Abstract
The past decade in Parkinson's disease (PD) research has been punctuated by numerous advances in understanding genetic factors that contribute to the disease. Common to most of the genetic models of Parkinsonian neurodegeneration are pathologic mechanisms of mitochondrial dysfunction, secretory vesicle dysfunction and oxidative stress that likely trigger common cell death mechanisms. Whereas presynaptic function is implicated in the function / dysfunction of α-synuclein, the first gene shown to contribute to PD, synaptic function has not comprised a major focus in most other genetic models. However, recent advances in understanding the impact of mutations in parkin and LRRK2 have also yielded insights into synaptic dysfunction as a possible early pathogenic mechanism. Autophagy is a common neuronal response in each of these genetic models of PD, participating in the clearance of protein aggregates and injured mitochondria. However, the potential consequences of autophagy upregulation on synaptic structure and function remain unknown. In this review, we discuss the evidence that supports a role for synaptic dysfunction in the neurodegenerative cascade in PD, and highlight unresolved questions concerning a potential role for autophagy in either pathological or compensatory synaptic remodeling.
Introduction
Parkinson's disease (PD) is a progressive, debilitating neurodegenerative disease characterized clinically by deterioration of motor capacities and, in many patients, dementia. The hallmark diagnostic pathology of PD is comprised of neuronal cell death and Lewy bodies/neurites in the substantia nigra, several additional brainstem nuclei and areas of the cerebral cortex. Although our understanding of the multifactorial etiology of PD is far from complete, numerous monogenic determinants of disease identified in kindreds with familial PD, together with a growing number of genetic risk factors identified in large cohorts of patients with sporadic PD, have provided opportunities to investigate the functions of proteins that likely play pivotal roles in the neurodegenerative cascade (Lesage and Brice, 2009).
Protein aggregation, mitochondrial dysfunction, secretory pathway organelle dysfunction and oxidative stress represent common factors in various models of Parkinsonian neurodegeneration (Cookson and van der Brug, 2008). Synaptic dysfunction, implicated in numerous studies of the first known genetic determinant of PD, α-synuclein, is beginning to emerge as a factor in other genetic models of PD. In this review, we will discuss evidence that the PD gene products LRRK2, parkin and α-synuclein play roles in regulating synaptic (dys)function. We will also highlight emerging questions concerning possible roles for autophagy in the neuritic/synaptic compartment in LRRK2, parkin and α-synuclein models of PD. Whereas autophagy functions in the clearance of protein aggregates (Wong and Cuervo, 2010), and in mitochondrial quality control (Zhu and Chu, 2010), the potential impact of autophagic proteolysis on synaptic structure and function remains unknown.
The ubiquitin-proteasome system (UPS) (Yi and Ehlers, 2005; Yi and Ehlers, 2007) and endocytic-lysosomal pathways (Groc and Choquet, 2006; Hirling, 2009) of protein degradation play important roles in the regulation of synaptic function and plasticity. In contrast, potential roles for autophagy in modulating synaptic structure and function have remained largely unexplored. Supporting the possibility that autophagy could modify synaptic structure and function is the presence of components of the autophagic machinery localized to the synaptic compartment (Seidenbecher et al., 2004). Microtubule associated protein 1 light chain 3 (LC3) and gamma-aminobutyric acid receptor-associated protein (GABARAP) are homologs of the yeast AuTophaGy protein 8 (Atg8). GABARAP regulates aspects of GABA receptor degradation in an invertebrate model of neuronal development (Bamber and Rowland, 2006; Rowland et al., 2006). Furthermore, autophagy interacts with both UPS (Kirkin et al., 2009; Korolchuk et al.) and the endocytic/lysosomal system (Fader and Colombo, 2009; Lee and Gao, 2008; Rusten and Simonsen, 2008). We propose that autophagy may play compensatory or deleterious roles in shaping synaptic structure/function in PD and other neurodegenerative diseases.
Leucine-Rich Repeat Kinase 2 (LRRK2)
Mutations in the LRRK2 gene underlie familial, autosomal dominant parkinsonism linked to the PARK8 chromosomal locus 12p11.23-q13.11 (Funayama et al., 2002) (Paisan-Ruiz et al., 2004; Zimprich et al., 2004). LRRK2 is a large (2527 amino acids, ~280 kDa), multidomain protein featuring two catalytic domains: a ras of complex proteins (Roc) GTPase domain and a kinase domain separated by a C-terminal of Ras (COR) domain (Mata et al., 2006). Disease segregating mutations in LRRK2 have been reported in the kinase domain (G2019S, I2020T), the Roc domain (R1441C/G) and in the COR domain (Y1699C) (reviewed in (Mata et al., 2006). The literature suggests that the G2019S mutation in the kinase domain increases LRRK2 kinase activity, whereas mutations in the Roc domain appear to decrease the GTPase activity of LRRK2, affect protein dimerization and may increase kinase activity (analyzed in more detail in a recent review by (Greggio and Cookson, 2009).
A more ubiquitous role for LRRK2 in neurodegenerative diseases associated with Parkinsonism is suggested by the identification of the G2019S mutation in Parkinsonism patients with no family history of disease (Gilks et al., 2005; Healy et al., 2008) and identification of variations in LRRK2 as important risk factors in two genome wide association studies of sporadic PD (Simon-Sanchez et al., 2009) (Satake et al., 2009). Furthermore, the neuropathology demonstrated in post-mortem brain examinations of patients with LRRK2 mutations most often involves synucleinopathy, but occasionally tauopathy, suggesting a role for LRRK2 that is upstream of protein inclusion pathology (Taymans and Cookson, 2010; Uitti et al., 2004; Wider et al., 2010; Wszolek et al., 1995).
Although mRNA expression studies show little concordance regarding the level of LRRK2 mRNA expression in the substantia nigra (Galter et al., 2006; Higashi et al., 2007; Melrose et al., 2006; Simon-Sanchez et al., 2006; Taymans et al., 2006), LRRK2 protein expression has been demonstrated in tyrosine-hydroxylase positive neurons of the substantia nigra pars compacta and in medium-sized spiny neurons of the striatum. Cortical regions that are affected in dementia associated with Parkinson's disease, including pyramidal neurons of the cerebral cortex and of Ammon's horn, also demonstrate relatively high levels of LRRK2 (Biskup et al., 2006; Higashi et al., 2007; Melrose et al., 2007; Paisan-Ruiz et al., 2004). LRRK2 immunoreactivity does not appear in Lewy bodies (Higashi et al., 2007; Melrose et al., 2007).
Functional impairments in nigrostriatal dopaminergic innervation and degeneration of the nigrostriatal projections have been demonstrated in R1441C LRRK2 homozygous knock-in mice (Tong et al., 2009) and in R1441C-LRRK2 BAC transgenic mice (Li et al., 2009), respectively. G2019S BAC transgenic mice show deficiencies in striatal dopamine release and enhanced striatal tau immunoreactivity without dopaminergic neuron loss in the substantia nigra (Li et al., 2010). Furthermore, LRRK2 overexpression appears to be a modifier of synucleinopathy in A53T transgenic mice (Lin et al., 2009). Homozygous LRRK2 knockout mice show no readily apparent brain pathology (Andres-Mateos et al., 2009; Lin et al., 2009; Tong et al., 2010). Taken together, these studies suggest a gain-of-function effect of mutant LRRK2.
In neurons and neuronal cell lines, overexpression of LRRK2 cDNAs containing PD-associated mutations are associated with a phenotype of neurite injury and retraction (Macleod et al., 2006; Plowey et al., 2008; Smith et al., 2006; Smith et al., 2005). Macleod and colleagues (Macleod et al., 2006) demonstrated that overexpression of mutant LRRK2 in primary cortical cultures results in gradual neurite injury and retraction that temporally precede apoptotic cell death in both in vitro and in vivo systems. Neurite injury is accompanied by induction of neuritic autophagy, which eventually contributes to neurite shortening (Plowey et al., 2008). This suggests that early events in the neurodegenerative cascade downstream of mutant LRRK2 likely target the neuritic/synaptic compartment.
Endogenous and overexpressed LRRK2 immunoreactivity has been demonstrated in association with membrane bound organelles of the secretory pathway, including the endoplasmic reticulum and Golgi apparatus (Biskup et al., 2006; Gloeckner et al., 2006; Hatano et al., 2007), structures of the endocytic pathway, including lipid rafts, caveolar necks, clathrin-coated endosomes and multivesicular endosomes (Alegre-Abarrategui et al., 2009; Biskup et al., 2006; Hatano et al., 2007; Shin et al., 2008) and microtubules (Biskup et al., 2006; Gloeckner et al., 2006). Localization to amphisomes, points of intersection of endocytic and autophagic degradation pathways, has also been reported (Alegre-Abarrategui et al., 2009), although it is unclear if LRRK2 is entrapped passively as cargo or in a regulatory capacity. Collectively, these immunolocalization studies implicate potential roles for LRRK2 in regulating protein trafficking through the secretory/endocytic pathways, critical avenues of synaptic regulation.
Several recent studies have provided some suggestion that LRRK2 impacts secretory and endocytic pathway function. Shin and colleagues (Shin et al., 2008) demonstrated that LRRK2 interacts with Rab5b, a regulator of endocytic vesicle trafficking, by yeast two-hybrid and co-immunoprecipitation techniques. In their primary neuron experiments, overexpression of wild type LRRK2, LRRK2 PD mutants and kinase impaired 1906M-LRRK2, as well as siRNA knockdown of LRRK2, were associated with decreases in pre-synaptic vesicle endocytosis rates in primary rat hippocampal neurons (Shin et al., 2008), prompting the authors to surmise that any change in LRRK2 expression or function may alter the balance of homeostatic interactions with other endocytosis proteins. More recently, Xiong and colleagues (2010) overexpressed wild type LRRK2 in primary neuronal cultures and documented reduced rates of synaptic vesicle endocytosis and exocytosis in hippocampal neurons (Xiong et al, 2010). LRK-1 - a homolog of human LRRK2 in Caenorhabditis elegans that is localized to the Golgi apparatus (Sakaguchi-Nakashima et al., 2007) – is necessary for appropriate axonal localization of synaptic vesicle proteins, suggesting a role for LRK-1 in Golgi cargo sorting (Sakaguchi-Nakashima et al., 2007). Although additional evidence is needed, an attractive hypothesis is that LRRK2 modifies synaptic structure and function through effects on synaptic protein trafficking and potentially degradation.
Mutant LRRK2 induced degeneration was first reported to be associated with membrane-bound, phospho-tau immunoreactive accumulations in dystrophic neurites (Macleod et al., 2006). Our group subsequently demonstrated a link between mutant LRRK2 induced neurite degeneration and autophagy: mutant LRRK2 expression in differentiated SH-SY5Y cells is associated with elevated neurite autophagosomes (Plowey et al., 2008). Furthermore, neuritic autophagy is responsible for neurite retraction because inhibition of the autophagic machinery via siRNA knockdown of LC3 or Atg7 reversed the mutant LRRK2 induced neurite retraction (Plowey et al., 2008). Other manipulations that suppress autophagy, such as phosphorylation of LC3, also prevent mutant LRRK2-mediated neurite retraction in primary cortical neuronal cultures (Cherra et al., 2010b). These data suggest that autophagy, which is thought to be protective in the many neuronal contexts, may induce neurite withdrawal in the process of promoting survival (Cherra et al., 2010a). An alternative hypothesis is that autophagy induced by mutant LRRK2 contributes to neuronal death as has been demonstrated in a MPP+ cell culture model of PD (Zhu et al., 2007).
Additional studies delineating whether the increase in neurite autophagy is due to direct or indirect effects of mutant LRRK2 overexpression may help in resolving these questions. Our group rarely sees colocalization of GFP-LC3 labeled autophagosomes with Hα-tagged LRRK2 (unpublished data), and protein aggregation is not a prominent ultrastructural feature in these cells. Thus, it is less likely that autophagy is induced as a nonspecific cellular response to degrade an overexpressed, dysfunctional protein. While LRRK2 has been suggested by some to function as a direct regulator of autophagy (Alegre-Abarrategui et al., 2009), a role for endogenous LRRK2 in modifying the activity of autophagy proteins by direct interaction or phosphorylation has yet to be demonstrated. It is also possible that autophagy is elicited as a compensatory response to upstream injury. Nevertheless, over time, even compensatory autophagy responses can result in pathologic dendrite remodeling.
In summary, several lines of emerging evidence suggest that mutant LRRK2 impacts the morphology and possibly the function of the neuritic/synaptic compartment in a potentially reversible manner that precedes neuronal cell death. These effects may be mediated through mutant LRRK2 effects on the secretory / endocytic pathways, through direct effects on autophagic neurite catabolism or via secondary autophagic responses to neurite injury. We favor a model in which neurite autophagy is a response to an as yet undefined alteration in dendritic/synaptic function (Fig. 1), although a direct effect on the autophagy machinery cannot be excluded. To date, few potential LRRK2 substrates have been reported: LRRK2 (autophosphorylation) (Gloeckner et al., 2006; Greggio et al., 2009; Greggio et al., 2008; Luzon-Toro et al., 2007; West et al., 2005), moesin (Jaleel et al., 2007; Parisiadou et al., 2009) and 4E-BP (Imai et al., 2008; Kumar et al., 2010). It will be of critical interest to determine which regulatory proteins in the secretory, endocytic or autophagic pathways, if any, are LRRK2 kinase substrates to lend insight into this issue.
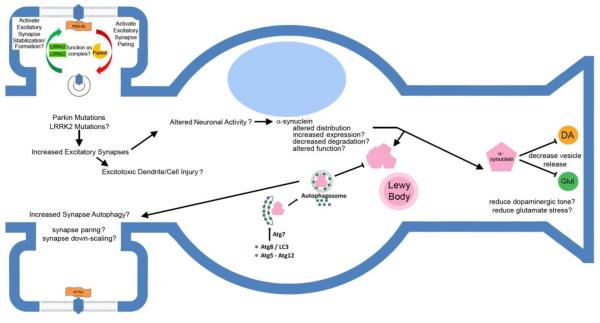
Figure 1. Schematic diagram showing hypothetical links for LRRK2, parkin and α-synuclein in synaptic dysregulation.
Parkin mutations have been reported by Helton and colleagues (Helton et al., 2008) to interrupt the ability of wild type parkin to pare excitatory synapses. Association of parkin and LRRK2 with elements of the secretory and endocytic pathways (see text) raise the possibility that they affect synaptic structure and function by regulating synaptic protein trafficking. LRRK2, which has been reported to interact with parkin (Smith et al., 2005), is hypothesized to induce the formation or stabilization of excitatory synapses. Downstream effects of LRRK2 and parkin mutations would thus increase vulnerability to synaptic glutamate stress (Helton et al., 2008) and could influence neuronal activity. Alterations in neuronal activity induce alterations in the distribution of α-synuclein, and thus may affect α-synuclein effects in the pre-synaptic terminal. Hypothetically, alterations in α-synuclein distribution and function could decrease pre-synaptic vesicle cycling to decrease dopaminergic oxidative stress and synaptic glutamate stress, but this function may be undermined by the propensity for α-synuclein to form cytosolic aggregates. The speculative linkages among these elements are indicated with question marks.
Parkin
Parkin mutations underlie disease in kindreds with autosomal recessive juvenile parkinsonism linked to the PARK2 locus on chromosome 6 (Kitada et al., 1998). Parkin is an E3 ubiquitin ligase (Shimura et al., 2000; Zhang et al., 2000), one of numerous ubiquitin ligases expressed in neurons that likely impact synaptic function via the UPS (Helton and Ehlers, 2008). Parkin mutations found in PD patients confer either decrements in ubiquitin ligase activity (Hampe et al., 2006; Shimura et al., 2000) or disrupt its localization, solubility or interactions with substrates (Hampe et al., 2006; Sriram et al., 2005; Wang et al., 2005). Most mouse models of parkin-deficient mice have demonstrated nigrostriatal synaptic deficits, mitochondrial dysfunction and deficits in learning without nigral dopaminergic cell loss (Goldberg et al., 2003; Itier et al., 2003; Kitada et al., 2009; Palacino et al., 2004). However, a recent report demonstrates hallmark Parkinson's disease features in BAC mutant Parkin-Q311X mice (Hart and Xu, 2009), including accumulation of protease resistant α-synuclein. Although synucleinopathy is not a typical feature of autosomal recessive juvenile parkinsonism in humans (Kitada et al., 1998), Lewy bodies have been observed in rare families (Farrer et al., 2001; Pramstaller et al., 2005).
In the post-synaptic compartment, parkin has been reported to function as a PDZ-binding protein via its C-terminus. Its association with CASK, a post-synaptic multidomain scaffolding protein, results in synaptic localization of parkin in cultured cortical neurons (Fallon et al., 2002). Loss of the C-terminus, as occurs with a common truncating mutation of parkin, could thus be hypothesized to reduce parkin localization to the post-synaptic scaffold and reduce ubiquitination of parkin's post-synaptic substrates. The C-terminus of parkin also appears to be important for parkin interactions with the BAR-SH3 domain of the endocytic protein endophilin-A1, suggesting a role for parkin in regulating membrane protein ubiquitination and endocytosis (Trempe et al., 2009). Immunoelectron microscopy experiments in rat brain demonstrate neuronal parkin expression to be localized prominently to membranous organelles (ER, Golgi) and to vesicular structures of dendrites and presynaptic nerve terminals (Mouatt-Prigent et al., 2004). Taken together, these findings suggest potential roles for parkin in regulating the trafficking of synaptic proteins through the secretory and endocytic pathways.
Several electrophysiologic studies in parkin knockout mice have suggested decreased excitatory post-synaptic responsiveness and plasticity in the striatum or CA1 hippocampal neurons (Goldberg et al., 2003; Hanson et al., 2010; Itier et al., 2003; Kitada et al., 2009). These studies were conducted in brain slices from animals ranging from 2-16 months old, during which compensatory mechanisms or chronic injury may counteract the loss of parkin function on excitatory synapse function. Furthermore, other possibilities should be considered. Upregulation of other ubiquitin ligases (Helton and Ehlers, 2008), decreased de-ubiquitination or decreases in parkin substrate synthesis may compensate for lifelong loss of parkin activity.
In contrast, acute modulation of parkin expression by knockdown of endogenous parkin or overexpression of mutant parkin potentiates glutamatergic post-synaptic function in primary hippocampal cultures with enhanced vulnerability to synaptic glutamate stress (Helton et al., 2008). Enhanced functional expression of excitatory synapses was observed, suggesting that one function of parkin may relate to removal of glutamatergic synapses. Acute overexpression of parkin has been shown to protect, and acute parkin knockdown has been shown to exacerbate, kainate-induced excitotoxicity in cultured cerebellar granule neurons and dopaminergic midbrain neurons (Staropoli et al., 2003). The anti-apoptotic effect was attributed to modulation of the levels of the candidate parkin substrate and potential pro-apoptotic factor cyclin E (Staropoli et al., 2003). A further investigation of the effects of parkin on kainate receptor expression in this model would be very interesting given the above discussion. Inhibitory synapse activity and density were unaffected by altering parkin expression (Helton et al., 2008). Since LRRK2 and parkin interact via the COR domain of LRRK2 and the C-terminal RING2 domain of Parkin (Smith et al., 2005), it is possible that LRRK2 and parkin function as part of a complex to regulate glutamatergic synapse formation/elimination and/or excitatory synapse protein trafficking and degradation (Fig. 1).
Mutant parkin effects on glutamate toxicity may be adversely complemented by decreased ubiquitination and degradation of phospholipase Cγ1 resulting in enhanced Ca+2 levels emanating from ryanodine-sensitive stores (Sandebring et al., 2009). Parkin also monoubiquitinates PICK1, resulting in reduced potentiation of acid-sensing ion channel (ASIC) currents by PICK1; loss of parkin function results in potentiation of excitotoxic ASIC currents via interactions with PICK1 (Joch et al., 2007). PICK1 is also implicated in activity-dependent internalization and retention of the GluR2 subunit of AMPA receptors (Lin and Huganir, 2007) and may represent a mechanism through which parkin impacts glutamate receptor trafficking. Parkin overexpression has been shown to potentiate ATP-induced inward currents conducted by P2X receptors in PC12 cells, an affect that is not seen with overexpression of parkin mutants (Sato et al., 2006).
Deficiencies in presynaptic dopamine release in parkin knockout mice (Goldberg et al., 2003; Itier et al., 2003; Kitada et al., 2009) have driven research into presynaptic parkin substrates. CDCrel-1, a septin GTPase that interacts with syntaxin to inhibit vesicle exocytosis, is ubiquitinated by Parkin (Zhang et al., 2000). CDCrel-1 was shown not to be essential for neuronal development or function in homozygotic CDCrel-1 null mice (Peng et al., 2002). However, dysfunctional mutant Parkin might therefore be hypothesized to lead to increased levels of CDCrel-1 and increased inhibition of presynaptic vesicle exocytosis, leading to inhibition of presynaptic dopaminergic function. Alternatively, as supported by the work of Son and colleagues (Son et al., 2005), this could lead to accumulation of CDCrel-1 as has been documented in proteomic studies of parkin knockout mice (Periquet et al., 2005). Overexpression of Septin 4, the Drosophila homologue of human CDCrel-1, is toxic to dopaminergic neurons (Munoz-Soriano and Paricio, 2007). Parkin-mediated ubiquitination of synaptotagmin XI (Huynh et al., 2003) and the orphan G protein-coupled receptor 37 (GPR37) (Marazziti et al., 2007), may impact presynaptic function through their effects on neurotransmitter release and dopamine transporter function, respectively. Finally, in addition to documented effects on protein accumulation and inclusion pathology, parkin ubiquitination of the glycosylated form of α-synuclein, Sp22 (Shimura et al., 2001), and the α-synuclein interacting protein synphilin-1(Chung et al., 2001) also may impact presynaptic functions of these proteins.
In addition to catalyzing the formation of K48 polyubiquitin chains that target substrates for proteasomal degradation, parkin has garnered recent attention for its potential roles in targeting substrates for autophagy via K63-linked polyubiquitin tagging (Doss-Pepe et al., 2005). Parkin recruits injured mitochondria for degradation via autophagy (mitophagy) as a mechanism of mitochondrial quality control (Narendra et al., 2008; Narendra et al., 2009) (Dagda et al., 2009). Parkin targets the damaged mitochondria for p62-dependent mitophagy via K63 and K27 polyubiquitin linkages (Geisler et al., 2010). Therefore, loss of parkin function may affect synaptic function indirectly by affecting the energy generating capacity in dendrites. Parkin has also been shown target some proteins, including the synaptic protein synphilin-1, for inclusion formation via K63-linked polyubiquitin linkages (Lim et al., 2005). Furthermore, K63 polyubiquitin linkages mediated by parkin or other ubiquitin ligases suggests the possibility synaptic modulation by autophagic proteolysis.
α-Synuclein
α-synuclein has been a major focus of PD genetic research since point mutations (Polymeropoulos et al., 1997) (Kruger et al., 1998) (Zarranz et al., 2004) and genetic locus multiplication (Singleton et al., 2003) in the α-synuclein gene were found to underlie rare forms of familial PD. Furthermore, the discovery that ubiquitinated aggregates of α-synuclein are a prominent component of Lewy bodies in post-mortem PD brains (Spillantini et al., 1997) has fueled the notion that α-synuclein aggregation plays a central role in the sporadic PD. α-synuclein is a 19kDa, 140 amino acid protein containing three domains including an N-terminal alpha-helical lipid binding domain that confers its ability to loosely bind to synaptic vesicles. The A30P substitution negates or reduces the ability to bind membranes (Cole et al., 2002; Fortin et al., 2004; Jensen et al., 1998; Jo et al., 2002; Perrin et al., 2000). The propensity of α-synuclein and its mutants to form toxic soluble oligomers and insoluble aggregates and the potential downstream disruption of various critical cell processes likely represents a common pathway of neurodegeneration in PD and are the subjects of several excellent reviews over the past decade (Cookson and van der Brug, 2008; Lotharius and Brundin, 2002; Sidhu et al., 2004a; Sidhu et al., 2004b). In our discussion, we will focus on evidence implicating roles for α-synuclein in synaptic regulation.
Consideration of the potential roles of α-synuclein in synaptic function has traditionally been focused on presynaptic function since several studies have demonstrated that the normal localization of α-synuclein is in the pre-synaptic terminal. α-Synuclein was first cloned from the a rat brain cDNA library by Maroteaux and colleagues (Maroteaux et al., 1988) who showed that endogenous α-synuclein is localized to presynaptic vesicles in cholinergic fibers innervating the electric organ of Torpedo californica. Ultrastructurally, immunoreactivity for endogenous α-synuclein in rat brain has been demonstrated in close association with the undocked, distal synaptic vesicle pool (Clayton and George, 1999; Iwai et al., 1995). Differential centrifugation experiments have also demonstrated the localization of α-synuclein expression to synaptosomal fractions (Kahle et al., 2000). Recombinant wild-type α-synuclein (Specht et al., 2005) and transgenic wild-type α-synuclein expression (Kahle et al., 2000) also localize to presynaptic terminals. Presynaptic localization appears to be dependent on large portions of the N-terminal and core domains but is independent of the C-terminus (Specht et al., 2005). Neural activity (Fortin et al., 2005) and depolarization (Tao-Cheng, 2006) have been reported to affect α-synuclein distribution in the pre-synaptic terminal albeit in opposite directions. Overexpression of α-synuclein mutants does not disturb delivery to presynaptic terminals (Kahle et al., 2000) despite abnormal cellular accumulations. A clue to the possible function of synuclein in regulating vesicle release comes from the CSPα knockout mice, a mouse with a severe neurodegenerative phenotype caused by dysfunction of SNARE complexes that mediate vesicle fusion/release (Chandra et al., 2005). Chandra and colleagues (Chandra et al., 2005) found that crossing transgenic wild-type α-synuclein mice, but not A30P α-synuclein transgenic mice, with CSPα knockout mice restores SNARE complex formation and rescues the progeny from the severe neurodegenerative phenotype, suggesting a role for α-synuclein in maintaining SNARE complexes. These studies suggest that α-synuclein impacts pre-synaptic function by regulating some aspect of synaptic vesicle fusion/release or the dynamics of the reserve pool of synaptic vesicles.
Studies of neurotransmitter release in catecholaminergic cells have lent support to the hypothesis that α-synuclein functions in the regulation of presynaptic neurotransmitter vesicle pools. Murphy and colleagues (Murphy et al., 2000) showed a robust decrease of vesicles in the distal synaptic pool but not in the docked pool in primary rat hippocampal cultures following a 50% knockdown in endogenous α-synuclein levels. In an α-synuclein knockout mouse, Abeliovich and colleagues (Abeliovich et al., 2000) reported the effect of increasing stimulated DA release in knockout mice striatal slices using a paired-pulse stimuli, suggesting that α-synuclein normally acts as activity-dependent negative regulator of DA neurotransmission. They found that knockout mice demonstrated reduced striatal DA content and decreased amphetamine-stimulated locomotion and proposed that the lack of negative α-synuclein regulation led to a decrease in the readily releasable pool of DA vesicles, although no apparent change in presynaptic vesicle pools in electron micrographs of striatum were seen (Abeliovich et al., 2000). Cabin and colleagues (Cabin et al., 2002) did show in a separate α-synuclein knockout mouse attenuated CA1 dendritic field EPSCs with stimulation of Schaffer collaterals in hippocampal slices of mice lacking a-synuclein and ultrastructural evidence of decreased presynaptic vesicles, suggesting decreased reserve storage pool of vesicles. In a double-knockout model of α- and β-synuclein, no changes in presynaptic vesicle density or any other presynaptic morphologic parameter was seen, nor were there any changes in tests of hippocampal synaptic plasticity, yet a modest 20% reduction in striatal dopamine content was reported (Chandra et al., 2004). Yavich, and colleagues (Yavich et al., 2004) employed in vivo voltammetry in α-synuclein null mice to document an increased readily releasable pool (altered dopamine compartmentalization) and increased facilitation, suggestive of increased refilling rate of the reserve pool, as a compensatory mechanism to maintain stable dopamine release during stimulation. Most recently, α-and γ-synuclein double knockout mice were reported to show increased evoked release of striatial dopamine that was not attributable to increased striatal DA or to decreased DA reuptake (Senior et al., 2008). Overexpression of wild-type or A30P synuclein in chromaffin cells and PC12 cells is associated with impairment of dopamine secretion despite robustly increased numbers of morphologically docked dense core vesicles and unaltered sensitivity to Ca+2, suggestive of inhibition of a step in exocytosis before Ca+2 induced fusion (Larsen et al., 2006). Overall, these studies support a model in which α-synuclein functions to inhibit DA vesicle release by downregulating vesicle access to the presynaptic terminal membrane.
In dopaminergic cells, α-synuclein has also been observed to suppress tyrosine hydroxylase activity, improve dopamine storage into vesicles and reduce the activity of the dopamine transporter, whereas mutant α-synuclein is impaired in many of these interactions (Sidhu et al., 2004a; Sidhu et al., 2004b). The cumulative effect of α-synuclein may therefore be to decrease the levels of cytoplasmic dopamine and oxidative stress in a neuroprotective manner (Sidhu et al., 2004a; Sidhu et al., 2004b). In consideration of recent findings in which afferent glutamatergic activity is enhanced in neurons with mutant parkin expression, we envision a model in which the function of α-synuclein may be to decrease synaptic dopamine turnover and oxidative stress, but which is undermined by the propensity of α-synuclein to aggregate (Fig. 1).
Potential functions of α-synuclein in cortical glutamatergic neurons are important to consider given proposed roles for α-synuclein in synaptic plasticity and the involvement of cortical synucleinopathy in patients with Parkinson's disease with dementia and dementia with Lewy bodies. Synelfin, a songbird homologue of α-synuclein, is expressed in the glutamatergic circuits that govern song learning (Mooney and Konishi, 1991), during the critical juvenile learning period (George et al., 1995), suggesting a role in learning and plasticity. Wu and colleagues (Wu et al., 2009) reported decreased miniature EPSCs in striatal medium-sized spiny neurons and increased thresholds for stimulating EPSCs from the corticostriatal afferents in transgenic mice overexpressing human WT α-synuclein. Overexpression of α-synuclein was also reported to inhibit the exocytosis of VGLUT1-expressing glutamatergic vesicles from both hippocampal and midbrain dopaminergic neurons secondary to decreased clustering of vesicles in the presynaptic terminal and reduction of the recycling pool (Nemani et al., 2010). From a presynaptic plasticity standpoint, however, Gurevicience and colleagues (Gureviciene et al., 2007) reported enhancement of LTP, a presynaptic phenomenon in mossy fiber-CA3 synapses with overexpression of α-synuclein, whereas KO mice also showed an LTP deficit in response to high frequency stimulation. α-synuclein enhancement of glutamate vesicle release from the presynaptic terminal was also reported by Liu and colleagues (Liu et al., 2007; Liu et al., 2004), who showed enhancement of mEPSC amplitudes and rates and amplitudes of evoked EPSCs in the post-synaptic cell of monosynaptically-connected pairs in hippocampal neuron cultures when they injected α-synuclein into the pre-synaptic neuron. Thus, whereas some studies recapitulate an inhibitory effect of α-synuclein on presynaptic vesicle release in glutamatergic synapses, a full understanding of α-synuclein in cortical synaptic plasticity is probably more complex and is important for our understanding of Lewy body dementia.
Autophagy contributes to the degradation of α-synuclein (Webb et al., 2003; Yu et al., 2009), providing another avenue through which autophagy may impact synaptic function. Expression of mutant α-synuclein increases cellular autophagosomes (Stefanis et al., 2001). Furthermore, chaperone mediated autophagy (CMA), which involves targeting proteins for lysosomal translocation by the LAMP2A receptor by cytosolic chaperone proteins, also contributes to α-synuclein degradation (Mak et al., 2010; Vogiatzi et al., 2008) and is impaired in models of synucleinopathy (Cuervo et al., 2004; Martinez-Vicente et al., 2008; Xilouri et al., 2009). Macroautophagy is induced to compensate for defective CMA, although this regulation may be altered in patients with synucleinopathy due to reduced levels of autophagy regulatory proteins Atg7 and mTOR (Crews et al., 2010). These findings raise the possibilities that autophagy may influence or regulate synaptic function through α-synuclein degradation.
Methods to enhance cellular autophagy as a means to dispose of accumulating toxic synuclein oligomers have been suggested as potential therapies for synucleinopathies and other neurodegenerative diseases characterized by protein aggregation (Ravikumar et al., 2008; Sarkar et al., 2007a; Sarkar et al., 2007b; Spencer et al., 2009). However, the potential for adverse functional effects of pharmacologically inducing autophagy needs to be carefully considered as well, particularly if synaptic remodeling may occur as an unintended side-effect of increased autophagic activity (Cherra et al., 2010a), or if there is relative impairment in autophagosome maturation, fusion or lysosomal degradation, as has been described in Alzheimer's disease (Boland et al., 2008) and several other neurodegenerative diseases (Wong and Cuervo, 2010).
Conclusions
Although a complete understanding of the functions of α-synuclein has not been achieved, it is implicated in inhibiting pre-synaptic vesicle release. Recent studies also suggest potential mechanisms by which parkin and LRRK2 could impact synaptic structure and function. Given these trends, construction of a unifying concept of Parkinsonian neurodegeneration should consider possible roles for these proteins in synaptic function and dysfunction as early events in the neurodegenerative cascade. Critical issues that remain to be addressed include: (1) differentiating effects on post-synaptic function versus pre-synaptic function, (2) whether observed effects are context dependent (acute knockdown/overexpression versus chronic compensatory effects), and (3) whether these effects are similar or dissimilar in dopaminergic, glutamatergic and other synapse types. Furthermore, the impact on synaptic function needs to be confirmed as primary early neurodegenerative events, versus secondary to other documented PD phenomena of protein aggregation, mitochondrial dysfunction or oxidative stress. Dysregulation of mitochondrial dynamics, trafficking or quality control, as implicated in autosomal recessive PD pathogenesis (Dagda and Chu, 2009), could indirectly affect synaptic maintenance and function as well. Lastly, as autophagy is heavily implicated as a neuritic response in each of these genetic models of PD, and as autophagy-modulating drugs garner increased attention as potential therapeutic agents in neurodegenerative diseases, it becomes critically important to study the potential impact of autophagy modulation on synaptic structure and function.
Acknowledgements
This work is supported in part by NIH Grants AG026389 and NS065789 to CTC. EDP was supported in part by T32NS007391.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeliovich A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–52. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Alegre-Abarrategui J, et al. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet. 2009;18:4022–34. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres-Mateos E, et al. Unexpected lack of hypersensitivity in LRRK2 knock-out mice to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) J Neurosci. 2009;29:15846–50. doi: 10.1523/JNEUROSCI.4357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamber BA, Rowland AM. Shaping cellular form and function by autophagy. Autophagy. 2006;2:247–9. doi: 10.4161/auto.2746. [DOI] [PubMed] [Google Scholar]
- Biskup S, et al. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- Boland B, et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J Neurosci. 2008;28:6926–37. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabin DE, et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, et al. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci U S A. 2004;101:14966–71. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, et al. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–96. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Cherra SJ, 3rd, et al. Review: autophagy and neurodegeneration: survival at a cost? Neuropathol Appl Neurobiol. 2010a;36:125–32. doi: 10.1111/j.1365-2990.2010.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherra SJ, 3rd, et al. Regulation of the autophagy protein LC3 by phosphorylation. J Cell Biol. 2010b;190:533–9. doi: 10.1083/jcb.201002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KK, et al. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med. 2001;7:1144–50. doi: 10.1038/nm1001-1144. [DOI] [PubMed] [Google Scholar]
- Clayton DF, George JM. Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:120–9. [PubMed] [Google Scholar]
- Cole NB, et al. Lipid droplet binding and oligomerization properties of the Parkinson's disease protein alpha-synuclein. J Biol Chem. 2002;277:6344–52. doi: 10.1074/jbc.M108414200. [DOI] [PubMed] [Google Scholar]
- Cookson MR, van der Brug M. Cell systems and the toxic mechanism(s) of alpha-synuclein. Exp Neurol. 2008;209:5–11. doi: 10.1016/j.expneurol.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, et al. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS One. 2010;5:e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cuervo AM, et al. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–5. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Dagda RK, et al. Loss of pink1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–55. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagda RK, Chu CT. Mitochondrial quality control: insights on how Parkinson's disease related genes PINK1, parkin, and Omi/HtrA2 interact to maintain mitochondrial homeostasis. J Bioenerg Biomembr. 2009;41:473–9. doi: 10.1007/s10863-009-9255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss-Pepe EW, et al. Alpha-synuclein and parkin contribute to the assembly of ubiquitin lysine 63-linked multiubiquitin chains. J Biol Chem. 2005;280:16619–24. doi: 10.1074/jbc.M413591200. [DOI] [PubMed] [Google Scholar]
- Fader CM, Colombo MI. Autophagy and multivesicular bodies: two closely related partners. Cell Death Differ. 2009;16:70–8. doi: 10.1038/cdd.2008.168. [DOI] [PubMed] [Google Scholar]
- Fallon L, et al. Parkin and CASK/LIN-2 associate via a PDZ-mediated interaction and are co-localized in lipid rafts and postsynaptic densities in brain. J Biol Chem. 2002;277:486–91. doi: 10.1074/jbc.M109806200. [DOI] [PubMed] [Google Scholar]
- Farrer M, et al. Lewy bodies and parkinsonism in families with parkin mutations. Ann Neurol. 2001;50:293–300. doi: 10.1002/ana.1132. [DOI] [PubMed] [Google Scholar]
- Fortin DL, et al. Neural activity controls the synaptic accumulation of alpha-synuclein. J Neurosci. 2005;25:10913–21. doi: 10.1523/JNEUROSCI.2922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DL, et al. Lipid rafts mediate the synaptic localization of alpha-synuclein. J Neurosci. 2004;24:6715–23. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama M, et al. A new locus for Parkinson's disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann Neurol. 2002;51:296–301. doi: 10.1002/ana.10113. [DOI] [PubMed] [Google Scholar]
- Galter D, et al. LRRK2 expression linked to dopamine-innervated areas. Ann Neurol. 2006;59:714–9. doi: 10.1002/ana.20808. [DOI] [PubMed] [Google Scholar]
- Geisler S, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–31. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- George JM, et al. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–72. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- Gilks WP, et al. A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet. 2005;365:415–6. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- Gloeckner CJ, et al. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet. 2006;15:223–32. doi: 10.1093/hmg/ddi439. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–35. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Greggio E, Cookson MR. Leucine-rich repeat kinase 2 mutations and Parkinson's disease: three questions. ASN Neuro. 2009;1 doi: 10.1042/AN20090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greggio E, et al. The Parkinson's disease kinase LRRK2 autophosphorylates its GTPase domain at multiple sites. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.08.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greggio E, et al. The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J Biol Chem. 2008;283:16906–14. doi: 10.1074/jbc.M708718200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Choquet D. AMPA and NMDA glutamate receptor trafficking: multiple roads for reaching and leaving the synapse. Cell Tissue Res. 2006;326:423–38. doi: 10.1007/s00441-006-0254-9. [DOI] [PubMed] [Google Scholar]
- Gureviciene I, et al. Role of alpha-synuclein in synaptic glutamate release. Neurobiol Dis. 2007;28:83–9. doi: 10.1016/j.nbd.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Hampe C, et al. Biochemical analysis of Parkinson's disease-causing variants of Parkin, an E3 ubiquitin-protein ligase with monoubiquitylation capacity. Hum Mol Genet. 2006;15:2059–75. doi: 10.1093/hmg/ddl131. [DOI] [PubMed] [Google Scholar]
- Hanson JE, et al. Altered hippocampal synaptic physiology in aged parkin-deficient mice. Neuromolecular Med. 2010;12:270–6. doi: 10.1007/s12017-010-8113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart MP, Xu A. Mice expressing mutant parkin exhibit hallmark features of Parkinson's disease. J Neurosci. 2009;29:7392–4. doi: 10.1523/JNEUROSCI.1719-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano T, et al. Leucine-rich repeat kinase 2 associates with lipid rafts. Hum Mol Genet. 2007;16:678–90. doi: 10.1093/hmg/ddm013. [DOI] [PubMed] [Google Scholar]
- Healy DG, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol. 2008;7:583–90. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton TD, Ehlers MD. Ubiquitin and protein degradation in synapse function. In: Hell JW, Ehlers MD, editors. Structural and functional organization of the synapse. Vol. 2008. Springer; New York: pp. 553–597. [Google Scholar]
- Helton TD, et al. Pruning and loss of excitatory synapses by the parkin ubiquitin ligase. Proc Natl Acad Sci U S A. 2008;105:19492–7. doi: 10.1073/pnas.0802280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi S, et al. Localization of Parkinson's disease-associated LRRK2 in normal and pathological human brain. Brain Res. 2007;1155:208–19. doi: 10.1016/j.brainres.2007.04.034. [DOI] [PubMed] [Google Scholar]
- Hirling H. Endosomal trafficking of AMPA-type glutamate receptors. Neuroscience. 2009;158:36–44. doi: 10.1016/j.neuroscience.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Huynh DP, et al. The autosomal recessive juvenile Parkinson disease gene product, parkin, interacts with and ubiquitinates synaptotagmin XI. Hum Mol Genet. 2003;12:2587–97. doi: 10.1093/hmg/ddg269. [DOI] [PubMed] [Google Scholar]
- Imai Y, et al. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. Embo J. 2008;27:2432–43. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier JM, et al. Parkin gene inactivation alters behaviour and dopamine neurotransmission in the mouse. Hum Mol Genet. 2003;12:2277–91. doi: 10.1093/hmg/ddg239. [DOI] [PubMed] [Google Scholar]
- Iwai A, et al. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–75. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Jaleel M, et al. LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson's disease mutants affect kinase activity. Biochem J. 2007;405:307–17. doi: 10.1042/BJ20070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PH, et al. Binding of alpha-synuclein to brain vesicles is abolished by familial Parkinson's disease mutation. J Biol Chem. 1998;273:26292–4. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- Jo E, et al. Defective membrane interactions of familial Parkinson's disease mutant A30P alpha-synuclein. J Mol Biol. 2002;315:799–807. doi: 10.1006/jmbi.2001.5269. [DOI] [PubMed] [Google Scholar]
- Joch M, et al. Parkin-mediated monoubiquitination of the PDZ protein PICK1 regulates the activity of acid-sensing ion channels. Mol Biol Cell. 2007;18:3105–18. doi: 10.1091/mbc.E05-11-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle PJ, et al. Subcellular localization of wild-type and Parkinson's disease-associated mutant alpha -synuclein in human and transgenic mouse brain. J Neurosci. 2000;20:6365–73. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkin V, et al. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–69. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–8. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kitada T, et al. Impaired dopamine release and synaptic plasticity in the striatum of parkin−/− mice. J Neurochem. 2009;110:613–21. doi: 10.1111/j.1471-4159.2009.06152.x. [DOI] [PubMed] [Google Scholar]
- Korolchuk VI, et al. Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett. 584:1393–8. doi: 10.1016/j.febslet.2009.12.047. [DOI] [PubMed] [Google Scholar]
- Kruger R, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–8. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kumar A, et al. The Parkinson's disease associated LRRK2 exhibits weaker in vitro phosphorylation of 4E-BP compared to autophosphorylation. PLoS One. 2010;5:e8730. doi: 10.1371/journal.pone.0008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen KE, et al. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–22. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Gao FB. Roles of ESCRT in autophagy-associated neurodegeneration. Autophagy. 2008;4:230–2. doi: 10.4161/auto.5384. [DOI] [PubMed] [Google Scholar]
- Lesage S, Brice A. Parkinson's disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18:R48–59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- Li X, et al. Enhanced striatal dopamine transmission and motor performance with LRRK2 overexpression in mice is eliminated by familial Parkinson's disease mutation G2019S. J Neurosci. 2010;30:1788–97. doi: 10.1523/JNEUROSCI.5604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson's disease. Nat Neurosci. 2009;12:826–8. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KL, et al. Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J Neurosci. 2005;25:2002–9. doi: 10.1523/JNEUROSCI.4474-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DT, Huganir RL. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J Neurosci. 2007;27:13903–8. doi: 10.1523/JNEUROSCI.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, et al. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson's-disease-related mutant alpha-synuclein. Neuron. 2009;64:807–27. doi: 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, et al. Alpha-synuclein involvement in hippocampal synaptic plasticity: role of NO, cGMP, cGK and CaMKII. Eur J Neurosci. 2007;25:3583–96. doi: 10.1111/j.1460-9568.2007.05569.x. [DOI] [PubMed] [Google Scholar]
- Liu S, et al. alpha-Synuclein produces a long-lasting increase in neurotransmitter release. Embo J. 2004;23:4506–16. doi: 10.1038/sj.emboj.7600451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotharius J, Brundin P. Pathogenesis of Parkinson's disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3:932–42. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- Luzon-Toro B, et al. Mechanistic insight into the dominant mode of the Parkinson's disease-associated G2019S LRRK2 mutation. Hum Mol Genet. 2007;16:2031–9. doi: 10.1093/hmg/ddm151. [DOI] [PubMed] [Google Scholar]
- Macleod D, et al. The Familial Parkinsonism Gene LRRK2 Regulates Neurite Process Morphology. Neuron. 2006;52:587–93. doi: 10.1016/j.neuron.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Mak SK, et al. Lysosomal degradation of alpha-synuclein in vivo. J Biol Chem. 2010;285:13621–9. doi: 10.1074/jbc.M109.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, et al. GPR37 associates with the dopamine transporter to modulate dopamine uptake and behavioral responses to dopaminergic drugs. Proc Natl Acad Sci U S A. 2007;104:9846–51. doi: 10.1073/pnas.0703368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux L, et al. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–15. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vicente M, et al. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–88. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata IF, et al. LRRK2 in Parkinson's disease: protein domains and functional insights. Trends Neurosci. 2006;29:286–93. doi: 10.1016/j.tins.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Melrose H, et al. Anatomical localization of leucine-rich repeat kinase 2 in mouse brain. Neuroscience. 2006;139:791–4. doi: 10.1016/j.neuroscience.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Melrose HL, et al. A comparative analysis of leucine-rich repeat kinase 2 (Lrrk2) expression in mouse brain and Lewy body disease. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Mooney R, Konishi M. Two distinct inputs to an avian song nucleus activate different glutamate receptor subtypes on individual neurons. Proc Natl Acad Sci U S A. 1991;88:4075–9. doi: 10.1073/pnas.88.10.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouatt-Prigent A, et al. Ultrastructural localization of parkin in the rat brainstem, thalamus and basal ganglia. J Neural Transm. 2004;111:1209–18. doi: 10.1007/s00702-004-0144-9. [DOI] [PubMed] [Google Scholar]
- Munoz-Soriano V, Paricio N. Overexpression of Septin 4, the Drosophila homologue of human CDCrel-1, is toxic for dopaminergic neurons. Eur J Neurosci. 2007;26:3150–8. doi: 10.1111/j.1460-9568.2007.05937.x. [DOI] [PubMed] [Google Scholar]
- Murphy DD, et al. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–20. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, et al. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, et al. Parkin-induced mitophagy in the pathogenesis of Parkinson disease. Autophagy. 2009;5:706–8. doi: 10.4161/auto.5.5.8505. [DOI] [PubMed] [Google Scholar]
- Nemani VM, et al. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisan-Ruiz C, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Palacino JJ, et al. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004;279:18614–22. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- Parisiadou L, et al. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J Neurosci. 2009;29:13971–80. doi: 10.1523/JNEUROSCI.3799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng XR, et al. The septin CDCrel-1 is dispensable for normal development and neurotransmitter release. Mol Cell Biol. 2002;22:378–87. doi: 10.1128/MCB.22.1.378-387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periquet M, et al. Proteomic analysis of parkin knockout mice: alterations in energy metabolism, protein handling and synaptic function. J Neurochem. 2005;95:1259–76. doi: 10.1111/j.1471-4159.2005.03442.x. [DOI] [PubMed] [Google Scholar]
- Perrin RJ, et al. Interaction of human alpha-Synuclein and Parkinson's disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J Biol Chem. 2000;275:34393–8. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- Plowey ED, et al. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J Neurochem. 2008;105:1048–56. doi: 10.1111/j.1471-4159.2008.05217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–7. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Pramstaller PP, et al. Lewy body Parkinson's disease in a large pedigree with 77 Parkin mutation carriers. Ann Neurol. 2005;58:411–22. doi: 10.1002/ana.20587. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, et al. Clearance of mutant aggregate-prone proteins by autophagy. Methods Mol Biol. 2008;445:195–211. doi: 10.1007/978-1-59745-157-4_13. [DOI] [PubMed] [Google Scholar]
- Rowland AM, et al. Presynaptic terminals independently regulate synaptic clustering and autophagy of GABAA receptors in Caenorhabditis elegans. J Neurosci. 2006;26:1711–20. doi: 10.1523/JNEUROSCI.2279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusten TE, Simonsen A. ESCRT functions in autophagy and associated disease. Cell Cycle. 2008;7:1166–72. doi: 10.4161/cc.7.9.5784. [DOI] [PubMed] [Google Scholar]
- Sakaguchi-Nakashima A, et al. LRK-1, a C. elegans PARK8-Related Kinase, Regulates Axonal-Dendritic Polarity of SV Proteins. Curr Biol. 2007 doi: 10.1016/j.cub.2007.01.074. [DOI] [PubMed] [Google Scholar]
- Sandebring A, et al. Parkin deficiency disrupts calcium homeostasis by modulating phospholipase C signalling. Febs J. 2009;276:5041–52. doi: 10.1111/j.1742-4658.2009.07201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, et al. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem. 2007a;282:5641–52. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- Sarkar S, et al. Small molecules enhance autophagy and reduce toxicity in Huntington's disease models. Nat Chem Biol. 2007b;3:331–8. doi: 10.1038/nchembio883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake W, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat Genet. 2009;41:1303–7. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- Sato A, et al. Parkin potentiates ATP-induced currents due to activation of P2X receptors in PC12 cells. J Cell Physiol. 2006;209:172–82. doi: 10.1002/jcp.20719. [DOI] [PubMed] [Google Scholar]
- Seidenbecher CI, et al. Caldendrin but not calmodulin binds to light chain 3 of MAP1A/B: an association with the microtubule cytoskeleton highlighting exclusive binding partners for neuronal Ca(2+)-sensor proteins. J Mol Biol. 2004;336:957–70. doi: 10.1016/j.jmb.2003.12.054. [DOI] [PubMed] [Google Scholar]
- Senior SL, et al. Increased striatal dopamine release and hyperdopaminergic-like behaviour in mice lacking both alpha-synuclein and gamma-synuclein. Eur J Neurosci. 2008;27:947–57. doi: 10.1111/j.1460-9568.2008.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimura H, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–5. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- Shimura H, et al. Ubiquitination of a new form of alpha-synuclein by parkin from human brain: implications for Parkinson's disease. Science. 2001;293:263–9. doi: 10.1126/science.1060627. [DOI] [PubMed] [Google Scholar]
- Shin N, et al. LRRK2 regulates synaptic vesicle endocytosis. Exp Cell Res. 2008;314:2055–65. doi: 10.1016/j.yexcr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Sidhu A, et al. The role of alpha-synuclein in both neuroprotection and neurodegeneration. Ann N Y Acad Sci. 2004a;1035:250–70. doi: 10.1196/annals.1332.016. [DOI] [PubMed] [Google Scholar]
- Sidhu A, et al. Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse? Faseb J. 2004b;18:637–47. doi: 10.1096/fj.03-1112rev. [DOI] [PubMed] [Google Scholar]
- Simon-Sanchez J, et al. LRRK2 is expressed in areas affected by Parkinson's disease in the adult mouse brain. Eur J Neurosci. 2006;23:659–66. doi: 10.1111/j.1460-9568.2006.04616.x. [DOI] [PubMed] [Google Scholar]
- Simon-Sanchez J, et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet. 2009;41:1308–12. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Smith WW, et al. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9:1231–3. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- Smith WW, et al. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci U S A. 2005;102:18676–81. doi: 10.1073/pnas.0508052102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JH, et al. Neurotoxicity and behavioral deficits associated with Septin 5 accumulation in dopaminergic neurons. J Neurochem. 2005;94:1040–53. doi: 10.1111/j.1471-4159.2005.03257.x. [DOI] [PubMed] [Google Scholar]
- Specht CG, et al. Subcellular localisation of recombinant alpha- and gamma-synuclein. Mol Cell Neurosci. 2005;28:326–34. doi: 10.1016/j.mcn.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Spencer B, et al. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. J Neurosci. 2009;29:13578–88. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Sriram SR, et al. Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. Hum Mol Genet. 2005;14:2571–86. doi: 10.1093/hmg/ddi292. [DOI] [PubMed] [Google Scholar]
- Staropoli JF, et al. Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from kainate excitotoxicity. Neuron. 2003;37:735–49. doi: 10.1016/s0896-6273(03)00084-9. [DOI] [PubMed] [Google Scholar]
- Stefanis L, et al. Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci. 2001;21:9549–60. doi: 10.1523/JNEUROSCI.21-24-09549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH. Activity-related redistribution of presynaptic proteins at the active zone. Neuroscience. 2006;141:1217–24. doi: 10.1016/j.neuroscience.2006.04.061. [DOI] [PubMed] [Google Scholar]
- Taymans JM, Cookson MR. Mechanisms in dominant parkinsonism: The toxic triangle of LRRK2, alpha-synuclein, and tau. Bioessays. 2010;32:227–35. doi: 10.1002/bies.200900163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taymans JM, et al. Distribution of PINK1 and LRRK2 in rat and mouse brain. J Neurochem. 2006;98:951–61. doi: 10.1111/j.1471-4159.2006.03919.x. [DOI] [PubMed] [Google Scholar]
- Tong Y, et al. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci U S A. 2009;106:14622–7. doi: 10.1073/pnas.0906334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, et al. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of {alpha}-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempe JF, et al. SH3 domains from a subset of BAR proteins define a Ubl-binding domain and implicate parkin in synaptic ubiquitination. Mol Cell. 2009;36:1034–47. doi: 10.1016/j.molcel.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Uitti RJ, et al. Is the neuropathological ‘gold standard’ diagnosis dead? Implications of clinicopathological findings in an autosomal dominant neurodegenerative disorder. Parkinsonism Relat Disord. 2004;10:461–3. doi: 10.1016/j.parkreldis.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Vogiatzi T, et al. Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem. 2008;283:23542–56. doi: 10.1074/jbc.M801992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, et al. Alterations in the solubility and intracellular localization of parkin by several familial Parkinson's disease-linked point mutations. J Neurochem. 2005;93:422–31. doi: 10.1111/j.1471-4159.2005.03023.x. [DOI] [PubMed] [Google Scholar]
- Webb JL, et al. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278:25009–13. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- West AB, et al. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–7. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wider C, et al. Leucine-rich repeat kinase 2 gene-associated disease: redefining genotype-phenotype correlation. Neurodegener Dis. 2010;7:175–9. doi: 10.1159/000289232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13:805–11. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wszolek ZK, et al. Western Nebraska family (family D) with autosomal dominant parkinsonism. Neurology. 1995;45:502–5. doi: 10.1212/wnl.45.3.502. [DOI] [PubMed] [Google Scholar]
- Wu N, et al. Alpha-synuclein overexpression in mice alters synaptic communication in the corticostriatal pathway. J Neurosci Res. 2009 doi: 10.1002/jnr.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xilouri M, et al. Abberant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PLoS One. 2009;4:e5515. doi: 10.1371/journal.pone.0005515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavich L, et al. Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24:11165–70. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JJ, Ehlers MD. Ubiquitin and protein turnover in synapse function. Neuron. 2005;47:629–32. doi: 10.1016/j.neuron.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Yi JJ, Ehlers MD. Emerging roles for ubiquitin and protein degradation in neuronal function. Pharmacol Rev. 2007;59:14–39. doi: 10.1124/pr.59.1.4. [DOI] [PubMed] [Google Scholar]
- Yu WH, et al. Metabolic activity determines efficacy of macroautophagic clearance of pathological oligomeric alpha-synuclein. Am J Pathol. 2009;175:736–47. doi: 10.2353/ajpath.2009.080928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarranz JJ, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–73. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci U S A. 2000;97:13354–9. doi: 10.1073/pnas.240347797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Chu CT. Mitochondrial dysfunction in Parkinson's disease. J Alzheimers Dis. 2010;20(Suppl 2):S325–34. doi: 10.3233/JAD-2010-100363. [DOI] [PubMed] [Google Scholar]
- Zhu JH, et al. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–7. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]