Abstract
Previous data suggest that overtraining can overcome fear conditioning deficits in rats with lesions of the basolateral complex of the amygdala (BLA). We have previously shown that the central nucleus of the amygdala (CEA) is essential for the acquisition and expression of conditional fear to both contextual and auditory conditioned stimuli (CSs) after overtraining. This provides strong evidence that the CEA can compensate for the loss of the BLA. Another brain area that may compensate for the loss of the BLA is the bed nucleus of the stria terminalis (BNST). We explored this possibility by examining the consequences of lesions or reversible inactivation of the BNST on the expression of overtrained fear in rats with BLA lesions. We demonstrate that lesions or inactivation of the BNST block the expression of freezing to the conditioning context, but not to an auditory conditional stimulus. These results reveal that the BNST has a critical role in the expression of contextual fear, but not fear to an auditory CS, and is therefore not the essential locus of compensation for fear learning in the absence of the BLA.
Keywords: Pavlovian fear conditioning, overtraining, amygdala, NBQX, NMDA, rats
1. Introduction
Neuroscientists have long sought to understand the neural basis of learning and memory. An influential approach to this problem pioneered by Richard F. Thompson and colleagues is the model-systems approach whereby the neural circuitry underlying a simple form of learning, such as classical conditioning, is dissected (Kim and Thompson, 1993). In recent years, Pavlovian fear conditioning has proved to be an important model for studying the neural mechanisms and circuitry of emotional learning and memory (Davis, 1992; LeDoux, 2000; Maren, 2001, 2005). In the paradigm, a conditioned stimulus (CS), such as the context of the conditioning chamber or a discrete auditory cue, is paired with an unconditioned stimulus (US), such as a mild footshock. After conditioning, the CS elicits a conditioned fear response (CR), characterized by an increase in heart rate, blood pressure, release of stress hormones, and somatomotor immobility (i.e. freezing).
Years of work have now revealed that the amygdala is essential for the acquisition and expression of Pavlovian fear memories (Fendt and Fanselow, 1999; LeDoux, 2000; Davis and Whalen, 2001; Maren, 2001). Specifically, the basolateral complex of the amygdala (BLA) is believed to be the critical site of CS-US convergence underlying the acquisition and of Pavlovian fear memories. However, we have discovered that deficits in fear conditioning in rats with BLA lesions can be overcome with overtraining (Maren, 1999a; Zimmerman et al., 2007). The capacity for fear learning in rats with BLA lesions suggests other brain areas are sufficient for the acquisition and expression of conditional fear. Indeed, we have recently shown that the amygdaloid central nucleus (CEA), which also receives CS and US information, is essential for the acquisition and expression of conditional fear in rats with BLA lesions. These findings suggest that the CEA mediates the acquisition of fear in rats with BLA lesions, although this memory requires many more trials to acquire (Maren, 1999a) and is short-lived (Poulos et al., 2009).
Another brain structure that might mediate fear conditioning in the absence of the BLA is the bed nucleus of the stria terminalis (BNST). The BNST possesses similar afferent and efferent connectivity to that of the CEA (Dong et al., 2001; Walker et al., 2003). Furthermore, Sullivan and colleagues recently demonstrated a role for the BNST in the expression of conditioned fear (Sullivan et al., 2004). Specifically, they found that lesions of the BNST block the expression of contextual fear, but not fear to an auditory CS (Sullivan et al., 2004; Waddell et al., 2006). It is possible therefore that the bed nucleus of the stria terminalis (BNST), like the CEA (Zimmerman et al., 2007), may be able to compensate for the loss of the BLA following overtraining.
In support of this possibility, Poulos and colleagues (2010) have reported that BNST lesions or inhibition of BNST protein synthesis impairs contextual fear conditioning in rats with BLA lesions. However, it is not clear whether the BNST has a global role in mediating fear in the absence of the BLA, or if it has a selective role in the expression of contextual fear independent of the BLA. The following experiments address this possibility. Rats received bilateral excitotoxic BLA lesions prior to overtraining in an auditory fear conditioning task. They then received either post-training lesions of the BNST or pre-testing infusions of the AMPA receptor antagonist NBQX into the BNST before retention tests in which conditional freezing to the shock-associated context and auditory CS was assessed. We report that although BNST lesions or inactivation disrupt the expression of context freezing in rats with BLA lesions, they did not effect the expression of fear to the auditory CS. These results reveal that although the BNST is critical for the expression of contextual fear, it is not the essential locus of compensation for fear learning in the absence of the BLA.
2. Materials and Methods
2.1 BNST lesions and the expression of overtrained fear in rats without a BLA
2.1.1 Subjects
The subjects were 66 male Long-Evans rats (200–224 g; Blue Spruce) obtained from a commercial supplier (Harlan Sprague Dawley, Indianapolis, IN). After arrival, the animals were individually housed in clear plastic cages hanging from a standard stainless-steel rack. The vivarium lights were on a 14:10 light:dark cycle (lights on at 7:00 am) and the rats had free access to food and tap water. After housing, the rats were handled (15–20 sec each) for five days to acclimate them to the experimenter. All experiments were carried out in accordance with guidelines approved by the University of Michigan University Committee on Use and Care of Animals.
2.1.2 Behavioral apparatus
Eight identical observation chambers (30 × 24 × 21 cm; Med-Associates, St. Albans, VT) were used for all phases of training and testing. The chambers were constructed from aluminum (two side walls) and Plexiglas (rear wall, ceiling, and hinged front door) and were situated in sound-attenuating chests located in an isolated room. The floor of each chamber consisted of 19 stainless-steel rods (4 mm diameter) spaced 1.5 cm apart (center to center). The rods were wired to a shock source and solid-state grid scrambler (Med-Associates) for delivery of the foot shock unconditioned stimulus (US) (1.0 mA, 2 sec). For “context A” (used for conditioning and context testing), background noise (65 dB) was provided by ventilation fans built into the chests, house lights within the chambers and fluorescent lights within the room provided illumination, the chest doors were left open, the chambers were cleaned with a 1% ammonium hydroxide solution, and the rats were transported in white 5-gallon buckets with bedding. For “context B” (used for tone testing), illumination was provided by incandescent red lights, the chest doors were closed, the ventilation fans were inactive, the chambers were cleaned with a 1% acetic acid solution, the floors were covered with black plastic panels, and the rats were transported in white 5-gallon buckets with bedding. Stainless steel pans containing a thin film of the corresponding cleaning solutions were placed underneath the grid floors before the animals were placed inside the boxes.
Each conditioning chamber rested on a load cell platform that was used to record chamber displacement in response to each rats’ motor activity. To ensure interchamber reliability, each load cell amplifier was calibrated to a fixed chamber displacement. The output of the load cell of each chamber was set to a gain that was optimized for detecting freezing behavior. Load cell amplifier output from each chamber was digitized and acquired on-line using Threshold Activity software (Med-Associates).
2.1.3 Surgery
After handling for at least five days, rats were treated with atropine sulfate (0.4 mg/kg body weight, i.p.) and sodium pentobarbital (65 mg/kg body weight, i.p.), and mounted in stereotaxic apparatus (David Kopf instruments, Tujunga, CA). The scalp was incised and retracted, and head position was adjusted to place bregma and lambda in the same horizontal plane. Small burr holes (2 mm in diameter) were drilled bilaterally in the skull for the temporary placement of 28-gauge cannula in the BLA (3.3 mm posterior to bregma, 5.0 mm lateral to the midline). Two 10 μl Hamilton syringes were mounted into an infusion pump (Harvard Apparatus, South Natick, MA) and connected to the injection cannula with polyethylene tubing. NMDA was dissolved in 100 mM PBS (20 mg/ml; ph 7.4; Sigma, St. Louis, MO). For BLA lesions, NMDA was infused (0.1 μl/min) at two sites: 8.0 mm ventral to brain surface (0.2 μl) and 7.5 mm ventral to brain surface (0.1 μl). Five minutes were allowed after each infusion for diffusion of the drug. Sham animals received a similar surgery and had small burr holes drilled bilaterally in their skulls, but injectors were not lowered into the brain. Additionally, five additional small burr holes were drilled in the skull for the bilateral placement of two 26-gauge guide cannula (cut at 11 mm below the pedestal; Plastics One, Roanoke, VA) in the BNST (0.5 mm posterior to bregma, 2.7 mm lateral to the midline, 7.4 mm ventral to bregma at a10 degree angle from vertical) and 3 small screws. Following implantation, dental acrylic was applied to the skull to hold the cannula in place. After surgery, dummy cannulae (33-gauge, 16 mm; Plastics One, Roanoke, VA) were inserted into the guide cannula, and the rats were allowed to recover from the anesthesia before being returned to their home cages. The dummy cannulae were replaced every other day during the week of recovery.
2.1.4 Procedure
After at least 7 days recovery from surgery, rats were transported to the laboratory in squads of eight and placed in the conditioning chambers for fear conditioning. The chamber position was counterbalanced for each squad and group. The rats received 75 tone (80 dB, 10 sec, 2 kHz) shock (1.0 mA, 2.0 sec) pairings (70 sec fixed intertrial interval) beginning 3 min after being placed in the chamber and ending 60 sec after the final shock (context A). The rats were then transported back to their home cages. Twenty-four hours after conditioning the rats were anesthetized as described above in order to receive an intracranial NMDA infusion (3.5 μg in 0.175 μl of 100 mM PBS at 0.1 μl/min; pH 7.4; Sigma) into the BNST. Bilateral BNST infusions were made using 10 μl Hamilton syringes mounted into an infusion pump (Harvard Apparatus, South Natick, MA) and connected to injection cannula (28 gauge; 16 mm; Plastics One, Roanoke, VA) with polyethylene tubing. After the infusion, five minutes was allowed for diffusion before removing the injection cannula. Rats receiving sham BNST lesions were anesthetized but received no infusions. After removing the internal cannulae, clean dummy cannulae were inserted into the guide cannula and rats were allowed to recover from the anesthesia before being returned to their home cages. After 3 days of recovery rats, were placed in the conditioning chambers in the absence of tones or foot-shocks for 10 min (context A) to test the level of conditioned fear to the conditioning context. Twenty-four hours after the context test, rats were transported back to the chambers and placed in a novel context (context B) for a tone test. Two minutes after placement in the chambers, the rats were presented with an 8-min continuous tone (80 dB, 2 kHz).
During the training and test sessions, each rat’s activity was monitored continuously using the data acquisition software described above. For each chamber, load cell activity was digitized at 5 Hz, yielding one observation per rat every 200 msec (300 observations per rat per minute). Load cell values ranged between 0 and 100, and this value was used to quantify locomotor activity. Freezing was quantified by computing the number of observations for each rat that had a load cell value less than the freezing threshold (threshold = 10). The freezing threshold was determined in a separate group of pilot animals by comparing load cell output with an observer’s rating of freezing behavior. To avoid counting momentary inactivity as freezing, an observation was only scored as freezing if it fell within a contiguous group of at least five observations that were all less than the freezing threshold. Thus, freezing was only scored if the rat was immobile for at least 1 sec. For each session, the freezing observations were transformed to a percentage of total observations.
2.1.5 Histology
Histological verification of lesions and cannula placements were performed after behavioral testing. Rats were perfused across the heart with 0.9% saline followed by 10% formalin. After extraction from the skull, the brains were post-fixed in 10% formalin for 2 days and 10% formalin and 30% sucrose until sectioning. Coronal sections (45 μm thick, taken every 135 μm) were cut on a cryostat (−20 °C) and wet mounted on glass microscope slides with 70% ethanol. After drying, the sections were stained with 0.25% thionin to visualize cell bodies. Lesions and cannula placements were verified by visual inspection of the stained brain sections.
2.1.6 Data analysis
For each session, the freezing data were transformed to a percentage of total observations, a probability estimate that is amenable to analysis with parametric statistics. These probability estimates of freezing were analyzed using ANOVA. Post-hoc comparisons in the form of Fisher’s PLSD tests were performed after a significant overall F ratio. All data are represented as means ± SEMs.
2.2 BNST inactivation and the expression of overtrained fear in rats without a BLA
2.2.1 Subjects
The subjects were 71 male Long-Evans rats (200–224 g; Blue Spruce) obtained and housed as described in Experiment 1.
2.2.2 Behavioral apparatus and surgery
The behavioral apparatus and surgical procedures are identical to those described in Experiment 1.
2.2.3 Procedure
After at least 7 days recovery from surgery, rats were acclimated to the infusion procedure by transporting them to the infusion room in identical white 5-gallon buckets in squads of eight (counterbalanced for each squad and group). Their dummy cannulae were replaced and the infusion pumps (Harvard Apparatus, South Natick, MA) were activated. After 3 minutes, the pumps were stopped and the animals were returned to their home cages. Twenty-four hours after acclimation, on the conditioning day, the rats were transported to the laboratory in squads of eight and placed in the conditioning chambers. Training was identical to that described in Experiment 1. Twenty-four hours after training, the rats were transported to the infusion room as described above. Infusions were delivered using 10 μl Hamilton syringes mounted into an infusion pump (Harvard Apparatus, South Natick, MA) and connected to the injection cannula (28 gauge; 16 mm; Plastics One, Roanoke, VA) with polyethylene tubing. Rats were infused with the AMPA receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX; 3.0 μg in 0.3 μl of 100 mM PBS at 0.1 μl/min) or 100 mM PBS (VEH; 0.3 μl at 0.1 μl/min). After the infusion, one minute was allowed for diffusion before removing the internal cannula. After removing the internal cannula, clean dummy cannula were inserted into the guide cannula and rats were immediately transported to the conditioning chambers for a context test as described in Experiment 1. Seventy-two hours after conditioning the rats were transported back to the infusion room where they received a second BNST infusion identical to that described above. The rats were then immediately transported back to the conditioning chambers for a tone test as described in Experiment 1.
2.2.4 Histology and Data Analysis
Histology and data analysis were performed as described in Experiment 1.
3. Results
3.1 BNST lesions and the expression of overtrained fear in rats without a BLA
3.1.1 Histology
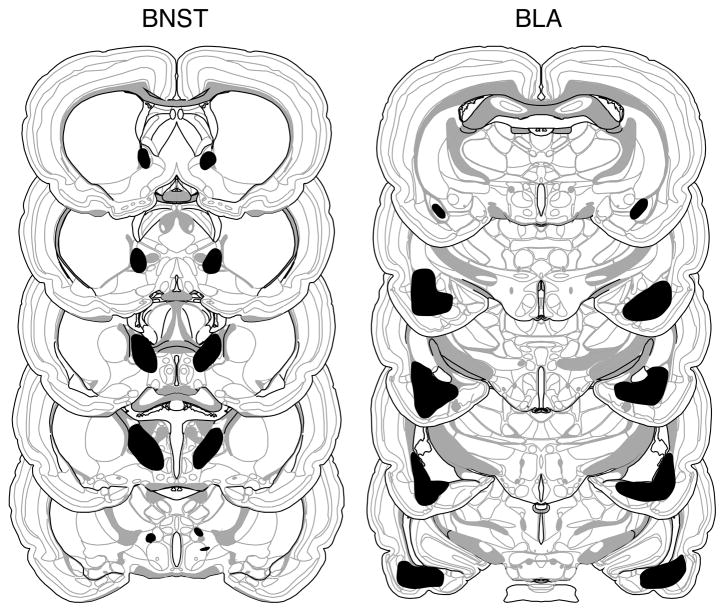
Based on the histological results, 10 of 66 rats were excluded. Rats were excluded if their lesions were larger than intended, misplaced, largely unilateral, or produced substantial damage in the CEA. This yielded the following group sizes: BLA-BNST (n = 13), BLA-SH (n = 14), SH-BNST (n = 11), and SH-SH (n = 18). The extent of the amygdala and BNST damage for rats included in the analyses are depicted in Figure 1. As can be seen damage was generally confined to the targeted structure and was estimated to include at least 80% of each structure. BLA lesions were associated with minimal damage to the most caudal aspect of the CEA.
Figure 1.
Schematic representation of the extent of NMDA lesions in the BNST (left panels) and BLA (right panels) (Experiment 1). A representative lesion (black shading) is shown from a rat in the BLA-BNST group. The extent of the BNST and BLA lesions in this rat were typical of those of others in the BLA-SH and SH-BNST groups. Coronal brain section images adapted from Swanson (2003).
3.1.2 Behavior
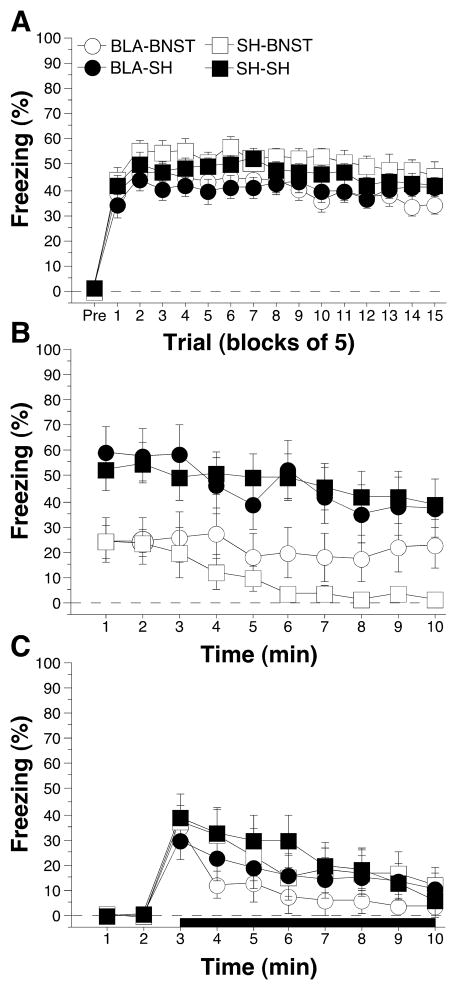
Post-shock freezing during the conditioning session is shown in Figure 2A. Note that at this point in the experiment, some of the rats had received BLA lesions (and others sham surgery), but none had received a BNST lesion. The data were analyzed using repeated measures ANOVA with between-subjects variables of pre-training lesion (SH or BLA) and post-training lesion (SH or BNST) and a repeated measure of trial (fifteen 5-trial blocks). During the pre-trial period rats displayed minimal levels of freezing (<5%) before footshock. After the onset of conditioning, rats exhibited robust freezing. The ANOVA revealed a main effect of trial [F(14,728) = 7.9; p < 0.0001] without a significant main effect or interaction for any other variable (p > 0.05 for all comparisons). There was a trend for BLA lesions to reduce post-shock freezing (p = .057). This indicates that all rats acquired similar levels of conditioned fear at similar rates.
Figure 2.
Conditioned freezing in rats with pre-training BLA lesions and post-training BNST lesions (Experiment 1). A, Mean percentage of freezing (± SEM) during the 75-trial training session (data are displayed with a 3-min pre-trial period followed by fifteen 5-trial blocks). Freezing was quantified before the first conditioning trial (Pre) and during the 1 min period after each conditioning trial; these values were averaged in 5-trial blocks. B, Mean percentage of freezing (± SEM) to contextual (10 min context extinction test) cues 4 days following training. C, Mean percentage of freezing (± SEM) to the auditory CS in a novel context 5 days following training. The auditory CS commenced 2 min after rats were placed in the chambers (horizontal bar indicates the CS). Data are shown for rats with pre-training lesions of the BLA (filled circles), pretraining lesions of the BLA and post-training lesions of the BNST (open circles), post-training lesions of the BNST (open squares), and intact rats (SH-SH; filled squares).
Long-term fear memories to the conditioning context and the auditory CS were assessed in separate retention tests conducted 4 and 5 days after conditioning, respectively. Figure 2B shows the freezing data during the context test. A repeated measures ANOVA for the 10 min context test with between-subjects variables of pre-training lesion (SH and BLA) and post-training lesion (SH and BNST), and a repeated measure of time (min 1–10) revealed significant main effects of post-training lesion [F(1,52) = 13.2; p < 0.001] and time [F(9,468) = 5.8; p < 0.0001] without a significant main effect of pre-training lesion [F(1,52) = 0.2; p > 0.6]. The ANOVA also revealed a significant three-way interaction of pre-training lesion X post-training lesion X time [F(9,468) = 2.0; p < 0.05]. No other interactions were significant (p > 0.56 for all comparisons). These data indicate that rats with BLA lesions exhibited similar degrees of conditioned fear to sham rats, and lesions of the BNST impaired the expression of contextual freezing in both sham rats and rats with BLA lesions.
Freezing during the tone test is shown in Figure 2C. A repeated measures ANOVA on conditional freezing during the tone with between-subjects variables of pre-training lesion (SH and BLA) and post-training lesion (SH and BNST), and a within-subject variable of time (min 3–10) revealed a significant main effect of time [F(7,364) = 18.9; p < 0.0001] without significant main effects of pre-training lesion (SH or BLA) [F(1,52) = 1.4; p = 0.25] or post-training lesion (SH or BNST) [F(1,52) = 0.4; p = 0.52]. The three-way ANOVA revealed no significant interactions (p > 0.19 for all comparisons). These data indicate that neither pre-training BLA lesions nor post-training lesions of the BNST blocked the expression of auditory cued fear.
3.2 BNST inactivation and the expression of overtrained fear in rats without a BLA
3.2.1 Histology
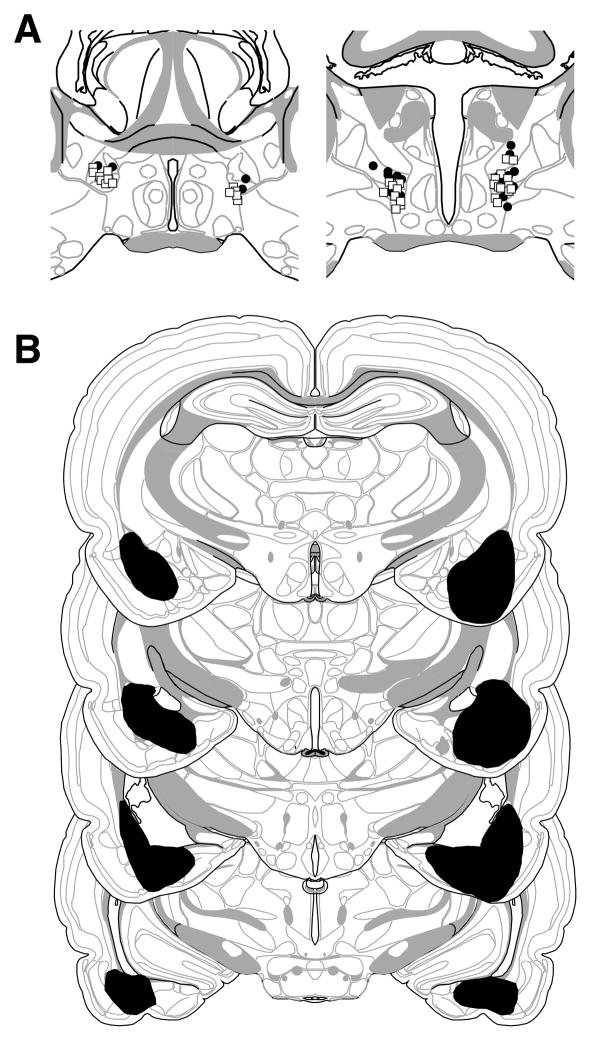
Based on the histological results, 43 of 71 rats were excluded. Rats were excluded if their cannulae were misplaced or lesions were larger than intended, misplaced, or largely unilateral. The large number of exclusion resulted from an unexpectedly high number of rats with BLA lesions that encroached upon the CEA. This yielded the following group sizes: BLA-NBQX (n = 6), BLA-VEH (n = 3), SH-NBQX (n = 11), and SH-VEH (n = 8). The extent of the amygdala lesions and cannulae placements for rats included in the analyses are depicted in Figure 3. As can be seen, cannula placements and damage were generally confined to the BNST. For lesions targeting the BLA, there was some damage to the rostral entorhinal cortex.
Figure 3.
Schematic representation of the locations of BNST cannula placements (A) and (B) extent of pre-training NMDA lesions of the BLA (black shading) (Experiment 2). Cannula placements for rats receiving NBQX or VEH in the BNST are indicated by open circles or filled squares, respectively. Coronal brain section images adapted from Swanson (2003).
3.2.2 Behavior
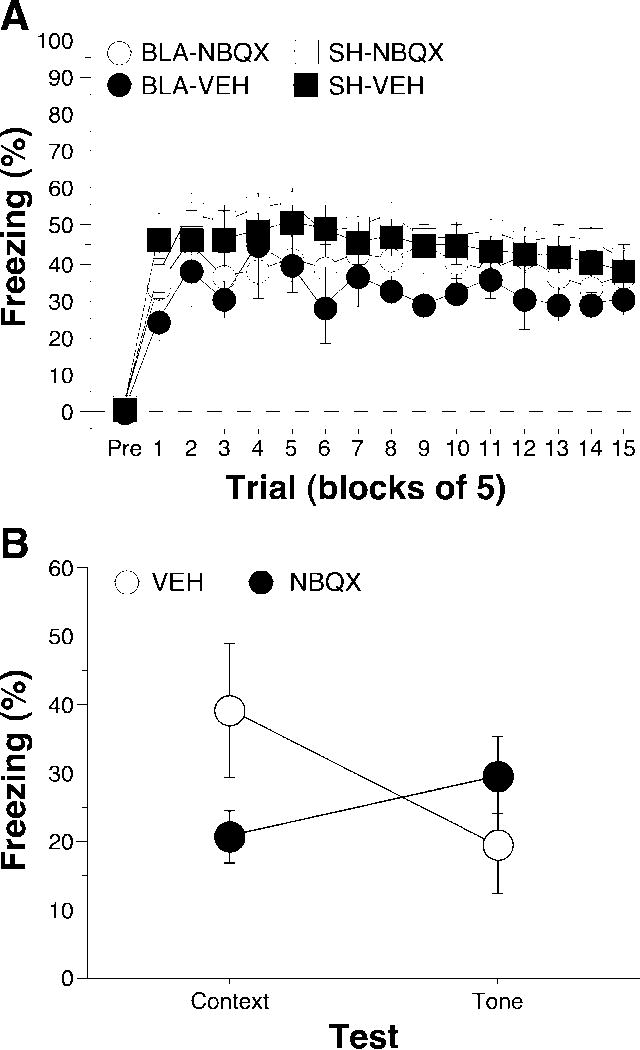
Post-shock freezing during the conditioning session is shown in Figure 4A. The data were analyzed using repeated measures ANOVA with between-subjects variables of lesion (SH or BLA) and drug (VEH or NBQX) and a within-subjects variable of trial (fifteen 5-trial blocks). During the pre-trial period rats displayed minimal levels of freezing (<5%) before footshock. After the onset of conditioning, rats exhibited robust freezing. The ANOVA revealed a main effect of lesion [F(1,24) = 5.6; p < 0.05] and a main effect of training trial [F(14, 336) = 4.0; p < 0.0001] without a significant main effect of drug [F(1, 24) = 1.2; p = 0.290] or significant interactions across all variables (p > 0.29 for all comparisons). This indicates that rats with pre-training BLA lesions froze significantly less than intact rats during the training session.
Figure 4.
Conditioned freezing in rats with pre-training BLA lesions and pre-test NBQX infusions into the BNST (Experiment 2). A, Mean percentage of freezing (± SEM) during the 75-trial training session (data are displayed with a 3-min pre-trial period followed by fifteen 5-trial blocks). Freezing was quantified before the first conditioning trial (Pre) and during the 1-min period after each conditioning trial; these values were averaged in 5-trial blocks. Data are shown for rats with pre-training sham surgeries receiving VEH in the BNST prior to testing (SH-VEH: filled squares), pre-training sham surgeries receiving NBQX in the BNST prior to testing (SH-NBQX: open squares), pre-training NMDA lesions of the BLA receiving VEH in the BNST prior to training (BLA-VEH: filled circles), or pre-training NMDA lesions of the BLA receiving NBQX in the BNST prior to training (BLA-NBQX: open circles). B, Mean percentage of freezing (± SEM) averaged across the context and tone tests for rats receiving infusions of NBQX or VEH into the BNST. Rats in the BLA and SHAM groups were collapsed as they did not differ from one another during the retention tests.
Long-term fear memories to the conditioning context and the auditory CS were assessed in separate retention tests conducted 24 and 72 hours after conditioning, respectively (Figure 4B). As in Experiment 1, there was no difference between BLA and SH rats in either contextual or auditory freezing; this variable was therefore collapsed in the analysis. Figure 4B shows the average freezing data across the context and tone tests. A repeated measures ANOVA with a within-subjects variable of CS (context and tone) and a between-subjects factor of drug (VEH and NBQX) revealed a significant interaction of CS and drug [F(1,26) = 20.0; p < 0.0001]; there were no significant main effects of either variable alone. Hence, rats receiving NBQX infusions in the BNST immediately before the context test showed significantly less freezing than those receiving vehicle, whereas freezing to the tone was spared. These data indicate the expression of overtrained contextual fear, but not cue fear, is dependent upon the activation of AMPA receptors within the BNST in rats without a BLA.
4. Discussion
The results of the present study indicate that BNST lesions or inactivation selectively impairs the expression of contextual fear after overtraining. This impairment was not observed to an auditory CS, and was manifest in both intact rats and rats with BLA lesions. Hence, the effect of BNST lesions on conditional freezing was not due to a performance deficit. These results are consistent with previous studies that have demonstrated that lesions of the BNST selectively disrupt contextual fear after limited training (Sullivan et al., 2004; Waddell et al., 2006). Similarly, lesions of the BNST do not affect fear-potentiated startle, a paradigm in which a discrete light CS paired with a shock unconditioned stimulus increases the acoustic startle reflex (Lee and Davis, 1997). Collectively, these data suggest that the BNST is critically involved in the expression of contextual fear after both limited and extensive training. Because BNST lesions or inactivation did not influence the expression of fear to an auditory CS in rats with BLA lesions, it does not serve a general role in compensating for the absence of the BLA to mediate fear conditioning. Indeed, we have previously reported that lesions of the central nucleus of the amygdala prevent the acquisition and expression of both contextual and auditory fear in rats with BLA lesions (Zimmerman et al., 2007). Together, these data reveal that the CEA compensates for the loss of the BLA to mediate fear conditioning.
The present data add to a growing body of evidence that the BNST has a special role in the expression of conditioned anxiety, rather than conditioned fear per se. For example, Walker and Davis (1997) have demonstrated that lesions of the BNST prevent light-enhanced startle, a model for unconditioned fear in which the presence of a continuous anxiogenic stimulus (bright light) enhances fear to a loud noise burst. Moreover, Waddell and colleagues (2006) have found that BNST lesion effect fear conditioning to long duration CSs relative to short duration CSs. It has been argued that shock-associated contexts, long duration CSs, and ambient bright light yield a state of conditioned anxiety because they signal that an aversive event is likely to occur, but not when it will happen (Walker et al., 2003; Davis, 2006; Waddell et al., 2006). The BNST is highly interconnected with hypothalamic nuclei involved in coordinating the release of stress hormones, and therefore may engage conditioned and unconditioned anxiety responses that prepare animals for potential threats in the environment. In contrast, the CEA is anatomically connected to brain stem systems involved in organizing conditioned fear responses, such as freezing, that anticipate imminent insult.
Much like the amygdala, the BNST receives input from the ventral hippocampus and ventral subiculum (primary output of the hippocampus) (Dong et al., 2001). Interestingly, these hippocampal subregions are implicated in the expression of conditioned fear and anxiety responses. Excitotoxic or electrolytic lesions of the ventral subiculum impair the acquisition and expression of conditioned fear (Maren, 1999b), much like lesions of the amygdala. Additionally, lesions of the ventral hippocampus produce an anxiolytic effect on tests of unconditioned anxiety (McHugh et al., 2004), an effect not seen in rats with amygdala lesions, but similar to the effects discussed above in rats with lesions of the BNST. Such findings suggest that the contextual information necessary for the expression of conditioned anxiety is likely mediated via input from the ventral subiculum and ventral hippocampus directly to the BNST.
In contrast to the BNST, lesions or inactivation of the CEA completely block the expression of conditioned freezing to both auditory CSs and shock-associated contexts (Zimmerman et al., 2007), as well as eliminating fear-potentiated startle to a visual CS (Walker and Davis, 1997). This suggests that the CEA is necessary for the acquisition and expression of conditioned fear in both intact rats and rats with BLA lesions. Interestingly however, Kim and Davis (1993) have shown that rats with CEA lesions can reacquire fear potentiated startle after extensive training as long as the CEA was intact during the initial acquisition of fear. In this case it is unclear whether the brain is compensating for the loss of the CEA during the reacquisition of fear, expression of fear, or both.
Interestingly, inactivation of the CEA does not affect light-enhanced startle, whereas inactivation of either the BLA or BNST impairs this effect (Walker and Davis, 1997; Walker et al., 2003). These data suggest that the CEA is essential for mediating conditional fear, while the BNST is required for conditioned anxiety (Walker et al., 2003; Davis, 2006; Waddell et al., 2006). Interestingly, lesions of either the CEA or BNST block the expression of contextual fear indicating that freezing to the conditioning context encompasses aspects of both fear and anxiety. Moreover, these data imply that both freezing and startle can index different psychological states (fear or anxiety) and that behavior under these different states is mediated by different neural systems.
In summary, our findings indicate that the BNST is necessary for the expression of contextual fear even after overtraining. However, BNST lesions did not prevent the expression of freezing to an auditory CS in either intact rats or rats with BLA lesions. Hence, it does not appear that the BNST functions as a surrogate for a damaged BLA. Rather, our previous work suggests that the CEA plays such a role, compensating for the BLA to mediate both context and CS fear after overtraining (Zimmerman et al., 2007). As has been previously suggested (Walker et al., 2003; Davis, 2006; Waddell et al., 2006), our data are consistent with a role for the CEA and BNST in mediating conditioned fear and anxiety, respectively. However, these systems are not mutually exclusive and interact to mediate contextual freezing, for example. Understanding the precise circumstances under which each system is utilized will require additional research.
5. Conclusions
Rats with basolateral amygdala lesions acquire both contextual and auditory fear after overtraining. The expression of overtrained contextual fear, but not tone fear, requires the bed nucleus of the stria terminalis. These results reveal that the bed nucleus of the stria terminalis has a selective role in the expression of context fear, rather than a general role in the associative processes mediating fear memory in the absence of the basolateral amygdala. This latter role appears to be mediated by the central nucleus of the amygdala (Zimmerman et al., 2007).
Acknowledgments
The research reported in this manuscript was supported by a grant from the National Institutes of Health (R01MH73655).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 1993;10:427–55. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Reviews Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Kim M, Davis M. Electrolytic lesions of the amygdala block acquisition and expression of fear-potentiated startle even with extensive training but do not prevent reacquisition. Behav Neurosci. 1993;107:580–595. doi: 10.1037//0735-7044.107.4.580. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci. 1999a;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlovian fear conditioning in rats. Behav Neurosci. 1999b;113:283–290. doi: 10.1037//0735-7044.113.2.283. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Building and burying fear memories in the brain. The Neuroscientist. 2005;11:89–99. doi: 10.1177/1073858404269232. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- Poulos AM, Li V, Sterlace SS, Tokushige F, Ponnusamy R, Fanselow MS. Persistence of fear memory across time requires the basolateral amygdala complex. Proc Natl Acad Sci U S A. 2009;106:11737–11741. doi: 10.1073/pnas.0905257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Academic Press; San Diego: 2003. [Google Scholar]
- Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Zimmerman JM, Rabinak CA, McLachlan IG, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Mem. 2007;14:634–644. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]