Abstract
BACKGROUND
Arterial calcifications are associated with increased cardiovascular risk, but the genetic basis of this association is unclear.
METHODS
We performed clinical, radiographic, and genetic studies in three families with symptomatic arterial calcifications. Single-nucleotide-polymorphism analysis, targeted gene sequencing, quantitative polymerase-chain-reaction assays, Western blotting, enzyme measurements, transduction rescue experiments, and in vitro calcification assays were performed.
RESULTS
We identified nine persons with calcifications of the lower-extremity arteries and hand and foot joint capsules: all five siblings in one family, three siblings in another, and one patient in a third family. Serum calcium, phosphate, and vitamin D levels were normal. Affected members of Family 1 shared a single 22.4-Mb region of homozygosity on chromosome 6 and had a homozygous nonsense mutation (c.662C→A, p.S221X) in NT5E, encoding CD73, which converts AMP to adenosine. Affected members of Family 2 had a homozygous missense mutation (c.1073G→A, p.C358Y) in NT5E. The proband of Family 3 was a compound heterozygote for c.662C→A and c.1609dupA (p.V537fsX7). All mutations found in the three families result in nonfunctional CD73. Cultured fibroblasts from affected members of Family 1 showed markedly reduced expression of NT5E messenger RNA, CD73 protein, and enzyme activity, as well as increased alkaline phosphatase levels and accumulated calcium phosphate crystals. Genetic rescue experiments normalized the CD73 and alkaline phosphatase activity in patients’ cells, and adenosine treatment reduced the levels of alkaline phosphatase and calcification.
CONCLUSIONS
We identified mutations in NT5E in members of three families with symptomatic arterial and joint calcifications. This gene encodes CD73, which converts AMP to adenosine, supporting a role for this metabolic pathway in inhibiting ectopic tissue calcification. (Funded by the National Human Genome Research Institute and the National Heart, Lung, and Blood Institute of the National Institutes of Health.)
Vascular calcification, arising either in the intima or media of vessels, is associated with an excess risk of cardiovascular events.1,2 This was initially considered a passive response to degenerative events, but mounting evidence suggests it is the result of a process that mimics active bone remodeling.3,4 Extracellular calcification is increasingly viewed as arising from a default biochemical pathway and requiring the constant stimulation of inhibitory systems to prevent its occurrence.
Only a single mendelian disorder of isolated vascular calcification — idiopathic infantile arterial calcification, now also referred to as generalized arterial calcification of infancy — has been described.5 This autosomal recessive disease, due to mutations in the ectonucleotide pyrophosphatase–phosphodiesterase 1 gene (ENPP1), often results in death during childhood, apparently owing to disruption of the trophic influences that inhibit vascular-cell calcification. We conducted a study to evaluate an adult-onset disorder in three families whose affected members had extensive calcifications of the lower-extremity arteries and small joint capsules and to investigate a possible genetic basis of the symptoms.
METHODS
PATIENTS
Three families were studied. Members of Family 1 and Family 3 were admitted to the National Institutes of Health (NIH) Undiagnosed Diseases Program and enrolled in clinical studies whose protocols had been approved by the institutional review board of the National Human Genome Research Institute. The genetic studies for Family 2 were approved by the institutional review board of Azienda Ospedaliera Universitaria San Giovanni Battista. All subjects provided written informed consent.
FIBROBLAST-CELL CULTURES
Fibroblast cultures were prepared from a 4-mm punch-biopsy skin specimen obtained from each patient and grown in Dulbecco’s modified Eagle’s medium containing 10% fetal-calf serum and 1% penicillin–streptomycin, as previously described.6 Cells were fed every other day and split 1:2 at confluence.
ANALYSIS OF SINGLE-NUCLEOTIDE POLYMORPHISMS (SNPS)
Genomic DNA in Family 1 was isolated from peripheral leukocytes and genotyped on a genotyping array (Human 1M Duo, Illumina). Homozygosity-allele plots were generated with the use of GenomeStudio software. Anomalous regions of homozygosity were identified visually and confirmed by means of haplotype imputation (ENT program). A lod score was established with the use of parametric multipoint linkage analysis, as previously described.7
MUTATION ANALYSIS OF CD73
Coding exons and intron–exon junctions of NT5E were amplified with the use of a touchdown polymerase-chain-reaction (PCR) assay of 50 ng of genomic DNA, 3 µM of sense and antisense oligonucleotides, and 5 µl of HotStart Master Mix (Qiagen) in a final volume of 10 µl. PCR products were sequenced in both directions with the use of the Big Dye terminator kit (version 1.1, Applied Biosystems) and an automated capillary sequencer (ABI PRISM 3130xl Genetic Analyzer, Applied Biosystems). We compared electrophoretogram-derived sequences with reference sequences for NT5E (Ensembl gene number ENSG00000135318) by using Sequencher software (version 4.8). Screening of 200 DNA samples from controls of white race (panel HD200CAU, Coriell Cell Repositories) was performed by means of the 5′–nuclease allelic discrimination (TaqMan) assay, as previously described.8 Details of the PCR amplification, primer sequences, and allelic discrimination assay are available in the Supplementary Appendix, available with the full text of this article at NEJM.org.
EXPRESSION STUDIES
RNA was isolated from cultured fibroblasts with the use of the RNeasy kit (Qiagen), and complementary DNA (cDNA) was generated from 1-µg RNA samples with the use of the SuperScript II Reverse Transcriptase kit (Invitrogen). Expression of NT5E was measured by means of a quantitative real-time PCR assay involving SYBR Green technology on a Chromo4 Real Time PCR Detection System (Bio-Rad). Expression levels were calculated on the basis of the 2−ΔCt method, in which the cycling threshold (Ct) of the candidate gene is compared with the Ct of 18S ribosomal RNA and expressed as a power of two (2(Ct of CD73−Ct of 18S)). Primer sequences for NT5E and 18S rRNA are provided in the Supplementary Appendix.
WESTERN BLOTTING FOR CD73
Western blotting methods are described in the Supplementary Appendix.
CD73 ENZYME ASSAY
Fibroblasts were washed with a solution of 2 mM magnesium chloride, 120 mM sodium chloride, 5 mM potassium chloride, 10 mM glucose, and 20 mM HEPES. Incubation buffer, consisting of the wash solution supplemented with 2 mM AMP, was added, and cells were incubated at 37°C for 10 minutes.9 The supernatant was removed, and inorganic phosphate was measured with the SensoLyte MG Phosphate Assay Kit (AnaSpec) according to the manufacturer’s instructions. Inorganic phosphate measurements were normalized to micrograms of protein.
CLONING OF MUTATIONS AND PRODUCTION OF LENTIVIRUS
The plasmid pCMV-Sport6 containing human CD73 cDNA was purchased from Open Biosystems. Mutations were introduced by means of the QuikChange XL Site-Directed Mutagenesis Kit (Stratagene). Primer sequences used for site-directed mutagenesis for each family are provided in the Supplementary Appendix.
Construct sequences were confirmed by sequencing in both directions. Since the pCMV-Sport6 vector contains Gateway cloning (Invitrogen) recombination sites, we used this cloning strategy to insert normal and mutated CD73 cDNA into the vector pLenti6.3/V5-DEST; lentivirus was generated with the use of the ViraPower HiPerform Lentiviral Gateway Expression Kit (Invitrogen). For transduction, viral particles were added to cells in growth medium containing 6 µg of polybrene per milliliter (Sigma). To select for cells transduced with virus, blasticidin (Invitrogen; 10 µg per milliliter) was added to growth medium 4 days after transduction.
TRANSFECTION INTO HEK293 CELLS
HEK293 cells (from human embryonic kidneys) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal-calf serum and 1% penicillin–streptomycin. One microgram of pCMV-Sport6 containing green fluorescent protein, wild-type NT5E cDNA, or mutated NT5E cDNA was transfected into HEK293 cells with the use of FuGENE 6 reagent (Roche) according to the manufacturer’s instructions. Three days after transfection, cells were analyzed for CD73 activity, as described above.
IN VITRO ALKALINE PHOSPHATASE AND CALCIUM ASSAYS
Staining for alkaline phosphatase was performed by means of the Alkaline Phosphatase Detection Kit (Millipore). Assay of alkaline phosphatase was performed with the use of the Quantitative Alkaline Phosphatase ES Characterization Kit (Millipore) according to the manufacturer’s instructions. Briefly, cells were trypsinized and collected in aliquots of 60,000 cells per reaction in p-nitrophenol–phosphate substrate. Alkaline phosphatase was quantified by measuring the amount of p-nitrophenol produced, as gauged by absorption at 405 nm.
A modified protocol for in vitro calcification was used for fibroblasts obtained from patients and controls.10,11 Cultures were treated with 0.1 µM dexamethasone, 50 µM ascorbic acid–2-phosphate, and 10 mM β-glycerol phosphate in alpha minimal essential medium supplemented with 10% fetal-calf serum and 1% penicillin–streptomycin for 21 days, with replenishment of the medium every 4 or 5 days. On day 21, cells were washed with phosphate-buffered saline and fixed in 10% formalin for 10 minutes. After washing with water, a solution of 2% alizarin red S, pH 4.2, was used to stain calcium phosphate crystals.12
RESULTS
CASE REPORTS
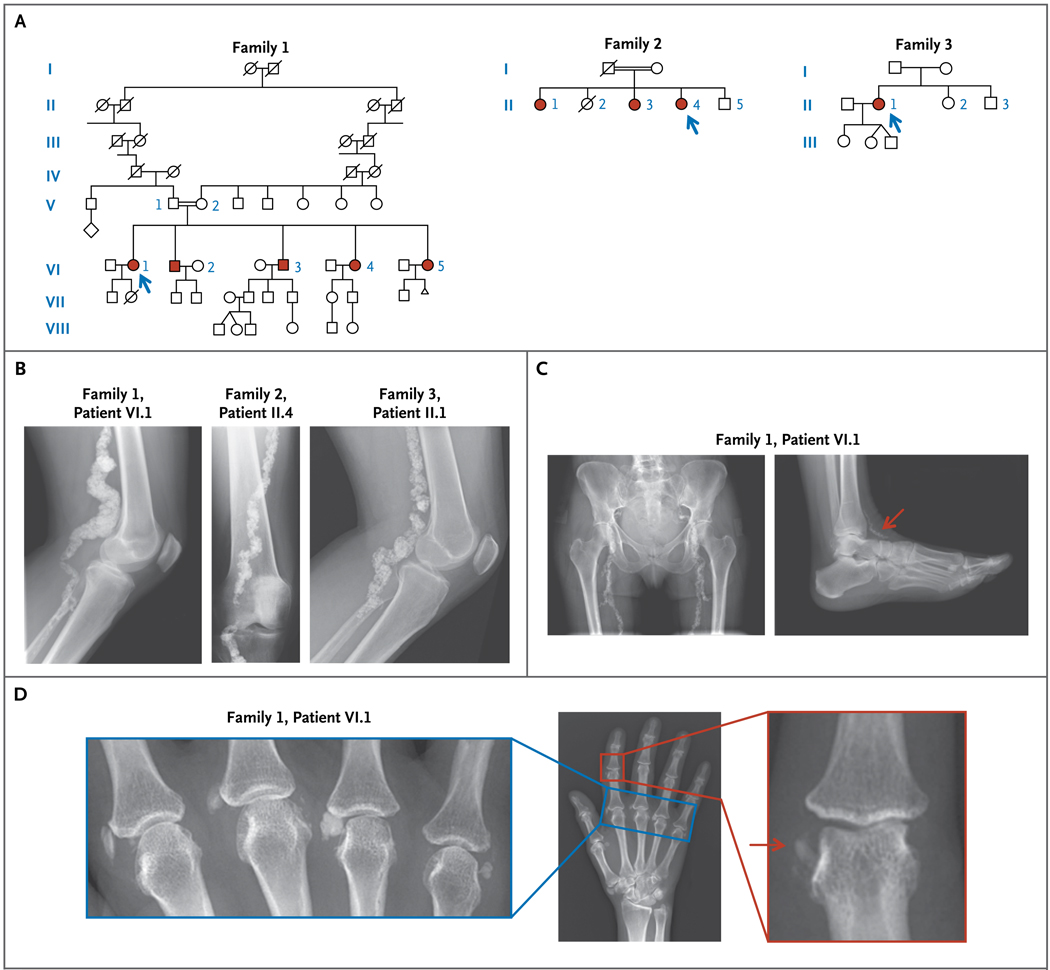
Full clinical details of the study families are provided in the Supplementary Appendix. Family 1 was of English descent. The proband (Fig. 1A), Patient VI.1, was a 54-year-old woman with a 20-year history of intermittent claudication of the calves, thighs, and buttocks and chronic ischemic pain in the feet at rest. Her parents were third cousins. On evaluation at the NIH, her ankle–brachial blood-pressure index values were markedly reduced, but the levels of serum calcium, phosphate, vitamin D, alkaline phosphatase, creatinine, and cholesterol, and other chemical values were normal (Table 1). Contrast-enhanced magnetic-resonance angiography revealed extensive occlusion of the iliac, femoropopliteal, and tibial arteries, with extensive collateralization. Plain radiography of the lower extremities revealed heavy calcification with areas of arteriomegaly (Fig. 1B and 1C); chest radiography revealed no vascular calcifications above the diaphragm. Radiography also revealed juxta-articular joint-capsule calcifications of the fingers (Fig. 1D), wrists, ankles, and feet.
Figure 1. Pedigrees of the Study Patients and Radiographic Findings.
Panel A shows the pedigrees of the three study families. Open symbols indicate unaffected family members, and solid red symbols affected members. Arrows indicate the probands. Squares indicate male family members, circles female members, slashes deceased members, and double horizontal lines consanguinity. The diamond indicates offspring of unknown number, and the triangle a lost pregnancy. Panel B shows plain radiographs of popliteal arteries of the three probands. Panel C shows plain radiographs of the pelvis and femurs (left) and ankle (right) of Patient VI.1 of Family 1, revealing calcified arteries (arrow). Panel D shows radiographs of metacarpal phalangeal and interphalangeal joint calcification (arrow) in Patient VI.1 of Family 1.
Table 1.
Clinical Characteristics of Affected Members of Family 1.*
| Characteristic | Patient VI.1 | Patient VI.2 | Patient VI.3 | Patient VI.4 | Patient VI.5 | Unaffected State |
|---|---|---|---|---|---|---|
| Age (yr) | 54 | 53 | 51 | 49 | 44 | |
| Sex | F | M | M | F | F | |
| Calcification | ||||||
| Coronary arteries | Normal | Moderate | Normal | Normal | Normal | Normal |
| Aorta | Normal | Normal | Normal | Normal | Normal | Normal |
| Iliac arteries | Calcified, occluded | Mildly calcified | Tortuous, mildly calcified | Minimally calcified | Calcified but not obstructed | Normal |
| Femoral arteries | Calcified, occluded; popliteal arteriomegaly | Calcified, occluded; femoropopliteal arteriomegaly | Calcified, occluded | Calcified, occluded; femoropopliteal arteriomegaly | Calcified, occluded; popliteal arteriomegaly | Normal |
| Tibial arteries | Calcified, occluded | Calcified | Normal | Calcified, occluded | Calcified, proximal occlusion | Normal |
| Diabetes mellitus | No | No | No | No | No | No |
| White cells (per mm3) | 3570 | 5200 | 7390 | 4150 | 3560 | 3980–10,040 |
| Hemoglobin (g/dl) | 13.5 | 15.7 | 13.9 | 12.4 | 12.3 | 11.2–15.7 |
| Calcium (mmol/liter) | 2.31 | 2.30 | 2.42 | 2.29 | 2.29 | 2.05–2.50 |
| Phosphate (mg/dl) | 3.8 | 3.2 | 3.7 | 3.8 | 3.4 | 2.5–4.8 |
| Alkaline phosphatase (U/liter) | 70 | 69 | 62 | 62 | 65 | 37–116 |
| Parathyroid hormone (pg/ml) | 102 | 59.4 | 18.6 | 39.7 | 24.9 | 16.0–87.0 |
| Vitamin D (pg/ml) | 72 | 55 | 37 | 53 | 58 | 18–78 |
| Creatinine (mg/dl) | 0.70 | 0.82 | 0.73 | 0.40 | 0.54 | 0.70–1.30 |
| Cholesterol (mg/dl) | 182 | 109 | 143 | 204 | 153 | <200 |
| Ankle–brachial blood-pressure index | ||||||
| Right | 0.4 | 0.8 | 0.7 | 0.3 | 0.7 | 1.0–1.3 |
| Left | 0.4 | 0.5 | 0.7 | 0.3 | 0.8 | 1.0–1.3 |
To convert values for calcium to milligrams per deciliter, divide by 0.250. To convert values for phosphate to millimoles per liter, multiply by 0.3229. To convert values for creatinine to micromoles per liter, multiply by 88.4. To convert values for cholesterol to millimoles per liter, multiply by 0.02586.
All four siblings of Patient VI.1 had disabling intermittent claudication (ability to walk only 1 to 6 blocks) and hemodynamically significant lower-extremity obstructive peripheral artery disease, with resting ankle–brachial blood-pressure index values between 0.3 and 0.8 (normal range, 1.0 to 1.3). All had extensive femoropopliteal occlusion evident on magnetic-resonance arteriography, with diffuse, mild aneurysmal remodeling in a pattern of arteriomegaly. Computed tomographic (CT) angiography in three of the siblings showed that the obstructive lesions were diffusely and heavily calcified. Whole-body CT scanning for calcium, performed in one sister (Patient VI.5), showed prominent circumferential vascular calcifications in the lower extremities; CT angiography revealed extensive vascular obstruction with diffuse calcification (see the video, available at NEJM.org).
The proband of Family 2 (Fig. 1A), Patient II.4, was a 68-year-old northern Italian woman whose mother’s surname was the same as that of some of her father’s relatives four generations ago. She reported having intense joint pain in her hands that was unresponsive to glucocorticoids administered from 14 to 27 years of age. Radiographs of the lower limbs revealed calcifications (Fig. 1B), initially diagnosed as chondrocalcinosis. Serum electrolyte, calcium, and cholesterol levels were normal. Two sisters, 73 and 70 years of age (Fig. 1A), also had lower-extremity pain and had vascular calcifications that were similar to those of the proband.
The proband of Family 3 (Fig. 1A), Patient II.1, was a 44-year-old woman with an English father and a French mother. At 42 years of age, mild paresthesias in the lower legs prompted an evaluation that revealed extensive calcifications of the distal arteries (Fig. 1B), with sparing of the carotid arteries, aorta, and coronary arteries. Extensive rheumatologic evaluations were negative. Concern about impending ischemia in the right leg prompted a femoral–popliteal bypass at 43 years of age. Serum C-reactive protein, cholesterol, lipid, calcium, phosphate, and vitamin D levels were within the normal range.
None of the nine affected patients and none of their parents or children had abnormal bone morphologic characteristics, type 2 diabetes mellitus, or decreased kidney function. The parents of the five siblings in Family 1 had no clinically significant calcifications in their lower extremities or joint capsules.
SNPs, MUTATION ANALYSES, AND EXPRESSION STUDIES
We identified biallelic nonsense, missense, and single-nucleotide insertion–frameshift mutations in the ecto-5′-nucleotidase gene NT5E, encoding the CD73 enzyme, which generates extracellular adenosine, directly downstream of ENPP1 in the extracellular ATP–degradation pathway.
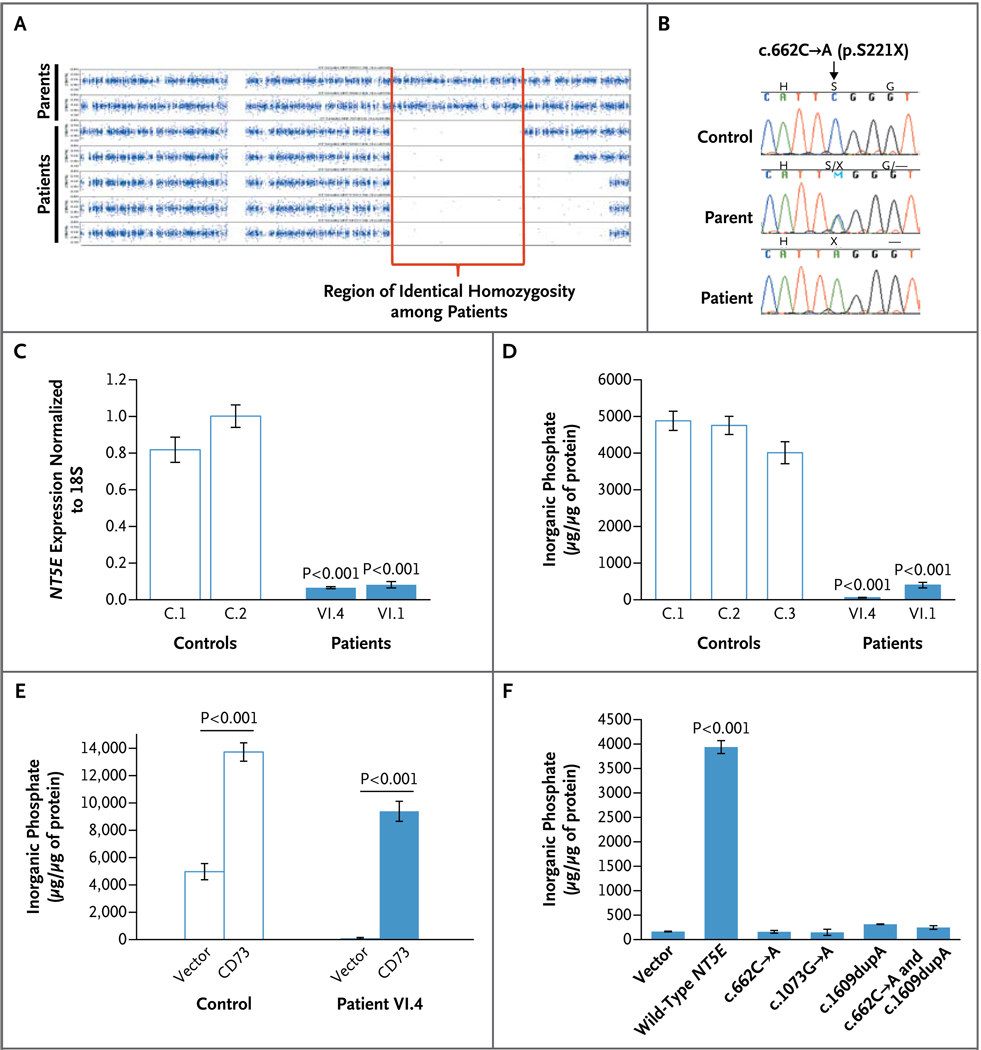
The consanguineous pedigree of Family 1, with disease confined to one generation (Fig. 1A), suggested autosomal recessive inheritance. Therefore, we searched for a region of the genome in which all five affected siblings were homozygous and identical by means of descent but in which both parents were heterozygous. There was only one such region in the entire genome: a 22.4-Mb region (Fig. 2A) on chromosome 6q14 (86,157,551 to 108,573,717 bp), containing 7977 genotyped SNPs and 92 genes. The lod score for the region in this family, calculated with the use of parametric multipoint linkage analysis, was 4.81.
Figure 2. Results of Genetic and Enzyme Studies in Family 1.
Panel A shows single-nucleotide polymorphism (SNP)–array homozygosity plots for the five affected siblings in Family 1 and their parents. The five affected siblings shared a region in which heterozygosity was absent, reflecting identity by means of descent in this consanguineous family. Panel B shows sequence chromatograms for a control, a parent of an affected member of Family 1, and an affected member; the nonsense mutation (p.S221X) is indicated. Panel C shows NT5E messenger RNA (complementary DNA [cDNA]) expression in Patients VI.4 and VI.1 as compared with controls. Panel D shows a deficiency in CD73 enzyme activity (represented by AMP-dependent inorganic phosphate production) in cultured fibroblasts from Patient VI.4 and Patient VI.1 of Family 1. Panel E shows CD73 enzyme activity in fibroblasts from controls and from Patient VI.4 after the fibroblasts were transduced with either an empty vector (containing green fluorescent protein only) or a CD73-containing vector. The CD73 vector increased CD73 activity (represented by AMP-dependent inorganic phosphate production) in both the control cells and the patients’ cells. Panel F shows CD73 activity (represented by AMP-dependent inorganic phosphate production) in HEK293 cells transfected with an empty vector or a vector containing wild-type NT5E or NT5E with the c.622C→A, c.1073G→A, or c.1609dupA mutation or both the c.622C→A and c.1609dupA mutations (in a 1:1 ratio, simulating the state in Patient II.1 of Family 3). In Panels C, D, and F, data are the means of three experiments and were analyzed with the use of one-way analysis of variance with Dunnett’s multiple-comparison post hoc test. In Panel E, data are the means of three experiments and were analyzed with the use of an unpaired Student’s t-test. P values are shown for the comparison with controls in Panels C and D and with the various mutated cDNA in Panel F. In Panels C through F, I bars indicate the standard deviations.
Of the 92 genes in this region, 3 were evaluated: ATG5 and CASP8AP2 because they are involved in degenerative cellular processes that could lead to calcification, and NT5E because its enzyme substrate is the product of ENPP1, mutations in which cause arterial calcifications in infants.5 Direct sequencing identified a homozygous nonsense mutation (c.662C→A, resulting in p.S221X) in exon 3 of the NT5E gene in all five siblings of Family 1 and the same nonsense mutation in the heterozygous state in both parents (Fig. 2B). Quantitative PCR analysis documented decreased expression of NT5E messenger RNA in the fibroblasts of Patients VI.1 and VI.4 in Family 1 (Fig. 2C). Affected members of Family 2 were homozygous for a missense mutation, c.1073G→A (p.C358Y), in exon 5 of NT5E; the mother was heterozygous for this variant of the amino acid, which is conserved across 16 species (Fig. 1A in the Supplementary Appendix). The affected member of Family 3 was a compound heterozygote for the c.662C→A nonsense mutation found in Family 1 and a c.1609dupA (V537fsX7) mutation leading to a premature stop codon in exon 9 of NT5E (Fig. 1B in the Supplementary Appendix). None of these mutations was present in 400 alleles in ethnically matched controls.
PROTEIN AND ENZYME ACTIVITY
NT5E encodes CD73, a membrane-bound ecto-5′-nucleotidase (specifically, 5′–ribonucleotide phosphohydrolase; EC 3.1.3.5) involved in extracellular ATP metabolism. The enzyme preferentially binds AMP and converts it to adenosine and inorganic phosphate.13
Protein analysis involving Western blotting of fibroblast extracts from Patients VI.1 and VI.4 of Family 1 revealed markedly reduced expression of CD73 protein, as compared with normal controls (Fig. 2 in the Supplementary Appendix). An enzymatic assay of CD73 in fibroblasts from our patients revealed nearly absent activity (Fig. 2D); values for fibroblasts from the patients’ parents were approximately 72% of the control level (Fig. 3 in the Supplementary Appendix). Genetic rescue with a CD73-encoding lentiviral vector reestablished normal AMP-dependent inorganic phosphate production (Fig. 2E). The enzymatic activities of normal and mutant CD73 constructs were tested in HEK293 cells, which have low endogenous CD73 activity. Transfection with normal NT5E cDNA resulted in abundant CD73 activity, whereas transfection with the c.662C→A NT5E, c.1073G→A NT5E, or c.1609dupA NT5E yielded negligible production of AMP-dependent inorganic phosphate (Fig. 2F).
CELLULAR STUDIES
A key enzyme in tissue calcification in vitro and in vivo is tissue-nonspecific alkaline phosphatase (TNAP).14 After 3 days of calcific stimulation, fibroblasts from Patient VI.4 of Family 1 stained abundantly for TNAP, as compared with control cells; treatment with adenosine substantially reduced the amount of TNAP staining (Fig. 3A). TNAP activity was also assayed in the lysates of fibroblasts grown in calcifying medium. As compared with control cells, cells from Patient VI.4 showed high levels of TNAP that were significantly reduced after transduction with CD73-encoded lentiviral vector or by adenosine treatment (Fig. 3B). Three weeks of calcific stimulation resulted in abundant calcium phosphate crystal formation in mutant fibroblasts (from Patient VI.4) but no formation in normal fibroblasts (Fig. 3C). Calcium phosphate crystal formation was prevented in cells transduced with a CD73-encoding lentiviral vector but not control vector expressing β-galactosidase. Adenosine treatment largely abrogated the calcification process, and the noncompetitive alkaline phosphatase inhibitor levamisole completely prevented calcification in the mutant cells (Fig. 3C).
Figure 3. Studies of Fibroblasts Obtained from Patient VI.4 of Family 1.
Panel A shows the results of staining for tissue-nonspecific alkaline phosphatase (TNAP) in fibroblasts from a control and from Patient VI.4 after incubation for 3 days in calcifying medium. The increased staining in the patient’s cells (middle image) was reduced by adding 30 µM adenosine to the incubation medium daily (right image). Panel B shows TNAP activity in fibroblasts from a control and from Patient VI.4 after incubation for 3 days in calcifying medium. Transduction with a control vector expressing β-galactosidase had little effect on alkaline phosphatase activity, whereas transduction with a CD73-encoding vector reduced alkaline phosphatase activity significantly; incubation in 30 µM adenosine produced TNAP levels similar to those seen in control cells. The data shown are the means of three experiments and were analyzed by means of analysis of variance with Dunnett’s multiple-comparison post hoc test. P values are shown for the comparison with no treatment. I bars indicate standard deviations. Panel C shows the effects of interventions on calcium phosphate crystal formation in fibroblasts from a control and from Patient VI.4. Staining for calcium with alizarin red S showed abundant staining in the patient’s cells, as compared with no staining in control cells, after 21 days in calcifying medium. Calcium staining was prevented by transduction with a CD73-encoding lentiviral vector, but not a control vector expressing β-galactosidase, and by treatment every fourth day with 1 mM levamisole; daily treatment with 30 µM adenosine partially abrogated the calcification process.
DISCUSSION
Medial arterial calcification of the lower extremities with periarticular calcification was described first by Magnus-Levy15 in 1914 and again by Levitin16 in 1945. The familial nature of this condition was first suggested in a report on two affected siblings by Sharp17 in 1954, leading to a subsequent record in the Online Mendelian Inheritance in Man database (OMIM number, 211800). Other, single cases were described by Nosaka and colleagues18 and Mori and coworkers,19 yielding a total of seven cases published to date. Here, we describe the molecular and enzymatic basis of this disorder in nine patients with three different mutations in NT5E.
Considerable evidence supports the association of these families’ vascular disease with mutations in the NT5E gene. The results of segregation analysis were consistent among our families, and the nonsense mutation (p.S221X) and single-nucleotide insertion (p.V537fsX7) predict truncated CD73 proteins. The missense mutation (p.C358Y), which was not found in 200 unaffected persons, predicts a pathologic change in an amino acid conserved through evolution and is located in the critical nucleotidase domain of CD73. Furthermore, the nonsense mutation resulted in markedly reduced levels of CD73 mRNA and protein in cultured cells. Enzyme activity was virtually absent in fibroblasts from affected members of Family 1 and was rescued by transduction of a lentiviral vector expressing NT5E. Each of the three different mutations in the three families produced essentially nonfunctional CD73.
CD73 participates in the extracellular pathway that converts ATP to adenosine on the surface of various types of cells, as follows. First, ENPP1 produces AMP and pyrophosphate from ATP; then CD73 produces adenosine and inorganic phosphate from AMP (Fig. 4). Cellular calcification depends critically on levels of pyrophosphate, a strong inhibitor of calcification, and TNAP, which degrades pyrophosphate.14 In patients with hypophosphatasia due to TNAP deficiency, increased levels of pyrophosphate result in defective bone mineralization.20 In patients with generalized arterial calcification of infancy, ENPP1 deficiency leads directly to decreased pyrophosphate levels,21 causing early-onset vascular calcification, myocardial infarction, and often death in infancy.5 In our adult patients, CD73 deficiency may not lead directly to decreased pyrophosphate levels, but the consequent reduction in extracellular adenosine levels apparently enhances TNAP activity; adenosine supplementation reversed the increase in TNAP activity in CD73-deficient cells. We hypothesize that increased TNAP activity reduces pyrophosphate levels, leading to calcification; indeed, levamisole, an inhibitor of TNAP, prevented calcium crystal formation by CD73-deficient fibroblasts. The selective involvement of lower-extremity arteries may be related to the particular distribution of adenosine receptors in these tissues.22
Figure 4. Proposed Mechanism of Mineralization Due to CD73 Deficiency from an NT5E Mutation.
On the surface of vascular cells, ENPP1 (the protein encoded by the ectonucleotide pyrophosphatase–phosphodiesterase 1 gene) converts ATP to AMP and pyrophosphate (PPi), and CD73 converts AMP to adenosine and inorganic phosphate (Pi). Pyrophosphate inhibits calcification, tissue-nonspecific alkaline phosphatase (TNAP) degrades pyrophosphate, and adenosine inhibits TNAP. Deficiency of CD73 results in decreased adenosine levels, eliminating the inhibition of TNAP from the pathway either directly or by way of adenosine receptor signaling. Increased TNAP from the pathway activity results in decreased pyrophosphate and increased cell calcification.
Knowledge of the basic defect in our patients allows for consideration of therapeutic interventions. Bisphosphonates, which are pyrophosphate analogues and potent inhibitors of tissue calcification, have been successfully used to treat ENPP1 deficiency and might prove beneficial in patients with CD73 deficiency.23,24 Dipyridamole, an antithrombotic drug used successfully in patients with aneurysmal vascular remodeling, could provide adenosine rescue, since it inhibits cellular reuptake of adenosine (and subsequent degradation by adenosine deaminase) both in vitro and in vivo.25 Other therapeutic possibilities include the use of adenosine-receptor agonists or a direct inhibitor of TNAP such as lansoprazole.26,27 The potential efficacy of such interventions can be investigated in cultured cells, which exhibit both TNAP and calcification phenotypes that are abrogated by transduction with a CD73-encoded lentiviral vector. Mice with CD73 deficiency can also be studied, if a calcification phenotype can be discerned,28,29 to elucidate the role of adenosine in regulating vascular calcification, influencing bone mineralization, and modulating ectopic calcium deposition.
In summary, we identified mutations in NT5E in members of three families with symptomatic arterial and joint calcifications. This gene encodes CD73, a nucleotidase that converts AMP to adenosine. Thus, our results support a role for this metabolic pathway in inhibiting ectopic tissue calcification.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Programs of the National Human Genome Research Institute (NHGRI) and the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health.
We thank Annette Stine, Victor Wright, Anne Madeo, Dr. Michael Collins, and the referring physician, Dr. Karen B. Saylor, for the evaluation of Family 1; the NHGRI Genomics Core for providing data on SNPs, and Dr. Christian A. Combs and Dr. Daniela Malide at the NHLBI Light Microscopy Core for providing the microscopical images.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 2.Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification: a neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 3.Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res. 2004;95:1046–1057. doi: 10.1161/01.RES.0000149165.99974.12. [DOI] [PubMed] [Google Scholar]
- 4.Boström K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutsch F, Ruf N, Vaingankar S, et al. Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet. 2003;34:379–381. doi: 10.1038/ng1221. [DOI] [PubMed] [Google Scholar]
- 6.Normand J, Karasek MA. A method for the isolation and serial propagation of keratinocytes, endothelial cells, and fibroblasts from a single punch biopsy of human skin. In Vitro Cell Dev Biol Anim. 1995;31:447–455. doi: 10.1007/BF02634257. [DOI] [PubMed] [Google Scholar]
- 7.Bockenhauer D, Feather S, Stanescu HC, et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 9.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding HT, Wang CG, Zhang TL, Wang K. Fibronectin enhances in vitro vascular calcification by promoting osteoblastic differentiation of vascular smooth muscle cells via ERK pathway. J Cell Biochem. 2006;99:1343–1352. doi: 10.1002/jcb.20999. [DOI] [PubMed] [Google Scholar]
- 11.Hoemann CD, El-Gabalawy H, McKee MD. In vitro osteogenesis assays: influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol Biol (Paris) 2009;57:318–323. doi: 10.1016/j.patbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87:1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 13.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hessle L, Johnson KA, Anderson HC, et al. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycon protein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnus-Levy A. Ueber ungewöhnliche Verkalkung der Arterien (Arterien-verkalkung ohne primäre Arteriosklerose?) Dtsch Med Wochenschr. 1914;40:1305–1309. [Google Scholar]
- 16.Levitin J. A case of arterial and periarticular calcinosis of unknown etiology. Radiology. 1945;44:489–494. [Google Scholar]
- 17.Sharp J. Heredo-familial vascular and articular calcification. Ann Rheum Dis. 1954;13:15–27. doi: 10.1136/ard.13.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nosaka H, Yamasaki G, Yamamoto K, Hayashi Y. A case of extensive calcification of the peripheral arteries. Yonago Acta Med. 1974;18:191–197. [Google Scholar]
- 19.Mori H, Yamaguchi K, Fukushima H, et al. Extensive arterial calcification of unknown etiology in a 29-year-old male. Heart Vessels. 1992;7:211–214. doi: 10.1007/BF01744607. [DOI] [PubMed] [Google Scholar]
- 20.Henthorn PS, Raducha M, Fedde KN, Lafferty MA, Whyte MP. Different missense mutations at the tissue-nonspecific alkaline phosphatase gene locus in autosomal recessively inherited forms of mild and severe hypophosphatasia. Proc Natl Acad Sci U S A. 1992;89:9924–9928. doi: 10.1073/pnas.89.20.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmey D, Hessle L, Narisawa S, Johnson KA, Terkeltaub R, Millán JL. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. 2004;164:1199–1209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang D, Zhang Y, Nguyen HG, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramjan KA, Roscioli T, Rutsch F, Sillence D, Munns CF. Generalized arterial calcification of infancy: treatment with bisphosphonates. Nat Clin Pract Endocrinol Metab. 2009;5:167–172. doi: 10.1038/ncpendmet1067. [DOI] [PubMed] [Google Scholar]
- 24.Rutsch F, Böyer P, Nitschke Y, et al. Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet. 2008;1:133–140. doi: 10.1161/CIRCGENETICS.108.797704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi E, Maeda T, Shinozuka K. Adenosine and dipyridamole: actions and interactions on the contractile response of guinea-pig ileum to high frequency electrical field stimulation. Br J Pharmacol. 1985;84:765–771. doi: 10.1111/j.1476-5381.1985.tb16159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts S, Narisawa S, Harmey D, Millán JL, Farquharson C. Functional involvement of PHOSPHO1 in matrix vesicle-mediated skeletal mineralization. J Bone Miner Res. 2007;22:617–627. doi: 10.1359/jbmr.070108. [DOI] [PubMed] [Google Scholar]
- 27.Delomenède M, Buchet R, Mebarek S. Lansoprazole is an uncompetitive inhibitor of tissue-nonspecific alkaline phosphatase. Acta Biochim Pol. 2009;56:301–305. [PubMed] [Google Scholar]
- 28.Koszalka P, Ozüyaman B, Huo Y, et al. Targeted disruption of cd73/ecto-5′-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ Res. 2004;95:814–821. doi: 10.1161/01.RES.0000144796.82787.6f. [DOI] [PubMed] [Google Scholar]
- 29.Castrop H, Huang Y, Hashimoto S, et al. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Invest. 2004;114:634–642. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.