Abstract
Purpose
To determine the safety, target inhibition, and signals of clinical activity of tipifarnib in combination with bortezomib in patients with advanced acute leukemias.
Experimental Design
In a 3+3 design, patients received escalating doses of tipifarnib (days 1–14) and bortezomib (days 1, 4, 8, 11) every 3 weeks until maximum tolerated dose was reached. Peripheral blood mononuclear cells (PBMCs) were collected at days 1, 8, and 22 for measurement of chymotrypsin-like and farnesyltransferase activity. Purified bone marrow leukemic blasts were collected at baseline and at day 8 for measurement of NF-kB activity.
Results
The combination was well-tolerated, and maximum tolerated dose was not reached. Dose-limiting toxicities included diarrhea, fatigue, and sensorimotor neuropathy. Chymotrypsin-like and farnesyltransferase activity within PBMCs were decreased in a majority of patients at day 8. NF-kB activity within leukemic blasts was decreased in a majority of patients at day 8. Complete response with incomplete count recovery was observed in 2 patients, and an additional 5 patients had stable disease.
Conclusions
Tipifarnib and bortezomib combination in patients with advanced leukemias was well-tolerated, demonstrated relevant target inhibition, and was associated with signals of clinical activity in patients with advanced and refractory acute leukemias. Future studies of this combination may be warranted in more selected groups of patients in whom these molecular targets are of particular importance.
Keywords: acute leukemia, NF-kappa B, farnesyltransferase inhibitor, proteasome inhibitor, phase 1
INTRODUCTION
Despite recent advances in therapy for adult patients with acute leukemias, the overall cure rate remains only 25–30%. Outcomes are worse in older patients, patients who evolved from previous myelodysplastic syndromes, and patients whose disease is linked to environmental and occupational exposures (1, 2). These patients frequently relapse, and the remission duration is generally short. Thus, new strategies, including combinations of agents with non-overlapping mechanisms of action and toxicities, are needed to improve clinical outcomes in adult leukemia patients, especially in those who are refractory to current standard treatment regimens.
Bortezomib (PS-341, Velcade, Millennium Pharmaceuticals, Cambridge, MA) is a reversible inhibitor of the chymotrypsin-like activity of the proteasome, a key complex in the ubiquitin-proteasomal system (UPS). The UPS is responsible for the degradation of proteins involved in the regulation of important physiological processes such as cell cycle division, cell death, and apoptosis (i.e., p53, Bax, and IκBα). For example, inhibition of the proteasome results in decreased degradation of IκBα levels and leads to inactivation of nuclear factor κB (NF-κB), which regulates many genes that code for mediators of immune responses, inflammatory responses, and cell survival. NF-κB is constitutively active in acute myelogenous leukemia (AML) (3), chronic myelogenous leukemia (CML), and acute lymphoblastic leukemia (ALL) progenitor cells, making it an attractive target in these diseases. Preclinical studies have also demonstrated that inhibition of NF-κB with a proteasome inhibitor induces rapid cell death in leukemic progenitor cells with little effect on normal stem cells (3). Other critical regulatory proteins thought to be regulated by the UPS include cyclins, cyclin-dependent kinases, and p53 (4, 5). Although bortezomib currently has FDA-approved indications only for the treatment of multiple myeloma and mantle cell lymphoma, it has also been studied in refractory acute leukemias. It has been shown to be well-tolerated as a single agent in a phase I study that also demonstrated proteasomal inhibition (6).
Tipifarnib (R115777, Zarnestra, Johnson & Johnson) is an orally available selective inhibitor of farnesyltransferase (FTase), an enzyme that catalyzes the transfer of a 15 carbon farnesyl moiety at a cysteine near the carboxyl terminus of a polypeptide, with the ability to disrupt the prenylation of Ras and many other intracellular proteins, including Rheb and Lamin proteins (7–9). FTase inhibitors have also been shown to target the phosphoinositide 3-OH kinase/Akt pathway (10), which appears to be critical in the transcription activation of NF-κB (11). Multiple clinical studies have evaluated tipifarnib in AML, with complete response rates in the range of 10–15% (12–14). A pivotal phase III trial, however, did not demonstrate a survival advantage for elderly patients with newly diagnosed AML treated with tipifarnib versus best supportive care (15).
Because bortezomib and tipifarnib act upon distinct, non-overlapping targets, a combination of these agents was evaluated for synergistic anti-tumor activity; Yanamandra et al have previously demonstrated synergistic activity between tipifarnib and bortezomib in multiple myeloma and AML cell lines. Furthermore, this combination was shown to be particularly synergistic in a fibronectin-adhesion model, a setting that is thought to confer properties of drug resistance (16).
In this phase I combination trial (ClinicalTrials.gov identifier NCT00383474), we studied the effect of combination treatment of tipifarnib and bortezomib in patients with advanced acute leukemias. The primary objectives were to determine the toxicity and maximum tolerated doses of tipifarnib and bortezomib in combination. Secondary endpoints were to determine the effect of combined therapy on putative drug targets, including FTase, CTL proteasomal activity, and NF-κB activity within leukemic blasts.
PATIENTS AND METHODS
Patient Eligibility and Selection
Patients above the age of 18 years with relapsed or refractory AML (non M3 subtype), ALL, and blast-phase CML were eligible for enrollment. Patients who had previously untreated AML who were either not eligible or refused conventional cytotoxic therapy were also eligible. All patients had to meet the following criteria: Eastern Cooperative Oncology Group performance status 0–2, normal bilirubin, hepatic enzymes twice normal or less, serum creatinine 1.5 times normal or less, and left ventricular ejection fraction ≥ 40%. Patients were ineligible if they had a peripheral blast count of ≥ 30,000/μL (cytoreduction with hydroxyurea was permitted up to 24 hours prior to beginning treatment); acute promyelocytic leukemia (M3); disseminated intravascular coagulation; active central nervous system leukemia; concomitant radiation therapy, chemotherapy, or immunotherapy; coexisting medical or psychiatric conditions that could interfere with study procedures; known allergy to imidazoles; or prior therapy with bortezomib or tipifarnib. Pregnant or lactating women were ineligible. All patients were given written informed consent with the approval of our Institutional Review Boards and according to the guidelines of each participating institution.
Treatment Regimen
The planned dose-escalation schema is depicted in Table 1. A “3 + 3” dose-escalation design was used. If dose-limiting toxicity (DLT) occurred in 1 of 3 patients, the group was expanded to 6 patients. If no further toxicity was seen, 3 patients were enrolled at the next dose level. Maximum tolerated dose (MTD) was defined as the dose level below the one in which 2 or more DLTs occurred. Each treatment cycle was 21 days. Bortezomib was administered as an intravenous infusion over 3–5 seconds on days 1, 4, 8, and 11 of each cycle. Tipifarnib was administered every 12 hours by mouth (with food) on days 1–14 of each cycle. The first 3 patients received tipifarnib at a dose of 300 mg every 12 hours by mouth for 14 days (days 1–14) and bortezomib 1.0 mg/m2 for 4 doses on days 1, 4, 8, and 11. If a DLT occurred at the initial dosing level, then a dose level minus (−) 1 was permitted. An expansion of up to 10 patients at the MTD or the final dosing cohort was planned in order to gain additional experience and knowledge about the toxicity and pharmacodynamic effects of this regimen.
Table 1.
Dose escalation schema
| Dose Level | Dose Escalation Schedule | |
|---|---|---|
| R115777 (Tipifarnib) | PS-341 (Bortezomib) | |
| Level (−) 1 | 200 mg BID | 0.7 mg/m2 twice weekly |
| Level 1 | 300 mg BID | 1 mg/m2 twice weekly |
| Level 2 | 400 mg BID | 1 mg/m2 twice weekly |
| Level 3 | 600 mg BID | 1 mg/m2 twice weekly |
| Level 4 | 600 mg BID | 1.3 mg/m2 twice weekly |
BID, twice daily.
Assessment of Response and Toxicity
All adverse events were graded according to the NCI Common Terminology Criteria for Adverse Events version 3.0. A DLT was defined as any grade 3–4 drug-related non-hematologic toxicity with the exception of alopecia, diarrhea, mucositis, nausea or vomiting, and infection or febrile neutropenia adequately controlled with supportive measures. Observations for DLT were performed during cycle 1 for a minimum period of 21 days. Hematologic DLT was defined as grade 3–4 neutropenia and/or thrombocytopenia (thought to be due to marrow hypoplasia and NOT leukemic burden) that did not recover to grade ≤ 2 by day 56.
Translational/Correlative Studies
Measurement of NF-κB
Nuclear extracts were prepared from 2 million Ficoll-purified bone marrow nucleated cells using a nuclear extraction kit (Panomics Inc., Fremont, CA). DNA binding activity was assayed using an NF-κB p50 DNA binding ELISA (Panomics Inc.). The manufacturer’s suggested methods were used with the addition of poly d(IC) added to the assay buffer to prevent nonspecific binding. Excess cold probe was used in control wells.
Measurement of proteasome chymotrypsin-like activity
Chymotrypsin-like activity levels on day 8 pre- and post-bortezomib (1 hour) treatment were measured by incubating the PBMC lysates in the presence of purified fluorogenic 20S proteasome peptide substrate Suc-Leu-Leu-Val-Tyr-7-amido-4-methyl-coumarin for 2 hours at 37° C. After incubation, chymotrypsin-like activity was determined by measuring the amount of hydrolyzed 7-amido-4-methyl-coumarin groups using a fluorometer with an excitation filter of 380 nm and an emission filter of 460 nm. The inhibitory activity of bortezomib on day 8 for each patient was then reported as the percentage measured 1 hour after the day 8 bortezomib dose compared with that of one hour before the day 8 bortezomib dose.
Measurement of FTase activity
The ability of FTase to transfer [3H]farnesyl from [3H]farnesyl pyrophosphate to recombinant H-Ras was determined by adding equal protein amounts from each patient’s PMBC lysate to 50 mM Tris-HCL (pH 7.5), 50 μM ZnCl2, 20 mM KCl, 1 mM DTT, and 3 mM MgCl2 in the presence of 1 μCi [3H]farnesyl pyrophosphate and 20 μg H-Ras (FTase) and incubating at 37° C for 30 min. After the reaction was stopped with 4% SDS, the proteins were precipitated with 30% TCA and collected on glass microfiber filters as described previously (17). Baseline FTase activity (without exogenous H-Ras) was also determined for each sample. Activity of each sample was determined by scintillation counting, and basal FTase activity was subtracted out of each sample to give total sample activity. Day 8 (pre- and 1 hour post-bortezomib) and day 22 samples were compared to baseline (before initial treatment day 1, cycle 1) and reported as percent of baseline activity.
Statistics
The unpaired Student’s t test was used to analyze in vivo results for NF-κB, FTase activity, and chymotrypsin-like enzyme activity. Statistical analysis was performed using Microsoft Excel software.
RESULTS
Thirty-one patients were consented for this study, of which 27 were treated. The remaining four patients were deemed ineligible, due to either rapid disease progression prior to starting study therapy (2 patients) or development of intercurrent severe medical illness precluding initiation of study therapy (2 patients). All 27 treated patients were evaluated for toxicity and response. Patient demographics are shown in Table 2. Most patients had recurrent AML, the majority of whom had received extensive prior therapy (median of 2 prior regimens).
Table 2.
Patient characteristics
| Number of patients | 27 |
|---|---|
| Median age, years (range) | 70 (47–82) |
| Sex, no. of patients (%) | |
| Male | 21 (78) |
| Female | 6 (22) |
| Race or ethnicity, no. of patients (%) | |
| Hispanic | 1 (4) |
| Black or African American | 2 (7) |
| White | 24 (89) |
| ECOG performance status, no. of patients (%) | |
| 0–1 | 26 (96) |
| 2 | 1 (4) |
| Diagnosis, no. of patients (%) | |
| AML | 26 (87) |
| ALL | 1 (13) |
| Median number of prior chemotherapy regimens | 2 (0–2) |
AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; ECOG, Eastern Cooperative Oncology Group.
Toxicities
A summary of all non-hematologic toxicities is shown in Table 3. The most common toxicities that were observed included diarrhea, infection/febrile neutropenia, nausea/vomiting, fatigue, and anorexia, most of which were grade 1 or 2. Three patients experienced DLT, each occurring in different dosing cohorts. An 81-year-old female in cohort 1 with AML who had been previously treated with low-dose cytarabine with no response experienced recurrent grade 2 diarrhea on day 5, requiring hospitalization, with resolution 1 day later. This event reached DLT criteria (per protocol definition) because it recurred and resulted in > 1 occurrence of dose interruption or delay. A 65-year-old female in cohort 3 with refractory AML developed severe fatigue beginning on day 10, which improved after discontinuation of the study regimen, but the patient developed progressive disease shortly thereafter. Finally, a 67-year-old male in cohort 4, with no baseline neurologic deficits, experienced grade 3 sensorimotor neuropathy starting on day 21, which improved 2 months later, but never resolved. None of the 3 patients with DLTs received re-treatment with the study combination following these events. Despite the occurrences of these DLTs, the trial completed accrual to the final planned cohort.
Table 3.
Treatment-emergent adverse events (≥10% of patients)
| Number of Patients With Specified Adverse Event |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose Level 1 (n=6) | Dose Level 2 (n=5) | Dose Level 3 (n=6) | Dose Level 4 (n=10) | Total (%)(n = 27) | Total (%) Grade 3/4 | |||||
| Total | Grade 3/4 | Total | Grade 3/4 | Total | Grade 3/4 | Total | Grade 3/4 | |||
| Diarrhea | 3* | 0 | 2 | 1 | 5 | 0 | 8 | 1 | 18 (67) | 2 (7) |
| Infection/febrile | ||||||||||
| neutropenia | 5 | 5 | 5 | 3 | 2 | 2 | 4 | 2 | 16 (59) | 12 (4) |
| Nausea/vomiting | 2 | 0 | 2 | 1 | 5 | 0 | 7 | 0 | 16 (59) | 1 (4) |
| Fatigue | 2 | 0 | 5 | 2 | 8 | 5* | 0 | 0 | 15 (56) | 7 (26) |
| Anorexia | 3 | 0 | 3 | 1 | 3 | 0 | 4 | 0 | 13 (48) | 1 (4) |
| Muscle weakness | 2 | 1 | 1 | 1 | 3 | 0 | 4 | 0 | 10 (37) | 2 (7) |
| Dizziness | 1 | 0 | 1 | 0 | 3 | 0 | 3 | 0 | 8 (30) | 0 (0) |
| Dyspnea | 3 | 1 | 1 | 1 | 2 | 0 | 2 | 0 | 8 (30) | 2 (7) |
| Abdominal pain | 1 | 0 | 2 | 0 | 0 | 0 | 4 | 1 | 7 (26) | 1 (4) |
| Dehydration | 1 | 0 | 1 | 0 | 2 | 0 | 3 | 0 | 7 (26) | 0 (0) |
| Rash | 1 | 0 | 1 | 0 | 2 | 0 | 3 | 0 | 7 (26) | 0 (0) |
| Hypokalemia | 1 | 1 | 1 | 0 | 2 | 0 | 2 | 1 | 6 (22) | 2 (7) |
| Insomnia | 0 | 0 | 1 | 0 | 2 | 0 | 3 | 0 | 6 (22) | 0 (0) |
| Bone pain | 0 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 5 (19) | 0 (0) |
| Limb pain | 0 | 0 | 3 | 1 | 1 | 0 | 1 | 1 | 5 (19) | 2 (7) |
| Mucositis | 2 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 5 (19) | 1 (4) |
| Rigors/chills | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 5 (19) | 0 (0) |
| Sensory neuropathy | 1 | 1 | 1 | 0 | 1 | 0 | 2 | 1* | 5 (19) | 2 (7) |
| Altered mental status | 0 | 0 | 2 | 1 | 0 | 0 | 2 | 0 | 4 (15) | 1 (4) |
| Constipation | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 4 (15) | 0 (0) |
| Cough | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 4 (15) | 0 (0) |
| Dyspepsia | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 4 (15) | 0 (0) |
| Joint pain | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 4 (15) | 0 (0) |
| Cardiac arrhythmia | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 3 (11) | 0 (0) |
| Creatinine | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 (11) | 0 (0) |
| Dysphagia | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 3 (11) | 1 (4) |
| Edema | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 (11) | 0 (0) |
| Hyperbilirubinemia | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 3 (11) | 1 (4) |
| Motor neuropathy | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 3 (11) | 2 (7) |
| Muscle pain | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 (11) | 0 (0) |
| Pleural effusion | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 3 (11) | 0 (0) |
Dose-limiting toxicity.
Correlative Laboratory Studies
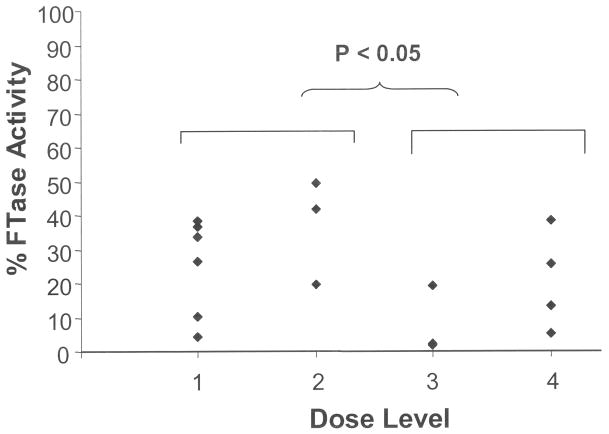
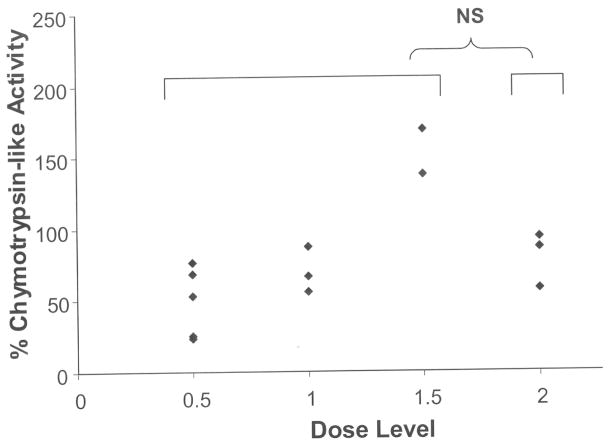
FTase activity within peripheral blood mononuclear cells (PBMC) was measured serially in 16 patients, with a median of 77.5% inhibition (range 50.7% to 98.8%) from baseline to day 8 (Figure 1). Patients in dosing cohorts 3 and 4 (tipifarnib 600 mg twice daily) had significantly decreased FTase activity at day 8 (average 14.9% of baseline, 95% confidence interval 1.3% – 28.5%) compared with patients treated with lower doses of tipifarnib (300 mg or 400 mg twice daily) in cohorts 1 and 2 (average 28.9% of baseline, 95% confidence interval 13.9% – 43.9%) (p = 0.038), suggesting more potent FTase inhibition with higher doses of tipifarnib. Interestingly, 5 out of 8 evaluable patients had sustained inhibition at day 22 (data not shown). We also found that the reduced FTase activity levels in 7 evaluable patient samples did not change significantly 1 hour post-bortezomib infusion on day 8 compared with pre-infusion, indicating that the ability of tipifarnib to inhibit its primary target was not affected by bortezomib. The 20S proteasomal function within PBMCs, as measured by chymotrypsin-like enzyme activity, was evaluated in 13 patient PBMC samples obtained pre-infusion and 1 hour post-infusion of bortezomib on day 8. Of these 13 samples, 11 showed a reduction in chymotrypsin-like enzyme activity by a median of 33.3% (range 6.2% to 76.9%), whereas two samples showed an increase in activity at day 8 (Figure 2). In addition, chymotrypsin-like enzyme activity within PBMCs at day 22 decreased from baseline in 5 out of 7 patient samples tested by a median of 45% (range 8.8% to 77.8%). There was no significant difference in the chymotrypsin-like enzyme activity in dosing cohorts 1–3 (bortezomib 1.0 mg/m2) compared to dosing cohort 4 (bortezomib 1.3 mg/m2).
Fig. 1.
FTase activity in PBMCs on day 8, compared with at baseline.
Fig. 2.
Residual chymotrypsin-like activity in PBMCs on day 8, 1 hour after treatment with bortezomib.
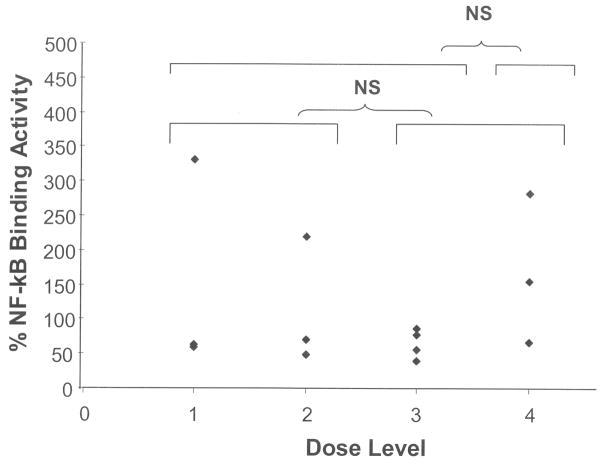
In an attempt to assess the effects of this drug combination on a critical apoptotic regulator, we measured NF-κB binding activity within leukemic marrow blasts. Figure 3 shows that NF-κB activity decreased by a median of 39% (range 14% – 60%) from baseline to day 8 in 10 out of 14 paired samples tested. Again, there was no significant difference in the NF-κB binding activity level at day 8 (compared with baseline) amongst any of the dosing cohort tested (groups 1+2 versus 3+4 or groups 1+2+3 versus 4).
Fig. 3.
NF-κB binding activity in leukemic blasts on day 8 compared with baseline.
Anti-Tumor Efficacy
Two patients on the study demonstrated complete response with incomplete count recovery (CRi); both received treatment on dose cohort 2 (i.e., tipifarnib 400 mg twice daily and bortezomib 1.0 mg/m2 twice weekly). The first was a 70-year-old male with AML (normal cytogenetics) in first relapse after 2 courses of induction chemotherapy, with a baseline bone marrow aspirate sample revealing 54% blasts. CRi was achieved following cycle 2 of the study regimen and lasted for 21 weeks. The patient was removed from study after 3 cycles due to low-grade persistent sensory neuropathy and subsequently relapsed. The second patient was also a 70-year-old male with AML (normal cytogenetics) in second relapse. Bone marrow blast clearance and CRi were achieved following cycle 3, without peripheral count recovery. The patient went on to receive 6 cycles (total) of study treatment before relapse occurred. CRi lasted 14 weeks. Five additional patients had stable disease following cycle 1 and were able to receive treatment for a total of 2 cycles or more. In comparing patients with CRi or stable disease (i.e., received 2 or more cycles) with those patients who had progressive disease or received < 2 cycles of treatment, we did not observe any significant baseline-to-day 8 changes in NF-κB binding activity, FTase activity, or chymotrypsin-like enzyme activity, although the number of patients with serial samples available for correlative assays was relatively limited. Similarly, baseline NF-κB and FTase activity levels did not differ significantly between these groups of patients.
DISCUSSION
Intersecting inhibition of key regulatory proteins with small molecule inhibitors offers the potential for more targeted therapy with less toxicity. However, signaling inhibitors are often ineffective when used as single agents, possibly because malignancies develop as a consequence of multiple genetic alterations leading to many aberrant signal transduction pathways. Therefore, combination therapy of agents with non-overlapping mechanisms of action is attractive for the purposes of increasing efficacy. Because of the putative effects of tipifarnib and bortezomib on critical intracellular targets of proliferation and survival (e.g., Ras, NF-κB), along with demonstration of synergy in preclinical models, and the verified single-agent clinical anti-leukemic activity of tipifarnib in AML, we chose to evaluate this combination in a phase 1 trial. In this study, the combination of tipifarnib and bortezomib was generally well-tolerated across all treatment cohorts with limited non-hematologic toxicity. DLTs included fatigue, diarrhea, and sensorimotor neuropathy. The acceptable side effect profile compares well with those seen in previous phase I and II studies using tipifarnib or bortezomib as single agents in acute leukemias (6, 12, 13). Of note, MTD was not reached with this combination, in contrast to other targeted therapy combination regimens, where significant toxicities were observed, precluding maximum dose elevation or the ability to administer the agents together chronically (18, 19). Previous DLTs reported for tipifarnib not seen in this study included enterocolitis, arrhythmias, and delayed hematologic recovery after consolidation (12, 20). Clinical anti-leukemic activity was modest, as expected, in this primarily heavily pre-treated cohort of patients; however, bona fide responses (CRi) were observed in 2 patients. An additional 5 patients maintained stable disease beyond 2 cycles, indicating the potential to utilize this regimen on a more prolonged or chronic basis.
The putative drug targets for these agents, FTase and chymotrypsin-like activity of the UPS, were inhibited by the combination at all doses tested, and FTase inhibition persisted for several days beyond drug discontinuation in several patients. Inhibition of chymotrypsin-like activity at day 8 was more modest, even at the maximum dose of 1.3 mg/m2, but further dose escalation of bortezomib is not likely possible, based on the previously described MTD of this agent (21). Specifically, within leukemic blasts, NF-κB binding activity decreased following treatment in the majority of patient samples tested, indicating the ability of this combination to inhibit an important leukemic progenitor cell survival pathway that may be the focus of future studies in AML. While the small number of responding patients, along with a molecularly unselected overall population of patients, precluded an ability to correlate response with degree of target inhibition in this study, the ability to inhibit these targets in a majority of patients indicates that future studies of these agents, either alone or in combination, will be viable in prospectively selected patient populations in whom inhibition of these targets is determined to be desirable.
The future for successful small molecule and targeted therapy approaches in leukemia, and cancer in general, likely depends on the ability to predict which patients express a molecular profile that predicts response to a particular drug. In the case of tipifarnib, meaningful strides have been undertaken to identify a molecular signature associated with response to this agent. In their analysis of gene expression profiles from bone marrow of AML patients treated with tipifarnib on 2 clinical studies, Raponi et al found that the expression ratio of 2 genes, RASGRP1 (encodes for a guanine nucleotide exchange factor that activates Ras) and APTX (encodes for a protein involved with DNA excision repair), both positively and negatively predicted response to single-agent tipifarnib (22, 23). Using the same gene panel, investigators in Italy have also demonstrated a high response rate to tipifarnib combined with bortezomib in patients with leukemia cells exhibiting the proper expression ratio (24). As such, a bortezomib-tipifarnib combination to induce response may be feasible as primary therapy in preselected patients or as adjunctive therapy via inhibition of targets such as NF-κB.
In summary, combination therapy with tipifarnib and bortezomib in advanced acute leukemias is well-tolerated, induces target protein inhibition, and produces modest anti-leukemic clinical activity in patients with advanced, poor-risk acute leukemia. The results from this trial may facilitate future development of this or similar combinations in selected patients with an appropriate leukemic phenotype.
Acknowledgments
FINANCIAL SUPPORT: This work was supported by National Cancer Institute Grant N01 CM-62208 and National Cancer Institute/CTEP Translational Research Initiative Contract 26XXS043 TO2.
The authors gratefully acknowledge the dedicated nurses at all 4 institutions who provided exemplary care for the patients.
The authors also wish to thank Ms. Rasa Hamilton, Moffitt Cancer Center, for her invaluable assistance in preparing the manuscript.
Finally, we are deeply indebted to the patients and their families, whose participation made this study possible.
References
- 1.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–5. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantarjian H, O'Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–8. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 3.Guzman ML, Neering SJ, Upchurch D, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–7. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 4.Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol. 2001;8:739–58. doi: 10.1016/s1074-5521(01)00056-4. [DOI] [PubMed] [Google Scholar]
- 5.Voorhees PM, Dees EC, O'Neil B, Orlowski RZ. The proteasome as a target for cancer therapy. Clin Cancer Res. 2003;9:6316–25. [PubMed] [Google Scholar]
- 6.Cortes J, Thomas D, Koller C, et al. Phase I study of bortezomib in refractory or relapsed acute leukemias. Clin Cancer Res. 2004;10:3371–6. doi: 10.1158/1078-0432.CCR-03-0508. [DOI] [PubMed] [Google Scholar]
- 7.Rowinsky EK, Windle JJ, Von Hoff DD. Ras protein farnesyltransferase: A strategic target for anticancer therapeutic development. J Clin Oncol. 1999;17:3631–52. doi: 10.1200/JCO.1999.17.11.3631. [DOI] [PubMed] [Google Scholar]
- 8.End DW, Smets G, Todd AV, et al. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 2001;61:131–7. [PubMed] [Google Scholar]
- 9.Nardella C, Chen Z, Salmena L, et al. Aberrant Rheb-mediated mTORC1 activation and Pten haploinsufficiency are cooperative oncogenic events. Genes & Development. 2008;22:2172–7. doi: 10.1101/gad.1699608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang K, Coppola D, Crespo NC, et al. The phosphoinositide 3-OH kinase/AKT2 pathway as a critical target for farnesyltransferase inhibitor-induced apoptosis. Mol Cell Biol. 2000;20:139–48. doi: 10.1128/mcb.20.1.139-148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madrid LV, Wang CY, Guttridge DC, Schottelius AJ, Baldwin AS, Jr, Mayo MW. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol Cell Biol. 2000;20:1626–38. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karp JE, Lancet JE, Kaufmann SH, et al. Clinical and biologic activity of the farnesyltransferase inhibitor R115777 in adults with refractory and relapsed acute leukemias: a phase 1 clinical-laboratory correlative trial. Blood. 2001;97:3361–9. doi: 10.1182/blood.v97.11.3361. [DOI] [PubMed] [Google Scholar]
- 13.Lancet JE, Gojo I, Gotlib J, et al. A phase 2 study of the farnesyltransferase inhibitor tipifarnib in poor-risk and elderly patients with previously untreated acute myelogenous leukemia. Blood. 2007;109:1387–94. doi: 10.1182/blood-2006-04-014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harousseau JL, Lancet JE, Reiffers J, et al. A phase 2 study of the oral farnesyltransferase inhibitor tipifarnib in patients with refractory or relapsed acute myeloid leukemia. Blood. 2007;109:5151–6. doi: 10.1182/blood-2006-09-046144. [DOI] [PubMed] [Google Scholar]
- 15.Harousseau JL, Martinelli G, Jedrzejczak WW, et al. A randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood. 2009;114:1166–73. doi: 10.1182/blood-2009-01-198093. [DOI] [PubMed] [Google Scholar]
- 16.Yanamandra N, Colaco NM, Parquet NA, et al. Tipifarnib and bortezomib are synergistic and overcome cell adhesion-mediated drug resistance in multiple myeloma and acute myeloid leukemia. Clin Cancer Res. 2006;12:591–9. doi: 10.1158/1078-0432.CCR-05-1792. [DOI] [PubMed] [Google Scholar]
- 17.Nigam M, Seong CM, Qian Y, Hamilton AD, Sebti SM. Potent inhibition of human tumor p21ras farnesyltransferase by A1A2-lacking p21ras CA1A2X peptidomimetics. Journal of Biological Chemistry. 1993;268:20695–8. [PubMed] [Google Scholar]
- 18.Azad NS, Posadas EM, Kwitkowski VE, et al. Combination Targeted Therapy With Sorafenib and Bevacizumab Results in Enhanced Toxicity and Antitumor Activity. J Clin Oncol. 2008;26:3709–14. doi: 10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel P, Senico P, Curiel R, Motzer R. Phase I Study Combining Treatment with Temsirolimus and Sunitinib Malate in Patients with Advanced Renal Cell Carcinoma. Clinical Genitourinary Cancer. 2009;7:24–7. doi: 10.3816/CGC.2009.n.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandwein JM, Leber BF, Howson-Jan K, et al. A phase I study of tipifarnib combined with conventional induction and consolidation therapy for previously untreated patients with acute myeloid leukemia aged 60 years and over. Leukemia. 2009;23:631–4. doi: 10.1038/leu.2008.341. [DOI] [PubMed] [Google Scholar]
- 21.Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. Journal of Clinical Oncology. 2002;20:4420–7. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 22.Raponi M, Harousseau JL, Lancet JE, et al. Identification of molecular predictors of response in a study of tipifarnib treatment in relapsed and refractory acute myelogenous leukemia. Clin Cancer Res. 2007;13:2254–60. doi: 10.1158/1078-0432.CCR-06-2609. [DOI] [PubMed] [Google Scholar]
- 23.Raponi M, Lancet JE, Fan H, et al. A 2-gene classifier for predicting response to the farnesyltransferase inhibitor tipifarnib in acute myeloid leukemia. Blood. 2008;111:2589–96. doi: 10.1182/blood-2007-09-112730. [DOI] [PubMed] [Google Scholar]
- 24.Paolini S, Ottaviani E, Parisi S, et al. RASGRP1/APTX Ratio Strongly Correlates with Clinical Response and Survival in AML Patients Treated with Tipifarnib-Bortezomib Combination. ASH Annual Meeting Abstracts. 2009;114:1028. [Google Scholar]