Abstract
Hypoglycemia is a common complication for insulin treated people with diabetes. Severe hypoglycemia, which occurs in the setting of excess or ill-timed insulin administration, has been shown to cause brain damage. Previous pre-clinical studies have shown that memantine (an N-methyl-D-aspartate receptor antagonist) and erythropoietin can be neuroprotective in other models of brain injury. We hypothesized that these agents might also be neuroprotective in reponse to severe hypoglycemia-induced brain damage. To test this hypothesis, 9-week old, awake, male Sprague-Dawley rats underwent hyperinsulinemic (0.2 U.kg−1.min−1) hypoglycemic clamps to induce severe hypoglycemia (blood glucose 10–15 mg/dl for 90 minutes). Animals were randomized into control (vehicle) or pharmacological treatments (memantine or erythropoietin). One week after severe hypoglycemia, neuronal damage was assessed by Fluoro-Jade B and hematoxylin and eosin staining of brain sections. Treatment with both memantine and erythropoietin significantly decreased severe hypoglycemia-induced neuronal damage in the cortex by 35% and 39%, respectively (both p<0.05 vs controls). These findings demonstrate that memantine and erythropoietin provide a protective effect against severe hypoglycemia-induced neuronal damage.
Keywords: hippocampus, cortex, insulin, memantine, erythropoietin
Introduction
Hypoglycemia is the major obstacle in achieving tight glycemic control in people with diabetes [1]. Intensive insulin therapy increases the risk of severe hypoglycemia [2]. By depriving the brain of glucose, severe hypoglycemia can cause brain damage leading to long-term impairments in learning and memory [3;4]. Episodes of severe hypoglycemia have been shown to cause significant cognitive damage in many [5–12] but not all [13–18] studies. Unlike retrospective clinical studies, carefully controlled prospective pre-clinical studies have unambiguously demonstrated that acute severe hypoglycemia induces neuronal damage, especially to the vulnerable neurons in the cortex and hippocampus [3;19–25]. Deficits in learning and memory have been shown to be a direct consequence of this severe hypoglycemia-induced hippocampal neuronal damage [3;21;22;25].
Mechanistically, similarities and differences have been noted between neuronal injury induced by hypoglycemia and neuronal damage caused by other forms of acute neurologic insult [26]. Both ischemic and hypoglycemic neuronal damage are caused by an excitotoxic amino acid mediated increase in intracellular calcium, production of reactive oxygen species and apoptosis [27;28]. Agents that have been shown to be neuroprotective in animal models of brain ischemia warrant investigation to determine if they would be neuroprotective in severe hypoglycemia-induced brain injury. There are many optimistic reasons to think that neuroprotective agents would be particularly efficacious to prevent severe hypoglycemia-induced brain damage. Since cerebral blood flow is not impeded during hypoglycemia (as it is with a stroke), administered neuroprotective agents should have unrestricted ability to reach vulnerable brain areas. Neuroprotective agents used in pre-clinical hypoglycemia models may translate well into clinical trials given the unique nature of insulin-induced hypoglycemic brain damage which is unlike the heterogeneous types of stroke damage (i.e., lacunar, hemorrhagic, or embolic with or without reperfusion). Finally, the inherent delay in administering neuroprotective agents in the clinical setting of a stroke (due to transportation to a hospital or waiting for a brain imaging) could be minimized in a patient recovering from severe hypoglycemia if a neuroprotective agent could be administered on-site, simultaneously with a rescue intravenous bolus of glucose.
In this study, we tested memantine (an N-methyl-D-aspartate receptor antagonist) and erythropoietin as potential neuroprotective agents in a model of severe hypoglycemia. Previously, an older N-methyl-D-aspartate (NMDA) receptor antagonist MK-801 (dizocilpine), has been shown to be neuroprotective in response to ischemia [29] and severe hypoglycemia [30] but the neurobehavioral changes induced by this older NMDA agonist (ie, induction of cataplexy) limit its potential clinical utility. Memantine, a lower affinity NMDA receptor antagonist already in clinical use with limited side effects [31] has been shown to be neuroprotective in models of focal cerebral ischemia [29] and traumatic brain injury [32].
Erythropoietin, in addition to its role in the regulation of erythropoiesis, has neuroprotective, anti-inflammatory and neurotrophic properties and has been found to play a role in neurogenesis [33]. When recombinant erythropoietin was given intraperitoneally 24 hours prior to, and up to six hours after focal brain ischemia in rats, infarct volume was decreased by 50–75% [34]. Similarly, infusion of erythropoietin into the lateral ventricle of gerbils prevented ischemia-induced cell death in the hippocampus [35].
The goals of these studies are to determine whether these pharmacological agents, previously shown to be neuroprotective in pre-clinical models of ischemia, would be neuroprotective in response to brain damage induced by severe hypoglycemia.
Methods
Animals
Nine week old male Sprague-Dawley rats (Charles River Laboratories) were individually housed in a temperature and light controlled environment maintaining the animal’s diurnal cycle (12hrs light, 12hrs dark), and with an ad lib diet. All studies were done in accordance with the Animal Studies Committee at the Washington University School of Medicine and the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Implantation of arterial and venous catheters
Anesthesia was induced with an intraperitoneal injection of ketamine 87 mg/kg and xylazine 2.6 mg/kg. A Micro-Renathane brand (Braintree Scientific, Boston, MA) catheter was inserted into the left common carotid artery and two catheters were implanted into the right jugular vein. To prevent clotting, catheters were filled with a 40% polyvinylpyrrolidone (Sigma, St. Louis, MO) heparin (1000 USP U/ml) solution (Baxter Healthcare Corporation, Deerfield, IL).
Severe Hypoglycemia Clamp
Clamp experiments were performed after the rats were allowed to recover for one week following implantation of arterial and venous catheters. After an overnight fast, vascular catheters were externalized and attached to infusion pumps. After a basal period to allow the rats to acclimate, insulin (Humulin R, Eli Lilly and Co., Indianapolis, IN) was infused intravenously (0.2 U.kg−1.min−1) into awake, unrestrained rats. Throughout the clamp, blood glucose was measured from the arterial cannula every fifteen minutes using Ascensia Contour blood glucose monitors (Bayer Healthcare, LLC, Mischawake, IN). Previous studies determined the nadir hypoglycemia necessary to reproducibly cause neuronal damage was a glucose level <15 mg/dl [24;25]. Once the blood glucose dropped to below 15 mg/dl, hypoglycemia was maintained at 10–15 mg/dl for ninety minutes by adjusting the rate of continuous intravenous glucose infusion (50% dextrose). After the ninety minutes of severe hypoglycemia, hypoglycemia was terminated with infusions of dextrose to restore euglycemia. Throughout this recovery period blood glucose readings were made every 15 minutes and the rates of glucose infusion were slowly decreased as the glucose-lowering effects of insulin waned.
Experimental groups
Memantine group
Based on previous neuroprotective studies [32;36;37], memantine hydrochloride (Sigma, St. Louis, MO) was dissolved in saline. Memantine (20mg/kg) (n=8) or saline vehicle (n=7) was injected intraperitoneally immediately following 90 minutes of severe hypoglycemia.
Erythropoietin group
Vehicle solution was made with 5.8mg of sodium citrate (Sigma, St. Louis, MO), 5.8mg of sodium chloride (Sigma, St. Louis, MO), 0.06mg of citric acid (Sigma, St. Louis, MO), and 2.5mg of albumin from human serum (Sigma, St. Louis, MO) dissolved in 1ml of water. Based on previous neuroprotective studies [38–43] animals were given 5000IU/kg (n=11) of Epoetin alfa (Amgen, Thousand Oaks, CA) or vehicle solution (n=10) on three occasions; intraperitoneally 24 hours prior to the hypoglycemic clamp, intravenously immediately following 90 minutes of severe hypoglycemia, and intraperitoneally 24 hours after the hypoglycemic clamp.
Histology
One week following the episode of severe hypoglycemia, rats were anesthetized with isoflurane (Butler Animal Health Supply, Dublin, OH) and then transcardially perfused with 100 ml of 0.01 M phosphate buffered saline (Sigma) and 100 ml of 4% paraformaldehyde (PFA) (Electron Microscopy Scientific). Perfused brains were left in 4% PFA overnight in 4 °C and then cryoprotected in 30% sucrose (Sigma, St. Louis, MO). Brains were then set in Tissue-Tek OCT Compound (Sakura Finetek, Torrance, CA) and stored at −20 °C. Using a Leica CM1850 cryostat (Leica Microsystems Inc., Bannockburn, IL), 6–10 coronal sections (10 and 20μm) taken 120 μm apart were collected starting 2.8m posterior to the bregma. Sections were stained with either Fluoro-Jade B (Chemicon International, Inc., CA) and Dapi (Sigma, St. Louis, MO) or hematoxylin and eosin (H&E, Sigma, St. Louis, MO) and examined with an epifluorescent microscope (Zeiss) using fluorescein isothiocynate (FITC) (for Fluoro-Jade B).
Microscopy and Cell Counting
H&E staining was used to identify the different brain regions and identify neuronal cell damage as noted by pyknotic cells. For quantification purposes, the number of Fluoro-Jade B positive cells per high power field was assessed by an observer blinded to the treatment conditions. Using two sections per animal, the data is expressed as the average number of Fluoro-Jade B positive cells per brain region of interest.
Statistical Analysis
All data is expressed as mean ± SEM. Statistical significance was determined by a two-tailed student t-test, p<0.05.
Results
Memantine
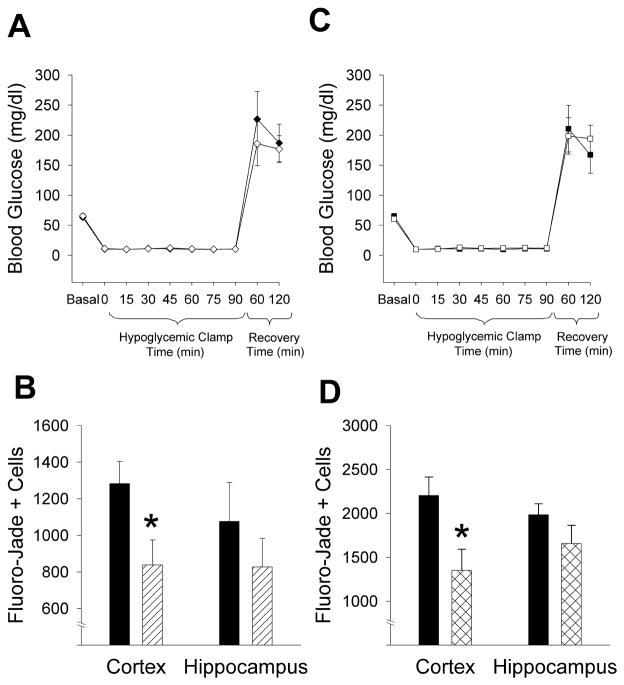
In the memantine and control groups, there was no difference in blood glucose values at baseline (MEM 66 ± 4 mg/dl vs. CONT 63 ± 3 mg/dl, p=NS), during ninety minutes of severe hypoglycemia (MEM 11 ± 0.5 mg/dl vs. CONT 11 ± 0.2 mg/dl, p=NS), or during recovery (MEM 177 ± 22 mg/dl vs. CONT 187 ± 31 mg/dl, p=NS) (Fig 1A). Treatment with memantine decreased hypoglycemia-induced neuronal damage in the cortex by 35% (MEM 838 ± 137 vs. CONT 1282 ± 121 cells, p=0.02)(Fig 1B, 2). Although memantine treatment also decreased the amount of neuronal damage in the hippocampus (MEM 827 ± 155 vs. CONT 1075 ± 213 cells, p=NS), these results were not statistically significant (Fig 1B).
Figure 1. Memantine and Erythropoietin attenuate cortical brain damage after 90 minutes of severe hypoglycemia.
(A) During a hyperinsulinemic severe hypoglycemic (10–15 mg/dl) clamp, blood glucose was not significantly different between vehicle-treated (n=7; ◆) and memantine-treated (n=8; ◇) rats before, during or after 90 minutes of severe hypoglycemia. (B) Neuronal damage resulting from severe hypoglycemia was quantified using Fluoro-Jade B staining in vehicle treated (black bar) and memantine-treated (lined bar) rats. Treatment with memantine significantly reduced the number of degenerating neurons compared to vehicle-treated animals in the cortex (*p=0.02) but not in the hippocampus (p=NS). (C) During a hyperinsulinemic severe hypoglycemic (10–15 mg/dl) clamp, blood glucose was not significantly different between vehicle-treated (n=10; ■) and erythropoietin-treated (n=11; □) rats before, during or after 90 minutes of severe hypoglycemia. (D) Neuronal damage resulting from severe hypoglycemia was quantified using Fluoro-Jade B staining in vehicle-treated (black bar) and erythropoietin-treated (cross-hatched bar) rats. Treatment with erythropoietin significantly reduced the number of degenerating neurons compared to vehicle-treated animals in the cortex (*p=0.01) but not in the hippocampus (p=NS).
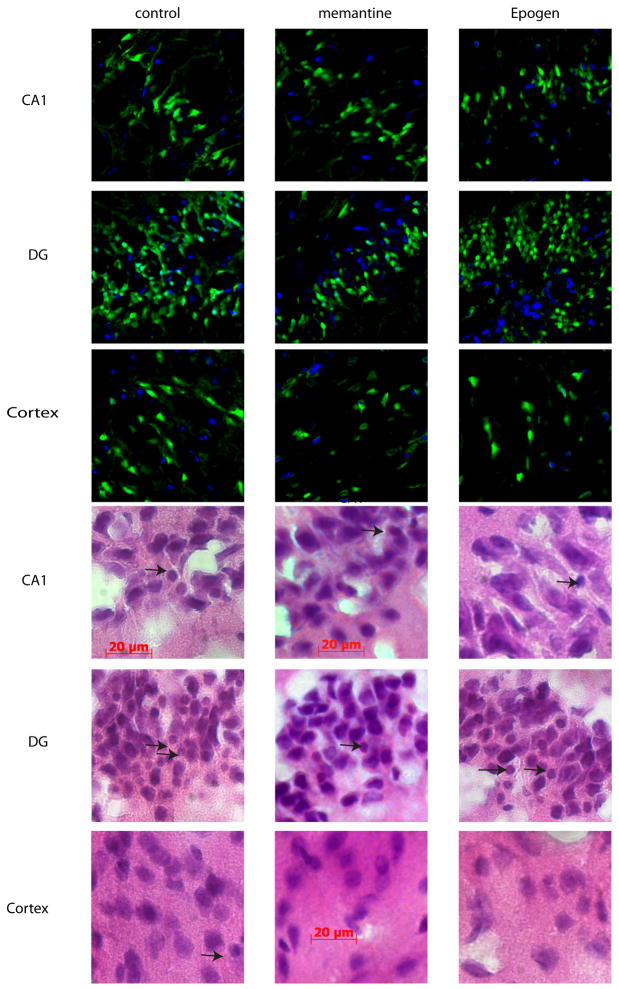
Figure 2. Neuronal damage as assessed by hematoxylin and eosinophilic staining and by Fluoro-Jade B staining.
In the top 9 photomicrographs Fluoro-Jade B-positive (green) and Dapi (blue) staining are shown and in the bottom 9 photomicrographs Hematoxylin and eosinophilic staining are shown from representative sections in the hippocampus [CA 1 and dentate gyrus (DG)] and ectorhinal cortex superior to the rhinal fissure viewed at X 400 magnification in control, memantine-treated, and erythropoietin (Epogen) treated animals. Brain cells damaged by severe hypoglycemia are characterized by condensed shrunken morphology and pyknotic nuclei with eosinophilic staining (arrows). Scale bar indicates 20 microns.
Erythropoietin
There was no difference in glucose levels at baseline (EPO 61 ± 4 mg/dl vs. CONT 65 ± 5 mg/dl, p=NS), during severe hypoglycemia (EPO 12 ± 0.4 mg/dl vs. CONT 11 ± 0.2 mg/dl, p=NS), or during recovery (EPO 194 ± 23 mg/dl vs. 167 ± 31 mg/dl, p=NS) (Fig 1C). In the cortex, animals treated with erythropoietin had a 39% decrease in the amount of hypoglycemia-induced neuronal damage (EPO 1350 ± 241 vs. CONT 2202 ± 211 cells, p=0.01)(Fig 1D, 2). Although erythropoietin also attenuated damage in the hippocampus, results were not statistically significant (EPO 1655 ± 209 vs. CONT 1982 ± 126 cells, p=NS) (Fig 1D).
Discussion
In this study we tested whether or not the administration of pharmacologic agents shown to be neuroprotective in other models of acute neuronal injury would be similarly neuroprotective against the brain cell damage caused by severe hypoglycemia. We demonstrate that memantine and erythropoietin protect against severe hypoglycemia-induced cortical brain damage.
In this study a hyperinsulinemic hypoglycemic clamp technique was used to precisely match for duration (90 minutes) and nadir of hypoglycemia (10–15 mg/dl). Our lab [24;25] and others [3;19–23] have previously shown that this degree of hypoglycemia consistently and reproducibly causes neuronal brain damage in the cortex and hippocampus. We also noted that there was equal damage in both the left and right hemispheres (data not shown) in response to global severe hypoglycemia indicating that cannulation of the left common carotid artery did not appear to have any significant detrimental effects.
Although anesthesia is often used in severe hypoglycemia experiments; to create a more real-world model of hypoglycemia, our experiments were performed in awake, freely mobile rats. We were thus able to circumvent the confounding effects of anesthesia including, 1) reduced brain glucose uptake which may increase brain damage, 2) abrogated seizure activity which is often associated with severe hypoglycemia, 3) induced hypothermia, 4) impaired increase blood pressure (Cushing) response to hypoglycemia, and 5) neurotoxicity [44–48].
During the ninety minutes of severe hypoglycemia, animals went into a coma (as evidenced by the fact that they loss their righting reflex) and exhibited characteristic tonic-clonic seizure-like behavior noted by characteristic brief (5–10 seconds) neck extensions, tonic stretching, uncontrolled limb movements, and spontaneous spinning [24;25]. In the absence of electroencephalogram (EEG) monitoring, the effect of subclinical seizures (i.e. seizures not associated with noticeable motor activity) on brain damage could not be assessed. Also, since potassium was not measured, we could not rule out the possibility that the effects of insulin induced hypoglycemia on brain damage were mediated, at least in part, by insulin induced hypokalemia.
Rather than employing a pre-treatment protocol, memantine were administered following the episode of hypoglycemia, at the time of glucose administration. This post-hypoglycemia administration of memantine was designed to mimic real-world conditions where severe hypoglycemia would be terminated in the Emergency Room with the infusion of glucose and a potential neuroprotective agent. Since severe hypoglycemia causes neuronal damage via excitotoxicity [26], memantine’s efficacy in this study is likely attributed to its mechanism of action as a low-affinity, uncompetitive NMDA blocker that blocks receptor in situations of excessive or pathologic activity [31] [29;32].
The mechanism of erythropoietin-induced neuroprotection in the cortex of rats in this study is likely multifactorial and may be related to its inhibition of proinflammatory cytokines [49], inhibition of nitric oxide synthesis [35], stimulation of anti-apoptotic proteins [43;50] or protection against reactive oxygen species and free radicals [51]. Since studies have shown erythropoietin to be neuroprotective when given both before and after several models of brain injury [35;38–43;49;51]; we chose to administer erythropoietin both prior to and following severe hypoglycemia in order to increase the likelihood of observing an effect. It remains to be determined if erythropoietin would be similarly neuroprotective if given only after an episode of severe hypoglycemia.
Both memantine and erythropoietin significantly protected against cortical brain damage, but the trend towards neuroprotection against hippocampal brain damage did not reach significance suggesting that there may be a regional susceptibility to hypoglycemia induced brain damage and/or regional responsiveness to neuroprotective agents. In previous studies, the cortex has consistently been shown to be more vulnerable than the hippocampus to glucose deprivation [24;25;52–54]. It is therefore speculated that differential susceptibilities to damage and differential responses to neuroprotective agents may be due to regional variations in antioxidant defenses [53–55], mitochondria bioenergetics [27;52], and/or distribution of NMDA receptors [56].
In summary, the administration of memantine and erythropoietin significantly decreased severe hypoglycemia-induced cortical neuronal damage by 35% and 39% respectively in our model (p<0.05 vs controls). Further studies are needed to determine the mechanisms by which neuronal damage is caused by severe hypoglycemia and identify the pathways by which neuroprotective pharmacological agents act to ameliorate damage. It is concluded that memantine and erythropoietin have the potential to be clinically efficacious agents that confer neuroprotection in response to severe hypoglycemia induced brain damage.
Acknowledgments
We would like to extend thanks to Dr. K. Yamada for assistance with Fluoro-Jade staining. We gratefully acknowledge research support from the NIH (DK073683, NS070235) and the core grant support from the Washington University’s Neuroscience Blueprint Core (NS057105), Diabetes Research and Training Center (DK020579) and Clinical Nutrition Research Unit (DK056341).
Abbreviation
- NMDA
N-methyl-D-aspartate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57:3169–3176. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Effects of intensive diabetes therapy on neuropsychological function in adults in the Diabetes Control and Complications Trial. Ann Intern Med. 1996;124:379–388. doi: 10.7326/0003-4819-124-4-199602150-00001. [DOI] [PubMed] [Google Scholar]
- 3.Auer RN. Hypoglycemic brain damage. Metab Brain Dis. 2004;19:169–175. doi: 10.1023/b:mebr.0000043967.78763.5b. [DOI] [PubMed] [Google Scholar]
- 4.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117:910–918. doi: 10.1172/JCI30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorgaas M, Gimse R, Vik T, Sand T. Cognitive function in type 1 diabetic children with and without episodes of severe hypoglycaemia. Acta Paediatr. 1997;86:148–153. doi: 10.1111/j.1651-2227.1997.tb08856.x. [DOI] [PubMed] [Google Scholar]
- 6.Hershey T, Lillie R, Sadler M, White NH. Severe hypoglycemia and long-term spatial memory in children with type 1 diabetes mellitus: a retrospective study. J Int Neuropsychol Soc. 2003;9:740–750. doi: 10.1017/S1355617703950077. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman FR, Epport K, Engilman R, Halvorson M. Neurocognitive functioning in children diagnosed with diabetes before age 10 years. J Diabetes Complications. 1999;13:31–38. doi: 10.1016/s1056-8727(98)00029-4. [DOI] [PubMed] [Google Scholar]
- 8.Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, Jimerson DC, Hennen J, Renshaw PF, Jacobson AM. Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes. 2006;55:326–333. doi: 10.2337/diabetes.55.02.06.db05-0520. [DOI] [PubMed] [Google Scholar]
- 9.Northam EA, Anderson PJ, Werther GA, Warne GL, Andrewes D. Predictors of change in the neuropsychological profiles of children with type 1 diabetes 2 years after disease onset. Diabetes Care. 1999;22:1438–1444. doi: 10.2337/diacare.22.9.1438. [DOI] [PubMed] [Google Scholar]
- 10.Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA. Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care. 2001;24:1541–1546. doi: 10.2337/diacare.24.9.1541. [DOI] [PubMed] [Google Scholar]
- 11.Rovet JF, Ehrlich RM. The effect of hypoglycemic seizures on cognitive function in children with diabetes: a 7-year prospective study. J Pediatr. 1999;134:503–506. doi: 10.1016/s0022-3476(99)70211-8. [DOI] [PubMed] [Google Scholar]
- 12.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–1572. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin EJ, Deary IJ. Effects of repeated hypoglycemia on cognitive function: a psychometrically validated reanalysis of the Diabetes Control and Complications Trial data. Diabetes Care. 1999;22:1273–1277. doi: 10.2337/diacare.22.8.1273. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842–1852. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer L, Fasching P, Madl C, Schneider B, Damjancic P, Waldhausl W, Irsigler K, Grimm G. Previous episodes of hypoglycemic coma are not associated with permanent cognitive brain dysfunction in IDDM patients on intensive insulin treatment. Diabetes. 1998;47:1909–1914. doi: 10.2337/diabetes.47.12.1909. [DOI] [PubMed] [Google Scholar]
- 16.Schoenle EJ, Schoenle D, Molinari L, Largo RH. Impaired intellectual development in children with Type I diabetes: association with HbA(1c), age at diagnosis and sex. Diabetologia. 2002;45:108–114. doi: 10.1007/s125-002-8250-6. [DOI] [PubMed] [Google Scholar]
- 17.Strudwick SK, Carne C, Gardiner J, Foster JK, Davis EA, Jones TW. Cognitive functioning in children with early onset type 1 diabetes and severe hypoglycemia. J Pediatr. 2005;147:680–685. doi: 10.1016/j.jpeds.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Wysocki T, Harris MA, Mauras N, Fox L, Taylor A, Jackson SC, White NH. Absence of adverse effects of severe hypoglycemia on cognitive function in school-aged children with diabetes over 18 months. Diabetes Care. 2003;26:1100–1105. doi: 10.2337/diacare.26.4.1100. [DOI] [PubMed] [Google Scholar]
- 19.Butcher SP, Sandberg M, Hagberg H, Hamberger A. Cellular origins of endogenous amino acids released into the extracellular fluid of the rat striatum during severe insulin-induced hypoglycemia. J Neurochem. 1987;48:722–728. doi: 10.1111/j.1471-4159.1987.tb05576.x. [DOI] [PubMed] [Google Scholar]
- 20.Nellgard B, Wieloch T. Cerebral protection by AMPA- and NMDA- receptor antagonists administered after severe insulin-induced hypoglycemia. Exp Brain Res. 1992;92:259–266. doi: 10.1007/BF00227969. [DOI] [PubMed] [Google Scholar]
- 21.Suh SW, Aoyama K, Chen Y, Garnier P, Matsumori Y, Gum E, Liu J, Swanson RA. Hypoglycemic neuronal death and cognitive impairment are prevented by poly(ADP-ribose) polymerase inhibitors administered after hypoglycemia. J Neurosci. 2003;23:10681–10690. doi: 10.1523/JNEUROSCI.23-33-10681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suh SW, Fan Y, Hong SM, Liu Z, Matsumori Y, Weinstein PR, Swanson RA, Liu J. Hypoglycemia induces transient neurogenesis and subsequent progenitor cell loss in the rat hippocampus. Diabetes. 2005;54:500–509. doi: 10.2337/diabetes.54.2.500. [DOI] [PubMed] [Google Scholar]
- 23.Wieloch T, Engelsen B, Westerberg E, Auer R. Lesions of the glutamatergic cortico-striatal projections in the rat ameliorate hypoglycemic brain damage in the striatum. Neurosci Lett. 1985;58:25–30. doi: 10.1016/0304-3940(85)90323-4. [DOI] [PubMed] [Google Scholar]
- 24.Bree AJ, Puente ET, Daphna-Iken D, Fisher S. Diabetes Increases Brain Damage Caused by Severe Hypoglycemia. Am J Physiol Endocrinol Metab. 2009;297:E194–E201. doi: 10.1152/ajpendo.91041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puente EC, Silverstein J, Bree AJ, Musikantow DR, Wozniak DF, Maloney S, Daphna-Iken D, Fisher SJ. Recurrent moderate hypoglycemia ameliorates brain damage and cognitive dysfunction induced by severe hypoglycemia. Diabetes. 2010;59:1055–1062. doi: 10.2337/db09-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auer RN, Siesjo BK. Biological differences between ischemia, hypoglycemia, and epilepsy. Ann Neurol. 1988;24:699–707. doi: 10.1002/ana.410240602. [DOI] [PubMed] [Google Scholar]
- 27.Suh SW, Hamby AM, Swanson RA. Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia. 2007;55:1280–1286. doi: 10.1002/glia.20440. [DOI] [PubMed] [Google Scholar]
- 28.Taoufik E, Probert L. Ischemic neuronal damage. Curr Pharm Des. 2008;14:3565–3573. doi: 10.2174/138161208786848748. [DOI] [PubMed] [Google Scholar]
- 29.Gorgulu A, Kins T, Cobanoglu S, Unal F, Izgi NI, Yanik B, Kucuk M. Reduction of edema and infarction by Memantine and MK-801 after focal cerebral ischaemia and reperfusion in rat. Acta Neurochir (Wien ) 2000;142:1287–1292. doi: 10.1007/s007010070027. [DOI] [PubMed] [Google Scholar]
- 30.Papagapiou MP, Auer RN. Regional neuroprotective effects of the NMDA receptor antagonist MK-801 (dizocilpine) in hypoglycemic brain damage. J Cereb Blood Flow Metab. 1990;10:270–276. doi: 10.1038/jcbfm.1990.44. [DOI] [PubMed] [Google Scholar]
- 31.Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- 32.Rao VL, Dogan A, Todd KG, Bowen KK, Dempsey RJ. Neuroprotection by memantine, a non-competitive NMDA receptor antagonist after traumatic brain injury in rats. Brain Res. 2001;911:96–100. doi: 10.1016/s0006-8993(01)02617-8. [DOI] [PubMed] [Google Scholar]
- 33.Rabie T, Marti HH. Brain protection by erythropoietin: a manifold task. Physiology (Bethesda) 2008;23:263–274. doi: 10.1152/physiol.00016.2008. [DOI] [PubMed] [Google Scholar]
- 34.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Block F, Schwarz M. Memantine reduces functional and morphological consequences induced by global ischemia in rats. Neurosci Lett. 1996;208:41–44. doi: 10.1016/0304-3940(96)12545-3. [DOI] [PubMed] [Google Scholar]
- 37.Volbracht C, van Beek J, Zhu C, Blomgren K, Leist M. Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur J Neurosci. 2006;23:2611–2622. doi: 10.1111/j.1460-9568.2006.04787.x. [DOI] [PubMed] [Google Scholar]
- 38.Xiong Y, Lu D, Qu C, Goussev A, Schallert T, Mahmood A, Chopp M. Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice. J Neurosurg. 2008;109:510–521. doi: 10.3171/JNS/2008/109/9/0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- 41.Yatsiv I, Grigoriadis N, Simeonidou C, Stahel PF, Schmidt OI, Alexandrovitch AG, Tsenter J, Shohami E. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB J. 2005;19:1701–1703. doi: 10.1096/fj.05-3907fje. [DOI] [PubMed] [Google Scholar]
- 42.Verdonck O, Lahrech H, Francony G, Carle O, Farion R, Van de LY, Remy C, Segebarth C, Payen JF. Erythropoietin protects from post-traumatic edema in the rat brain. J Cereb Blood Flow Metab. 2007;27:1369–1376. doi: 10.1038/sj.jcbfm.9600443. [DOI] [PubMed] [Google Scholar]
- 43.Liao ZB, Zhi XG, Shi QH, He ZH. Recombinant human erythropoietin administration protects cortical neurons from traumatic brain injury in rats. Eur J Neurol. 2008;15:140–149. doi: 10.1111/j.1468-1331.2007.02013.x. [DOI] [PubMed] [Google Scholar]
- 44.Alkire MT, Pomfrett CJ, Haier RJ, Gianzero MV, Chan CM, Jacobsen BP, Fallon JH. Functional brain imaging during anesthesia in humans: effects of halothane on global and regional cerebral glucose metabolism. Anesthesiology. 1999;90:701–709. doi: 10.1097/00000542-199903000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Canal CE, McNay EC, Gold PE. Increases in extracellular fluid glucose levels in the rat hippocampus following an anesthetic dose of pentobarbital or ketamine-xylazine: an in vivo microdialysis study. Physiol Behav. 2005;84:245–250. doi: 10.1016/j.physbeh.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Jeong YB, Kim JS, Jeong SM, Park JW, Choi IC. Comparison of the effects of sevoflurane and propofol anaesthesia on regional cerebral glucose metabolism in humans using positron emission tomography. J Int Med Res. 2006;34:374–384. doi: 10.1177/147323000603400406. [DOI] [PubMed] [Google Scholar]
- 47.Johnson SA, Young C, Olney JW. Isoflurane-induced neuroapoptosis in the developing brain of nonhypoglycemic mice. J Neurosurg Anesthesiol. 2008;20:21–28. doi: 10.1097/ANA.0b013e3181271850. [DOI] [PubMed] [Google Scholar]
- 48.Nakao Y, Itoh Y, Kuang TY, Cook M, Jehle J, Sokoloff L. Effects of anesthesia on functional activation of cerebral blood flow and metabolism. Proc Natl Acad Sci U S A. 2001;98:7593–7598. doi: 10.1073/pnas.121179898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, Viviani B, Marinovich M, Cerami A, Coleman TR, Brines M, Ghezzi P. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198:971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wen TC, Sadamoto Y, Tanaka J, Zhu PX, Nakata K, Ma YJ, Hata R, Sakanaka M. Erythropoietin protects neurons against chemical hypoxia and cerebral ischemic injury by up-regulating Bcl-xL expression. J Neurosci Res. 2002;67:795–803. doi: 10.1002/jnr.10166. [DOI] [PubMed] [Google Scholar]
- 51.Wakida K, Shimazawa M, Hozumi I, Satoh M, Nagase H, Inuzuka T, Hara H. Neuroprotective effect of erythropoietin, and role of metallothionein-1 and -2, in permanent focal cerebral ischemia. Neuroscience. 2007;148:105–114. doi: 10.1016/j.neuroscience.2007.04.063. [DOI] [PubMed] [Google Scholar]
- 52.Cardoso S, Santos MS, Seica R, Moreira PI. Cortical and hippocampal mitochondria bioenergetics and oxidative status during hyperglycemia and/or insulin-induced hypoglycemia. Biochim Biophys Acta. 2010;1802:942–951. doi: 10.1016/j.bbadis.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Xu L, Sapolsky RM, Giffard RG. Differential sensitivity of murine astrocytes and neurons from different brain regions to injury. Exp Neurol. 2001;169:416–424. doi: 10.1006/exnr.2001.7678. [DOI] [PubMed] [Google Scholar]
- 54.Srivastava A, Shivanandappa T. Hexachlorocyclohexane differentially alters the antioxidant status of the brain regions in rat. Toxicology. 2005;214:123–130. doi: 10.1016/j.tox.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Jiang X, Mu D, Manabat C, Koshy AA, Christen S, Tauber MG, Vexler ZS, Ferriero DM. Differential vulnerability of immature murine neurons to oxygen-glucose deprivation. Exp Neurol. 2004;190:224–232. doi: 10.1016/j.expneurol.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Bernal F, Saura J, Ojuel J, Mahy N. Differential vulnerability of hippocampus, basal ganglia, and prefrontal cortex to long-term NMDA excitotoxicity. Exp Neurol. 2000;161:686–695. doi: 10.1006/exnr.1999.7293. [DOI] [PubMed] [Google Scholar]