Abstract
Ultraviolet (UV) radiation, in particular the mid-wavelength range (UVB; 290–320 nm), is one of the most significant risk factors for the development of non-melanoma skin cancer. UVB radiation-induced immunosuppression, which occurs in both humans and laboratory animals, contributes to their pathogenesis. However, there are conflicting reports on the relative role of CD4+ and CD8+ T-cells in UVB induced skin cancer. The purpose of this study was to delineate the contribution of these two cell subpopulations to UVB induced immunosuppression and tumor development using C3H/HeN (WT), CD4 knockout (CD4−/−) and CD8 knockout (CD8−/−) mice. We observed that UVB induced skin carcinogenesis was retarded in terms of number of tumors per group, tumor volume and percentage of mice with tumors, in mice deficient in CD4+ T-cells compared to wild-type mice, whereas significantly greater (p<0.05) numbers of tumors occurred in CD8−/− mice. These results indicate that, CD4+ T-cells promote tumor development while CD8+ T-cells have the opposite effect. Further, we found that CD4+ T cells from tumor-bearing mice produced IL-4, IL-10, and IL-17 whereas CD8+ T cells produced IFN-γ. Manipulation of T-cell subpopulations that are induced by UVB radiation could be a means of preventing skin cancers caused by this agent.
INTRODUCTION
Ultraviolet (UV) radiation, particularly wavelengths in the UVB (290–320nm) range, produces molecular changes in the skin that ultimately lead to marked deviations in the induction of cell-mediated immune responses (1, 2). As currently conceptualized, following UVB exposure a marked alteration in the composition and function of antigen presenting cells occurs in the skin. This includes direct inhibitory effects on the immuno-stimulatory functions of epidermal Langerhans cells and other cutaneous antigen presenting cells, recruitment of immunosuppressive CD11b+ macrophages into the skin and alterations in the cytokine milieu which is important in determining the cell mediated immune response (3). With respect to cytokines, UVB exposure increases the production of the immunosuppressive mediators interleukin (IL)-10 and TGF-β and reduces the levels of immuno-stimulatory molecules like IL-12 and IL-23 (4). When haptens are applied to UVB-irradiated skin, these changes eventuate in greater numbers of regulatory T-cells (T-regs) and smaller number of effector T-cells, resulting in a shift in the balance from T-cell-mediated immunity to immunosuppression (5). In vivo treatment with either anti-CD4 or anti-CD8 antibodies results in CD8+ protective immunity with only a minor contribution from CD4+ T-cells. Effector cells for hapten-induced T-cell mediated immunity have been shown to secrete IFN-γ (6), whereas UV-induced T-regs express CD4 and CD25, and exert their suppressive activity through the release of IL-10 (7).
In addition to its photo-immunological effects, UVB radiation is a major risk factor for non-melanoma skin cancers. Their growth is controlled primarily by T-cell mediated immune responses. This hypothesis originated from observations in therapeutically immunosuppressed organ transplant recipients, in whom there is a 30- fold increase in the numbers of cutaneous tumors that occur in sun-exposed skin (8–10). In murine models, a number of studies have shown that most UV-induced skin tumors are immunogenic and accordingly are rejected upon transplantation into immunocompetent hosts. However, if the recipient animal is immunosuppressed by medication, UV radiation or gamma radiation, unrestricted growth of these tumors occurs (11–13). Similar to UV-induced suppression of hapten-induced T-cell responses, it has been reported that in vivo treatment with either anti-CD4 or anti-CD8 antibodies, results in CD8+ cell mediated protective immunity in the effector phase, with only a minor contribution from CD4+ cells. (14). However, it has also been reported that both CD8+and CD4+ T-cells contribute to control of UV-induced tumor growth (15). UV-induced T regulatory cells have been detected in UV-treated mice, which apparently promote tumor growth by suppressing anti-tumor effector functions (7).
In contrast to the knowledge that has been generated on the effects of T-cells on the growth of UV-induced tumors, the contribution of T-cells to the development of these tumors has received much less attention, although it is known that regulatory T-cells facilitate and effector T-cells restrict UV-induced tumor cell development. It has been reported that specific depletion of CD4+ T-cells increased both the UVB-induced inflammatory responses as well as the number of UVB-induced tumors suggesting that CD4+ lymphocytes protect against UV-induced skin tumor development (16). The cytokines that the effector and regulatory T-cells produce is unknown, although it is known that UV-induced tumor cell development is decreased in IL-10 knockout mice (7).
The purpose of this study was to delineate the role of CD4+ and CD8+ T-cells in tumor growth and development using CD4 gene knockout (CD4−/−) and CD8 gene knockout (CD8−/−) mice and to determine the cytokines that these cells produce. We observed that the T-cells that mediate UVB-induced immunosuppression and tumor development share complementary characteristics. CD8+ T-cells which produce IFN-γ are effector cells in contact hypersensitivity and inhibit tumor development, whereas CD4+ T-cells which secrete IL-4, IL-10 and IL-17, have opposite effect.
MATERIALS & METHODS
Animals and reagents
Wild-type female C3H/HeN (WT) mice 6–8 weeks of age were purchased from Charles River Laboratories (Wilmington, MA). CD4−/− and CD8−/− mice on a C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, ME) and were backcrossed onto a C3H/HeN background as described in our earlier report (17). All animal procedures were performed according to National Institutes of Health guidelines under protocols approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. The UV-2240 cell line is a fibrosarcoma derived by chronically exposing C3H/HeN mice to UVB radiation, was a kind gift from Dr. HN Ananthaswamy, Houston TX. Mouse anti-CD3e antibody was purchased from Pharmingen (San Diego, CA). The 2,4 dinitrofluorobenzene (DNFB) was purchased from Sigma Chemical Co. (St. Louis, MO). Magnetic beads for purification of CD4+, CD8+, CD11c+and CD4+CD25+ T-cells were purchased from Miltenyi Biotech (Auburn, CA).
UVB light source and irradiation of mice
The clipper-shaved dorsal skin of the mice was exposed to UVB radiation (200 mJ/cm2) from a bank of four UVB lamps (Daavlin, UVA/UVB Research Irradiation Unit, Bryan, OH) equipped with an electronic controller to regulate UVB dosage at the fixed distance of 24 cm from the lamps to the dorsal skin surface of the mice. Wavelengths <290 nm were filtered out using Kodacel cellulose film (Eastman Kodak Co., Rochester, NY). The majority of the resulting wavelengths were in the UVB (290–320 nm; ~80%) and UVA (~20%) range, with peak emission at 314 nm as monitored regularly.
UV-induced local immunosuppression
UV radiation is known to cause immunosuppression which can be seen when the hapten DNFB is applied to the irradiated skin (18). The shaved dorsal skin of CD4−/−, CD8−/− and WT C3H/HeN mice were exposed to UVB radiation (100mJ/cm2) for 4 successive days on the shaved back to induce immunosuppression in mice as described earlier with some modifications (19). During UVB irradiation, the ears of mice were protected from UVB using opaque black tape, which was removed after exposure. One day later, the mice were treated topically with 50 μl of 0.5% DNFB in acetone/olive oil (4:1, v/v) at the UVB-irradiated site. The contact hypersensitivity response was elicited 5 days later by challenging both surfaces of the ears of each mouse with 20 μl of 0.2% DNFB in acetone/olive oil (4:1, v/v). The ear thickness was measured 24 hours after the challenge using an engineer's micrometer (Mitutoyo, Tokyo, Japan) and was compared with the ear thickness just before the challenge. Mice that received the same treatment with DNFB but were not UVB irradiated served as positive controls. Non-UVB-irradiated mice that received only ear challenge without sensitization with DNFB served as negative controls.
Adoptive transfer
Donor mice were UVB irradiated as described above. After 5 days, mice were sacrificed and lymph nodes were removed and CD4+ and CD8+ T-cells were isolated from them. 200 μl containing 1× 107 cells of either CD4+ or CD8+ cells or 1× 106 cells of CD4+CD25+ cells were injected i.v. into the recipient mice, which were then sensitized 24h later. After 5 days mice were challenged on the ear as described above.
Photocarcinogenesis protocol
Female mice, 6 to 8 weeks old, were divided into the different treatment groups with 15 mice in each group. The control groups of mice were age- and sex-matched with the experimental group. A long-term photocarcinogenesis protocol was used as described previously (20) in which mice were irradiated with the same dose of UVB three times weekly with UVB (200 mJ/cm2) until the end of the protocol. The UVB-irradiated backs of the mice were examined on a weekly basis for papillomas, or tumor development. Growths >1 mm in diameter, that persisted for at least for 2 weeks, were defined as tumors and were recorded.
Preparation of tissue lysates and western blotting
Lysates were prepared from skin and tumor samples of UVB induced tumor bearing mice as described earlier (21). For western blot analysis 50μg protein was loaded in each well and resolved on 10% SDS-polyacrylamide gel and transferred onto nitrocellulose membranes. Membranes were incubated in blocking buffer for 2 h and then incubated with the primary antibodies in blocking buffer for 2h at room temperature or overnight at 4°C. The membrane was then washed with TBST and incubated with secondary antibody conjugated with horseradish peroxidase. Protein bands were visualized using the enhanced chemiluminescence detection system (Amersham Life Science, Inc., Piscataway, NJ). To verify equal protein loading and transfer of proteins from gel to membrane, the blots were stripped and reprobed for β-actin.
Tumor growth in vivo
The UV-2240 fibrosarcoma tumor cell line derived from WT C3H/HeN mice is highly antigenic and is routinely rejected when transplanted into normal syngeneic recipients, but grows progressively in immunodeficient mice (22). UV-2240 tumor cells grown to 70% confluence as a monolayer in DMEM containing 10% fetal bovine serum were harvested by trypsinization, washed, and re-suspended in serum free DMEM. Cells (5×106) were injected subcutaneously into the dorsal skin of CD4−/−, CD8−/−, and WT C3H/HeN mice. Tumor volumes (mm3) were measured every 3 days for nearly 3 weeks using a caliper. Mice were euthanized when the tumor reached a size >400mm3 in accordance with the IACUC policy at UAB.
Purification of cells and stimulation for cytokine production and ELISA
CD11c+, CD4+ T cells and CD8+ T cells were purified from draining lymph nodes and spleens of mice by MACS (Miltenyi Biotech). Regulatory and effector T-Cells were also purified from draining lymph nodes and spleen of mice using MACS T-regulatory cell purification kit according to the manufacture's protocol. Purification of T-cell subpopulations was determined by flow cytometric analysis using specific antibodies to target cells. The efficiency of purification was >95%. The cells (2×105 in 200μl) were cultured in anti-CD3 antibody coated 96 well plates. The plates were incubated with anti-CD3 antibody (25μg/ml; 30μl/well) at 37°C for 90 min and then washed with PBS before adding the cell suspensions. Supernatants were harvested 48h later for quantification of cytokines by enzyme linked immunosorbent assay (ELISA) kits from Invitrogen using standard protocols. To check the specificity of T-cells against 2240 tumor cells, C3H/HeN mice were injected with the cell line subcutaneously as described above and 10 days later CD4+ and CD8+ T-cells were isolated from draining lymph nodes. CD11c+ cells were isolated from spleens of tumor bearing and naïve mice and later co-cultured with CD4+ and CD8+ T-cells from tumor bearing mice for 72h and cytokines detected by ELISA as described above.
RNA Extraction and Real-time RT-PCR
The total RNA was extracted from the mouse tumor samples using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The concentration of total RNA was determined by measuring the absorbance at 260 nm using a BioRad Smart Spec spectrophotometer. Purity of isolated RNA was determined with the ratio of absorbance 260nm/280nm >1.9. cDNA was synthesized from 1 μg RNA using Reverse transcriptase kit (Promega) according to the manufacturer's instructions.
Using iQ™ SYBR Green Master Mix (Biorad), cDNA was amplified by real-time PCR with a Bio-Rad MyiQ thermocycler and SYBR Green detection system (Bio-Rad) (23). The primers used for the following genes have been described: Foxp3 (24); T-bet (25); IL-12p35 and IL-12p40 (26); IL-23p19 (27) TGF-β (28); ROR-γt (29); GAPDH (30). The primers for STAT-3 Forward 5'-CAATACCATTGACCTGCCGAT-3' and Reverse 5'-GAGCGACTCAAACTGCCCT-3' (Accession no. BC003806); CD25 Forward 5'-ACTCCCATGACAAATCGAGAAAG-3' and reverse 5'-TCTCTTGGTGCATAGACTGTGT-3' (Accession no. NM_008367); IL-10 Forward 5'-GCTCTTACTGACTGGCATGAG-3' and Reverse 5'-CGCAGCTCTAGGAGCATGTG-3' (Accession no. NM_010548) were retrieved from Primer Bank (31). The standard PCR conditions were 95°C for 10 min and then 40 cycles at 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s. The expression levels of genes were normalized to the expression level of the GAPDH mRNA in each sample. For mRNA analysis, the calculations for determining the relative level of gene expression were made using the cycle threshold (Ct) method. The mean Ct values from duplicate measurements were used to calculate the expression of the target gene with normalization to a housekeeping gene used as internal control and using the formulae 2−ΔΔCT.
Statistical Analysis
The differences between experimental groups for CHS and cytokine ELISAs were analyzed using the Student's t-test. A simple ANOVA test followed by Tukey's test was employed for analysis of the tumors per group and the percentage of mice with tumors. In all cases, a p<0.05 was considered significant.
RESULTS
CD4+ T-cells cause UVB-induced local immunosuppression
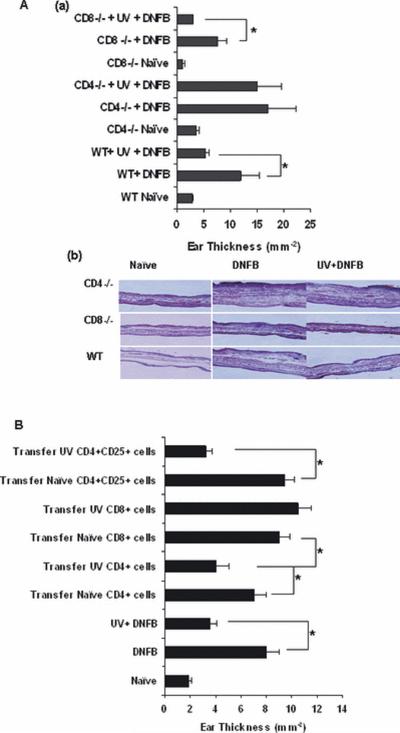
Initial studies were conducted to determine whether CD4+ or CD8+ T-cells are responsible for UVB-induced immunosuppression using CD4−/− and CD8−/− mice. We found that in WT C3H/HeN mice, as expected, the development of DNFB contact hypersensitivity was suppressed by UVB exposure (Figure 1A). In un-irradiated CD8−/− mice, the contact hypersensitivity response was reduced but not completely abolished. Similar to its effect on WT C3H/HeN mice, induction of contact hypersensitivity was significantly reduced (p<0.05) in UV-irradiated CD8−/− mice. The DNFB contact hypersensitivity response in CD4−/− mice was higher compared to WT mice. However, there was no difference in the contact hypersensitivity response to DNFB in UVB treated CD4−/− mice compared to non-UVB treated CD4−/− mice (Figure 1A). These data indicate that in this system CD4+ T-cells are responsible for UVB induced immunosuppression in mice.
Figure. 1.
(A) (a) The development of DNFB contact hypersensitivity responses in UVB treated and non-UVB treated WT, CD4−/− and CD8−/− mice. There were 3 mice per group and experiment was repeated twice with similar results. (b) Histology of ear sections from day 2 after DNFB application on ear as described in the immunosuppression protocol in materials and methods section. Paraffin sections were stained with H & E and pictures were taken to show the ear swelling in corresponding groups (B) The development of DNFB CHS responses after adoptive transfer of 1×107 CD4+ or CD8+ T-cells or 1×106 CD4+CD25+ regulatory T-cells (*, p<0.05). The experiments were repeated twice with similar results. The data are represented as mean ± SEM.
CD4+ T cells from UVB-irradiated mice suppress CHS responses by adoptive transfer
The CHS response was used to determine whether CD4+ T cells or CD8+ T-cells from UVB-irradiated mice could alter DNFB specific immune responses. As described in materials and methods section 1.0×107 CD4+ or CD8+ T-cells or 1.0×106 CD4+CD25+ regulatory T-cells were adoptively transferred via tail-vein injection into naive C3H/HeN mice. Irradiation of mice with UVB significantly suppressed the ear-swelling response by 50% as compared with responses observed in non-irradiated control mice (Figure. 1B). Adoptive transfer of 1.0×107 CD4+ T cells from UVB-irradiated mice significantly reduced ear swelling by 40% in comparison with responses in mice that had received CD4+ cells from non-irradiated mice (Figure 1B). Similarly, CD8+ T cells (1.0 ×107) from the UVB-irradiated mice did not reduce ear swelling and was comparable to the positive control. How ever transfer of regulatory T-cells (1.0 ×106) decreased the responses to 60% indicating that this subset of CD4+ T-cells is responsible for the UVB induced immunosuppression.
Cytokine production by T-cell response in UVB induced immunosuppression
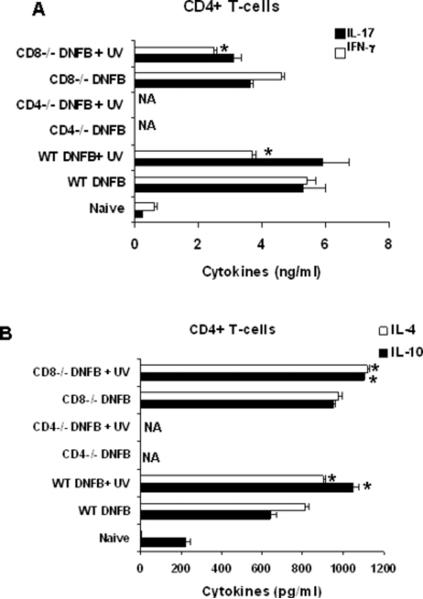
Since the adoptive transfer of CD4+ T-cells resulted in decrease of CHS response we got interested in the nature of cytokines these cells produce upon UVB exposure. The T-cells were purified from draining lymph nodes of the mice that had been UVB-irradiated and treated with DNFB as described in materials and methods. The cytokines in the CD4+ and CD8+ T-cell subpopulations were then examined by ELISA. CD8+ T-cells secreted higher levels of IFN-γ and smaller amounts of IL-17, as compared to CD4+ T-cells. More over CD4+ T-cells secreted significantly high levels of IL-4 and IL-10 while the CD8+ T-cells did not secrete detectable levels of these two cytokines (data not shown). Upon UVB exposure there was no effect on IFN-γ and IL-17 levels in CD8+ T-cells (data not shown). In case of CD4+ T-cells there was not any significant effect on the levels of IL-17 after UVB exposure in CD4+ T-cells (Figure 2 A). Interestingly, after UVB exposure CD4+ T-cells produced lower IFN-γ than the positive control indicating that the cause of immunosuppression being the lowering of IFN-γ (Figure 2 A). This decrease may be attributed to the elevated levels of IL-10 and IL-4 after UVB exposure in CD4+ T-cells (Figure 2 B).
Figure 2.
Cytokine profiles (A) IL-17 and IFN-γ (B) IL-10 and IL-4 of CD4+ T-cells isolated from draining lymph nodes of WT and CD8−/− mice after mice were subjected to UVB immunosuppression protocol. NA is not available. (*, p<0.05). Samples were pooled from each group with three mice and were run in duplicates and results are expressed as Mean± SD.
Enhanced development of UVB-induced skin cancer in CD8−/− mice
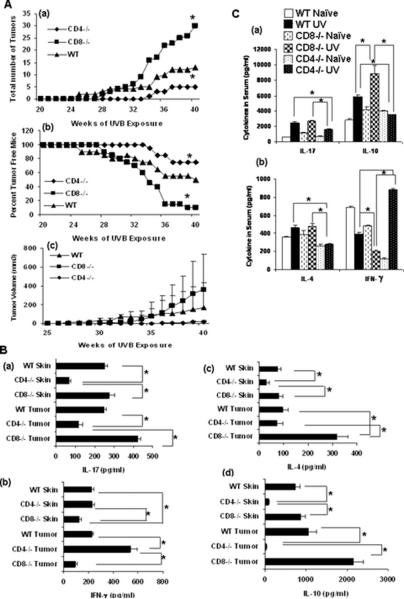
To determine the role of T-cells in the development of UV-induced cutaneous malignancies, groups of CD4−/−, CD8−/−, and WT mice were chronically irradiated with UVB on their shaved backs and tumor development was documented over time. Upon irradiation, the vast majority of CD8−/− mice developed UV-induced skin tumors. Significant numbers of WT mice also developed cutaneous tumors, but less than CD8−/− mice. In contrast, CD4−/− mice developed significantly few skin tumors after 40 weeks of UVB treatment (Figure. 3A a). The latency period for tumor development was 25 weeks in CD8−/− mice, 28 weeks in WT mice, and 34 weeks in CD4−/− mice. A similar pattern was observed for the percent tumor free mice in the three groups (Figure 3 A b). This pattern was also visible in the tumor volumes of the groups (Figure 3A c). These findings indicate that the CD4+ T-cells facilitate UV-induced skin carcinogenesis in mice, and CD8+ T-cells have a protective effect. We did not perform adoptive transfer experiments for photocarcinogenesis because it not be possible to interpret the results. After the adoptive transfer, the recipient mice still contain CD4 and CD8 T-cells. Chronic exposure to UVB radiation during photo-carcinogenesis protocol generates CD4+CD25+ regulatory T-cells. If we transfer CD4+ T-cells in CD8 knockout mice and then expose them to UVB, the mice will generate more CD4+ T-cells after exposure to UVB radiation. This would greatly complicate interpretation of results. This is why we turned to CD4 and CD8 knockout mice.
Figure 3.
(A) Mice were subjected to a complete UVB cutaneous carcinogenesis protocol (n=15) using 200mJ/cm2 for 40 weeks, (a) the number of tumors per group was plotted as a function of the number of weeks on the test, (b) the percent tumor free mice were plotted as a function of the number of weeks on the test and (c) the tumor volume was also plotted as a function of the number of weeks on the test. (*, p < 0.05) (B) Cytokine profiles of (a) IL-17 and (b) IFN-γ in (c) IL-4 and (d) IL-10 in tumor and skin lysates from CD4−/−, CD8−/−, and WT mice after 40 wk of UVB induced cutaneous carcinogenesis. Cytokine levels were measured by ELISA. (*p < 0.05). (C) Cytokine profiles of (a) IL-17 and IL-10 and (b) IFN-γ and IL-4 in the serum of CD4−/−, CD8−/−, and WT mice after 40 wk of UVB induced cutaneous carcinogenesis. (*p < 0.05). Samples from five mice per group were pooled and run in duplicates and results are expressed as mean±SD.
Analysis of cytokine production after photocarcinogenesis
After the end of photocarcinogenesis protocol, the cytokines in serum, skin and tumor lysates were examined by ELISA for production of IFN-γ, IL-4, IL-10 and IL-17 (Figure 3B&C). Compared to WT mice, there were high levels of IFN-γ, but significantly smaller amounts of IL-4, IL-10 and IL-17 in serum, skin, and tumor lysates of CD4−/− mice. In contrast, there were high levels of IL-4, IL-10 and IL-17 detected in these samples isolated from CD8−/− mice as compared to WT mice. However, there was significantly less IFN-γ present in the serum, skin and tumor samples of CD8−/− mice as compared to WT mice.
Regulation of UVB induced T-cell response at the transcriptional level
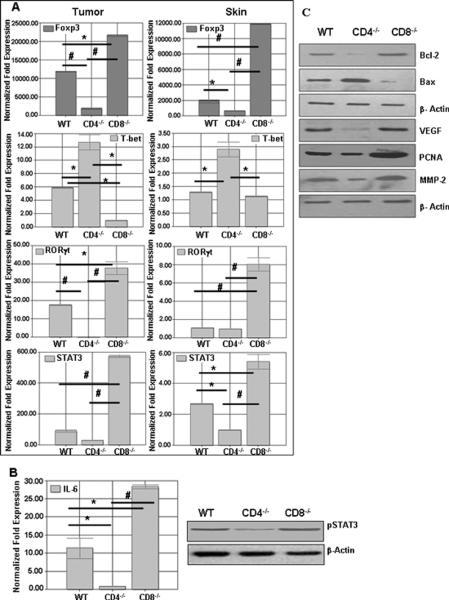
Previously it has been reported that STAT3 is important for development and differentiation of Th17 cells (32) and that T-bet negatively regulates IL-17 production by direct regulation of transcription of the IL-23R, and hence influences the outcome of Th17 cells, which depend on optimal IL-23 production for survival (33). In this study, we found that there were higher levels of STAT3 in CD8−/− mice and lower levels in CD4−/− mice, while WT mice had higher levels than CD4−/− mice and lower levels than CD8−/− mice (Figure 4 A). Further, the expression of ROR-γt that also supports Th-17 development was higher in CD8−/− mice as compared to WT or CD4−/− mice. Similarly, T-bet expression which is an important regulator of IFN-γ were lower in CD8−/− mice and higher in CD4−/− mice, while C3H/HeN mice had higher levels of T-bet than CD8−/− mice and lower levels than CD4−/− mice as predicted. These corroborated our cytokine data that CD4+ T-cells produce higher levels of IL-17 and CD8+ T-cells produce higher amounts of IFN-γ (Figure 4 A).
Figure 4.
(A) Expression of transcription factors from tumors and Skin of CD4−/−, CD8−/−, and WT mice after 40 wk of UVB induced cutaneous carcinogenesis. CD4−/− mice have higher levels of transcription factor T-bet, and CD8−/− mice have higher levels of STAT3 in their tumors. Also, CD8−/− mice show higher levels of Foxp3 when subjected to UVB induced cutaneous carcinogenesis. (*. p<0.05., #, p<0.01) (B) Higher levels of IL-6 and pSTAT3 in tumors of CD8−/− mice as compared to WT and CD4−/− mice. (C) Downstream molecules of STAT3 signaling were detected using western blotting. Proapoptotic and proangiogenic molecules were found to be highly expressed in CD8−/− mice tumors as compared to WT and CD4−/− mice tumors.
Next we wanted to investigate the levels of Foxp3 which is an important transcription factor of CD4+CD25+ regulatory T-cells. We found higher levels of Foxp3 in CD8−/− mice and lower levels in CD4−/− mice (Figure 4 A). The expression of Foxp3 in the case of CD4−/− mice may be due to CD8+Foxp3+ which have a role similar to CD4+FoxP3+ regulatory T-cells but are less frequent (34).
Promotion of tumor growth by IL-17 through the STAT3 and IL-6 pathway
Stat3 activation in tumor cells as well as tumor-associated inflammatory cells plays a critical role in tumor development (35) and since higher levels of IL-17 positively correlated with elevated STAT3 expression, indicating that IL-17 is activating STAT3. We observed more phospho-STAT 3 levels in tumors from CD8−/− mice compared with WT controls and CD4−/− mice (Figure 4B). In addition, STAT3 activity was drastically decreased in tumors grown in CD4−/− mice where there was higher T-bet and IFN-γ expression, suggesting an inhibitory role of IFN-γ on tumor STAT3 activation. Collectively, these results indicated that IL-17 enhances STAT3 activity while IFN-γ reduces tumor STAT3. Further analysis of downstream STAT3 genes indicated IL-17 expression affected these genes as well (Figure 4C). We found that Bcl-2 up-regulated in tumors from CD8−/− mice while Bax was higher in CD4−/− mice. In addition, vascular endothelial growth factor and PCNA were up-regulated in tumors from CD8−/− mice, and their expression was reduced in tumors from WT and CD4−/− mice (Figure 4 C). These results suggested that IL-17 promotes STAT3 activity in tumors, leading to up-regulation of antiapoptotic and angiogenic genes.
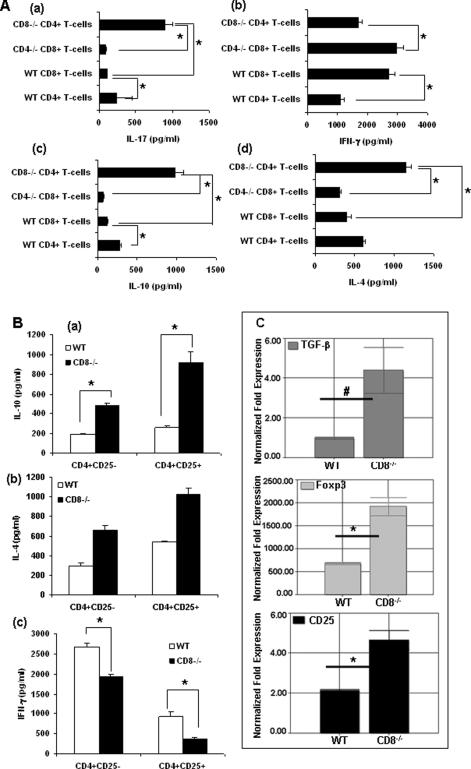
Cytokine production by T-cell response in UVB induced cutaneous carcinogenesis
To further analyze the types of cytokines produced by CD4+ and CD8+ T-cells after UVB treatment, T-cell suspensions were prepared from lymph nodes of mice on photocarcinogenesis protocol. There were low levels of IL-4, IL-17 (p<0.05) and IL-10 (p<0.001) and high levels of IFN-γ in supernatants from CD8+ cells derived from CD4−/− mice (Figure 5A). On the other hand, compared to CD4+ T-cells from WT mice, IL-4, IL-10 and IL-17 levels were elevated in CD4+ cells derived from CD8−/− mice, indicating that CD4+ T-cells were primarily responsible for the IL-4, IL-10 and IL-17 production. In contrast, in CD4+ T-cells from CD8−/− mice, low levels of IFN-γ concentrations (Figure 5 A b) were up regulated (p<0.05). Investigation of the expression of transcription factors in T-cells showed the same trend in the T-cells as was for tumor and skin samples (data not shown). Further effector T-cells (CD4+CD25−) and regulatory T-cells (CD4+CD25+) from WT and CD8−/− mice were purified and then stimulated with anti-CD3 antibody and IL-10, IL-4 and IFN-γ was measured in supernatants by ELISA. Regulatory as well as effector T-cells from CD8−/− mice secreted higher levels of immunosuppressive IL-10 and IL-4 than that of WT mice (Figure 5B). More IFN-γ was secreted by WT effector and regulatory T-cells than that of CD8−/− mice.
Figure 5.
(A) Cytokine profiles of T-cells isolated from draining lymph nodes of CD4−/−, CD8−/−, and WT mice after 40 wk of UVB induced cutaneous carcinogenesis. Cytokine levels were measured in supernatants by ELISA after 48h of CD3 capture. (*p < 0.05). There were three mice per group and results are expressed as mean ± SD. (B) Production of IL-10, IL-4 and IFN-γ from regulatory T-cells (Tregs) and T-effector cells from CD8−/−, and WT mice after 40 wk of UVB induced cutaneous carcinogenesis. Effector (CD4+CD25−) and regulatory T-cells (CD4+CD25+) purified from draining lymph nodes of the mice were then stimulated with anti-CD3 antibody. IL-10, IL-4 and IFN-γ were measured in supernatants by ELISA. (C) Expression of immunosuppressive TGF-β, CD25 and Foxp3 in regulatory T-cells from CD8−/− and WT mice by Real-time PCR.
TGF-β has an important role in the generation and expansion of regulatory T-cells. TGF-β induces CD4+ T cells to become CD4+ CD25+ T-cells, suppresses T-cell cytotoxic activity and together with. IL-10 induces alloreactive CD4+ CD25+ T cells (36). The regulatory T-cells secreted elevated levels of TGF-β in CD8−/− mice as compared to WT mice indicating more suppressive function of CD8−/− regulatory T-cells (Figure 5 C). Foxp3 is a key regulatory gene for the development and function of regulatory T-cells (36) and because of this we were interested in the expression of Foxp3 and CD25 on the regulatory T-cells. We found elevated levels of CD25 and Foxp3 on CD8−/− regulatory T-cells as compared to WT regulatory T-cells (Figure 5C).
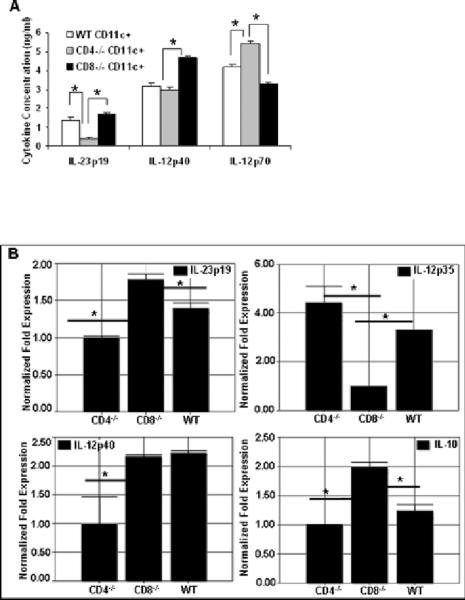
CD8−/−CD11c+ cells are poor IL-12 producers
Mouse spleens contain at least three subsets of DCs: CD8+ DC, CD4+ DC, and CD4−CD8−[double negative (DN)] DC. All these subsets vary in the expression of CD4 and CD8 receptors.
We observed higher expression of IL-12p70 (Figure 6 A) and lower expression of IL-10 (Figure 6 B) in the CD4−/− DC group which will have predominantly more CD8+ and CD8−CD4− subset while lower levels were observed in CD8−/− DC group which will be lacking CD8+CD4− subset (Figure 6). Previous findings support our data where they have found that CD8+CD4− DCs are efficient in polarizing the immune system to TH1 while the CD8− DCs polarize the immune system to TH2 (37, 38).
Figure 6.
(A) CD11c+ cells were isolated as described in materials and methods and ELISA was done for IL-12p40, IL-12p70 and IL-23p19. Higher levels of IL-23 were found in CD8−/− CD11c+ cells as compared to CD4−/−CD11c+ cells. (B) Real-time PCR analysis showing gene expression of IL-23p19, IL-12p35, IL-12p40 and IL-10 in CD11c+ cells isolated from WT, CD4−/− and CD8−/−mice.
CD4+ T-cells increase the growth of UV induced regressor tumors in mice
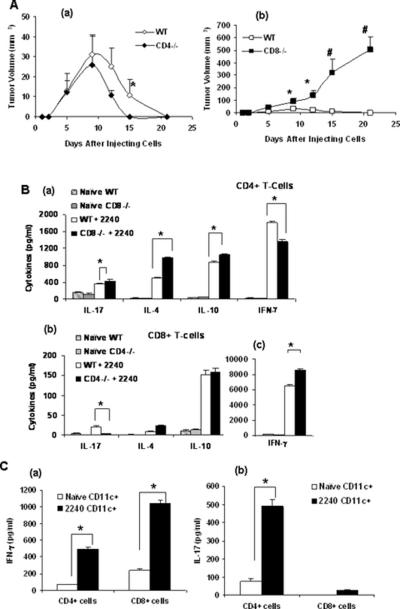
In addition to assessing the effects of CD4+ and CD8+ T-cells and the cytokines that they produce during photocarcinogenesis (i.e. skin cancer development), experiments were conducted to examine their role in the growth of tumors once they had developed. For this purpose, we used the UVB-induced UV-2240 cell line. Injection of the UV-2240 tumor cell line into WT mice gave rise to tumors that grew initially, and then began to regress after approximately 10 days and resolved in approximately 20 days (Figure 7A a). The tumors in the CD4−/− mice also followed a similar pattern, although were slightly smaller in size (Figure 7A a). In contrast, when the UV-2240 cell line was injected subcutaneously into CD8−/− mice, a large-sized tumor grew continuously without regression (Figure 7 A b). The spontaneous tumor regression in CD4−/− and WT mice and continuous progression in CD8−/− mice indicated that CD8+ T-cells have an anti-tumor effect and protect against tumor growth.
Figure 7.
(A) Injection of the UV-2240 tumor cell line into WT, CD4−/− and CD8−/− mice (a & b). Tumor growth was monitored till 3 weeks. The tumor in CD8−/− mice grew continuously without regression (*, p<0.05., #, p<0.01). (B) (a) Cytokines analysis (IL-17, IFN-γ, IL-10 and IL-4) of CD4+ T-cells from WT and CD8−/− mice after 3 weeks of UV-2240 injection. Cytokines analysis (b) (IL-17, IL-10 and IL-4) (c) IFN-γ of CD8+ T-cells from WT and CD4−/− mice after 3 weeks of UV-2240 injection. Purified T-cell suspensions were prepared from spleen and lymph nodes and were then re-stimulated in culture for 48 hours in anti-CD3 coated plates. (*, p<0.05) There were five mice per group and results are expressed as mean ± SD. T (C) To check the specificity of T-cells against 2240 tumor cells, WT mice were injected with the cell line subcutaneously and 10 days later CD4+ and CD8+ T-cells were isolated from draining lymph nodes. CD11c+ cells were isolated from spleens of tumor bearing and naïve mice and later co-cultured with CD4+ and CD8+ T-cells from tumor bearing mice for 72h and (a) IFN-γ or (b) IL-17 levels detected by ELISA.
Cytokine production by T-cell response in regression of subcutaneous UV-2240 tumors
The types of cytokines produced by CD4+ and CD8+ T-cells were examined approximately 3 weeks after development of UV-2240 tumors. Purified T-cell suspensions were prepared from spleen and lymph nodes and were then re-stimulated in culture for 48 hours in anti-CD3 coated plates. There were low levels of IL-17 (p<0.05) and high levels of IFN-γ in supernatants from CD8+ cells of CD4−/− mice. The IL-17 levels were elevated in CD4+ cells from CD8−/− mice, indicating that CD4+ T-cells were responsible for the IL-17 that was produced. In contrast, in CD4+ T-cells from CD8−/− mice, low levels of IFN-γ concentrations were observed (p<0.05) (Figure 7B). Further the levels of IL-4 and IL-10 were high in CD8−/− mice indicating that CD4+ cells secrete more IL-10 and IL-4 and that they have a part to play not only in immunosuppression but also in tumor inhibition.
To check the specificity of T-cells against 2240 tumor cells, CD4+ and CD8+ T-cells were isolated from draining lymph nodes and co-cultured with naïve and 2240 cell line specific CD11c+ cells. The T-cells that were co-cultured with 2240 specific CD11c+ cells produced more cytokines as compared to T-cells that were co-cultured with naïve CD11c+ cells indicating that the T-cells produced were tumor specific (Figure 7C).
DISCUSSION
Cutaneous exposure to UVB radiation modifies the initial events involved in cell-mediated immune responses culminating in a disproportionate increase in the development of regulatory T-cells. Primarily employing in vivo models of contact and delayed type hypersensitivity, the types of T-cells activated by UVB radiation have been characterized extensively. In murine models of contact hypersensitivity, they are CD4+, CD25+, CTLA-4 and produce the cytokine IL-10 (39, 40). In delayed type hypersensitivity studies employing Candida albicans and sheep red blood cells, they have been found to be NKT cells which display the DX5 marker, secrete large amounts of IL-4 and are TCRαβint (41). Photoimmunological perturbations have been exploited to treat a variety of immunologically mediated cutaneous diseases (42, 43), but have also been shown to facilitate the growth and development of UV-induced non-melanoma skin cancers (12, 44). The T-cells responsible for promoting the development of UVB induced tumors and their growth once they have occurred has received less attention. In one of the few studies that have been performed in this regard, NKT cells, when adoptively transferred to syngeneic recipients facilitated the growth of transplanted UV-induced tumors (41).
Employing CD4−/− and CD8−/− mice on a C3H/HeN background, we found striking similarities among the cells that are responsible for immunosuppression, tumor development, and tumor growth. All three biologic effects of ultraviolet radiation – immunosuppression, tumor development and tumor growth – are enhanced by CD4+ T-cells. On the other hand, CD8+ T-cells were found to impede both tumor development and growth. Despite the fact that CD4+ T-cells, which had the same cytokine profile, were responsible for all the immunosuppressive activities in contact hypersensitivity, tumor development and tumor growth, it is important to recognize that the growth and development of UV-induced tumors is a complex process and that the antigenic moieties to which they are directed may be different and upon further characterization may involve different subpopulations of these cells.
Similar to what was found in this study, CD4−/− mice on a C3H/HeN background were resistant, and CD8−/− mice were susceptible to tumor development when subjected to a DMBA chemical skin carcinogenesis protocol. CD4+ T-cells in those mice produced greater amounts of IL-4, IL-10, and IL-17, whereas CD8+ T-cells produced more IFN-γ (17). This suggests that at least in this strain of mice, that the mechanisms responsible for protection against tumor development and growth are common for both chemical and UV carcinogenesis.
In these studies, we observed that CD8+ T-cells that secrete IFN-γ are effector cells for host defenses against UVB induced skin cancers. A question that arises is how these CD8+ T-cells mediate their effects. It has been recently suggested that the relation between the immune system and tumors can be separated into three distinct stages-elimination, equilibrium, and escape (45, 46). It is tempting to speculate that one function of these CD8+ T-cells is to identify and eradicate the neoplastic cells. However, it is also possible that immune mechanisms might have a static effect on tumors, operating to control the proliferation of cells so they do not become clinically apparent tumors but not eradicating them either. By promoting the development of CD4+ T-cells, the photoimmunological effects of UV radiation may shift the balance to one of “escape”, in which tumor cell growth exceeds the capacity of the immune system to restrict it (45, 46).
There is convincing evidence that IL-10 enhances the development and growth of UV-induced tumors. Because CD4+ UV-induced regulatory T cells produce IL-10 upon stimulation, it was suggested that IL-10 has a regulatory function. UV radiation is able to inhibit the Ag-presenting function directly via UV-induced cytotoxicity as well as indirectly via the release of immunosuppressive cytokines such as IL-10 (3, 47). From several reports it was concluded that UV-induced expression of IL-10 contributes to the development of photocarcinogenesis by suppressing protective cellular immune responses.
In this study we found that both acute and chronic UVB exposure led to an increase in IL-17 producing cells in the skin, lymph nodes and in UV-induced tumors. TH17 cells, which produce IL-17, have been suggested to be important in the pathogenesis of a variety of autoimmune diseases, but an examination of their role in cancer has led to conflicting results. This cytokine has been reported to increase the growth of a number of tumors, at least in part because of its pro-angiogenic effects. However, in other systems, it has had a protective effect (21). The role that UVB radiation plays in the induction of IL-17 producing T-cells has received little attention, but in this study we observed that IL-17 production by T-cells was increased and that CD4+ T-cells, which promote UV-induced tumor growth and development, were primarily responsible. Additional experiments will need to be conducted to specifically evaluate the role of IL-17 in UVB-induced immunosuppression, tumor growth and development.
The findings of this study are consistent with what we know about the T-cell response to non-melanoma skin cancers in humans, the vast majority of which are caused by excessive exposure to ultraviolet radiation. Immunohistochemical studies have shown that CD4+CD25+Foxp3+ T-cells can be found intermingled with human basal cell carcinoma along with the TH2 cytokines IL-4 and IL-10. In the tumor microenvironment of cutaneous squamous cell carcinomas, increased IL-10, TGF-β and VEGF-A can be observed (48).
Several studies indicate that UVB induced suppression is unique and the immune profile of patients immunosuppressed by UVB radiation may also be different (49–53). Also, studies have also shown that there is a Th2 type predominance in human cutaneous squamous cell and basal cell carcinomas which is consistent with our findings (48, 54).
Furthermore, we plan to re-populate CD4+ T-cells from UVB treated and untreated mice in CD4−/− mice to generate chimeric mice and subject these mice to UVB induced tumor development. This will further enhance our understanding of how CD4+ T-cells from untreated mice behave differently from CD4+ T-cells from UVB treated mice (due to generation of CD4+CD25+ regulatory T-cells) in promoting UVB induced cutaneous tumors.
Our current observations support the concept that CD4+ and CD8+ T-cells have opposing actions in UV-induced tumor growth and development. CD4+ T-cells promote their growth and development whereas CD8+ T-cells have an important role to play in protective tumor immunity. Efforts to diminish regulatory CD4+ T-cells or to enhance the protective CD8+ T-cells may prove fruitful for the immunoprevention and/or therapy of this common type of neoplasm.
ACKNOWLEDGEMENTS
This work was supported by Pilot & Feasibility Study to NY from NIH funded UAB Skin Disease Research Center grant P30AR050948. The authors would like to thank Prof. Craig A Elmets, MD for his critical review of the manuscript.
REFERENCES
- 1.Elmets CA, Bergstresser PR. Ultraviolet radiation effects on immune processes. Photochem. Photobiol. 1982;36:715–719. doi: 10.1111/j.1751-1097.1982.tb09494.x. [DOI] [PubMed] [Google Scholar]
- 2.Ullrich SE. Sunlight and skin cancer: lessons from the immune system. Mol. Carcinog. 2007;46:629–633. doi: 10.1002/mc.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loser K, Apelt J, Voskort M, Mohaupt M, Balkow S, Schwarz T, Grabbe S, Beissert S. IL-10 Controls Ultraviolet-Induced Carcinogenesis in Mice. J. Immunol. 2007;179:365–371. doi: 10.4049/jimmunol.179.1.365. [DOI] [PubMed] [Google Scholar]
- 4.Zhou L, Nazarian AA, Smale ST. Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol. Cell Biol. 2004;24:2385–2396. doi: 10.1128/MCB.24.6.2385-2396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochem. Photobiol. 2008;84:10–18. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, DiIulio N, Fairchild R. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon γ-producing (Tc1) effector CD8+ T cells and interleukin (IL) 4/IL-10-producing (Th2) negative regulatory CD4+ T cells. J. Exp. Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda A, Beissert S, Schwarz T, Schwarz A. Phenotypic and functional characterization of ultraviolet radiation-induced regulatory T cells. J. Immunol. 2008;180:3065–3071. doi: 10.4049/jimmunol.180.5.3065. [DOI] [PubMed] [Google Scholar]
- 8.Penn I. Depressed immunity and skin cancer. Immunol. Today. 1984;5:291–293. doi: 10.1016/0167-5699(84)90152-X. [DOI] [PubMed] [Google Scholar]
- 9.Boyle J, MacKie RM, Briggs JD, Junor BJ, Aitchison TC. Cancer, warts, and sunshine in renal transplant patients. A case–control study. Lancet. 1984;1:702–705. doi: 10.1016/s0140-6736(84)92221-9. [DOI] [PubMed] [Google Scholar]
- 10.Euvrard S, Kanitakis J, Pouteil-Noble C, Claudy A, Touraine JL. Skin cancers in organ transplant patients. Ann. Transplant. 1997;2:28–32. [PubMed] [Google Scholar]
- 11.Kripke ML, Lofgreen JS, Beard J, Jessup JM, Fisher MS. In vivo immune responses of mice during carcinogenesis by ultraviolet irradiation. J. Natl. Cancer Inst. 1977;59:1227–1230. doi: 10.1093/jnci/59.4.1227. [DOI] [PubMed] [Google Scholar]
- 12.Fisher MS, Kripke ML. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogeneis. Proc. Natl. Acad. Sci. USA. 1977;74:1688–1692. doi: 10.1073/pnas.74.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeFabo EC, Kripke ML. Dose–response characteristics of immunologic unresponsiveness to UV-induced tumors produced by UV irradiation of mice. Photochem. Photobiol. 1979;30:385–390. doi: 10.1111/j.1751-1097.1979.tb07372.x. [DOI] [PubMed] [Google Scholar]
- 14.Cavanagh LL, Halliday GM. Dendritic epidermal T cells in ultravioletirradiated skin enhance skin tumor growth by inhibiting CD4+ T-cell-mediated immunity. Cancer Res. 1996;56:2607–2615. [PubMed] [Google Scholar]
- 15.Vanbuskirk A, Oberyszyn TM, Kusewitt DF. Depletion of CD8+ or CD4+ lymphocytes enhances susceptibility to transplantable ultraviolet radiation-induced skin tumors. Anticancer. Res. 2005;25:1963–1967. [PubMed] [Google Scholar]
- 16.Hatton JL, Parent A, Tober KL, Hoppes T, Wulff BC, Duncan FJ, Kusewitt DF, VanBuskirk AM, Oberyszyn TM. Depletion of CD4+ cells exacerbates the cutaneous response to acute and chronic UVB exposure. J. Invest. Dermatol. 2007;127:1507–1515. doi: 10.1038/sj.jid.5700746. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf N, Nasti TH, Katiyar SK, Jacobs MK, Seibert MD, Ginsburg AC, Timares L, Xu H, Elmets CA. Antagonistic roles of CD4+ and CD8+ T-cells in 7,12-dimethylbenz(a)anthracene cutaneous carcinogenesis. Cancer Res. 2008;68:3924–3930. doi: 10.1158/0008-5472.CAN-07-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penn I. Depressed immunity and skin cancer. Immunol. Today. 1984;5:291–293. doi: 10.1016/0167-5699(84)90152-X. [DOI] [PubMed] [Google Scholar]
- 19.Thatcher TH, Luzina I, Fishelevich R, Tomai MA, Miller RL, Gaspari AA. Topical imiquimod treatment prevents UV-light induced loss of contact hypersensitivity and immune tolerance. J Invest. Dermatol. 2006;126:821–831. doi: 10.1038/sj.jid.5700167. [DOI] [PubMed] [Google Scholar]
- 20.Kripke ML. Photoimmunology. Photochem. Photobiol. 1990;52:919–924. doi: 10.1111/j.1751-1097.1990.tb08703.x. [DOI] [PubMed] [Google Scholar]
- 21.Yusuf N, Nasti TH, Long JA, Naseemuddin M, Lucas AP, Xu H, Elmets CA. Protective Role of Toll-like Receptor 4 during the initiation stage of cutaneous chemical carcinogenesis. Cancer. Res. 2008;68:615–622. doi: 10.1158/0008-5472.CAN-07-5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ananthaswamy HN. Relationship between expression of tumor-specific transplantation antigens and neoplastic transformation in an ultraviolet radiation-induced murine skin cancer. Cancer Res. 1986;46:6322–6326. [PubMed] [Google Scholar]
- 23.Yusuf N, Nasti TH, Meleth S, Elmets CA. Resveratrol enhances cell-mediated immune response to DMBA through TLR4 and prevents DMBA induced cutaneous carcinogenesis. Mol. Carcinog. 2009;48:713–723. doi: 10.1002/mc.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YM, Zhang GY, Wang Y, Hu M, Wu H, Watson D, Hori S, Alexander IE, Harris DC, Alexander SI. Foxp3-transduced polyclonal regulatory T cells protect against chronic renal injury from adriamycin. J. Am. Soc. Nephrol. 2006;17:697–706. doi: 10.1681/ASN.2005090978. [DOI] [PubMed] [Google Scholar]
- 25.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 26.Honda K, Sakaguchi S, Nakajima C, Watanabe A, Yanai H, Matsumoto M, Ohteki T, Kaisho T, Takaoka A, Akira S, Seya T, Taniguchi T. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. 2003;100:10872–10877. doi: 10.1073/pnas.1934678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waibler Z, Kalinke U, Will J, Juan MHS, Pfeilschifter JM, Radeke HH. TLR-ligand stimulated interleukin-23 subunit expression and assembly is regulated differentially in murine plasmacytoid and myeloid dendritic cells. Molecular Immunology. 2007;44:1483–1489. doi: 10.1016/j.molimm.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz PA, Shkoda A, Kim SC, Sartor RB, Haller D. IL-10 gene-deficient mice lack TGF-beta/Smad-mediated TLR2 degradation and fail to inhibit proinflammatory gene expression in intestinal epithelial cells under conditions of chronic inflammation. Ann. Acad. Sci. N.Y. 2006;1072:389–394. doi: 10.1196/annals.1326.023. [DOI] [PubMed] [Google Scholar]
- 29.van Hamburg JP, de Bruijn MJ, Ribeiro de Almeida C, van Zwam M, van Meurs M, de Haas E, Boon L, Samsom JN, Hendriks RW. Enforced expression of GATA3 allows differentiation of IL-17-producing cells, but constrains Th17-mediated pathology. Eur. J. Immunol. 2008;38:2573–2586. doi: 10.1002/eji.200737840. [DOI] [PubMed] [Google Scholar]
- 30.Shibata M, Hariya T, Hatao M, Ashikaga T, Ichikawa H. Quantitative polymerase chain reaction using an external control mRNA for determination of gene expression in a heterogeneous cell population. Toxicol. Sci. 1999;49:290–296. doi: 10.1093/toxsci/49.2.290. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Research. 2003;31(e154):1–8. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 Direct development of IL-17-Secreting Th Cells1. J. Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 33.Gocke AR, Cravens PD, Ben LH, Hussain RZ, Northrop SC, Racke MK, Lovett-Racke AE. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J. Immunol. 2007;178:1341–1348. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- 34.Jiang L, Yang P, He H, Li B, Lin X, Hou S, Zhou H, Huang X, Aize K. Increased expression of Foxp3 in splenic CD8+ T cells from mice with anterior chamber-associated immune deviation. Mol. Vis. 2007;13:968–974. [PMC free article] [PubMed] [Google Scholar]
- 35.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 36.Fu S, Zhang N, Yopp AC, Chen D, Mao M, Chen D, Zhang H, Ding Y, Bromberg JS. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 − precursors. Am. J. Transplant. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 37.Schnorrer P, Behrens GMN, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, Davey G, Kupresanin F, Li M, Maraskovsky E, Belz GT, Carbone FR, Shortman K, Heath WR, Villadangos JA. The dominant role of CD8+dendritic cells in cross-presentation is not dictated by antigen capture. Proc. Natl. Acad. Sci. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 39.Atarashi K, Kabashima K, Akiyama K, Tokura Y. Skin application of the nonsteroidal anti-inflammatory drug ketoprofen downmodulates the antigen-presenting ability of Langerhans cells in mice. Br. J. Dermatol. 2008;159:306–313. doi: 10.1111/j.1365-2133.2008.08683.x. [DOI] [PubMed] [Google Scholar]
- 40.Ring S, Schäfer SC, Mahnke K, Lehr HA, Enk AH. CD4+ CD25+ regulatory T cells suppress contact hypersensitivity reactions by blocking influx of effector T cells into inflamed tissue. Eur. J. Immunol. 2006;36:2981–2992. doi: 10.1002/eji.200636207. [DOI] [PubMed] [Google Scholar]
- 41.Moodycliffe AM, Nghiem D, Clydesdale G, Ullrich SE. Immune suppression and skin cancer development: regulation by NKT cells. Nature Immunol. 2000;1:521–525. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- 42.Scherschun L, Kim JJ, Lim HW. Narrow-band ultraviolet B is a useful and well-tolerated treatment for vitiligo. J. Am. Acad. Dermatol. 2001;44:999–1003. doi: 10.1067/mjd.2001.114752. [DOI] [PubMed] [Google Scholar]
- 43.Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest. Dermatol. 1981;76:359–362. doi: 10.1111/1523-1747.ep12520022. [DOI] [PubMed] [Google Scholar]
- 44.Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression. Carcinogenesis. 2005;26:1657–1667. doi: 10.1093/carcin/bgi123. [DOI] [PubMed] [Google Scholar]
- 45.Dunn GP, Old LJ, Schreiber RD. The Three Es of cancer immunoediting. Annu. Rev. Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 46.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 47.Kitajima T, Ariizumi K, Bergstresser PR, Takashima A. Ultraviolet B radiation sensitizes a murine epidermal dendritic cell line (XS52) to undergo apoptosis upon antigen presentation to T cells. J. Immunol. 1996;157:3312–3316. [PubMed] [Google Scholar]
- 48.Kaporis HG, Guttman-Yassky E, Lowes MA, Haider AS, Fuentes-Duculan J, Darabi K, Ertelt J, Khatcherian A, Cardinale I, Novitskaya I, Krueger JG, Carucci JA. Human basal cell carcinoma is associated with Foxp3+ T cells in a Th2 dominant microenvironment. J. Invest. Dermatol. 2007;127:2391–2398. doi: 10.1038/sj.jid.5700884. [DOI] [PubMed] [Google Scholar]
- 49.Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982;216:1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- 50.Schwarz A, Maeda A, Wild MK, Kernebeck K, Gross N, Aragane Y, Beissert S, Vestweber D, Schwarz T. Ultraviolet radiation-induced regulatory T cells not only inhibit the induction but can suppress the effector phase of contact hypersensitivity. J. Immunol. 2004;172:1036–1043. doi: 10.4049/jimmunol.172.2.1036. [DOI] [PubMed] [Google Scholar]
- 51.Elmets CA, Bergstresser PR, Tigelaar RE, Wood PJ, Streilein JW. Analysis of mechanism of unresponsiveness produced by haptens painted on skin exposed to low dose ultraviolet radiation. J. Exp. Med. 1983;158:781–794. doi: 10.1084/jem.158.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwarz T. 25 years of UV-induced Immunosuppression Mediated by T Cells; From Disregarded T Suppressor Cells to Highly Respected Regulatory T Cells. Photochem. Photobiol. 2008;84:10–18. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 53.Beissert S, Hosoi J, Kühn R, Rajewsky K, Müller W, Granstein RD. Impaired immunosuppressive response to ultraviolet radiation in interleukin-10-deficient mice. J. Invest. Dermatol. 1996;107:553–557. doi: 10.1111/1523-1747.ep12582809. [DOI] [PubMed] [Google Scholar]
- 54.Kosmidis M, Dziunycz P, Suárez-Fariñas M, Mühleisen B, Schärer L, Läuchli S, Hafner J, French LE, Schmidt-Weber C, Carucci JA, Hofbauer GF. Immunosuppression affects CD4+ mRNA expression and induces Th2 dominance in the microenvironment of cutaneous squamous cell carcinoma in organ transplant recipients. J. Immunother. 2010;33:538–546. doi: 10.1097/CJI.0b013e3181cc2615. [DOI] [PubMed] [Google Scholar]