Abstract
Background
Estrogen receptor (ER) is expressed in normal and malignant breast epithelium, and expression levels have been found to increase with age in normal breast epithelium but not in atypical hyperplasia (AH) and carcinoma in situ. Here we assess ER expression in AH and its association with later breast cancer.
Methods
ER expression was assessed immunohistochemically in archival sections from 246 women with AH who had open benign breast biopsy from 1967–1991. The ACISRIII (Dako, Carpinteria, CA) was utilized to calculate ER expression in all atypical foci. Using multivariate linear regression, we examined associations of ER expression with age at biopsy, indication for biopsy, type of atypia, number of atypical foci, involution status, and family history. Breast cancer risk across levels of ER expression was also assessed compared to the Iowa SEER control population.
Results
Among 246 women, 87 (35%) had atypical ductal hyperplasia (ADH), 141 (57%) had atypical lobular hyperplasia (ALH), and 18 (7%) had both. Forty-nine (20%) developed breast cancer (median follow-up of 14.4 years). Multivariate analysis indicated that type of atypia and age at diagnosis were significantly associated with ER percent staining and intensity [p<0.05]. ER expression was increased in women with ADH and/or those over age 55. ER expression did not significantly impact breast cancer risk in patients diagnosed with atypia.
Conclusion
We found increasing ER expression in atypical hyperplasia with increasing age. ER expression in atypical hyperplasia does not further discriminate breast cancer risk in women with atypia.
Keywords: atypical hyperplasia, estrogen receptor, breast cancer, risk
INTRODUCTION
Estrogen plays a major role in promoting normal growth of breast epithelium and is thought to be important in the pathogenesis of breast cancer via the uptake into the cell through the mechanism of the estrogen receptor (ER).(1) Its association with many of the epidemiological risk factors for breast carcinoma (i.e. age of menarche, first child, menopause, and the use of oral contraceptive or hormone replacement therapy) is well known.(2) ER expression is present in both normal (3–5) and to a greater degree in malignant breast epithelium.(6) ER expression is clinically assessed with immunostaining, specifically to the alpha variant of the estrogen receptor. ER expression appears to increase with age in normal breast epithelium as well as in ductal hyperplasia of usual type.(7) Several studies have shown that ER expression is relatively low in normal ductal epithelium and higher in proliferative breast disease, particularly when associated with atypia and carcinoma in situ.(8, 9) One case control study has also reported that increased ER expression in usual ductal hyperplasia is associated with breast cancer risk,(10) but the impact of ER expression on breast cancer risk in women with atypical hyperplasia (a known higher risk group) has not been reported.
In one of the largest, prospective breast cancer prevention trials, NSABP P-1, long term follow up has shown a risk reduction of breast cancer in women with prior atypical hyperplasia taking tamoxifen, presumably acting through the ER.(11) These clinical data combined with published results on ER expression in other benign breast epithelium suggest that ER expression in atypical hyperplasia may be a marker of subsequent breast cancer risk. The goal of this study was to evaluate ER expression in atypical hyperplasia and examine possible associations with age at biopsy, indication for biopsy, type of atypia, number of atypical foci, involution status, and family history, as well as to assess any association between level of ER expression in atypia and subsequent breast cancer risk.
MATERIALS AND METHODS
Study Population
Entry criteria for the study cohort have been described previously.(12, 13) Briefly, this study comprises an institutional review board-approved study of women aged 18–85 years who had a surgical benign breast biopsy at Mayo Clinic between January 1, 1967 and December 31, 1991. The initial cohort included 9087 women.(12) With additional follow-up, data for 9376 women were available for this analysis, 334 (3.6%) of whom had atypical hyperplasia. Adequate archival paraffin-embedded formalin-fixed tissue for ER staining was available for 246 of the 334 women.
Risk Factor Information and Follow-up
Follow-up for breast cancer events (including both invasive cancer and ductal carcinoma in situ) and risk-factor information were obtained for all women with atypia through the Mayo medical record and a study questionnaire.(12, 13) Family history was collected via respondent questionnaires and medical record abstraction and classified as negative, weak, or strong. The criteria for a strong family history were at least one first-degree relative with breast cancer before the age of 50 years or two or more relatives with breast cancer, with at least one being a first-degree relative. Any lesser degree of family history was considered to be weak.(12)
Histology
All archival hematoxylin and eosin-stained sections were evaluated by a study breast pathologist (DWV), without knowledge of the original histologic diagnosis or patient outcome. Each case was reviewed a second time by an additional breast pathologist (CAR) at the time of marking atypical foci for quantitative analysis. A diagnosis of atypical ductal hyperplasia (ADH) or atypical lobular hyperplasia (ALH) was based on the criteria of Page et al (14) and Page and Rogers.(15) Multifocal atypia required the identification of atypical hyperplasia in more than one terminal duct lobular unit (TDLU), as defined by clear separation from another TDLU by nonspecialized interlobular mammary stroma.(13) The extent of involution was assessed in the normal background breast tissue on a hematoxylin and eosin-stained slide. The degree of involution was classified into three categories: “no involution” (0% involuted TDLUs), “partial” (1 to 74% involuted TDLUs), or “complete” (≥ 75% involuted TDLUs).(16)
Immunostaining of ER
Five-micrometer sections of formalin-fixed, paraffin-embedded samples were deparaffinized with three changes of xylene, rehydrated in a series of graded alcohols (100% ethanol, 95% ethanol, and 70% ethanol), and rinsed well in running distilled water. Slides were then placed in a preheated epitope retrieval buffer (1mM EDTA, pH 8.0) for 30 minutes, cooled in the buffer for 5 minutes, and then followed by a 5 minute rinse in running distilled water. Slides were then placed on an automated slide stainer (AS100 Autostainer Plus, DAKO, Carpinteria, CA) at room temperature as follows. Sections were first incubated with 3% H2O2 in ethanol for 5 minutes to inactivate the endogenous peroxides and then incubated in primary mouse anti-human estrogen receptor alpha, Clone 1D5, monoclonal antibody (1:100 dilution; M7047, DAKO, Carpinteria, CA) for 30 minutes. Sections were rinsed with TBST wash buffer (S3006, tris-buffered saline with Tween 20, DAKO, Carpinteria, CA). Sections were then incubated with a peroxidase-labeled polymer conjugated to goat anti-mouse, goat anti-rabbit immunoglobulins (EnVision+ Dual Link System-HRP, K4061, DAKO, Carpinteria, CA) for 15 minutes. The slides were rinsed with TBST wash buffer, incubated in 3,3’-diaminobenzidine (K3468, DAB+ Substrate Chromogen, DAKO, Carpinteria, CA) for 5 minutes, and counterstained with modified Schmidts’ hematoxylin for 5 minutes and rinsed for 3 minutes in tap water to set the hematoxylin counterstain. Specimens were dehydrated through a series of graded alcohols (100% ethanol, 95% ethanol, and 70% ethanol), cleared in three changes of xylene, and mounted with a permanent mounting medium. The positive control was ER positive breast cancer tissue; the negative control was ER positive breast cancer tissue without the primary antibody. All samples were stained over a two day period with appropriate positive and negative controls.
Evaluation of ER Immunostaining
All ER immunostained slides were scanned on the Automated Cellular Image SystemRIII (ACISRIII) for quantification by digital image analysis. After the slides were scanned, the study breast pathologist (CAR) circled all foci of ADH in red and/or all foci of ALH in blue on the ER immunostained slides. This marking system was utilized to indicate the type of atypia and to identify the region(s) for quantification by digital image analysis. If there was no staining in the atypical hyperplasia, an internal control (i.e. benign lobules) was identified to ensure adequate staining. There were two cases in which ER expression was not identified in either atypical hyperplasia or benign lobules; both were excluded from the study.
Without knowledge of patient outcome, the cytotechnologist (EGBF) trained in breast histomorphology used the ACIS free-form tool on the digital image to outline the regions of atypical hyperplasia based on the pathologist’s marking system (stroma was excluded). The ACISRIII generated a percent of staining and intensity value for each region of atypical hyperplasia that was outlined. The percent of staining represents the proportion of nuclear area that stained positively for ER, multiplied by 100. The intensity value represents the average brown stain concentration over the region on a scale of 0 to 255. For women with more than one ACIS region of atypical hyperplasia, per-woman summary measures of percent staining and intensity of staining were calculated using a weighted average of the multiple values, with weights proportional to the size of each given area. Additional analysis of percent staining and intensity of staining using maximum values were also performed.
Statistical Analyses
In order to confirm that the subset of women with atypical hyperplasia and available tissue were representative of the entire 334 women with atypical hyperplasia, we compared distributions of demographic and clinical variables by tissue availability for ER staining using chi-square tests of significance. We compared distributions of ER percent staining and intensity of staining across levels of demographic and clinical characteristics using linear regression models. Variables considered included age at initial biopsy (<45 years, 45–54, 55 and older); indication for biopsy (lump or mammogram); type of atypia (ALH, ADH, both); number of atypical foci (1, 2, 3 or more); involution status (none, partial, complete); and family history of breast cancer (none, weak, strong). Two sets of analyses were carried out. First, we assessed univariate associations for each attribute using a series of simple linear regression models. We then examined the independent effects of each attribute on ER staining levels by including each in a multivariate linear regression analysis. Least squares means and corresponding 95% confidence intervals were calculated based on the resulting parameter estimates.
The length of follow-up for each woman in the study was calculated as the number of days from her benign biopsy to the date of her breast cancer diagnosis, death, lobular carcinoma in situ diagnosis, prophylactic mastectomy, or last contact. We compared the observed number of incident breast cancer events in our cohort, overall and by strata of ER expression levels and type of atypia, to that expected in the general population using standardized incidence ratios (SIRs). Expected values were calculated by apportioning each woman’s person-years of follow-up into 5-year age and calendar-period categories, multiplying each by the corresponding SEER breast cancer incidence rates. Thus, all SIRs account for the potentially confounding effects of age and calendar period. The Iowa Surveillance, Epidemiology, and End Results (SEER) registry was used as the standard population, due to the proximity of its participants to the Mayo Clinic catchment area and racial/ethnic similarities to our cohort.
Separate analyses were carried out for percent staining, intensity of staining, and the percent-by-intensity product term, using both the weighted averages as well as maximal values for percent staining, staining intensity, and the percent-intensity product. We tested for differences in the standardized incidence ratios across strata defined by ER expression levels using Poisson regression models that accounted for the population-based expected event rate for each individual using an offset term. This approach facilitates the calculation of SIRs with the added flexibility that generalized linear models provide, such as covariate adjustment and formal assessment of heterogeneity. The following strata were used for SIR calculations: percent staining 0–19%, 20–39%, 40–59%, 60–79%, 80% or greater; intensity of staining 0–99, 100–119, 120–139, 140 or greater; percent-intensity product 0–19%, 20–39%, 40–59%, 60–79%, 80% or greater. All statistical tests were two-sided, and all analyses were conducted using the SAS (SAS Institute, Inc., Cary, NC) software system.
RESULTS
Characteristics of Patients and Pathologic Specimens
Among the original cohort of 334 women with atypical hyperplasia,(13) the distributions of breast cancer status, family history of cancer, and (for breast cancer patients) time to diagnosis were not different between the 246 patients with formalin-fixed tissue available for ER staining and the 88 patients without available tissue (p > 0.05 for each attribute). Among the 246 women diagnosed with atypical hyperplasia, 87 (35%) had ADH, 141 (57%) had ALH, and the remaining 18 (7%) had both ADH and ALH. At the time of diagnosis of atypical hyperplasia, approximately half of the women were older than 55 (53%), 34% were between 45–54 years and 13% were less than 45 years. We have previously reported the increased breast cancer risk associated with atypical hyperplasia, which was approximately four-fold and similar regardless of histologic subtype (ADH versus ALH).(13)
ER Expression in Atypical Hyperplasia
In univariate analysis, age at diagnosis, indication for biopsy, type of atypia, and involution status were significant predictors of ER percent staining and intensity (p <0.01, data not shown). In multivariate analysis that included all significant univariate predictors and after adjusting for all factors, only type of atypia and age at diagnosis were significant predictors of ER percent staining and intensity (p <0.05, see Table 1).
Table 1.
Multivariate Linear Regression Analysis for Prediction Estrogen Receptor (ER) Mean Percent Staining and Intensity in Atypical Hyperplasia
| N (%) | Mean ER Percent Staining (95% CI)* | P-value* | Mean ER Intensity (95% CI)* | P-value* | |
|---|---|---|---|---|---|
| Age | |||||
| <45 | 32 (13.0) | 38 (27 – 50) | 0.011 | 45 (29 – 62) | 0.008 |
| 45 – 54 | 84 (34.1) | 38 (30 – 46) | 48 (36 – 59) | ||
| 55+ | 130 (52.8) | 48 (40 – 56) | 61 (50 – 73) | ||
| Indication for Biopsy | |||||
| Lump | 93 (38.1) | 42 (33 – 50) | 0.706 | 52 (40 – 64) | 0.868 |
| Mammogram | 151 (61.9) | 41 (32 – 49) | 51 (39 – 63) | ||
| Type of Atypia | |||||
| ADH | 87 (35.4) | 56 (47 – 65) | <.001 | 79 (66 – 92) | <.001 |
| ALH | 141 (57.3) | 28 (21 – 36) | 30 (20 – 41) | ||
| Both | 18 ( 7.3) | 39 (27 – 52) | 46 (28 – 63) | ||
| Number of Foci | |||||
| 1 | 132 (53.7) | 39 (31 – 47) | 0.584 | 47 (36 – 59) | 0.374 |
| 2 | 68 (27.6) | 43 (34 – 51) | 53 (41 – 66) | ||
| 3+ | 46 (18.7) | 42 (31 – 53) | 54 (39 – 69) | ||
| Involution | |||||
| None | 18 ( 7.5) | 34 (22 – 47) | 0.277 | 44 (27 – 62) | 0.378 |
| Partial | 188 (78.7) | 43 (36 – 50) | 52 (41 – 62) | ||
| Complete | 33 (13.8) | 46 (35 – 57) | 58 (42 – 74) | ||
| Family History | |||||
| None | 136 (58.1) | 42 (35 – 50) | 0.899 | 52 (41 – 62) | 0.993 |
| Weak | 43 (18.4) | 41 (30 – 51) | 52 (37 – 66) | ||
| Strong | 55 (23.5) | 41 (31 – 50) | 51 (38 – 65) | ||
Least squares means and p-values, adjusting for age, indication for biopsy, type of atypia, number of foci, involution, and family history
Association of ER Expression with Type of Atypia
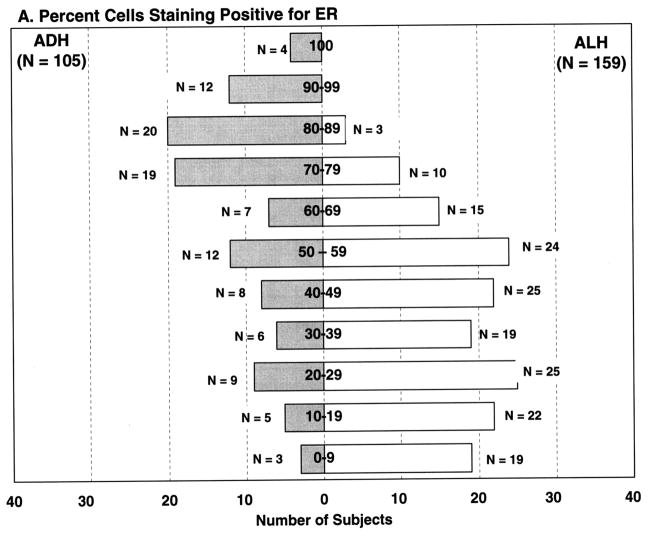
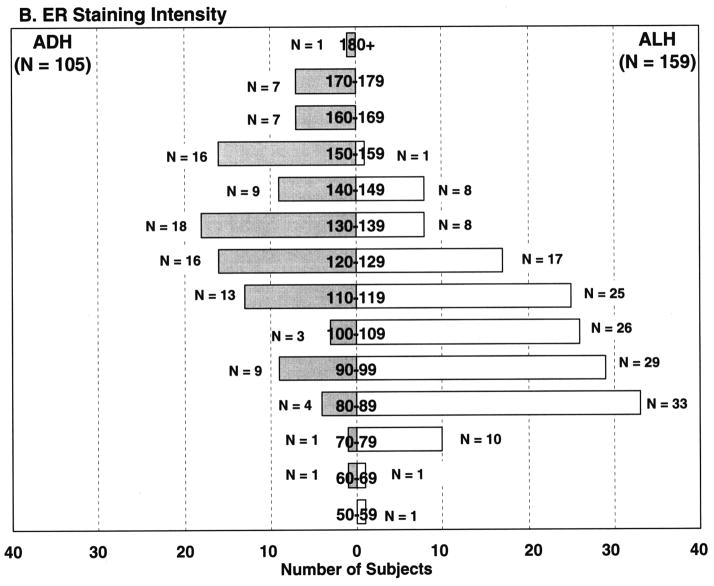
ER percent staining and intensity of staining were statistically significantly associated with the type of atypical hyperplasia (ADH vs ALH, p <0.001). ADH was more likely to show increased ER percent cells staining, with mean percent cells staining of 55.7%. In contrast, ALH showed less ER staining (mean percent cells staining 28.4%) (Figures 1–2). As can be seen in Figure 2A, 74/105 (70.5%) of the ADH lesions had ER staining in over half of the atypical cells. Conversely, only 52/159 (32.7%) of ALH lesions demonstrated ER expression in over 50% of the atypical cells. Similar findings were seen for staining intensity (Figure 2B).
Figure 1.
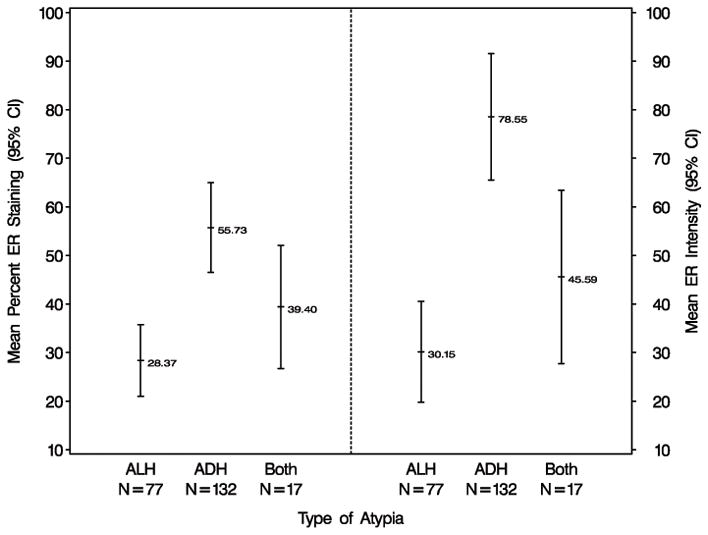
Relationship between ER expression and atypia type in women with atypical hyperplasia. *Least squares means and corresponding 95% confidence intervals are presented based on multivariate linear regression analysis adjusting for the effects of age at biopsy, indication for biopsy, number of atypical foci, involution status, and family history of breast cancer.
Figure 2.
Frequency of ER expression in women with atypical ductal hyperplasia and atypical lobular hyperplasia.
Association of ER Expression with Age
Women older than 55 years with atypical hyperplasia had increased ER expression in comparison to those women 45 to 54 years of age or less than 45 years of age (p <0.01, see Table 1). The increased ER expression was seen in both percent ER staining and ER intensity (Figure 3).
Figure 3.
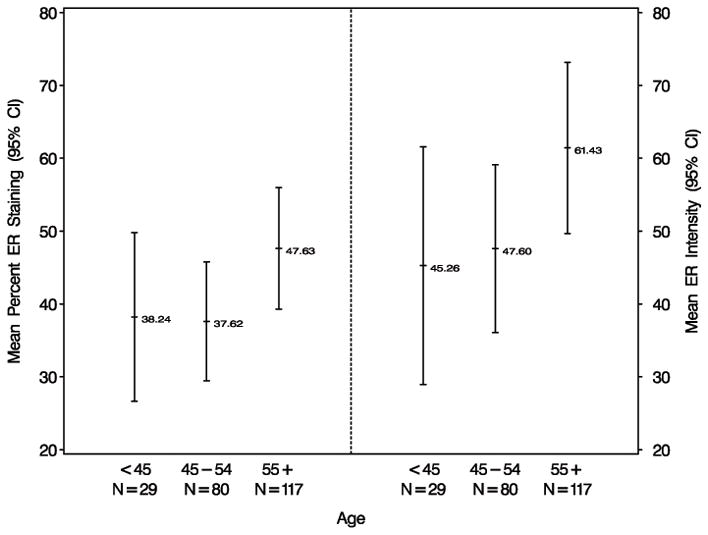
Relationship between ER expression and age in women with atypical hyperplasia. *Least squares means and corresponding 95% confidence intervals are presented based on multivariate linear regression analysis adjusting for the effects of age at biopsy, indication for biopsy, number of atypical foci, involution status, family history of breast cancer, and type of atypia (ALH or ADH).
Association of ER Expression with Breast Cancer Risk
Forty-nine (20%) of the 246 women with atypical hyperplasia in this study developed breast cancer during a median follow-up of 14.4 years. The standardized incidence ratio for all 246 subjects was 3.8 (95% CI 2.8–5.0), reflecting the increased breast cancer risk for women with atypical hyperplasia. Among all subjects, risk of breast cancer did not differ across levels of ER expression (tests for heterogeneity p-values > 0.05 for percent staining, intensity of staining, and the product of the two, Table 2). When the analyses were repeated using maximum values for percent staining, intensity of staining, and the product of the two, the findings were the same, ie there was no association of breast cancer risk and degree of ER staining (data not shown). There was some evidence of heterogeneity for intensity of staining among women with ADH (p=0.01), but the numbers of events were small and the patterns of risk difficult to interpret biologically. No heterogeneity of risk was observed in women with ALH for varying degrees of ER expression.
Table 2.
Risk of breast cancer in atypical hyperplasia by ER expression levels and type of atypia
| All Subjects | ADH1 | ALH1 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Obs | Exp | SIR (95% CI)2 | P- value | N | Obs | Exp | SIR (95% CI)2 | P- value | N | Obs | Exp | SIR (95% CI)2 | P- value | |
| Percent staining | 0.97 | 0.39 | 0.80 | ||||||||||||
| 0–19 | 61 | 13 | 3.2 | 4.01 (2.33–6.90) | 7 | 1 | 0.4 | 2.29 (0.32–16.3) | 51 | 11 | 2.6 | 4.23 (2.34–7.64 | |||
| 20–39 | 54 | 9 | 2.6 | 3.43 (1.79–6.59) | 13 | 3 | 0.7 | 4.60 (1.48–14.3) | 38 | 5 | 1.8 | 2.85 (1.18–6.84) | |||
| 40–59 | 53 | 10 | 2.8 | 3.53 (1.90–6.56) | 11 | 0 | 0.6 | NA | 35 | 7 | 1.9 | 3.69 (1.76–7.73) | |||
| 60–79 | 53 | 12 | 2.7 | 4.39 (2.49–7.74) | 33 | 5 | 1.7 | 3.01 (1.25–7.24) | 15 | 3 | 0.8 | 3.54 (1.14–11.0) | |||
| 80+ | 25 | 5 | 1.5 | 3.27 (1.36–7.84) | 23 | 5 | 1.4 | 3.62 (1.51–8.71) | 2 | 0 | 0.2 | NA | |||
| Intensity of Staining | 0.25 | 0.01 | 0.71 | ||||||||||||
| 0–99 | 99 | 20 | 4.8 | 4.21 (2.71–6.52) | 11 | 1 | 0.5 | 2.03 (0.29–14.4) | 84 | 16 | 4.1 | 3.93 (2.41–6.41) | |||
| 100–119 | 70 | 17 | 3.7 | 4.54 (2.82–7.30) | 19 | 6 | 1.0 | 6.12 (2.75–13.6) | 41 | 7 | 2.1 | 3.26 (1.55–6.84) | |||
| 120–139 | 38 | 4 | 2.3 | 1.71 (0.64–4.56) | 21 | 0 | 1.3 | NA | 14 | 3 | 0.9 | 3.45 (1.11–10.7) | |||
| 140+ | 39 | 8 | 2.1 | 3.76 (1.88–7.52) | 36 | 7 | 1.9 | 3.69 (1.76–7.74) | 2 | 0 | 0.2 | NA | |||
| Product of Percent, Intensity | 0.97 | 0.66 | 0.77 | ||||||||||||
| 0–19 | 68 | 16 | 3.7 | 4.38 (2.68–7.14) | 10 | 1 | 0.6 | 1.66 (0.23–11.8) | 54 | 13 | 2.8 | 4.70 (2.73–8.09) | |||
| 20–39 | 41 | 6 | 1.9 | 3.24 (1.45–7.20) | 6 | 2 | 0.2 | 8.84 (2.21–35.3) | 33 | 4 | 1.5 | 2.69 (1l01–7.16) | |||
| 40–59 | 43 | 8 | 2.2 | 3.66 (1.83–7.31) | 11 | 1 | 0.5 | 1.90 (0.27–13.5) | 28 | 4 | 1.5 | 2.59 (0.97–6.91) | |||
| 60–79 | 27 | 5 | 1.5 | 3.34 (1.39–8.02) | 7 | 1 | 0.4 | 2.44 (0.34–17.3) | 15 | 3 | 0.8 | 3.92 (1.26–12.1) | |||
| 80+ | 67 | 14 | 3.8 | 3.72 (2.20–6.27) | 53 | 9 | 2.9 | 3.07 (1.60–5.91) | 11 | 2 | 0.7 | 2.89 (0.72–11.6) | |||
N, number of subjects; Obs, observed number of breast cancer events; Exp, expected number of breast cancer events; SIR, standardized incidence ratio; CI, confidence interval
Subjects with both ALH and ADH excluded from histology specific analysis
SIRs and corresponding 95% confidence intervals compare the observed number of breast cancer events to those expected based on incidence rates from Iowa SEER data. Analyses account for the effects of age and calendar period. P-values test for heterogeneity in the SIRs across levels of ER expression.
DISCUSSION
We quantified ER expression utilizing computerized digital image analysis in a well-characterized cohort of 246 women with atypical hyperplasia who were evaluated for breast cancer events with a median follow-up of 14.4 years. In multivariate analysis after adjusting for all factors (age at biopsy, indication for biopsy, type of atypia, number of atypical foci, involution status, and family history) among the 246 women with atypical hyperplasia, only type of atypia and age at diagnosis were significant predictors of ER percent staining and intensity. The relationship between ER percent staining and intensity and breast cancer risk in patients diagnosed with atypical hyperplasia was not significant.
It has been reported that virtually all cells in atypical ductal hyperplasia(17) and low grade ductal carcinoma in situ are ER positive.(17, 18) The literature on this topic is limited, although one study of 23 cases of ADH demonstrated ER staining in at least 95% of epithelial cells in all 23 cases.(7) However, in another very small sample, Schmitt et al showed ER staining in only three out of 5 cases of ADH.(9) Our large cohort study with digital quantification of ER expression in ADH reveals heterogeneity of ER expression in these lesions. Seventy percent of the atypical ductal hyperplasias in our cohort demonstrated staining in 50% or more of the cells. The remaining 30% had less ER expression. This proportion of ADH samples with low ER staining differs from the small prior published series. Nevertheless, we believe these samples represent true negatives since all demonstrated the usual pattern of scattered cells with nuclear ER staining in benign lobules as a positive internal control. Furthermore, neither intensity nor percent of ER staining correlated with year of diagnosis, which implies that technical factors such as fixation or tissue deterioration did not impair staining sensitivity (data not shown).
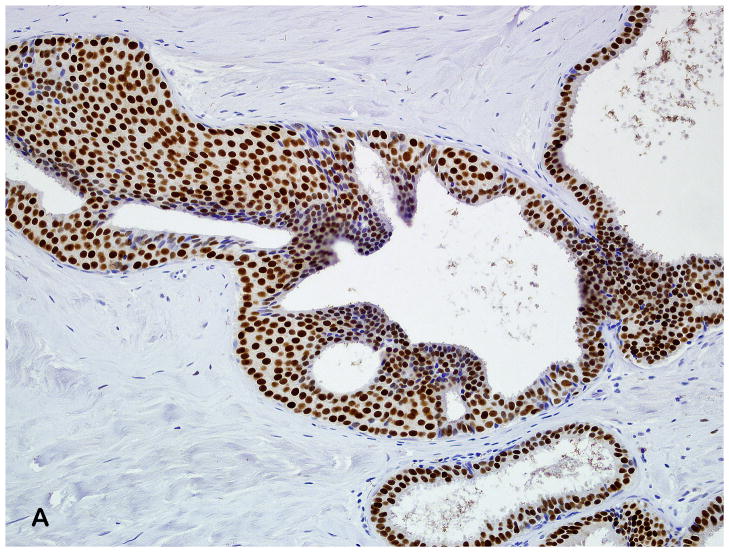
In our cohort, the percent of ER staining and intensity was greater in atypical ductal hyperplasia (mean 56%) than atypical lobular hyperplasia (mean 28%). A few recent studies have examined ER expression in lobular neoplasia.(19–20) Nonni and colleagues(19) examined ER expression in 30 patients with either lobular carcinoma in situ and/or atypical lobular hyperplasia, but the exact number of patients with each lesion is unknown. They found that in all 30 samples, areas of lobular neoplasia demonstrated ER alpha staining, both by percent cells positive (mean 68%) and by Allred score (mean 6.9). In another study, Middleton and colleagues showed that the median percentage of cells that stained for ER alpha in lobular carcinoma in situ was 75%, with an intensity range of 25% weakly positive to 100% strongly positive.(20) We found that atypical ductal hyperplasia demonstrated increased intensity in the majority of cases, while atypical lobular hyperplasia showed relatively less ER alpha expression (both percent staining and intensity). Lesser ER scores in ALH could potentially reflect relatively higher proportions of residual normal acinar epithelium or myoepithelial cells, since mixed populations of cells can be seen in lobular neoplasia lesions as opposed to the more uniform epithelial proliferation in ADH (Figure 4). Of interest, in our cohort of women with atypia, atypical ductal hyperplasia and atypical lobular hyperplasia had a unimodal distribution but differed in expression frequency.
Figure 4.
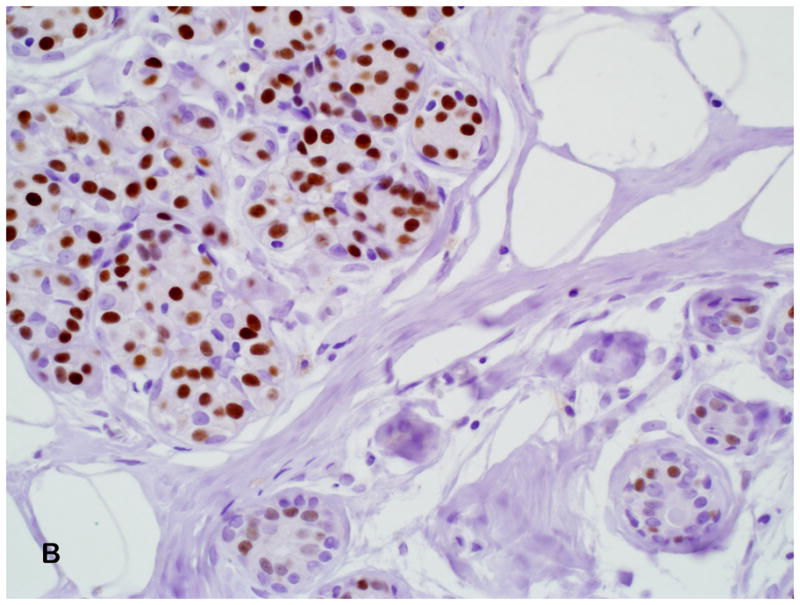
(A) Diffuse and strong ER expression in atypical ductal hyperplasia (Estrogen receptor antibody, 200X). (B) Moderate ER expression in atypical lobular hyperplasia; high power magnification demonstrates some heterogeneity in intensity of nuclear staining with the ALH lesion, and low intensity and percent nuclear staining in adjacent normal lobule (Estrogen receptor antibody, 400X).
Another interesting finding in our study is that women greater than 55 years of age with atypia had increased ER expression compared to younger women with atypia. This differs from the previous report by Shoker and colleagues(7) who found that ER expression was high in atypical ductal hyperplasia, ductal carcinoma in situ and lobular neoplasia regardless of age. The number of samples in that study was somewhat small; 23 cases of atypical hyperplasia, 43 cases of ductal carcinoma in situ, and 32 cases of lobular neoplasia. The authors suggested that in atypia and carcinoma in situ the regulation of ER expression may escape the normal age-related regulatory mechanisms. ER expression and relationship to age has also been studied in hyperplastic enlarged lobular units, a common alteration of the normal terminal duct lobular unit and potential precursor of breast cancer. Lee and colleagues(21) have shown that ER expression in hyperplastic enlarged lobular units is increased in post-menopausal woman compared to pre-menopausal women. Their study included 398 hyperplastic enlarged lobular units from 262 breasts.
Women with atypical hyperplasia have an approximate four-fold increase in breast cancer risk.(12, 13, 22, 23) Large, prospective breast cancer risk reduction trials of selective ER modulators (i.e., tamoxifen and raloxifene) have shown decreased breast cancer incidence in high risk women, especially those with atypia.(11, 24, 25) Therefore, it is reasonable to hypothesize that cancer risk should be higher in women whose benign breast tissues have higher ER expression. Most studies attempting to evaluate the association between ER expression in benign breast tissue and breast cancer risk have approached the problem by studying benign, premalignant lesions that are in proximity to concomitant malignancy.(6, 7) Although this is important information, it cannot evaluate the risk for a future cancer associated with the precursor lesion. To our knowledge, there are only a few previously published studies that have evaluated the association of ER expression in benign breast tissue without a concomitant cancer and future breast cancer risk. One report from Shaaban et al was a case control study of 44 women with hyperplasia of the usual type- 25 who later developed breast cancer and 19 who did not.(10) They found that ER-alpha expression was significantly higher (median 57% of cells) among women who later developed cancer compared to those who did not (median 30% of cells). It is interesting to note that in usual hyperplasia without atypia, ER positive cells were intermingled with ER negative cells. In the second study by Fabian et al, ER expression in random periareolar fine needle aspirates in high risk women was associated with future breast cancer risk by univariate analysis but not by multivariate analysis.(26)
Our study is unique because it represents the only report in women with atypical hyperplasia that evaluates association of ER expression with risk for a breast cancer in subsequent years. In contrast to the findings of Shaaban et al on risk and ER expression in usual ductal hyperplasia,(10) we found that ER expression in atypical hyperplasia did not correlate significantly with breast cancer risk. It is possible that there may be a small magnitude association of ER expression and risk that we could not detect with our sample size (we had adequate statistical power to detect approximately two-fold differences in risk across expression categories). Nevertheless, our cohort is one of the largest groups of atypical hyperplasia that has longterm follow-up on breast cancer events. It is also possible that ER expression in atypia does not correlate with breast cancer risk because whatever underlying aberration in estrogen regulation contributed to the development of atypia is already accounted for by the presence of the atypia itself.
In addition to the large size of our study cohort, another strength of our study is central pathology review, which is important because the analysis of atypical hyperplasia can be difficult due to these lesions being focal and relatively uncommon. We also used digital image analysis to quantify ER expression and the inclusion of all foci of atypia for assessment. ER has been used for predicting response to endocrine therapy of breast cancer for many years.
Immunohistochemistry is the major assay used and interpretation is usually done manually, thus dependent on the expertise and the ability of the interpreter. Computerized image analysis systems have been used to provide a more accurate means of quantification of ER. Analysis and quantification of immunohistochemical stains using digital microscopy is more likely to be accurate and reproducible in comparison to manual interpretation. Previous studies utilizing the ACIS instrument for analysis of HER-2/neu staining in breast carcinoma specimens have demonstrated improved objectivity and accuracy with this methodology over manual microscopy.(27–29) Our study allowed a more accurate way of measuring percent staining and intensity of ER expression among multiple foci of atypical hyperplasia.
CONCLUSION
Estrogen plays a major role in breast cancer development. ER expression is present in benign proliferative breast disease as well as in atypical hyperplasias and in situ carcinomas. We quantified ER expression in a large cohort of women with atypical hyperplasia and showed that ER expression is higher in atypical ductal hyperplasia than in atypical lobular hyperplasia. In contrast to other studies, we found that the expression of ER increased with age at diagnosis of atypical hyperplasia. Despite evidence that estrogen exposure is related to breast cancer risk and atypical hyperplasia has higher ER expression compared to normal breast epithelium, we found that degree of ER expression in atypical hyperplasia does not correlate with breast cancer risk.
Acknowledgments
This work was supported by NCI R01 grant CA132879 and the Mayo Clinic Breast SPORE CA116201. Amy C. Degnim is supported by the CA90628-08 Paul Calabresi Award for Clinical-Translational Research (K12) via the Mayo Clinic Cancer Center. We thank Joel Worra for database development; Teresa Allers, Joanne Johnson, Mary Campion, Melanie Kasner, and Romayne Thompson for data collection; Linda Murphy for immunostaining; and Patricia Haugen for her perspective as a patient advocate.
References
- 1.Howell A. Clinical evidence for the involvement of oestrogen in the development and progression of breast cancer. Proceeding of the Royal Society of Edinburgh. 1989;95:49–57. [Google Scholar]
- 2.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiologic reviews. 1993;15(1):36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 3.Allegra JC, Lippman ME, Green L, et al. Estrogen receptor values in patients with benign breast disease. Cancer. 1979;44(1):228–31. doi: 10.1002/1097-0142(197907)44:1<228::aid-cncr2820440137>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Petersen OW, Hoyer PE, van Deurs B. Frequency and distribution of estrogen receptor-positive cells in normal, nonlactating human breast tissue. Cancer Research. 1987;47(21):5748–51. [PubMed] [Google Scholar]
- 5.Ricketts D, Turnbull L, Ryall G, et al. Estrogen and progesterone receptors in the normal female breast. Cancer research. 1991;51(7):1817–22. [PubMed] [Google Scholar]
- 6.Visscher DW, Padaiyar N, Long D, Tabaczka P. Immunohistologic analysis of estrogen receptor expression in breast carcinoma precursor lesions. The Breast Journal. 1998;4:447–51. [Google Scholar]
- 7.Shoker BS, Jarvis C, Sibson DR, Walker C, Sloane JP. Oestrogen receptor expression in the normal and pre-cancerous breast. The Journal of Pathology. 1999;188(3):237–44. doi: 10.1002/(SICI)1096-9896(199907)188:3<237::AID-PATH343>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Shoker BS, Jarvis C, Clarke RB, et al. Estrogen receptor-positive proliferating cells in the normal and precancerous breast. The American Journal of Pathology. 1999;155(6):1811–5. doi: 10.1016/S0002-9440(10)65498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitt FC. Multistep progression from an oestrogen-dependent growth towards an autonomous growth in breast carcinogenesis. Eur J Cancer. 1995;31A(12):2049–52. doi: 10.1016/0959-8049(95)00430-0. [DOI] [PubMed] [Google Scholar]
- 10.Shaaban AM, Sloane JP, West CR, Foster CS. Breast cancer risk in usual ductal hyperplasia is defined by estrogen receptor-alpha and Ki-67 expression. The American Journal of Pathology. 2002;160(2):597–604. doi: 10.1016/s0002-9440(10)64879-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. Journal of the National Cancer Institute. 2005;97(22):1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. The New England Journal of Medicine. 2005;353(3):229–37. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 13.Degnim AC, Visscher DW, Berman HK, et al. Stratification of breast cancer risk in women with atypia: a Mayo cohort study. J Clin Oncol. 2007;25(19):2671–7. doi: 10.1200/JCO.2006.09.0217. [DOI] [PubMed] [Google Scholar]
- 14.Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985;55(11):2698–708. doi: 10.1002/1097-0142(19850601)55:11<2698::aid-cncr2820551127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Page DL, Rogers LW. Combined histologic and cytologic criteria for the diagnosis of mammary atypical ductal hyperplasia. Human Pathology. 1992;23(10):1095–7. doi: 10.1016/0046-8177(92)90026-y. [DOI] [PubMed] [Google Scholar]
- 16.Milanese TR, Hartmann LC, Sellers TA, et al. Age-related lobular involution and risk of breast cancer. Journal of the National Cancer Institute. 2006;98(22):1600–7. doi: 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- 17.Allred DC, Mohsin SK, Fuqua SA. Histological and biological evolution of human premalignant breast disease. Endocrine-related Cancer. 2001;8(1):47–61. doi: 10.1677/erc.0.0080047. [DOI] [PubMed] [Google Scholar]
- 18.Roger P, Daures JP, Maudelonde T, et al. Dissociated overexpression of cathepsin D and estrogen receptor alpha in preinvasive mammary tumors. Human Pathology. 2000;31(5):593–600. doi: 10.1053/hp.2000.6687. [DOI] [PubMed] [Google Scholar]
- 19.Nonni A, Zagouri F, Sergentanis TN, Lazaris AC, Patsouris ES, Zografos GC. Immunohistochemical expression of estrogen receptors alpha and beta in lobular neoplasia. Virchows Arch. 2007;451(5):893–7. doi: 10.1007/s00428-007-0504-6. [DOI] [PubMed] [Google Scholar]
- 20.Middleton LP, Perkins GH, Tucker SL, Sahin AA, Singletary SE. Expression of ERalpha and ERbeta in lobular carcinoma in situ. Histopathology. 2007;50(7):875–80. doi: 10.1111/j.1365-2559.2007.02689.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Mohsin SK, Mao S, Hilsenbeck SG, Medina D, Allred DC. Hormones, receptors, and growth in hyperplastic enlarged lobular units: early potential precursors of breast cancer. Breast Cancer Res. 2006;8(1):R6. doi: 10.1186/bcr1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. The New England Journal of Medicine. 1985;312(3):146–51. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 23.London SJ, Connolly JL, Schnitt SJ, Colditz GA. A prospective study of benign breast disease and the risk of breast cancer. JAMA. 1992;267(7):941–4. [PubMed] [Google Scholar]
- 24.Vogel VG. The NSABP Study of Tamoxifen and Raloxifene (STAR) trial. Expert review of anticancer therapy. 2009;9(1):51–60. doi: 10.1586/14737140.9.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 26.Fabian CJ, Kimler BF, Zalles CM, et al. Short-Term Breast Cancer Prediction by Random Periareolar Fine-Needle Aspiration Cytology and the Gail Risk Model. Journal of the National Cancer Institute. 2000;92(15):1217–27. doi: 10.1093/jnci/92.15.1217. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Saboorian MH, Frenkel EP, et al. Assessment of HER-2/neu status in breast cancer. Automated Cellular Imaging System (ACIS)-assisted quantitation of immunohistochemical assay achieves high accuracy in comparison with fluorescence in situ hybridization assay as the standard. American Journal of Clinical Pathology. 2001;116(4):495–503. doi: 10.1309/TMUW-G4WB-LXJ2-FUDN. [DOI] [PubMed] [Google Scholar]
- 28.Bloom K, Harrington D. Enhanced accuracy and reliability of HER-2/neu immunohistochemical scoring using digital microscopy. American Journal of Clinical Pathology. 2004;121(5):620–30. doi: 10.1309/Y73U-8X72-B68T-MGH5. [DOI] [PubMed] [Google Scholar]
- 29.Tawfik OW, Kimler BF, Davis M, et al. Comparison of immunohistochemistry by automated cellular imaging system (ACIS) versus fluorescence in-situ hybridization in the evaluation of HER-2/neu expression in primary breast carcinoma. Histopathology. 2006;48(3):258–67. doi: 10.1111/j.1365-2559.2005.02322.x. [DOI] [PubMed] [Google Scholar]