concentrically organized lobes of feeding generalist scarab beetles (Larsson et al., 2004; Farris, 2008b).
Finally, some degree of metamorphosis of the mushroom bodies also appears to be characteristic of holometabolous insects. Reorganization of the calyces, in which the microglomerular structure is lost in the prepupa and early pupa and reformed later in the pupal stage is observed in Manduca and in most other holometabolous species studied to date (present account; Zhu et al., 2003; Marin et al., 2005; Zhao et al., 2008). Given the dramatic differences in the sensory ecologies of the two active life stages of holometabolous insects, it is not surprising that a restructuring of sensory inputs to a higher brain center like the mushroom bodies would occur during the transition from the larval to the adult stage. The only exception observed thus far is in the aculeate Hymenoptera in which the development of the entire mushroom body is delayed until the late larval stage so that calyx microglomerular formation occurs for the first time only during the prepupal stage (Farris et al., 1999; Farris et al., 2004; Ganeshina et al., 2006; Groh and Rössler, 2008). The larvae of aculeate Hymenoptera, which are provisioned or provided with food and protected within a nest, lead a behaviorally simple life relative to the active larvae of other holometabolous species. Hymenopteran larvae are likely to have little need for the kinds of higher processing functions performed by the mushroom bodies, so that the development of these structures may be delayed, similar to what is observed in other animals that provide extensive parental care such as altricial rodents and birds (Sánchez-Villagra and Sultan, 2002).
Lobe metamorphosis shows more variation across the Holometabola, ranging from no observable changes aside from the addition of subcompartments composed of adult-specific Kenyon cells in the beetle Tribolium castaneum (Zhao et al., 2008) to restructuring of the axon projections of a single Kenyon cell type in Drosophila (Armstrong et al., 1998; Lee et al., 1999) to massive restructuring of the entire lobe neuropil in the paper wasp Polistes apachiensis (Farris et al., 2004). Lobe metamorphosis in Manduca consists of a relatively subtle change in shape of the larval-formed γ lobes, occurring alongside the addition of Kenyon cells making up theα’/β’ and α/β lobes (present account; Huetteroth et al., 2010).
We observed lifelong neurogenesis in the mushroom bodies of adult Manduca sexta, although the rate of neurogenesis was slow (approximately 1-2 progeny per neuroblast per 24h). Adult mushroom body neurogenesis has been previously observed in another moth, the noctuid Agrotis ipsilon; however, neurogenesis in this species ceased after 3 days of adult life (Dufour and Gadenne, 2006). Adult neurogenesis is only observed in a few other insect species (Cayre et al., 1996), but in crickets, where adult neurogenesis is extensive, Kenyon cell production is linked to sensory experience and enhances performance in a learning and memory assay using olfactory and visual cues (Cayre et al., 2007). The moths used in the present study were housed individually in paper bags throughout adulthood, which likely constitutes extremely sensory impoverished conditions. It is therefore possible that adult neurogenesis in Manduca may be more dramatic under natural conditions, in which the insect is moving about its environment in search of food, mates and oviposition sites, and is exposed to the associated chemosensory and visual cues. If so, adult neurogenesis may play a similar role in behavioral plasticity as observed in insects like the cricket.
1.2 Diversity in mushroom body architecture and development: Class III Kenyon cells
The most divergent aspect of the Manduca mushroom bodies from the above-discussed groundplans is the presence and morphogenesis of Class III Kenyon cells (Figure 8a, b). The axons of Class III Kenyon cells in Manduca and other lepidopterans form a Y tract and lobelets that are structurally separate from the lobe system formed by the γ, α’/β’, and α/β Kenyon cells (present account; Pearson, 1971; Sjöholm et al., 2005; Fukushima and Kanzaki, 2009; Huetteroth et al., 2010). In Manduca, Class III Kenyon cell dendrites may also form a separate accessory calyx; this is typical of Class III Kenyon cells in other insect species (Farris, 2005b), although in the other lepidopterans studied to date Class III dendrites are integrated into the primary calyx neuropil formed by the other Kenyon cell types. As in other insects with Class III Kenyon cells, the putative Manduca accessory calyx receives input from projection neurons originating in gustatory centers (present account; Farris, 2008c); it is likely that Class III Kenyon cells in other lepidopteran species receive similar inputs.
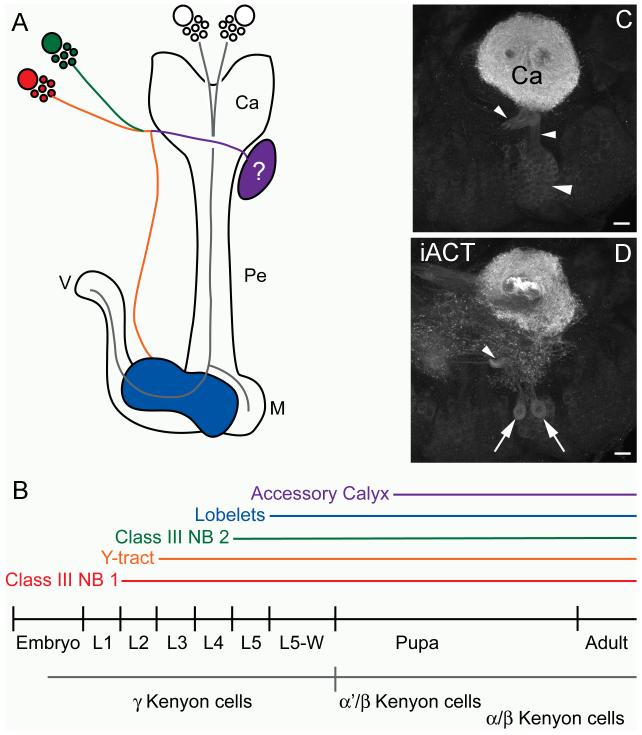
Figure 8. Schematic of Class III Kenyon cell development and potential non-Kenyon cell progeny of Class III neuroblasts.
A. Schematic diagram of Manduca sexta mushroom bodies and Class III Kenyon cells in sagittal view. Components of the Class III Kenyon cell system are color coded according to their appearance and formation during development, which is indicated on the timeline in B. Other Kenyon cell types are shown in grey and their birthdates indicated by the grey line in B. C. Anti-DC0 labeling of Class III Kenyon cells projecting into the calyx (Ca) and Y tract (arrowheads). D. More ventral view of (C) shows two large neurons with strong affinity for the anti-DC0 antibody (arrows) ventral to the Class III Kenyon cell bodies providing a diffuse network of processes anterior and ventral to the calyx. The location of these large neurons suggests that they may also be progeny of the Class III neuroblasts. Ca- calyx, iACT- inner antennocerebral tract, M- medial lobe, Pe- pedunculus, V- vertical lobe. Scale bars = 20 μm for all panels.
The most unusual aspect of Manduca Class III Kenyon cells, and of the Manduca mushroom bodies as a whole, is the generation of these neurons by two protocerebral neuroblasts residing outside of the mushroom bodies, anterior to the mushroom body calyces (hereafter referred to as the Class III neuroblasts). Production of Kenyon cells by any progenitors aside from the canonical mushroom body neuroblasts (the two single neuroblasts or neuroblast clusters residing dorsal to the calyces) has not been previously observed in any other insect. In Manduca, one Class III neuroblast begins to proliferate in the second larval instar, and the axons of newborn Class III neurons begin to enter the calyx and form the Y tract in the third instar. A second Class III neuroblast begins contributing neurons to the Y tract in the fifth larval instar. The lobelet neuropils formed by Class III Kenyon cell axons become visible in the wandering fifth instar. An accessory calyx-like structure, putatively supplied by the dendrites of Class III Kenyon cells and receiving input from gustatory centers, arises in the mid-pupal stage. Neurogenesis by both the Class III neuroblasts and the canonical mushroom body neuroblasts continues through the pupal stage and into adulthood.
Class III Kenyon cells occur sporadically across the insects, and have thus far only been observed in species belonging to the orders Dictyoptera, Dermaptera, Orthoptera, Hemiptera, Neuroptera and Lepidoptera (Farris, 2005b). Their defining morphological features (accessory calyx, lobelets, etc.) make Class III Kenyon cells unique among mushroom body intrinsic neurons, yet until now they have been believed to share the same developmental origin as other Kenyon cells: produced by the canonical mushroom body neuroblasts or neuroblast clusters located at the center of each calyx. The location of Class III Kenyon cell bodies at the periphery of the Kenyon cell body region has been interpreted to mean that they are the earliest-born population of Kenyon cells (Farris and Strausfeld, 2003); in Manduca, however, their peripheral position is actually due to their production by the Class III neuroblasts outside of the mushroom bodies. Future studies may reveal whether the same developmental mechanism, recruitment of protocerebral neuroblasts, has been employed to generate Class III Kenyon cells throughout the Lepidoptera as well as in other taxa.
1.3 Neuroblast recruitment for the generation of novel brain structures
The recruitment of nearby protocerebral neuroblasts represents a novel evolutionary mechanism for generating new structural and functional modules in the mushroom bodies, and has likely occurred in other brain regions as well. For example, a comparison of neuroblasts in the thoracic ganglia of a primitively wingless insect, the silverfish Ctenolepisma longicaudata, with that of winged insects revealed that no new neuroblasts have been acquired during the evolution of winged species, despite the acquisition of many additional neurons comprising the sensory and motor circuits necessary for flight (Truman and Ball, 1998). Instead, some neuroblasts were observed to have prolonged proliferation durations in winged insects, during which neurons making up the flight circuitry were likely produced. Thus, a population of thoracic neuroblasts has been recruited to produce novel neuronal populations during the evolution of winged insects. It is not known, however, whether these neuroblasts have been entirely co-opted to the production of flight circuit neurons, or if they also produce neurons belonging to ancestral thoracic circuits (say, sensory and motor neurons associated with the walking legs); the relative contribution of neuroblasts to new and old circuits may even vary according to species.
Similar questions about the origin, identity and progeny of Class III neuroblasts in Manduca await future studies. Mushroom body neuroblasts and their progeny have characteristic patterns of gene expression, most notably those of the eyeless regulatory cascade (Kurusu et al., 2000); expression of these genes by the Class III neuroblasts may indicate that they have taken on the identity of canonical mushroom body neuroblasts, while expression of other transcription factors may assign identity to a neuroblast field adjacent to the mushroom bodies (Urbach and Technau, 2003a; Urbach and Technau, 2003b). A related question regards the origin of the Class III neuroblasts: are they recruited from an embryonic neuroblast field adjacent to the mushroom bodies, or do they arise de novo with no homologs in insects outside of the Lepidoptera? If the Class III neuroblasts have homologs in insect lacking Class III Kenyon cells, it would be interesting to know what neurons they produce, and whether they still produce these neurons in addition to Class III Kenyon cells in Lepidoptera. A tantalizing clue to this question may be observed in the P0-P1 Manduca pupa, in which two large neurons with a high affinity for the anti-DC0 antibody and extensive dendritic arborizations anterior to the calyx are observed immediately ventral to the somata of Class III Kenyon cells (Figure 8c, d). While tracing the origin and eventual fate of these neurons is beyond the scope of the present study, the location of their cell bodies suggests that they may be derived from the Class III neuroblasts. Perhaps these two neurons represent progeny ancestrally produced by these neuroblasts, prior to their recruitment to Class III Kenyon cell production. Further studies of the developmental history of these neurons may thus shed light upon the identity of the Class III neuroblasts and the regulation of mechanisms for neuroblast recruitment and the generation of novelty in mushroom body structure and function.
Acknowledgements
The authors would like to thank Dr. Ronald J. Bayline for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Armstrong JD, deBelle JS, Wang Z, Kaiser K. Metamorphosis of the mushroom bodies; large-scale rearrangements of the neural substrates for associative learning and memory in Drosophila. Learning and Memory. 1998;5:102–114. [PMC free article] [PubMed] [Google Scholar]
- Bell RA, Joachim FG. Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Annals of the Entomologial Society of America. 1976;69:365–372. [Google Scholar]
- Blum AL, Li W, Cressy M, Dubnau J. Short- and long-term memory in Drosophila require cAMP signaling in distinct neuron types. Curr Biol. 2009;19:1341–1350. doi: 10.1016/j.cub.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayre M, Scotto-Lomassese S, Malaterre J, Strambi C, Strambi A. Understanding the regulation and function of adult neurogenesis: contribution from an insect model, the house cricket. Chemical Senses. 2007;32:385–395. doi: 10.1093/chemse/bjm010. [DOI] [PubMed] [Google Scholar]
- Cayre M, Strambi C, Charpin P, Augier R, Meyer MR, Edwards JS, Strambi A. Neurogenesis in adult insect mushroom bodies. Journal of Comparative Neurology. 1996;371:300–310. doi: 10.1002/(SICI)1096-9861(19960722)371:2<300::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Skoulakis EMC, Han K-A, Kalderon D, Davis RL. Tripartite mushroom body architecture revealed by antigenic markers. Learning and Memory. 1998;5:38–51. [PMC free article] [PubMed] [Google Scholar]
- Daly KC, Christensen TA, Lei H, Smith BH, Hildebrand JG. Learning modulates the ensemble representations for odors in primary olfactory networks. Proceedings of the National Academy of Sciences USA. 2004;101:10476–10481. doi: 10.1073/pnas.0401902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour MC, Gadenne C. Adult neurogenesis in a moth brain. Journal of Comparative Neurology. 2006;495:635–643. doi: 10.1002/cne.20909. [DOI] [PubMed] [Google Scholar]
- Ehmer B, Hoy RR. Mushroom bodies of vespid wasps. Journal of Comparative Neurology. 2000;416:93–100. doi: 10.1002/(sici)1096-9861(20000103)416:1<93::aid-cne7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Farris SM. Developmental organization of the mushroom bodies of Thermobia domestica (Zygentoma, Lepismatidae): insights into mushroom body evolution from a basal insect. Evolution of Development. 2005a;7:150–159. doi: 10.1111/j.1525-142X.2005.05017.x. [DOI] [PubMed] [Google Scholar]
- Farris SM. Evolution of insect mushroom bodies: Old clues, new insights. Arthropod Structure and Development. 2005b;34:211–234. [Google Scholar]
- Farris SM. Evolutionary convergence of higher brain centers spanning the protostome-deuterostome boundary. Brain Behavior and Evolution. 2008a;72:106–122. doi: 10.1159/000151471. [DOI] [PubMed] [Google Scholar]
- Farris SM. Structural, functional and developmental convergence of the insect mushroom bodies with higher brain centers of vertebrates. Brain Behavior and Evolution. 2008b;72:1–15. doi: 10.1159/000139457. [DOI] [PubMed] [Google Scholar]
- Farris SM. Tritocerebral tract input to the insect mushroom bodies. Arthropod Structure and Development. 2008c;37:492–503. doi: 10.1016/j.asd.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Farris SM, Abrams AI, Strausfeld NJ. Development and morphology of Class II Kenyon cells in the mushroom bodies of the honey bee, Apis mellifera. Journal of Comparative Neurology. 2004;474:325–339. doi: 10.1002/cne.20146. [DOI] [PubMed] [Google Scholar]
- Farris SM, Robinson GE, Davis RL, Fahrbach SE. Larval and pupal development of the mushroom bodies in the honey bee, Apis mellifera. Journal of Comparative Neurology. 1999;414:97–113. doi: 10.1002/(sici)1096-9861(19991108)414:1<97::aid-cne8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Farris SM, Sinakevitch I. Development and evolution of the insect mushroom bodies: Towards the understanding of conserved developmental mechanisms in a higher brain center. Arthropod Structure and Development. 2003;32:79–101. doi: 10.1016/S1467-8039(03)00009-4. [DOI] [PubMed] [Google Scholar]
- Farris SM, Strausfeld NJ. Development of laminar organization in the mushroom bodies of the cockroach: Kenyon cell proliferation, outgrowth, and maturation. Journal of Comparative Neurology. 2001;439:331–351. doi: 10.1002/cne.1354. [DOI] [PubMed] [Google Scholar]
- Farris SM, Strausfeld NJ. A unique mushroom body substructure common to both basal cockroaches and to termites. Journal of Comparative Neurology. 2003;456:305–320. doi: 10.1002/cne.10517. [DOI] [PubMed] [Google Scholar]
- Frambach I, Schürmann F. Separate distribution of deutocerebral projection neurons in the mushroom bodies of the cricket brain. Acta Biol Hung. 2004;55:21–29. doi: 10.1556/ABiol.55.2004.1-4.4. [DOI] [PubMed] [Google Scholar]
- Fukushima R, Kanzaki R. Modular subdivision of mushroom bodies by Kenyon cells in the silkmoth. Journal of Comparative Neurology. 2009;513:315–330. doi: 10.1002/cne.21946. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Vorobyev M, Menzel R. Synaptogenesis in the mushroom body calyx during metamorphosis in the honeybee Apis mellifera: an electron microscopic study. Journal of Comparative Neurology. 2006;497:876–897. doi: 10.1002/cne.21033. [DOI] [PubMed] [Google Scholar]
- Groh C, Rössler W. Caste-specific postembryonic development of primary and secondary olfactory centers in the female honeybee brain. Arthropod Structure and Development. 2008;37:459–468. doi: 10.1016/j.asd.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Gundersen RW, Larsen JR. Postembryonic development of the lateral protocerebral lobes, corpora pedunculata, deutocerebrum and tritocerebrum of Phormia regina Meigen (Diptera: Calliphoridae) Journal of Insect Morphology and Embryology. 1978;7:467–477. [Google Scholar]
- Huetteroth W, el Jundi B, el Jundi S, Schachtner J. 3D-reconstructions and virtual 4D-visualization to study metamorphic brain development in the sphinx moth Manduca sexta. Frontiers in Systems Neuroscience. 2010;4:7. doi: 10.3389/fnsys.2010.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito I, Bazhenov M, Ong RC-Y, Raman B, Stopfer M. Frequency transitions in odor-evoked neural oscillations. Neuron. 2009;64:692–706. doi: 10.1016/j.neuron.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development. 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- Ito K, Hotta Y. Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Developmental Biology. 1992;149:134–148. doi: 10.1016/0012-1606(92)90270-q. [DOI] [PubMed] [Google Scholar]
- Jørgensen K, Kvello P, Almaas TJ, Mustaparta H. Two closely located areas in the suboesophageal ganglion and the tritocerebrum receive projections of gustatory receptor neurons lovated on the antennae and the proboscis in the moth Heliothis virescens. Journal of Comparative Neurology. 2006;496:121–134. doi: 10.1002/cne.20908. [DOI] [PubMed] [Google Scholar]
- Kent KS, Hildebrand JG. Cephalic sensory pathways in the central nervous system of larval Manduca sexta (Lepidoptera: Sphingidae) Philosophical Transactions of the Royal Society of London B. 1987;315:1–36. doi: 10.1098/rstb.1987.0001. [DOI] [PubMed] [Google Scholar]
- Kurusu M, Awasaki T, Masuda-Nakagawa LM, Kawauchi H, Ito K, Furukubo-Tokunaga K. Embryonic and larval development of the Drosophila mushroom bodies: concentric layer subdivisions and the role of fasciclin II. Development. 2002;129:409–419. doi: 10.1242/dev.129.2.409. [DOI] [PubMed] [Google Scholar]
- Kurusu M, Nagao T, Walldorf U, Flister S, Gehring WJ, Furukuo-Tokunaga K. Genetic control of development of the mushroom bodies, the associative learning centers in the Drosophila brain, by the eyeless, twin of eyeless, and dachshund genes. Proceedings of the National Academy of Sciences USA. 2000;97:2140–2144. doi: 10.1073/pnas.040564497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvello P, Almaas TJ, Mustaparta H. A confined taste area in a lepidopteran brain. Arthropod Structure and Development. 2006;35:35–45. doi: 10.1016/j.asd.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Larsson MC, Hansson BS, Strausfeld NJ. A simple mushroom body in an African scarabid beetle. Journal of Comparative Neurology. 2004;478:219–232. doi: 10.1002/cne.20284. [DOI] [PubMed] [Google Scholar]
- Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126:4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- Lei H, Riffel JA, Gage SL, Hildebrand JG. Contrast enhancement of stimulus intermittency in a primary olfactory network and its behavioral significance. J Biol. 2009;8:21. doi: 10.1186/jbiol120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-S, Strausfeld NJ. Multimodal efferent and recurrent neurons in the medial lobes of cockroach mushroom bodies. J Comp Neurol. 1999;409:647–663. doi: 10.1002/(sici)1096-9861(19990712)409:4<647::aid-cne9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Malaterre J, Strambi C, Chiang A-S, Aouane A, Strambi A, Cayre M. Development of cricket mushroom bodies. Journal of Comparative Neurology. 2002;452:215–227. doi: 10.1002/cne.10319. [DOI] [PubMed] [Google Scholar]
- Marin EC, Watts RJ, Tanaka NK, Ito K, Luo L. Developmentally programmed remodeling of the Drosophila olfactory circuit. Development. 2005;132:725–737. doi: 10.1242/dev.01614. [DOI] [PubMed] [Google Scholar]
- O’Shea M, Adams M. Pentapeptide (proctolin) associated with an identified neuron. Science. 1981;213:567–569. doi: 10.1126/science.6113690. [DOI] [PubMed] [Google Scholar]
- Panov AA. General structure of the mushroom body calyx in Brachycera Orthorrhapha flies (Diptera) Biological Bulletin. 2009a;36:267–276. [PubMed] [Google Scholar]
- Panov AA. How many neuroblasts build mushroom bodies in Lucilia caesar L. and Musca domestica L. (Diptera, Brachycera Cyclorrhapha)? Biological Bulletin. 2009b;36:598–606. [PubMed] [Google Scholar]
- Pearson L. The corpora pedunculata of Sphinx ligustri L. and other Lepidoptera: an anatomical study. Philosophical Transactions of the Royal Society of London B. 1971;259:477–516. [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representation in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- Rössler W, Oland LA, Higgins MR, Hildebrand JG, Tolbert LP. Development of a glia-rich axon-sorting zone in the olfactory pathway of the moth Manduca sexta. Journal of Neuroscience. 1999;19:9865–9877. doi: 10.1523/JNEUROSCI.19-22-09865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Villagra MR, Sultan F. The cerebellum at birth in therian mammals, with special reference to rodents. Brain Behavior and Evolution. 2002;59:101–113. doi: 10.1159/000064158. [DOI] [PubMed] [Google Scholar]
- Sjöholm M, Sinakevitch I, Ignell R, Strausfeld NJ, Hansson BS. Organization of Kenyon cells in subdivisions of the mushroom bodies of a lepidopteran insect. Journal of Comparative Neurology. 2005;491:290–304. doi: 10.1002/cne.20698. [DOI] [PubMed] [Google Scholar]
- Sjöholm M, Sinakevitch I, Strausfeld NJ, Ignell R, Hansson BS. Functional division of intrinsic neurons in the mushroom bodies of male Spodoptera littoralis revealed by antibodies against aspartate, taurine, FMRF-amide, Mas-allatotropin and DC0. Arthropod Structure and Development. 2006;35:153–168. doi: 10.1016/j.asd.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Skoulakis EMC, Kalderon D, Davis RL. Preferential expression in mushroom bodies of the catalytic subunit of protein kinase A and its role in learning and memory. Neuron. 1993;11:197–208. doi: 10.1016/0896-6273(93)90178-t. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ. Organization of the honey bee mushroom body: representation of the calyx within the vertical and gamma lobes. Journal of Comparative Neurology. 2002;450:4–33. doi: 10.1002/cne.10285. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ, Hansen L, Li Y-S, Gomez RS, Ito K. Evolution, discovery, and interpretations of arthropod mushroom bodies. Learning and Memory. 1998;5:11–37. [PMC free article] [PubMed] [Google Scholar]
- Strausfeld NJ, Li Y-S. Organization of olfactory and multimodal afferent neurons supplying the calyx and pedunculus of the cockroach mushroom bodies. J Comp Neurol. 1999;409:603–625. [PubMed] [Google Scholar]
- Strausfeld NJ, Sinakevitch I, Vilinsky I. The mushroom bodies of Drosophila melanogaster: an immunocytological and Golgi study of Kenyon cell organization in the calyces and lobes. Microscopy Research and Technique. 2003;62:151–169. doi: 10.1002/jemt.10368. [DOI] [PubMed] [Google Scholar]
- Truman JW, Ball E. Patterns of embryonic neurogenesis in a primitive wingless insect, the silverfish, Ctenolepisma longicaudata: comparison with those seen in flying insects. Development Genes and Evolution. 1998;208:357–368. doi: 10.1007/s004270050192. [DOI] [PubMed] [Google Scholar]
- Truman JW, Bate M. Spatial and temporal patterns of neurogenesis in the CNS of Drosophila melanogaster. Developmental Biology. 1988;125:146–157. doi: 10.1016/0012-1606(88)90067-x. [DOI] [PubMed] [Google Scholar]
- Urbach R, Technau GM. Early steps in building the insect brain: neuroblast formation and segmental patterning in the developing brain of different insect species. Arthropod Structure and Development. 2003a;32:103–123. doi: 10.1016/S1467-8039(03)00042-2. [DOI] [PubMed] [Google Scholar]
- Urbach R, Technau GM. Molecular markers for identified neuroblsts in the developing brain of Drosophila. Development. 2003b;130:3621–3637. doi: 10.1242/dev.00533. [DOI] [PubMed] [Google Scholar]
- van Swinderen B, Brembs B. Attention-like deficit and hyperactivity in a Drosophila memory mutant. J Neurosci. 2010;30:1003–1014. doi: 10.1523/JNEUROSCI.4516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss MJ. Structural patterns in the corpora pedunculata of Orthoptera: a reduced silver analysis. Journal of Comparative Neurology. 1981;203:515–553. doi: 10.1002/cne.902030312. [DOI] [PubMed] [Google Scholar]
- Zhao X, Coptis V, Farris SM. Metamorphosis and adult development of the mushroom bodies of the red flour beetle, Tribolium castaneum. Developmental Neurobiology. 2008;68:1487–1502. doi: 10.1002/dneu.20669. [DOI] [PubMed] [Google Scholar]
- Zhu S, Chiang A-S, Lee T. Development of the Drosophila mushroom bodies: elaboration, remodeling and spatial organization of dendrites in the calyx. Development. 2003;130:2603–2610. doi: 10.1242/dev.00466. [DOI] [PubMed] [Google Scholar]