Abstract
Understanding the function of Langerhans cells (LC) in vivo has been complicated by conflicting results from LC-deficient mice. HuLangerin-DTA mice constitutively lack LC and develop exaggerated contact-hypersensitivity (CHS) responses. MuLangerin-DTR mice allow for the inducible elimination of LC and Langerin+ dermal DC (dDC) after administration of diphtheria toxin (DT) which results in reduced CHS. When Langerin+ dDC have partially repopulated the skin but LC are still absent, CHS returns to normal. Thus, LC appear to be suppressive in huLangerin-DTA and redundant in muLangerin-DTR. To determine whether inducible vs. constitutive LC ablation explains these results, we engineered huLangerin-DTR mice in which DT ablates LC without affecting Langerin+ dDC. The inducible ablation of LC in huLangerin-DTR mice resulted in increased CHS. Thus, LC-mediated suppression does not require their absence during ontogeny or during the steady state and is consistent with a model in which LC actively suppress antigen specific CHS responses.
Introduction
Langerhans cells (LC) reside in the epidermis of the skin and are considered to be the archetypal tissue-resident DC. In the steady-state, they acquire local antigen. Inflammation induces their migration from the skin to T cell areas in the regional LN where they present processed antigen to T cells (1, 2). In the classic model of LC function, LC were thought to initiate adaptive immune responses. However, this view has been recently challenged based on in vivo studies using LC-deficient mice (3).
MuLangerin-DTR (muDTR) mice were generated by two independent groups using “knock-in” technology to introduce the primate receptor for diphtheria toxin (DT) into the endogenous murine langerin gene(4, 5). Langerin is expressed by LC, Langerin+ dermal DC (dDC) as well as DC subsets in other tissues (4, 6–10). Injection of DT leads to rapid and efficient ablation of these cells(4–6). At early time points after DT administration, all Langerin-expressing DC are absent and contact hypersensitivity reactions (CHS) are diminished (5, 6). However, Langerin+ dDC and Langerin+ DC in other organs repopulate much more quickly than LC after DT administration. At 7 or 13 days after DT administration, LC are still absent but Langerin+ dDC have partially recovered(6). CHS responses in this setting are normal suggesting that Langerin+ dDC are required for CHS responses but LC appear to be largely redundant(6, 11).
HuLangerin-DTA (huDTA) mice express the active subunit of diphtheria toxin (DTA) under control of the human langerin genomic locus which leads to a constitutive ablation of epidermal LC(12). Other Langerin-expressing cells including Langerin+ dDC are unaffected presumably due to variations between the human and mouse langerin promoters (6, 12). Unlike muDTR mice, huDTA mice develop exaggerated CHS responses which suggests that LC actually function to suppress the CHS response(12). Similar evidence of LC-mediated suppression was also observed with minor-mismatched skin grafts and T cell responses to tick infestation(13, 14). Examination of the mechanism has revealed that LC-mediated suppression of CHS occurs during the sensitization phase and requires direct cognate interaction between LC and CD4 cells as well as LC-derived IL-10 (15).
Although muDTR mice 7-13 days after DT injection and huDTA both selectively lack LC, CHS responses are divergent. This is a major inconsistency between the two LC-deficient models. One difference is the constitutive absence of LC from birth in huDTA but not muDTR mice. LC migrate to skin-draining LN in the steady-state and have been proposed to participate in the maintenance of peripheral tolerance(16, 17). The absence of this process in huDTA mice may account for the observed exaggerated CHS responses. Alternatively, Langerin+ dDC in muDTR mice 7–13 days after DT injection are still reduced in number which may lessen CHS responses that otherwise would be exaggerated in the absence of LC.
To distinguish between these possibilities, we have generated huLangerin-DTR (huDTR) mice. These mice allow us to selectively ablate only epidermal LC just prior to immunization and test whether the acute ablation of LC affects cutaneous immune responses.
Materials and methods
Generation of huDTR mice
The primate DTR receptor cDNA was generated by PCR as described(18). Recombination into the 3’utr of Langerin in BAC RP11-504o1 was performed as described(12). Primers used for generation of the recombination cassette: 5’A box – 5 - ttaaggcgcgccggattccaggtgagcccaac-3; 3’A box 5 – agcagcttcatggttgtggccatattatcatcgtg-3; 5’ B box 5 – aattcccactgaatgactttgcacgttaatttttcttgc-3; 3’ B box 5 – tattaaggccggcccgtgacattggagaccttgc-3; 5’ I box 5 –atggccacaaccatgaagctgctg ccgtcggtggt -3; 3’ I box 5 – cgtgcaaagtcattcagtgggaattagtcatg-3. Transgenic injections were performed into pronuclei of C57BL/6 and FVB by the University of Minnesota Mouse Genetics Laboratory.
Mice
huDTR mice generated in FVB and C57BL/6 backgrounds were maintained on FVB or C57BL/6 wild-type mice obtained from Jackson Laboratories (Bar Harbor, ME), respectively. All experiments were performed on age (7–12 weeks) and sex matched mice. Mice were housed in micro-isolator cages and fed irradiated food. The institutional animal care and use committee approved all mouse protocols.
Antibodies
The following antibodies were used: muLangerin-bio and anti huLangerin-AF647 (Dendritics, Lyon, France); CD11c-FITC, CD11c-PacBlue, CD11b-PacBlue, CD45-PacBlue, MHC II-AF700 (BioLegend, San Diego, CA); and CD103-PE (eBioscience, San Diego, CA). Cells were stained for extracellular and intracellular markers as described(12). All flow cytometry was performed using a LSR II (BD Biosciences, San Jose, CA).
Flow Cytometry
Single cell preparations from LN, spleen, thymus, dermis and epidermis were prepared as previously described(12). In brief, LN, spleen and thymus were digested with collagenase D (Roche, Indianapolis, IN). Epidermis was digested with 0.3% trypsin for 120 minutes and removed from the dermis. The dermis was further digested with collagenase XI (4830 U/ml) and hyaluronidase (260 U/ml) (both Sigma, St. Louis, MO) for 120 minutes. Data were analyzed with FlowJo software (Treestar, Ashland, OR).
Immunofluorescence
For immunofluorescence, epidermal sheets were prepared by treating mouse ears with Nair (Chursh and Dwight Co, Princeton, NJ) for 3 min followed by affixing them to slides (epidermis side down) with double sided adhesive (3M, St. Paul, MN). Slides were incubated in 10 mM EDTA in PBS for 1 hr at 37°C followed by physical removal of the dermis. Tissue was fixed and stained as previously described (19).
Contact Hypersensitivity
Allergic contact dermatitis was induced in mice as previously described(12). Mice were sensitized with 0.5% DNFB (2,4 dinitroflurobenzene, Sigma) in acetone:olive oil (4:1) and challenged using 10 µl of 0.2% DNFB. Ears were measured daily after challenge and data are expressed as the ear size minus the baseline thickness.
Epicutaneous immunization
CD44low (naive phenotype) CD8+ Thy1.1+ T cells were purified from OT-I TCR tg mice by negative selection using magnetic cell sorting (Miltenyi Biotec) as previously described(20). Recipient mice (C57BL/6, muLangerin-DTR, or huLangerin-DTR) were shaved and transferred with 2.5 X 105 naïve OT-I cells one day before immunization. Langerin + cells were ablated in some mice by injection of 1 mg DT i.p. one day before immunization. On the day of immunization mice were anesthetized with ketamine and xylazine (100/10 µg/Kg body weight) and the flanks were hydrated for 15 min with water. Ovalbumin (500 µg in 25 µl of PBS, Sigma-Aldrich) was applied topically on the flank. After 20 minutes the skin area was washed and covered with an occlusive patch (DuoDERM Extra Thin, ConvaTec) for two days post-immunization. Five days after immunization, OT-I expansion was evaluated in spleen and DLN. Control mice received PBS on the flank.
Statistics
Statistical comparisons between groups were made with a Student's two-tailed t test.
Results
Generation and validation of huDTR mice
Using an approach similar to the one used to develop Langerin-DTA mice(12), human bacterial artificial chromosome RP11-504O1 was modified by homologous recombination in E. coli. A cassette encoding an IRES sequence followed by cDNA for the primate diphtheria toxin receptor (DTR) was introduced into the 3’ UTR of the langerin gene (Figure 1a). Successful recombination was confirmed by PCR and restriction digest (unpublished observations). A 73kb Not-I linear fragment containing 26 kb upstream of the langerin gene was used to generate 4 transgenic founders. The founders were phenotypically similar and data from a single founder is presented below.
Figure 1. DTR expression in huDTR mice is specific for LC and allows for efficient ablation.
A) Targeting construct for homologous recombination introducing the diphtheria toxin receptor (DTR) into the 3’UTR of langerin in human BAC RP11-504O1. B) Flow cytometry of epidermal single cell suspension from untreated huDTR mice stained with MHC II and CD45 to identify LC (CD45+, MHC-II+) and DETC (CD45+, MHC-II−). C) huLangerin expression in LC as gated in (B) in huDTR mice (solid line) and littermate control (shaded). D) As in (B), huDTR mice 2 days after DT administration. E) Percentage of LC and DETC among total epidermal cells in huDTR or control mice untreated or 2 days after DT administration, as specified. F) Immunofluorescence of epidermal whole mounts stained for MHC-II (red) or CD3 (green) from huDTR mice untreated or 2 days after DT administration. All data is representative of at least 3 independent experiments.
To assess transgene expression in the epidermis, a single cell suspension of epidermal cells was prepared and stained for MHC II, CD45, and huLangerin. Since the insertion of DTR did not disrupt the langerin gene, the expression of huLangerin as detected with a species-specific antibody was used to identify cells expressing the transgene. All LC (CD45+, MHC-II+) from huDTR mice demonstrated clear expression of transgenic huLangerin compared with transgene negative littermate controls (Fig. 1b and c). Expression of huLangerin by CD45+, MHC-II-dendritic epidermal T cells (DETC) was not observed (unpublished observation). Administration of 1µg of diphtheria toxin (DT) to huDTR mice efficiently eliminated virtually all LC but did not alter numbers of DETC (Fig. 1d and e). Immunofluorescent imaging of whole-mounted epidermal sheets stained for MHC-II (red) revealed a complete absence of LC in DT treated huDTR mice (Fig. 1f). As expected, the density and distribution of LC in untreated mice and the density and distribution of DETC expressing CD3 (green) in both treated and untreated mice was unaltered. Thus, transgene expression is specific for LC which are efficiently ablated by administration of DT.
Langerin+ DC unaffected in huDTR
In the dermis, in addition to LC migrating from the epidermis to the cutaneous LN (CLN), Langerin is also expressed by a subset of dDC. We have previously shown in huDTA mice that transgene expression is limited to epidermal LC and does not target Langerin+ dDC(6). To determine whether Langerin+ dDC are ablated in huDTR mice, we generated single cell suspensions from the dermis of WT, huDTR and muDTR mice that had been treated with DT. As expected, cells gated based on expression of muLangerin were virtually absent in muDTR mice compared to WT (Figure 2a). In huDTR mice, Langerin+ dDC, identified as CD103bright and CD11bdim are unaffected while epidermal LC (CD103dim, CD11bbright) are absent.
Figure 2. DT ablates LC leaving other Langerin+ DC unaffected.
A) Flow cytometry of dermal single cell suspensions showing expression of CD11b and CD103 from WT, huDTR, and muDTR mice 2 days after DT administration. Cells were gated on muLangerin+, CD11c+. Numbers represent the percentage of cells in the indicated gate. B) Cutaneous LN (CLN), spleen and thymus from huDTR + DT, huDTR –DT and WT+DT stained for huLangerin and CD11c. C) CLN from untreated huDTR mice gated on muLangerin showing expression of huLangerin, CD103 and CD8. D) The depletion of huLangerin+ cells from epidermis and CLN at the indicated times after DT administration normalized to littermate controls. All data is representative of at least 3 independent experiments.
DC expressing Langerin are found in tissues other than the skin. In the spleen and thymus, Langerin is expressed primarily by CD8+ DC(4, 10, 21–23). Expression of huLangerin is not evident in either tissue (Fig. 2b). In the CLN, Langerin is expressed by LC, Langerin+ dDC and CD8+ DC. CD11c+ DC expressing huLangerin are present in the CLN. These cells represent skin migratory LC based on the absence of CD8 and CD103 expression (Fig. 2c). As was observed in the epidermis and dermis, DT administration efficiently ablates LC in the CLN. Other DC and lymphoid subsets (i.e. B cells, T cells, macrophages) in the secondary lymphoid tissues are unaffected in DT treated huDTR mice (unpublished observations). Thus, DT administration efficiently and selectively ablates LC in huDTR mice.
Time course of LC ablation
To determine the optimal time after DT administration to test the effects of LC ablation, the kinetics of LC repopulation after DT administration was next examined. On Day +2, there was a dramatic decrease in the number of LC found in the epidermis and CLN which continues through day +7 (Figure 2d). By day +14, a small number of LC can be observed in the epidermis which increases to approximately 50% by day +28. Repopulation in the LN is delayed compared to the epidermis. This is consistent with nascent LC repopulating the epidermis prior to migrating to the CLN. The rate of LC repopulation is similar to that observed in muDTR mice(4, 5). Importantly, there is a window from day +2 through at least day +7 when LC are absent during which functional assays can be performed.
Langerin+ dDC functionally intact in huDTR mice
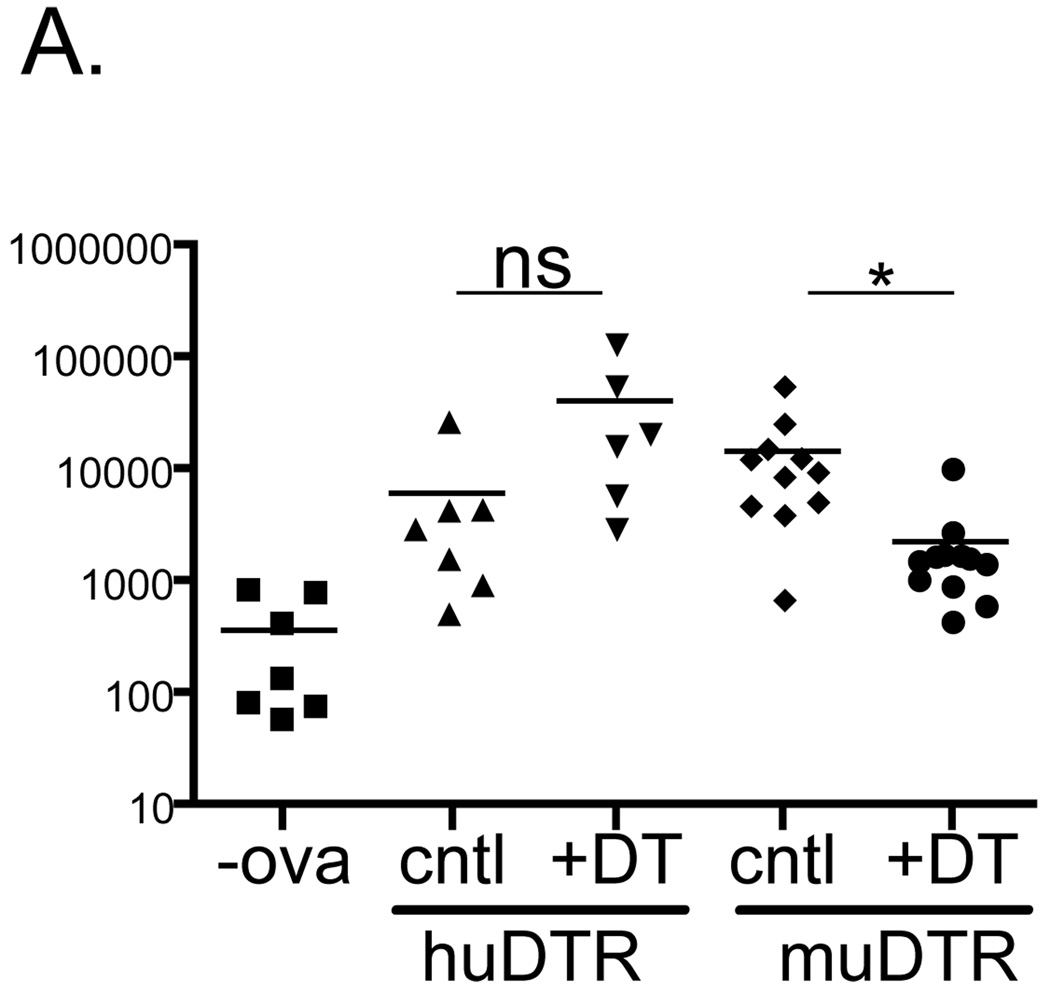
Hogquist and colleagues have recently demonstrated that optimal epicutaneous immunization of whole protein on the flanks of mice requires the presence of Langerin+ dDC(24). To determine whether Langerin+ dDC are functionally altered in huDTR mice, congenic CD90.1+ OT-I T cells were adoptively transferred into huDTR, muDTR and littermate control (cntl) mice. Mice were treated with DT and immunized with ovalbumin on day +2. Five days later, CLN were harvested and the number of OT-I cells was determined by flow cytometry based on a CD90.1 and CD8 gate. As expected muDTR mice showed an approximately 10 fold decrease in OT-I expansion (Fig. 3). In DT treated huDTR, OT-I expansion was not inhibited by the ablation of LC. Rather, there was a non-significant trend towards increased numbers of OT-I. Thus, Langerin+ dDC do not appear to be functionally compromised in huDTR mice.
Figure 3. Langerin+ dDC are functionally intact in huDTR mice.
2.5x105 CD90.1 OT-I CD8 T cells were adoptive transferred into huDTR, muDTR and control mice. One day after administration of DT, mice were epicutaneouly immunized with 500 µg ovalbumin or vehicle (-ova). Five days later the number of OT-I cells from CLN was determined based on expression CD90.1. * p<0.05
CHS increased in huDTR mice
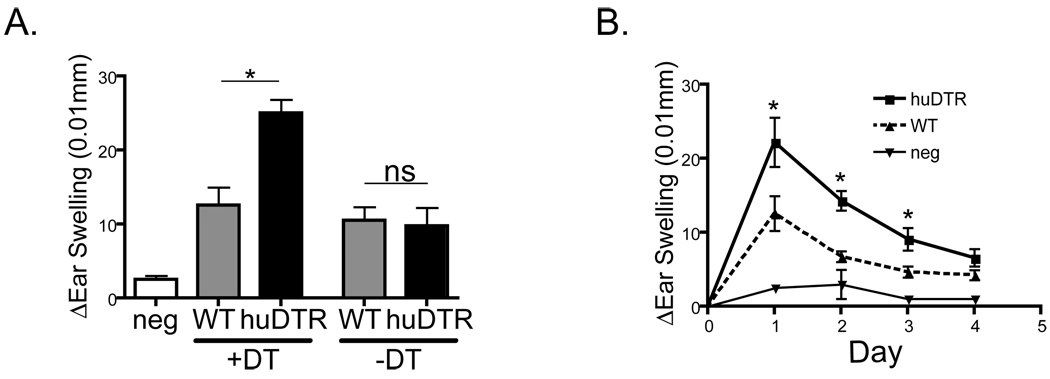
The standard assay used extensively to study LC function is contact hypersensitivity against cutaneously applied haptens. HuDTR and littermate controls (WT) mice were sensitized with 0.5% dinitro-fluorbenzene (DNFB) 2 days after injection of 1 µg DT. Five days later the mice were challenged with 0.2% DNFB and ear swelling was monitored. HuDTR mice treated with DT displayed approximately a 2-fold increase in ear swelling one day after challenge (Fig. 4a). This increase in CHS response persisted for several days and returned to baseline by day +4 (Fig. 4b). This pattern of increased CHS closely mimics our previously reported CHS responses in huDTA (12, 15). Importantly, huDTR mice not treated with DT and WT mice treated with DT did not develop increased CHS (Fig. 4a). Expression of the transgene or injection of DT alone does not alter CHS responses. Thus, the acute ablation of LC in a setting in which other Langerin+ DC are intact leads to exaggerated CHS responses and clearly demonstrates that the acute ablation of LC leads to exaggerated CHS responses.
Figure 4. Acute depletion of epidermal LC leads to enhanced CHS.
A) WT and huDTR mice were sensitized with 0.5% DNFB on day+2 after DT or PBS administration. Five days later mice were challenged with 0.2% DNFB on the ear. Ear swelling one day after challenge (A) and over time (B) was measured. All data is representative of at least 3 independent experiments. * p<0.05
Discussion
Herein, we described the generation of huLangerin-DTR (huDTR) mice that allow for the highly selective and efficient inducible ablation of LC. Langerin+ dDC are not depleted and remain functionally intact. The acute ablation of LC promotes increased CHS responses and recapitulates our previous findings with constitutive depletion of LC in huLangerin-DTA (huDTA) mice. These results clearly demonstrate that the absence of LC during ontogeny and/or the steady-state is not required for exaggerated CHS responses. Rather, LC-mediated suppression occurs at the time of immunization.
We have recently demonstrated that LC suppress CHS responses and the development of hapten-specific effectors via a mechanism that requires cognate interaction with CD4 T cells and elaboration of IL-10(15). We favor a model in which LC and Langerin+ dDC both transport haptenated antigen to the LN. Dermal DC arrive in the LN within 24 hours and initiate anti-hapten responses. LC arrive approximately 72–96 hours after sensitization and actively limit the development of antigen specific effectors.
Although the duration of LC ablation was an attractive hypothesis to explain the different CHS responses in huDTA and muDTR mice, our current results exclude this possibility. Thus, the question why enhanced CHS responses have not been observed in muDTR mice remains. As has been discussed, functional analysis of LC in muDTR mice is complicated by the concomitant ablation of Langerin+ dDC. This has been overcome by relying on differences in the rate of repopulation of LC vs. Langerin+ dDC after DT administration(6). CHS on day +2 after DT administration when LC and Langerin+ DC are absent is greatly reduced. CHS is normal on days +7 and +13 when LC are absent but other Langerin+ DC have partially recovered. A key caveat, however, is that at day +7 and +13 Langerin+ dDC are still only 30 and 50% their pre-depletion levels, respectively. The still-decreased numbers of Langerin+ dDC would be expected to reduce in the total pro-inflammatory capacity of Langerin+ dDC. We suggest that the resulting decreased response may compensate for the increased response due to the absence of LC and results in CHS that appears unchanged.
It is important to note that the mechanism of LC-suppression has only been carefully studied in CHS to DNFB and in response to tick bites which are predominantly Th1/Th17 type response(14, 15). In both settings, the extent of Th1/17 effectors was exaggerated by the absence of LC. A compensatory increase or decrease in Th2 cytokines, e.g. IL-4 and IL-13, was not observed suggesting that LC only affect Th1/17 responses. However, the possibility that LC may be specialized to promote Th2 responses in other settings cannot be excluded. Indeed, selectively targeting antigen to LC with a gene gun led to the production of Th2-related antibody isotypes(25). LC stimulated in vitro also promoted the development of Th2 cells(26, 27) and topical application of a vitamin D analog appears to selectively activate LC to promote Th2-type responses(28). In addition, CHS responses to low doses of Oxazalone are reduced in the absence of LC, though the effect on Th differentiation has not been reported(29). Examination of antigen-specific effects from LC depletion in models other than CHS may help address this issue.
Recently, experiments using bone-marrow chimeric mice to selectively deplete Langerin+ dDC suggests that LC may be capable of priming CHS responses in certain circumstances(11). Thus, a trend that is becoming increasingly clear is that LC behavior can depend on the methods used to assay their function. Seemingly small differences in technique can give diametrically opposite results. This is abundantly evident from work using different LC-deficient mouse strains. The generation of huLangerin-DTR along with huLangerin-DTA mice provide tools to examine the effects of LC depletion without having to rely on manipulations that affect other DC populations. We expect that these mouse lines, in combination with novel methods to immunize mice through intact epidermis, can define additional functions of LC in vivo.
Acknowledgements
We thank the University of Minnesota Research Animal Resources staff for outstanding animal care.
Supported by NIH R01-AR056632 (DHK) and P01-AI35296 (KAH)
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Romani N, Ebner S, Tripp CH, Flacher V, Koch F, Stoitzner P. Epidermal Langerhans cells--changing views on their function in vivo. Immunol Lett. 2006;106:119–125. doi: 10.1016/j.imlet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 4.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and Function of Langerhans Cells In Vivo Dermal Dendritic Cells Colonize Lymph Node AreasDistinct from Slower Migrating Langerhans Cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, Clausen BE. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, Snoeck H, Randolph G, Merad M. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valladeau J, Clair-Moninot V, Dezutter-Dambuyant C, Pin JJ, Kissenpfennig A, Mattei MG, Ait-Yahia S, Bates EE, Malissen B, Koch F, Fossiez F, Romani N, Lebecque S, Saeland S. Identification of mouse langerin/CD207 in Langerhans cells and some dendritic cells of lymphoid tissues. J Immunol. 2002;168:782–792. doi: 10.4049/jimmunol.168.2.782. [DOI] [PubMed] [Google Scholar]
- 10.Douillard P, Stoitzner P, Tripp CH, Clair-Moninot V, Ait-Yahia S, McLellan AD, Eggert A, Romani N, Saeland S. Mouse lymphoid tissue contains distinct subsets of langerin/CD207 dendritic cells, only one of which represents epidermal-derived Langerhans cells. J Invest Dermatol. 2005;125:983–994. doi: 10.1111/j.0022-202X.2005.23951.x. [DOI] [PubMed] [Google Scholar]
- 11.Honda T, Nakajima S, Egawa G, Ogasawara K, Malissen B, Miyachi Y, Kabashima K. Compensatory role of Langerhans cells and langerin-positive dermal dendritic cells in the sensitization phase of murine contact hypersensitivity. J Allergy Clin Immunol. 2010;125:1154–1156. doi: 10.1016/j.jaci.2009.12.005. e1152. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Obhrai JS, Oberbarnscheidt M, Zhang N, Mueller DL, Shlomchik WD, Lakkis FG, Shlomchik MJ, Kaplan DH. Langerhans cells are not required for efficient skin graft rejection. J Invest Dermatol. 2008;128:1950–1955. doi: 10.1038/jid.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vesely DL, Fish D, Shlomchik MJ, Kaplan DH, Bockenstedt LK. Langerhans cell deficiency impairs Ixodes scapularis suppression of Th1 responses in mice. Infect Immun. 2009 doi: 10.1128/IAI.00030-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igyarto BZ, Jenison MC, Dudda JC, Roers A, Muller W, Koni PA, Campbell DJ, Shlomchik MJ, Kaplan DH. Langerhans cells suppress contact hypersensitivity responses via cognate CD4 interaction and langerhans cell-derived IL-10. J Immunol. 2009;183:5085–5093. doi: 10.4049/jimmunol.0901884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 17.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ. Autocrine/paracrine TGF{beta}1 is required for the development of epidermal Langerhans cells. J Exp Med. 2007;204:2545–2552. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayerova D, Wang L, Bursch LS, Hogquist KA. Conditioning of Langerhans cells induced by a primary CD8 T cell response to self-antigen in vivo. J Immunol. 2006;176:4658–4665. doi: 10.4049/jimmunol.176.8.4658. [DOI] [PubMed] [Google Scholar]
- 21.McLellan AD, Kapp M, Eggert A, Linden C, Bommhardt U, Brocker EB, Kammerer U, Kampgen E. Anatomic location and T-cell stimulatory functions of mouse dendritic cell subsets defined by CD4 and CD8 expression. Blood. 2002;99:2084–2093. doi: 10.1182/blood.v99.6.2084. [DOI] [PubMed] [Google Scholar]
- 22.Flacher V, Douillard P, Ait-Yahia S, Stoitzner P, Clair-Moninot V, Romani N, Saeland S. Expression of langerin/CD207 reveals dendritic cell between inbred mouse strains. Immunology. 2008;123:339–347. doi: 10.1111/j.1365-2567.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Bursch LS, Kissenpfennig A, Malissen B, Jameson SC, Hogquist KA. Langerin Expressing Cells Promote Skin Immune Responses Defined Conditions. J Immunol. 2008;180:4722–4727. doi: 10.4049/jimmunol.180.7.4722. [DOI] [PubMed] [Google Scholar]
- 25.Nagao K, Ginhoux F, Leitner WW, Motegi S, Bennett CL, Clausen BE, Merad M, Udey MC. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci U S A. 2009;106:3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, Reiter Y, Banchereau J, Ueno H. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding W, Stohl LL, Wagner JA, Granstein RD. Calcitonin gene-related peptide biases Langerhans cells toward Th2-type immunity. J Immunol. 2008;181:6020–6026. doi: 10.4049/jimmunol.181.9.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elentner A, Finke D, Schmuth M, Chappaz S, Ebner S, Malissen B, Kissenpfennig A, Romani N, Dubrac S. Langerhans cells are critical in the development of atopic dermatitis-like inflammation and symptoms in mice. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett CL, Noordegraaf M, Martina CA, Clausen BE. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179:6830–6835. doi: 10.4049/jimmunol.179.10.6830. [DOI] [PubMed] [Google Scholar]