Abstract
When comparing a cumulative dose-response curve for endothelin-1 (ET-1)-induced mechanical hyperalgesia to the effect of individual doses (1 ng, 10 ng, 100 ng and 1 µg) administered in separate groups of rats, a marked difference was observed in the peak magnitude of hyperalgesia. Hyperalgesia was measured as decrease in the threshold for mechanically-induced withdrawal of the hind paw. The cumulative dosing protocol produced markedly greater maximum hyperalgesia. To determine whether this was due to the cumulative dosing protocol or to the repeated exposure to the mechanical test stimulus, we evaluated the impact of repeated testing on ET-1-induced mechanical hyperalgesia. While ET-1-induced mechanical hyperalgesia was dose- and time-dependent, repeated testing of nociceptive threshold, at 5 minute intervals, following a single dose of ET-1, produced further decrease in nociceptive threshold. This mechanical stimulation-induced enhancement of ET-1 hyperalgesia lasted only 3–4 hrs, while the hyperalgesia lasted in excess of 5 days. The stimulation-enhanced hyperalgesia also occurred after a second injection of ET-1, administered 24 hours after the initial dose. That this phenomenon is unique to ET-1 is suggested by the observation that while five additional, direct-acting hyperalgesic agents — PGE2, NGF, GDNF, IL-6 and TNFα — induced robust mechanical hyperalgesia, none produced mechanical stimulation-enhanced hyperalgesia.
Keywords: Endothelin, ET-1, Hyperalgesia, Stimulus-induced Enhancement, Vasoconstrictor, Pain
Endothelins 1–3 are a family of 21-amino acid isopeptides produced in large part by vascular endothelium (Butt et al., 2010). Endothelins act as structurally and pharmacologically distinct potent vasoconstrictors (Yanagisawa et al., 1988, Inoue et al., 1989). Endothelin receptors (i.e., ETA and ETB) are located on sensory neurons (Laziz et al., 2010, Werner et al., 2010), as well as in blood vessels (Sanchez et al., 2010), where endothelin-1 (ET-1) acts to sensitize and, at high concentrations activate nociceptors (Khodorova et al., 2009). Given the close proximity of nociceptor terminals to blood vessels, it is not surprising that the role of ET-1 in pain has been most closely associated with clinical conditions, in which vascular pathophysiology has been strongly implicated, such as unstable (Killip, 1980, Krishnan et al., 2010) and variant (Jang et al., 2008, Lee et al., 2008) angina, Raynaud’s syndrome (Bottomley and Goodfield, 1994, Edwards et al., 1999) sickle cell crisis (Angerio and Lee, 2003) and complex regional pain syndrome (Noori and Kabbani, 2003, Groeneweg et al., 2008, Millecamps et al., 2010). To further elucidate the sensitization induced by ET-1, we studied ET-1-induced mechanical hyperalgesia in the skin, a tissue in which neurovascular interactions are known to be important (Cameron and Cotter, 1996, 1997). We now describe a novel phenomenon associated with ET-1-induced mechanical hyperalgesia, marked enhancement by repeated testing with threshold intensity noxious mechanical stimuli, and characterize its dose-dependence and temporal relationship with respect to ET-1- induced mechanical hyperalgesia.
EXPERIMENTALPROCEDURES
Animals
Experiments were performed on adult male Sprague–Dawley rats (220–250 g; Charles River, Hollister, CA). They were housed three per cage, under a 12-h light/dark cycle, in a temperature and humidity controlled environment. Food and water were available ad libitum. All nociceptive testing was done between 10:00 am and 4:00 pm. All experimental protocols were approved by the UCSF Committee on Animal Research and conformed to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
Nociceptive testing
The nociceptive flexion reflex was quantified with an Ugo Basile Analgesymeter® (Stoelting, Chicago, IL, USA), which applies a linearly increasing mechanical force to the dorsum of the rat’s hind paw. Nociceptive threshold was defined as the force, in grams, at which the rat withdrew its paw, but in no case did the applied mechanical force exceed 200 g (cutoff). Hyperalgesia was defined as decrease in nociceptive threshold as a percent of the baseline threshold. Rats were lightly restrained in cylindrical transparent acrylic restrainers that have triangular windows on the side, which allow extension of the hind leg from the restrainer for testing nociceptive threshold. To acclimatize rats to the testing environment, they were brought to the experimental area in their home cages 15–30 minutes prior to placing them in the restrainers. Another 15–30 minutes elapsed before starting the experiment. Experiments were begun only after they were quiet in their restrainers. This acclimatization procedure consistently results in baseline paw withdrawal thresholds of 100–110 g for rats weighing 220–250 g, the body weight range for the rats used in this study. Actual baseline paw withdrawal thresholds in this study were 106.37±0.95 g (mean ± s.e.m.). Each paw was treated as an independent measure and both paws of the same rat received the same treatment. Each experiment was performed on separate groups of rats, and no paw was treated with a second injection except in the cases of cumulative dose-response curve and the tachyphylaxis experiment (see Results).
Drugs
The drugs employed in this study were: endothelin-1 (ET-1), prostaglandin E2 (PGE2) and interleukin-6 (IL-6) (Sigma-Aldrich, St. Louis, MO, USA), tumor necrosis factor alpha (TNFα) and nerve growth factor (NGF) (R&D Systems, Minneapolis, MN, USA), and glia-derived neurotrophic factor (GDNF) (EMD Bioscience, Pacific Center, San Diego, CA, USA). All drugs were dissolved in saline and administered by intradermal injection on the dorsum of the hind paw. The doses for ET-1 were determined from two dose-response studies conducted as part of this study (see Results). For all other drugs the doses employed in this study were based on our previous studies (Aley and Levine, 1999, Parada et al., 2003, Malik-Hall et al., 2005, Bogen et al., 2008, Summer et al., 2008). All drugs were administered intradermally in a volume of 5 µl using a 30-gauge hypodermic needle attached to a micro-syringe (Hamilton, Reno, NV) by PE-10 tubing.
Statistical analysis
For the single dose per animal dose-response experiment, one-way between-subjects ANOVA followed by Scheffé post hoc tests was employed. For the remaining experiments one-way or two-way repeated measures ANOVAs were employed. If there was a significant group × time interaction, multivariate analyses (i.e., one-way ANOVAs) were performed for all time points in order to determine which points accounted for the interaction. In these cases, a Bonferroni correction was applied in order to account for multiple comparisons. For within-subjects effects the Mauchly criterion was used to determine if the assumption of sphericity was met; if not, Greenhouse-Geiser p-values are presented. Statistical significance (i.e. the α-level) was set at p < 0.05.
Results
Dose dependence of ET-1 hyperalgesia
The dose-dependence of ET-1 mechanical hyperalgesia was assessed by two methods. In the cumulative dosing protocol, a single group of rats (n=4) received four logarithmically increasing doses (1 ng – 1 µg; 0.4 pmol – 400 pmol) with a 30 minute interval between each dose. In the single dose per animal protocol each of the four doses was administered to a different groups of rats (n=4 per group).
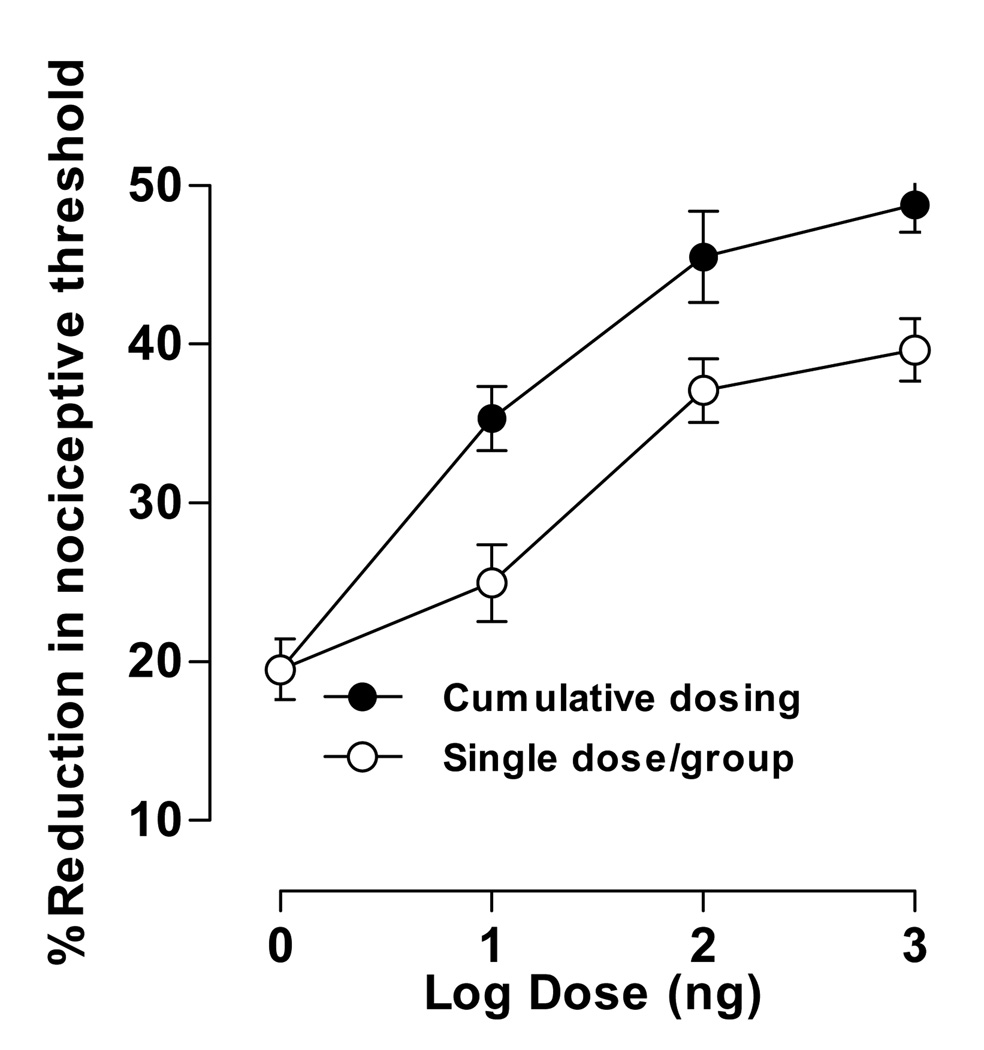
In the single dose per animal protocol, the lowest dose of ET-1 tested (1 ng) produced a 20±1.4% decrease in nociceptive threshold; higher doses produced monotonically greater decreases in threshold; the highest dose (1 µg) produced a 40±2.7% decrease in threshold (Fig. 1). The decrease in threshold appears to be asymptotic at the two highest ET-1 doses.
Figure 1. Dose-response of ET-1-induced hyperalgesia, effect of cumulative dosing compared to single dose per animal.
Four logarithmically increasing doses of ET-1 (1 ng, 10 ng, 100 ng and 1 µg) were administered in both dosing regimens. For cumulative dosing, a single group of rats received all four doses administered at 30 minute intervals; separate groups of rats received each dose in the single dose per animal regimen. Each data point represents the average paw withdrawal threshold measured at 15, 20, and 25 minutes after ET-1 administration. For the cumulative dosing group one-way repeated measures ANOVA showed a significant effect of dose (F3,9=32.492; p<0.001); simple contrasts showed that all doses were significantly different from the first dose (p=0.012, p=0.010, p=0.003, respectively). For the single dose groups one-way between subjects ANOVA showed a significant main effect (F3,12=21.485; p<0.001); Scheffé post hocs showed that the lowest dose (1 ng) did not differ significantly from the second lowest (10 ng) dose (p=0.367) but did differ significantly from the two highest doses (p=0.001, p<0.001, respectively). To compare the effects of cumulative and single dosing at each time point, univariate analyses showed: 1 ng (p=1.000), 10 ng (p=0.017), 100 ng (p=0.053), 1 µg (p=0.013), suggesting that cumulative dosing induces greater hyperalgesia than a single dose per animal protocol. In this and subsequent figures data are plotted as mean ± s.e.m.
In the cumulative dosing protocol, the lowest dose of ET-1 (1 ng) produced a decrease in nociceptive threshold (20±1.4%) similar to that observed in the single dose per animal protocol. However, for each of the higher doses the magnitude of the hyperalgesia was greater in the cumulative dosing group. The highest dose (1 µg) decreased nociceptive threshold 49±3.2% in the cumulative dosing protocol, compared to 40±2.7% in the single dose per animal protocol (Fig. 1).
Time course of ET-1 hyperalgesia
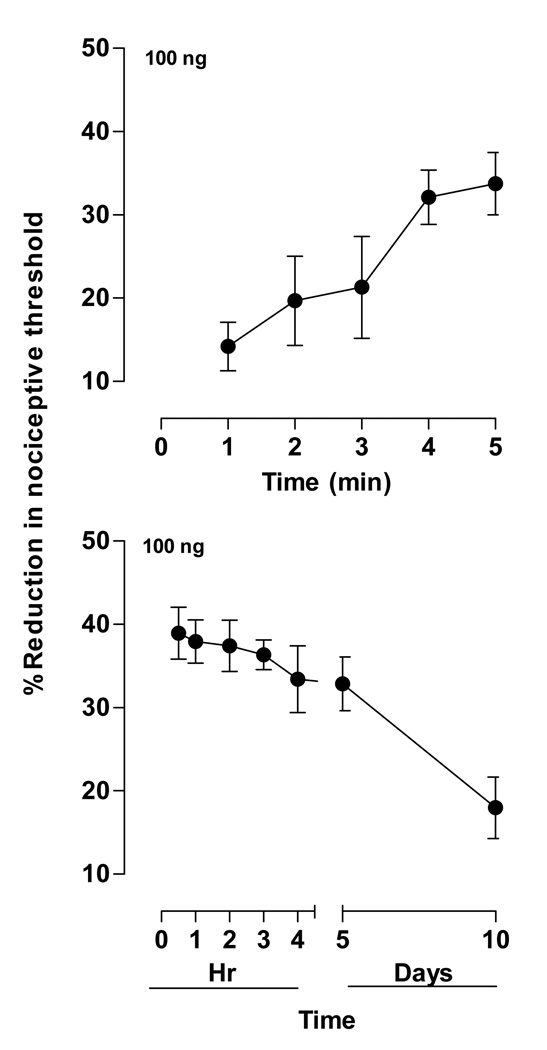
To characterize the time course of ET-1-induced hyperalgesia, a single dose of ET-1 (100 ng) was administered to two separate groups of rats (n=4 per group). Onset of ET-1-induced hyperalgesia was examined in a group in which testing began one minute after administration (Fig. 2A), and duration of hyperalgesia was examined in another group in which testing began 30 minutes after ET-1 administration and continued out 10 days (Fig. 2B).
Figure 2. Time course of ET-1-induced hyperalgesia.
A. Onset. Paw withdrawal thresholds measured at one minute intervals after ET-1 (100 ng) administration showed a steep rise in hyperalgesia to near-maximal levels within five minutes. One-way repeated measures ANOVA showed a significant effect of time (F4,20=16.921; p=0.002).
B. Duration. Paw withdrawal thresholds measured at 0.5, 1, 2, 3, 4, and 5 hours, and 5 and 10 days after ET-1 (100 ng) administration showed a gradual decrease in hyperalgesia to near-baseline levels by day 10. One-way repeated measures ANOVA showed a significant effect of time (F6,30=7.276; p=0.005).
Onset
Decreased mechanical nociceptive threshold (14.1±2.9%; n=4) was already present at the first time point assayed, 1 minute after administration (Fig. 2A). The decrease in threshold was maximal (33±3.7%) by approximately 5 minutes after administration.
Duration
Decrease in mechanical threshold persisted at near maximal levels out to five days and were still significant though diminished at 10 days after ET-1 administration, the last time point sampled in this experiment (Fig. 2B).
Effect of repeated stimulation on ET-1-induced hyperalgesia
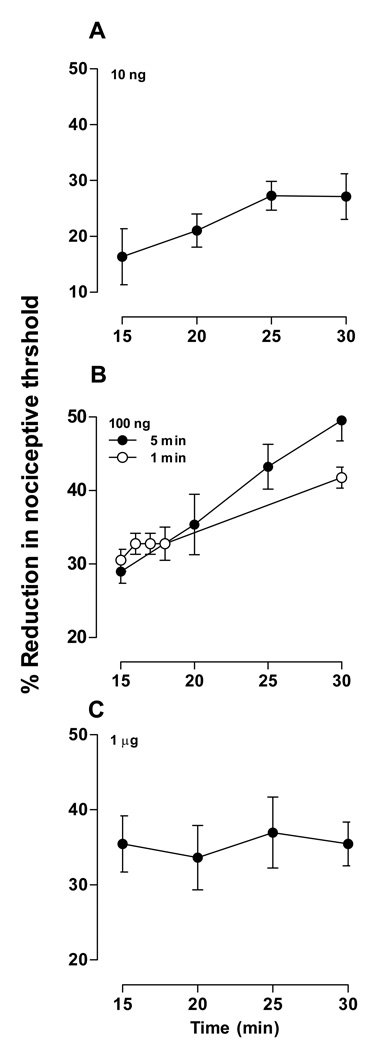
Although the enhanced hyperalgesia observed in the cumulative dosing protocol (Fig. 1) might be due to the accumulation of ET-1 in the paw, this explanation seems unlikely because the accumulated amount of all previous doses would constitute only slightly more than 10% of each succeeding dose. Another difference between the cumulative dosing and the single dose per animal protocols was that the cumulative dosing group received repeated paw withdrawal testing. Therefore, to determine if the repeated mechanical stimulation was responsible for the markedly greater hyperalgesia observed, we tested the effect of repeated stimulation after the administration of a single dose of ET-1. Three doses of ET-1 (10 ng, n = 6; 100 ng, n = 6; and 1 µg, n = 6) were administered in separate groups (Fig. 3A–C). Paw withdrawal thresholds were measured at five minute intervals for 15 minutes, starting 15 minutes after the second ET-1 administration. One additional group that received ET-1 (100 ng, n = 4) was measured at one minute intervals for four minutes, starting 15 minutes after administration, and then one last measurement at 30 minutes (Fig. 3B).
Figure 3. Effect of repeated stimulation on ET-1-induced hyperalgesia.
Paw withdrawal thresholds were measured every five minutes starting 15 minutes after ET-1 administration. Different doses were administered to different groups of rats. Low dose (10 ng). The hyperalgesic effect of ET-1 did not change significantly with stimulation repeated at five minute intervals at this dose (F3,15=1.987; p=0.188). Medium dose (100 ng). Two stimulation protocols (“5 min” and “1 min”) were tested at this dose in different groups of rats. The hyperalgesic effect of ET-1 differed significantly depending on the repeated stimulation protocol. Two-way repeated measures ANOVA showed a significant group × time interaction (F1,8=11.302; p=0.010) as well as a significant main effect of time (F1,8=131.542; p<0.001), but not a significant main effect of group (F1,8=1.301; p=0.287). Based on the significant interaction, one-way repeated measures ANOVAs were performed for each group. For the five minute interval group, this analysis showed a significant effect of time (F3,15=33.716; p<0.001), indicating that repeated stimulation significantly increased the hyperalgesic effect of ET-1. For the one minute interval group, this analysis was not significant (F4,12=6.333; p<0.069), indicating that enhancement of ET-1 hyperalgesia is frequency dependent.
High dose (1 µg). The hyperalgesic effect of ET-1 did not change significantly with stimulation repeated at five minute intervals at this dose (F3,15=0.129; p=0.942).
Low dose ET-1 (10 ng)
Although ET-1-induced hyperalgesia tended to increase with successive testing, the effect did not reach statistical significance (Fig. 3A).
Middle dose ET-1 (100 ng)
Repeated testing significantly enhance ET-1-induced hyperalgesia in the group that was tested at five minute intervals, but not in the group that was tested at 1 minute intervals (Fig. 3B).
High dose ET-1 (1 ug)
Repeated testing failed to enhance ET-1-induced hyperalgesia in this group (Fig. 3C).
Taken together, these results suggest that ET-1 not only induces hyperalgesia, but also produces a second hyperalgesia effect related to repeated testing. Furthermore, these effects are additive. The lack of significant stimulation-enhanced hyperalgesia in Figs. 3A, 3C, and in the one minute interval group in Fig. 3B, indicates that stimulation-enhanced ET-1 hyperalgesia is dependent of both drug dose and frequency of stimulation.
Duration of stimulation-enhanced hyperalgesia
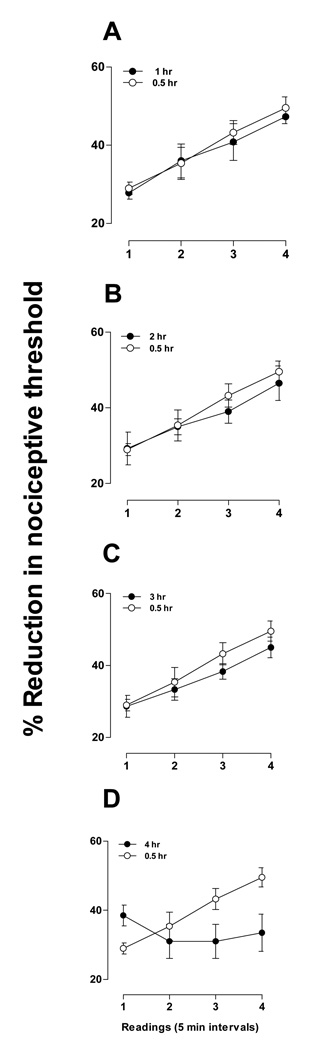
To determine the time course over which ET-1 produces stimulation-enhanced hyperalgesia, we administered ET-1 to four groups and tested each group at successively longer intervals after administration (Fig. 4A – C). The dose of ET-1 (100 ng, n = 6) and the stimulation protocol (five minute intervals for 15 minutes) were the same as the group shown in Fig. 3B that exhibited stimulation-enhanced hyperalgesia. Groups tested within the first three hours (Fig. 3A – C; n = 6, n = 4, n = 6, respectively) all showed stimulation-enhanced hyperalgesia that was not significantly different from the originally-observed group in Fig. 3B, which was tested in the first 30 minutes after ET-1 administration. The group tested in the fourth hour (n = 4), however, failed to demonstrate stimulation-enhanced hyperalgesia. These results indicate that, although ET-1-induced hyperalgesia lasts several days (Fig. 2), stimulation-enhanced hyperalgesia is a shorter duration phenomenon that disappears within four hours of ET-1 administration.
Figure 4. Duration of stimulation-enhanced hyperalgesia.
Separate groups of rats were tested for stimulation-induced hyperalgesia at increasing intervals of one hour after ET-1 (100 ng) administration (1 – 4 hours, panels A – D). (Note: groups are designated by the time of the last measurement since ET-1 administration.) Each group was tested at five minute intervals for 15 minutes (Readings 1 – 4), similar to the 5 minute interval 30 minute group shown in Fig. 3B (replotted in this figure for comparison). Two-way repeated measures ANOVA with one within-subjects factor (time) and one between-subjects factor (group) was performed for each of the four experiments. The 1 hour, 2 hour, or 3 hour groups each showed a significant main effect of time (F3,30=32.035; p<0.001), (F3,24=36.971; p<0.001), and (F3,30=29.956; p<0.001), respectively, but not a significant group × time interaction (F3,30=0.210; p=0.832), (F3,24=0.653; p=0.533), and (F3,30=0.558; p=0.592), nor a significant main effect of group (F1,10=0.118; p=0.738), (F1,8=0.196; p=0.669), and (F1,10=0.826; p=0.385). Thus, while there was an overall change over time for these three groups, there was no significant difference between the groups. The 4 hour group showed a significant main effect of time (F3,24=7.286; p<0.001) as well as a significant group × time interaction (F3,24=15.808; p<0.001), but not a significant main effect of group (F1,8=1.539; p=0.250). Based on the significant interaction, separate one-way repeated measures ANOVAs were performed for each of the two groups. The effect of time for the four hour group was not significant (F3,9=1.800; p=0.217), indicating that stimulation-enhanced hyperalgesia was no longer present at the four hour time point. See Fig. 3B for the ANOVA result for the 30 minute group.
Lack of tachyphylaxis of stimulation-enhanced hyperalgesia
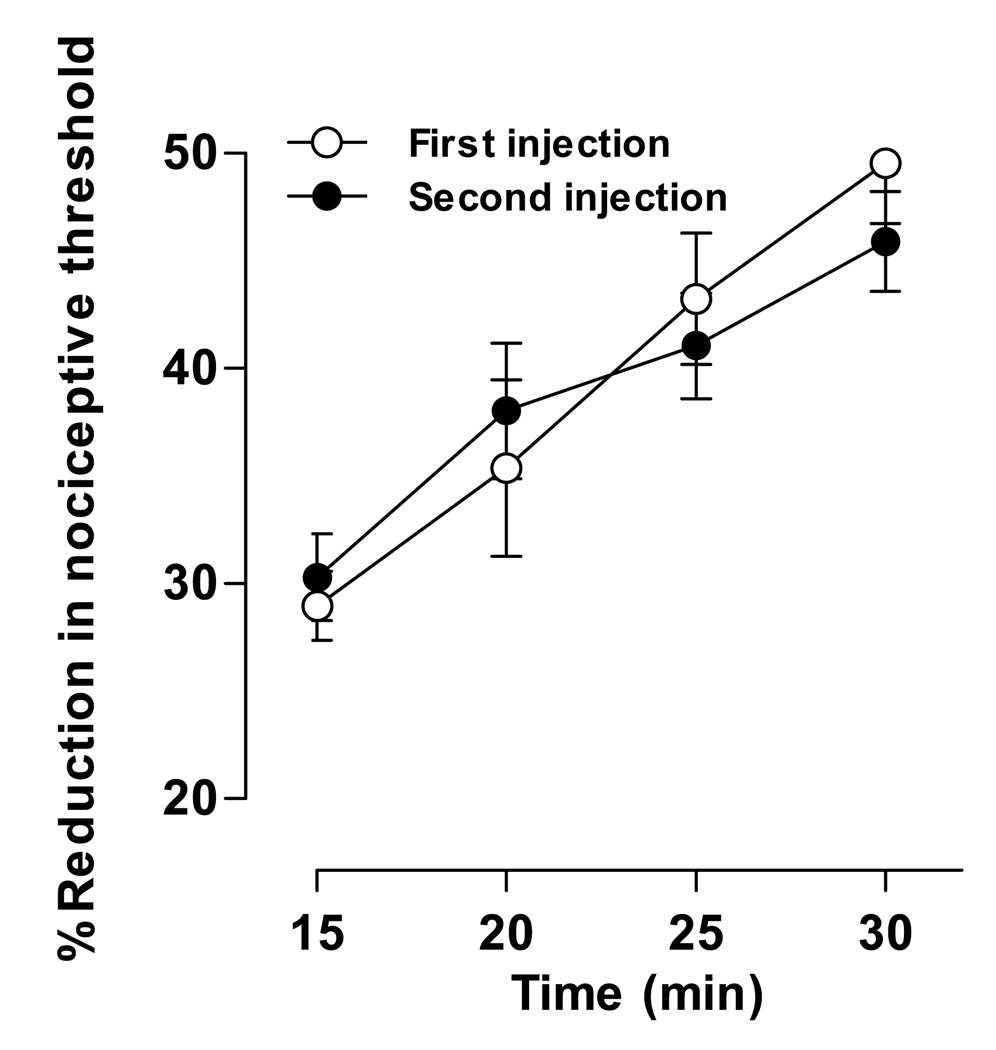
Because the duration of stimulation-enhanced hyperalgesia is much shorter than that of ET-1 hyperalgesia, we determined if stimulation-enhanced hyperalgesia undergoes tachyphylaxis. ET-1 (100 ng, n = 6) was administered twice, with the second injection 24 hours after the first. Stimulation-enhanced hyperalgesia following the second ET-1 administration was indistinguishable from that following a single injection (Fig. 5), suggesting that tachyphylaxis for stimulation-enhanced hyperalgesia does not occur with repeated administration.
Figure 5. The effect of repeated ET-1 administration on stimulation–enhanced hyperalgesia.
ET-1 (100 ng) was administered twice, the second dose 24 hours after the first. Paw withdrawal thresholds were measured at five minute intervals for 15 minutes, starting 15 minutes after the second ET-1 administration. Data for the 5 minute interval 30 minute group shown in Fig. 3B is replotted in this figure for comparison. Two-way repeated measures ANOVA with one within-subjects factor (time) and one between-subjects factor (group) showed no significant differences between the two groups. Main effect of group was F1,10=0.022; p=0.886; and the group × time interaction was F3,30=1.129; p=0.349. These results indicate that stimulation-enhanced hyperalgesia does not undergo tachyphylaxis with repeated ET-1 administration.
Effect of other proalgesic substances
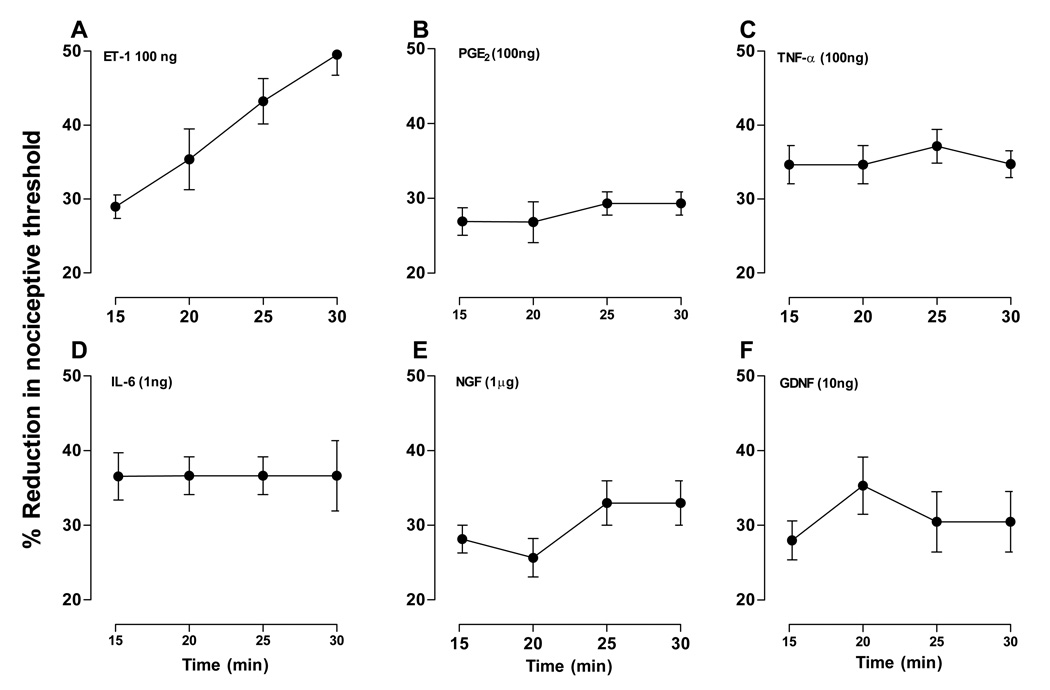
Since stimulation-enhanced hyperalgesia has not, to our knowledge, been previously reported for any algesic compound, we investigated whether other compounds known to produce hyperalgesia also induce this novel phenomenon (Fig. 6A – F). The following compounds were tested: prostaglandin E2 (PGE2, n = 4), tumor necrosis factor alpha (TNFα, n = 4), interleukin-6 (IL-6, n = 4), nerve growth factor (NGF, n = 4) and glia-derived neurotrophic factor (GDNF, n = 4). Paw withdrawal thresholds were tested at 5 minute intervals for 15 minutes starting 15 minutes after administration, the same stimulation protocol used in the group shown in Fig. 3B that exhibited stimulation-enhanced hyperalgesia (data replotted in Fig. 6A). Although all compounds showed hyperalgesia (Fig. 6B – F), in no case did repeated stimulation enhance this effect.
Figure 6. Effect of repeated stimulation on hyperalgesia for other algesic agents.
The ability of other proalgesic substances to induce stimulation-enhanced hyperalgesia was tested. Prostaglandin E2 (PGE2, 100 ng, panel B), tumor necrosis factor alpha (TNFα, 100 ng, panel C), interleukin-6 (IL-6, 1 ng, panel D), nerve growth factor (NGF, 1 µg, panel E) and glia-derived neurotrophic factor (GDNF, 10 ng, panel F) were administered. Paw withdrawal thresholds were measured at five minute intervals for 15 minutes, starting 15 minutes after administration. Data for the 5 minute interval 30 minute group that received ET-1, shown in Fig. 3B, is replotted in this figure (panel A) for comparison. One-way repeated measures ANOVA showed there was no significant effect of time in any of these experiments: PGE2 (F3,9=0.600; p=0.631), TNF (F3,9=0.225; p=0.804), IL-6 (F3,9=0.007; p=1.000), NGF (F3,9=1.670; p=0.281), GNDF (F3,9=0.659; p=0.510). These results suggest that stimulation-enhanced hyperalgesia is a novel property of ET-1.
Discussion
Two effects of ET-1 on the peripheral terminal of the primary afferent nociceptor have been described, sensitization produced by low-to-moderate doses of ET-1, and activation produced by high doses (Khodorova et al., 2009). In the present study we characterized the sensitizing effects of ET-1 in the skin on the dorsum of the rat’s hind paw, a well vascularized tissue, where nociceptors might be expected to be exposed to, at the very least, sensitizing concentrations of ET-1.
While we have previously studied the effects of algesic compounds on nociceptor function using cumulative dosing protocols, (Khasar et al., 1995, Parada et al., 2003, Bogen et al., 2008) in preliminary studies different groups of animals are often exposed to single doses of the algesic compounds. When this was done for ET-1, we observed a phenomenon that we had not previously observed for any of a large number of other algesic compounds or for which we could find any previous examples in the literature, namely that the higher doses of ET-1 in the cumulative dose-response curve produced markedly greater hyperalgesia than when each dose of ET-1 was administered to separate groups of rats. This observation was not simply due to the effects of repeated injections because repeated injections of saline did not induce hyperalgesia (unpublished observation). Unexpectedly, our analysis demonstrated that it was the repeated mechanical stimulation rather than the previous lower dose of ET-1 that was responsible for the enhanced hyperalgesia observed in the cumulative dosing protocol. To further characterize this phenomenon, which we refer to as stimulation-enhanced hyperalgesia, we first examined the impact of the dose of ET-1 injected. The lowest dose of ET-1 (10 ng) did not induce significant hyperalgesia, nor did the highest dose (1 µg). In fact, the maximum hyperalgesia induced by the fourth stimulus was greater for the 100 ng dose of ET-1 than for the 1 µg dose, suggesting inhibition of stimulation-induced hyperalgesia with the higher dose. The mechanism by which high dose ET-1 can shut off stimulation-enhanced hyperalgesia, while still producing mechanical hyperalgesia, remains to be established; however, it has been shown that ET-1 can evoke the release of β-endorphin from keratinocytes through an action at the ETB receptor (Khodorova et al., 2009, Quang and Schmidt, 2010), suggesting one possible mechanism. That stimulation-induced enhancement of ET-1 hyperalgesia can be distinguished from ET-1 hyperalgesia is also supported by our finding that while stimulation-enhanced hyperalgesia had a duration of 3–4 hours following ET-1 administration, ET-1 hyperalgesia persisted for at least 5 days. Also, a second injection of ET-1, 24 hours after the first injection, again produced robust stimulation-enhanced hyperalgesia.
Since we could not locate prior reports of an effect similar to stimulation-enhanced hyperalgesia, for other proalgesic substances, and this phenomenon was not previously reported as an effect of ET-1, we used the protocol established for stimulation-induced enhancement of ET-1 hyperalgesia, to determine if this phenomenon is produced by several other direct-acting hyperalgesic mediators (i.e., PGE2, NGF, GDNF, IL-6 and TNFα). Of note, these compounds include proalgesic mediators that sensitize the TrkA (+)/IB4 (−) peptidergic (i.e., NGF) and Ret (−)/IB4 (+) non-peptidergic (i.e., GDNF) populations of nociceptors. While all five proalgesic substances produced robust mechanical hyperalgesia, none produced even a trend toward stimulation-enhanced hyperalgesia. In addition to confirming the uniqueness of stimulation-enhanced hyperalgesia as a proalgesic effect of ET-1, these findings, along with the temporal dissociation of ET-1-induced hyperalgesia and stimulation-induced enhancement of ET-1 hyperalgesia, support the suggestion that stimulation-enhanced hyperalgesia is an indirect effect, that is one that is not due to action of ET-1 on the peripheral terminals of nociceptor in the injected skin. This further distinguishes stimulation-enhanced hyperalgesia from ET-1-induced hyperalgesia, for which there is considerable evidence that it is produced by a direct action of ET-1 on the peripheral terminals of the primary afferent nociceptor (Hamamoto et al., 2008, Imamachi et al., 2009). Future studies will be required to determine if stimulation-enhanced hyperalgesia produced by ET-1 is mediated by its acting directly on nociceptor second messenger signaling pathways, indirectly by vascular mechanisms, or by other indirect effects of ET-1. If the effect of ET-1 is indirect then it will also be important to determine the substance released from the indirect target, by the mechanical stimulation, which acts on the primary afferent nociceptor to enhance mechanical hyperalgesia. Another important question for future experiments relates to how this unique effect of ET-1 might interact with hyperalgesia induced by other compounds of the inflammatory soup, since ET-1 has also been implicated in neuropathic pain in preclinical studies (Klass et al., 2005).
Why stimulation-enhanced hyperalgesia by ET-1 has not been described previously is currently unknown. Certainly if it is restricted to the algesic effects of ET-1, experimental protocols with this compound may have been markedly influenced by the experimental design used to study the hyperalgesic effects of a large number of other mediators that produce hyperalgesia but not stimulation-enhanced hyperalgesia. For example, threshold readings may have only been recorded after stable responses were observed, after 2 or 3 pre-readings. And, the time between test stimuli influences one’s ability to detect stimulation-enhanced hyperalgesia (Fig. 3A–C). Relatively, low neurovascular function of tissues in which nociceptive testing was performed might also prevent detection of stimulation-enhanced hyperalgesia. Furthermore, the complex dose-dependence of the nociceptive effects of ET-1, producing dose-dependent hyperalgesia at low- and mid-concentration range, and overt pain at high concentrations, may have obscured detection of stimulation-enhanced hyperalgesia in studies of ET-1-associated pain.
In conclusion, we report a novel dimension of ET-1-induced pain, one in which mechanical stimulation, at or below nociceptive threshold (which decreases from one stimulus to the next) produces a marked enhancement of ET-1-induced mechanical hyperalgesia in a highly vascular tissue, the skin on the dorsum of the hind paw. Importantly, the intensity of the stimulus needed to elicit this effect is, at most, only mildly noxious, being at or below nociceptive threshold. The fact that activity markedly enhances vascular pain syndromes (Cameron and Cotter, 1996) supports the suggestion that stimulation-enhanced hyperalgesia may have a prominent role in pain arising from the blood vessels. However, until such time as the mechanism of stimulation-enhanced hyperalgesia is elucidated it will be hard to specify its precise role in ET-1-dependent pain syndromes.
Acknowledgements
This work was funded by a grant from the NIH. The authors have no actual or potential conflict of interest including financial.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth K. Joseph, Email: elizabeth.joseph@ucsf.edu.
Robert W. Gear, Email: robert.gear@ucsf.edu.
Jon D. Levine, Email: jon.levine@ucsf.edu.
References
- Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angerio AD, Lee ND. Sickle cell crisis and endothelin antagonists. Crit Care Nurs Q. 2003;26:225–229. doi: 10.1097/00002727-200307000-00008. [DOI] [PubMed] [Google Scholar]
- Bogen O, Joseph EK, Chen X, Levine JD. GDNF hyperalgesia is mediated by PLCgamma, MAPK/ERK, PI3K, CDK5 and Src family kinase signaling and dependent on the IB4-binding protein versican. Eur J Neurosci. 2008;28:12–19. doi: 10.1111/j.1460-9568.2008.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley W, Goodfield M. A pathogenic role for endothelin in Raynaud's phenomenon? Acta Derm Venereol. 1994;74:433–434. doi: 10.2340/0001555574433434. [DOI] [PubMed] [Google Scholar]
- Butt M, Dwivedi G, Blann A, Khair O, Lip GY. Endothelial Dysfunction: Methods of Assessment & Implications for Cardiovascular Diseases. Curr Pharm Des. 2010 doi: 10.2174/138161210793563383. (in press) [DOI] [PubMed] [Google Scholar]
- Cameron NE, Cotter MA. Effects of a nonpeptide endothelin-1 ETA antagonist on neurovascular function in diabetic rats: interaction with the reninangiotensin system. J Pharmacol Exp Ther. 1996;278:1262–1268. [PubMed] [Google Scholar]
- Cameron NE, Cotter MA. Metabolic and vascular factors in the pathogenesis of diabetic neuropathy. Diabetes. 1997;46 Suppl 2:S31–S37. doi: 10.2337/diab.46.2.s31. [DOI] [PubMed] [Google Scholar]
- Edwards CM, Marshall JM, Pugh M. Cardiovascular responses evoked by mild cool stimuli in primary Raynaud's disease: the role of endothelin. Clin Sci (Lond) 1999;96:577–588. [PubMed] [Google Scholar]
- Groeneweg G, Niehof S, Wesseldijk F, Huygen FJ, Zijlstra FJ. Vasodilative effect of isosorbide dinitrate ointment in complex regional pain syndrome type 1. Clin J Pain. 2008;24:89–92. doi: 10.1097/AJP.0b013e318156db3b. [DOI] [PubMed] [Google Scholar]
- Hamamoto DT, Khasabov SG, Cain DM, Simone DA. Tumor-evoked sensitization of C nociceptors: a role for endothelin. J Neurophysiol. 2008;100:2300–2311. doi: 10.1152/jn.01337.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SN, Her SH, Do KR, Kim JS, Yoon HJ, Lee JM, Jin SW. A case of congenital bilateral coronary-to-right ventricle fistula coexisting with variant angina. Korean J Intern Med. 2008;23:216–218. doi: 10.3904/kjim.2008.23.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Ouseph AK, Chou B, Ho T, Green PG, Levine JD. Is there more than one prostaglandin E receptor subtype mediating hyperalgesia in the rat hindpaw? Neuroscience. 1995;64:1161–1165. doi: 10.1016/0306-4522(94)00423-3. [DOI] [PubMed] [Google Scholar]
- Khodorova A, Montmayeur JP, Strichartz G. Endothelin receptors and pain. J Pain. 2009;10:4–28. doi: 10.1016/j.jpain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killip T. Unstable angina - an overview. Herz. 1980;5:72–78. [PubMed] [Google Scholar]
- Klass M, Hord A, Wilcox M, Denson D, Csete M. A role for endothelin in neuropathic pain after chronic constriction injury of the sciatic nerve. Anesth Analg. 2005;101:1757–1762. doi: 10.1213/01.ANE.0000180766.74782.7E. [DOI] [PubMed] [Google Scholar]
- Krishnan U, Win W, Fisher M. First report of the successful use of bosentan in refractory vasospastic angina. Cardiology. 2010;116:26–28. doi: 10.1159/000313365. [DOI] [PubMed] [Google Scholar]
- Laziz I, Larbi A, Grebert D, Sautel M, Congar P, Lacroix MC, Salesse R, Meunier N. Endothelin as a Neuroprotective Factor in the Olfactory Epithelium. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.10.063. (in press) [DOI] [PubMed] [Google Scholar]
- Lee J, Cheong SS, Kim J. Association of endothelin-1 gene polymorphisms with variant angina in Korean patients. Clin Chem Lab Med. 2008;46:1575–1580. doi: 10.1515/CCLM.2008.313. [DOI] [PubMed] [Google Scholar]
- Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neurosci. 2005;21:3387–3394. doi: 10.1111/j.1460-9568.2005.04173.x. [DOI] [PubMed] [Google Scholar]
- Millecamps M, Laferriere A, Ragavendran JV, Stone LS, Coderre TJ. Role of peripheral endothelin receptors in an animal model of complex regional pain syndrome type 1 (CRPS-I) Pain. 2010;151:174–183. doi: 10.1016/j.pain.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori A, Kabbani S. Endothelins and coronary vascular biology. Coron Artery Dis. 2003;14:491–494. doi: 10.1097/00019501-200311000-00003. [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- Quang PN, Schmidt BL. Peripheral endothelin B receptor agonist-induced antinociception involves endogenous opioids in mice. Pain. 2010;149:254–262. doi: 10.1016/j.pain.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Recio P, Orensanz LM, Bustamante S, Navarro-Dorado J, Climent B, Benedito S, Garcia-Sacristan A, Prieto D, Hernandez M. Mechanisms involved in the effects of endothelin-1 in pig prostatic small arteries. Eur J Pharmacol. 2010;640:190–196. doi: 10.1016/j.ejphar.2010.04.059. [DOI] [PubMed] [Google Scholar]
- Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Werner MF, Trevisani M, Campi B, Andre E, Geppetti P, Rae GA. Contribution of peripheral endothelin ETA and ETB receptors in neuropathic pain induced by spinal nerve ligation in rats. Eur J Pain. 2010;14:911–917. doi: 10.1016/j.ejpain.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]