Summary
Mechanisms of hydrogen peroxide generation in Escherichia coli were investigated using a strain lacking scavenging enzymes. Surprisingly, the deletion of many abundant flavoenzymes that are known to auto-oxidize in vitro did not substantially lessen overall H2O2 formation. However, H2O2 production diminished by 25-30% when NadB turnover was eliminated. The flavin-dependent desaturating dehydrogenase, NadB uses fumarate as an electron acceptor in anaerobic cells. Experiments showed that aerobic NadB turnover depends upon its oxidation by molecular oxygen, with H2O2 as a product. This reaction appears to be mechanistically adventitious. In contrast, most desaturating dehydrogenases are associated with the respiratory chain and deliver electrons to fumarate anaerobically or oxygen aerobically without the formation of toxic byproducts. Presumably, NadB can persist as an H2O2-generating enzyme because its flux is limited. The anaerobic respiratory enzyme fumarate reductase uses a flavoprotein subunit that is homologous to NadB and accordingly forms substantial H2O2 upon aeration. This tendency is substantially suppressed by cytochrome oxidase. Thus cytochrome d oxidase, which is prevalent among anaerobes, may diminish intracellular H2O2 formation by the anaerobic respiratory chain, whenever these organisms encounter oxygen. These two examples reveal biochemical and physiological arrangements through which evolution has minimized the rate of intracellular oxidant formation.
Introduction
Whether a microbe can occupy a given habitat depends in part upon whether it can withstand the local concentration of oxygen. While microbes differ enormously in their tolerance for oxygen, the bases of these differences is not fully understood. Some bacteria and archaea rely for their metabolism upon enzymes that are directly poisoned by oxygen (Wagner et al., 1992; Pan and Imlay, 2001; Martins et al., 2004). Many other organisms, however, are threatened not by molecular oxygen itself but rather by reactive oxygen species (ROS)—superoxide and hydrogen peroxide—that are derived from it.
Bacteria universally express catalases and/or peroxidases to scavenge intracellular H2O2, and they employ superoxide dismutases and/or reductases to scavenge superoxide (Imlay, 2008). Nevertheless, high rates of intracellular ROS formation can elevate these species to toxic concentrations, and cell damage and dysfunction can result. For this reason, it seems plausible that differences in oxygen tolerance might stem in part from differences in the rates of intracellular ROS formation when various microbes enter aerobic habitats. To evaluate this idea, it is necessary to identify the mechanisms by which ROS are generated in vivo.
ROS are formed when molecular oxygen collides with redox enzymes and steals their electrons. In vitro surveys have identified a variety of flavoenzymes that display this behavior (Massey et al., 1969; Geary and Meister, 1977; Messner and Imlay, 1999; Grinblat et al., 1991; Messner and Imlay, 2002; Kussmaul and Hirst, 2006). Typically their FAD or FMN moiety is solvent-exposed, a trait which is catalytically essential because it enables the flavin to exchange electrons with soluble substrates. Oxygen is small enough to enter the active site and contact the reduced flavin, thereby eliciting the inadvertent transfer of either a single electron or two consecutive electrons. The former generates superoxide, which in the cell would ultimately be converted by dismutation to hydrogen peroxide; the latter event generates hydrogen peroxide directly. The rate at which an enzyme reacts with oxygen depends upon the degree to which the flavin is solvent-exposed, the electron residence time on the flavin during the catalytic cycle, and the reduction potential of the flavin. Because of these factors, the inherent autoxidation rates of enzymes range over several orders of magnitude (Massey et al., 1969; Messner and Imlay, 2002).
The overall rate of H2O2 formation inside E. coli can be directly measured using a strain that lacks catalases and NADH peroxidase (katG katE ahp) (Seaver and Imlay, 2001b). H2O2 that is formed inside this mutant diffuses across the membrane to the external medium, and so the gradual rise in external H2O2 allows one to calculate the rate of its internal formation. Rates of 10-15 micromolar/s (normalized to the cytoplasmic volume of the cells) have been observed for cells grown in air-saturated glucose medium (Seaver and Imlay, 2004). The rates increase when oxygen levels are raised, which is consistent with the notion that the H2O2 is formed by adventitious processes that are proportionate to the intracellular oxygen concentration. This result also implies that aerobic microbes can minimize their stress by occupying habitats in which oxygen levels are sufficient to saturate their cytochrome oxidases (Km < 5 micromolar) but do not approach full aeration (210 micromolar).
Because both the autoxidation behaviors and titers of various flavoenzymes vary widely, we thought it statistically likely that a few enzymes would be responsible for most of the H2O2 that is formed inside E. coli. If so, then the H2O2 efflux should be diminished in mutant strains that lack these enzymes. Surprisingly, a previous study showed that cellular H2O2 production was not lessened by a mutation that eliminated NADH dehydrogenase II, which in vitro was the most autoxidizable component of the electron transport chain (Seaver and Imlay, 2004). A sixteen-fold overproduction of the enzyme was enough to boost total H2O2 formation only four-fold, which indicated that although the enzyme does autoxidize, it normally is only a minor source of cellular H2O2.
The goals of the work reported here were to test our notion that a few ROS sources predominate in vivo, to determine whether they are flavoproteins, and to evaluate whether the total ROS yield depends upon growth conditions. We found that two fumarate-reducing flavoenzymes have the capacity to generate substantial H2O2 in E. coli and that their contributions are strongly affected by the medium composition. The autoxidation of one of these enzymes is substantially suppressed through its interaction with the respiratory chain. This observation suggests that other flavin-dependent dehydrogenases are commonly associated with the chain not for the sake of energy conservation but rather to avoid the production of ROS. Further, the prevalence of cytochrome oxidases among apparent anaerobes might reflect its ability to suppress ROS formation by discharging the reducing capacity of anaerobic respiratory chains.
Results
Measurements of intracellular H2O2 formation
Mutants that lack catalases and NADH peroxidase (katG katE ahp) release H2O2 at a steady rate into the growth medium (Seaver and Imlay, 2004). As our standard conditions we selected defined glucose medium that contains all 20 amino acids. In this medium the catalase/peroxidase mutant grows with a doubling time (44 min) that is close to that of its wild-type parent (37 min). Cells were precultured in this aerobic medium for at least five generations, and they were then centrifuged and resuspended to a relatively low cell density (0.020 OD600) in fresh warm medium of the same composition. At intervals aliquots of the culture were centrifuged to remove cells, and the amount of accumulated H2O2 in the medium was determined. H2O2 accumulation typically was linear for the first 25-30 minutes (e.g., Fig. 1); at longer times, the rate of H2O2 accumulation gradually diminished, due to pyruvate that collected in the medium at concentrations of up to 0.3 mM. Pyruvate chemically degrades H2O2 (O’Donnell-Tormey et al., 1987) at a substantial rate (k = 1.4 M−1 s−1 in our growth medium at room temperature).
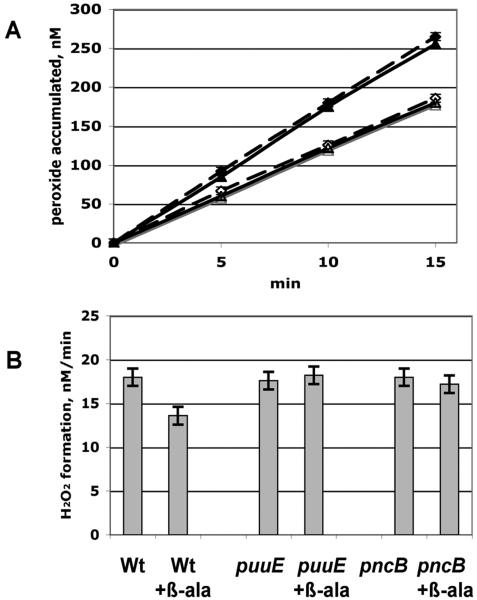
Figure 1.
Suppression of H2O2 formation by β-alanine occurs through catabolism rather than pantothenate synthesis. A. In vivo H2O2 production by the catalase/peroxidase-deficient parent strain (LC106, triangles) and a panC mutant (SSK62, diamonds). Open symbols: 25 mM β-alanine was included in the medium. B. Rates of H2O2 formation by the parent LC106 strain and its puuE (SSK96) and pncB (SSK102) derivatives.
Rates of intracellular H2O2 formation were calculated from the rates of H2O2 accumulation in the medium and the total intracellular volume (Materials and Methods). H2O2 was formed in the catalase/peroxidase mutant at a reproducible rate of 12 μM/s when it was cultured in air-saturated medium. This rate is in excellent agreement with the results of a previous study (Seaver and Imlay, 2001b).
H2O2 production by mutants lacking prominent flavoenzymes
The working hypothesis was that one or more flavoproteins might be responsible for a substantial fraction of the total H2O2 that is generated inside cells. Given the precision with which H2O2 formation can be measured, we anticipated that we could reliably detect a diminution of 15% or more of the H2O2 flux, corresponding to about 2 μM/s H2O2. The number of E. coli pathways that carry at least 2 μM/s flux under our growth conditions is limited (Materials and Methods) and involves only a small group of flavoenzymes. Therefore we created a set of catalase/peroxidase mutants with null mutations in many of these: nuo (encoding the respiratory NADH dehydrogenase I), ndh (respiratory NADH dehydrogenase II), gdhA (glutamate dehydrogenase), gltBDF (glutamate synthase), gor (glutathione reductase), trxB (thioredoxin reductase), glpD (respiratory glycerol-3-phosphate dehydrogenase), lpd (lipoamide dehydrogenase of the pyruvate and 2-oxoglutarate dehydrogenase complexes), sdh (succinate dehydrogenase), dld (respiratory D-lactase dehydrogenase), pyrD (dihydroorotate dehydrogenase), and ldhA (soluble D-lactate dehydrogenase). None of these mutations diminished H2O2 formation (Table 1). In fact, H2O2 production was actually elevated by a few mutations, notably ones that might cause the accumulation of NADH in the cell (ndh, glpD, ldhA). H2O2 production was especially large in cyd cyo and ndh nuo double mutants, which have < 10% of the normal respiratory rate and accumulate several-fold more NADH than a wild-type cell (Woodmansee and Imlay, 2002). We suspect that when NADH levels are anomalously high, a not-yet-identified NADH-reducible enzyme might leak electrons to oxygen. This notion was not pursued further in this work.
Table 1.
Rates of H2O2 formation inside strains lacking prominent flavoproteins
| Mutation | Cytosolic H2O2 formation, μM/s |
|---|---|
| LC106, as a wild type | 12.0 +/− 0.6 |
| gdhA | 11.5 +/−1 |
| gltBDF | 11.6 +/−1 |
| gor | 10.5 +/−1 |
| dld | 11.0 +/−1 |
| lpd | 10.0 +/−1 |
| sdhABCD | 11.8 +/−1 |
| pyrD | 10.9 +/−1 |
| trxB | 18.0 +/−1 |
| glpD | 17.6 +/−1 |
| ldhA | 20.0 +/−1 |
| ndh | 15.3 +/−2 |
| nuo | 12.0 +/−1 |
| ndh nuo | 23.8 +/−1 |
| cyo cyd | 23.5 +/−1 |
Thus the mutant screen did not identify any prominent source of H2O2. As an alternative, less-biased approach, we varied the composition of the growth medium, in hopes that by inducing or repressing various biosynthetic or assimilatory pathways we might detect any contributions to H2O2 formation made by the enzymes that belong to them. We did not see any response to the presence or absence of amino acids, purines, pyrimidines, or to most nitrogen sources (data not shown). Most cofactor biosynthetic pathways are expected to have fluxes substantially less than 1 μM/s and so are unlikely to be sources of substantial H2O2.
However, supplementation of the medium with β-alanine consistently diminished the rate of H2O2 formation by 25-30% (Fig. 1A). This effect required high concentrations of β-alanine (25 mM), and the effect was most pronounced after cells had grown in its presence for up to 25 generations.
β-alanine suppresses H2O2 formation by serving as a source of nicotinamide
E. coli synthesizes β-alanine as a precursor for pantothenic acid; β-alanine has no other known role in metabolism. However, deletions of panC, which encodes the enzyme that incorporates β-alanine into pantothenate, did not alter the rate of H2O2 production, and H2O2 formation by this mutant was still suppressed by β-alanine, indicating that its effects on H2O2 formation had nothing to do with its involvement in this pathway (Fig. 1A). We speculated that β-alanine might be catabolized; if so, it seemed likely to occur through non-specific actions of enzymes devoted to other substrates, since the amount of β-alanine needed for H2O2 suppression was very high. Amino acid transaminases are notably non-specific. The puuE gene product is a transaminase that operates on 4-aminobutyrate, a structural homologue of β-alanine (Kurihara et al., 2005), and so might catalyze the conversion of β-alanine to malonic semialdehyde. Indeed, we found that β-alanine supplements could not diminish H2O2 formation in mutants that lack puuE (Fig. 1B).
The subsequent amination of malonic semialdehyde by free intracellular ammonium would reversibly form the Schiff’s base iminopropionate. Such reactions occur easily in vitro, and it is reasonable to expect them to occur in vivo as well, if NH4+ levels are high. Consistent with this requirement, we noted that B-alanine failed to suppress H2O2 formation in cells that were grown with glutamine, rather than ammonia, as a nitrogen source (data not shown).
Iminopropionate is a homologue of iminosuccinate, the first intermediate in the de novo biosynthesis of nicotinamide (Fig. 2). In this pathway iminosuccinate is condensed with dihydroxyacetone phosphate by quinolinate synthase, encoded by nadA. If this enzyme were to recognize iminopropionate as an alternative substrate, the product would be nicotinic acid, an intermediate in the nicotinamide salvage pathway. The notion seemed plausible, since iminopropionate differs from iminosuccinate only in having an additional carboxylic acid residue, which is uninvolved in the quinolinate synthase chemistry. These considerations prompted us to test whether β-alanine might serve as an alternative source of nicotinamide that would by-pass the initial steps of the conventional pathway.
Figure 2.
Pathways of NAD+ biosynthesis. Top: NadB-dependent formation of NAD+ from aspartate. Bottom: Proposed conversion of β-alanine supplements to NAD+. The requirement for PuuE and PncB was demonstrated; the involvement of NadA is speculative (see text).
Mutants that lack enzymes in the de novo pathway of nicotinamide biosynthesis can grow if nicotinic acid is supplied in the growth medium. β-alanine did not support the growth of nadB mutants when nicotinic acid was withdrawn from liquid cultures; however, when this experiment was repeated on solid medium, suppressor mutants did arise whose growth was dependent on β-alanine (data not shown). This observation, plus the fact that β-alanine exposure must be protracted to influence H2O2 formation, suggested that the ability to assimilate β-alanine into nicotinamide is an inefficient process that might have regulatory barriers. The pncB gene product is essential for the salvaging of nicotinic acid, and we found that β-alanine could not suppress H2O2 formation in a pncB mutant (Fig. 1B). Collectively, these data indicate that β-alanine can be assimilated into nicotinamide through this pathway and that it is through this process that β-alanine supplements suppress H2O2 formation.
Measurements of the activity of NadB, the first enzyme in the standard nicotinamide biosynthetic pathway, showed that β-alanine supplements ultimately suppressed NadB content by > 95%. (Supplements reduced the activity from 0.1 mU/mg in a wild-type strain, and 0.95 U/mg when overproduced from its own promoter on a plasmid, to < 0.01 mU/mg in both cases.)
These results suggested that circumvention of the initial part of the pathway might be the cause of the diminution of H2O2. To test this idea more directly, cultures were grown with nicotinic acid, quinolinic acid, or NAD itself, which also bypass this section of the pathway (Fig. 2). All three supplements suppressed H2O2 formation by 25-30%, matching the effect of β-alanine (Fig. 3A). There was no further suppression of H2O2 formation when β-alanine was added to cultures that already included the other nicotinamide precursors (data not shown).
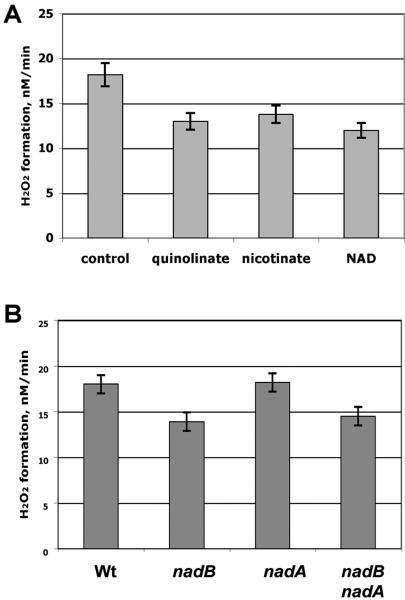
Figure 3.
H2O2 formation is suppressed by nicotinamide precursors and depends on NadB. A. H2O2 formation was measured for the catalase/peroxidase-deficient parent strain LC106 grown aerobically in the presence of 0.6 mM of the indicated supplements. B. H2O2 formation by LC106 (catalase/peroxidase mutant) and its nadB (SSK84), nadA (JI426), and nadB nadA (SSK91) derivatives. All strains were supplemented with 1.6 μM nicotinic acid.
Thus our analysis of the β-alanine phenomenon led us to the recognition that nicotinamide precursors suppress H2O2 formation. The pathway of β-alanine catabolism that is proposed in Fig. 2 was not tested further, as the focus of our investigation turned to the mechanism of H2O2 production.
NadB is responsible for H2O2 formation in vivo
The proposed scheme suggests that β-alanine supplements might by-pass two steps in the standard biosynthetic pathway, those catalyzed by NadB and NadA. To genetically test the roles of these two enzymes in H2O2 formation, it was necessary to find a supplement protocol that would allow growth of nad mutants but which would not suppress expression of NadB and NadA. We found that this goal could be achieved through supplementation with modest (1.6 micromolar) levels of nicotinic acid. This dose did not diminish H2O2 production by wild-type cells.
Under these growth conditions, mutants lacking nadB generated 25% less H2O2 than did their nad+ parent (Fig. 3B). In contrast, the nadA mutant produced usual levels of H2O2. The nadB nadA double mutant released the same diminished H2O2 as the nadB strain. These data specifically implicated NadB in H2O2 production, and it indicated that NadB has this effect whether or not flux through this enzyme ultimately leads to nicotinamide formation.
Fumarate and oxygen compete to oxidize NadB
NadB is a flavoenzyme (Messner and Imlay, 2002). The high reduction potential of its FAD cofactor allows it to desaturate aspartate; the reduced enzyme is then recycled when the electrons are transferred to an oxidant. The reduction potential of NAD is too low for it to serve as this acceptor. Genetic studies showed that nadB is essential for nicotinamide biosynthesis under both aerobic and rigorously anaerobic conditions (Messner and Imlay, 2002), which appeared to rule out molecular oxygen as the physiological oxidant. Indeed, Tedeschi et al. demonstrated that fumarate is a capable acceptor, suggesting that it is likely to be the native substrate (eq. 1) (Tedeschi et al., 1996).
| (1) |
However, the capacity of molecular oxygen to serve as an acceptor in vitro has long been recognized, and in fact the enzyme was originally denoted as aspartate oxidase because of this activity (eq. 2).
| (2) |
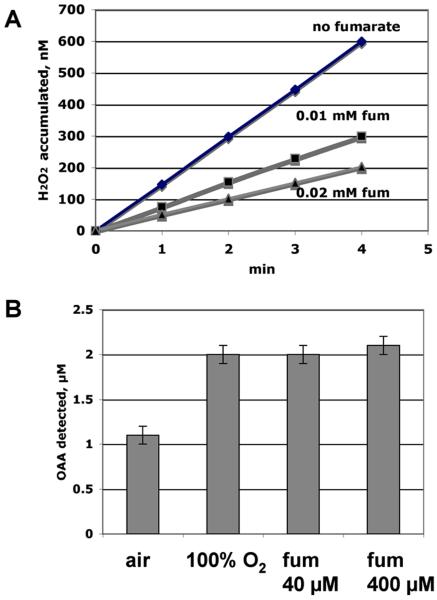
NadB was purified. Fig. 4A represents the ability of the isolated enzyme to oxidize aspartate and utilize oxygen as the electron acceptor, thereby generating H2O2. Fumarate is a more-efficient electron acceptor, allowing the enzyme to turn over twice as fast (Fig 4B). When the enzyme was incubated in air-saturated medium (210 micromolar oxygen), the addition of low levels of fumarate diminished H2O2 production (Fig. 4A). Interestingly, the suppressive effect of fumarate was reversed when pure oxygen was bubbled through the reaction mixture (not shown). Together these data suggest that fumarate is a conventional substrate for the enzyme and is kinetically superior to oxygen. The enzyme must not have a saturable binding site for oxygen, since its turnover number was enhanced by supraphysiological concentrations (Fig. 4B). By contrast, the two cytochrome oxidases of E. coli bind oxygen with high affinity, with dissociation constants in the range of 1 micromolar or less (Rice and Hempfling, 1978; D’mello et al., 1996).
Figure 4.
Molecular oxygen is a poorer electron acceptor for purified NadB than is fumarate. A. Rate of reduction of oxygen to H2O2 by NadB in the absence and presence of fumarate. The dissolved oxygen concentration was 260 μM. B. NadB turnover rate, measured as oxaloacetate (OAA) production, in buffer saturated with air (260 μM oxygen), with 100% oxygen (1.1 mM), or with fumarate in the absence of oxygen.
If NadB contributes substantially to cellular H2O2 formation, then oxygen must outcompete fumarate for the reduced enzyme in vivo. Mass spectrometric analysis indicated that in anaerobic glucose-grown cells the fumarate concentration was 300 (+/− 10) micromolar, a level that would saturate the enzyme in vivo and preclude its oxidation by molecular oxygen. However, when cells were cultured aerobically, intracellular fumarate was much more scarce: levels were detectable but near the quantification limits and did not exceed 10 micromolar. The in vitro data imply that under this condition molecular oxygen is likely to be the predominant electron acceptor from the enzyme.
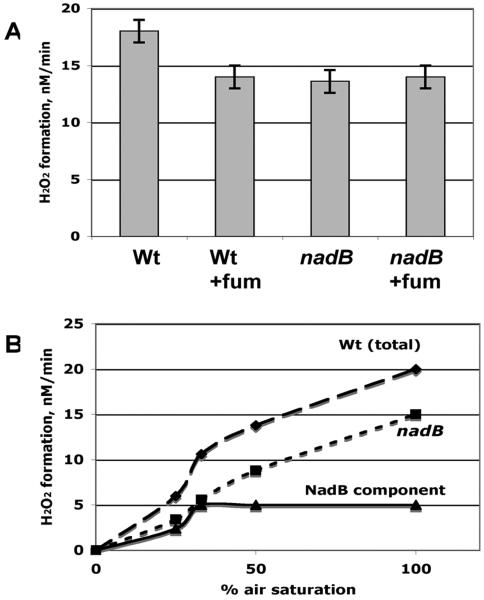
Two experiments were conducted to perturb the relative concentrations of fumarate and oxygen in vivo, so that we could appraise the effects of their competition for NadB. First, supplementation of growth medium with fumarate eliminated the NadB contribution to cellular H2O2 production (Fig 5A). Second, the oxygen level in the gas flow to cultures was varied, for both wild-type and nadB null mutants. Subtraction of the resultant curves revealed that the contribution of NadB to H2O2 formation increased with the oxygen concentration up to approximately 70 micromolar oxygen (30% air saturation) (Fig. 5B). It is not clear whether this latter effect depends upon direct competition for the enzyme, metabolic effects on intracellular fumarate pools, or both. We conclude that when fumarate levels are low, as in aerobic glucose medium, NadB stoichiometrically generates H2O2 as it turns over. In contrast, when intracellular fumarate levels are high—in anaerobic or fumarate-supplemented cells—fumarate, rather than oxygen, is the electron acceptor. The evolution of this unusual arrangement is considered in the Discussion.
Figure 5.
Molecular oxygen and fumarate compete as NadB substrates in vivo. A. H2O2 production was measured for the catalase/peroxidase-deficient parent strain LC106 and its nadB derivative (SSK84) in the presence and absence of 50 mM fumarate supplements. B. Rates of H2O2 production as a function of oxygen concentration for catalase/peroxidase deficient LC106 parent strain (diamonds) and its nadB derivative (SSK84, squares). By subtraction, the NadB-dependent component of LC106 H2O2 formation was derived (triangles).
Fumarate reductase also generates H2O2
Succinate dehydrogenase and fumarate reductase employ a succinate/fumarate oxidoreductase subunit (SdhA, FumA) that is structurally and evolutionarily related to NadB (Mattevi et al., 1999). Our previous in vitro studies showed that succinate/fumarate oxidoreductases are prone to autoxidation if electrons accumulate on their solvent-exposed flavin (Messner and Imlay, 2002). The flavin is the only redox moiety in NadB, and so its oxidation rate is high; in reduced succinate dehydrogenase, however, the electron density is shifted away from the flavins and onto the adjacent iron-sulfur clusters, and as a consequence the autoxidation rate is low and the energy of activation is high. Fumarate reductase behaves more like NadB, because the reduction potentials favor electron density on the flavin rather than the iron-sulfur clusters. In fact, phenotypic studies of strains with limited superoxide dismutase indicated that fumarate reductase may be a major source of superoxide (Imlay, 1995). Because fumarate reductase is an alternative respiratory oxidase that is primarily synthesized under anaerobic conditions, the particular circumstance in which it might contribute to oxidative stress is when anaerobic cultures enter an aerobic environment.
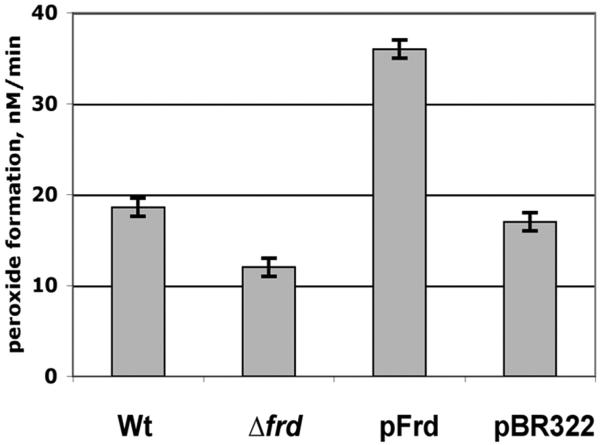
These considerations suggested that fumarate reductase might be an important source of H2O2 when anaerobic cultures are abruptly aerated. Indeed, under these circumstances H2O2 production was diminished in an frd null mutant (Fig. 6). Conversely, H2O2 production was substantially elevated in an frd-overexpressing strain.
Figure 6.
Fumarate reductase generates substantial H2O2 when E. coli is transferred from anaerobic to aerobic medium. Strains were the catalase/peroxidase-deficient parent strain LC106, its Δfrd derivative (LC126), a fumarate-reductase overproducer (LC106 +pFrd), and a vector control strain (LC106).
The respiratory quinone pool pulls electrons away from fumarate reductase and diminishes its formation of H2O2
However, in contrast to NadB, the rate at which fumarate reductase reduced oxygen to H2O2 in aerobic cells was far less than the rate at which it reduced fumarate to succinate in anaerobic cells. In large part, this disparity can be ascribed to its preference for fumarate. However, we wondered whether the association of fumarate reductase with the respiratory chain might also suppress its autoxidation rate. This would occur if reduced fumarate reductase were to transfer electrons back to the quinone pool more rapidly than to molecular oxygen. It has been shown that if frd is manipulated so that it is expressed in aerobic cultures, it can complement the growth defect that an sdh mutant normally manifests on succinate medium (Guest, 1981). We reproduced this result: an sdh mutant was able to grow on aerobic succinate plates if frd was expressed from a plasmid (data not shown). This phenomenon did not occur in mutants that lack either menaquinone (ΔmenA) or ubiquinone (ΔubiCA). This result indicates that upon aeration, succinate can back-reduce fumarate reductase, which in this circumstance acts as a succinate dehydrogenase. Importantly, electrons evidently flow from the reduced fumarate reductase complex to its menaquinone partner, and the reduced menaquinone can transfer electrons to the ubiquinone pool and ultimately to cytochrome oxidase. Therefore, the action of cytochrome oxidase as an electron sink might serve to pull electrons away from fumarate reductase and thereby diminish the rate at which this enzyme generates H2O2 when erstwhile anaerobic cells are exposed to oxygen.
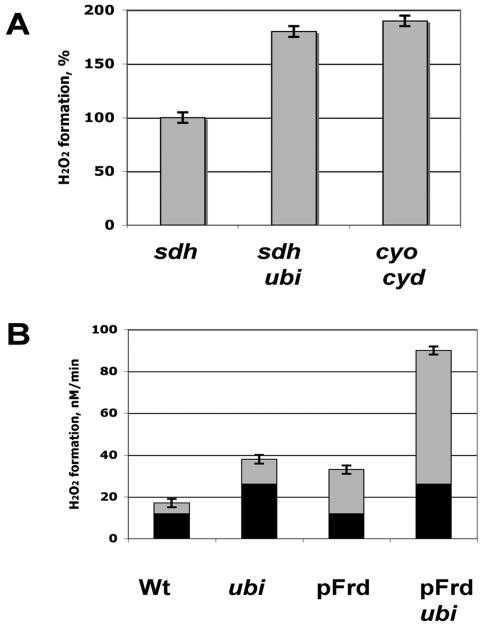
To test this notion in vitro, we prepared inverted membrane vesicles from anaerobically grown strains, including strains that lacked ubiquinone and cytochrome oxidases. When fumarate reductase was directly reduced by succinate, it generated substantial H2O2 (Fig. 7A). Strikingly, membranes that lacked either ubiquinone or cytochrome oxidases produced H2O2 at a much higher rate. We infer that electrons from the reduced enzyme can move to the menaquinone pool and thence through the higher potential ubiquinone to cytochrome oxidase. This process is competitive with direct transfer from fumarate reductase to oxygen.
Figure 7.
The aerobic respiratory system suppresses H2O2 formation by reduced fumarate reductase. A. Inverted membrane vesicles (10 μg/ml) were prepared from anaerobic cells that contain fumarate reductase. The rates of H2O2 formation were determined during back-reduction of fumarate reductase with succinate, using a catalase/peroxidase-deficient sdhABCD strain with an intact aerobic respiratory system (SSK90) and ones that lacked ubiquinone (ubi, SSK124) or cytochrome d and o oxidases (SSK53). B. H2O2 formation by growing cells upon shift from anaerobic to aerobic medium. The full bar indicates the rate of H2O2 formation by strains synthesizing fumarate reductase. The black part of the bar indicates the rate of H2O2 by matched frd-deficient strains; by subtraction, the gray bar indicates the fumarate-reductase-dependent rate of H2O2 formation. The ubi mutation increased frd-dependent H2O2 production three-fold, both with normal and overexpressing strains.
Finally, analogous experiments were conducted in vivo. In mutants that lacked ubiquinone, fumarate reductase generated H2O2 at a three-fold higher rate than it did in strains that contained ubiquinone (Fig. 7B). Thus the autoxidation rate of this enzyme, like that of NadB, depends upon the competition between oxygen and other physiological oxidants—in this case, menaquinone. This observation suggests that the autoxidation of other redox enzymes may be similarly suppressed through their association with the respiratory chain (Discussion).
Discussion
The purpose of this study was to identify predominant sources of hydrogen peroxide inside aerobic E. coli. Previous in vitro studies had showed that a variety of reduced flavoenzymes can adventitiously generate H2O2 in vitro (Massey et al., 1969; Geary and Meister, 1977; Messner and Imlay, 1999; Grinblat et al., 1991; Messner and Imlay, 2002; Kussmaul and Hirst, 2006), and we anticipated that the most abundant of these were likely to be significant contributors to the total H2O2 flux in vivo, too. We were surprised when that turned out not to be the case. Mutant analyses suggested that none of the flavoenzymes involved in central metabolism or in respiration was responsible for > 10% of the overall yield. There is a caveat: in principle, it is possible that deletion of a major autoxidizable enzyme had the effect of shifting metabolic flux to another, equally autoxidizable enzyme, thereby obscuring the role of the first enzyme in H2O2 formation. However, given the disparity in oxidation turnover numbers, this possibility seems small. We conclude that the tendency of certain flavoenzymes to produce H2O2 is suppressed in the living cell by the short residence time of electrons on their flavin subunits. In fact, a previous study showed that NADH dehydrogenase II oxidation, which is rapid in vitro, is substantial in vivo only if electron transfer to the quinone pool is blocked (Seaver and Imlay, 2004). These results underscore the need to test presumptive ROS sources in the context of living cells.
The behavior of NadB is unique
In the end we made progress through the non-hypothesis-driven approach of testing whether H2O2 yield might be perturbed by changes in nutritional supplements. Gratifyingly, this strategy ultimately identified another flavoenzyme, NadB, as a significant H2O2 source. The oxidizability of this enzyme in vitro was already well-known; what was not clear was that it quantitatively uses molecular oxygen, rather than fumarate, as its electron acceptor when E. coli grows in air-saturated habitats. This behavior, to our knowledge, is unprecedented: in anaerobic habitats the enzyme uses a classic organic substrate, while in aerobic environments a tiny inorganic molecule is substituted. This strategy is evidently forced upon the cell when fumarate levels drop as a consequence of aerobic metabolism, which consumes NADH and by mass action reverses the flux through the fumarate-generating branch of the anaerobic TCA cycle. Deprived of its native oxidant, reduced NadB nevertheless manages to turn over by the slightly less efficient transfer of electrons to oxygen.
Several features of this reaction convince us that evolution has not substantially honed the enzyme for its reaction with oxygen. First, even in air-saturated buffers the turnover is limited by the secondary substrate, which is an unusual property for a pathway enzyme. Second, there is no evidence of an oxygen binding site—NadB turnover in vitro does not become optimal unless pure oxygen is bubbled into the reaction, achieving a level of oxygen that exists nowhere in the natural world. Third, the oxidation reaction generates a mixture of superoxide and hydrogen peroxide (Messner and Imlay, 2002), indicating that the oxidative half-reaction is not directed well enough to ensure consistency in the number of electrons that are transferred to the acceptor. And finally, fumarate reductase, which shares with NadB a common structure and catalytic behavior, also reduces oxygen with a similar kinetic efficiency (Messner and Imlay, 2002), even though its physiological function is not helped whatsoever by its doing so. Instead, it appears that evolution designed NadB to function in the anaerobic world, with fumarate as a conventional substrate. When it was introduced into aerobic environments, with the consequent drop in fumarate concentration, the tendency of its flavin towards adventitious oxidation was sufficient to support its continued physiological function.
One might imagine that the H2O2 that this reaction creates might prompt aerobic organisms to develop a less-hazardous method of aspartate oxidation. However, any selective pressure is mitigated by the fact that most cellular H2O2 still comes from other sources, so that modification of the NAD biosynthetic pathway would have only a minor effect on oxidative stress. Moreover, the turnover of NadB itself is tightly controlled, at both transcriptional and activity levels, by the cellular NAD content (Nasu et al., 1982). Thus, although aspartate levels always exceed the dissociation constant of NadB, electron transfer to the enzyme—and thus the amount of H2O2 that it forms—is precisely limited by the cellular demand for more NAD(P). A further control is exerted through product inhibition: iminosuccinate binds tightly to the enzyme active site (Mortarino et al., 1996) and does not allow further turnover until it is consumed by NadA. In fact, because of this last effect, H2O2 formation is not significantly increased in cells that overproduce NadB (data not shown).
Where does the rest of the H2O2 come from?
Under our growth conditions, NadB accounts for about one-quarter of the H2O2 that is generated endogenously in aerobic E. coli. Menaquinone autoxidation accounts for another 5-10% (Korshunov and Imlay, 2006). We do not yet know the source of the remaining two-thirds. Its first-order dependence upon oxygen concentration (Fig. 5B) indicates that the NadB-independent ROS arises from adventitious reactions; it is not due to metabolic reactions that, like NadB, generate H2O2 stoichiometrically. We are working to identify these sources.
This study verified several hypotheses: (1) that flavoenzymes can generate H2O2 in vivo as well as in vitro; (2) that a few enzymes contribute a substantial fraction of the total ROS yield; (3) that the rate of H2O2 yield depends upon growth conditions. The last fact is determined both because the titers of autoxidizable enzymes, such as fumarate reductase and NadB, are regulated in response to growth conditions, and because the levels of competing metabolites influence the rate at which these enzymes transfer electrons to oxygen. A corollary is that two microbes that have different metabolic plans—and that therefore employ distinct types of enzymes—might generate ROS at different rates even if they occupy the same environment. Thus oxygenation might be more stressful to some organisms than to others.
Linkage of redox enzymes to the respiratory chain avoids ROS formation
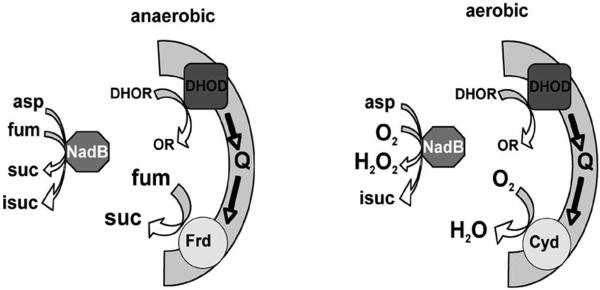
The metabolic pathways that are employed by E. coli evolved in the anaerobic world, and E. coli continues to dwell primarily in the anoxic gastrointestinal tract. Desaturation reactions require a high-potential electron acceptor, and so NadB employs a flavin (E°’ ~ −0.22 V). It follows that the electrons must next be transferred to good oxidants like fumarate (+ 0.033 V) rather than NAD (−0.320 V). Several other pathways in E. coli involve analogous desaturation reactions, including the oxidations of dihydroorotate in the pyrimidine biosynthetic pathway and of proline in its catabolic pathway. In contrast to NadB, however, dihydroorotate dehydrogenase and proline dehydrogenase (PutA) do not directly reduce fumarate; instead, they bind to the cytoplasmic membrane and transfer the electrons to the quinone pool. Under anaerobic conditions these electrons are ultimately delivered to fumarate—thereby accomplishing in several steps the same thing that NadB does all by itself. So why do these other enzymes use the respiratory chain as a bridge to fumarate? A traditional answer might be that under aerobic conditions the electrons will flow to oxygen instead of fumarate, allowing some energy to be conserved when cytochrome o oxidase pumps protons. (Neither of the dehydrogenases themselves translocate protons.) We suggest an alternative idea (Fig. 8). If dihydroorotate dehydrogenase and proline dehydrogenase were soluble enzymes that transferred electrons directly to fumarate in anaerobic habitats—in the fashion of NadB—then upon entry into an aerobic environment, one might by analogy expect them to transfer the electrons to oxygen, generating H2O2. While this outcome might be acceptable for NadB, which has a limited metabolic flux, the rates of pyrimidine synthesis and of proline catabolism can be at least two orders of magnitude higher. Such rates of intracellular H2O2 formation would presumably be intolerable. Instead, the linkage of the contemporary enzymes to the respiratory chain ensures that the electrons flow to oxygen through cytochrome oxidases, without ROS formation.
Figure 8.
Model: Association with the respiratory chain avoids H2O2 production by the desaturating dehydrogenases. A. Under anaerobic conditions, both soluble NadB and membrane-bound dihydroorotate dehydrogenase (DHOD) deliver electrons to fumarate. B. Upon aeration, fumarate levels fall, and NadB transfers electrons directly to oxygen, generating H2O2. Electrons from dihydroorotate dehydrogenase are delivered to oxygen through cytochrome oxidase, without H2O2 formation. (Abbreviations: DHO, dihydroorotate; OR, orotate; Q, the quinone pool; Frd, fumarate reductase; fum, fumarate; suc, succinate; asp, asparate; isuc, iminosuccinate.)
This logic implies that stoichiometric H2O2 production will be tolerated only from low-flux pathways. In E. coli a few metabolic pathways aside from nicotinamide biosynthesis are believed to include enzymes that form H2O2 quantitatively. Each of these handle a very low flux (e.g., pyridoxine-5-phosphate oxidase, putrescinglutamyl oxidase, and protoporphyrinogen oxidase, in pyridoxine salvage, putrescine degradation, and heme biosynthesis, respectively) or act outside the cytoplasm (monoaminoxidase, localized in the periplasm). Thus none has much impact upon cytoplasmic ROS levels.
Why do anaerobes contain cytochrome oxidase?
The aeration of the Earth created a need for succinate:quinone oxidoreductase activity, and this is a role that fumarate reductase can fulfill (Guest, 1981). However, fumarate reductase generates ROS in vitro as rapidly as does NadB, so its deployment in aerobic habitats would have been hazardous for cells. Evolution circumvented this threat by modifying the enzyme to the form that we now know as succinate dehydrogenase: the elevation of its iron-sulfur cluster potentials and the addition of an accessory heme pull electron density away from the flavin and thereby strongly suppresses its oxidation rate (Messner and Imlay, 2002; Yandovskaya et al., 2003).
The autoxidizability of fumarate reductase is, of course, not a problem in anaerobic environments, and in many anaerobes it is an abundant, key enzyme. Nevertheless, anaerobes that rely upon it potentially face a problem when they are exposed to aerobic fluids. However, this study found that ROS formation by fumarate reductase is suppressed by its back-oxidation through the quinone pool, with cytochrome oxidase acting as an ultimate electron sink.
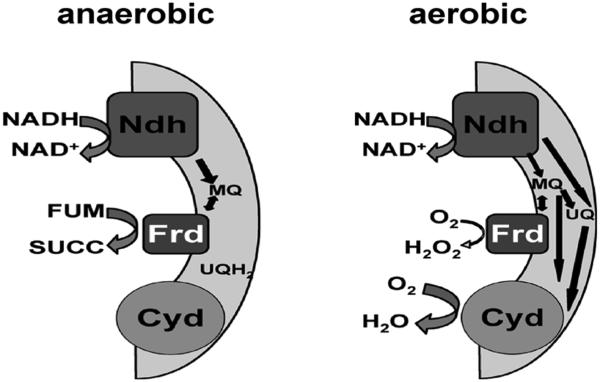
In E. coli this effect might be regarded as a fortuitous consequence of its facultative lifestyle, which links both aerobic and anaerobic respiratory complexes to a common quinone pool. However, we surveyed the sequenced members of the 33 bacterial genera annotated as non-facultative anaerobes by the Integrated Microbial Genomics web site (http://img.jgi.doe.gov/cgi-bin/pub/main.cgi), and we found that 60% encode clear cytochrome d oxidase homologues. This widespread phenomenon includes members of five phyla (Actinobacteria, Bacteroidetes, Chlorobi, Firmicutes and Proteobacteria) and all the anaerobes among the four branches of Proteobacteria. Why do apparent anaerobes synthesize a cytochrome oxidase? One explanation is that when oxygenated fluids invade self-contained microhabitats such as biofilms, cytochrome d oxidase activity may help to reestablish local anaerobiosis and thereby allow oxygen-sensitive metabolisms to resume. However, we wonder whether an alternative (or additional) role of this non-translocating oxidase is to quickly drain electrons from their anaerobic respiratory chains (Fig. 9). This arrangement would minimize the autoxidation of classic anaerobic terminal oxidases such as fumarate reductase, as it did in E. coli. By doing so, the oxidase may suppress the formation of intracellular H2O2 and enable the organism to survive transient oxygen exposure. Efforts to test this notion are underway.
Figure 9.
Model: Upon aeration, the aerobic respiratory chain pulls electrons away from fumarate reductase and thereby suppresses H2O2 formation. (Abbreviations: Cyd, cytochrome d oxidase; Frd, fumarate reductase; Ndh, NADH dehydrogenase; MQ, menaquinone; UQ and UQH2, oxidized and reduced ubiquinone; fum, fumarate; succ, succinate.)
Methods
Growth conditions
For most experiments cells were grown in minimal A glucose/amino acids medium (Miller, 1972) containing 140 mM KPi (pH 7.2), 7 mM ammonium sulfate, 2 mM sodium citrate, 0.5 mM magnesium sulfate, 0.2% glucose, 0.2% casamino acids, and 0.1 mM tryptophan. Where indicated, the cultures were supplemented with 1.6 μM or 0.6 mM nicotinic acid, 25 mM β-alanine, 2 mM uracil, 2 mM guanine, 0.1 mM pantothenate, 0.6 mM quinolinic acid, 0.6 mM NAD+, 50 mM fumarate, or 25 mM succinate. Ampicillin (0.1 mg/ml) was included in cultures of plasmid-bearing strains. Ampicillin, aspartic acid, β-alanine, casamino acids (an acid hydrolysate of casein), FAD, fumaric acid, H2O2, quinolinic acid, sodium sulfate, succinic acid, and tryptophan were from Sigma. Guanine, nicotinic acid, pantothenic acid and uracil were from Aldrich.
The ability of fumarate reductase to complement sdh mutations was demonstrated by the growth of frd-plasmid-containing strains on aerobic minimal salts plates that contained succinate as the sole carbon source. The ability of β-alanine to serve as a source of nicotinamide was tested by growing nadB null mutants on nicotinate-free glucose/amino acids plates (above). Outgrowing colonies were subsequently able to grow in liquid medium only when it was supplemented with either nicotinic acid or β-alanine.
Aerobic liquid cultures were grown in well-shaken flasks at cell densities that did not exceed OD600 = 0.25. Anaerobic growth occurred in an anaerobic chamber (Coy Laboratories) under an atmosphere of 90% nitrogen, 5% hydrogen and 5% CO2. All anaerobic media and solutions were degassed for at least 20 hours before use.
Bacterial strains
Strains and plasmids used in this study are listed in the supplementary table S1. Bacterial strains were derived from the MG1655 K-12 strain. Gene deletions were generated by Red/Gam recombination (Datsenko and Wanner, 2000) and transduced into strains of interest (Miller, 1972). Primers for deletions were from Fisher. Null mutations in gor, trxB, gdhA, ldhA and nadB nadA knock-outs were verified by enzymatic assays, while nadB, nadA, pncB, pyrD, gltBDF, lpd, dld, glpD, sdh, frd, ubi and men mutations were confirmed by phenotype. The puuE deletion was verified by PCR.
The nadB structural gene was cloned using following primers: GAACACACGAAGCTTAGCTGCAATTTGAGCAAGCAAAGGGTTTAATAACATGCATGCGGGCCAGACCAGAACTATTCCGAAGCG. The cloned region included non-coding 352 bp upstream of the gene. The linear PCR product was digested with HindIII and SphI restriction sites and inserted into the matching sites of the pBR322 vector. Overexpression of the enzyme was verified by complementing of nadB growth phenotype and by assaying aspartate-dependent H2O2 production in cell crude extracts.
Measurements of H2O2 formation by growing cells
Cells were grown to 0.2 OD in glucose/amino acids medium, and 2 ml of cell culture were then centrifuged at room temperature for 1 min (10000 rpm). The pellet was resuspended in freshly made room-temperature medium, centrifuged again, and finally resuspended in 1 ml of fresh medium. The cell suspension was diluted to OD 0.02, and 10 ml of this suspension were placed in a 50 ml flask and incubated with shaking at 37 °C for 5 min. Subsequently, 1.2-ml aliquots were removed every 5 minutes for analysis. Cells were briefly spun down in table centrifuge, and 1 ml of supernatant was mixed with 0.5 ml of Amplex UltraRed fluorescent dye (Invitrogen, 50 μg/ml) and 0.5 ml of horseradish peroxidase (Sigma, 10 U/ml). Peroxidase and Amplex UltraRed were dissolved in 50 mM K2HPO4, pH 7.8. Fluorescence was measured using 520/620 nm wavelength pair. Control measurements were conducted in the absence of cells. H2O2 levels were determined using a calibration curve derived from authentic H2O2. For experiments that tested the effect of nitrogen sources on H2O2 formation, measurements were performed in amino-acid free medium that contained sodium sulfate in place of ammonium sulfate.
The concentration of dissolved oxygen was varied using a V-shaped tubing system attached to air- and nitrogen-containing cylinders. Air was supplied at a constant rate, while nitrogen flow varied from 0 to 300%, as determined by flow meters. The gas mixture was humidified by passage through two water-filled containers at 37°C. Growing cell suspensions (10 ml) were placed in a tall 50-ml flask at 37 °C and bubbled vigorously with gas mix for 10 s. Thereafter a constant moderate flow of gas mix into the flask was maintained for 5 min and during all subsequent measurements.
Detection of excreted pyruvate
Pyruvate was detected using the NADH-lactic dehydrogenase system. Two ml of cell suspension were filtered through Millex-GP syringe filters (0.22 μm, Millipore Express) every 5 min and stored on dry ice. Filtrate (0.25 ml) was then mixed with 0.75 mL of 150 μM of NADH in 100 mM KPi buffer (pH 7.2), 20 U of lactic dehydrogenase (Sigma) were added, and NADH consumption was monitored by decrease in absorbance at 340 nm.
Studies of purified NadB
NadB was purified in our laboratory previously (Messner and Imlay, 2002). Enzyme turnover was measured by the accumulation of oxaloacetate, a hydrolysis product of iminosuccinate. NadB reactions were performed at 37 °C in 100 mM KPi buffer (pH 7.6) containing 20 μM FAD and 5 mM aspartate. Succinate (2 mM) was included in the system to help displace iminosuccinate from the NadB active site (Tedeschi et al., 1996). The reaction mixtures (10 ml) were placed inside 50-ml tubes. The mixtures were bubbled with air or oxygen for 10 s, and then a constant flow of humidified, pre-warmed gas was maintained approximately 2 cm above the surface. At the reaction end point, 100 μM NADH was added, and the absorbance at 340 nm was determined before and after the addition of malic dehydrogenase (Sigma, 10 U/ml). The amount of NADH that was consumed was stoichiometric with the amount of oxaloacetate that was derived from iminosuccinate hydrolysis.
H2O2 formation by NadB was measured by removing aliquots from the enzyme reaction and combining them with 0.5 ml of Amplex UltraRed and 0.5 ml of horseradish peroxidase, as described above.
H2O2 formation by inverted membrane vesicles
Inverted vesicles were prepared from 200 ml of anaerobic cell culture (0.2 OD). Cells were chilled anaerobically on ice for 5 min, centrifuged aerobically for 5 min at 10000 x g, then washed once by ice-cold 100 mM KPi washing buffer (pH 7.2), and resuspended in 3 ml of the same buffer. The resulting suspension was lysed by French press, and crude debris was pelleted by centrifuging for 12 min at 10000 x g. The supernatant was centrifuged in an ultracentrifuge at 100,000 x g for 90 min at 4 °C. The resulting pellet was resuspended in 1 ml of washing buffer.
Intracellular fumarate measurements
Cells were grown either aerobically in flasks or anaerobically in the chamber to OD 0.2. To 500 ml of cell culture was mixed 500 ml of 60% methanol (−50 °C). The mixture was centrifuged for 5 min (7000 rpm), washed once with ice-cold 30% methanol, and resuspended in 8 ml of 60% methanol. Eight ml of chloroform were added, the suspension was vigorously shaken for 10 minutes and centrifuged, and the upper layer was collected for analysis. Mass spectrometry was conducted at the Metabolomics Center of Roy J. Carver Biotechnology Center at UIUC (MSD 5975C, GC7890A, autosampler 7683B, Agilent Technologies, USA).
Intracellular fluxes
To calculate the rate of intracellular H2O2 production, the overall rate of H2O2 formation in a cell culture was normalized to the cumulative cytoplasmic volume of the cells, using a standard ratio of 0.47 μL of internal volume per 1 ml of 1.0 OD of E. coli, as described previously (Seaver and Imlay, 2001b).
To consider whether a biosynthetic pathway is capable of generating 2 μM/s H2O2, which approached our detection limit, we considered the growth rate of cells (k = 2.64 × 10−4 s−1 for a doubling time of 44 min). At this growth rate, a 2 μM/s pathway flux would generate enough product to maintain a steady-state concentration of 7 mM, if product degradation were not considered. In growing E. coli the steady-state concentration of the average amino acid is 75 mM (mostly as protein), while purines and pyrimidines are each about 100 mM (mostly as nucleic acids). Thus their biosynthetic pathways operate at a sufficient flux that even partial electron leakage to oxygen might generate detectable yields of H2O2. In contrast, most protein-bound cofactors such as heme and pyridoxamine are micromolar in concentration, and even stoichiometric H2O2 formation by their biosynthetic pathways would contribute << 5% of the H2O2 yield that was detected. NAD(P)(H) is exceptional, with a steady-state intracellular concentration in glucose medium of approximately 6 mM. The NadB product, iminosuccinate, is unusually unstable (Nasu et al., 1982), and if a substantial fraction is hydrolyzed in vivo, then the flux through NadB must be higher.
The distribution of protein orthologs
A preliminary analysis was performed using the EcoCyc web site (http://ecocyc.org/), with further inspection via the BLAST protein database (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Bacteria were checked for presence of linked cydA and cydB orthologs in the case of cytochrome d oxidase and for both frdA and frdB orthologs in case of fumarate reductase. Organisms were grouped as aerobes, facultative aerobes, or anaerobes according to the identifiers at the Integrated Microbial Genomics web site (http://img.jgi.doe.gov/cgi-bin/pub/main.cgi). These labels should be regarded as tentative, since it remains possible that untested conditions might permit the growth of some putative anaerobes.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant GM49640.
We are also very thankful to Zhong Li and Alexander Ulanov from Metabolomics Center of Roy J. Carver Biotechnology Center at UIUC for the help in experiments involving mass-spectrometry and to Kevin Messner for isolation and purification of NadB.
References
- Blaut M, Whittaker K, Valdovinos A, Ackrell BAC, Gunsalus RP, Cecchini G. Fumarate reductase mutants of Escherichia coli that lack covalently bound flavin. J Biol Chem. 1989;264:13599–13604. [PubMed] [Google Scholar]
- Bolivar F, Rodriguez RL, Green PJ, Betlach MC, Heyneker HL, Boyer HW, Crosa JH, Falkow S. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of plasmid pMB9. Gene. 1977;2:95–113. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Bunch PK, Mat-Jan F, Lee N, Clark DP. The ldhA gene encoding fermentative lactate dehydrogenase of Escherichia coli. Microbiology. 1997;143:187–195. doi: 10.1099/00221287-143-1-187. [DOI] [PubMed] [Google Scholar]
- D’mello R, Hill S, Poole RK. The cytochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two oxygen-binding haems: implications for regulation of activity in vivo by oxygen inhibition. Microbiology. 1996;142:755–763. doi: 10.1099/00221287-142-4-755. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary LE, Meister A. On the mechanism of glutamine-dependent reductive amination of α-ketoglutarate catalyzed by glutamate synthase. J Biol Chem. 1977;252:3501–3508. [PubMed] [Google Scholar]
- Grinblat L, Sreider CM, Stoppani AO. Superoxide anion production by lipoamide dehydrogenase redox-cycling: effect of enzyme modifiers. Biochem Int. 1991;23:83–92. [PubMed] [Google Scholar]
- Guest JR. Partial replacement of succinate dehydrogenase function by phage- and plasmid-specified fumarate reductase in Escherichia coli. Microbiology. 1981;122:171–179. doi: 10.1099/00221287-122-2-171. [DOI] [PubMed] [Google Scholar]
- Imlay JA. A metabolic enzyme that rapidly produces superoxide, fumarate reductase of Escherichia coli. J Biol Chem. 1995;270:19767–19777. [PubMed] [Google Scholar]
- Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Ann Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunov S, Imlay JA. Detection and quantification of superoxide formed within the periplasm of Escherichia coli. J Bacteriol. 2006;188:6326–6334. doi: 10.1128/JB.00554-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara S, Oda S, Kato K, Kim HG, Koyanagi T, Kumagai H, Suzuki H. A novel putrescine utilization pathway involves gamma-glutamylated intermediates of Escherichia coli K-12. J Biol Chem. 2005;280:4602–4608. doi: 10.1074/jbc.M411114200. [DOI] [PubMed] [Google Scholar]
- Kussmaul L, Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci USA. 2006;103:7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins BM, Dobbek H, Cinkaya I, Buckel W, Messerschmidt A. Crystal structure of 4-hydroxybutyryl-CoA dehydratase: radical catalysis involving a [4Fe-4S] cluster and flavin. Proc Natl Acad Sci USA. 2004;101:15645–15649. doi: 10.1073/pnas.0403952101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V, Strickland S, Mayhew SG, Howell LG, Engel PC, Matthews RG, Schuman M, Sullivan PA. The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem Biophys Res Commun. 1969;36:891–897. doi: 10.1016/0006-291x(69)90287-3. [DOI] [PubMed] [Google Scholar]
- Mattevi A, Tedeschi G, Bacchella L, Coda A, Negri A, Ronchi S. Structure of L-aspartate oxidase: implications for the succinate dehydrogenase/fumarate reductase oxidoreductase family. Structure. 1999;7:745–756. doi: 10.1016/s0969-2126(99)80099-9. [DOI] [PubMed] [Google Scholar]
- Messner KR, Imlay JA. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J Biol Chem. 1999;274:10119–10128. doi: 10.1074/jbc.274.15.10119. [DOI] [PubMed] [Google Scholar]
- Messner KR, Imlay JA. Mechanism of superoxide and hydrogen peroxide formation by fumarate reductase, succinate dehydrogenase, and aspartate oxidase. J Biol Chem. 2002;277:42563–42571. doi: 10.1074/jbc.M204958200. [DOI] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y.: 1972. [Google Scholar]
- Mortarino M, Negri A, Tedeschi G, Simonic T, Duga S, Gassen HG, Ronchi S. L-Aspartate oxidase from Escherichia coli. I. Characterization of coenzyme binding and product inhibition. Eur J Biochem. 1996;239:418–426. doi: 10.1111/j.1432-1033.1996.0418u.x. [DOI] [PubMed] [Google Scholar]
- Nasu S, Wicks FD, Gholson RK. L-aspartate oxidase, a newly discovered enzyme of Eshcerichia coli, is the B protein of quinolinate synthetase. J Biol Chem. 1982;257:626–632. [PubMed] [Google Scholar]
- O’Donnell-Tormey J, Nathan CF, Lanks K, DeBoer CJ, de la Harpe J. Secretion of pyruvate. An antioxidant defense of mammalian cells. J Exp Med. 1987;165:500–514. doi: 10.1084/jem.165.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Imlay JA. How does oxygen inhibit central metabolism in the obligate anaerobe Bacteroides thetaiotaomicron? Mol Microbiol. 2001;39:1562–1571. doi: 10.1046/j.1365-2958.2001.02343.x. [DOI] [PubMed] [Google Scholar]
- Prinz WA, Aslund F, Holmgren A, Beckwith J. The role of the thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds in the Escherichia coli cytoplasm. J Biol Chem. 1997;272:15661–15667. doi: 10.1074/jbc.272.25.15661. [DOI] [PubMed] [Google Scholar]
- Rice CW, Hempfling WP. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol. 1978;134:115–124. doi: 10.1128/jb.134.1.115-124.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaver LC, Imlay JA. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol. 2001a;183:7173–7181. doi: 10.1128/JB.183.24.7173-7181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaver LC, Imlay JA. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J Bacteriol. 2001b;183:7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaver LC, Imlay JA. Are respiratory enzymes the primary sources of intracellular hydrogen peroxide? J Biol Chem. 2004;279:48742–48750. doi: 10.1074/jbc.M408754200. [DOI] [PubMed] [Google Scholar]
- Tedeschi G, Negri A, Mortarino M, Ceciliani F, Simonic T, Faotto L, Ronchi S. L-Aspartate oxidase from Escherichia coli. II. Interaction with C4 dicarboxylic acids and identification of a novel L-aspartate:fumarate oxidoreductase activity. Eur J Biochem. 1996;239:427–433. doi: 10.1111/j.1432-1033.1996.0427u.x. [DOI] [PubMed] [Google Scholar]
- Wagner AF, Frey M, Neugebauer FA, Schafer W, Knappe J. The free radical in pyruvate formate-lyase is located on glycine-734. Proc Natl Acad Sci USA. 1992;89:996–1000. doi: 10.1073/pnas.89.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodmansee AN, Imlay JA. Reduced flavins promote oxidative DNA damage in non-respiring Escherichia coli by delivering electrons to intracellular free iron. J Biol Chem. 2002;277:34055–34066. doi: 10.1074/jbc.M203977200. [DOI] [PubMed] [Google Scholar]
- Yandovskaya V, Horsefield R, Tomroth S, Luna-Chavez C, Miyoshi H, Leger C, Byrne B, Cecchini G, Iwata S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 2003;299:700–704. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.