Abstract
Background
Although high body mass index (BMI) is a risk factor for hypertension, diabetes, and cardiovascular disease, limited data exist on the association of overweight and obesity with early stages of kidney disease.
Methods
Cross-sectional data for 5083 participants of the nationally representative Third National Health and Nutrition Examination Survey with an estimated glomerular filtration rate ≥ 60 ml/min/1.73m2 without micro- or macro-albuminuria were analyzed to determine the association between BMI and elevated serum cystatin C. Normal weight, overweight, class I obesity, and class II–III obesity were defined as a BMI of 18.5 to 24.9 kg/m2, 25.0 to 29.9 kg/m2, 30.0 to 34.9 kg/m2 and ≥ 35.0 kg/m2, respectively. Elevated serum cystatin C was defined as ≥ 1.09 mg/L (≥99th percentile for participants 20 to 39 years of age without diabetes, hypertension, micro- or macro-albuminuria or stage 3–5 chronic kidney disease).
Results
The age-standardized prevalence of elevated serum cystatin C was 9.6%, 12.9%, 17.4%, and 21.5% among adults of normal weight, overweight, class I obesity, and class II–III obesity, respectively (p-trend<0.001). After multivariate adjustment for demographics, behaviors, systolic blood pressure and serum biomarkers and compared to normal weight participants, the odds ratio (95% confidence interval) of elevated serum cystatin C was 1.46 (1.02–2.10) for overweight, 2.36 (1.56–3.57) for class I obesity, and 2.82 (1.56–5.11) for class II–III obesity.
Conclusion
A graded association exists between higher BMI and elevated serum cystatin C. Further research is warranted to assess whether reducing BMI favorably impacts elevated serum cystatin C and the development of chronic kidney disease.
Overweight and obesity affect more than two-thirds of US adults1. Several studies have reported a direct association between body mass index (BMI) and chronic kidney disease, 2–4 whereas other studies demonstrate a J-shaped or an inverse association5,6. Interpreting the results from studies of BMI and advanced chronic kidney disease can be difficult as this association may be confounded by accompanying wasting and malnutrition. Epidemiological studies using biomarkers of early kidney disease provide the opportunity to address the association between BMI and kidney function with less concern for confounding.
Shlipak and colleagues have identified an early stage of kidney disease defined as elevated serum cystatin C in the absence of stage-3 through 5 chronic kidney disease7. This condition, “pre-clinical kidney disease”, was demonstrated to be associated with an increased risk of chronic kidney disease progression and cardiovascular disease morbidity and mortality7. As many interventions to slow the deterioration of kidney function have not proven effective in randomized trials, identifying the causes of the earliest stages of kidney disease may have important implications and provide approaches to prevent the initiation of chronic kidney disease. Overweight and obesity are associated with many chronic kidney disease risk factors. Therefore, addressing the role of excess BMI in the development of the early stages of kidney disease seems especially important.
The purpose of the current analysis is to evaluate the cross-sectional association between overweight and obesity and elevated serum cystatin C, a potential marker of early kidney disease, in a nationally representative sample of US adults. To do so, we analyzed data on adults without stage 3 through 5 chronic kidney disease or micro- or macro-albuminuria who participated in the Third National Health and Nutrition Examination Survey (NHANES III). Due to higher BMI levels among women, a higher percent body fat for any degree of BMI in women compared to men, and higher serum cystatin C levels among men, we performed analyses stratified by sex.
METHODS
NHANES III was conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention between 1988 and 19948. The current analysis was limited to adult NHANES III participants aged 20 years and older. Cystatin C was measured on a sample of 6951 participants including all NHANES III participants 60 years and older and a random sample of those 20 to 59 years of age. Additionally, cystatin C was measured in all men and women with a serum creatinine ≥1.2 and 1.0 mg/dL, respectively. Of these participants, 21 missing a valid height and/or weight measurement and 136 participants who were underweight (BMI<18.5 kg/m2) were excluded from the current analyses. In order to focus on persons without established kidney disease, NHANES participants without valid serum creatinine or urinary albumin and creatinine measurements (n=266) or with chronic kidney disease (n=1445) according to the National Kidney Foundation definition were excluded9. After these exclusions, our main analyses were based on the experience from 5083 participants.
NHANES III data were collected by administration of a standardized questionnaire during a home interview followed by conduct of a detailed physical examination with collection of blood specimens at a mobile examination center or the participant’s home. Questionnaire data included self-reported age, race-ethnicity, gender, years of education completed, physical activity, history of cigarette smoking, and use of anti-diabetic and anti-hypertensive medications.
Body weight and height were measured according to a standard protocol. Height was measured with participants standing on the floor using a fixed stadiometer with a vertical backboard and movable headboard. Weight was taken by asking each participant to stand on the center of the platform of a Toledo digital scale while wearing underwear, a disposable gown, and foam slippers. BMI was calculated as weight in kilograms divided by height in meters squared and analyzed as a continuous variable and after categorization using National Heart Lung and Blood Institute defined cut-points (18.5 to <25 kg/m2 - normal weight; 25 to <30 kg/m2 – overweight; 30 to <35 kg/m2 – class I obesity; and ≥ 35 kg/m2 – class II–III obesity). Based on the average of six systolic and diastolic blood pressure readings, hypertension was defined as a mean systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg and/or use of antihypertensive medication.
Blood samples were obtained during the clinical examination. Serum cystatin C was measured using an automated particle-enhanced nephelometric assay on the Dade Behring Nephelometer II. This assay maintains a range from 0.23 to 7.25 mg/L and intra-assay and inter-assay coefficients of variation of 2.0% to 3.0% and 3.2% to 4.4%, respectively. Elevated serum cystatin C was defined as ≥ 1.09 mg/L. This level represents the 99th percentile of the serum cystatin C distribution for NHANES III participants 20 to 39 years of age without hypertension, diabetes mellitus, micro- or macro-albuminuria or stages 3–5 chronic kidney disease. Serum creatinine was measured by the modified kinetic Jaffe reaction and re-calibrated in order to calculate estimated glomerular filtration rate using the Modification of Diet in Renal Disease Study formula10. During the physical examination, a random spot urine sample was obtained from all adults by using clean-catch techniques and sterile containers. Microalbuminuria was defined as a urinary albumin/creatinine ratio of 30–299 mg/g and macroalbuminuria was defined as a urinary albumin/creatinine ratio of ≥ 300 mg/g. Diabetes mellitus was defined as a plasma glucose ≥ 126 mg/dL for participants who fasted 8 hour or longer, ≥ 200 mg/dL for non-fasting individuals, and/or current anti-diabetes medication use. Total- and high density lipoprotein (HDL)- cholesterol were measured using the Hitachi 704 Analyzer. Serum C-reactive protein levels were measured using latex-enhanced nephelometry. C-reactive protein levels were categorized as being undetectable (<0.22 mg/dl), detectable (0.22–0.99 mg/dl), or clinically elevated (≥1.00 mg/dl).
The protocol for NHANES III was approved by the National Center for Health Statistics of the Centers for Disease Control and Prevention Institutional Review Board. Informed consent was obtained from each NHANES participant.
Statistical Analysis
Participant characteristics were calculated by BMI category and sex. The age-standardized mean serum cystatin C and prevalence of elevated serum cystatin C was calculated by BMI category, overall, and, for men and women, separately. Odds ratios of elevated serum cystatin C associated with BMI category were calculated, adjusted initially for age, race-ethnicity, and sex with subsequent models including additional adjustment for education, physical inactivity, current and former smoking, systolic blood pressure, diabetes mellitus, total to HDL-cholesterol ratio, and detectable and elevated C-reactive protein. To further explore the relationship between BMI and elevated serum cystatin C, we used a restricted quadratic spline with knots at the 10th, 50th, and 90th percentiles of the BMI distribution (20.9, 25.7, and 33.8 kg/m2, respectively). Finally, the association between BMI as a continuous variable and elevated serum cystatin C was determined overall and for sub-groups defined by age, race-ethnicity, sex, education, physical inactivity, cigarette smoking, total-to-HDL cholesterol ≥ 4.0, elevated C-reactive protein, hypertension, and diabetes mellitus. These variables were selected as potential confounders or effect modifiers in the association between BMI and serum cystatin C levels. For analyses as a continuous variable, the odds ratios of elevated serum cystatin C are presented for 5 kg/m2 higher BMI (i.e., approximately one standard deviation).
Sensitivity analyses were conducted by defining elevated serum cystatin C as levels ≥ 1.00 mg/L. This cut-point was applied in a previous analysis of serum cystatin C with kidney disease progression and cardiovascular events in the Cardiovascular Health Study7.
Data were analyzed using SUDAAN (version 9.0; Research Triangle Institute, Research Triangle Park, NC) to account for the complex NHANES sampling design including unequal probabilities of selection, over-sampling, non-response, and measurement of serum cystatin C in a sub-sample of NHANES participants.
RESULTS
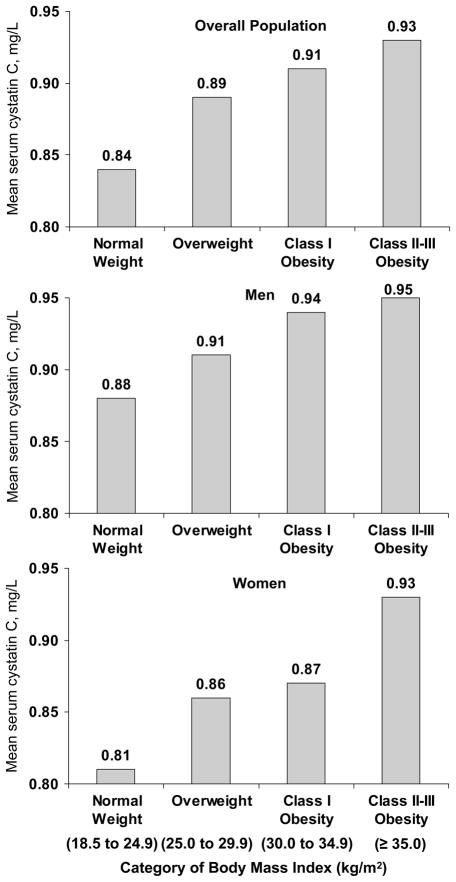
The distribution of characteristics for men and women included in the current study are presented by BMI category in Table 1. Men and women with higher BMI were younger and more likely to be non-Hispanic black. Current smoking was less common, and physical inactivity, hypertension, diabetes mellitus, and elevated C-reactive protein were more common, at higher BMI. Mean systolic and diastolic blood pressure, and serum cholesterol levels, were higher at higher BMI levels. In contrast, HDL-cholesterol was lower at higher BMI levels. Lower estimated glomerular filtration rates were present among men and women with elevated serum cystatin C. Specifically, mean age-standardized estimated glomerular filtration rate was 82.4 and 81.0 ml/min/1.73m2 among men and women with elevated serum cystatin C and 101.9 and 101.5 ml/min/1.73m2 among men and women without elevated serum cystatin C (each p<0.001). Serum cystatin C levels increased with higher BMI in the overall population and among men and women, separately (Figure 1; each p=trend<0.001).
Table 1.
Baseline characteristics of NHANES III participants without chronic kidney disease.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic* | Normal Weight (n=898 ) | Overweight (n =1,099) | Obesity (n=397) | Morbid Obesity (n=117) | Normal Weight (n=992 ) | Overweight (n=856) | Obesity (n=434) | Morbid Obesity (n=290) |
| Age, years | 49.0 (1.0) | 43.7 (0.9) | 44.5 (2.1) | 42.2 (1.7) | 41.3 (0.8) | 48.5 (1.0) | 47.0 (1.4) | 44.3 (1.1) |
| Non-Hispanic White, % | 72.6 | 78.6 | 76.9 | 72.2 | 81.3 | 75.2 | 69.6 | 68.5 |
| Non-Hispanic Black, % | 10.4 | 8.9 | 9.9 | 13.7 | 8.3 | 12.5 | 17.5 | 19.8 |
| Mexican American, % | 5.6 | 6.0 | 5.7 | 4.9 | 3.4 | 5.7 | 7.4 | 6.0 |
| High school graduate, % | 75.3 | 77.2 | 79.4 | 79.1 | 83.2 | 77.3 | 70.3 | 71.9 |
| Weight, kg | 70.1 (0.7) | 84.3 (0.4) | 99.5 (1.1) | 122.2 (3.2) | 58.2 (0.3) | 71.2 (0.4) | 83.3 (0.8) | 101.9 (1.3) |
| Height, cm | 175.5 (0.7) | 176.0 (0.4) | 176.4 (0.9) | 176.2 (1.3) | 162.4 (0.4) | 161.8 (0.4) | 161.0 (0.5) | 161.3 (0.7) |
| BMI, kg/m2 | 22.7 (0.1) | 27.2 (0.1) | 31.9 (0.1) | 39.2 (0.6) | 22.0 (0.1) | 27.1 (0.1) | 32.1 (0.2) | 39.1 (0.2) |
| Current cigarette smokers, % | 39.5 | 28.9 | 27.2 | 26.0 | 28.1 | 22.5 | 21.1 | 27.8 |
| Physically inactive, % | 13.9 | 14.9 | 13.5 | 21.5 | 19.9 | 29.6 | 35.4 | 36.9 |
| Systolic BP, mmHg | 119.7 (0.7) | 124.3 (0.8) | 126.9 (1.8) | 133.3 (1.4) | 114.1 (0.8) | 121.0 (1.0) | 124.2 (1.1) | 125.5 (1.6) |
| Diastolic BP, mmHg | 73.4 (0.7) | 77.9 (0.4) | 80.8 (0.8) | 83.0 (0.7) | 69.5 (0.4) | 72.9 (0.6) | 74.7 (0.7) | 76.8 (1.0) |
| Total cholesterol, mg/dL | 190.6 (2.1) | 205.1 (1.8) | 205.1 (5.1) | 213.9 (3.7) | 191.8 (2.1) | 218.0 (2.4) | 216.5 (3.4) | 211.9 (3.5) |
| HDL-cholesterol, mg/dL | 50.5 (0.9) | 44.3 (0.6) | 37.6 (1.6) | 38.4 (1.5) | 58.6 (0.7) | 53.8 (0.5) | 51.8 (1.6) | 46.8 (1.1) |
| Elevated C-reactive protein, % | 2.0 | 5.2 | 3.9 | 10.3 | 3.2 | 6.3 | 17.3 | 26.7 |
| Hypertension, %† | 9.8 | 18.0 | 30.4 | 37.7 | 11.8 | 23.0 | 30.4 | 37.0 |
| History of diabetes, % | 2.5 | 4.7 | 9.3 | 7.6 | 1.5 | 3.3 | 4.7 | 10.7 |
Characteristics are mean (SE) or percentage
Weight categories were defined as BMI ranges of: normal weight − 18.5 to 24.9 kg/m2, overweight − 25.0 to 29.9 kg/m2, obesity − 30.0 to 34.9 kg/m2. and morbid obesity – ≥ 35.0 kg/m2
Figure 1.
Age-standardized mean serum cystatin C by category of body mass index.
Elevated serum cystatin C defined as levels ≥ 1.09 mg/L
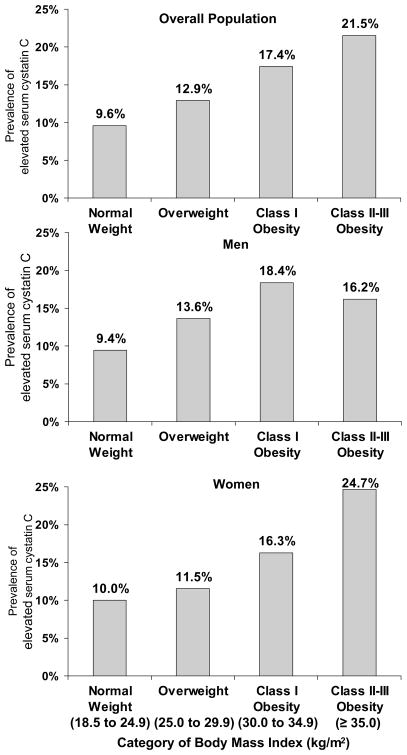
The prevalence of elevated serum cystatin C in the study population was 8.1% (9.2% among men and 7.0% among women). After age-standardization, a graded association existed between higher BMI and a higher prevalence of elevated serum cystatin C (Figure 2). This association was present overall as well as among men and women, separately (each p-trend<0.001). This association remained present after demographic and multivariate adjustment (Table 2).
Figure 2.
Age-standardized prevalence of elevated serum cystatin C by category of body mass index.
Elevated serum cystatin C defined as levels ≥ 1.09 mg/L
Table 2.
Demographic and multivariate adjusted odds ratio of elevated serum cystatin C (≥1.09 mg/L) associated with body mass index categories
| BMI Category, kg/m2 | Demographic Adjusted Odds Ratio (95% CI) | Multivariate Adjusted Odds Ratio (95% CI) |
|---|---|---|
| Total | ||
| Normal weight (18.5–24.9) | 1.00 (ref) | 1.00 (ref) |
| Overweight (25.0–29.9) | 1.54 (1.11 – 2.13)* | 1.46 (1.02 – 2.10)* |
| Class I Obesity (30.0 – 34.9) | 2.26 (1.59 – 3.23)*** | 2.36 (1.56 – 3.57)*** |
| Class II–III Obesity (≥35.0) | 2.89 (1.78 – 4.67)*** | 2.82 (1.56 – 5.11)*** |
| p-value for trend | <0.001 | <0.001 |
| Men | ||
| Normal weight (18.5–24.9) | 1.00 (ref) | 1.00 (ref) |
| Overweight (25.0–29.9) | 1.73 (1.12 – 2.68)* | 1.85 (1.12 – 3.08)* |
| Class I Obesity (30.0 – 34.9) | 2.90 (1.61 – 5.24)*** | 3.59 (1.78 – 7.25)*** |
| Class II–III Obesity (≥35.0) | 2.51 (1.33 – 4.75)** | 2.69 (1.21 – 5.95)* |
| p-value for trend | <0.001 | <0.001 |
| Women | ||
| Normal weight (18.5–24.9) | 1.00 (ref) | 1.00 (ref) |
| Overweight (25.0–29.9) | 1.14 (0.69 – 1.88) | 1.08 (0.61 – 1.90) |
| Class I Obesity (30.0 – 34.9) | 1.56 (0.87 – 2.80) | 1.43 (0.75 – 2.71) |
| Class II–III Obesity (≥35.0) | 3.61 (1.66 – 7.83)** | 2.80 (1.08 – 7.26)* |
| p-value for trend | <0.001 | 0.045 |
p<0.05;
p<0.01;
p<0.001
CI – confidence interval
Demographic adjusted for age, race-ethnicity and gender for the total population and for age and race-ethnicity in the gender-stratified analyses.
Multivariate adjusted for age, race, education, physical inactivity, current and former smoking, systolic blood pressure, serum total-to-HDL cholesterol ratio, diabetes mellitus, and detectable and elevated C-reactive protein.
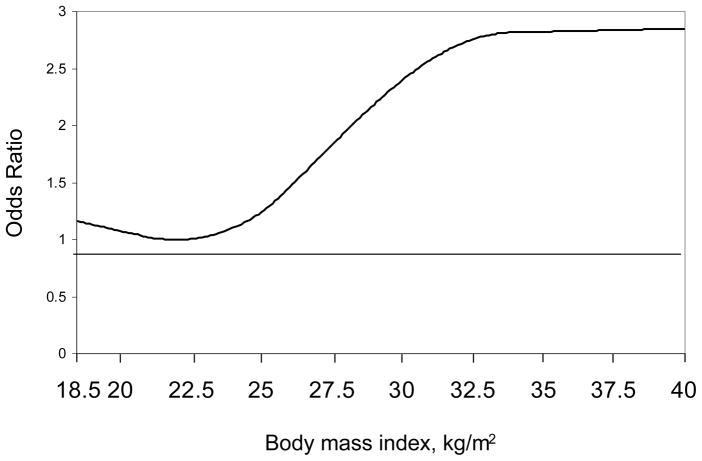
In spline regression models, the increase in elevated serum cystatin C was evident at BMI levels above 22.5 kg/m2 (Figure 3). After multivariate adjustment, higher BMI was associated with an increased odds ratio of elevated serum cystatin C, overall, and among men and women (Table 3). Although some of the 95% confidence intervals crossed the null, higher BMI levels were associated with increased odds ratios of elevated serum cystatin C in every sub-group investigated except non-Hispanic black men. Differences in the association between BMI and elevated serum cystatin C were not statistically significant across sub-group (all p-interaction>0.05).
Figure 3.
Multivariate adjusted odds ratio of elevated serum cystatin C from a spline analysis
Elevated serum cystatin C defined as levels ≥ 1.09 mg/L
Adjusted for age, race, education physical inactivity, current and former smoking, hypertension, diabetes, serum total to HDL cholesterol ratio, and detectable and elevated C-reactive protein.
Knots at 10th, 50th, and 90th percentile (body mass index of 20.9, 25.6, and 33.8 kg/m2, respectively)
Table 3.
Multivariate adjusted odds ratio of elevated serum cystatin C (≥1.09 mg/L) associated with 5 kg/m2 higher body mass index, overall and by subgroup.
| Men | Women | |
|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | |
| Overall | 1.37 (1.09 – 1.73) | 1.38 (1.09 –1.74) |
| Subgroup | ||
| < 60 years of age | 1.61 (1.27 – 2.03) | 1.68 (1.41 – 2.01) |
| ≥60 years of age | 1.29 (0.91 – 1.84) | 1.05 (0.56 – 1.96) |
| Non-Hispanic Whites | 1.38 (1.05 – 1.82) | 1.37 (1.03 – 1.81) |
| Non-Hispanic Blacks | 0.92 (0.60 – 1.41) | 1.21 (0.94 – 1.54) |
| Mexican Americans | 1.60 (0.97 – 2.65) | 1.28 (0.80 – 2.06) |
| Less than High School Education | 1.49 (1.10 – 2.00) | 1.35 (1.00 – 1.81) |
| High school education | 1.36 (1.02 – 1.82) | 1.38 (0.97 – 1.97) |
| Physically Inactive | 1.45 (0.95 – 2.23) | 1.18 (0.84 – 1.67) |
| Physically Active | 1.38 (1.03 – 1.84) | 1.58 (1.21 – 2.06) |
| Current smoker | 1.56 (0.96 – 2.52) | 1.13 (0.75 – 1.70) |
| Former Smoker | 1.70 (1.28 – 2.28) | 1.38 (1.02 – 1.87) |
| Never Smoker | 1.06 (0.76 – 1.48) | 1.60 (1.13 – 2.25) |
| Total/HDL cholesterol ratio ≥ 4.0 | ||
| No | 1.44 (1.05 – 1.98) | 1.59 (1.29 – 1.95) |
| Yes | 1.27 (1.02 – 1.58) | 1.17 (0.89 – 1.53) |
| Elevated C-reactive protein | ||
| No | 1.29 (1.06 – 1.57) | 1.28 (1.10 – 1.48) |
| Yes | 1.15 (0.86 – 1.54) | 1.17 (0.91 – 1.52) |
| Hypertension | ||
| No | 1.41 (1.04 – 1.92) | 1.10 (0.76 –1.59) |
| Yes | 1.58 (1.22 – 2.05) | 1.52 (1.19 –1.95) |
| Diabetes Mellitus | ||
| No | 1.38 (1.11 – 1.72) | 1.38 (1.07 –1.77) |
| Yes | 1.82 (0.77 – 4.28) | 1.50 (1.05 –2.15) |
Multivariate adjusted for age, race, education, physical inactivity, current and former smoking, systolic blood pressure, serum total-to-HDL cholesterol ratio, diabetes mellitus, and detectable and elevated C-reactive protein. The overall model includes additional adjustment for sex.
Sensitivity Analyses
Results were markedly consistent when elevated serum cystatin C was defined as levels ≥ 1.0 mg/L. The multivariate adjusted odds ratios (95% confidence interval) of a serum cystatin C ≥ 1.0 mg/L were 1.32 (0.86, 2.04), 1.98 (1.24, 3.18), and 3.55 (1.84, 6.86) for individuals who were overweight, class I, and class II–III obesity, compared to normal weight. Analogous odds ratios (95% confidence intervals) were 1.21 (0.68, 2.16), 2.32 (1.28, 4.21), and 2.93 (0.89, 9.66) for men and 1.64 (1.11, 2.42), 1.65 (0.91, 2.99), and 5.21 (2.84, 9.56) for women when elevated serum cystatin C was defined as levels ≥ 1.0 mg/L.
DISCUSSION
In this large nationally-representative sample of participants free of micro- and macro-albuminuria and stage 3–5 chronic kidney disease, overweight and obesity maintained a strong association with elevated serum cystatin C. This association was strong, graded, and present in the overall population and among men and women, separately. Furthermore, this relationship was independent of systolic blood pressure, total to HDL-cholesterol ratio, elevated C-reactive protein, and diabetes mellitus, suggesting that excess BMI may be an independent risk factor for early kidney disease.
A graded relationship between elevated BMI and end-stage renal disease risk was identified in over 320,252 Kaiser Permanente members followed for up to 21 years2. In this study, the relative hazards of developing end-stage renal disease, compared to normal weight individuals, was 1.8, 3.6, 7.3, and 9.4 for overweight, class I, II, and III obesity, respectively, among men, and 2.2, 3.6, 5.4, and 6.5, respectively, among women. In contrast, no association was present between BMI and end-stage renal disease among participants of the Hypertension Detection Follow-up Study5. Among 100,753 members of a screened Japanese cohort, baseline BMI predicted future risk for end-stage renal disease in men but not women11.
In the Framingham Heart Study, each standard deviation higher BMI was associated with 23% higher odds of developing incident kidney disease over 18.5 years of follow-up3. However, sex-specific associations were not presented for this cohort. In the Physician’s Health Study, a study of male physicians, a graded association existed between higher baseline BMI and the presence of chronic kidney disease at follow-up12. Additionally, a graded association was present between higher BMI and higher risk of elevated serum creatinine, defined as a serum creatinine > 3.4 mg/dL among men and > 2.8 among women, in a case-control study conducted in Sweden4. The current study extends these previous findings and demonstrates that a graded association exists between higher BMI and elevated serum cystatin C, in the context of individuals without chronic kidney disease.
At least one study has assessed the association between body size and serum cystatin C13. Specifically, serum cystatin C was measured among 8058 community-dwelling adults, aged 31 to 71 years, from Groningen, The Netherlands. A significant correlation (ρ=0.22; p<0.001) between body weight and serum cystatin C was observed in this study. Our findings extend this prior work with data on the multivariate-adjusted association between overweight and obesity and elevated serum cystatin C, gender stratified results, and the association for participants without stage-3 chronic kidney disease or albuminuria.
The challenges involved in epidemiological studies of chronic kidney disease and end-stage renal disease have been documented previously14,15. Many of these challenges are relevant to studying overweight and obesity as a risk factor for kidney disease. Excess BMI is associated with hypertension and diabetes mellitus, the two leading causes of end-stage renal disease16. Therefore, understanding the causal relationship between BMI and kidney disease, independent of these risk factors, is difficult. Nonetheless, lowering BMI reduces blood pressure, is effective in preventing diabetes mellitus, and, therefore, may reduce the development and progression of kidney disease17–19. Additional challenges of studying the relationship between BMI and chronic kidney disease include malnutrition and wasting that occurs in chronic kidney disease, the slow progression of chronic kidney disease in many patients, and competing mortality risks15. Studying elevated serum cystatin C, a potential marker of early kidney disease, may overcome several of these challenges.
The limitations of using mild elevations in serum creatinine and small reductions in estimated glomerular filtration rate as markers of kidney disease are well documented20. Many studies, including two meta-analyses, have reported serum cystatin C, compared to serum creatinine, may be a better marker of kidney function22,23. While elevated serum cystatin C is demonstrated to be associated with increased chronic kidney disease and cardiovascular disease risk, there remains a great need for future studies to elucidate the value of serum cystatin C as a marker of kidney function. There are several lines of evidence that suggest serum cystatin C may have extra-renal sources, particularly in pathways that may associate with body weight. Serum cystatin C has been shown to be associated with thyroid function, corticosteroid administration, and C reactive protein, conditions that are associated with alterations in body weight24–26. Thus, there may be additional potential mechanisms for the association between BMI and serum cystatin C, observed in the current study, above and beyond kidney function.
Limitations
The data for the current analysis were derived from a cross-sectional study. Although it seems that higher BMI leads to elevated serum cystatin C levels, caution is recommended when interpreting the direction of causality in cross-sectional studies. Unfortunately, longitudinal data on BMI are not available in NHANES III. Additionally, despite the use of a large study population, a relatively small percentage of these participants were morbidly obese.
Strengths
The current results extend the findings from previous studies in several important ways. One key advantage is the large nationally-representative sample of participants without chronic kidney disease, as defined by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative guidelines9. Prior to conducting the current analyses, we were able to exclude study participants with stage 3 through 5 chronic kidney disease and/or micro- or macro-albuminuria. Furthermore, the large sample size permitted the investigation of the association of BMI with elevated serum cystatin C in multiple sub-groups. The consistency of results within these sub-groups is noteworthy. Additionally, all study measures were collected using standardized protocols with rigorous quality control procedures.
Conclusions
In conclusion, data from the current study demonstrate a strong graded association between BMI and elevated serum cystatin C. Further research is needed to assess whether reducing BMI has a favorable impact on the development of elevated serum cystatin C and prevalence of subsequent chronic kidney disease and end-stage renal disease.
Reference List
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 3.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 4.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyren O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17:1695–1702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 5.Perry H, Miller J, Fornoff J, et al. Early Predictors of 15-Year End-Stage Renal Disease in Hypertensive Patients. Hypertension. 1995;25:587–594. doi: 10.1161/01.hyp.25.4.587. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez SP, McClellan W, Port FK, Hsu SI. Risk factors for proteinuria in a large, multiracial, southeast Asian population. J Am Soc Nephrol. 2002;13:1907–1917. doi: 10.1097/01.asn.0000018406.20282.c8. [DOI] [PubMed] [Google Scholar]
- 7.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics. Plan and operation of the third National Health and Nutrition Examination survey, 1988–1994. US Dept of Health and Human Services publication 94–1308. 1994:1. [Google Scholar]
- 9.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int. 2004;65:1870–1876. doi: 10.1111/j.1523-1755.2004.00582.x. [DOI] [PubMed] [Google Scholar]
- 12.Gelber RP, Kurth T, Kausz AT, et al. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46:871–880. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 14.Perneger TV, Brancati FL, Whelton PK, Klag MJ. Studying the causes of kidney disease in humans: a review of methodologic obstacles and possible solutions. Am J Kidney Dis. 1995;25:722–731. doi: 10.1016/0272-6386(95)90548-0. [DOI] [PubMed] [Google Scholar]
- 15.Hsu CY, Chertow GM, Curhan GC. Methodological issues in studying the epidemiology of mild to moderate chronic renal insufficiency. Kidney Int. 2002;61:1567–1576. doi: 10.1046/j.1523-1755.2002.00299.x. [DOI] [PubMed] [Google Scholar]
- 16.United States Renal Data System (USRDS). USRDS. Annual Data Report: Atlas of End-Stage Renal Disease in the United States. 2006. Bethesda, MD: National Instistutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2005. [Google Scholar]
- 17.Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr. 2003;22:331–339. doi: 10.1080/07315724.2003.10719316. [DOI] [PubMed] [Google Scholar]
- 18.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42:878–884. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 19.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 21.Madero M, Sarnak MJ, Stevens LA. Serum cystatin C as a marker of glomerular filtration rate. Curr Opin Nephrol Hypertens. 2006;15:610–616. doi: 10.1097/01.mnh.0000247505.71915.05. [DOI] [PubMed] [Google Scholar]
- 22.Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 23.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 24.Wiesli P, Schwegler B, Spinas GA, Schmid C. Serum cystatin C is sensitive to small changes in thyroid function. Clin Chim Acta. 2003;338:87–90. doi: 10.1016/j.cccn.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Cimerman N, Brguljan PM, Krasovec M, Suskovic S, Kos J. Serum cystatin C, a potent inhibitor of cysteine proteinases, is elevated in asthmatic patients. Clin Chim Acta. 2000;300:83–95. doi: 10.1016/s0009-8981(00)00298-9. [DOI] [PubMed] [Google Scholar]
- 26.Nyrnes A, Jorde R, Sundsfjord J. Serum TSH is positively associated with BMI. Int J Obes (Lond) 2006;30:100–105. doi: 10.1038/sj.ijo.0803112. [DOI] [PubMed] [Google Scholar]