Abstract
Multiple systems atrophy (MSA) is a neurodegenerative disorder characterized by oligodendrocytic accumulations of alpha-synuclein (αsyn). Oxidative stress is a key mechanism proposed to underlie MSA pathology. To address the role of αsyn modifications, over and above general oxidative modifications, this study examined the effects of 3-nitropropionic acid (3NP) administration, a technique used to model MSA, in knock-out mice lacking αsyn (αsynKO). Although susceptible to 3NP-induced oxidative stress, αsynKO mice display reduced neuronal loss and dendritic pathology. The αsynKO mice are resistant to 3NP-induced motor deficits and display attenuated loss of tyrosine hydroxylase and dopamine transporter striatal immunoreactivity. The results suggest that deficits in MSA are not due to general oxidative protein modification but in addition may be related to specific αsyn modifications.
Keywords: alpha-synucleinopathy, neurodegeneration, oxidative stress
Introduction
Multiple system atrophy (MSA) is a sporadic neuro degenerative disease of the central nervous system and neuronal loss predominantly in the basal ganglia, brainstem, and cerebellum is a key feature of MSA [1,2]. Pathologically, MSA is characterized primarily by alpha-synuclein (αsyn)-positive glial cytoplasmic inclusions, though neuronal αsyn inclusions have also been reported [2].
Administration of 3-nitropropionic acid (3-NP), a mitochondrial complex II inhibitor mimics MSA by aggravating nigrostriatal and olivopontocerebellar degeneration in transgenic mice expressing αsyn under the oligodendrocytic proteolipid promoter [1], highlighting the role of mitochondrial dysfunction and oxidative stress in MSA pathology. Oxidative stress has also been associated with increased risk of MSA [2-4] and has been reported to posttranslationally modify αsyn and enhance αsyn fibrillogenesis and aggregation [5].
When evaluating oxidative stress, the relative importance of αsyn modifications over a background of general protein oxidation must be determined to assess whether deficits are a direct result of the αsyn or merely attributable to general protein modifications.
This study examined the effect of 3NP administration on αsyn-deficient knock-out mice (αsynKO) compared with those overexpressing human αsyn under the oligodendrocytic-specific myelin basic protein (MBP) promoter (MBP-hαsyn Tg mice). The results show that while 3NP administration induced protein oxidation in the both the αsynKO and MBP-hαsyn Tg mice, the neuropathological and behavioral deficits in MBP-hαsyn Tg mice were absent in the αsynKO mice suggesting that, rather than a generalized response to protein oxidation, 3NP-induced deficits in the MBP-hαsyn Tg mice [6] are linked to the presence of αsyn.
Materials and methods
Animals and treatment
The MBP human αsyn transgenic [MBP-hαsyn Tg mice (MBP1 line)] have been previously described [7], they develop oligodendrocytic αsyn inclusions along axonal tracts in the brainstem, basal ganglia, cerebellum, corpus callosum, and neocortex and display myelin loss and astrogliosis [7]. They have been used to model MSA [7–9] and are susceptible to 3NP-induced neurotoxicity [6]. The αsynKO were obtained from Jackson Laboratories (ID:003692, Jackson Laboratories, Maine, USA). A total of 36 mice with a mean age of 8.9 months were used. The mice of each genotypes (MBP-hαsyn Tg, n = 12, αsynKO, n = 12), and non-transgenic littermates (NTg, n = 12) were divided into saline-treated and 3NP-treated groups (n = 6 in each group). The 3NP (Sigma, St Louis, Missouri, USA) was dissolved in saline and pH 7.4 was adjusted with 1 M NaOH. The 3NP (or saline) was administered intraperitoneally twice daily over 6 days; 10 mg/kg (days 1 and 2), 20 mg/kg (days 3 and 4), 30 mg/kg (days 5 and 6), total dose 240 mg/kg in 6 days. Behavioral analysis was conducted 3 weeks post-final injection, the mice were sacrificed after behavioral testing.
Behavioral testing
Motor behavior was assessed using the Pole test [10]. The animals were placed upwards on a vertical wooden pole 50 cm long (1 cm in diameter). Time taken to descend the pole (T-total) was measured. Mice received three training trials and five test trials on the same day with a resting period between trials.
Tissue processing
Following NIH guidelines for the humane treatment of animals, under anesthesia mice were sacrificed and brains removed. The right hemibrain was immersion-fixed in 4% paraformaldehyde in pH 7.4 PBS and serially sectioned at 40 μm with the Vibratome (Leica, Deerfield, Illinois, USA) for subsequent analysis of neurodegeneration. The left hemibrain was kept at −80°C for biochemical analysis.
Immunohistochemistry
Immunohistochemistry was conducted on 40 μm vibratome sections, immunolabeled overnight with antibodies against the dendritic marker microtubule-associated protein-2 (MAP2, 1:50, Chemicon, Temecula, California, USA), the neuronal marker NeuN (1:1000, Chemicon), the astroglial marker glial fibrillary acidic protein (GFAP, 1:500, Millipore, Temecula, California, USA), the dopaminergic marker tyrosine hydroxylase (TH, 1:200, Chemicon), the dopamine transporter (DAT, 1:500, Chemicon) and αsyn (1:500, Chemicon) followed by incubation with species-appropriate secondary antibodies (1:2000, Vector Laboratories). The NeuN, αsyn, and DAT immunolabeled sections were reacted with diaminobenzidine tetrahydrochloride containing 0.001% H2O2, whereas tetrahydrochloride (TH) immunolabeled sections were reacted with diaminobenzi-dine containing 0.001% H2O2 and nickel chloride for visualization. The MAP2 and GFAP were incubated with fluorochrome-labeled secondary antibodies. The sections were transferred to SuperFrost slides (Fisher Scientific, Tustin, California, USA) and mounted under glass coverslips with antifading media (Vector Laboratories). The sections were analyzed with the laser scanning confocal microscope (MRC1024, BioRad, Hercules, California, USA).
Stereological analysis
An unbiased stereological estimation of striatal αsyn and NeuN immunoreactive cells was performed using the disector method with the Stereo-Investigator system (MBS Technology) and expressed as cells per unit volume.
Oxyblot analysis of protein oxidation
Level of protein oxidation in brain homogenates from MBP-hαsyn Tg mice, αsynKO mice, and NTg mice were analyzed using the Oxyblot Protein Oxidation Detection Kit (Chemicon) as per manufacturer's instructions. The samples were transferred to an Immobilon membrane, which was blocked with 3% bovine serum albumin in phosphate-buffered solution with 0.01% Tween-20 (0.05%), incubated with the anti-2,4-dinitrophenylhydrazone antibody, followed by incubation with secondary antibody, then reacted with chemi-illuminescence (ECL, Pierce) and developed on the VersaDoc gel-imaging machine (BioRad).
Analysis of mitochondrial complex II activity
In a 96-well plate, sample wells contained 195 μl of Tris-SO4, 0.1 M, pH 7.4, Horse heart cytochrome c (Sigma type II) 1.6 mg/ml, sodium cyanide, 1 mM, 2.5 μl of sodium succinate, 20%, pH 7.5 and 2 μl of the protein sample (30 μg, from detergent insoluble, membrane fraction). Reference wells contained all the above plus 2 μl of sodium malonate 20%, pH 7.5. The samples were left at room temperature for 5 min then absorbance was measured at 550 nm (DTX 880, Beckman Coulter, Brea, California, USA).
Western blot analysis
The levels of total αsyn and nitrosylated proteins were determined by immunoblot analysis. Tissue was processed as described earlier [7], protein (20 μg) was loaded in 4–12% Bis-Tris (Invitrogen) SDS-PAGE gels, transferred onto Immobilon membranes, blocked in 3% bovine serum albumin (Fisher Scientific) in phosphate-buffered solution with 0.01% Tween-20 (Sigma), incubated with antibodies against total αsyn (1:1000; Chemicon) and (Syn211, 1:1000, Sigma) and nitro-tyrosine (1:1000, Chemicon). After overnight incubation, the membranes were incubated in secondary antibodies, reacted with ECL (Pierce), and developed on a VersaDoc gel-imaging machine (BioRad). Anti-β-actin (1:1000; Sigma) antibody was used to confirm equal loading.
Statistical methods
The group differences were tested using one and two factor analysis of variance with Fisher's protected least significance difference post hoc tests. Additional preliminary analysis between control and treated groups was by unpaired, two-tailed, Student's t-test. All results are expressed as mean ± SEM.
Results
Induction of oxidative stress αsynKO and MBP-hαsyn Tg mice after 3NP administration
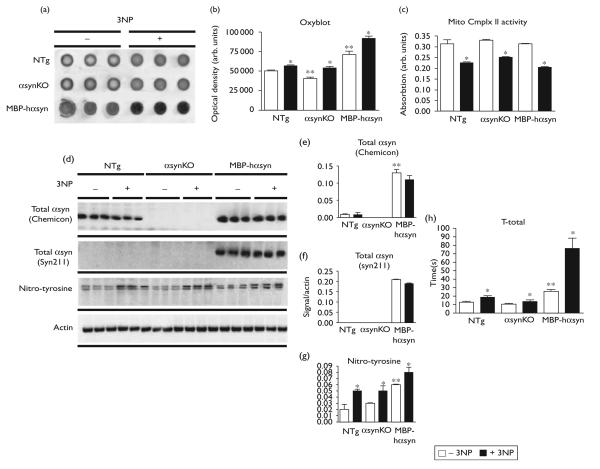
An Oxyblot assay conducted to assess the oxidative effects of 3NP administration (Fig. 1a, quantified in Fig. 1b) showed increased levels of protein oxidation in all groups (Fig. 1a). The levels of protein oxidation in saline-treated MBP-hαsyn Tg mice were significantly higher than both the saline-treated αsynKO and NTg, whereas those in the saline-treated αsynKO mice were significantly lower than the saline-treated NTg mice (Fig. 1a, quantified in Fig. 1b). To assess the activity of 3NP, an analysis of mitochondrial complex II activity was conducted (Fig. 1c) and reduced activity was observed in all lines (Fig. 1c).
Fig. 1.
Analysis of oxidative stress, alpha-synuclein (α-syn) levels and behavioral deficits upon 3-nitropropionic acid (3NP) administration. Oxyblot analysis of global protein oxidation levels in non-transgenic littermates (NTg), knock-out mice lacking αsyn (αsynKO), and myelin basic protein (MBP)-hαsyn Tg mice (a), analyzed in (b). Assessment of mitochondrial complex II (Mitc Cmplx II) activity levels after 3NP administration in the NTg, αsynKO, and MBP-hαsyn Tg mice (c). Immunoblot analysis of total αsyn levels, using two different antibodies and nitrotyrosine levels 3NP administration in the NTg, αsynKO, and MBP-hαsyn Tg mice (d), quantitative analysis (e–g). Motor behavior was assessed using the pole test (h). Mice were placed at the top of a vertical pole and total time to descend (T-total) was measured. *Significant difference (P < 0.05, one-way analysis of variance and post hoc Fisher's test) between saline and 3NP-treated members of the same genotype (saline-treated NTg vs. 3NP-treated NTg mice). **Significant difference (P < 0.05, one-way analysis of variance and post hoc Fisher's test) between mice of different genotype (saline-treated NTg vs. saline-treated αsynKO mice). Arb., arbitrary.
Characterization of αsyn expression in αsynKO and MBP-hαsyn Tg mice after 3NP administration
The analysis of total αsyn levels was conducted using two antibodies, the first (Chemicon) showed an increase in total αsyn level in the MBP-hαsyn Tg mice in comparison to NTg mice, as reported earlier [7] (Fig. 1d, analyzed in Fig. 1e). The second antibody (Syn211, specific for human αsyn) also showed high levels of αsyn expression in the MBP-hαsyn Tg mice (Fig. 1d, analyzed in Fig. 1f). No αsyn expression was observed in the αsynKO mice (Fig. 1d). 3NP administration had no effect on levels of total αsyn in the MBP-hαsyn Tg mice [6]. Analysis of nitro-tyrosine expression (a marker of general protein oxidation and nitration) across the lines showed an increase upon 3NP administration in all of the groups (Fig. 1d, analyzed in Fig. 1g). The saline-treated MBPhαsyn Tg mice displayed higher levels of nitro-tyrosine in comparison to saline-treated NTg and αsynKO mice whereas the saline-treated NTg and αsynKO mice expressed similar levels of nitro-tyrosine expression.
Behavioral analysis of αsynKO and MBP-hαsyn Tg mice after 3NP administration
The pole test was conducted to assess the impact of 3NP administration upon motor behavior (Fig. 1h). Consistent with earlier reports, the saline-treated MBP-hαsyn mice took much longer to descend the pole in comparison to NTg mice [9], indicative of motor deficit, 3NP administration significantly increased this time (Fig. 1h) [8]. In contrast, T-total for saline-treated αsynKO did not differ from the saline-treated NTg; 3NP did not have a significant effect. 3NP administration increased the time taken by NTg mice to descend the pole, but this increase was not to the same degree as seen in the MBP-hαsyn (Fig. 1h). These results show that while 3NP administration can induce and exacerbate motor deficits in the NTg and MBP-hαsyn Tg mice, αsynKO mice appear resistant to 3NP-induced deficits.
Neuropathological correlates of 3NP administration in αsynKO and MBP-hαsyn Tg mice
Immunohistochemical analysis of the striatum was conducted with antibodies against human αsyn, TH, and DAT (Fig. 2). Consistent with earlier reports [6,7] saline-treated MBP-hαsyn Tg mice displayed significant oligodendrocytic hαsyn immunoreactivity in comparison to saline-treated NTg mice (Figs 2a and e, analyzed in Fig. 2g), 3NP administration did not significantly increase level of total human αsyn in the MBP-hαsyn Tg mice. No hαsyn immunoreactivity was detected in saline-treated or 3NP-treated NTg or αsynKO mice (Figs 2a–d, analyzed in Fig. 2g).
Fig. 2.
Immunohistochemical analysis of alpha-synuclein (α-syn) and dopamine markers in non-transgenic littermates (NTg), knock-out mice lacking αsyn (αsynKO), and myelin basic protein (MBP)-hαsyn Tg mice after 3-nitropropionic acid (3NP) administration. Immunohistochemical analysis was performed to examine the number of αsyn immunoreactive cells in the striatum of vehicle-treated and 3NP-treated NTg mice (a and b), αsynKO mice (c and d) and MBP-hαsyn mice (e and f), analyzed in (g). To assess the effect of 3NP administration on dopamine markers immunohistochemical analysis was performed to examine the number of tyrosine hydroxylase (TH) and dopamine transporter (DAT) immunoreactive cells in the striatum of vehicle-treated and 3NP-treated NTg mice (h, i, o, and p, respectively), αsynKO mice (j, k, q, and r, respectively) and MBP-hαsyn mice (l, m, s, and t, respectively), analyzed in (n) and (u). Scale bar = 50 μM. *Significant difference (P < 0.05, one-way analysis of variance and post hoc Fisher's test) between saline and 3NP-treated members of the same genotype (saline-treated NTg vs. 3NP-treated NTg mice). **Significant difference (P < 0.05, one-way analysis of variance and post hoc Fisher's test) between mice of different genotype (saline-treated NTg vs. saline-treated αsynKO mice).
Immunohistochemical analysis TH and DAT showed a significant decrease in levels of both in the saline-treated MBP-hαsyn Tg mice in comparison with both saline-treated NTg and αsynKO mice (Figs 2h, j and l, analyzed in Fig. 2n). The 3NP administration significantly reduced levels of TH and DAT immunoreactivity in both the NTg and MBP-hαsyn Tg mice (Figs 2h, i, l and m, analyzed in Fig. 2n). In contrast 3NP had no effect on levels of TH and DAT immunoreactivity in αsynKO mice, both saline-treated and 3NP-treated αsynKO mice displayed comparable levels of TH and DAT immunoreactivity to saline-treated NTg mice.
Analysis of striatal neuronal density and dendritic complexity as evidenced by NeuN and MAP2 immuno-reactivity, respectively (Fig. 3), showed a significant reduction in neuronal number and area of MAP2-immunoreactive neutrophil in the saline-treated MBPhαsyn Tg mice in comparison to both saline-treated NTg and αsynKO mice (Figs 3a and e, analyzed in Figs 3g, h and l, analyzed in Fig. 3n), this is consistent with published reports [6,7]. 3NP administration had no effect on neuronal density and dendritic complexity in the MBP-hαyn Tg mice (Figs 3e and f, analyzed in Figs 3g, l and m, analyzed in Fig. 3n), but did appear to decrease dendritic complexity in the NTg mice, as evidenced by a decrease in MAP2 immunoreactive neuropil in the NTg mice (Figs 3h and i, analyzed in Fig. 3n). The 3NP administration did not effect neuronal number and dendritic complexity in the αsynKO mice, with both saline-treated and 3NP-treated αsynKO mice displaying similar levels of NeuN and MAP2 immunoreactivity to saline-treated NTg controls (Figs 3c and d, analyzed in Figs 3g, j and k, analyzed in Fig. 3n).
Fig. 3.
The knock-out mice lacking αsyn (αsynKO) display attenuated neuropathological deficits after 3-nitropropionic acid (3NP) administration. Immunohistochemical analysis of neuronal marker (NeuN) positive cells in the striatum was conducted in order to investigate the effects of 3NP administration on neuronal density in saline-treated and 3NP treated non-transgenic littermates (NTg) mice (a and b), αsynKO mice (c and d) and myelin basic protein (MBP)-hαsyn Tg mice (e and f), analyzed (g). Dendritic pathology was assessed by the percentage of microtubule-associated protein-2 (MAP2) immunoreactive area of neutrophil in the striatum of saline-treated and 3NP treated NTg mice (h and i), αsynKO mice (j and k) and MBP-hαsyn Tg mice (l and m), analyzed in (n). Astrogliosis after 3NP administration was assessed through glial fibrillary acidic protein (GFAP) immunoreactivity in the striatum of saline-treated and 3NP treated NTg mice (o and p), αsynKO mice (q and r), and MBP-hαsyn Tg mice (s and t), analyzed (u). Scale bar (a–f and o–t) = 50 μM, scale bar (h–m) = 20 μM. *Significant difference (P < 0.05, one-way analysis of variance and post hoc Fisher's test) between saline and 3NP-treated members of the same genotype (saline-treated NTg vs. 3NP-treated NTg mice). **Significant difference (P < 0.05, one-way analysis of variance and post hoc Fisher's test) between mice of different genotype (saline-treated NTg vs. saline-treated αsynKO mice). Arb., arbitrary.
Analysis of astrogliosis, as evidenced by GFAP immunoreactivity, showed a significant increase in GFAP-immunoreactivity in the saline-treated MBP-hαsyn Tg mice in comparison to both saline-treated NTg and αsynKO mice (Figs 3o, q and s, analyzed in Fig. 3u). 3NP had no significant effect on GFAP immunoreactivity in the MBP-hayn Tg mice (Figs 3s and t, analyzed in Fig. 3u) but did increase GFAP immunoreactivity in the NTg mice (Figs 3o and p, analyzed in Fig. 3u). The 3NP did not affect GFAP immunoreactivity in the αsynKO mice, with saline-treated and 3NP-treated αsynKO mice displaying similar levels to saline-treated NTg controls.
Discussion
This study shows for the first time that, compared with MBP-hαsyn Tg mice, αsynKO mice are resistant to neuropathological and behavioral deficits associated with 3NP administration. The 3NP resistance of the αsynKO mice is consistent with earlier studies where αsyn deficient mice have been shown to exhibit reduced susceptibility to 1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine [11-13], 6-hydroxydopamine [14,15], and 3NP [16]. Earlier work on αsynKO mice has focused on Parkinson's disease-related toxins, dopamine metabolism, and toxin-induced lesion formation in αsynKO mice whereas data regarding neuropathological measures such as neuronal density and dendritic complexity were sparse. This study sought to bridge this gap by investigating these mice in relation to MSA and shows that while αsynKO mice are susceptible to 3NP-induced mitochondrial complex II activity inhibition and display a global increase in protein oxidation as evidenced by the Oxyblot assay and nitrotyrosine immunoblot results, they do not display the same reduced neuronal density or dendritic loss that is evident in the MBP-hαsyn Tg mice upon 3NP administration. In addition, αsynKO mice are resistant to motor deficits as evidence by the pole test, consistent with the attenuated loss of striatal dopamine markers TH and DAT, this study is the first to report an attenuation of a disease-related behavioral phenotype in the αsynKO mice.
Conclusion
Oxidative stress may underlie disease progression in a synucleinopathies such as MSA [17] and emerging data suggests that posttranslational oxidative modifications of αsyn are linked to αsyn accumulation and inclusion formation [5,18–22]. Results from this study are consistent with this and suggest that, while oxidative stress, resulting from mitochondrial dysfunction, can induce widespread oxidative protein changes, the presence of αsyn plays a key role in the neurodegeneration and behavioral deficits observed.
Acknowledgement
This work was funded by NIH grants AG18440, NS044233, and AG 022074.
References
- 1.Stefanova N, Reindl M, Neumann M, Haass C, Poewe W, Kahle PJ, et al. Oxidative stress in transgenic mice with oligodendroglial alpha-synuclein overexpression replicates the characteristic neuropathology of multiple system atrophy. Am J Pathol. 2005;166:869–876. doi: 10.1016/s0002-9440(10)62307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanna PA, Jankovic J, Kirkpatrick JB. Multiple system atrophy: the putative causative role of environmental toxins. Arch Neurol. 1999;56:90–94. doi: 10.1001/archneur.56.1.90. [DOI] [PubMed] [Google Scholar]
- 3.Nee LE, Gomez MR, Dambrosia J, Bale S, Eldridge R, Polinsky RJ. Environmental-occupational risk factors and familial associations in multiple system atrophy: a preliminary investigation. Clin Auton Res. 1991;1:9–13. doi: 10.1007/BF01826052. [DOI] [PubMed] [Google Scholar]
- 4.Vanacore N, Bonifati V, Fabbrini G, Colosimo C, De Michele G, Marconi R, et al. Case-control study of multiple system atrophy. Mov Disord. 2005;20:158–163. doi: 10.1002/mds.20303. [DOI] [PubMed] [Google Scholar]
- 5.Norris EH, Giasson BI, Ischiropoulos H, Lee VM. Effects of oxidative and nitrative challenges on alpha-synuclein fibrillogenesis involve distinct mechanisms of protein modifications. J Biol Chem. 2003;278:27230–27240. doi: 10.1074/jbc.M212436200. [DOI] [PubMed] [Google Scholar]
- 6.Ubhi K, Lee PH, Adame A, Inglis C, Mante M, Rockenstein E, et al. Mitochondrial inhibitor 3-nitroproprionic acid enhances oxidative modification of alpha-synuclein in a transgenic mouse model of multiple system atrophy. J Neurosci Res. 2009;87:2728–2739. doi: 10.1002/jnr.22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shults CW, Rockenstein E, Crews L, Adame A, Mante M, Larrea G, et al. Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. J Neurosci. 2005;25:10689–10699. doi: 10.1523/JNEUROSCI.3527-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, et al. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24:9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ubhi K, Rockenstein E, Mante M, Patrick C, Adame A, Thukral M, et al. Rifampicin reduces alpha-synuclein in a transgenic mouse model of multiple system atrophy. Neuroreport. 2008;19:1271–1276. doi: 10.1097/WNR.0b013e32830b3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuura K, Kabuto H, Makino H, Ogawa N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods. 1997;73:45–48. doi: 10.1016/s0165-0270(96)02211-x. [DOI] [PubMed] [Google Scholar]
- 11.Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, et al. Resistance of alpha-synuclein null mice to the Parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drolet RE, Behrouz B, Lookingland KJ, Goudreau JL. Mice lacking alpha-synuclein have an attenuated loss of striatal dopamine following prolonged chronic MPTP administration. Neurotoxicology. 2004;25:761–769. doi: 10.1016/j.neuro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Fountaine TM, Venda LL, Warrick N, Christian HC, Brundin P, Channon KM, et al. The effect of alpha-synuclein knockdown on MPP + toxicity in models of human neurons. Eur J Neurosci. 2008;28:2459–2473. doi: 10.1111/j.1460-9568.2008.06527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez-Fischer D, Henze C, Strenzke C, Westrich J, Ferger B, Hoglinger GU, et al. Characterization of the striatal 6-OHDA model of Parkinson's disease in wild type and alpha-synuclein-deleted mice. Exp Neurol. 2008;210:182–193. doi: 10.1016/j.expneurol.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Robertson DC, Schmidt O, Ninkina N, Jones PA, Sharkey J, Buchman VL. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J Neurochem. 2004;89:1126–1136. doi: 10.1111/j.1471-4159.2004.02378.x. [DOI] [PubMed] [Google Scholar]
- 16.Klivenyi P, Siwek D, Gardian G, Yang L, Starkov A, Cleren C, et al. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis. 2006;21:541–548. doi: 10.1016/j.nbd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Jenner P. Oxidative stress in Parkinson's disease and other neurodegenerative disorders. Pathol Biol (Paris) 1996;44:57–64. [PubMed] [Google Scholar]
- 18.Duda JE, Giasson BI, Chen Q, Gur TL, Hurtig HI, Stern MB, et al. Widespread nitration of pathological inclusions in neurodegenerative synucleinopathies. Am J Pathol. 2000;157:1439–1445. doi: 10.1016/S0002-9440(10)64781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 20.Ischiropoulos H. Oxidative modifications of alpha-synuclein. Ann N Y Acad Sci. 2003;991:93–100. doi: 10.1111/j.1749-6632.2003.tb07466.x. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi A, Takeda A, Onodera H, Kimpara T, Hisanaga K, Sato N, et al. Systemic increase of oxidative nucleic acid damage in Parkinson's disease and multiple system atrophy. Neurobiol Dis. 2002;9:244–248. doi: 10.1006/nbdi.2002.0466. [DOI] [PubMed] [Google Scholar]
- 22.Rockenstein EM, McConlogue L, Tan H, Power M, Masliah E, Mucke L. Levels and alternative splicing of amyloid beta protein precursor (APP) transcripts in brains of APP transgenic mice and humans with Alzheimer's disease. J Biol Chem. 1995;270:28257–28267. doi: 10.1074/jbc.270.47.28257. [DOI] [PubMed] [Google Scholar]