Abstract
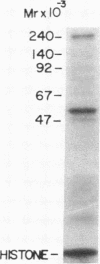
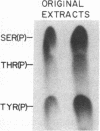
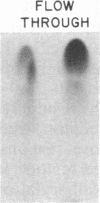
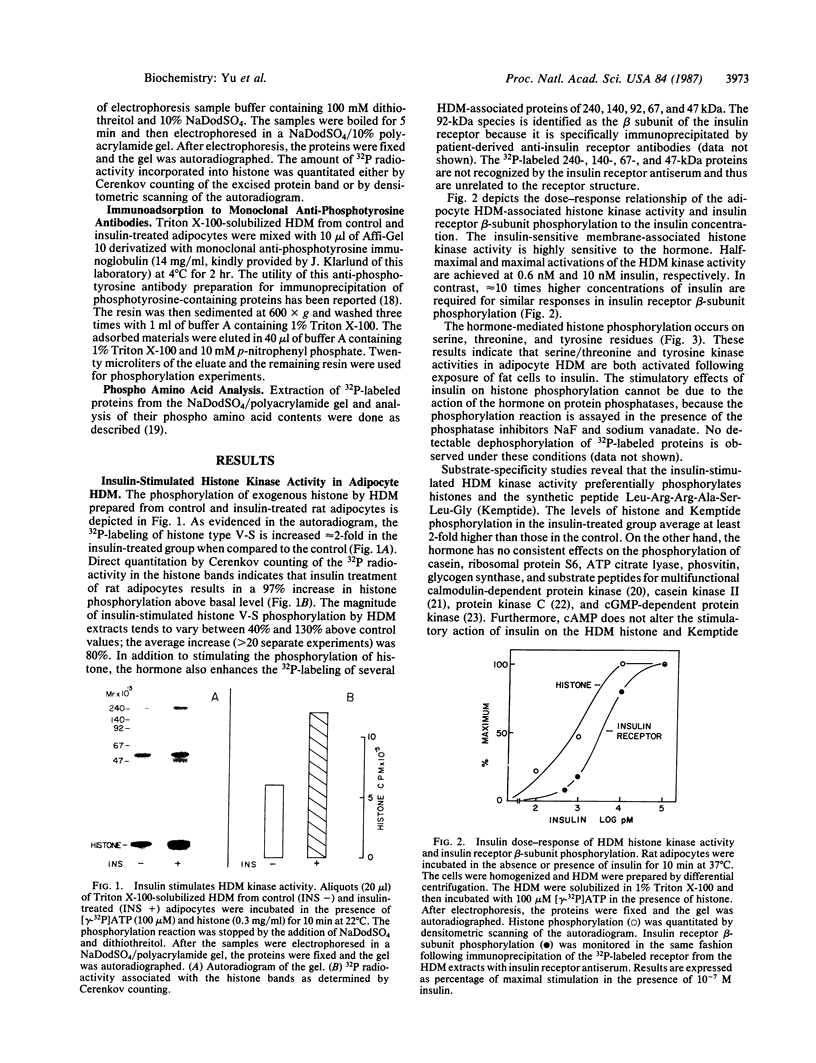
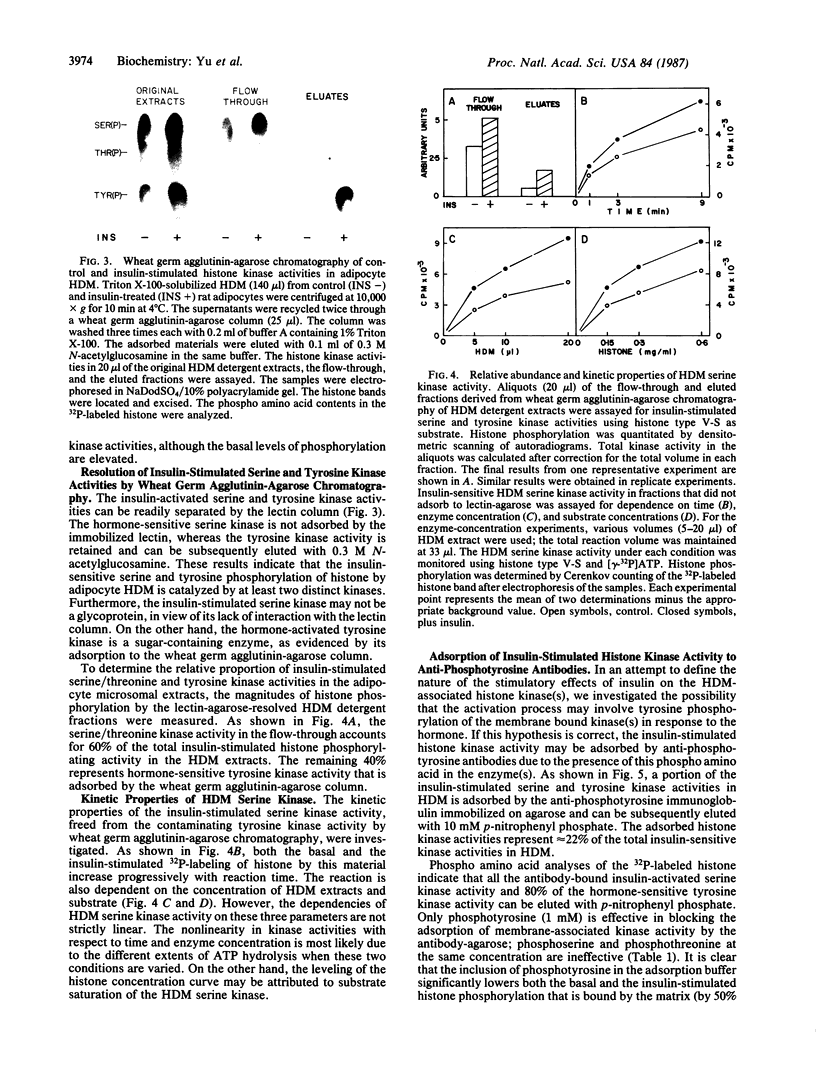
Triton X-100-solubilized high-density microsomes from insulin-treated rat adipocytes exhibit a marked increase in serine/threonine and tyrosine kinase activities toward exogenous histone when compared to controls. The insulin-dependent activation of microsomal histone kinase activities occurs within the physiological range of hormone concentrations (ED50 = 0.6 nM). The hormone-enhanced histone phosphorylation by the high-density microsomes appears to be catalyzed by two distinct kinases, based on their differential interaction with wheat germ agglutinin-agarose. The insulin-sensitive serine/threonine kinase is not retained by The insulin-sensitive serine/threonine kinase is not retained by the lectin column, whereas the tyrosine kinase appears to be a glycoprotein as evidenced by its adsorption to the immobilized lectin. The insulin-stimulated serine/threonine kinase exhibits preferential phosphorylation of histone and Kemptide (synthetic Leu-Arg-Arg-Ala-Ser-Leu-Gly) compared to a number of other peptide substrates. The substrate specificity of this serine/threonine kinase shows that it is distinct from the kinases that phosphorylate ribosomal protein S6, casein, phosvitin, ATP citrate lyase, and glycogen synthase and from multifunctional calmodulin-dependent, cAMP- and cGMP-dependent, and Ca2+/phospholipid-dependent protein kinases. Furthermore, 22% of the insulin-sensitive serine/threonine kinase activity can be adsorbed by monoclonal anti-phosphotyrosine antibodies immobilized on agarose. Its adsorption is specifically inhibited by excess free phosphotyrosine but not phosphoserine or phosphothreonine. The data suggest that this insulin-stimulated serine/threonine kinase in adipocyte high-density microsomes is tyrosine-phosphorylated, consistent with the hypothesis that the stimulatory action of insulin on this kinase may be mediated by tyrosine phosphorylation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cobb M. H., Rosen O. M. Description of a protein kinase derived from insulin-treated 3T3-L1 cells that catalyzes the phosphorylation of ribosomal protein S6 and casein. J Biol Chem. 1983 Oct 25;258(20):12472–12481. [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Glass D. B., Krebs E. G. Phosphorylation by guanosine 3':5'-monophosphate-dependent protein kinase of synthetic peptide analogs of a site phosphorylated in histone H2B. J Biol Chem. 1982 Feb 10;257(3):1196–1200. [PubMed] [Google Scholar]
- Hunter T., Ling N., Cooper J. A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984 Oct 4;311(5985):480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Fujita-Yamaguchi Y., Blithe D. L., Kahn C. R. Tyrosine-specific protein kinase activity is associated with the purified insulin receptor. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2137–2141. doi: 10.1073/pnas.80.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blith D. L., Karlsson F. A., Häring H. U., Kahn C. R. Insulin stimulation of phosphorylation of the beta subunit of the insulin receptor. Formation of both phosphoserine and phosphotyrosine. J Biol Chem. 1982 Sep 10;257(17):9891–9894. [PubMed] [Google Scholar]
- Klarlund J. K. Transformation of cells by an inhibitor of phosphatases acting on phosphotyrosine in proteins. Cell. 1985 Jul;41(3):707–717. doi: 10.1016/s0092-8674(85)80051-9. [DOI] [PubMed] [Google Scholar]
- Klein H. H., Freidenberg G. R., Kladde M., Olefsky J. M. Insulin activation of insulin receptor tyrosine kinase in intact rat adipocytes. An in vitro system to measure histone kinase activity of insulin receptors activated in vivo. J Biol Chem. 1986 Apr 5;261(10):4691–4697. [PubMed] [Google Scholar]
- Kuenzel E. A., Krebs E. G. A synthetic peptide substrate specific for casein kinase II. Proc Natl Acad Sci U S A. 1985 Feb;82(3):737–741. doi: 10.1073/pnas.82.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J. L., Pike L. J., Freidenberg G. R., Cordera R., Stith B. J., Olefsky J. M., Krebs E. G. Increased phosphorylation of ribosomal protein S6 following microinjection of insulin receptor-kinase into Xenopus oocytes. Nature. 1986 Apr 3;320(6061):459–461. doi: 10.1038/320459a0. [DOI] [PubMed] [Google Scholar]
- McKeel D. W., Jarett L. Preparation and characterization of a plasma membrane fraction from isolated fat cells. J Cell Biol. 1970 Feb;44(2):417–432. doi: 10.1083/jcb.44.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., Ho L., Korn L. J., Roth R. A. Insulin action is blocked by a monoclonal antibody that inhibits the insulin receptor kinase. Proc Natl Acad Sci U S A. 1986 Jan;83(2):328–332. doi: 10.1073/pnas.83.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak-Hofer I., Thomas G. Epidermal growth factor-mediated activation of an S6 kinase in Swiss mouse 3T3 cells. J Biol Chem. 1985 Aug 25;260(18):10314–10319. [PubMed] [Google Scholar]
- Oka Y., Mottola C., Oppenheimer C. L., Czech M. P. Insulin activates the appearance of insulin-like growth factor II receptors on the adipocyte cell surface. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4028–4032. doi: 10.1073/pnas.81.13.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. B., Woodgett J. R., Cohen P., Kemp B. E. Substrate specificity of a multifunctional calmodulin-dependent protein kinase. J Biol Chem. 1985 Nov 25;260(27):14471–14476. [PubMed] [Google Scholar]
- Perisic O., Traugh J. A. Protease-activated kinase II as the potential mediator of insulin-stimulated phosphorylation of ribosomal protein S6. J Biol Chem. 1983 Aug 25;258(16):9589–9592. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Smith C. J., Rubin C. S., Rosen O. M. Insulin-treated 3T3-L1 adipocytes and cell-free extracts derived from them incorporate 32P into ribosomal protein S6. Proc Natl Acad Sci U S A. 1980 May;77(5):2641–2645. doi: 10.1073/pnas.77.5.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarini D., Heinrich J., Rosen O. M. Activation of S6 kinase activity in 3T3-L1 cells by insulin and phorbol ester. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4369–4373. doi: 10.1073/pnas.82.13.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S., White M. F., Lauris V., Kahn C. R. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Martin-Pérez J., Siegmann M., Otto A. M. The effect of serum, EGF, PGF2 alpha and insulin on S6 phosphorylation and the initiation of protein and DNA synthesis. Cell. 1982 Aug;30(1):235–242. doi: 10.1016/0092-8674(82)90029-0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of insulin receptor beta subunit activates the receptor tyrosine kinase in intact H-35 hepatoma cells. J Biol Chem. 1986 Apr 5;261(10):4715–4722. [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]