Abstract
Summary
Development of optimal skeletal strength should decrease adult bone fragility. Nongymnasts (NON) were compared with girls exposed to gymnastics during growth (EX/GYM), using peripheral quantitative computed tomography (pQCT) to evaluate postmenarcheal bone geometry, density, and strength. Pre- and perimenarcheal gymnastic loading yields advantages in indices of postmenarcheal bone geometry and skeletal strength.
Introduction
Two prior studies using pQCT have reported bone density and size advantages in Tanner I/II gymnasts, but none describe gymnasts’ bone properties later in adolescence. The current study used pQCT to evaluate whether girls exposed to gymnastics during late childhood growth and perimenarcheal growth exhibited greater indices of distal radius geometry, density, and skeletal strength.
Methods
Postmenarcheal subjects underwent 4% and 33% distal radius pQCT scans, yielding: 1) vBMD and cross-sectional areas (CSA) (total bone, compartments); 2) polar strength-strain index; 3) index of structural strength in axial compression. Output was compared for EX/GYM vs. NON, adjusting for gynecological age and stature (maturity and body size), reporting means, standard errors, and significance.
Results
Sixteen postmenarcheal EX/GYM (age 16.7 years; gynecological age 3.4 years) and 13 NON (age 16.2 years; gynecological age 3.6 years) were evaluated. At both diaphysis and metaphysis, EX/GYM exhibited greater CSA and bone strength indices than NON; EX/GYM exhibited 79% larger intramedullary CSA than NON (p<0.05). EX/GYM had significantly higher 4% trabecular vBMD; differences were not detected for 4% total vBMD and 33% cortical vBMD.
Conclusions
Following pre-/perimenarcheal gymnastic exposure, relative to nongymnasts, postmenarcheal EX/GYM demonstrated greater indices of distal radius geometry and skeletal strength (metaphysis and diaphysis) with greater metaphyseal trabecular vBMD; larger intramedullary cavity size was particularly striking.
Keywords: Adolescence, Bone geometry, Bone strength, Female, Mechanical loading, pQCT
Introduction
Artistic gymnastics provides a prime model of mechanical loading, generating forces of up to ten times body weight [1] via extreme weight bearing and impact loading. Dual energy X-ray absorptiometry (DXA) studies have shown high areal bone mineral density (aBMD) and bone mineral content (BMC) in gymnasts [2–9]. However, DXA does not measure bone cross-sectional geometry, or volumetric bone mineral density (vBMD); thus, results cannot distinguish bone size differences from bone density differences. In addition, DXA yields mean aBMD and BMC; it does not assess cross-sectional bone architecture or compartmental vBMD. In contrast, peripheral quantitative computed tomography (pQCT) assesses cross-sectional bone geometry and compartmental density, characterizing bone size, tissue distribution, and vBMD. However, because pQCT samples a narrower region of interest, care must be taken in positioning scans, particularly across the variable metaphyseal region.
Recently, our group applied formulae to distal radius DXA data from premenarcheal females, deriving bone mineral apparent density (BMAD) as well as indices of bone geometry and skeletal strength [10–12]. Gymnast vs. nongymnast comparisons indicated significantly greater bone size and mass in gymnasts, yielding greater indices of metaphyseal and diaphyseal bone strength. Gymnast BMAD was greater only at the metaphysis. Results suggested that gymnastic loading increases bone size and vBMD in a site-specific manner, as previously shown in racquet sport models [13–15]. However, DXA-derived indices rely upon geometric simplifications to approximate cross-sectional geometry and vBMD [10].
Because gymnastics loads the forearm in a manner that is not duplicated by other activities, the radius provides an indication of skeletal adaptation to weight bearing and impact loading [10, 16]. In addition, the radius contains regions of cortical (diaphyseal) and corticocancellous (metaphyseal) composition, allowing assessment of bone tissue-specific adaptation using pQCT [10] (Fig. 1). Few publications have reported pQCT-measured bone properties in gymnasts and nongymnasts [16–20]. Most have evaluated the radius in prepubertal and/or early pubertal subjects. One study (recently published by our group) focused on the explanatory value of muscular indices [muscle cross-sectional area (CSA) and arm fat-free mass (FFM)] vs. gymnastic exposure for radius outcomes in postmenarcheal girls, without characterizing bone outcomes for gymnasts relative to nongymnasts [16]. Another publication has identified significant advantages for pQCT parameters in adult former gymnasts compared with age-matched nongymnasts [20]. In the present analysis, we tested the hypothesis that gymnastics exposure from late childhood through menarche is linked to greater indices of bone geometry, density, and theoretical strength after menarche. We expected that advantages in cortical CSA would predominate at the diaphysis and that advantages in trabecular vBMD would predominate at the metaphysis.
Fig. 1.
For 33% diaphysis (a, c) and 4% metaphysis (b) sites, sample pQCT scans are presented, indicating bone compartments in gymnasts and nongymnasts. For both sites, the “total” compartment includes both cortical and intramedullary/trabecular compartments. Thus, total cross-sectional area (CSA) is the sum of cortical/subcortical and intra-medullary/trabecular CSAs. Total volumetric density (vBMD) is the mean vBMD over the total CSA. For a and b, although randomly selected, the gymnast and nongymnast were well-matched for age and body size (33% SSI and 4% IBS are 69% and 100% greater in the gymnast than the nongymnast, respectively). For c, the scans were specifically selected to illustrate the wide variability in intramedullary cavity characteristics; the gymnast was 5.5 cm taller, but lighter, younger, and less physically mature than the nongymnast (gymnast SSI =271% greater)
Methods
Postmenarcheal subjects from an ongoing longitudinal study of gymnastics-related bone accrual were recruited to undergo pQCT scans of the distal radius. Subjects/guardians provided written assent/consent using a document approved by the SUNY Upstate Medical University Institutional Review Board, in compliance with the US bioethical legislation and the Declaration of Helsinki. Gymnasts/ex-gymnasts (EX/GYM) were defined as girls who had participated in at least 5 h per week (hours per week) of gymnastic activity for at least 2 years between age 8 years and menarche, including both elite and nonelite gymnasts. All subjects within the EX/GYM group had participated in gymnastics through early puberty or later (to at least 4 months premenarche). Subjects who discontinued gymnastics at an earlier maturity phase were excluded from analyses (n=3, ceased gymnastics 4.7, 1.8, and 1.7 years premenarche). This ensured that all subjects in the EX/GYM group were exposed to gymnastics in the context of increasing estrogen exposure, coincident with accelerated perimenarcheal bone mineral accrual [21].
All subjects had participated in the longitudinal DXA study for the previous 5–10 years [8]. Nongymnasts were recruited from local private schools, athletic groups, and the university community. At initial enrollment, nongymnasts were matched for age, maturity, and body size to gymnasts from local clubs (future ex-gymnasts n=6 and gymnasts n= 10). Semiannual data were collected, as follows [8]. Stature (in centimeters) was measured using wall-mounted rulers. Calcium intake was assessed using food frequency questionnaires; estimated mean intake was calculated for the period 18 months prior to and including the pQCT scan. Self-assessed Tanner breast stage was recorded. Gynecological age (years postmenarche) was calculated relative to self-reported menarche date. Annual means for self-reported gymnastic activity (current gymnasts only) and total weight-bearing activity (nongymnasts and ex-gymnasts) were calculated for the year up to and including the pQCT scan, based on semiannual questionnaires. Early in the longitudinal study, gymnasts’ self-reported annual physical activity means were validated against coaches’ training logs (r>0.97, p<0.001, unpublished results).
Densitometric data were analyzed from a single session of contemporaneous DXA and pQCT scans. A whole-body DXA scan [Hologic QDR 4500W, software 8.26a, coefficient of variation (CV) <1.0%] measured total body FFM (in kilograms). Forearm pQCT scans were performed on the nondominant arm (Norland-Stratec XCT 2000). Machine in vivo forearm pQCT scan precision was determined in an unrelated adult sample at the 4% metaphysis and 30% diaphysis. Using the analysis protocol detailed below, coefficients of variation for pQCT parameters were as follows: metaphyseal total CSA=2.44%; metaphyseal total vBMD=2.15%; metaphyseal trabecular CSA=3.41%; metaphyseal trabecular vBMD=1.21%; metaphyseal cortical-subcortical CSA=2.29%; metaphyseal IBS=2.22%; diaphyseal total CSA=0.96%; diaphyseal total vBMD= 0.50%; diaphyseal cortical-subcortical CSA=0.94%; diaphyseal cortical vBMD=0.63%; diaphyseal IMCSA=2.34%; diaphyseal polar SSI=2.96%.
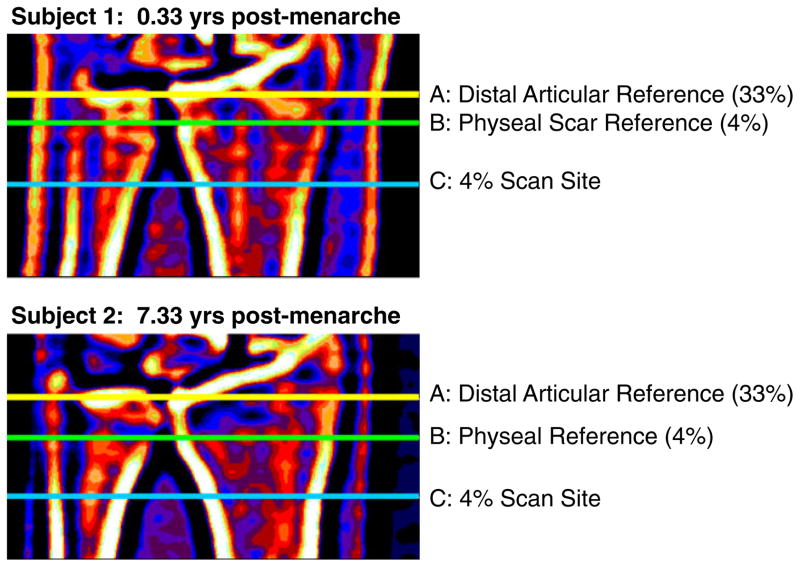
Scout views were performed for reference line placement (Fig. 2). For metaphyseal assessments, scans were performed at 4% of ulnar length from the reference line, set at the proximal border of the physis or physeal scar on the ulnar side of the radius [22]. Positioning relative to the physis has been recommended to improve agreement of metaphyseal scans between individuals, as the degree of metaphyseal inwaisting and maturity of bone tissue varies according to distance from the physis [23–25]. Ulnar length (mm) was measured from the olecranon to the ulnar styloid with a ruler and rounded to the nearest 5 mm increment (CV=4%). To assess metaphyseal bone geometry and vBMD throughout the bone cross-section, 4% scans were analyzed based upon vBMD thresholds (contour mode 3, peel mode 4, inner threshold 450 g/cm3, threshold 169 mg/cm3, and peel by 5%) [22]. This contrasts with analysis modes that only analyze the central 45% of the trabecular compartment [24]. For diaphyseal assessments, scans were performed at 33% of ulnar length from a distal articular reference (Fig. 2), as this region of interest is less variable, and the tissue is more uniformly mature than at the metaphysis [22–25]. For analysis of 33% region scans, we used analysis modes as previously reported (CALCBD: contour mode 1, peel mode 2, inner threshold 540 mg/cm3, threshold 711 mg/cm3; CORTBD and strength-strain index (SSI): threshold 711, separation mode 2) [22]. Cortical vBMD was measured at the 33% site; mean cortical thickness exceeded 2.5 mm (>4 mm), limiting the influence of partial volume effects [23].
Fig. 2.
A scout view is shown to depict pQCT scan reference line placement for 33% scans (articular reference, A) and 4% scans (physeal/scar reference, B)
Cortical thickness may be low at the 4% site and therefore more strongly influenced by partial volume effects [23]. Nonetheless, 4% cortical CSA was measured for comparison with other studies [15, 26]. Similarly, the utility of total vBMD has been questioned as a meaningful index of bone strength at the diaphysis, and we note that total vBMD may vary independently of bone strength indices, depending upon the underlying bone geometric adaptation. For example, high cortical CSA combined with low intramedullary CSA is associated with thick cortices, yielding high total vBMD and SSI. In contrast, high cortical CSA combined with high intramedullary CSA may be associated with relatively thin cortices, yielding low total vBMD but high SSI. It is important to consider this phenomenon when interpreting DXA areal BMD for the radial diaphysis, as it may mask differences in theoretical bone strength and hamper evaluation of fracture risk. Therefore, we report diaphyseal total vBMD results to illustrate this underlying variability and yield a means of comparison with other skeletal sites. To evaluate metaphyseal bone strength, index of structural strength in axial compression (IBS) was calculated as total CSA×vBMD2 (elsewhere, bone strength index) [15, 22, 23, 27]. To assess theoretical resistance to fracture in the event of a low trauma fall, fall strength was calculated using polar SSI (33%) or IBS (4%) divided by the product of forearm length and total body weight (fall SSI, fall IBS)[10, 22, 25, 28]. Throughout the paper, cortical vBMD represents the mean vBMD for the cortical compartment only, whereas cortical CSA represents cortical/subcortical cross-sectional area (such that total CSA=intramedullary CSA + cortical CSA).
Variables were screened for normality; natural logarithmic transformation was applied to nonnormal distributions [33% intramedullary cross-sectional area (IMCSA) only]. To evaluate bone traits attributed to gymnastic loading during growth, gymnasts and ex-gymnasts were grouped together (EX/GYM) for comparison against nongymnasts (NON). To specifically evaluate the influence of gynecological age on physeal (4% reference) location and bone outcomes, Pearson correlations were applied to all outcomes except 33% IMCSA (Spearman correlations, ln-transformed data; alpha=0.05). To account for potential variation in biological maturity and body size, analysis of covariance (ANCOVA) adjusted for gynecological age and stature, accounting statistically for the linear correlations between these covariates and each outcome. The resultant adjusted means, standard errors, effect sizes (Cohen’s d [29]) and significance are reported (alpha=0.05).
Power analyses were based on forearm areal BMD comparisons for ex-gymnasts vs. nongymnasts at approximately 18 months postmenarche, in order to gauge effect sizes for maturity-specific group differences [30]. On this basis, minimum cell size for 80% power was determined to be seven; cell sizes of n=16 and n=13 were deemed adequate to detect significant differences if effect sizes were similar for pQCT and DXA differences. Post hoc power analyses using the pQCT comparisons of Eser et al. (2010, [20]) indicate that cell sizes of n=16 and n=13 should be adequate to detect significant differences for total CSA (4% and 66%/33%), 66%/33% medullary CSA, and 66%/33% SSI with 80% power [31]. However, to detect differences of Eser’s observed magnitude, cell sizes of 33 to 63 would be required for 4% trabecular vBMD and 66% cortical CSA and cortical vBMD; cell sizes of over 2,000 would be required for 4% total vBMD (80% power).
Results
Subjects included 16 EX/GYM and 13 NON, with a mean chronological age of 16.5 years (13.3 to 20.4 years) and a mean gynecological age of 3.5 years (0.25 to 7.3 years). During 5 to 8 years of observation prior to the pQCT scans, all subjects reported normal health, barring occasional sports injuries. NON and EX/GYM were well matched, only differing significantly by physical activity history (see Table 1). Self-assessed Tanner breast stage distributions were not significantly different between groups (NON TB3= 15%, TB4=31%, TB5=54%; EX/GYM TB3=19%, TB4=44%, TB5=38%). Rates of oral contraceptive use were similar between groups [NON 3/13 (23%); EX/GYM 2/16 (13%)]. All subjects were white except one nongymnast (1 Asian). All EX/GYM were exposed to gymnastics prior to puberty and continued gymnastics to at least 4 months before menarche. The majority of EX/GYM continued gymnastics up to or beyond menarche (81%), with mean exposure to 1.25 years post-menarche [standard deviation (SD)=1.3 years]. All EX/GYM had participated in gymnastics (>5 h/week) for at least 2 of the past 10 years (3–9 years). The grand mean for gymnastic participation per year of gymnastic activity (mean of all annual means of all EX/GYM) was 13.3 h/week (6.6–17.9 h/week). Ex-gymnast gymnastic exposure (at level ≥5 h/week) averaged 5.1 years (SD=1.7, 3–8 years), at an average of 13.7 h/week (SD=3.2, 10–17.9 h/week). Current gymnasts had been exposed to gymnastics (at level ≥5 h/week) for 6.1 years (SD=1.5, 4–9 years), at an average of 12.9 h/week (SD=3.0, 6.6–16.1 h/week).
Table 1.
Subject characteristics by gymnastic exposure group
| Variable | Ex/gymnasts (n=16) |
Nongymnasts (n = 13) |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 16.7 | 2.1 | 16.2 | 2.2 |
| Gynecological age (years postmenarche) | 3.4 | 2.4 | 3.6 | 2.2 |
| Age at menarche (years) | 13.2 | 1.0 | 12.6 | 0.9 |
| Tanner breast stage (3 to 5) | 4.2 | 0.8 | 4.4 | 0.8 |
| Height (m) | 1.61 | 0.08 | 1.62 | 0.05 |
| Weight (kg) | 55.2 | 6.4 | 55.9 | 5.4 |
| DXA fat-free mass (kg) | 39.9 | 3.8 | 39.3 | 2.1 |
| BMI (kg/m2) | 21.4 | 1.7 | 21.1 | 1.6 |
| % body fat | 22.7 | 4.8 | 25.1 | 4.2 |
| Calcium intake (mg/day) | 731.2 | 323.5 | 718.7 | 330.8 |
| Weight-bearing activity (h/week) | 6.8 (EX n=7) | 3.9 (EX n=7) | 5.9 | 4.1 |
| Gymnastic activity (h/week) | 6.9 (GYM n=9) | 7.7 (GYM n=9) | NA | NA |
Means and standard deviations are provided for gymnast/ex-gymnast and nongymnast groups. No significant differences were identified between means (ANOVA p>0.05)
NA not applicable
Nongymnasts were active in various physical activities during late childhood and perimenarcheal growth; most participated in multiple activities in a given year. Similarly, ex-gymnasts also participated in a variety of activities after cessation of gymnastic training (during peri-/postmenarcheal growth). For the year prior to the pQCT scan, nongymnasts and ex-gymnasts did not differ for annual mean weight-bearing activity (Table 1, p=0.64).
For the pQCT scans, quality was generally high; nonetheless, for the 33% site, two scans were excluded for movement errors (one gymnast, one nongymnast). For the 4% site, reference distance (distance of scan reference line from articular surface) was correlated with gynecological age (r=+0.45, p<0.02); there was a strong trend for a positive correlation between gynecological age and 4% scan distance (distance of scan site from the articular surface, r=+0.31, p<0.11). Forearm length, 4% reference distance, and 4% scan distance were compared for EX/GYM and NON. There were no significant gymnastic group differences in any of these parameters by analysis of variance (ANOVA) or ANCOVA adjusting for gynecological age (although for scan distance from the articular reference, ANOVA p=0.07, ANCOVA p=0.05, EX/GYM > NON).
Diaphysis, 33% site
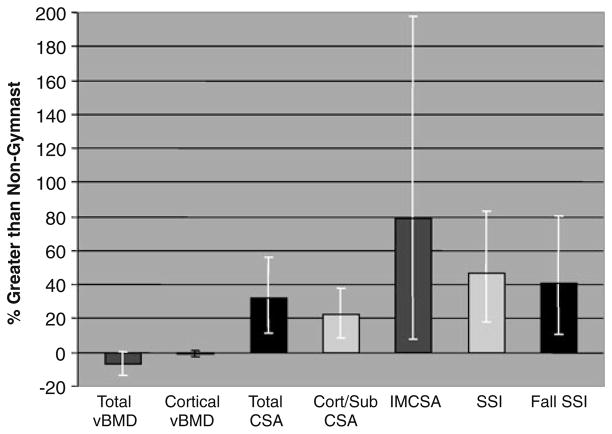
At the diaphysis, EX/GYM adjusted means were higher than NON for total, cortical, and intramedullary CSA (p<0.005; Table 2, pFigs. 1a, c and 3). Accordingly, SSI and fall SSI were higher in EX/GYM than NON (ANCOVA <0.001). Conversely, NON had higher 33% total vBMD than EX/GYM (ANCOVA p<0.02); significant differences in 33% cortical vBMD were not detected (p>0.30). Gynecological age correlated with total and cortical vBMD (r=+0.43, p< 0.02; r=+0.79, p<0.000, respectively), acting as a significant covariate for these parameters. No other diaphyseal parameter correlated with gynecological age, but lnIMCSA suggested a negative trend (rho=−0.32, p=0.11).
Table 2.
Adjusted bone parameters by gymnastic exposure group (ANCOVA)
| Site | Parameter | Mean (SEM) |
Absolute difference (95% CI) | Effect size (d) | |
|---|---|---|---|---|---|
| Ex/gymnasts (33%, n=15) (4%, n=16) | Nongymnasts (33%, n=12) (4%, n=13) | ||||
| 33% radius diaphysis | Total CSA (mm2) | 110.0 (3.6) | 83.5 (4.0) | 26.5*** (10.7–42.3) | 2.04 large |
| Cortical (mm2 CSA ) | 87.3 (2.1) | 71.5 (2.4) | 15.9*** (6.6–25.1) | 2.09 large | |
| Intramedullary CSA (mm2) | 20.3 (1.1) | 11.3 (1.1) | 9.0** (1.1–17.1) | 1.39 large | |
| Total vBMD (g/cm3) | 0.942 (0.017) | 1.011 (0.019) | −0.069* (−0.144–0.005) | 1.13 large | |
| Cortical vBMD (g/cm3) | 1.160 (0.005) | 1.169 (0.006) | −0.009 (−0.033–0.016) | 0.43 medium | |
| Polar SSI (mm3) | 252.2 (10.1) | 172.2 (11.3) | 80.0*** (35.7–124.2) | 2.20 large | |
| 4% radius metaphysis | Total CSA (mm2) | 255.2 (8.5) | 202.6 (9.5) | 52.6*** (15.4–89.7) | 1.64 large |
| Cortical CSA (mm2) | 106.4 (2.8) | 85.0 (3.1) | 21.4*** (9.4–33.5) | 2.06 large | |
| Trabecular CSA (mm2) | 148.8 (8.4) | 117.6 (9.3) | 31.1*** (−5.4–67.7) | 0.99 large | |
| Total vBMD (g/cm3) | 0.451 (0.016) | 0.439 (0.018) | 0.013 (−0.058 –0.084) | 0.21 small | |
| Trabecular vBMD (g/cm3) | 0.229 (0.011) | 0.193 (0.012) | 0.037* (−0.010–0.084) | 0.92 large | |
| IBS (g2/cm4) | 0.510 (0.022) | 0.381 (0.025) | 0.129** (0.032–0.225) | 1.54 large | |
For gymnast/ex-gymnast and nongymnast groups, means, standard errors of the mean, differences, and 95% confidence intervals for the difference are presented for bone parameters by site
ANCOVA analysis of covariance, SEM standard error of the mean, d Cohen’s d, CSA cross-sectional area, vBMD volumetric bone mineral density, SSI polar strength strain index, IBS index of structural strength in axial compression, NS not significant (p≥0.20)
p<0.05;
p≤0.01;
p≤0.001
Fig. 3.
Percent advantages for ex/gymnasts relative to nongymnasts are depicted for each pQCT parameter at the 33% site. Means are adjusted for gynecological age and height; bars represent 95% confidence intervals. For all parameters, ANCOVA p<0.05, except cortical vBMD (not significant). Cort/Sub cortical/subcortical, CSA cross-sectional area, IMCSA intramedullary CSA, SSI polar strength–strain index
Metaphysis, 4% site
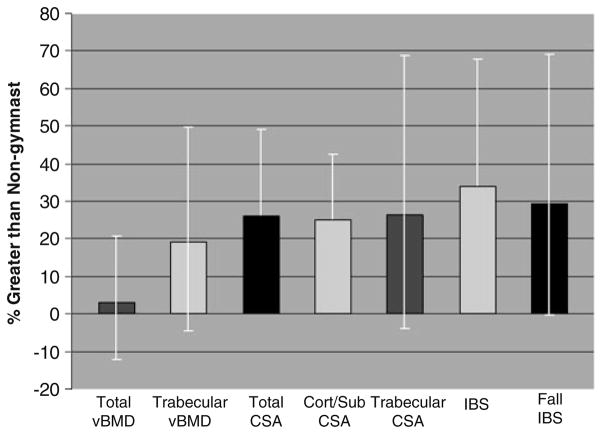
At the metaphysis, compared with NON, EX/GYM adjusted means were higher for CSA (total, trabecular, and cortical/subcortical compartments), SSI, fall SSI, and IBS (ANCOVA p<0.03; Table 2, pFigs. 1b and 4). Trabecular vBMD was also higher in EX/GYM than NON (ANCOVA <0.03), but differences in total vBMD were not detected (small effect size d=0.21). Gynecological age was positively correlated with total vBMD, IBS, and IBS fall strength (r=+0.64, p< 0.000; r=+0.51, p=0.005; r=+0.42, p<0.03, respectively). In contrast, gynecological age was negatively correlated with total CSA and trabecular CSA (r=−0.38, p< 0.05; r=−0.46, p<0.02, respectively). Gynecological age was a significant covariate for 4% total CSA, total vBMD, trabecular CSA, and IBS.
Fig. 4.
Percent advantages for ex/gymnasts relative to nongymnasts are depicted for each pQCT parameter at the 4% site. Means are adjusted for gynecological age and height; bars represent 95% confidence intervals. For all parameters, ANCOVA p<0.05, except total vBMD (not significant). CSA cross-sectional area, Cort/Sub cortical/subcortical compartment, Trabecular trabecular compartment
Discussion
In these postmenarcheal girls, distal radius bone size and strength were at least 20% greater for EX/GYM compared with NON, at both metaphyseal and diaphyseal sites. At the diaphysis, gymnastic loading was associated with marked intramedullary cavity expansion and moderate cortical shell expansion, reflected by lower total vBMD in EX/GYM compared with NON (no cortical vBMD difference was detected). Similarly, at the metaphysis, EX/GYM did not exhibit an advantage over NON for total vBMD, despite trabecular vBMD and cortical CSA advantages of approximately 20%. Overall, exposure to pre- and perimenarcheal gymnastic loading was associated with greater theoretical bone strength; this advantage resulted from larger bone geometry at both diaphyseal and metaphyseal sites, with unaltered cortical vBMD and high metaphyseal trabecular vBMD. Our group has previously noted that both high mineral content and efficient mineral distribution underlie gymnast advantages in bone geometry and bone strength indices [10, 16].
Aside from our muscle index work [16], studies by Dyson, Ward et al., and now Eser et al. (2010) are the only published reports that have used pQCT to compare the radii of gymnasts and nongymnasts [17–20]. In contrast to our postmenarcheal subjects, both Dyson and Ward et al. evaluated subjects who were, on the whole, prepubertal (Ward et al.=Tanner I at baseline; Dyson et al.=majority Tanner I/some Tanner II) [17–19]. Our EX/GYM and the adult ex-gymnasts of Eser et al. [20] were also exposed to gymnastic loading during late childhood/early puberty, but loading exposure was extended, continuing into perimenarcheal growth. This additional loading, over a more advanced maturational phase, would be expected to either increase gymnast/nongymnast differentials or induce maturity-specific differences.
At the metaphysis, our postmenarcheal results contrast with Dyson/Ward premenarcheal results and are similar to those of Eser et al. [17, 18, 20]. Specifically, differentials varied for metaphyseal total CSA and vBMD. Dyson’s premenarcheal results suggested some gymnast advantage in total CSA over nongymnasts, although the differential was smaller than observed postmenarcheal advantages (gymnast advantage, Dyson=11%, not significant (NS); EX/GYM=26%, p<0.05; Eser=25%, p<0.05) [17, 20]. In contrast, Ward’s gymnasts and nongymnasts did not differ for total CSA (gymnast advantage, Ward=−0.4%, NS) [18]. For total vBMD, both Dyson and Ward et al. reported significant premenarcheal gymnast advantages, whereas no significant postmenarcheal difference has been detected (Dyson=20%; Ward=17%; EX/GYM=3%, NS; Eser=−1.1%, NS) [17, 18, 20]. For trabecular vBMD, Dyson’s and Ward’s premenarcheal gymnasts demonstrated greater advantages than either postmenarcheal cohort (Ward=21%; Dyson=27%; EX/GYM= 19%, p<0.05; Eser=9%, NS; absolute differences: 40–44 mg/cm3 vs. 12–17 mg/cm3) [17, 18, 20]. We observed a gymnast advantage in trabecular compartment CSA. Dyson et al. noted similar findings in their discussion, but Dyson, Ward, and Eser et al. do not report trabecular CSA results for comparison [17, 18, 20].
At the metaphysis, discrepancies between pre- and postmenarcheal results may stem from at least two hypothetical sources. Relative to prepubertal loading, continued gymnastic loading during puberty may have induced greater metaphyseal expansion in all compartments. This emphasis on geometric expansion may limit total vBMD advantages due to wider distribution of bone mass, so that prepubertal advantages in vBMD appear larger. In addition, during puberty, nongymnasts may have experienced maturity-specific increases in total vBMD, partially offsetting earlier loading advantages in postmenarcheal gymnasts. Eser et al. examined adult ex-gymnasts to specifically evaluate maintenance of benefits 3+years after discontinuation of gymnastics (mean 6 years; 3–18 years). Their metaphyseal vBMD advantages were lower than advantages in the younger pre- and postmenarcheal gymnast/ex-gymnast cohorts, suggesting partial deterioration of benefits after gymnastic cessation [20].
Ward et al. reported significant advantages in diaphyseal parameters at the 50% midradius in their premenarcheal cohort of girls and boys. However, compared with postmenarcheal gymnast advantages, Ward’s gymnast advantages were lower for total CSA (Ward=9%, p<0.05; EX/GYM= 32%, p<0.05; Eser=32%, p<0.05), cortical CSA (Ward= 8%, p<0.05; EX/GYM=22%, p<0.05; Eser=13%, p<0.05), and intramedullary CSA (Ward=10%, NS; EX/GYM=79%, p<0.05; Eser=56%, p<0.05), particularly compared with our EX/GYM [18, 20]. In our postmenarcheal cohort, the large advantages in intramedullary cavity expansion are particularly striking. This differential is corroborated in the adult sample of Eser et al. [20], but it is not significant in Ward’s mixed cohort of prepubertal males and females [18]. The coupling of large intramedullary cavities with large periosteal cross-sectional areas occurs with peripheral distribution of diaphyseal BMC from the bone centroid. Theoretically, this efficient and effective adaptation yields higher bending/torsional strength independent of BMC, increasing skeletal strength even if mineral resources are limited (mean calcium intake <1,200 mg).
On the whole, discrepancies between Ward’s and Dyson’s Tanner I/II comparisons and the postmenarcheal results of our group and Eser et al. [10, 18, 20] suggest a heightened geometric response to mechanical loading during perimenarcheal growth. However, the noted discrepancies may also be partially attributed to site difference (50% vs. 33% and 66%) and/or Ward’s representation of males and females. Alternatively, in Ward’s and Dyson’s younger subjects, loading exposure may have been too short to accumulate the high levels of adaptation observed in postmenarcheal subjects. Further research is necessary to examine the relative importance of mechanical loading during specific developmental phases.
Loading-enhanced geometric expansion has been described in other populations [13–15, 26, 32]. In particular, adult females exposed to weight lifting (WTLIFT) for 6.5 to 14 years (apparently postmenarche) exhibited similar patterns and magnitudes of distal radius adaptation to our postmenarcheal EX/GYM [26]. Diaphyseal advantages were comparable for cortical CSA (WTLIFT=26%, EX/GYM=25%; unadjusted WTLIFT=16.4mm2, EX/GYM=20.9mm2) and cortical vBMD (WTLIFT =−0.3%, EX/GYM =−0.7%; unadjusted WTLIFT=−3.7 mg/cm3, EX/GYM=−11.1 mg/cm3). For the metaphysis, EX/GYM loading advantages were similar to weight lifters for total CSA (WTLIFT=19%, EX/GYM=26%; unadjusted WTLIFT=45.1 mm2, EX/GYM=48.5 mm2) and SSI (WTLIFT=41%, EX/GYM=43%; unadjusted WTLIFT=91.6 mm3, EX/GYM =112.4 mm3) but were slightly lower in EX/GYM for cortical CSA (WTLIFT=38%, EX/GYM=25%; unadjusted WTLIFT=27.4 mm2, EX/GYM=15.7 mm2). In contrast, EX/GYM trabecular vBMD relative and absolute advantages were more than double the weight lifter advantages (WTLIFT=9%, EX/GYM=19%; unadjusted WTLIFT=17.8 mg/cm3, EX/GYM= 40.4 mg/cm3). Unfortunately, intramedullary CSA was not reported for comparison [26].
Bone geometric advantages have also been reported for the playing vs. nonplaying arm in postmenarcheal racquet sport players (mean ages 14.5 to 26.5 years.), with lower advantages than in our postmenarcheal EX/GYM [13–15]. Compared with postmenarcheal tennis players (started tennis premenarche), EX/GYM distal radius advantages were two to ten times higher than playing arm advantages for diaphyseal radius cortical geometry [14, 15] and diaphyseal radius/humerus total geometry [13–15]. At the radial metaphysis, EX/GYM advantages for trabecular vBMD and strength (IBS) were 2.5 to four times the playing arm advantage in older tennis players [15]. EX/GYM advantages in distal radius diaphyseal strength were 1.5 to three times greater than playing arm advantages at the diaphyseal humerus [13, 15].
Trends toward differences in cortical vBMD were strikingly similar for comparisons involving tennis players (playing vs. nonplaying arm: 50% humerus −0.7%, NS [15]; distal humerus −2.2% [32]; distal radius −0.8% [33]), weight lifters (distal radius: −0.3%) [26], premenarcheal gymnasts (mid-radius: −0.9%, NS) [18], our postmenarcheal EX/GYM (distal radius: −0.7%, NS) and Eser’s adult ex-gymnasts (proximal radius: −2.6%, p<0.05). These similarities imply minimal cortical vBMD adaptation in response to loading across maturational levels and loading modalities. Alternatively, because pQCT cannot discern variation in tissue microarchitecture or composition, it is possible that cortical vBMD adaptations to loading may have escaped detection in most studies. The results of Eser et al. suggest that mechanical loading may actually reduce cortical vBMD [20]; our study and the others discussed may have been under-powered to detect loading-related differences in cortical vBMD. Further human research is necessary to elucidate the nature of bone adaptation to loading at the microarchitectural level.
Overall, the large (79%) EX/GYM advantages in intramedullary canal dimensions are the most intriguing findings reported here (Fig. 1c). Furthermore, intramedullary CSA was hypervariable, as indicated by large confidence intervals for the gymnast advantage, suggesting genetic diversity for this parameter. This observation was corroborated by both Eser and Ward et al. In contrast, Ward et al. did not corroborate our large, significant EX/GYM advantages in intramedullary dimensions [18]. Certainly, sexual dimorphism may explain this disparity, as Ward’s sample included both genders, and they discuss strong, nonsignificant trends toward both a sex interaction and a female gymnast advantage (+22%) [10, 18]. However, we surmise that discordance between our intramedullary results and Ward’s is primarily due to maturity-specific variation in loading response, with midpubertal loading promoting intramedullary canal expansion. This hypothesis is supported by the results of Eser et al., which demonstrated large gymnast advantages in IMCSA. Nonetheless, within the limited maturity range represented by our subjects (gynecological age, 0.25 to 7.33 years), gynecological age was not significantly correlated with 33% radius IMCSA and did not act as a significant covariate in IMCSA ANCOVA. However, as our analyses did not include prepubertal girls, we cannot rule out a relationship between these variables across a broader maturational spectrum. Clearly, in our young postmenarcheal girls, the significant correlations between gynecological age and other bone outcomes highlight the importance of accounting for this variable in this maturity group.
Racquet sport athletes demonstrate variable advantages in intramedullary dimensions [14]. At the distal radius, Ducher et al. reported a moderate playing arm advantage in adult tennis players for intramedullary volume (MRI, 13.3%) [14]. In contrast, at the midhumerus, Bass et al. demonstrated playing arm advantages in IMCSA of only 3–4% in premenarcheal (pre/early pubertal) tennis players, with no advantage at the distal humerus [13]. Postmenarcheal comparisons (mean age 14.5 years) indicated smaller intramedullary dimensions in the loaded humerus at both distal and middiaphyses, leading Bass et al. to infer that peri- and postmenarcheal loading induce endocortical contraction [13]. In a study of adult racquet sport players (loaded premenarche), Kontulainen et al. detected no significant difference, but reported a trend toward lower mid-humeral intramedullary dimensions [15]. Corroborating results from all three studies, Haapasalo evaluated adult male tennis players, demonstrating no playing arm advantage at the midhumerus, but significant intramedullary expansion at the 30% radius (26%) [32]. Furthermore, comparisons of dominant arm advantages for players vs. controls indicated relative intramedullary expansion in the impact-loaded radius (23%) but a trend toward relatively lower intramedullary expansion in the humerus [32]. Similarly, Eser’s results in adult ex-gymnasts demonstrate no significant advantage in humeral intramedullary width (+3.9%, p>0.58), but cortical thickness was greater (+14.7%, p<0.0001); at the radius, ex-gymnasts had larger intramedullary cavities (56%, p<0.05), without thicker cortices (−4.6%, p>0.28) [20]. All of the above suggest a bone-specific response to loading, in which the humerus exhibits intracortical contraction or limited intramedullary expansion, contrasting with marked radial intramedullary expansion.
The large advantages in bone parameters exhibited by our EX/GYM are particularly striking because our sample is comprised of both elite and nonelite gymnasts. In addition, many EX/GYM (44%) are no longer active gymnasts; 31% of EX/GYM retired more than 5 years (5.4 to 7.6 years) prior to the pQCT scans, with recent physical activity patterns similar to those of the nongymnast group. Of the ex-gymnasts, all retired circum-menarche (4 months premenarche to 16 months postmenarche), suggesting that pre- and perimenarcheal gymnastic exposure may generate benefits that persist over the short to medium term, transcending continued growth and maturation. It is possible that greater benefits would be observed in elite gymnasts who maintain loading exposure throughout development. However, the similar scale of advantages demonstrated by the ex-gymnasts of Eser et al. suggests that postpubertal loading may not contribute substantively to adult benefits. It should be noted that artistic gymnastics provides an excellent model of mechanical loading but is not recommended as a widespread intervention to improve bone health.
Limitations
Caution should be exercised with respect to the interstudy comparisons made in this discussion, as different pQCT methodologies for scan placement and analysis may generate discrepant results. Scan position may be particularly influential for pQCT of the radial metaphysis. Specifically, we evaluated pQCT positional variation in the context of physical maturity. Gynecological age was positively correlated with 4% scan distance from the articular reference, suggesting continued physeal growth/bone lengthening in the early postmenarcheal years. Use of a physeal reference should have accounted for this maturational variability. However, if developmental differences were inadequately addressed by this technique, girls of higher gynecological ages may have been measured closer to the diaphysis, lowering CSA results. Our adjusted results account for gynecological age, and by proxy, scan placement. Supplemental ANCOVAs, substituting scan distance for gynecological age, identified similar or larger gymnast advantages for all comparisons except fall IBS (p=0.14), supporting use of the physis/scar reference in this cohort. Finally, Eser’s results, using an articular reference in older postmenarcheal subjects, corroborate our large gymnast advantages. In future work, the use of two metaphyseal scans may further improve reliability of metaphyseal assessment [34].
The relatively small number of subjects in this analysis should be considered a limitation. A larger sample size might have yielded differences with narrower confidence intervals and a lower likelihood of both type I and II errors. Sample size calculations based on Eser’s results suggest that our metaphyseal vBMD comparisons were underpowered (required sample size 2000). However, Eser’s negligible effect sizes indicate no actual difference between adult retired gymnasts and non-gymnasts for this variable. As previously noted, in a slightly larger sample, Ward et al. demonstrated a significant total vBMD advantage in pre-pubertal gymnasts. These contrasting results suggest that during puberty, there is a counterbalance between geometric expansion and enhancement of total vBMD in gymnasts. Our cohort is postpubertal (lower gynecological age than Eser’s cohort) and is comprised of both active and retired gymnasts (low mean retirement interval vs. Eser’s cohort). In this context, our intermediate results for metaphyseal vBMD (small effect) may reflect pubertal expansion effects and/or partial maintenance of benefit due to lower mean post-retirement interval. A moderately larger sample size may support this hypothesis.
In our observational study, causality cannot be determined by correlations and observed interindividual differences. Genetic differences, self-selection bias, or unmeasured variables may explain some of the variation attributed to gymnastic exposure in this cohort. Finally, actual growth processes cannot be assessed effectively by this cross-sectional design. Future longitudinal studies should examine maturity-specific growth under contrasting loading conditions in a large number of subjects, applying consistent pQCT protocols and observing the same individuals over time.
Conclusion
These results demonstrate advantages in bone geometry, density, and theoretical strength for postmenarcheal girls exposed to gymnastic loading during childhood and perimenarcheal growth. In particular, perimenarcheal gymnastic exposure appears to generate large advantages in radius intramedullary cavity dimensions that had not been previously reported in gymnasts but have recently been corroborated by Eser et al. [20]. These results suggest that continued mechanical loading through menarche is particularly advantageous, enhancing bone development to yield larger bones with greater theoretical strength. Additional research is necessary to evaluate mode of adaptation and persistence of skeletal benefits attributed to mechanical loading during specific phases of growth.
Acknowledgments
We are extremely grateful to our subjects and their parents for their dedication; without them, we would have no results. We would also like to thank Jill Kanaley, Cathy Riley, Sue Hemingway, and Rebecca Hickman for their assistance, as well as Joseph Spadaro, who provided the pQCT scanner. Dan Schiferl (Bone Diagnostic, Inc.) provided technical support for maintenance and use of the pQCT scanner. This study was funded by the State University of New York Upstate Medical University (Bridge Grant).
Footnotes
Conflicts of interest None.
Contributor Information
J. N. Dowthwaite, Email: dowthwaj@upstate.edu, Department of Orthopedic Surgery, SUNY Upstate Medical University, Institute for Human Performance, 505 Irving Avenue, Rm 3206, Syracuse, NY 13210, USA
T. A. Scerpella, Department of Orthopedic Surgery, SUNY Upstate Medical University, Institute for Human Performance, 505 Irving Avenue, Rm 3206, Syracuse, NY 13210, USA
References
- 1.Daly RM, Rich PA, Klein R, Bass S. Effects of high-impact exercise on ultrasonic and biochemical indices of skeletal status: a prospective study in young male gymnasts. J Bone Miner Res. 1999;14 (7):1222–1230. doi: 10.1359/jbmr.1999.14.7.1222. [DOI] [PubMed] [Google Scholar]
- 2.Bass S, Pearce G, Bradney M, Hendrich E, Delmas PD, Harding A, Seeman E. Exercise before puberty may confer residual benefits in bone density in adulthood: studies in active prepubertal and retired female gymnasts. J Bone Miner Res. 1998;13:500–507. doi: 10.1359/jbmr.1998.13.3.500. [DOI] [PubMed] [Google Scholar]
- 3.Cassell C, Benedict M, Specker B. Bone mineral density in elite 7 to 9 year-old female gymnasts and swimmers. Med Sci Sports Exerc. 1996;28:1243–1246. doi: 10.1097/00005768-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Dowthwaite JN, DiStefano JG, Ploutz-Snyder RJ, Kanaley JA, Scerpella TA. Maturity and activity-related differences in bone mineral density: Tanner I vs II and gymnasts vs. non-gymnasts. Bone. 2006;39:895–900. doi: 10.1016/j.bone.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Gero N, Cole J, Kanaley J, van der Meulen M, Scerpella T. Increased bone accrual in premenarcheal gymnasts: a longitudinal study. Ped Ex Sci. 2005;17:43–55. [Google Scholar]
- 6.Laing EM, Wilson AR, Modlesky CM, O’Connor PJ, Hall DB, Lewis RD. Initial years of recreational artistic gymnastics training improves lumbar spine bone mineral accrual in 4- to 8-year-old females. J Bone Miner Res. 2005;20:509–519. doi: 10.1359/JBMR.041127. [DOI] [PubMed] [Google Scholar]
- 7.Nurmi-Lawton JA, Baxter-Jones AS, Mirwald RL, Bishop JA, Taylor P, Cooper C, New SA. Evidence of sustained skeletal benefits from impact-loading exercise in young females: a 3-year longitudinal study. J Bone Miner Res. 2004;19:314–322. doi: 10.1359/JBMR.0301222. [DOI] [PubMed] [Google Scholar]
- 8.Scerpella TA, Davenport M, Morganti CM, Kanaley JA, Johnson LM. Dose related association of impact activity and bone mineral density in pre-pubertal girls. Calcif Tissue Int. 2003;72:24–31. doi: 10.1007/s00223-001-1131-x. [DOI] [PubMed] [Google Scholar]
- 9.Taaffe DR, Robinson TL, Snow CM, Marcus R. High-impact exercise promotes bone gain in well-trained female athletes. J Bone Miner Res. 1997;12(2):255–260. doi: 10.1359/jbmr.1997.12.2.255. [DOI] [PubMed] [Google Scholar]
- 10.Dowthwaite JN, Flowers PPE, Spadaro JA, Scerpella TA. Bone geometry, density, and strength indices of the distal radius reflect loading via childhood gymnastic activity. J Clin Densitom. 2007;10(1):65–75. doi: 10.1016/j.jocd.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin RB. Determinants of the mechanical properties of bones. J Biomech. 1991;24(Suppl 1):79–88. doi: 10.1016/0021-9290(91)90379-2. [DOI] [PubMed] [Google Scholar]
- 12.Sievänen H, Kannus P, Nieminen V, Heinonen A, Oja P, Vuori I. Estimation of various mechanical characteristics of human bones using dual energy X-ray absorptiometry: methodology and precision. Bone. 1996;18:17S–27S. doi: 10.1016/8756-3282(95)00376-2. [DOI] [PubMed] [Google Scholar]
- 13.Bass SL, Saxon L, Daly RM, Turner CH, Robling AG, Seeman E, Stuckey S. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res. 2002;17(12):2274–2280. doi: 10.1359/jbmr.2002.17.12.2274. [DOI] [PubMed] [Google Scholar]
- 14.Ducher G, Courteix D, Même S, Magni C, Viala JF, Benhamou CL. Bone geometry in response to long-term tennis playing and its relationship with muscle volume: a quantitative magnetic resonance imaging study in tennis players. Bone. 2005;37(4):457–466. doi: 10.1016/j.bone.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Kontulainen S, Sievänen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res. 2003;18:352–359. doi: 10.1359/jbmr.2003.18.2.352. [DOI] [PubMed] [Google Scholar]
- 16.Dowthwaite JN, Kanaley JA, Hickman RM, Spadaro JA, Scerpella TA. Muscle indices do not fully account for enhanced upper extremity bone mass and strength in gymnasts. J Musculoskelet Neuronal Interact. 2009;9(1):2–14. [PubMed] [Google Scholar]
- 17.Dyson K, Blimkie CJR, Davison KS, Webber CE, Adachi JD. Gymnastic training and bone density in pre-adolescent females. Med Sci Sports Exerc. 1997;29(4):443–450. doi: 10.1097/00005768-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Ward KA, Roberts SA, Adams JE, Mughal MZ. Bone geometry and density in the skeleton of pre-pubertal gymnasts and school children. Bone. 2005;36:1012–1018. doi: 10.1016/j.bone.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Ward KA, Roberts SA, Adams JE, Lanham-New S, Mughal MZ. Calcium supplementation and weight bearing physical activity—do they have a combined effect on the bone density of pre-pubertal children? Bone. 2007;41:496–504. doi: 10.1016/j.bone.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Eser P, Hill B, Ducher G, Bass SL. Skeletal benefits after long-term retirement in former elite female gymnasts. J Bone Miner Res. 2010 doi: 10.1359/jbmr.090521. [DOI] [PubMed] [Google Scholar]
- 21.Bailey DA. The Saskatchewan pediatric bone mineral accrual study: bone mineral acquisition during the growing years. Int J Sports Med. 1997;18:S191–S194. doi: 10.1055/s-2007-972713. [DOI] [PubMed] [Google Scholar]
- 22.Dowthwaite JN, Hickman RM, Ploutz-Snyder RJ, Kanaley JA, Spadaro JA, Scerpella TA. Distal radius strength: a comparison of DXA-derived vs. pQCT-measured parameters in adolescent females. J Clin Densitom. 2009;12(1):42–53. doi: 10.1016/j.jocd.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Zemel B, Bass S, Binkley T, Ducher G, MacDonald H, McKay H, Moyer-Mileur L, Shepherd J, Specker B, Ward K, Hans D. Peripheral quantitative computed tomography in children and adolescents: the 2007 ISCD pediatric official positions. J Clin Densitom. 2008;11(1):59–74. doi: 10.1016/j.jocd.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Rauch F, Schoenau E. Peripheral quantitative computed tomography of the distal radius in young subjects—new reference data and interpretation of results. J Musculoskelet Neuronal Interact. 2005;5:119–126. [PubMed] [Google Scholar]
- 25.Rauch F, Neu C, Manz F, Schoenau E. The development of metaphyseal cortex—implications for distal radius fractures during growth. J Bone Miner Res. 2001;16(8):1547–1555. doi: 10.1359/jbmr.2001.16.8.1547. [DOI] [PubMed] [Google Scholar]
- 26.Heinonen A, Sievänen H, Kannus P, Oja P, Vuori I. Site specific skeletal response to long-term weight training seems to be attributable to principal loading modality: a pQCT study of female weightlifters. Calcif Tissue Int. 2002;70:469–474. doi: 10.1007/s00223-001-1019-9. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald H, Kontulainen S, Petit M, Janssen P, McKay H. Bone strength and its determinants in pre- and early pubertal boys and girls. Bone. 2006;39(3):598–608. doi: 10.1016/j.bone.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 28.Ruff C. Growth tracking of femoral and humeral strength from infancy through late adolescence. Acta Paediatr. 2005;94:1030–1037. doi: 10.1111/j.1651-2227.2005.tb02041.x. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J. Statistical power analysis for the behavioral sciences. 2. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. p. 532. [Google Scholar]
- 30.Scerpella TA, Dowthwaite JN, Gero N, Kanaley JA, Ploutz-Snyder RJ. Skeletal benefits of premenarcheal gymnastic activity are retained after activity cessation. Pediatr Exerc Sci. 2010;22(1):21–33. doi: 10.1123/pes.22.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenth RV. Java applets for power and sample size (computer software) 2006 Retrieved November 13, 2009 from http://www.stat.uiowa.edu/~rlenth/Power.
- 32.Haapasalo H, Kontulainen S, Sievänen H, Kannus P, Järvinen M, Vuori I. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone. 2000;27(3):351–357. doi: 10.1016/s8756-3282(00)00331-8. [DOI] [PubMed] [Google Scholar]
- 33.Ashizawa N, Nonaka K, Michikami S, Mizuki T, Amagai H, Tokuyama K, Suzuki M. Tomographical description of tennis-loaded radius: reciprocal relation between bone size and volumetric BMD. J Appl Physiol. 1999;86(4):1347–1351. doi: 10.1152/jappl.1999.86.4.1347. [DOI] [PubMed] [Google Scholar]
- 34.Marjanovic EJ, Ward KA, Adams JE. The impact of accurate positioning on measurements made by peripheral QCT in the distal radius. Osteoporos Int. 2009;20:1207–1214. doi: 10.1007/s00198-008-0778-9. [DOI] [PubMed] [Google Scholar]