Abstract
Purpose
Androgen-deprivation therapy (ADT) is associated with greater risk of incident coronary heart disease and hospital admission for myocardial infarction; treatment-related increases in serum lipids may contribute to greater cardiovascular disease risk. We evaluated the effects of toremifene, a selective estrogen-receptor modulator, on fasting serum lipid levels in men receiving ADT for prostate cancer.
Patients and Methods
In an ongoing, multicenter, double-blind, placebo-controlled phase III fracture-prevention study, 1,389 men receiving ADT for prostate cancer were randomly assigned to receive toremifene (80 mg/d) or placebo. In this interim analysis of 188 patients, changes in fasting serum lipids from baseline to month 12 were compared between the placebo and toremifene groups.
Results
Changes in serum lipids differed significantly between the groups. Mean (± SE) total cholesterol decreased by 1.0% ± 1.7% from baseline to month 12 in the placebo group and decreased by 8.1% ± 1.4% in the toremifene group (P = .001 for between group comparison). Low-density lipoprotein (LDL) cholesterol increased by 0.8% ± 2.5% in the placebo group and decreased by 8.2% ± 2.5% in the toremifene group (P = .003). In contrast, high-density lipoprotein (HDL) cholesterol decreased by 4.9% ± 1.2% in the placebo group and increased by 0.5% ± 2.2% in the toremifene group (P = .018). Triglycerides increased by 6.9% ± 4.2% in the placebo group and decreased by 13.2% ± 3.6% in the toremifene group (P = .003).
Conclusion
Toremifene significantly decreased total cholesterol, LDL cholesterol, and triglycerides, and increased HDL cholesterol in men receiving ADT for prostate cancer.
INTRODUCTION
Androgen-deprivation therapy (ADT), by bilateral orchiectomy or administration of a gonadotropin-releasing hormone (GnRH) agonist, is the core treatment for metastatic prostate cancer.1 In addition, GnRH agonists are routinely administered to many men with locally advanced or recurrent disease. Approximately one third of the estimated two million prostate cancer survivors in the United States currently receive treatment with a GnRH agonist.2,3
ADT has a variety of adverse effects including vasomotor flushing, gynecomastia, obesity, and osteoporosis.1 In addition, GnRH agonists have recently been associated with greater risk of incident coronary heart disease and hospital admission for myocardial infarction.4 Several mechanisms may contribute to greater risk for cardiovascular disease during ADT. GnRH agonists increase fat mass and decrease insulin sensitivity.5-8 GnRH agonists also increase serum cholesterol and triglycerides.5,9 In a prospective 12-month study of 40 men with non-metastatic prostate cancer, for example, GnRH-agonist therapy increased serum total cholesterol by 9.0% and triglycerides by 26.5%.5
Toremifene is a second-generation selective estrogen-receptor modulator (SERM) in development for the prevention of osteoporosis and other adverse effects resulting from ADT in men with prostate cancer.10 In an ongoing, multicenter, phase III study, 1,389 men receiving ADT for prostate cancer were assigned to receive either placebo or toremifene (80 mg/d) for 2 years. Toremifene significantly improved serum lipid profiles in postmenopausal women11-14 but its effects on serum lipids in men are unknown. We now report the results of interim analysis to evaluate the effects of toremifene on serum lipids in men receiving ADT for prostate cancer.
PATIENTS AND METHODS
Participants
The ongoing phase III study is a 24-month, double-blind, randomized, placebo-controlled trial of toremifene to prevent incident fractures in men receiving ADT for prostate cancer. Between July 2003 and November 2005, 1,389 participants from centers in the United States and Mexico were enrolled onto the study. All participants were men at least 50 years old with histologically documented prostate cancer and a serum prostate-specific antigen (PSA) no greater than 4 ng/mL. All participants had been treated with a GnRH agonist continuously for at least 6 months or intermittently for at least 12 months, or underwent bilateral orchiectomies at least 6 months before study entry. All participants had an increased risk for fracture based on either age at least 70 years, or low bone mineral density (BMD) of the lumbar spine or hip as assessed by Hologic or Lunar dual-energy x-ray absorptiometry (DXA). Low BMD was defined as BMD at or lower than the following thresholds: lumbar spine 0.926 g/cm2 for Hologic and 1.050 g/cm2 for Lunar, and femoral neck 0.717 g/cm2 for Hologic and 0.840 g/cm2 for Lunar.
Participants receiving prescription treatment for osteoporosis (bisphosphonates, SERM, parathyroid hormone, and calcitonin) or treatment with oral glucocorticoids or androgen-modulating treatments (finasteride, dutasteride, danazol, or testosterone-like supplements) within 45 days were excluded from the study. Participants with more than four vertebral fragility fractures, Paget's disease of bone, or any history of thromboembolic disease (including deep vein thrombosis or pulmonary embolus) were also excluded.
An institutional review board for each of the participating institutions approved the study. Participants provided written informed consent before study participation.
Study Design
Eligible participants were randomly assigned to receive toremifene (80 mg/d by mouth) or placebo for 2 years. Random assignment was performed with blocking for study center; there was no stratification. All patients and study personnel were blinded to treatment assignments. All participants continued ADT throughout the study.
The primary end point for the phase III study is the proportion of vertebral morphometric fractures in the toremifene group versus the placebo group after 24 months. In a protocol-specified interim analysis of the first 200 participants, changes in BMD from baseline to 12 months were compared between the toremifene and placebo groups.15 After a landmark study reported that ADT is associated with greater risk for incident coronary heart disease,4 we conducted this interim analysis to evaluate the effects of toremifene on serum lipoproteins. To minimize any potential bias, the lipid analyses were performed using data from the same 200 participants and follow-up period as specified for the interim BMD analyses. Twelve participants were excluded from the analyses because of missing data. The efficacy variables for this interim analysis were the percentages of change in fasting serum lipoproteins from baseline to month 12. Participants were classified as users or nonusers of cholesterol-lowering medications according to medication history at baseline and month 12.
Study End Points
Serum was collected from fasting participants at baseline and month 12. Serum total cholesterol, low-density lipoprotein (LDL) cholesterol and triglycerides were measured using a Roche enzymatic method. High-density lipoprotein (HDL) cholesterol was measured using a Roche Direct Homogeneous assay. All assays were performed at a central laboratory (CRL Medinet Inc, Lenexa, KS) by technicians who were blinded to treatment assignment.
Statistical Analyses
The purpose of these interim analyses was to determine the effects of toremifene on serum lipids in men receiving ADT for prostate cancer. The percentage of change in fasting serum total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and total cholesterol/HDL cholesterol ratio were computed for each patient as the difference between the lipid level at the 12-month assessment minus the baseline value then divided by the baseline value and converted to a percentage. These percentage changes were compared between the treatment arm and the placebo arm using a generalized linear model that allows for imbalance in the two arms; these analyses were also performed within the subsets of participants using and not using cholesterol-lowering medications. Complete data were available for all 188 participants included in these analyses. Statistical analyses were performed using SAS version 8 (SAS Institute, Cary, NC). Values are reported as mean ± 1 standard deviation. All P values are two-sided, and P values less than .05 were considered significant, no adjustments were made for multiple testing.
RESULTS
These interim analyses included 188 participants assigned to receive either placebo (n = 99) or toremifene (n = 89). Baseline characteristics were similar between the groups (Table 1). Mean (± standard deviation) age was 78 ± 6 years for men in the placebo group and 77 ± 6 years for men in the toremifene group. Approximately one half of participants in each group were using a cholesterol-lowering medication at baseline and/or study completion.
Table 1.
Baseline Characteristics
| Characteristic | Placebo (n = 99) | Toremifene (n = 89) |
|---|---|---|
| Age, years | ||
| Mean | 78 | 77 |
| SD | 6 | 6 |
| Median | 79 | 77 |
| Range | 61-90 | 61-89 |
| Cholesterol-lowering medication | ||
| No. | 51 | 43 |
| % | 52 | 48 |
| Body mass index, kg/m2 | ||
| Mean | 28.3 | 28.6 |
| SD | 4.5 | 4.3 |
| Median | 27.2 | 27.5 |
| Range | 20.9-42.8 | 20.4-41.6 |
| Total cholesterol, mg/dL* | ||
| Mean | 184 | 190 |
| SD | 31 | 42 |
| Median | 184 | 185 |
| Range | 103-253 | 111-324 |
| LDL cholesterol, mg/dL* | ||
| Mean | 103 | 104 |
| SD | 25 | 36 |
| Median | 104 | 98 |
| Range | 50-159 | 40-235 |
| HDL cholesterol, mg/dL* | ||
| Mean | 50 | 52 |
| SD | 12 | 13 |
| Median | 49 | 51 |
| Range | 27-88 | 25-105 |
| Triglycerides, mg/dL† | ||
| Mean | 160 | 166 |
| SD | 84 | 67 |
| Median | 134 | 157 |
| Range | 45-540 | 34-362 |
| Total/HDL cholesterol ratio | ||
| Mean | 3.8 | 3.8 |
| SD | 0.8 | 0.8 |
| Median | 3.7 | 3.7 |
| Range | 2.1-5.9 | 1.8-5.7 |
Abbreviations: SD, standard deviation; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
To convert from mg/dL to mmol/L, multiply by 0.0259.
To convert from mg/dL to mmol/L, multiply by 0.0113.
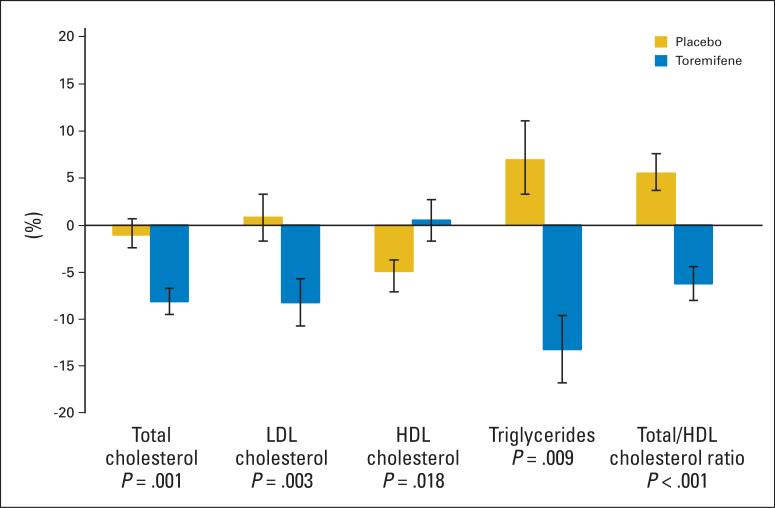
Changes in serum lipoproteins from baseline to month 12 differed significantly between the placebo and toremifene groups (Fig 1). Mean (± SE) total cholesterol decreased by 1.0% ± 1.7% from baseline to month 12 in the placebo group and decreased by 8.1% ± 1.4% in the toremifene group (P = .001 for between group comparison). LDL cholesterol increased by 0.8% ± 2.5% in the placebo group and decreased by 8.2% ± 2.5% in the toremifene group (P = .003). In contrast, HDL cholesterol decreased by 4.9% ± 1.2% in the placebo group and increased by 0.5% ± 2.2% in the toremifene group (P = .018). Consistent with the observed changes, the ratio of total to HDL cholesterol increased by 5.5% ± 2.1% in the placebo group and improved by 6.2% ± 1.8% in the toremifene group (P < .001). Triglycerides increased by 6.9% ± 4.2% in the placebo group and decreased by 13.2% ± 3.6% in the toremifene group (P = .003).
Fig 1.
Mean (± SE) percent changes in lipids in men with prostate cancer. P values are for between-group comparisons of changes from baseline to month 12. LDL, low-density lipoprotein; HDL, high-density lipoprotein.
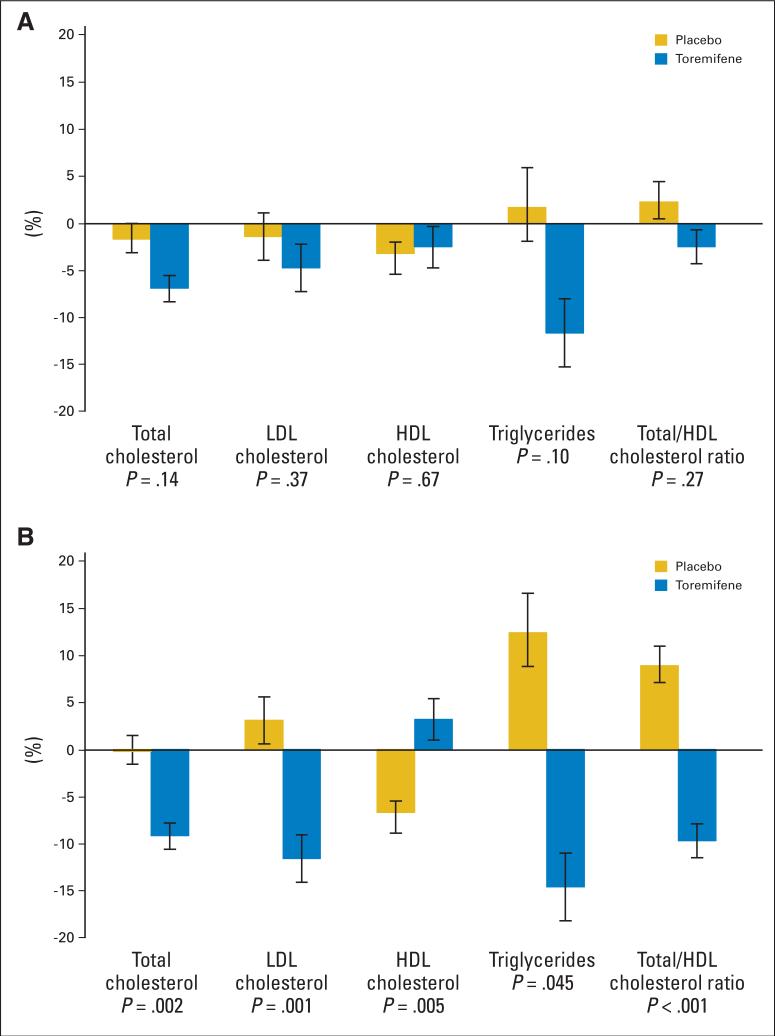
We conducted subset analyses to evaluate the effects of toremifene on serum lipids among users and nonusers of other cholesterol-lowering medications. For men who were taking a cholesterol-lowering medication at either baseline or month 12, toremifene tended to improve total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and total/HDL cholesterol ratio compared with placebo, but the changes were not statistically significant (Fig 2A). In contrast, among men who were not taking a cholesterol-lowering medication at baseline or month 12, changes in serum lipoproteins differed significantly between the placebo and toremifene groups (Fig 2B). In the subset of nonusers of cholesterol-lowering medications, total cholesterol decreased by 0.2% ± 2.2% from baseline to month 12 in the placebo group and decreased by 9.2% ± 1.7% in the toremifene group (P = .002). LDL cholesterol increased by 3.1% ± 3.3% in the placebo group and decreased by 11.6% ± 3.1% in the toremifene group (P = .001). HDL cholesterol decreased by 6.7% ± 1.9% in the placebo group and increased by 3.2% ± 3.2% in the toremifene group (P = .005). Triglycerides increased by 12.4% ± 6.9% in the placebo group and decreased by 14.6% ± 5.2% in the toremifene group (P = .045). The ratio of total to HDL cholesterol increased by 8.9% ± 3.1% in the placebo group and decreased by 9.7% ± 2.3% in the toremifene group (P < .001).
Fig 2.
Mean (± SE) percent changes in lipids in subset of men (A) receiving and (B) not receiving a cholesterol-lowering medication during the study period. P values are for between-group comparisons of changes from baseline to month 12. LDL, low-density lipoprotein; HDL, high-density lipoprotein.
DISCUSSION
In this interim analysis of a large, randomized, placebo-controlled, phase III fracture-prevention study, toremifene (80 mg/d) significantly improved serum lipoprotein profiles in men receiving ADT for prostate cancer. Compared with placebo, toremifene significantly increased HDL cholesterol and significantly decreased total cholesterol, LDL cholesterol, triglycerides, and total/HDL cholesterol ratio.
To the best of our knowledge, this interim analysis represents the largest evaluation of the effects of a SERM on serum lipids in hypogonadal men. In a smaller randomized controlled trial of 48 men receiving chronic GnRH-agonist therapy for prostate cancer, raloxifene did not significantly alter serum lipids.16 The smaller sample size of that study seems sufficient to explain the absence of an observed effect, although additional studies would be required to compare the effects of different SERMs on serum lipids in hypogonadal men. Additional studies are necessary to evaluate the effects of toremifene and other SERMs on other markers of cardiovascular disease risk in hypogonadal men.
In postmenopausal women, SERMs, including toremifene, tamoxifen, and raloxifene, significantly decrease total cholesterol and LDL cholesterol.11,17,18 In contrast, the various SERMs seem to differ in their effects on serum HDL cholesterol and triglycerides. In two of three randomized controlled trials in postmenopausal women, for example, toremifene significantly increased HDL cholesterol and decreased triglycerides compared with tamoxifen.12-14 Consistent with the favorable effects of toremifene on lipid profiles in postmenopausal women, toremifene significantly increased HDL cholesterol and significantly decreased total cholesterol, LDL cholesterol, and triglycerides in this interim analysis of men receiving ADT for prostate cancer. Additional studies are needed to determine the potential clinical implications of the favorable effects of toremifene on lipid profiles in men receiving ADT.
In a large randomized controlled trial of postmenopausal women with coronary heart disease or multiple risk factors for coronary heart disease, raloxifene had no effect on incident coronary events (including death resulting from coronary causes, myocardial infarction, or hospitalization for an acute coronary syndrome) or all-cause mortality.19 To date, no study has assessed the effects of toremifene or other SERMs on cardiovascular outcomes in men. The observed beneficial effects of toremifene on lipid profiles seem to provide a clear rationale to conduct exploratory analyses of cardiovascular outcomes when the ongoing fracture prevention study is completed. The results of those analyses will help determine whether additional clinical trials to evaluate the effects of toremifene on incident coronary events in men receiving ADT for prostate cancer are warranted.
GnRH agonists increase total cholesterol, LDL cholesterol, and triglycerides.5,9 In a prospective 12-month study of men with prostate cancer, for example, GnRH agonists increased serum total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides by 9.0%, 7.3%, 11.3%, and 26.5%, respectively.5 Notably, most but not all of the observed long-term adverse effects on serum lipids are apparent within the first 3 months of treatment.8 All participants in our study were receiving ADT for at least 6 months at study entry and continued ADT throughout the study period. In the placebo group, triglycerides and total/HDL ratio tended to increase from baseline to month 12. Compared with the marked changes in lipids observed with initial ADT,5,9 however, the changes in the placebo group were relatively small and seem consistent with the expected effects of chronic ADT.
The Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults has provided updated recommendations for cholesterol testing and management.20 The panel identifies LDL cholesterol as the primary target for cholesterol-lowering therapy and defines an LDL cholesterol level less than 100 mg/dL as optimal and 100 to 129 mg/dL as near optimal. Compared with placebo, toremifene in this interim analysis decreased LDL cholesterol by 9% overall and by nearly 15% in participants not receiving another cholesterol-lowering medication. By comparison, statins lower LDL cholesterol levels by at least 30%.21 Toremifene may be sufficient to achieve optimal or near-optimal LDL cholesterol levels in some but not all men. Accordingly, treatment with toremifene does not eliminate the need for cholesterol testing and management according to established guidelines.
In addition to osteoporosis and alterations in serum lipoproteins, ADT has a variety of other adverse effects, including gynecomastia, mastodynia, and vasomotor flushing.1 Toremifene may also decrease some of these other adverse effects of ADT. Toremifene is an estrogen antagonist in breast tissue and is approved for the treatment of metastatic breast cancer in postmenopausal women.22 The antagonist activity of toremifene on breast tissue may reduce breast enlargement and tenderness during ADT. Toremifene seems to act as a partial agonist on the hypothalamic-pituitary axis23 and may decrease vasomotor flushing. The effects of toremifene on breast symptoms and vasomotor flushing will be assessed in the ongoing phase III study.
In conclusion, toremifene (80 mg/d) significantly improved lipid profiles in men receiving ADT for prostate cancer. Compared with placebo, toremifene significantly decreased total cholesterol, LDL cholesterol, and triglycerides, and increased HDL cholesterol. These observations provide a solid rationale to evaluate the effects the effects of toremifene on cardiovascular outcomes in hypogonadal men.
Footnotes
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: K. Gary Barnette, GTx Inc (C); Domingo Rodriquez, GTx Inc (C); Mitchell S. Steiner, GTx Inc (C) Consultant or Advisory Role: Matthew R. Smith, GTx Inc (C); Paul Sieber, GTx Inc (C) Stock Ownership: Franklin Chu, GTx Inc; K. Gary Barnette, GTx Inc; Domingo Rodriquez, GTx Inc; Mitchell S. Steiner, GTx Inc Honoraria: Matthew R. Smith, GTx Inc; Paul Sieber, GTx Inc Research Funding: S. Bruce Malkowicz, GTx Inc; Franklin Chu, GTx Inc; John Forrest, GTx Inc; Paul Sieber, GTx Inc Expert Testimony: None Other Remuneration: None
REFERENCES
- 1.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 2.Barry MJ, Delorenzo MA, Walker-Corkery ES, et al. The rising prevalence of androgen deprivation among older American men since the advent of prostate-specific antigen testing: A population-based cohort study. BJU Int. 2006;98:973–978. doi: 10.1111/j.1464-410X.2006.06416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahinian VB, Kuo YF, Freeman JL, et al. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103:1615–1624. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 4.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 5.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 6.Smith JC, Bennett S, Evans LM, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86:4261–4267. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 7.Dockery F, Bulpitt CJ, Agarwal S, et al. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyper-insulinaemia. Clin Sci (Lond) 2003;104:195–201. doi: 10.1042/CS20020209. [DOI] [PubMed] [Google Scholar]
- 8.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305–1308. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 9.Eri LM, Urdal P, Bechensteen AG. Effects of the luteinizing hormone-releasing hormone agonist leuprolide on lipoproteins, fibrinogen and plasminogen activator inhibitor in patients with benign prostatic hyperplasia. J Urol. 1995;154:100–104. [PubMed] [Google Scholar]
- 10.Smith MR. Selective estrogen receptor modulators to prevent treatment-related osteoporosis. Rev Urol. 2005;7(suppl):S30–S35. [PMC free article] [PubMed] [Google Scholar]
- 11.Erkkola R, Mattila L, Powles T, et al. Bone mineral density and lipid changes during 5 years of follow-up in a study of prevention of breast cancer with toremifene in healthy, high-risk pre- and post-menopausal women. Breast Cancer Res Treat. 2005;93:277–287. doi: 10.1007/s10549-005-5701-x. [DOI] [PubMed] [Google Scholar]
- 12.Joensuu H, Holli K, Oksanen H, et al. Serum lipid levels during and after adjuvant toremifene or tamoxifen therapy for breast cancer. Breast Cancer Res Treat. 2000;63:225–234. doi: 10.1023/a:1006465732143. [DOI] [PubMed] [Google Scholar]
- 13.Saarto T, Blomqvist C, Ehnholm C, et al. Antiatherogenic effects of adjuvant antiestrogens: A randomized trial comparing the effects of tamoxifen and toremifene on plasma lipid levels in postmenopausal women with node-positive breast cancer. J Clin Oncol. 1996;14:429–433. doi: 10.1200/JCO.1996.14.2.429. [DOI] [PubMed] [Google Scholar]
- 14.Kusama M, Miyauchi K, Aoyama H, et al. Effects of toremifene (TOR) and tamoxifen (TAM) on serum lipids in postmenopausal patients with breast cancer. Breast Cancer Res Treat. 2004;88:1–8. doi: 10.1007/s10549-004-4384-z. [DOI] [PubMed] [Google Scholar]
- 15.Smith MR, Malkowicz SB, Chu F, et al. Toremifene increases bone mineral density in men receiving androgen deprivation therapy for prostate cancer: Interim analysis of a multicenter phase 3 clinical study. J Urol. 2008;179:152–155. doi: 10.1016/j.juro.2007.08.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith MR, Fallon MA, Lee H, et al. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: A randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841–3846. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 17.Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641–1647. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- 18.Walsh BW, Kuller LH, Wild RA, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445–1451. doi: 10.1001/jama.279.18.1445. [DOI] [PubMed] [Google Scholar]
- 19.Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 20.Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): Final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 21.Gotto AM., Jr Cholesterol management in theory and practice. Circulation. 1997;96:4424–4430. doi: 10.1161/01.cir.96.12.4424. [DOI] [PubMed] [Google Scholar]
- 22.Fareston (toremifene citrate) prescribing information [package insert] GTx Inc; Memphis, TN: 2007. [Google Scholar]
- 23.Ellmén J, Hakulinen P, Partanen A, et al. Estrogenic effects of toremifene and tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res Treat. 2003;82:103–111. doi: 10.1023/B:BREA.0000003957.54851.11. [DOI] [PubMed] [Google Scholar]