Abstract
Multiple neurotrophic factors play a role in proliferation, differentiation and survival in the olfactory epithelium; however, the signaling cascade has not been fully elucidated. We tested the hypotheses that ATP induces the synthesis and secretion of two neurotrophic factors, fibroblast growth factor 2 (FGF2) and transforming growth factor alpha (TGFα), and that these neurotrophic factors have a role in inducing proliferation. Protein levels of FGF2 and TGFα were increased 20 h post-intranasal instillation of ATP compared to vehicle control in adult Swiss Webster mice. Pre-intranasal treatment with purinergic receptor antagonist pyridoxalphosphate-6-azophenyl-20,40-disulfonic acid (PPADS) significantly blocked this ATP-induced increase, indicating that upregulation of FGF2 and TGFα expression is mediated by purinergic receptor activation. However, in neonatal mouse, intranasal instillation of ATP significantly increased the protein levels of FGF2, but not TGFα. Likewise, ATP evoked the secretion of FGF2, but not TGFα, from neonatal mouse olfactory epithelial slices and PPADS significantly blocked ATP-evoked FGF2 release. To determine the role of FGF2 and TGFα in inducing proliferation, 5-bromo-2-deoxyuridine (BrdU) incorporation was examined in adult olfactory epithelium. Intranasal treatment with FGF receptor inhibitor PD173074 or epidermal growth factor receptor inhibitor AG1478 following ATP instillation significantly blocked ATP-induced BrdU incorporation. Collectively, these data demonstrate that ATP induces proliferation in adult mouse olfactory epithelium by promoting FGF2 and TGFα synthesis and activation of their receptors. These data suggest that different mechanisms regulate neurogenesis in neonatal and adult OE, and FGF2 and TGFα may have different roles throughout development.
Keywords: purinergic receptor, cell proliferation, progenitor cell, FGF2, TGFα, sustentacular cell
In the mammalian olfactory epithelium (OE), neurogenesis begins during embryogenesis, (embryonic day 10 through birth) persists during an expansion period (birth to post-natal day 30) and continues through adulthood when needed to replace dead or dying neurons (post-natal day 30 to death) (Murdoch and Roskams, 2007). Even though each neurogenic phase has different spatiotemporal patterns, the rate of neurogenesis is tightly regulated by multiple chemical signals produced by the different cell types in the OE (Mackay-Sim and Chuah, 2000, Kawauchi et al., 2004). The olfactory epithelium is pseudostratified with the apical layer containing the sustentacular and microvillous cell somas, and the dendrites of the olfactory sensory neurons, the middle portion containing the olfactory sensory neuron somas, and the basal layer containing the multipotent progenitor cells and the endfeet processes of the sustentacular cells.
ATP works in synergy with growth factors to promote cell survival, mitogenesis and differentiation in the central nervous system. ATP, found in millimolar levels in all cells, is released by injured cells and acts as a positive regulator of cell proliferation by triggering localized neurotrophic factor release in the central nervous system (Rathbone et al., 1992, Neary et al., 1996, Rathbone et al., 1999). In the central nervous system, ATP acts in concert with growth factors, such as FGF2, epidermal growth factor, platelet-derived growth factor and nerve growth factor and potentiates the trophic effects (Wang et al., 1990, Neary et al., 2005). The potentiating effects of ATP on mitogenesis could occur at the purinergic receptor level and/or at downstream targets (Neary and Zimmermann, 2009). For example, activation of purinergic receptors expressed on the basal progenitor cells (Hegg et al., 2003) directly induces the cell to proliferate and differentiate into mature neurons in mouse OE (Jia et al., 2009). In addition, Hassenklover et al. (2009) found that activation of P2Y receptors in the basal cells of amphibian OE induces calcium signaling that leads to increased basal cell proliferation. Alternatively, activation of purinergic receptors in the glial-like sustentacular cells or the microvillous cells could induce the release of trophic factors to promote basal cell proliferation.
We hypothesize that ATP via activation of purinergic receptors upregulates the expression and evokes the release of growth factors that subsequently activates their respective receptors in the basal cells. We monitored two neurotrophic factors, FGF2 and TGFα that are expressed in the adult OE, have receptors in the OE, and have a putative role in OE neuroproliferation (Plendl et al., 1999, Newman et al., 2000). FGF receptors FGFR1 and FGFR2 have been located in the OE via RT-PCR and the FGF receptor ligand FGF2 is expressed in apical regions of olfactory sensory neurons and sustentacular cells (Hsu et al., 2001). FGF stimulates proliferation of basal cells (Nakamura et al., 2002) and neuronal differentiation (MacDonald et al., 1996). Epidermal growth factor receptor (EGFR) mRNA and protein has been localized in the basal and sustentacular cells (Plendl et al., 1999, Newman et al., 2000). The EGFR ligand, TGFα, is expressed in adult basal and sustentacular cells and stimulates proliferation (Farbman and Buchholz, 1996, Farbman and Ezeh, 2000).
EXPERIMENTAL PROCEDURES
Animals
Adult male (6-8 weeks) and neonatal (postnatal days 1-10) Swiss Webster mice were obtained from Charles River Laboratories (Portage, MI). All efforts were made to minimize the number of animals used and their suffering. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals as approved by Michigan State University Institutional Animal Care and Use Committee.
Measurement of trophic factor upregulation
Anesthetized adult mice (4% isoflurane) were intranasally instilled with the selective P2 purinergic receptor antagonist pyridoxalphosphate-6-azophenyl-20, 40-disulfonic acid (PPADS, 160 nmoles/kg body weight) or an equivalent volume (50 μl) of saline, and followed by ATP (400 nmoles/kg) or vehicle saline 30 min later. In some experiments, unanesthetized neonates (post-natal day 10) were intranasally instilled with ATP (400 nmoles/kg) or vehicle saline. 20 h after ATP instillation, tissue was collected. The adult mice were anesthetized (65 mg/kg ketamine + 5 mg/kg xylazine, i.p.), transcardially perfused with ice-cold 0.1 M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde and decapitated. The lower jaw and skin was removed and tissue was postfixed overnight in 4% paraformaldehyde, rinsed in PBS, placed in RDO Rapid Decalcifier for 4 h (Apex Engineering Products, Aurora, IL). The neonates were quickly decapitated and heads were post-fixed for 2 h in 4% paraformaldehyde. Adult and neonatal OE tissues were cryoprotected with 20% sucrose and embedded in Tissue Tek OCT (Sakura Finetek, Torrance, CA). Frozen coronal sections of OE (20 μm) were collected from levels 2-6 of the mouse nasal cavity (Young, 1981).
Immunohistochemistry
Tissue sections were rehydrated with PBS, permeabilized with 0.02% triton x-100 and blocked with 1% blocking reagent. The sections were incubated with rabbit anti-TGFα (1:200, Abcam, Cambridge, MA) or rabbit anti-FGF2 antibody (1:200, Abcam, Cambridge, MA). Immunoreactivity was detected using a tyramide signal amplification kit (Invitrogen/Molecular Probes, Eugene, OR). For double-labeling immunofluorescence, the sections were processed as described above for TGFα or FGF2 immunoreactivity and then the sections were washed, blocked and incubated with goat anti-olfactory marker protein (OMP, 1:1000, Wako Chemical, Plano, TX), rabbit anti-calnexin (1:500, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-notch 2 (1:200, Calbiochem, Gibbstown, NJ), rabbit anti-IP3R3 (1:1000, Millipore, Bedford, MA) or rabbit anti-PLCβ2 antibody (1:50, Santa Cruz Biotechnology, Santa Cruz, CA) followed by TRITC-conjugated donkey anti-goat or anti-rabbit immunoglobin (1:50 or 1:200, Jackson Immunoresearch Lab, West Grove, PA). Immunoreactivity was visualized on an Olympus FV1000 Confocal laser scanning microscope (Olympus America Inc., Center Valley, PA). Antibody specificity was examined by omitting the primary antibody or secondary antibody. No immunoreactivity was observed in any of the controls.
Western blot
Following treatment as described in Measurement of trophic factor upregulation, neonates and anesthetized adult mice (65 mg/kg ketamine + 5 mg/kg xylazine, i.p.) were decapitated. The olfactory epithelia were dissected immediately and stored at −80 °C. The OE tissues were processed following the protocol described previously (Jia et al., 2009). Briefly, tissues were homogenized by sonication in Tris buffer. Homogenates were resolved on 15% gels and transferred to nitrocellulose membranes. After incubation with blocking buffer (0.2% I-Block, Millipore, Bedford, MA), the membranes were probed with goat anti-TGFα (1:1000, Abcam, Cambridge, MA) or goat anti-FGF2 (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA) antibody overnight at 4°C. After washing, the membranes were incubated with HRP-labeled secondary antibody (Jackson Laboratory, West Grove, PA, USA). Immunoreactive proteins were detected with a chemiluminescence reagent (ECL, Amersham Biosciences, Piscataway, NJ) and then exposed to Kodak X-ray film. For quantitative analyses, the membranes were reprobed with mouse anti-actin antibody (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA). Films were analyzed by Image J (NIH). Integrated optimal densities (IOD)/μg protein were expressed as percentile changes from IOD/μg protein values of vehicle-treated animals. The value of FGF2 and TGFα for each animal was then normalized to the value of actin. Each sample was measured on three independent gels.
Measurement of BrdU incorporation
The use of BrdU as a marker for cell proliferation was validated previously for our model system using proliferating cell nuclear antigen (Jia et al., 2009). The adult mice were intranasally instilled with saline vehicle or ATP (400 nmoles/kg) followed by intranasal instillation of selective FGF receptor inhibitor PD173074 (0.2 μmoles/kg) and/or non-specific EGF receptor inhibitor AG1478 (100 μmoles/kg) at 30 min, 12 h and 24 h. Mice were injected three times with BrdU (i.p., 180 mg/kg total) between 42 and 46 h and tissues were collected at 48 h post-instillation of ATP, as described in Measurement of trophic factor upregulation. BrdU immunohistochemistry and analysis were performed following the protocol described previously (Jia et al., 2009). Briefly, tissue sections were rehydrated with PBS, permeabilized with 0.3% triton x-100, blocked with 10% normal donkey serum, incubated in 2M HCl to denature DNA, and then incubated with rat anti-BrdU immunoglobin G (1:100, Abcam, Cambridge, MA in blocking reagent) followed by FITC-conjugated secondary antibody. The number of BrdU+ cells per linear millimeter of ecto- and endo-turbinate 2 on three consecutive coronal sections of OE at level 4 in each animal were counted by an investigator that was blinded to the treatments and then normalized to the length of OE on which the BrdU+ cells were scored.
Measurement of trophic factor release
Olfactory epithelium slices (300 μm) were made from neonate mice (post natal day 0-6) following a previously described protocol (Kanekar et al., 2009). Briefly, mice were quickly decapitated. The skin and lower jaw were removed. The tissue was embedded in a carrot and mounted onto a vibratome and 300 μm OE slices were made. Slices were incubated in neurobasal media with 0.02 g/L B-27 serum-free supplement, 0.01 g/L penicillin/streptomycin and 0.01 g/L L-glutamine (Gibco, Carlsbad, CA) in the absence or presence of ATP (100 μM) and purinergic receptor antagonists: specific non-selective antagonists PPADS (25 μM) or reactive blue 2 (RB2; 50 μM), or non-specific non-selective antagonist suramin (100 μM) for 24 hours at 37 °C with 5% CO2. Our calcium imaging data suggests that PPADS and suramin inhibit the same subset of purinergic receptors expressed in the olfactory epithelium as the inhibition of the antagonists presented separately and together is 90-95% (Hegg et al., 2003). Conditioned media were then collected and concentrated with a SVC200H Speed Vac Concentrator (Savant Industries Inc., Farmingdale, NY). Growth factor secretion was quantified using human FGF2 and TGFα ELISA kits (R&D Systems, Minneapolis, MN) following manufacturer’s protocols. Experiments were repeated 4 times (n = 4). We performed two control experiments without OE slices incubated in the media to validate the ELISA assay kit (data not shown). First, we tested the FGF2 and TGFα protein levels in the media in the presence of ATP, PPADS, suramin or RB2. None of the media solutions exhibited detectable levels of FGF2 and TGFα proteins. Second, the media was incubated with known concentrations of FGF2 or TGFα and the above compounds. There was no interference observed among FGF2, TGFα and these compounds. These data support the use of ELISA assay kits to detect FGF2 and TGFα proteins in the presence of these compounds. We measured trophic factor release only in neonatal mouse since the bones supporting the OE in adult mouse are already calcified and obtaining viable OE slices is impossible.
Statistical Analysis
Student’s t-test or two-way ANOVA followed by the Bonferroni post-hoc test were performed using Prism 5 (GraphPad Software, San Diego, CA).
RESULTS
ATP upregulates FGF2 and TGFα expression in adult mouse OE
Extracellular ATP evokes the synthesis and/or release of neurotrophic factors from neurons and non-neuronal cells (Neary et al., 1996) and acts synergistically with these polypeptide growth factors to enhance mitogenesis (Wang et al., 1990). In the present study, we tested whether extracellular ATP had the similar effects in the OE. We measured FGF2 and TGFα expression in adult and neonatal mouse OE after intranasal instillation of ATP via immunohistochemistry at three distinct levels of the nasal cavity. There was very low endogenous FGF2-immunoreactivity (FGF2-IR) observed in vehicle-treated animals, mainly in the apical layer, at three different levels in the nasal cavity (Figure 1A1, 2A-C). FGF2-IR was robustly increased after intranasal instillation of ATP (Figure 1A2, B-G, 2 D-F). ATP-induced upregulation of FGF2 was observed in ectoturbinate 1 (Figure 1B, 2D,E), ectoturbinate 2 (Figure 1C, 2D-E), endotubinate II (Figure 1D, 2D-E), ectoturbinates 3 (Figure 1E, 2E), endoturbinate III (Figure 1F, 2E-F), endoturbinate IV (Figure 2F) and septum (Figure 1G, 2D-E). Pre-intranasal treatment with purinergic receptor antagonist PPADS resulted in a low level of FGF2-IR in the vehicle-treated animals (Figure 1A3, 2G-I). However, PPADS treatment did reduce the increased expression of FGF2 in ATP-instilled mice (Figure 1A4, 2J-L), suggesting that activation of purinergic receptors mediates FGF2 upregulation.
Figure 1. ATP instillation increases FGF2-immunoreactivity in adult mouse OE.
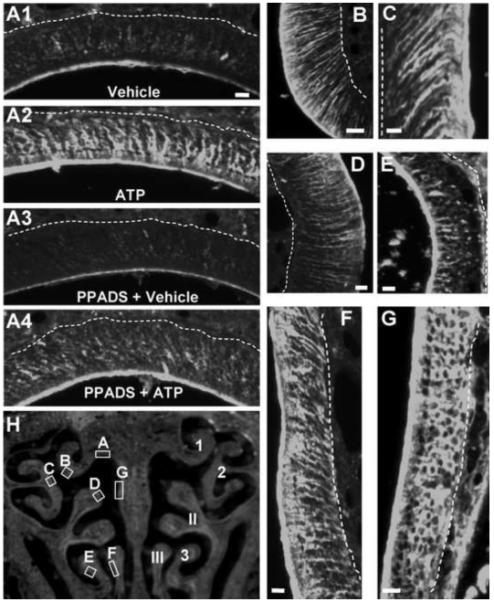
(A-G) FGF2-IR was assessed 48 hr post-instillation of vehicle (A1), ATP (A2, B-G), PPADS and vehicle (A3), or PPADS and ATP (A4) at level 4 in the nasal cavity. Images are Z-stack projections. Dashed white lines indicate basement membrane. Scale bar = 5μm. (H) Representative OE section depicting the regions of ATP-induced FGF2-IR shown in A to G (left) and previously established numbering of ectoturbinates (1-3) and endoturbinates (I-III) (right).
Figure 2. ATP upregulates FGF2 expression at 3 distinct levels of the OE via activation of purinergic receptors.
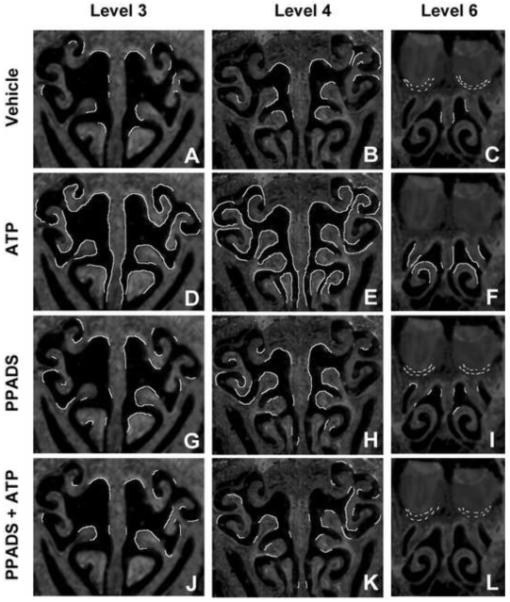
Mice were treated with vehicle (A-C) ATP (D-F) PPADS (G-I) and ATP + PPADS (J-L). FGF2 immunoreactivity (IR) was assessed 48 hr post-instillation at levels 3,4,and 6 in the nasal cavity defined by the presence of specific anatomical features: (3) the start of endoturbinate II (A,D,G,J), (4) the presence of endoturbinate III (B,E,H,K), and (6) the fusion of endoturbinate III (C,F,I,L). Regions exhibiting FGF2-IR were traced as white lines onto representative sections.
Consistent with the observations of FGF2, there was very little endogenous TGFα-IR in vehicle-treated animals at three different levels in the nasal cavity (Figure 3A1, 4A-C). ATP instillation dramatically increased TGFα-IR (Figure 3A2, B-G, 4D-F) in ectoturbinate 1 (Figure 3B, 4D-E), ectoturbinate 2 (Figure 3A2, 4D-E), endotubinate II (Figure 3C-D, 4D-E), ectoturbinate 3 (Figure 3E, 4E), endoturbinate III (Figure 3F, 4E-F), endoturbinate IV (Figure 4E-F) and septum (Figure 3G, 4D-E). Pre-treatment with PPADS resulted in a low level of TGFα-IR in the vehicle-treated animals (Figure 3A3, 4G-I), but prevented ATP from increasing TGFα expression (Figure 3A4, 4J-L). These data indicate that activation of purinergic receptors via intranasal instillation of ATP upregulates both FGF2 and TGFα expression in adult mouse OE.
Figure 3. ATP instillation increases TGFα -immunoreactivity in adult mouse OE.
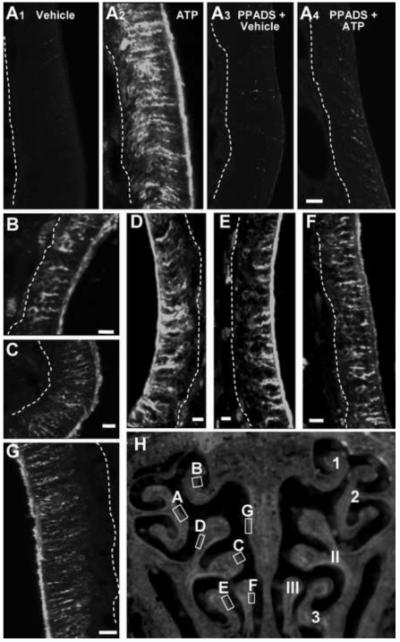
(A-G) TGFα-IR was assessed 48 hr post-instillation of vehicle (A1), ATP (A2, B-G), PPADS and vehicle (A3), or PPADS and ATP (A4) at level 4 in the nasal cavity. Images are Z-stack projections. Dashed white lines indicate basement membrane. Scale bar = 5μm. (H) Representative OE section depicting the regions of ATP-induced TGFα-IR shown in A to G (left) and previously established numbering of ectoturbinates (1-3) and endoturbinates (I-III) (right).
Figure 4. ATP upregulates TGFα expression at 3 distinct levels of the OE via activation of purinergic receptors.
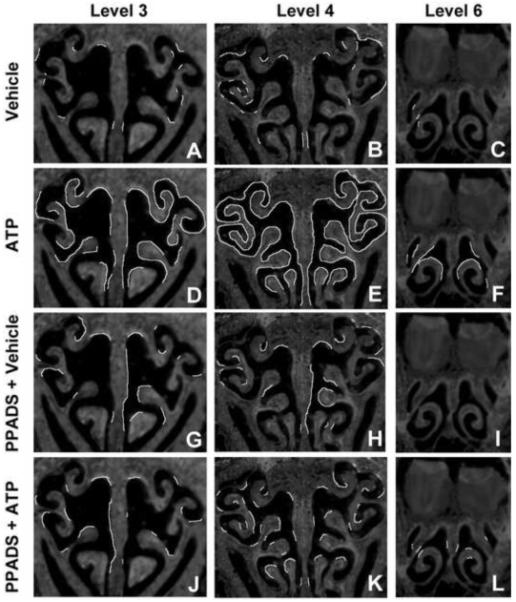
Mice were treated with vehicle (A-C) ATP (D-F) PPADS (G-I) and ATP + PPADS (J-L). TGFα-IR was assessed 48 hr post-instillation at levels 3, 4 and 6 in the nasal cavity defined by the presence of specific anatomical features: (3) the start of endoturbinate II (A,D,G,J), (4) the presence of endoturbinate III (B,E,H,K), and (6) the fusion of endoturbinate III (C,F,I,L). Regions exhibiting TGFα-IR were traced as white lines onto representative sections.
FGF2 was previously reported to be expressed in the olfactory sensory neurons, sustentacular and basal cells in adult rodent OE (Hsu et al., 2001). In order to identify the cell types in which ATP induced FGF2 expression, we performed immunohistochemistry with antibodies directed against FGF2 and sustentacular cell markers calnexin (Czesnik et al., 2006) or notch 2 (Carson et al., 2006), microvillous cell markers IP3R3 or PLCβ2 (Elsaesser et al., 2005), or the olfactory sensory neuron marker olfactory marker protein (OMP). FGF2-IR was co-localized with OMP-IR in the dendrites, cell soma, and in the axon bundles traveling to the olfactory bulb (Figure 5A1-A3). FGF2-IR also co-localized with notch 2-IR in the sustentacular cell somas, cytoplasmic extensions and endfeet processes (Figure 5B1-B3), calnexin-IR in the sustentacular cell somas (data not shown), and both IP3R3-IR (Figure 5C1-C3) and PLCβ2-IR (Figure 5D1-D3) in the cell somas and cytoplasmic extensions of the microvillous cells. These data indicate that ATP induces FGF2 expression in sustentacular cells, microvillous cells and olfactory sensory neurons in adult mouse OE.
Figure 5. Localization of ATP-induced FGF2 and TGFα expression in adult mouse OE.
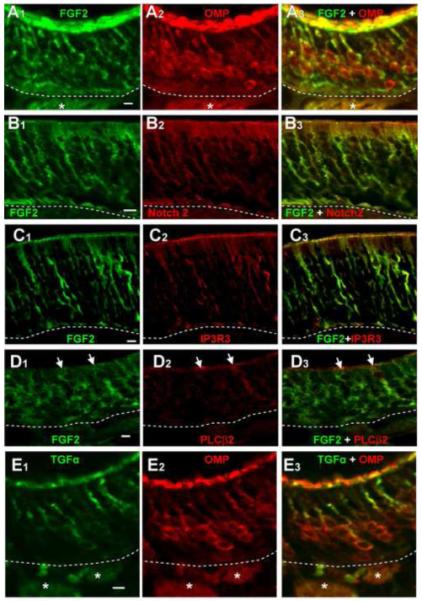
(A-D) FGF2-IR (A1, B1, C1, D1) in ATP-instilled animals co-localized with olfactory sensory neuron marker OMP-IR (A2-3), sustentacular cell marker Notch 2-IR (B2-3), and microvillous cell markers IP3R3-IR (C2-3) and PLCβ2-IR (D2-3). (F) TGFα-IR (F1) in ATP-instilled animals only co-localized with OMP-IR (E2-3). Images are Z-stack projections. *, nerve bundles. Arrows indicate immunoreactivity of representative cells. Dashed with lines indicate basement membrane. Scale bar = 5μm.
TGFα is expressed in the sustentacular and basal cells of adult rodent OE (Farbman and Buchholz, 1996, Plendl et al., 1999). In the present study, we observed that ATP instillation increased TGFα expression in cell somas located prominently in the middle olfactory sensory neuron layer of OE, and in dendrite-like processes in the apical layer (Figure 3). TGFα-IR on the apical surface of the epithelium in the ATP treated mice (Figure 3A2) is not present in the control-, the antagonist- and the ATP + antagonist-treated mice (Figure 3 A1, A3, A4), suggesting that ATP specifically increases the expression of TGFα in the apical layer, most likely in the dendritic knobs of the olfactory sensory neurons (See Figure 5E2). ATP-induced TGFα-IR co-localized with olfactory sensory neuron marker OMP in the dendrites, cell soma, and axon bundles of olfactory sensory neurons (Figure 5E1-E3). TGFα-IR did not co-localize with sustentacular cell markers calnexin and notch 2, and microvillous cell markers IP3R3 and PLCβ2 (data not shown). These data indicate that ATP induces TGFα expression in olfactory sensory neurons but not sustentacular and microvillous cells.
In order to quantify the effects of ATP-induced up-regulation of FGF2 and TGFα expression in adult mouse OE, we measured the protein levels following ATP instillation using the western blot technique. ATP instillation significantly increased the protein levels of both FGF2 (Figure 6A; 102.4 ± 13.5% vs. 215.8 ± 16.7%, p < 0.05) and TGFα (Figure 6B; 99.8 ± 20.0% vs. 164.1 ± 8.6%, p < 0.05) compared to the vehicle control. Pretreatment with PPADS did not alter the FGF2 and TGFα protein levels in vehicle-treated animals but significantly reduced ATP-induced increases of FGF2 and TGFα protein levels back to the control levels (Figure 6; FGF2: 215.8 ± 16.7% vs. 115.1 ± 25.0%, p < 0.05; TGFα: 164.1 ± 8.6% vs. 101.1 ± 12.5%, p < 0.05). These data indicate that ATP significantly increases the synthesis of FGF2 and TGFα proteins via activation of P2 purinergic receptors in adult mouse OE.
Figure 6. Quantification of ATP-evoked growth factor upregulation in adult mouse OE.
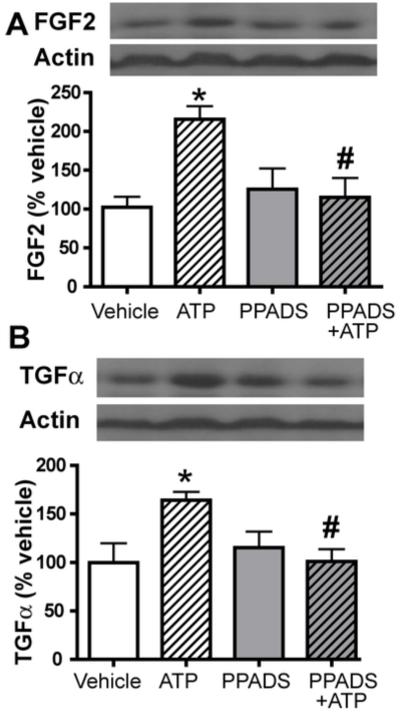
Mice were instilled with either PPADS or vehicle followed by either vehicle or ATP and tissue was collected 20 h post-ATP instillation and processed with western blot analysis for (A) FGF2 and (B) TGFα expression. Top panels show western blot and lower panels show bar graphs of normalized immunoreactivity. *, p<0.05 v. vehicle. #, p<0.05 v. ATP.
FGF and EGF receptors mediate ATP-induced neuroregeneration in adult mouse OE
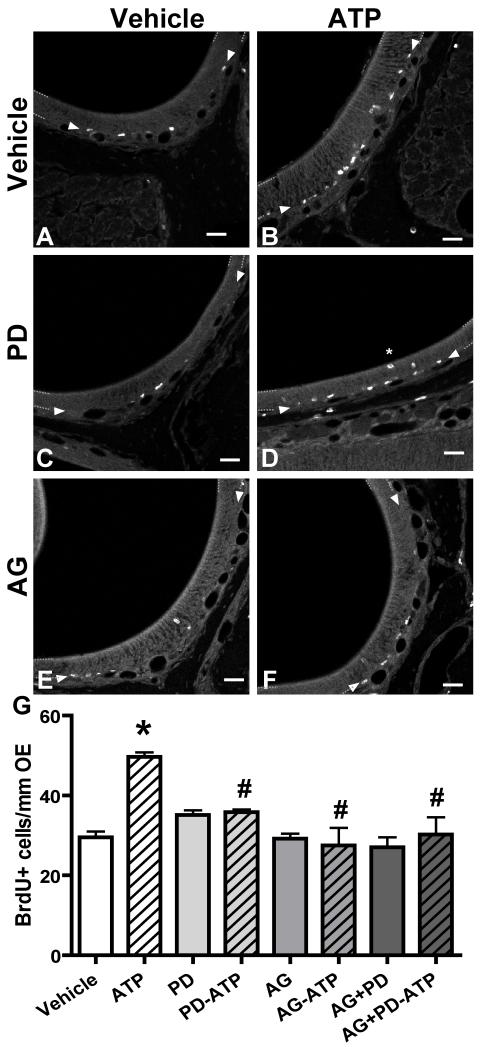
We next examined the involvement of the FGF receptor and the EGF receptor, of which TGFα is a ligand, in the ATP-induced mitogenesis in adult mouse OE. Mice were intranasally instilled with the specific FGF receptor inhibitor PD173074 (0.2 μmoles/kg) and/or the non-specific EGF receptor inhibitor AG1478 (100 μmoles/kg) at 30 min, 12 and 24 h and the levels of BrdU incorporation were measured 48 h after ATP instillation. Intranasal instillation of ATP significantly increased BrdU+ cells in the OE by 66.2% above the control (Figure 7 A,B,G; 29.9 ± 2.0 vs. 49.7 ± 1.1 cells/mm OE, p < 0.001, n = 3 animals, 9 tissue sections). Intranasal treatment with PD173074, AG1478, or both did not significantly alter BrdU incorporation in the vehicle-treated animals (Figure 7 C, E, G; PD173074: 35.1 ± 1.1 cells/mm OE, AG1478: 29.2 ± 1.2 cells/mm OE, PD173074+AG1478: 27.0 ± 2.5 cells/mm OE; p > 0.05, n = 3 animals, 9 slices for all groups) but significantly blocked the ATP-induced increase in BrdU+ cells (Figure 7 D, F, G; PD173074: 35.8 ± 0.7 cells/mm OE, AG1478: 27.5 ± 4.4 cells/mm OE, PD173074+AG1478: 30.2 ± 4.3 cells/mm OE; p < 0.001, n = 3 animals, 9 slices for all groups). These data indicate that FGF2 and TGFα receptors mediate ATP-induced mitogenesis in adult mouse OE.
Figure 7. FGF and EGF receptors mediate ATP-induced increase in BrdU incorporation in adult mouse OE.
Mice were instilled with vehicle (A, C, E) or ATP (B, D, F) followed by vehicle (A, B) FGF receptor inhibitor PD173074 (PD; C, D), TGFα receptor inhibitor AG1478 (AG; E, F) or both (G). Representative images of a single confocal plane depicting BrdU-IR from ectoturbinate 2 are shown. Dotted lines demark the OE. Arrowheads indicate the basal cell layer. Scale bar = 50μm. (G) Quantification of BrdU+ cells in endoturbinate II and ectoturbinate 2. * p<0.001 v. vehicle. #, p<0.001 v. ATP.
FGF2 expression was up-regulated by ATP in neonatal mouse OE
We also tested whether FGF2 and TGFα were expressed in neonatal mouse OE via western blot and immunohistochemistry. Intranasal instillation of ATP significantly increased the protein levels of FGF2 (Figure 8A, p < 0.05). The FGF2-IR in vehicle-treated animals was relatively low (Figure 8B). ATP robustly increased FGF2-IR throughout the OE and in the underlying olfactory nerve bundles (Figure 8C). TGFα expression could not be detected in either group with either method (data not shown). These data indicate that FGF2 is expressed in neonatal mouse OE and ATP upregulates its expression, while TGFα is not expressed in neonatal mouse OE or expressed below the levels detected by the methods we used, and ATP did not alter its expression.
Figure 8. ATP upregulates FGF2 expression in neonatal mouse OE.
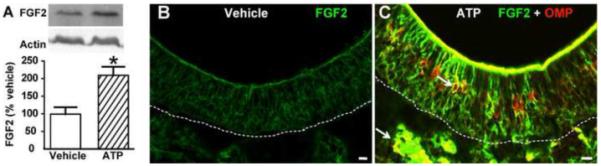
Neonatal mice were instilled with vehicle or ATP and 48 hr later assessed for (A) FGF2 protein levels or (B) FGF2- and OMP-IR. * p<0.05 (Student’s t-test). Arrows indicate representative co-localization. Dashed lines indicate basement membrane. Scale bar = 10μm.
ATP evoked FGF2 release in neonatal mouse OE
We next quantified the release of FGF2 and TGFα from neonatal mouse OE slices. Conditioned media was collected from slices incubated for 24 hours in the presence or absence of ATP and/or purinergic receptor antagonists PPADS, suramin and/or reactive blue 2 (RB2). The amount of FGF2 and TGFα in the conditioned media was quantified using an ELISA assay. We detected 219 ± 64 pg/ml FGF2 protein in the conditioned media in the presence of OE slices (vehicle control, n = 4 animals, ~20 slices). Addition of exogenous ATP (100 μM) significantly increased the amount of FGF2 proteins in the media by 21.4% over vehicle control (Figure 9, p < 0.05, n = 4 animals, ~20 slices). Incubation of slices with non-specific, but selective purinergic receptor antagonists suramin or RB2 alone did not alter FGF2 protein levels in the media (n = 4 animals, ~20 slices for each group) but reduced the ATP-induced FGF2 release to control levels (Figure 9, n = 4 animals, ~20 slices for each group). Incubation with selective purinergic receptor antagonist PPADS did not alter the levels of FGF2 proteins compared to the vehicle control (Figure 9, n = 3 animals, ~15 slices). However, incubation with PPADS significantly reduced ATP-induced FGF2 release (Figure 9, p < 0.05 v. ATP, n = 3 animals, ~15 slices). These data suggest that exogenous ATP induces FGF2 release from neonatal OE slices via activation of purinergic receptors. Consistent with immunohistochemistry and western blot observations, the concentration of TGFα in the media of any group was below the detection level of the ELISA kit (2.2 pg/ml). Slices incubated with ATP did not significantly enhance TGFα release in the media. These data indicate that the endogenous TGFα level in neonatal mouse OE is very low and exogenous ATP has no effect on its expression or release.
Figure 9. ATP induces FGF2 release from neonatal mouse OE slices.
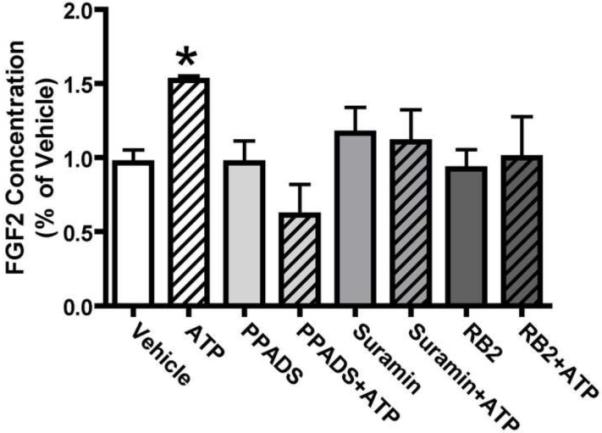
Neonatal OE slices were incubated in vehicle or ATP and/or purinergic receptor antagonists PPADS, suramin or reactive blue 2 (RB2). Conditioned media collected 24 hrs later was assayed for FGF2 content. *, p<0.05 v. vehicle.
DISCUSSION
The dynamic state of proliferation, differentiation and cell death in the OE are regulated by autocrine and paracrine mechanisms using multiple growth factors (Newman et al., 2000). Evidence suggests that mechanisms regulating adult neurogenesis and embryonic neurogenesis may be equivalent (Schwob, 2002, Beites et al., 2005, Murdoch and Roskams, 2007). However, it has not been established if regulatory signals are similar in the neonate. We previously determined that ATP induces neuroproliferation in the olfactory epithelium of neonatal and adult mice via purinergic receptor activation (Jia et al., 2009). Here, we investigate the mechanism further and show that ATP, acting on P2 purinergic receptors, increases the synthesis of growth factors TGFα in adults and FGF2 in both neonates and adults. We directly quantified the ATP-induced release of FGF2 in neonates. In adults, we indirectly verified the release of FGF2 and TGFα by the observation that EGF and FGF receptor inhibition blocks the ATP-induced increase in neuroproliferation. This study demonstrates that ATP differentially upregulates the synthesis of growth factors in neonates and adults, and that subsequent release of these growth factors induces the proliferation of neural progenitor cells.
ATP differentially induces the expression of FGF2 and TGFα in adult and neonate OE
Endogenous FGF2 expression in the OE varies among species. FGF2 mRNA is widely detected in all layers of adult mouse OE, with intense FGF2-IR located in the sustentacular cells and moderate IR distributed in olfactory sensory neurons and basal cells (Hsu et al., 2001). In the adult rat, FGF-IR is observed in mature olfactory sensory neurons and sustentacular cells (Goldstein et al., 1997). TGFα-IR is observed in the sustentacular and basal cells in the adult rat OE (Farbman and Buchholz, 1996), and to our knowledge, there is no published report indicating the cell types in which TGFα is expressed in adult mice. In the present study, we rarely observed FGF2-IR and TGFα-IR in the OE of vehicle-treated adult mice. Hsu and colleagues (2001) report that FGF2 immunoreactivity is greatly reduced when tissue is fixed with 4% paraformaldehyde, the same fixation that we used in this study. However, in the ATP-instilled adults, using the same 4% paraformaldehyde fixation protocol, FGF2-IR and TGFα-IR was robustly increased, an effect that was blocked by the broadly selective P2 receptor antagonist PPADS. Western analysis of the protein levels confirmed the immunohistochemical observations. ATP-induced increases of FGF2-IR and TGFα-IR were broadly distributed throughout the rostral-caudal axis of the nasal cavity, suggesting their actions may have a universal effect in the peripheral olfactory system. Similar to previous reports in adult rodent (Goldstein et al., 1997, Hsu et al., 2001), we observed intense FGF2-IR in the sustentacular cells and olfactory sensory neurons following ATP treatment. In addition, for the first time, we report that FGF2 is expressed in microvillous cells in ATP-instilled animals, suggesting that microvillous cells are involved in cell proliferation by stimulus-induced release of FGF2. TGFα-IR was observed following ATP-treatment in the olfactory sensory neurons rather than the sustentacular cells and basal cells as previous reported in untreated rat (Farbman and Buchholz, 1996). One possibility for this discrepancy is that the time course for ATP-induced upregulation of TGFα differs between species or between cell types. Collectively, these data demonstrate that ATP, via activation of P2 purinergic receptors, increases the synthesis of FGF2 and TGFα in adult mice.
In contrast to the adult OE, our data suggests that FGF2, but not TGFα, has a regulatory role in neonatal mouse OE. In the present study, we observed neither FGF2-IR nor TGFα-IR in vehicle-treated neonatal mouse OE. However, ATP instillation increased the expression of FGF2 in the neonatal olfactory sensory neurons and sustentacular cells, but not TGFα. TGFα mRNA and protein expression were previously reported in the embryonic rat OE from E17-E20, although it was not localized to a specific cell type (Huang et al., 1996). To our knowledge there have been no reports of TGFα expression in the neonatal rodent. In the neonatal rat, FGF2 was expressed extensively in the connective tissue residing in the lamina propria, and rarely, if at all, in the olfactory sensory neurons and the sustentacular cells (Chuah and Teague, 1999). Our observation in the vehicle-treated neonatal mice is in agreement with the lack of FGF2 expression in the neonatal rat OE. Our data suggests that TGFα may have different roles during embryogenesis, post-natal development and adulthood.
Growth factor release
In the present study we directly measure release of growth factors in neonates in vitro, and indirectly measure the evoked release of growth factors in adults in vivo. Our evidence demonstrates that activation of EGF and FGF receptors in adult mice mediate ATP-induced cell proliferation. Based on the immunohistochemical evidence, we predict that FGF2 is released from ATP-treated adult mice from olfactory sensory neurons, sustentacular cells and microvillous cells whereas TGFα is released only from olfactory sensory neurons. ATP stimulates transient increases in intracellular calcium in olfactory sensory neurons, sustentacular cells (Hegg et al., 2003, Hegg et al., 2009) and microvillous cells (unpublished observations). We hypothesize that intracellular calcium activates a cascade of intracellular signals and the induction of calcium-dependent release of neurotrophic factors, possibly from sustentacular cell endfeet, microvillous cell processes or neuronal axons. ATP increases the release of FGF2, but not TGFα, from neonatal mouse OE slices through a purinergic receptor dependent mechanism. We used broadly selective purinergic receptor antagonists suramin and PPADS, as well as reactive blue 2, an antagonist that can be P2Y selective, but does not adequately discriminate between P2X and P2Y receptor subtypes (Ralevic and Burnstock, 1998). As there are neither specific nor selective antagonists for the multiple types of P2 receptors, we are not able to definitively identify the subtype of purinergic receptor involved in evoking the release of growth factors. P2X receptors are expressed in olfactory sensory neurons and P2Y receptors are expressed on olfactory sensory neurons and sustentacular cells (Hegg et al., 2003), and FGF2 expression was upregulated in olfactory sensory neurons and sustentacular cells, suggesting that both purinergic receptor subtypes may have a role in ATP-induced growth factor release in the neonate.
Proliferation
We hypothesize that ATP induces cell proliferation via the paracrine release of a multiple neurotrophic factors including NPY (Kanekar et al., 2009, Jia and Hegg, 2010), FGF2 and TGFα. Inhibiting the signaling of these growth factors individually significantly reduces ATP-induced cell proliferation to the control levels. Notably inhibition of both the FGF receptor and the EGF receptor does not reduce the ATP-mediated proliferation significantly below the control levels. These results are consistent with other in vivo studies examining the effects of trophic factors in the olfactory epithelium. In mice lacking the leukemia inhibitor factor, a potent mitogenic factor upregulated in the olfactory system following injury, bulbectomy-induced increases in proliferation are reduced, however, in the normal olfactory epithelium, there is no significant change in proliferation (Bauer et al., 2003). This suggests that the normal olfactory epithelium maintains a stable level of cell proliferation that is tightly controlled through positive and negative regulation.
Interestingly, both FGF2 and TGFα were previously shown to differentially induce proliferation of two populations of basal cells in the OE: the quiescent multi-potent horizontal basal cells and the highly mitotic multi-potent globose basal cells. FGF2 stimulates the proliferation of globose basal cells in adult in vivo (Nishikawa et al., 2009) and in vitro (Newman et al., 2000), and in postnatal OE cell cultures (Barraud et al., 2007) and cell lines (Goldstein et al., 1997). In addition, FGF2 is released (Ensoli et al., 1998) and stimulates the proliferation of progenitor cells in embryonic cell cultures (DeHamer et al., 1994, Ensoli et al., 1998). Although we did not distinguish between basal cell subtypes, our observation that ATP-induces basal cell proliferation in adults corroborates these findings. Collectively with ATP-induced upregulation of FGF2 in adults and neonates and the release of FGF2 in neonates, these data suggest that FGF2 plays a role in proliferation of basal cells throughout embryogenesis, post-natal neuronal expansion and adulthood. In contrast, TGFα stimulates the proliferation of the horizontal basal cells in adult in vivo (Getchell et al., 2000), and in embryonic cell cultures in vitro (Farbman and Buchholz, 1996). Our data support these observations, and also indicate that TGFα may play a role in basal cell proliferation during embryogenesis and maintenance in adulthood, but not during the post-natal neuronal expansion phase of development. Indeed, the expression of TGFα in the post-natal mouse, during which time globose basal cells are actively proliferating, may not be necessary as the horizontal basal cells are quiescent and rarely divide in vivo.
Conclusions
In summary, the present study demonstrates that ATP induces proliferation in adult OE by promoting FGF2 and TGFα synthesis, release and activation of their respective receptors. In addition, the present study suggests that there may be different mechanisms to mediate the regulation of neuroproliferation in the OE during development and adulthood. The release of nucleotides from damaged or dying cells has functional implications for many types of CNS injuries and neurological diseases (Neary and Zimmermann, 2009). Application of ATP or other P2 receptor agonists can induce the phenomenon of cell proliferation in vitro and in vivo and P2 receptor antagonists reduce this effect (Neary and Kang, 2005, Franke and Illes, 2006, Di Virgilio et al., 2009). These data indicate that extracellular nucleotide and purinergic receptor signaling are important factors to mediate cell proliferation and neurogenesis in CNS injuries. Further studies should investigate the underlying signaling cascades of ATP, FGF2 and TGFα in the OE.
Abbreviations
- BrdU
5-bromo-2-deoxyuridine
- EGF
epidermal growth factor
- FGF2
fibroblast growth factor 2
- IR
immunoreactivity
- IOD
integrated optimal densities
- NPY
neuropeptide Y
- OE
olfactory epithelium
- OMP
olfactory marker protein
- PBS
phosphate-buffered saline
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate
- RB2
reactive blue 2
- TGFα
transforming growth factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barraud P, He X, Zhao C, Ibanez C, Raha-Chowdhury R, Caldwell MA, Franklin RJ. Contrasting effects of basic fibroblast growth factor and epidermal growth factor on mouse neonatal olfactory mucosa cells. Eur J Neurosci. 2007;26:3345–3357. doi: 10.1111/j.1460-9568.2007.05950.x. [DOI] [PubMed] [Google Scholar]
- Bauer S, Rasika S, Han J, Mauduit C, Raccurt M, Morel G, Jourdan F, Benahmed M, Moyse E, Patterson PH. Leukemia inhibitory factor is a key signal for injury-induced neurogenesis in the adult mouse olfactory epithelium. Journal of Neuroscience. 2003;23:1792–1803. doi: 10.1523/JNEUROSCI.23-05-01792.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beites CL, Kawauchi S, Crocker CE, Calof AL. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res. 2005;306:309–316. doi: 10.1016/j.yexcr.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Chuah MI, Teague R. Basic fibroblast growth factor in the primary olfactory pathway: mitogenic effect on ensheathing cells. Neuroscience. 1999;88:1043–1050. doi: 10.1016/s0306-4522(98)00277-2. [DOI] [PubMed] [Google Scholar]
- DeHamer MK, Guevara JL, Hannon K, Olwin BB, Calof AL. Genesis of olfactory receptor neurons in vitro: regulation of progenitor cell divisions by fibroblast growth factors. Neuron. 1994;13:1083–1097. doi: 10.1016/0896-6273(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009;32:79–87. doi: 10.1016/j.tins.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Ensoli F, Fiorelli V, Vannelli B, Barni T, De Cristofaro M, Ensoli B, Thiele CJ. Basic fibroblast growth factor supports human olfactory neurogenesis by autocrine/paracrine mechanisms. Neuroscience. 1998;86:881–893. doi: 10.1016/s0306-4522(98)00104-3. [DOI] [PubMed] [Google Scholar]
- Farbman AI, Buchholz JA. Transforming growth factor-alpha and other growth factors stimulate cell division in olfactory epithelium in vitro. J Neurobiol. 1996;30:267–280. doi: 10.1002/(SICI)1097-4695(199606)30:2<267::AID-NEU8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Farbman AI, Ezeh PI. TGF-alpha and olfactory marker protein enhance mitosis in rat olfactory epithelium in vivo. Neuroreport. 2000;11:3655–3658. doi: 10.1097/00001756-200011090-00051. [DOI] [PubMed] [Google Scholar]
- Franke H, Illes P. Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol Ther. 2006;109:297–324. doi: 10.1016/j.pharmthera.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Getchell TV, Narla RK, Little S, Hyde JF, Getchell ML. Horizontal basal cell proliferation in the olfactory epithelium of transforming growth factor-alpha transgenic mice. Cell Tissue Res. 2000;299:185–192. doi: 10.1007/s004419900149. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Wolozin BL, Schwob JE. FGF2 suppresses neuronogenesis of a cell line derived from rat olfactory epithelium. J Neurobiol. 1997;33:411–428. [PubMed] [Google Scholar]
- Hegg CC, Greenwood D, Huang W, Han P, Lucero MT. Activation of purinergic receptor subtypes modulates odor sensitivity. J Neurosci. 2003;23:8291–8301. doi: 10.1523/JNEUROSCI.23-23-08291.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegg CC, Irwin M, Lucero MT. Calcium store-mediated signaling in sustentacular cells of the mouse olfactory epithelium. Glia. 2009;57:634–644. doi: 10.1002/glia.20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P, Yu F, Feron F, Pickles JO, Sneesby K, Mackay-Sim A. Basic fibroblast growth factor and fibroblast growth factor receptors in adult olfactory epithelium. Brain Res. 2001;896:188–197. doi: 10.1016/s0006-8993(01)02173-4. [DOI] [PubMed] [Google Scholar]
- Huang L, Solursh M, Sandra A. The role of transforming growth factor alpha in rat craniofacial development and chondrogenesis. J Anat. 1996;189:73–86. [PMC free article] [PubMed] [Google Scholar]
- Jia C, Doherty JD, Crudgington S, Hegg CC. Activation of purinergic receptors induces proliferation and neuronal differentiation in Swiss Webster mouse olfactory epithelium. Neuroscience. 2009;163:120–128. doi: 10.1016/j.neuroscience.2009.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C, Hegg CC. NPY mediates ATP-induced neuroproliferation in adult mouse olfactory epithelium. Neurobiol Dis. 2010;38:405–413. doi: 10.1016/j.nbd.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C, Roman C, Hegg CC. Nickel sulfate induces location-dependent atrophy of mouse olfactory epithelium: protective and proliferative role of purinergic receptor activation. Toxicol Sci. 2010;115:547–556. doi: 10.1093/toxsci/kfq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekar S, Jia C, Hegg CC. Purinergic receptor activation evokes neurotrophic factor neuropeptide Y release from neonatal mouse olfactory epithelial slices. J Neurosci Res. 2009;87:1424–1434. doi: 10.1002/jnr.21954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S, Beites CL, Crocker CE, Wu HH, Bonnin A, Murray R, Calof AL. Molecular signals regulating proliferation of stem and progenitor cells in mouse olfactory epithelium. Dev Neurosci. 2004;26:166–180. doi: 10.1159/000082135. [DOI] [PubMed] [Google Scholar]
- MacDonald KP, Murrell WG, Bartlett PF, Bushell GR, Mackay-Sim A. FGF2 promotes neuronal differentiation in explant cultures of adult and embryonic mouse olfactory epithelium. J Neurosci Res. 1996;44:27–39. doi: 10.1002/(SICI)1097-4547(19960401)44:1<27::AID-JNR4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A, Chuah MI. Neurotrophic factors in the primary olfactory pathway. Prog Neurobiol. 2000;62:527–559. doi: 10.1016/s0301-0082(00)00009-5. [DOI] [PubMed] [Google Scholar]
- Murdoch B, Roskams AJ. Olfactory epithelium progenitors: insights from transgenic mice and in vitro biology. J Mol Histol. 2007;38:581–599. doi: 10.1007/s10735-007-9141-2. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Higuchi Y, Kondoh H, Obata M, Takahashi S. The effect of basic fibroblast growth factor on the regeneration of guinea pig olfactory epithelium. Eur Arch Otorhinolaryngol. 2002;259:166–169. doi: 10.1007/s00405-001-0430-1. [DOI] [PubMed] [Google Scholar]
- Neary JT, Kang Y. Signaling from P2 nucleotide receptors to protein kinase cascades induced by CNS injury: implications for reactive gliosis and neurodegeneration. Mol Neurobiol. 2005;31:95–103. doi: 10.1385/MN:31:1-3:095. [DOI] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Shi YF. Cell cycle regulation of astrocytes by extracellular nucleotides and fibroblast growth factor-2. Purinergic Signal. 2005;1:329–336. doi: 10.1007/s11302-005-8075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- Neary JT, Zimmermann H. Trophic functions of nucleotides in the central nervous system. Trends Neurosci. 2009;32:189–198. doi: 10.1016/j.tins.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Newman MP, Feron F, Mackay-Sim A. Growth factor regulation of neurogenesis in adult olfactory epithelium. Neuroscience. 2000;99:343–350. doi: 10.1016/s0306-4522(00)00194-9. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Doi K, Ochi N, Katsunuma S, Nibu K. Effect of intranasal administration of basic fibroblast growth factor on olfactory epithelium. Neuroreport. 2009;20:764–769. doi: 10.1097/WNR.0b013e32832b169e. [DOI] [PubMed] [Google Scholar]
- Plendl J, Stierstorfer B, Sinowatz F. Growth factors and their receptors in the olfactory system. Anat Histol Embryol. 1999;28:73–79. doi: 10.1046/j.1439-0264.1999.00165.x. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rathbone MP, Deforge S, Deluca B, Gabel B, Laurenssen C, Middlemiss P, Parkinson S. Purinergic stimulation of cell division and differentiation: mechanisms and pharmacological implications. Med Hypotheses. 1992;37:213–219. doi: 10.1016/0306-9877(92)90190-n. [DOI] [PubMed] [Google Scholar]
- Rathbone MP, Middlemiss PJ, Gysbers JW, Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P, Caciagli F. Trophic effects of purines in neurons and glial cells. Prog Neurobiol. 1999;59:663–690. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec. 2002;269:33–49. doi: 10.1002/ar.10047. [DOI] [PubMed] [Google Scholar]
- Wang DJ, Huang NN, Heppel LA. Extracellular ATP shows synergistic enhancement of DNA synthesis when combined with agents that are active in wound healing or as neurotransmitters. Biochem Biophys Res Commun. 1990;166:251–258. doi: 10.1016/0006-291x(90)91938-o. [DOI] [PubMed] [Google Scholar]
- Young JT. Histopathologic examination of the rat nasal cavity. Fundam Appl Toxicol. 1981;1:309–312. doi: 10.1016/s0272-0590(81)80037-1. [DOI] [PubMed] [Google Scholar]