Abstract
Calorie restriction (CR) improves obesity-related insulin resistance through undefined molecular mechanisms. Insulin receptor substrate (IRS)-1 serine/threonine kinases have been proposed to modulate insulin sensitivity through phosphorylation of IRS proteins. The aim of this study is to test the hypothesis that changes in the activity of IRS1 serine/threonine kinases may underlie the molecular mechanism of CR in improving insulin sensitivity. Obese and lean Zucker rats were subjected to 40% CR or allowed to feed ad libitum (AL) for 20 weeks; body weight and insulin sensitivity were monitored throughout this period. The activity of IRS1 serine/threonine kinases – including JNK, ERK, MTOR/p70S6K (RPS6KB1 as listed in the MGI Database), glycogen synthase kinase 3β (GSK3B), AMPK (PRKAA1 as listed in the MGI Database), and protein kinase Cθ (PRKCQ) in liver tissue extracts was measured by an in vitro kinase assay using various glutathione-S-transferase (GST)–IRS1 fragments as substrates, while phosphorylation of IRS1 and serine kinases was determined by western blotting using phosphospecific antibodies. CR in obese rats significantly reduced body weight and increased insulin sensitivity compared to AL controls. Serine kinase activity toward IRS1S612 (corresponding to S616 in human IRS1) and IRS1S632/635 (corresponding to S636/639 in human IRS1) was increased in obese rats compared to lean littermates, and was markedly decreased following CR. Concomitantly, obesity increased and CR decreased the activity of hepatic ERK and p70S6K against IRS1. The close association between the activity of hepatic ERK and p70S6K with insulin resistance suggests an important role for ERK and p70S6K in the development of insulin resistance, presumably via phosphorylation of IRS proteins.
Introduction
Calorie restriction (CR) may improve the outcome of obesity-associated diseases, including diabetes and cardiovascular disease. At the whole-body level, CR has been shown to reduce visceral fat (Barzilai et al. 1998), attenuate plasma dyslipidemia, and reduce oxidative stress, leading to improved insulin sensitivity (Ugochukwu & Figgers 2007). The underlying molecular mechanisms mediating these effects are not very clear. It has been observed that an elevation in the ratio of phosphatidylinositol-3-kinase (PI3K) catalytic subunits to regulatory subunits (McCurdy et al. 2005), downregulation of glycogen synthase kinase α (GSKA) in skeletal muscle, and suppression of GSK3B in adipocytes (Ciaraldi et al. 2006) may be involved. Increased AKT2 phosphorylation (McCurdy et al. 2003), augmented adiponectin levels, and enhanced AMP-activated protein kinase phosphorylation (Shinmura et al. 2007) have been observed following short-term CR.
Insulin receptor substrate proteins (IRS proteins) are important adaptor molecules that transmit signals from the insulin receptor tyrosine kinase on the plasma membrane to intracellular downstream effectors. Via phosphorylation of specific tyrosine residues, IRS proteins recruit SH2 domain-containing proteins, which in turn mediate further downstream signaling events (White 2006). There are over 50 potential serinine/threonine phosphorylation sites in both IRS1 and IRS2, and it has been reported in the earlier study that multiple serine/threonine sites in IRS1 are phosphorylated, indicating the important contributions of the serine phosphorylation of IRS protein in insulin signaling (Sun et al. 1992). Increased serine phosphorylation of IRS proteins has often been associated with insulin resistance in human as well as in animal models, and is thought to contribute to the development of insulin resistance (Zick 2004, Gual et al. 2005, White 2006).
To date, over ten serine/threonine phosphorylation sites in IRS1 have been identified in vivo and in vitro by different methods, and their roles in insulin resistance have been explored extensively. Among them are S302 (corresponding to S307 in human IRS1; Giraud et al. 2004), S307 (corresponding to S312 in human IRS1; Aguirre et al. 2000), S332 (corresponding to S337 in human IRS1; Liberman & Eldar-Finkelman 2005), S612 (corresponding to S616 in human IRS1; DeFea & Roth 1997), S632/635 (corresponding to S636/639 in human IRS1; Ozes et al. 2001), S789 (corresponding to S794 in human IRS1; Jakobsen et al. 2001, Qiao et al. 2002), and S1100 (corresponding to S1101 in human IRS1; Li et al. 2004, Tremblay et al. 2007). Candidate protein kinases that phosphorylate some of these sites have also been identified. These kinases include p70S6K for S302 (Harrington et al. 2004), JNK for S307 (Aguirre et al. 2000), GSK3 for S332 (Liberman & Eldar-Finkelman 2005), ERK for S612 (DeFea & Roth 1997), MTOR for 632/635 (Ozes et al. 2001), AMPK and SIK2 for 789 (Jakobsen et al. 2001, Horike et al. 2003), and protein kinase C (PKC) θ and p70S6K for S1100 (Li et al. 2004, Tremblay et al. 2007).
Studies have demonstrated the involvement of the aforementioned kinases in the induction and worsening of insulin resistance. Furthermore, data support the efficacy of inhibition of IRS1 serine/threonine kinases in the amelioration of resistance. Aspirin blocks serine phosphorylation of IRS1 in tumor necrosis factor-treated cells by suppressing the activity of multiple serine kinases (Gao et al. 2003). Tempering the actions of MTOR/p70S6K1 via rapamycin blunts insulin-induced S632/635 phosphorylation of IRS1 while increasing IRS1-associated PI3K activity and AKT phosphorylation (Khamzina et al. 2005). Repression of GSK3 has proven effective in promoting insulin-like effects and in potentiating insulin’s actions both in vitro and in vivo (Eldar-Finkelman & Ilouz 2003). Pharmacological manipulation of insulin sensitivity, however, does not allow for the determination of the importance of different IRS1 serine kinases during the development of insulin resistance.
In this study, we intend to identify the IRS protein kinase(s) whose activity is not only associated with obesity-induced insulin resistance, but also inversely associated with improved insulin sensitivity by means of CR. We selected Zucker fatty rats for this study because they are a well-characterized obese, insulin-resistant animal model, with typical hepatic insulin resistance including steatosis, dysregulated glucose production, and hyperinsulinemia (Zucker & Antoniades 1972). We compare the activity of several known IRS1 protein kinases via in vitro kinase assays in liver extracts prepared from lean and obese Zucker rats fed ad libitum (AL) as well as from obese and lean Zucker rats subjected to 20 weeks of CR. Among the candidate IRS protein kinases, our results reveal a close association between ERK and MTOR/p70S6K activities and insulin resistance. Our data lend additional credence to the value of CR as a therapy for improving obesity-induced insulin resistance, as well as implicating enhanced ERK and MTOR/p70S6K activities as potential mediating factors.
Materials and Methods
Reagents
Phospho-IRS1 (S302, S307, S332, S612, S636/639, S789, and S1101), phospho-SAPK/JNK (T183/Y185), JNK, phospho-p44/42 MAPK (T202/Y204), phospho-p70S6K (T421/S424), P70S6K (RPS6KB1 as listed in the MGI Database), phospho-AMPKα (T172), AMPK (PRKAA1 as listed in the MGI Database) phospho-GSK3B (S9), GSK3B, phospho-PKCθ (T538), PKCθ (PRKCQ as listed in the MGI Database), and MTOR antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA). ERK2 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All these antibodies recognize human, mouse, and rat proteins. Recombinant MTOR and p70S6K1 were obtained from HumanZyme Inc. (Chicago, IL, USA). All inhibitors including ERK inhibitor II, LY294002, and Y27632 were purchased from EMD Chemicals (San Diego, CA, USA).
Animals and CR
Four-week-old male obese Zucker (fa/fa) rats and their lean littermates were purchased from Charles River (Wilmington, MA, USA). All animals were individually housed in cages with free access to food for 1 week. Rats were then divided into four groups: obese-CR (O-CR), obese-AL (O-AL), lean-CR (L-CR), and lean-AL (L-AL) (n=8 per group). Starting at 5 weeks of age, O-AL and L-AL animals were allowed unlimited access to food (7017 NIH-31, Harlan Teklad, Indianapolis, IN, USA), and food intake was recorded daily. O-CR and L-CR animals were restricted to 60% of the food intake consumed by O-AL and L-AL animals respectively (a 40% reduction) with vitamin supplementation (7109 NIH-31/NIA Fortified Diet, Harlan Teklad). The body weight of all the rats was measured daily.
At 25 weeks of age, animals were anesthetized using pentobarbital, and insulin tolerance tests (ITTs) were conducted. Following a 1-week acclimation period, animals were killed after anesthetization. Livers were rapidly collected and washed briefly in PBS in preparation for tissue extraction. All care and treatment of animals were in accordance with the guidelines of the National Institutes of Health. The protocols were subjected to prior approval by the Institutional Animal Care and Use Committee of the University of Chicago.
Insulin tolerance test
ITTs were carried out following an overnight fast (16 h). The rats were injected i.p. with 1·5 U/kg insulin. Blood glucose was measured directly by a GM9D Glucose Analyzer (Analox Instruments, Lunenburg, MA, USA) using blood obtained from the tail vein at 0, 15, 30, 60, 90, and 120 min.
Tissue extract preparations
Liver tissue extracts (TEs) were prepared as described previously (Qiao et al. 1999). Briefly, rats were fasted for 4 h and anesthetized. Livers were rapidly removed and homogenized in a lysis buffer (10 mM Tris–HCl, pH 7·4, containing 250 mM sucrose, 100 mM NaF, 5 mM EDTA, 5 mM EGTA, 1 mM dithiothreitol (DTT), 0·5 mM phenylmethyl-sulphonyl fluoride (PMSF), 0 ·2 mM Na3VO4, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) (10 ml lysis buffer/g liver tissue) with a Brinkmann Polytron homogenizer, followed by centrifugation at 10 000 g for 20 min (Sorvall RC-5B). The supernatants were centrifuged at 100 000 g for 30 min in a Beckman L8-M ultracentrifuge, and proteins were precipitated with (NH4)2SO4 at 50% saturation. Samples were then centrifuged at 100 000 g for 30 min in a Beckman L8-M ultracentrifuge. (NH4)2SO4 precipitates were redissolved in the lysis buffer followed by centrifugation at top speed in a Biofuge (Heraeus, Waltham, MA, USA) centrifuge for 15 min. The recovered supernatants (TE) were adjusted to a protein concentration at 20 mg/ml and were stored at −80 °C for future use.
Subcloning of IRS1
Glutathione-S-transferase (GST)–IRS12–516, GST–IRS1526–859, and GST–IRS1900–1235 were prepared as described previously (Qiao et al. 1999). The DNAs encoding these regions of rat IRS1 were synthesized by PCR. PCR products were isolated, digested with appropriate restriction enzymes, subcloned into pGEX-2T, and transformed into Escherichia coli DH5. Positive clones were grown to an A600 nm of 0·6–0·8 in LB medium containing 0·1 mg/ml ampicillin and induced for 4 h with 0·5 mM isopropyl-β-D-thiogalactopyranoside. GST fusion proteins were purified by a glutathione–sepharose column (Amersham Biosciences) and eluted with glutathione.
In vitro kinase assay
In vitro kinase assays were carried out in a kinase buffer (20 mM HEPES, pH 7·4, 10 mM MgCl2, 1 mM DTT, 1 μg/ml okadaic acid, 2·5 μg/ml microcystein, and 100 μM cold ATP) at 30 °C for 60 min. TE (20 μg protein) was used as a kinase source and the GST–IRS1 fragments (1 μg) were used as substrates, or recombinant MTOR (50 ng) was used as a kinase and recombinant p70S6K1 (125 ng) as a substrate. In some cases, inhibitors, including ERK inhibitor II (5 μM), LY294002 (50 μM), and Y27632 (30 μM), were preincubated with liver extracts or a recombinant kinase for 30 min before adding substrates. Reactions were stopped by adding 6× Laemmli buffer containing 0·5 M DTT followed by boiling for 5 min. Proteins were separated by 10% SDS-PAGE; phosphorylated proteins were detected by immunoblotting.
Immunoblotting
Liver TE was solubilized in 1× Laemmli buffer and boiled for 5 min. Proteins in liver TE and in in vitro kinase reactions were separated by 10% SDS-PAGE. The separated proteins were transferred to a nitrocellulose membrane at 80 V for 90 min or at 100 V for 2 h for proteins less than or greater than 100 kDa in molecular mass respectively. Blots were blocked overnight in 5% nonfat milk in Tris-buffered saline with 0·05% Tween 20 (TBST) followed by incubation with the specified primary antibody/antibodies for 2 h. After immunoblotting, membranes were washed thrice (10 min/wash) with TBST and incubated with the appropriate secondary antibody conjugated with HRP for another hour. After washing off excess secondary antibody, blots were developed with enhanced chemiluminescence (ECL, Amersham Biosciences, Pittsburgh, PA, USA) and exposed using X-ray film. The results were quantified by scanning the films with a StudioStar Scanner (AGFA, Mortsel, Belgium) and Image J software http://rsb.info.nih.gov/ij.
Statistical analysis
ANOVA was performed to compare differences among three or more groups. Student’s t-test was used to analyze the differences between two groups. Statistical significance was set at a P value of <0·05.
Results
CR improves insulin sensitivity in obese Zucker rats
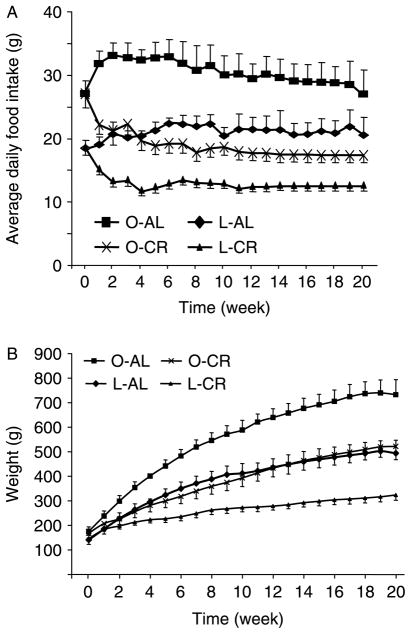
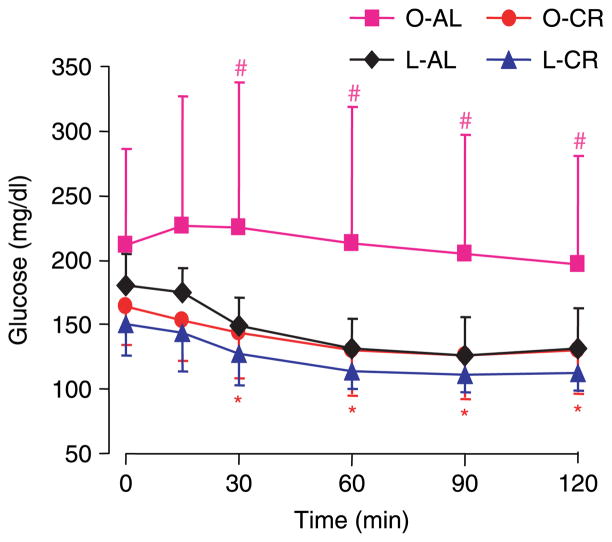
Obese Zucker (fa/fa) rats are a well-established and genetically distinct model for insulin resistance (Zucker & Antoniades 1972). They are obese and display hyperinsulinemia and hyperlipidemia but normo- or mild hyperglycemia (Pederson et al. 1991). Both food intake and body weight were higher in obese animals (1·42- and 1·15-fold respectively) at the beginning of the experiment when compared with their lean littermates (Fig. 1A and B, time 0). During the 20 weeks of 40% CR, all the four groups of rats (Zucker lean and Zucker obese (fa/fa) fed AL (L-AL and O-AL) and under CR (L-CR and O-CR)) continued to grow, although rats undergoing CR had reduced body weight. At the end of the 20 weeks, the body weight of obese rats fed AL was 1·5-fold greater than that of the L-AL controls (Fig. 1B). As expected, CR (Fig. 1A) led to a significant reduction of body weight in both obese (P<0·001) and lean rats (P<0·001) after 2 weeks when compared with their respective AL littermates (Fig. 1B). Interestingly, the growth curve of the CR obese rats could almost be superimposed onto that of the L-AL animals (Fig. 1B, O-CR versus L-AL). Insulin sensitivity was evaluated by an ITT (Fig. 2). Consistent with body weight, the curve of the ITT in restricted obese rats was nearly superimposable upon that of L-AL animals. Although the error bars for ITT data from the O-AL group were higher due, presumably, to variance in the degree of insulin resistance present in individual animals, analysis using ANOVA in the four groups indicated a significant difference (P<0·05 for all time points except for 0 and 15 min). Interestingly, there was no significant difference (P>0·1) when three groups (L-AL, O-CR, and L-CR), excluding O-AL group, were analyzed. The t-tests revealed significant differences between O-AL and O-CR groups as well as between O-AL and L-AL groups (Fig. 2). Sensitivity was moderately enhanced in restricted lean rats as well, but to a much lesser extent compared to their obese counterparts; the difference did not attain statistical significance when analyzed via t-test (P>0·05; Fig. 2). These data confirm that moderate CR over a period of 20 weeks improves insulin sensitivity even in animals genetically predisposed to obesity and insulin resistance.
Figure 1.
Average daily food intake and body weight of obese and lean Zucker rats. (A) Ad libitum (AL) animals were allowed unlimited access to food; food intake was measured every day. Calorie restriction (CR) was initiated (week 1) when animals were 5 weeks old; restricted animals were subjected to a 40% reduction in calories relative to that consumed by their ad libitum littermates. (B) Body masses were recorded daily. Data are means ± S.E.M., n=8 animals/group. O-CR, obese rats-calorie restriction; O-AL, obese rats-ad libitum; L-CR, lean rats-calorie restriction; L-AL, lean rats-ad libitum.
Figure 2.
Insulin tolerance test (ITT) in the four experimental groups following 20 weeks of ad libitum or restricted (CR) feeding. After an overnight fast (16 h), rats were injected i.p. with 1·5 U/kg insulin. Blood glucose was assayed from the tail vein at indicated times. Unpaired Student’s t-test with one-tailed distribution was used to compare the two different animal groups at each time point. *P<0·05 compared between O-CR and O-AL groups. #P<0·05 compared between O-AL and L-AL groups. Data are means ± S.E.M., n=8 animals/group.
Effect of obesity and CR on IRS1 kinase profiles
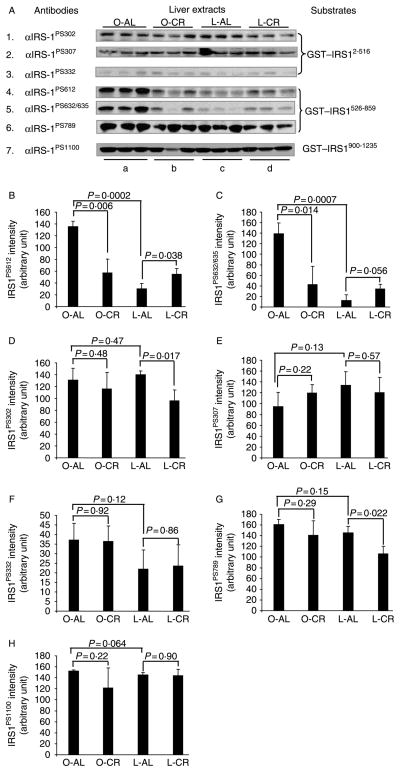
CR-induced improvements in insulin sensitivity in obese Zucker rats provided a good animal model for investigating the association between abnormal activation of IRS1 serine kinases and phosphorylation of IRS1 with respect to insulin sensitivity. To this end, we applied a well-established in vitro kinase assay using GST–IRS1 fragments as substrates and liver TEs as kinase sources (Qiao et al. 1999); in conjunction with western blotting analysis using commercially available phosphospecific antibodies against serine phosphorylation sites in rat IRS1 including S302, S307, S332, S612, S632/635, S789, and S1100, we were able to readily measure the activity of IRS1 kinases toward these phosphorylation sites in liver extracts (Fig. 3A). All these phosphorylation sites are well conserved between mice, rats, and human. Kinase activity toward S612 and S632/635 of IRS1 was low in normal rats, but was significantly higher in the liver of obese rats (Fig. 3A, panels 4 and 5, B and C), indicating an association between obesity and the activity of kinases involved in the phosphorylation of these sites. More interestingly, the phosphorylation of these sites returned to the same levels as seen in lean controls after 20 weeks of CR (Fig. 3A, panels 4 and 5, lane a versus lanes b and c, B and C). Statistical analysis using ANOVA showed a significant difference within all the four groups of animals (P<0·001 for both S612 and S632/635), and showed no significant difference if only three groups of animals were compared (L-AL, O-CR, and L-CR; P>0·1), excluding O-AL group. This suggests that the effect of CR on the activity of the IRS1 kinases that phosphorylate the aforementioned sites is specific for those kinases whose activity is influenced by obesity.
Figure 3.
Immunoblot analysis of serine phosphorylation in IRS1 by in vitro kinase assay. (A) After 20 weeks of ad libitum (AL) or calorie-restricted (CR) feeding, livers were excised and extracts were prepared for use as kinase sources (20 μg/lane); GST–IRS12–516, GST–IRS1526–859, and GST–IRS1900–1235 (1 μg/lane) were used as substrates. Specific phospho-IRS1 antibodies were used to detect serine phosphorylation of GST–IRS1 by western blot analysis. (B–H) Density of phosphoproteins in A was quantified and analyzed with Image J. P values were calculated using two-tailed Student’s t-test. Data are means ± S.E.M., n=3 animals/group.
Although evaluation by t-test showed a significant decrease of kinase activities toward S302 and S789 of IRS1 in lean Zucker rats, CR had little effect on kinase activity toward S302, S307, S332, S789, or S1100 in obese Zucker rats (Fig. 3A and D–H).
Obesity and CR modulate ERK and p70S6K activities
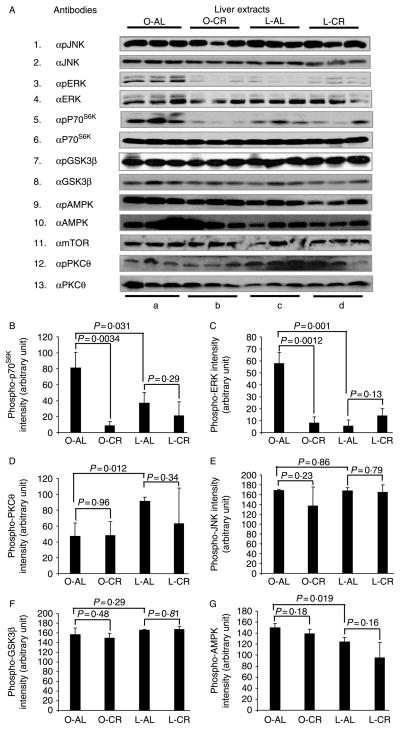
S612 and S632/635 in IRS1 are the sites for a number of serine kinases including ERK (DeFea & Roth 1997) and MTOR/p70S6K (Ozes et al. 2001). To validate the involvement of ERK and MTOR/p70S6K in the phosphorylation of these sites in liver TEs, we directly examined their activation by measuring their phosphorylation status with anti-phosphospecific antibodies. Consistent with the phosphorylation of S612 and S632/635 as shown in Fig. 3, phosphorylation of ERK and p70S6K (indicative of activation) in the liver of insulin-resistant (obese) rats was increased versus insulin-sensitive (lean) controls, and returned to levels similar to those observed in lean rats after 20 weeks of CR (Fig. 4A, panels 3–6, column a versus columns b and c, B, and C). Statistical analysis using ANOVA showed a significant difference within all the four groups of animals (P<0·01 for p70S6K and P<0·001 for ERK); when only three groups of animals were compared (L-AL, O-CR, and L-CR), excluding O-AL group, differences failed to attain statistical significance (P>0·1). CR had little effect on the phosphorylation of p70S6K and ERK in lean rats (Fig. 4A, panels 3–6, column c versus column d, B, and C). The quantity of tested kinases (i.e. kinase protein levels) was not affected by dietary treatment. The tight direct correlation between the phosphorylation (and therefore activation) of ERK and p70S6K with the phosphorylation of IRS1S612 and IRS1S632/635 implicates ERK and MTOR/p70S6K as the kinases responsible for the phosphorylation of these sites in the liver, with augmented activity observed in obesity-induced insulin resistance and diminished activity seen upon improved insulin sensitivity, secondary to CR.
Figure 4.
Immunoblot analysis of protein kinases in the liver of the four experimental groups. (A) After 20 weeks of ad libitum (AL) or calorie-restricted (CR) feeding, liver tissue extracts (20 μg/lane) from obese and lean rats were analyzed by western blotting. Phospho-MAPK antibody recognizes endogenous levels of p44 and p42 MAP kinase (Erk1 and Erk2) when phosphorylated either individually or dually at T202 and Y204 of ERK1 (T185 and Y187 of ERK2). Phospho-p70S6K antibody detects endogenous levels of p70S6K only when phosphorylated at T421/S424. Phospho-SAPK/JNK antibody detects endogenous levels of p46 and p54 SAPK/JNK dually phosphorylated at T183 and Y185. Phospho-GSK3B antibody detects endogenous levels of GSK3B phosphorylated at S9. Phospho-AMPKα antibody detects endogenous AMPKα phosphorylated at T172. (B–G) Density of phosphoproteins in A was quantified and analyzed with Image J. P values were calculated using two-tailed Student’s t-test. Data are means ± S.E.M., n=3 animals/group.
Other protein kinases including GSK3B, JNK, PKCθ, and AMPK are also known to phosphorylate rat IRS1 at S302, S307, S332, S612, S632/635, S789, and S1100 (Aguirre et al. 2000, Jakobsen et al. 2001, Li et al. 2004, Liberman & Eldar-Finkelman 2005). However, when the activity and/or protein level of the aforementioned kinases were examined in liver TEs, we did not observe any appreciable effect of CR on these kinases in obese animals (Fig. 4A, panels 1–2 and 7–13, lane b versus lane a; and Fig. 4D–G, O-AL versus O-CR). Interestingly, phosphorylation of PKCθ was significantly lower and the phosphorylation of AMPK was slightly higher in obese rats when compared with lean controls (Fig. 4A, panels 9, 10, 12, and 13, lane c versus lane a; and Fig. 4D and G, L-AL versus O-AL). However, CR had no effect on their phosphorylation levels in obese Zucker rats, suggesting that their activity was not associated with the improved insulin sensitivity observed following CR. JNK was given special attention based upon extensive data relating its activation to insulin resistance, presumably due to phosphorylation of IRS1307 (Aguirre et al. 2000, 2002, Hirosumi et al. 2002, Hilder et al. 2003). However, neither the protein level nor the phosphorylation status of JNK showed any change in obese rats when compared with lean littermates (Fig. 4A, panels 1–2, and E). This is consistent with the fact that we were unable to detect significant changes in IRS1S307 phosphorylation (Fig. 3A, panel 2, and E).
ERK, not MTOR/p70S6K, mediates the phosphorylation of IRS1 in the liver extracts
S612 in IRS1 has been identified as the phosphorylation site for ERK (DeFea & Roth 1997), and S632/635 is a target for multiple serine kinases including MTOR (upstream kinase of p70S6K), ERK, p70S6K, and ROCK (Ozes et al. 2001, Bouzakri et al. 2003, Gual et al. 2003, Furukawa et al. 2005). To determine the involvement of one kinase or both kinases in the changes in the phosphorylation of IRS1 (as shown in Fig. 3) in the livers from obese rats following CR, relatively selective inhibitors for these protein kinases were applied in the in vitro kinase assay. The ERK inhibitor II is a relatively specific inhibitor for ERK (Kelemen et al. 2002). It completely prevented the phosphorylation of both IRS1S612 and IRS1S632/635 induced by obesity (Fig. 5A, panels 2 and 3, lanes e–h versus lanes a–d), strongly suggesting that these are the phosphorylation sites for ERK. This effect was relatively specific in preventing phosphorylation of S612 and S632/635 since ERK inhibitor II had no effect on S302 (Fig. 5A, panel 1). With respect to MTOR, while rapamycin is a potent inhibitor in vivo, it does not inhibit MTOR activity in vitro (Oshiro et al. 2004). However, because MTOR belongs to the PI3K-related superfamily (Fingar & Blenis 2004), it can be inhibited in vitro by LY294002 in a cell-free system (Fig. 5B, lane c versus lane b; McMahon et al. 2005). Interestingly, neither phosphorylation of IRS1S612 nor that of IRS1S632/635 was affected by LY294002 (Fig. 5A, panels 2 and 3, lanes i–l versus lanes a–d), suggesting that MTOR/p70S6K may not be the kinase for these sites. Y27632 is a relatively specific inhibitor for ROCK, and it is able to prevent the phosphorylation of IRS1 at S632/635 by ROCK (Furukawa et al. 2005). Y27632 failed to inhibit the phosphorylation of S632/635 by liver extracts (Fig. 5A, panel 3, lanes m–p versus lanes a–d), suggesting that ROCK is not the kinase responsible for the phosphorylation of the aforementioned sites.
Figure 5.
Effects of various inhibitors on phosphorylation of IRS1 at S302, S612, and S632/635. (A) Liver extracts were made from rats after 20 weeks of ad libitum (AL) or calorie-restricted (CR) feeding. In vitro kinase assays were carried out using liver extracts (20 μg/lane) as kinase sources and GST–IRS12–516 (panel 1) or GST–IRS1526–859 (panels 2–3) (1 μg/lane) as substrates. Liver extracts were preincubated with various inhibitors for 30 min before the kinase assays were carried out. The concentrations of the inhibitors used in the in vitro kinase assay were 5 μM for ERK inhibitor II, 50 μM for LY294002, and 30 μM for Y27632. Phosphorylation of IRS1 at S302, S612, and S632/635 was determined by western blot analysis using specific phospho-IRS1 antibodies. (B) Inhibition of MTOR activity by LY294002 in vitro. In vitro kinase assays were carried out using recombinant MTOR (50 ng/assay, from HumanZyme) as a kinase and recombinant p70S6K1 (125 ng/assay, from HumanZyme) as a substrate. The reaction without ATP was used as a negative control. LY294002 (50 μM) was preincubated with MTOR for 30 min before ATP was added to initiate the reaction. Phosphorylation of p70S6K1 at T421/S424 was determined by western blot analysis. Density of p70S6K bands was quantified with Image J. Data are representative of at least two separate experiments.
Discussion
There is compelling evidence that CR and the consequent weight loss greatly improve glucose metabolism by augmenting insulin’s action. However, the molecular mechanism underlying the enhancement of insulin sensitivity by CR is unclear. Serine/threonine phosphorylation of IRS1 plays an important role in modulating insulin sensitivity, and it is thought to be the central molecular mechanism in insulin resistance (Zick 2004, Gual et al. 2005, White 2006). In the current study, we systematically examined the IRS protein kinase profiles in insulin-resistant animals (obese Zucker rats) subjected to CR. Using liver extracts as a kinase source for in vitro kinase assays, we have identified activation of ERK and MTOR/p70S6K and the phosphorylation of IRS1 at S612 and S632/635 to be tightly associated with the whole-body insulin resistance. We also found that S612 and S632/635 are phosphorylated by ERK and not by MTOR since an ERK inhibitor completely blocked the phosphorylation of both sites, while an inhibitor for MTOR did not. Our data imply that ERK is likely the key modulator that is involved in the development of insulin resistance, whereas the activation of other IRS protein kinases may be a subsequent event that contributes to the worsening of insulin resistance.
This study is focused on the liver because it plays a critical role in maintaining glucose homeostasis (Klover & Mooney 2004), and is an important contributor to the hyperglycemia of type 2 diabetes (Barthel & Schmoll 2003, Savage et al. 2007). The critical role of liver insulin’s action in maintaining glucose homeostasis was demonstrated in mice lacking the insulin receptor gene in the liver. These mice exhibit dramatic insulin resistance, which is evidenced by a failure of insulin to regulate hepatic glucose production and gene expression (Michael et al. 2000). Thus, defects in hepatic insulin signaling may precipitate systematic insulin resistance. Identification of key factors associated with insulin sensitivity in the liver may help us to understand the molecular mechanism of insulin resistance.
Many serine/threonine kinases have been recently identified as important molecules in the development of insulin resistance via phosphorylation of IRS proteins (Gual et al. 2005). However, due to the complexity of insulin resistance, changes in the activity of many serine/threonine kinases may be the consequence, rather than the cause, of resistance. A unique aspect of this study was the use of two criteria to establish association between changes in the activity of IRS1 serine/threonine kinases and insulin resistance: a) activity must increase during resistance, and b) activity must subside (to normal levels) following re-establishment of sensitivity. Much to our surprise, in the liver, among a handful of potential IRS1 serine/threonine kinases, the activity of only two, ERK and p70S6K, demonstrated tight association with whole-body insulin resistance. Our data provide compelling evidence that the effect of CR on the activity of IRS1 kinases is specific to ERK and p70S6K; our results further highlight that not all serine kinases are equally important in phosphorylating IRS1 and suppressing insulin signaling. We believe that this is the first study that compares the serine/threonine phosphorylation of IRS1 and associated kinase activity in insulin-resistant animals before and after the application of a treatment to improve insulin’s action, while demonstrating that CR can reverse the altered activity of specific protein kinases.
It has been known that S612 is the phosphorylation site for ERK, and S632/S635 for MTOR (upstream kinase for p70S6K) and ROCK (DeFea & Roth 1997, Ozes et al. 2001, Bouzakri et al. 2003, Gual et al. 2003, Furukawa et al. 2005). We found that not only S612 but also S632/S635 were completely suppressed by ERK inhibitor. In fact, based on in vitro kinase assay data, we observed that S635 was actually the strongest phosphorylation site in IRS1 for recombinant ERK when compared with S612 and S632 (unpublished data). In contrast, inhibition of MTOR and ROCK activities had no effect on the phosphorylation of S632/S635 of IRS1. These data further support that both S612 and S632/635 are most likely the ERK phosphorylation sites in liver, at least in our experimental system, and imply that ERK may have a more important role in modulating insulin sensitivity than originally expected.
Others have shown that JNK can phosphorylate IRS1 at S307, leading to attenuation of insulin signaling (Aguirre et al. 2000), and this has been proposed to be an important molecular mechanism with respect to obesity-induced insulin resistance (Aguirre et al. 2000, 2002, Hilder et al. 2003). In our study, phosphorylation of IRS1 at S307 measured by in vitro kinase assay did not show any significant change between normal and obese/insulin-resistant rats; CR also had no effect on the phosphorylation of S307. Consistent with these results, neither JNK protein level nor phosphorylation was associated with the degree of insulin sensitivity. It is possible that TEs may contain inhibitors that suppressed JNK kinase activity. However, we have fractionated lean and obese TEs using FPLC via anion-exchanged chromatography and tested each fraction for phosphorylation of IRS1 fusion proteins. None of the fractions showed increased activity toward S307, although increased S612 and S632/S635 kinase activities were detected in the fractions (unpublished data), indicating that the lack of S307 phosphorylation is not due to the presence of inhibitors in the crude extracts. The sum of these findings leads us to conclude that JNK, for yet unknown reasons, did not play a significant role in modulating insulin’s action in our experimental system. Nevertheless, we could not exclude the possibility that our in vitro kinase assay failed to detect the phosphorylation of S307 by GST fusion protein of IRS1.
S302 in IRS1 is another interesting site, the phosphorylation of which can lead to either enhancement or attenuation of insulin’s action (Giraud et al. 2004, Werner et al. 2004), presumably via different serine kinases. The kinases that phosphorylate S302 are unknown; thus far, JNK and IKK have been reported to phosphorylate this site. CR caused a significant reduction in kinase activity toward S302 in lean rats; however, this was not seen in obese Zucker rats. The answer for this phenomenon is unknown. We speculate that the combination of opposing kinase activities may lead to this unusual activity pattern.
GSK3B is a serine kinase that is negatively regulated by insulin (Richter et al. 1988). In addition to its role in the glycogen synthetic pathway, GSK3B plays roles in many other biological signaling networks including that of Wnt (crucial in development) and NFκB (a key mediator of the inflammatory response and listed as NFKB in the MGI Database; Frame & Cohen 2001, Lee & Kim 2007). GSK3B is also thought to be involved in insulin resistance. In type 2 diabetics, GSK3B activity is elevated twofold in both basal muscle and insulin-stimulated muscle (Nikoulina et al. 2000). Additionally, inhibition of GSK3B improves insulin’s action and glucose metabolism in human smooth muscle (Nikoulina et al. 2002). GSK3B has been shown to directly phosphorylate IRS1 in vitro and in vivo at serine332 and impair insulin signaling (Eldar-Finkelman & Krebs 1997, Liberman & Eldar-Finkelman 2005). In our system, we detected slightly reduced phosphorylation of GSK3B in obese rats, indicating mild activation; these data, however, did not reach statistical significance. Consistent with GSK3B activity, obese rats showed mild increased phosphorylation of S332, but again, this did not attain statistical significance. However, CR had no effect on phosphorylation of GSK3B and IRS1 at S332, suggesting a lack of association of GSK3B activity with insulin resistance based on our two-criterion approach. Thus, this enzyme may not have a critical role in controlling insulin sensitivity in obese Zucker rats or in modulating insulin signaling during CR.
Although our in vitro kinase assay provides an easy and reproducible way to examine the IRS protein kinases in TEs because of a well-controlled condition, it does have its limitations. First, due to the large sample numbers, all the in vitro kinase assays were carried out in the same condition. Although the condition used in this study generally works for many serine/threonine kinases, a specific condition is required for some kinases. Second, although phosphorylation sites for many serine kinases are determined by few adjacent amino acids, some serine kinases do require binding to their substrates before phosphorylation can take place. Therefore, we could not rule out the possibility that negative results such as S307, S302, and S332 may simply suggest that these sites are not phosphorylated in our condition. Future study with full-length IRS1 proteins in a kinase-specific reaction buffer should verify these results.
In summary, our results show that CR is effective in reversing insulin resistance, and that this reversal is tightly associated with a reduction in liver ERK activity, and possibly MTOR/p70S6K as well. We provide further evidence that ERK is likely the primary kinase responsible, in liver, for the phosphorylation of IRS1 (and possibly IRS-2 as well, since these phosphorylation sites are well conserved between the two isoforms) in obesity-induced insulin resistance. Given the central role of the liver in mediating glucose metabolism, combined with the fact that loss of insulin-regulated glucose output is a principle factor driving chronic hyperglycemia in the metabolic syndrome and type 2 diabetes, restraining ERK activity may have important implications for future therapies.
Acknowledgments
Funding
This research is partially supported by an NIH grant R01 DK062336 (XJS).
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) Journal of Biological Chemistry. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. Journal of Biological Chemistry. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. American Journal of Physiology. Endocrinology and Metabolism. 2003;285:E685–E692. doi: 10.1152/ajpendo.00253.2003. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. Journal of Clinical Investigation. 1998;101:1353–1361. doi: 10.1172/JCI485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, Riou JP, Laville M, Le Marchand-Brustel Y, Tanti JF, Vidal H. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes. 2003;52:1319–1325. doi: 10.2337/diabetes.52.6.1319. [DOI] [PubMed] [Google Scholar]
- Ciaraldi TP, Oh DK, Christiansen L, Nikoulina SE, Kong AP, Baxi S, Mudaliar S, Henry RR. Tissue-specific expression and regulation of GSK-3 in human skeletal muscle and adipose tissue. American Journal of Physiology. Endocrinology and Metabolism. 2006;291:E891–E898. doi: 10.1152/ajpendo.00176.2006. [DOI] [PubMed] [Google Scholar]
- DeFea K, Roth RA. Protein kinase C modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry. 1997;36:12939–12947. doi: 10.1021/bi971157f. [DOI] [PubMed] [Google Scholar]
- Eldar-Finkelman H, Ilouz R. Challenges and opportunities with glycogen synthase kinase-3 inhibitors for insulin resistance and type 2 diabetes treatment. Expert Opinion on Investigational Drugs. 2003;12:1511–1519. doi: 10.1517/13543784.12.9.1511. [DOI] [PubMed] [Google Scholar]
- Eldar-Finkelman H, Krebs EG. Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. PNAS. 1997;94:9660–9664. doi: 10.1073/pnas.94.18.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochemical Journal. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, et al. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metabolism. 2005;2:119–129. doi: 10.1016/j.cmet.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Gao Z, Zuberi A, Quon MJ, Dong Z, Ye J. Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. Journal of Biological Chemistry. 2003;278:24944–24950. doi: 10.1074/jbc.M300423200. [DOI] [PubMed] [Google Scholar]
- Giraud J, Leshan R, Lee YH, White MF. Nutrient-dependent and insulin-stimulated phosphorylation of insulin receptor substrate-1 on serine 302 correlates with increased insulin signaling. Journal of Biological Chemistry. 2004;279:3447–3454. doi: 10.1074/jbc.M308631200. [DOI] [PubMed] [Google Scholar]
- Gual P, Gremeaux T, Gonzalez T, Le Marchand-Brustel Y, Tanti JF. MAP kinases TOR, mediate insulin-induced phosphorylation of insulin receptor substrate-1 on serine residues 307, 612 and 632. Diabetologia. 2003;46:1532–1542. doi: 10.1007/s00125-003-1223-4. [DOI] [PubMed] [Google Scholar]
- Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. Journal of Cell Biology. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilder TL, Tou JC, Grindeland RE, Wade CE, Graves LM. Phosphorylation of insulin receptor substrate-1 serine 307 correlates with JNK activity in atrophic skeletal muscle. FEBS Letters. 2003;553:63–67. doi: 10.1016/s0014-5793(03)00972-4. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Horike N, Takemori H, Katoh Y, Doi J, Min L, Asano T, Sun XJ, Yamamoto H, Kasayama S, Muraoka M, et al. Adipose-specific expression, phosphorylation of Ser794 in insulin receptor substrate-1, and activation in diabetic animals of salt-inducible kinase-2. Journal of Biological Chemistry. 2003;278:18440–18447. doi: 10.1074/jbc.M211770200. [DOI] [PubMed] [Google Scholar]
- Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. Journal of Biological Chemistry. 2001;276:46912–46916. doi: 10.1074/jbc.C100483200. [DOI] [PubMed] [Google Scholar]
- Kelemen BR, Hsiao K, Goueli SA. Selective in vivo inhibition of mitogen-activated protein kinase activation using cell-permeable peptides. Journal of Biological Chemistry. 2002;277:8741–8748. doi: 10.1074/jbc.M108459200. [DOI] [PubMed] [Google Scholar]
- Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- Klover PJ, Mooney RA. Hepatocytes: critical for glucose homeostasis. International Journal of Biochemistry & Cell Biology. 2004;36:753–758. doi: 10.1016/j.biocel.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim MS. The role of GSK3 in glucose homeostasis and the development of insulin resistance. Diabetes Research and Clinical Practice. 2007;77:S49–S57. doi: 10.1016/j.diabres.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Li Y, Soos TJ, Li X, Wu J, Degennaro M, Sun X, Littman DR, Birnbaum MJ, Polakiewicz RD. Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101) Journal of Biological Chemistry. 2004;279:45304–45307. doi: 10.1074/jbc.C400186200. [DOI] [PubMed] [Google Scholar]
- Liberman Z, Eldar-Finkelman H. Serine 332 phosphorylation of insulin receptor substrate-1 by glycogen synthase kinase-3 attenuates insulin signaling. Journal of Biological Chemistry. 2005;280:4422–4428. doi: 10.1074/jbc.M410610200. [DOI] [PubMed] [Google Scholar]
- McCurdy CE, Davidson RT, Cartee GD. Brief calorie restriction increases Akt2 phosphorylation in insulin-stimulated rat skeletal muscle. American Journal of Physiology. Endocrinology and Metabolism. 2003;285:E693–E700. doi: 10.1152/ajpendo.00224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy CE, Davidson RT, Cartee GD. Calorie restriction increases the ratio of phosphatidylinositol 3-kinase catalytic to regulatory subunits in rat skeletal muscle. American Journal of Physiology. Endocrinology and Metabolism. 2005;288:E996–E1001. doi: 10.1152/ajpendo.00566.2004. [DOI] [PubMed] [Google Scholar]
- McMahon LP, Yue W, Santen RJ, Lawrence JC., Jr Farnesylthiosalicylic acid inhibits mammalian target of rapamycin (mTOR) activity both in cells and in vitro by promoting dissociation of the mTOR–raptor complex. Molecular Endocrinology. 2005;19:175–183. doi: 10.1210/me.2004-0305. [DOI] [PubMed] [Google Scholar]
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Molecular Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- Nikoulina SE, Ciaraldi TP, Mudaliar S, Mohideen P, Carter L, Henry RR. Potential role of glycogen synthase kinase-3 in skeletal muscle insulin resistance of type 2 diabetes. Diabetes. 2000;49:263–271. doi: 10.2337/diabetes.49.2.263. [DOI] [PubMed] [Google Scholar]
- Nikoulina SE, Ciaraldi TP, Mudaliar S, Carter L, Johnson K, Henry RR. Inhibition of glycogen synthase kinase 3 improves insulin action and glucose metabolism in human skeletal muscle. Diabetes. 2002;51:2190–2198. doi: 10.2337/diabetes.51.7.2190. [DOI] [PubMed] [Google Scholar]
- Oshiro N, Yoshino K, Hidayat S, Tokunaga C, Hara K, Eguchi S, Avruch J, Yonezawa K. Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes to Cells. 2004;9:359–366. doi: 10.1111/j.1356-9597.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, Donner DB. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. PNAS. 2001;98:4640–4645. doi: 10.1073/pnas.051042298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson RA, Campos RV, Buchan AM, Chisholm CB, Russell JC, Brown JC. Comparison of the enteroinsular axis in two strains of obese rat, the fatty Zucker and the JCR:LA-corpulent. International Journal of Obesity. 1991;15:461–470. [PubMed] [Google Scholar]
- Qiao LY, Goldberg JL, Russell JC, Sun XJ. Identification of enhanced serine kinase activity in insulin resistance. Journal of Biological Chemistry. 1999;274:10625–10632. doi: 10.1074/jbc.274.15.10625. [DOI] [PubMed] [Google Scholar]
- Qiao LY, Zhande R, Jetton TL, Zhou G, Sun XJ. In vivo phosphorylation of insulin receptor substrate 1 at serine 789 by a novel serine kinase in insulin-resistant rodents. Journal of Biological Chemistry. 2002;277:26530–26539. doi: 10.1074/jbc.M201494200. [DOI] [PubMed] [Google Scholar]
- Richter EA, Hansen BF, Hansen SA. Glucose-induced insulin resistance of skeletal-muscle glucose transport and uptake. Biochemical Journal. 1988;252:733–737. doi: 10.1042/bj2520733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiological Reviews. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Miralpeix M, Myers MG, Jr, Glasheen EM, Backer JM, Kahn CR, White MF. The expression and function of IRS-1 in insulin signal transmission. Journal of Biological Chemistry. 1992;267:22662–22672. [PubMed] [Google Scholar]
- Tremblay F, Brule S, Hee US, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, et al. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. PNAS. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugochukwu NH, Figgers CL. Attenuation of plasma dyslipidemia and oxidative damage by dietary caloric restriction in streptozotocin-induced diabetic rats. Chemico-Biological Interactions. 2007;169:32–41. doi: 10.1016/j.cbi.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Werner ED, Lee J, Hansen L, Yuan M, Shoelson SE. Insulin resistance due to phosphorylation of insulin receptor substrate-1 at serine 302. Journal of Biological Chemistry. 2004;279:35298–35305. doi: 10.1074/jbc.M405203200. [DOI] [PubMed] [Google Scholar]
- White MF. Regulating insulin signaling and b-cell function through IRS proteins. Canadian Journal of Physiology and Pharmacology. 2006;84:725–737. doi: 10.1139/y06-008. [DOI] [PubMed] [Google Scholar]
- Zick Y. Uncoupling insulin signalling by serine/threonine phosphorylation: a molecular basis for insulin resistance. Biochemical Society Transactions. 2004;32:812–816. doi: 10.1042/BST0320812. [DOI] [PubMed] [Google Scholar]
- Zucker LM, Antoniades HN. Insulin and obesity in the Zucker genetically obese rat “fatty”. Endocrinology. 1972;90:1320–1330. doi: 10.1210/endo-90-5-1320. [DOI] [PubMed] [Google Scholar]