Abstract
Background
Atrial fibrillation (AF) is common among patients with end-stage renal disease, but few data are available on its prevalence among adults with chronic kidney disease (CKD) of lesser severity.
Methods and Results
We evaluated the association of CKD with electrocardiogram-detected AF among 26,917 participants in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study, a population-based cohort of African-American and white US adults ≥45 years of age. Estimated glomerular filtration rate (eGFR) was calculated using the abbreviated Modification of Diet in Renal Disease study equation and albuminuria was defined as a urinary albumin to creatinine ratio ≥30 mg/g. Participants were categorized by renal function: no CKD (eGFR ≥60 ml/min/1.73m2 without albuminuria, n=21,081), stage 1–2 CKD (eGFR ≥60 ml/min/1.73m2 with albuminuria n=2,938), stage 3 CKD (eGFR 30 to 59 ml/min/1.73m2, n=2,683) and stage 4–5 CKD (eGFR <30 ml/min/1.73m2, n=215). The prevalence of AF among participants without CKD, and with stage 1–2, stage 3, and stage 4–5 CKD was 1.0%, 2.8%, 2.7% and 4.2%, respectively. Compared to participants without CKD, the age, race, sex adjusted odds ratios for prevalent AF were 2.67 (95% CI 2.04 – 3.48), 1.68 (95% CI 1.26 – 2.24) and 3.52 (95% CI 1.73–7.15) among those with stage 1–2, stage 3 and stage 4–5 CKD. The association between CKD and prevalent AF remained statistically significant after further multivariable adjustment and was consistent across numerous subgroups.
Conclusions
- Regardless of severity, CKD is associated with an increased prevalence of AF among US adults.
Keywords: chronic kidney disease, atrial fibrillation, electrocardiography
Atrial fibrillation (AF) is a frequent cardiac arrhythmia and confers a 2- to 3-fold increased risk of ischemic stroke and mortality.1–3 AF increases in prevalence with age, approaching 10% among adults ≥80 years old.4 Renal disease and AF share several risk factors, including hypertension, diabetes mellitus and coronary artery disease (CAD).5–7 A high prevalence of AF has been reported among patients with end-stage renal disease (ESRD) on dialysis.8–9 Patients with ESRD, however, represent a small fraction of individuals with chronic kidney disease (CKD) and the burden of AF among adults with less severe CKD has not been well investigated. Therefore, the goal of the current study was to evaluate the burden of AF in relation to renal function among US adults not on dialysis. Additionally, we determined demographic and clinical correlates of AF by CKD severity. To do so, we analyzed data from participants enrolled in the REasons for Geographic and Racial Differences in Stroke (REGARDS) study.
Methods
Study Participants
The REGARDS study is a population-based, longitudinal study of African-American and white U.S. adults aged ≥45 years of age, enrolled between January 2003 and October 2007.10 The study was designed to oversample African-Americans and to provide approximate equal representation of men and women; the final cohort included 26% African-American women, 16% African-American men, 29% white women, and 29% white men. By design, 56% (goal 50%) of the sample was recruited from the eight Southern U.S. states (North Carolina, South Carolina, Georgia, Alabama, Mississippi, Tennessee, Arkansas and Louisiana), commonly referred to as the “stroke belt”, with the remaining 44% of the sample recruited from the other 40 contiguous U.S. states. Individuals were identifiedfrom commercially available lists of residents and recruited through an initial mailing followed by telephone contacts. Overall, 30,239 individuals were enrolled in the REGARDS study. After excluding participants receiving hemodialysis (n=117), missing serum creatinine measurements (n=1,092), with implanted pacemakers or poor quality ECG recordings (n=846), and without urinary albumin and creatinine measurements (n=1,243) data from 26,917 participants were included in the present analysis. The REGARDS protocol was approved by the Institutional Review Boards governing research in human subjects at the participating centers and all participants provided verbal consent prior to the telephone interview and written consent prior to study examinations.
Data Collection
Baseline demographic and clinical data were collected through telephone interviews, self-administered questionnaires and in-home examinations. After obtaining consent, trained interviewers conducted computer-assisted telephone interviews to obtain participants’ demographic information, cigarette smoking status and self-reports of a prior diagnosis of major co-morbid conditions (diabetes, hypertension, myocardial infarction, stroke, coronary revascularization, and AF). A self-reported history of AF was defined as an affirmative response to the question: “Has a physician or a health professional ever told you that you had atrial fibrillation?
Trained staff then conducted in-home study visits 3–4 weeks after the interview where prescription and over-the-counter medications were documented via pill bottle review, a physical examination was performed, blood and urine samples were collected and a resting electrocardiogram (ECG) was recorded.
Systolic and diastolic blood pressure (BP) was estimated as the average of two measurements. Hypertension was defined as systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or the use of antihypertensive medication. Height and weight were measured during the study visit and body mass index (BMI)was calculated as weight in kilograms divided by height in meterssquared. Diabetes was defined as a serum glucose ≥126 mg/dL for participants who had fasted ≥8 hours prior to sampling, serum glucose ≥200 mg/dL for those who had not fasted, or self-report of a prior diagnosis of diabetes with current use of insulin or oral hypoglycemic medications. Of REGARDS study participants included in the current analysis, 87% had fasted more than 8 hours prior to their blood draw.
The first 8,459 REGARDS participants underwent 7-lead ECG recording acquired by applying the standard 4 limb electrodes and a mid-sternal electrode whereas the rest of REGARDS participants underwent standard 12-lead-ECG recording. The ECGs were read and coded at a central reading center by electrocardiographers blinded to the other REGARDS study data.
Left ventricular hypertrophy (LVH) was defined on the basis of the sex-specific Cornell voltage criteria in the 12-lead ECGs11 while a modified Cornell voltage criteria was used in the 7-lead ECGs.12
Symptoms of heart failure were defined as being present if participants reported having to sleep on two or more pillows to help breathing and awakening due to difficulty breathing. These criteria were associated with a specificity of approximately 83% for the detection of heart failure among community-dwelling adults.13 Prevalent CAD at baseline was defined based on a self-reported history of myocardial infarction or coronary revascularization (coronary angioplasty or bypass surgery). Hypercholesterolemia was defined as an LDL-cholesterol ≥160 mg/dL for those who fasted 8 or more hours prior to sampling, total serum cholesterol ≥240 mg/dL for those who hadn’t fasted, or current lipid-lowering medication use. High-sensitivity C-reactive protein (CRP) ≥3 mg/L was defined as elevated.
Using isotope-dilution mass spectrometry (IDMS) traceable serum creatinine, estimated GFR (eGFR) was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) study equation.14 All analyses were repeated using the CKD-EPI equation 15 with similar results (data not shown). Albuminuria was defined as a urinary albumin to creatinine ratio ≥30 mg/g.16 CKD stages were defined using the recommendations of the National Kidney Foundation:17 no CKD (eGFR ≥60 ml/min/1.73m2 without albuminuria), stage 1–2 CKD (eGFR ≥60 ml/min/1.73m2 with albuminuria), stage 3 CKD (eGFR of 30 to 59 ml/min/1.73m2) and stage 4–5 CKD (eGFR < 30 ml/min/1.73m2). Individuals with stage 1 and 2 CKD were grouped together to represent the presence of albuminuria with preserved GFR, and participants with stage 4 and 5 CKD were grouped together to provide an adequate sample size for stable estimates.
Statistical Analysis
The primary outcome was prevalent ECG-detected AF. Participant characteristics and the prevalence of ECG-detected AF were calculated by CKD stage. The statistical significance of linear trends for participant characteristics across CKD stages was tested by linear and logistic regression for continuous and dichotomous variables, respectively. The odds ratios for prevalent AF associated with stage 1–2, 3 and 4–5 CKD versus no CKD were calculated using logistic regression. An initial model included adjustment forage, race, and sex. A subsequent model includedadditional adjustment for other potential confounders, including geographic region of residence (inside or outside the stroke belt), current smoking, BMI, hypertension, diabetes, coronary disease, symptoms of heart failure, LVH, hypercholesterolemia, statin use, renin-angiotensin system inhibitor use, and elevated CRP. To assess the consistency of the associations, the multivariable-adjusted odds ratios for prevalent AF associated with CKD stage were calculated using logistic regression within participant subgroups. For the sub-group analyses, participants with stage 3, 4 or 5 CKD were combined due to the limited number of participants with stage 4 or 5 CKD. To assess the statistical significance of differences in the association of stage 1–2 and stage 3–5 CKD with AF across sub-group, interaction terms (e.g., stage 1–2 CKD * women gender and stage 3–5 CKD * women) were incorporated into the multivariable models.
Next, the age, race, sex adjusted odds ratios for prevalent AF associated with demographics and clinical risk factors were calculated for each CKD group (no CKD, stage 1–2 CKD, and stage 3–5 CKD) separately. Variables significantly associated with AF in age, race, and sex-adjusted analyses were included in multivariable-adjusted models. Additionally, the odds ratio for prevalent AF associated with albuminuria among participants with stage 3–5 CKD was calculated in a separate multivariable-adjusted model. For secondary analyses, the prevalence and odds ratios for AF, defined on the basis of self-report or ECG, were calculated by CKD stage. All analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
Results
Patient Characteristics
REGARDS participants with more advanced CKD were older and more likely to be African-American (Table 1). Diabetes, hypertension, CAD, symptoms of heart failure, LVH and elevated CRP were more frequent and BMI and serum cholesterol levels were higher among individuals with more advanced CKD. Additionally, statin and renin-angiotensin system inhibitor drug use were more common among participants with progressively worsening renal function.
Table 1.
Characteristics of REGARDS study participants stratified by chronic kidney disease stage
| Chronic Kidney Disease stage | |||||
|---|---|---|---|---|---|
| No CKD (n=21081) | Stage 1–2 (n=2938) | Stage 3 (n=2683) | Stage 4–5 (n=215) | p - trend | |
| Age, years | 64.2 (9.0) | 66.4 (9.5) | 71.2 (9.1) | 68.7 (9.7) | <0.001 |
| Male, % | 45.1 | 49.2 | 41.2 | 45.1 | 0.190 |
| African-American, % | 39.2 | 52.9 | 36.5 | 58.1 | <0.001 |
| Stroke belt, % | 34.8 | 34.6 | 32.3 | 29.8 | 0.005 |
| Diabetes, % | 16.3 | 39.6 | 31.1 | 55.4 | <0.001 |
| Hypertension, % | 53.6 | 74.7 | 79.9 | 86.9 | <0.001 |
| Current smoking, % | 14.2 | 20.6 | 11.0 | 13.1 | 0.565 |
| Body mass index, kg/m2 | 29.1 (6.0) | 30.4 (6.7) | 29.6 (6.4) | 31.0 (7.1) | <0.001 |
| Coronary artery disease†, % | 11.1 | 17.3 | 23.5 | 33.0 | <0.001 |
| Symptoms of heart failure††, % | 4.4 | 5.8 | 6.0 | 11.2 | <0.001 |
| Elevated serum cholesterol, % | 38.9 | 44.3 | 52.5 | 56.7 | <0.001 |
| Statins, % | 28.7 | 34.3 | 44.4 | 48.4 | <0.001 |
| Renin-angiotensin system inhibitors, % | 29.2 | 43.6 | 52.4 | 57.2 | <0.001 |
| ECG-Left ventricular hypertrophy, % | 4.2 | 9.6 | 8.0 | 14.6 | <0.001 |
| C-reactive protein ≥3 mg/L, % | 37.8 | 50.3 | 46.4 | 57.6 | <0.001 |
CKD - chronic kidney disease.
Numbers in table are mean (standard deviation) or percentage.
Prior self-reported myocardial infarction, coronary stenting or coronary bypass.
Based on self-reported symptoms of having to sleep on two or more pillows to help breathing or awakening due to difficulty breathing.
Prevalence of ECG-detected AF
There were 198 cases of ECG-detected AF among adults without CKD, and 83, 71 and 9 cases among adults with stage 1–2, stage 3 and stage 4–5 CKD, respectively. The prevalence of ECG-detected AF increased across worsening CKD stages. Specifically, the prevalence of AF was 1.0% among adults without CKD, and 2.8%, 2.7% and 4.2% among adults with stage 1–2, stage 3 and stage 4–5 CKD, respectively. After adjustment for age, race and sex, the odds ratios for AF were 2.67 (95% CI 2.04–3.48), 1.68 (95% CI 1.26–2.24) and 3.52 (95% CI 1.73–7.15) for those with stage 1–2, stage 3 and stage 4–5 CKD, respectively, compared to participants without CKD (Table 2). These associations were attenuated, but remained statistically significant, after further multivariable adjustment.
Table 2.
Odds Ratio (95% confidence interval) for atrial fibrillation defined by electrocardiogram
| CKD Stage | ||||
|---|---|---|---|---|
| No CKD | Stage 1–2 | Stage 3 | Stage 4–5 | |
| n=21081 | n=2938 | n=2683 | n=215 | |
| Cases of AF, n | 198 | 83 | 71 | 9 |
| Age, race, sex adjusted odds ratio (95% CI) | 1 (ref) | 2.67 (2.04, 3.48) *** | 1.68 (1.26, 2.24)*** | 3.52 (1.73, 7.15)*** |
| Multivariable adjusted odds ratio (95% CI) † | 1 (ref) | 2.20 (1.64, 2.94) *** | 1.51 (1.12, 2.05) ** | 2.86 (1.38, 5.92)** |
CKD, chronic kidney disease; AF, atrial fibrillation; CI, confidence interval.
Adjusted for age, race, sex, geographic region (stroke belt versus non-stroke belt), current smoking, body mass index, hypertension, diabetes, coronary artery disease, symptoms of heart failure, ECG-detected left ventricular hypertrophy, elevated cholesterol, statin and renin-angiotensin-system inhibitor use, and C-reactive protein ≥3 mg/L.
p-value<0.05
p-value<0.01
p-value<0.001.
Subgroup analysis
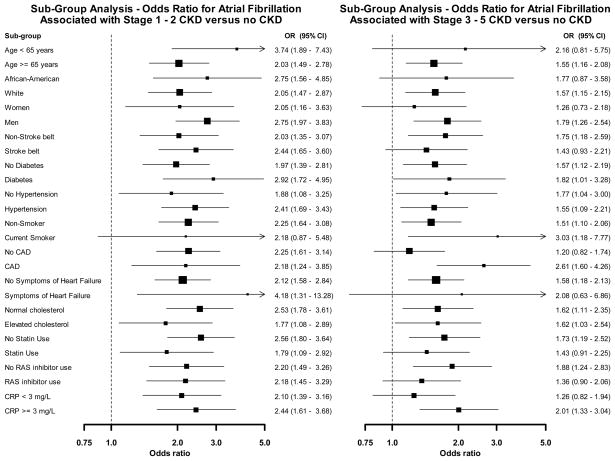
Figure 1 shows the multivariable adjusted odds ratios for prevalent AF associated with stage 1–2 CKD and stage 3–5 CKD, separately, versus no CKD within sub-groups of REGARDS participants. The associations were consistent (all p-values for interaction >0.10) with odds ratios greater than 1 in all subgroups.
Figure 1.
Odds ratios for atrial fibrillation associated with stage 1 – 2 and stage 3 – 5 versus no chronic kidney disease within sub-groups of REGARDS study participants. Odds ratios are from a multivariable adjusted model including age, race, gender, region of residence, diabetes, hypertension, smoking, CAD, symptoms of heart failure, elevated cholesterol, statin use, RAS inhibitor use, LVH, and CRP ≥3 mg/L. Boxes in the graph represent the odds ratio and lines/arrows represent the 95% confidence interval. OR indicates odds ratio; CI, confidence interval; CAD, coronary artery disease; RAS, renin angiotensin system; LVH, left ventricular hypertrophy; CRP, C-reactive protein.
Risk factors for Prevalent AF
The following variables were associated with prevalent AF in age, race and sex adjusted models among all participants: age, male sex, African-American race, elevated CRP, LVH and symptoms of heart failure. Higher BMI and renin-angiotensin-system inhibitors were significantly associated with prevalent AF among those without CKD while CAD was significantly associated with AF among those with stage 3–5 CKD. Hypertension was not significantly associated with prevalent AF in age, race and sex adjusted models.
The multivariable-adjusted associations between demographic and clinical variables and prevalent AF among those with no CKD, stage 1–2 CKD and stage 3–5 CKD, separately, are shown in Table 3. Regardless of CKD stage, older age and male sex were associated with an increased odds ratio for AF, while African-American race was associated with a lower odds ratio for AF. There was no evidence of effect modification by CKD on the association between demographic and clinical variables and prevalent AF as all interaction terms were > 0.10. Among those with stage 3–5 CKD, the multivariable-adjusted odds ratio for prevalent AF associated with albuminuria was 2.13 (95% CI 1.32–3.42).
Table 3.
Odds ratio (95% confidence interval) for atrial fibrillation defined by electrocardiogram associated with demographics and clinical risk factors for REGARDS study participants with no, stage 1–2, and stage 3–5 chronic kidney disease, separately.
| Multivariable adjusted odds ratios‡ (95% CI) | |||
|---|---|---|---|
| No CKD | Stage 1–2 CKD | Stage 3–5 CKD | |
| Age per 10 years | 2.84 (2.39, 3.387)*** | 2.08 (1.57, 2.76)*** | 2.05 (1.52, 2.76)*** |
| Male | 3.16 (2.25, 4.44)*** | 3.03 (1.71, 5.36)*** | 2.94 (1.75, 4.91)*** |
| African-American | 0.34 (0.23, 0.51)*** | 0.43 (0.25, 0.72)** | 0.34 (0.18, 0.65)*** |
| Body mass index per 5 kg/m2 | 1.26 (1.10, 1.46)** | 1.04 (0.83, 1.29) | 1.10 (0.88, 1.36) |
| Coronary artery disease† | 1.05 (0.73, 1.51) | 1.07 (0.62, 1.84) | 1.92 (1.20, 3.09)* |
| Symptoms of heart failure†† | 1.40 (0.68, 2.90) | 2.26 (0.93, 5.51) | 1.45 (0.59, 3.55) |
| Renin-angiotensin system inhibitors | 1.26 (0.92, 1.71) | 1.41 (0.87, 2.29) | 1.06 (0.66, 1.69) |
| C-reactive protein ≥3, mg/L | 1.28 (0.94, 1.74) | 1.42 (0.87, 2.32) | 1.93 (1.20, 3.09)** |
| Left ventricular hypertrophy | 1.56 (0.80, 3.02) | 2.46 (1.21, 5.01)* | 1.64 (0.75, 3.57) |
Abbreviations: CKD, chronic kidney disease; CI, confidence interval.
Age, sex, race, body mass index, coronary artery disease, symptoms of heart failure, renin-angiotensin- system inhibitor use, C-reactive protein ≥3 mg/L and ECG-detected left ventricular hypertrophy were included in three separate models; one each for No CKD,1–2 CKD and stage 3–5 CKD.
Prior self-reported myocardial infarction, coronary stenting or coronary bypass.
Based on self-reported symptoms of having to sleep on two or more pillows to help breathing or awakening due to difficulty breathing
p-value<0.05
p-value<0.01
p-value<0.001.
Self-reported or ECG-detected AF
There were 1478 cases of AF defined by self-report or ECG among those without CKD and 308, 321 and 34 cases among adults with stage 1–2, stage 3 and stage 4–5 CKD, respectively. The prevalence of self-report of ECG-detected AF was 7.1% (95% CI 6.7% - 7.5%) among adults without CKD and 10.7% (9.5% – 11.9%), 12.2% (11.0% – 13.4%), and 16.0% (11.1% – 20.9%) among those with stage 1–2, stage 3 and stage 4–5 CKD, respectively (p-trend <0.001). After adjustment for age, race and sex, the odds ratios for self-report or ECG-detected AF were 1.52 (95% CI: 1.33–1.73), 1.52 (95% CI: 1.33–1.74) and 2.29 (95% CI: 1.58–3.33) for those with stage 1–2, stage 3 and stage 4–5 CKD respectively, compared to participants without CKD. The multivariable-adjusted odds ratios for these groups were 1.29 (95% CI: 1.12–1.49), 1.21 (95% CI: 1.05–1.39) and 1.34 (95% CI: 0.88–2.03), respectively.
Discussion
In the present analysis of almost 27,000 U.S. adults, CKD was associated with an increased prevalence of AF. The prevalence of AF was highest among those with stage 4 or 5 CKD, and the association between CKD stage and AF persisted after multivariable adjustment and was consistent across all of the subgroups examined. In addition, the association between CKD and AF was present whether AF was detected via ECG or self-report of a prior diagnosis of the arrhythmia.
In previous studies involving patients on hemodialysis, the prevalence of AF ranged from 5 to 27%, depending upon the duration of dialysis therapy, associated risk factors and patterns of AF (paroxysmal, persistent or permanent).8–9, 18–19 In a prospectively followed cohort of 190 patients on chronic hemodialysis, AF was associated with a 2- to 3- fold increased risk of mortality and stroke over four years compared to dialysis patients without AF, a magnitude of incremental risk similar to that observed in patients without ESRD.1–2 Our findings extend these observations, suggesting that the prevalence of AF is increased even in persons with less advanced CKD.
Community-based studies in the Netherlands and Japan have evaluated the associations between albuminuria and reduced eGFR, separately, and AF.20–21 In both studies, participants were recruited by mail and underwent examinations in outpatient clinics or health screening programs. In the Netherlands study, microalbuminuria was associated with an odds ratio for prevalent AF of 1.93 (95% CI: 1.10–3.37), similar in magnitude to the association we observed for stage 1–2 CKD. In the Japanese study, the odds ratio for prevalent AF among participants in the lowest versus highest eGFR tertile (<62.6 ml/min/m2 versus >75.5 ml/min/m2) was 1.91 (95% CI: 1.54–2.38). The current results extend these earlier observations to a large and racially diverse population-based sample of US adults.
In addition to evaluating the prevalence of AF, we compared clinical correlates of AF among participants with and without CKD. Established risk factors for AF, such as advanced age, male sex, symptoms of heart failure, LVH and CAD 2, 5, 22 were associated with prevalent AF among those with CKD in the REGARDS cohort. The association between symptoms of heart failure, CAD and AF was less precise and weaker in magnitude compared to earlier studies. This finding may be due to limited statistical power when conducting subgroup analyses. Participants may also have been misclassified as having either symptoms of heart failure or CAD as these conditions were based on subjective criteria or self-report. Elevated CRP, a biomarker associated with an increased risk for cardiovascular events in both the general and CKD populations, was associated with AF within each CKD strata.
In addition, albuminuria was strongly associated with AF among participants with stage 3–5 CKD, suggesting that the burden of AF among individuals with both albuminuria and reduced eGFR may be substantially higher than in those with only one of these abnormalities. The current findings are also consistent with previous reports that other manifestations of CVD, such as peripheral arterial disease,23 are more frequent among individuals with both reduced eGFR and albuminuria compared to those with only one of these abnormalities.
Hypertension did not emerge as a significant correlate of AF, even among those without CKD. This is unexpected, given the established association of hypertension with AF in other studies.2, 5 This discrepancy may be due to differences in the prevalence of hypertension and racial diversity in the REGARDS cohort compared to earlier studies. The prevalence of hypertension is 57% in the REGARDS study.24 The high prevalence of hypertension may have limited our ability to detect an association between hypertension and AF. Longitudinal follow-up of the REGARDS cohort, including the ascertainment of incident AF, might clarify the relationship between hypertension and AF.
The association between CKD and AF was large and statistically significant across multiple subgroups and after adjustment for various risk factors. This suggests that other factors may contribute to the development of AF among individuals with CKD. Certain echocardiographic features, including left atrial dilation and mitral annular calcification, for example, are associated with incident AF in the general population 6 and these are more common among patients with CKD.25–27 However, echocardiograms were not obtained in the REGARDS study. Other important cardiac functional and structural abnormalities might also lead to a greater burden of AF among CKD individuals. Diastolic dysfunction, for example, increases the risk for developing AF in the general population28 and is more common among individuals with versus without CKD.29 Alterations of myocardial tissue, including interstitial collagen deposition, have been demonstrated early in the course of CKD30 and might also contribute to AF by enhancing intra-atrial re-entry.31
Quantifying the burden of AF among the population with CKD is clinically relevant and maintains public health importance, since approximately 26 million U.S. adults (~ 13%) have CKD.32 The prevalence of CKD is projected to increase as the U.S. population ages and the prevalence of risk factors for the development of renal disease increases.32 In contrast to other manifestations of CVD, such as aortic stiffness, vascular calcification and left ventricular hypertrophy, the association between CKD and AF has not been extensively studied. AF may emerge as an important prognostic marker or even as a mediator of excess cardiovascular risk among patients with CKD. The relatively high prevalence of AF combined with its impact on morbidity and mortality due to CVD, highlight the importance of AF detection, but additional studies are needed to elucidate the mechanisms responsible for development of AF among people with CKD and to develop preventive strategies.
This study should be interpreted in the context of certain limitations. The most important may be its cross-sectional observational design. This precludes inferences about the temporal relation between CKD and AF. Furthermore, serum creatinine and albuminuria measurements were performed only once for each participant. The same was true of ECG recordings, raising the likelihood that many cases of paroxysmal AF were not detected. There were a limited number of cases of AF among participants with stage 4–5 CKD, rendering prevalence estimates for this group less reliable than those for other CKD stages. Insufficient power may have precluded our ability to detect important associations between various risk factors for AF among participants in different CKD stages. Multiple statistical tests performed in our subgroup analyses might have also resulted in Type I error inflation. In addition, both recall and misclassification bias might have confounded the association between various risk factors and AF as many medical conditions were based on self-reported history alone. Not only are we unable to establish causality between CKD and AF based on the present investigation, our cross-sectional design is also limited by inherent selection and survival biases. Despite these weaknesses, this may be the largest and most racially diverse population-based sample yet to assess the relationship between renal function and ECG-documented AF. Moreover, measurements of both serum creatinine and albuminuria allowed for estimation of the association of AF with renal impairment across the entire spectrum of CKD.
In conclusion, CKD was associated with an increased prevalence of AF in this large population-based sample of U.S. adults. This association was present among individuals with stage 1 or 2 CKD, stage 3 CKD and stage 4 or 5 CKD, remained consistent across several subgroups and persisted after adjustment for multiple potential confounders. Given the large number of U.S. adults with CKD and their high risk of CVD, these findings have important clinical implications. Additional prospective studies are needed to determine the mechanisms responsible for this association and incremental risk for stroke associated with AF among individuals with CKD.
Acknowledgments
Funding Sources: This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.
Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation. Amgen did not have any role in the design and conduct of the study, the collection, management, data analysis, or interpretation of the data, or the preparation or approval of the manuscript.
Dr. Muntner had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflict of Interest Disclosures: Drs. Warnock and McClellan receive research support and serve as consultants for AMGEN, Inc. Dr. Halperin has received honoraria from Portola Pharmaceuticals, Inc. and has received consulting fees from Astellas Pharma, U.S., Bayer AG HealthCare, Boehringer Ingelheim, Daiichi Sankyo, Johnson & Johnson, Sanofi-Aventis and Biotronik, Inc.
References
- 1.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: The framingham study. N Engl J Med. 1982;306:1018–1022. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 2.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: Incidence, risk factors, and prognosis in the manitoba follow-up study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 4.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 5.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The framingham heart study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 6.Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The framingham heart study. Circulation. 1994;89:724–730. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 7.Weiner DE, Tabatabai S, Tighiouart H, Elsayed E, Bansal N, Griffith J, Salem DN, Levey AS, Sarnak MJ. Cardiovascular outcomes and all-cause mortality: Exploring the interaction between ckd and cardiovascular disease. Am J Kidney Dis. 2006;48:392–401. doi: 10.1053/j.ajkd.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Genovesi S, Pogliani D, Faini A, Valsecchi MG, Riva A, Stefani F, Acquistapace I, Stella A, Bonforte G, DeVecchi A, DeCristofaro V, Buccianti G, Vincenti A. Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis. 2005;46:897–902. doi: 10.1053/j.ajkd.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 9.Vazquez E, Sanchez-Perales C, Borrego F, Garcia-Cortes MJ, Lozano C, Guzman M, Gil JM, Borrego MJ, Perez V. Influence of atrial fibrillation on the morbido-mortality of patients on hemodialysis. Am Heart J. 2000;140:886–890. doi: 10.1067/mhj.2000.111111. [DOI] [PubMed] [Google Scholar]
- 10.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: Objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 11.Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS, Phillips MC. Electrocardiographic detection of left ventricular hypertrophy: Development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572–580. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 12.Soliman EZ, Howard G, Prineas RJ, McClure LA, Howard VJ. Calculating cornell voltage from nonstandard chest electrode recording site in the reasons for geographic and racial differences in stroke (regards) study. J Electrocardiol. 2009 doi: 10.1016/j.jelectrocard.2009.10.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekundayo OJ, Howard VJ, Safford MM, McClure LA, Arnett D, Allman RM, Howard G, Ahmed A. Value of orthopnea, paroxysmal nocturnal dyspnea, and medications in prospective population studies of incident heart failure. Am J Cardiol. 2009;104:259–264. doi: 10.1016/j.amjcard.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.K/doqi clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National kidney foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 18.Genovesi S, Vincenti A, Rossi E, Pogliani D, Acquistapace I, Stella A, Valsecchi MG. Atrial fibrillation and morbidity and mortality in a cohort of long-term hemodialysis patients. Am J Kidney Dis. 2008;51:255–262. doi: 10.1053/j.ajkd.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Vazquez E, Sanchez-Perales C, Lozano C, Garcia-Cortes MJ, Borrego F, Guzman M, Perez P, Pagola C, Borrego MJ, Perez V. Comparison of prognostic value of atrial fibrillation versus sinus rhythm in patients on long-term hemodialysis. Am J Cardiol. 2003;92:868–871. doi: 10.1016/s0002-9149(03)00904-4. [DOI] [PubMed] [Google Scholar]
- 20.Asselbergs FW, van den Berg MP, Diercks GF, van Gilst WH, van Veldhuisen DJ. C-reactive protein and microalbuminuria are associated with atrial fibrillation. Int J Cardiol. 2005;98:73–77. doi: 10.1016/j.ijcard.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 21.Iguchi Y, Kimura K, Kobayashi K, Aoki J, Terasawa Y, Sakai K, Uemura J, Shibazaki K. Relation of atrial fibrillation to glomerular filtration rate. Am J Cardiol. 2008;102:1056–1059. doi: 10.1016/j.amjcard.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Abbott RD, Savage DD, McNamara PM. Coronary heart disease and atrial fibrillation: The framingham study. Am Heart J. 1983;106:389–396. doi: 10.1016/0002-8703(83)90208-9. [DOI] [PubMed] [Google Scholar]
- 23.Mostaza JM, Suarez C, Manzano L, Cairols M, Garcia-Iglesias F, Sanchez-Alvarez J, Ampuero J, Godoy D, Rodriguez-Samaniego A, Sanchez-Zamorano MA. Relationship between ankle-brachial index and chronic kidney disease in hypertensive patients with no known cardiovascular disease. J Am Soc Nephrol. 2006;17:S201–205. doi: 10.1681/ASN.2006080915. [DOI] [PubMed] [Google Scholar]
- 24.Wadley VG, McClure LA, Howard VJ, Unverzagt FW, Go RC, Moy CS, Crowther MR, Gomez CR, Howard G. Cognitive status, stroke symptom reports, and modifiable risk factors among individuals with no diagnosis of stroke or transient ischemic attack in the reasons for geographic and racial differences in stroke (regards) study. Stroke. 2007;38:1143–1147. doi: 10.1161/01.STR.0000259676.75552.38. [DOI] [PubMed] [Google Scholar]
- 25.Fox CS, Larson MG, Vasan RS, Guo CY, Parise H, Levy D, Leip EP, O'Donnell CJ, D'Agostino RB, Sr, Benjamin EJ. Cross-sectional association of kidney function with valvular and annular calcification: The framingham heart study. J Am Soc Nephrol. 2006;17:521–527. doi: 10.1681/ASN.2005060627. [DOI] [PubMed] [Google Scholar]
- 26.Moran A, Katz R, Jenny NS, Astor B, Bluemke DA, Lima JA, Siscovick D, Bertoni AG, Shlipak MG. Left ventricular hypertrophy in mild and moderate reduction in kidney function determined using cardiac magnetic resonance imaging and cystatin c: The multi-ethnic study of atherosclerosis (mesa) Am J Kidney Dis. 2008;52:839–848. doi: 10.1053/j.ajkd.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nardi E, Palermo A, Mule G, Cusimano P, Cottone S, Cerasola G. Left ventricular hypertrophy and geometry in hypertensive patients with chronic kidney disease. J Hypertens. 2009;27:633–641. doi: 10.1097/HJH.0b013e3283220ecd. [DOI] [PubMed] [Google Scholar]
- 28.Vasan RS, Larson MG, Levy D, Galderisi M, Wolf PA, Benjamin EJ. Doppler transmitral flow indexes and risk of atrial fibrillation (the framingham heart study) Am J Cardiol. 2003;91:1079–1083. doi: 10.1016/s0002-9149(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 29.Hung MJ, Yang NI, Wu IW, Cheng CW, Wu MS, Cherng WJ. Echocardiographic assessment of structural and functional cardiac remodeling in patients with predialysis chronic kidney disease. Echocardiography. 2010;27:621–629. doi: 10.1111/j.1540-8175.2009.01122.x. [DOI] [PubMed] [Google Scholar]
- 30.Salvetti M, Muiesan ML, Paini A, Monteduro C, Bonzi B, Galbassini G, Belotti E, Movilli E, Cancarini G, Agabiti-Rosei E. Myocardial ultrasound tissue characterization in patients with chronic renal failure. J Am Soc Nephrol. 2007;18:1953–1958. doi: 10.1681/ASN.2006050462. [DOI] [PubMed] [Google Scholar]
- 31.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 32.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the united states. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]