Abstract
Although drugs of abuse have different chemical structures and interact with different protein targets, all appear to usurp common neuronal systems that regulate reward and motivation. Addiction is a complex disease that is thought to involve drug-induced changes in synaptic plasticity due to alterations in cell signaling, gene transcription, and protein synthesis. Recent evidence suggests that drugs of abuse interact with and change a common network of signaling pathways that include a subset of specific protein kinases. The best studied of these kinases are reviewed here and include extracellular signal-regulated kinase, cAMP-dependent protein kinase, cyclin-dependent protein kinase 5, protein kinase C, calcium/calmodulin-dependent protein kinase II, and Fyn tyrosine kinase. These kinases have been implicated in various aspects of drug addiction including acute drug effects, drug self-administration, withdrawal, reinforcement, sensitization, and tolerance. Identifying protein kinase substrates and signaling pathways that contribute to the addicted state may provide novel approaches for new pharma-cotherapies to treat drug addiction.
Keywords: protein kinase, addiction, extracellular signal-regulated kinase, cAMP-dependent protein kinase, cyclin-dependent protein kinase 5, protein kinase C, calcium/calmodulin-dependent protein kinase II, Fyn tyrosine kinase
Introduction
The development of addiction involves pathological drug-induced changes in cellular and molecular mechanisms of synaptic plasticity that underlie normal learning and memory.1–3 Common neuronal pathways and molecular adaptations to different drugs of abuse in the limbic system have been identified.4 Recently, a large genetic meta-analysis has identified some common genes and signaling pathways in drug addiction, and protein kinases are central components in this postulated common molecular network.5 Abused drugs stimulate signaling pathways that activate protein kinases, leading to altered gene transcription and protein synthesis, which contribute to long-term changes in synaptic function and neural networks that ultimately result in addiction. Here we review some of the best-studied protein kinases that appear to be pivotal in addiction: extracellular signal-regulated kinase, cAMP-dependent protein kinase, cyclin-dependent protein kinase 5, protein kinase C, calcium/calmodulin-dependent protein kinase II, and Fyn tyrosine kinase. These protein kinases are all widely expressed throughout the brain and in the limbic system.6
Addictive substances can be generally grouped into drugs that interact with monoamine transporters, activate Gi/o-coupled protein receptors, or alter the function of ion channels and ionotropic receptors.7 The psychostimulants cocaine and amphetamines both increase extracellular levels of monoamines, such as dopamine, but they act through different molecular mechanisms: cocaine inhibits the cell surface dopamine transporter (DAT), while amphetamines inhibit DAT and also stimulate dopamine release from vesicles allowing reverse transport of dopamine through DAT. Nicotine also has stimulatory effects through activation of nicotinic acetylcholinergic (nACh) receptors, which are ligand-gated cation channels. Opiates and cannabinoids activate different Gi/o-coupled protein receptors and appear to activate some similar downstream signaling events. Ethanol alters the function of several ion channels and ionotropic receptors and often produces different effects compared with other addictive drugs. We will examine the effects of cocaine, amphetamines, nicotine, opiates, cannabinoids, and ethanol on protein kinases, summarizing the available studies. Importantly, we will also review the effects of manipulating protein kinases in animal models with an emphasis on identifying signaling pathways that could provide new targets for treatment of addiction.
Extracellular Signal-Regulated Kinases
ERKs are serine-threonine protein kinases that are members of the mitogen-activated protein kinase (MAPK) family. Two isoforms have been identified, p44 ERK1 and p42 ERK2, which share similar, though not identical, functions. ERKs are involved in many aspects of neuronal function including control of cell growth, cell differentiation, neuronal survival, and synaptic plasticity.8–10 ERK1 and ERK2 are widely expressed throughout the brain, including in the mesolimbic dopaminergic system. Both are strongly expressed in the amygdala and prefrontal cortex and moderately expressed in the ventral tegmental area (VTA), nucleus accumbens (NAc), and midbrain.6 ERKs are activated by a Ras-Raf-MEK signaling cascade that involves sequential phosphorylation of upstream kinases. Several growth factors such as the brain-derived neurotrophic factor (BDNF) activate receptor tyrosine kinases that recruit the adapter proteins Grb2 and Shc, in turn leading to activation of the GTPase Ras. Ras can also be activated by calcium influx through L-type calcium channels or NMDA receptors through stimulation of Ras protein-specific guanine-nucleotide releasing factor (Ras-GRF1), a Ca2+-activated guanine nucleotide exchange factor expressed in neurons of the central nervous system.11 Ca2+ influx can be enhanced by Gs-coupled neurotransmitter receptors such as the dopamine D1 receptor, which increase protein kinase A (PKA)-mediated phosphorylation and ion channel function.12 Activated Ras in turn activates Raf, which phosphorylates and activates MAPK/ERK kinases (MEK1 and MEK2) that phosphorylate and activate the ERKs.13 ERK activity is also modulated by other signal transduction molecules, such as PKA and dopamine- and cAMP-regulated phospho-protein of 32kDa (DARPP-32, also called PPP1R1B; protein phosphatase 1, regulatory [inhibitor] subunit 1B),12 (Fig. 1) as discussed later. As ERK activity is regulated by dopamine and glutamate receptor stimulation and incorporates signals from many second messenger pathways, ERKs may function as coincidence detectors that combine rewards and contextual information in learning processes.8 Thus, ERK activity has been proposed to play integrative roles in the development of drug addictions.
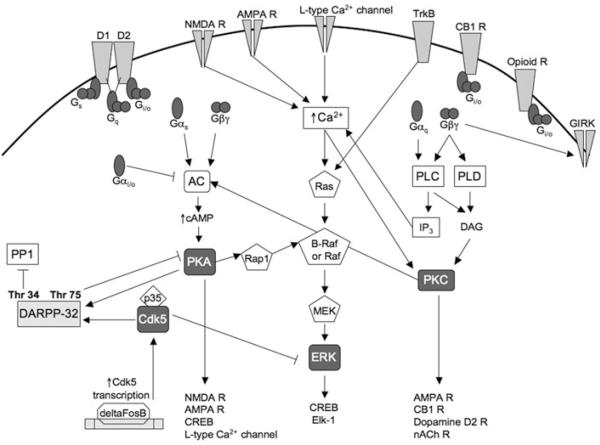
Figure 1.
Diagram of the major kinase signaling cascades involved in drug addiction. Dopamine receptors activate heterotrimeric G-proteins. D1 receptors activate stimulatory Gs, D2 receptors activate inhibitory Gi/o, and D1/D2 heteromers activate stimulatory Gq. Cannabinoid CB1 receptors and opioid receptors also activate Gi/o. Gαs and Gβγ released from Gi/o activate adenylyl cyclase, leading to increased cAMP levels which activate PKA. Induction of the transcription factor deltaFosB increases Cdk5 expression. Differential phosphorylation of DARPP-32 by PKA and Cdk5 inhibits or facilitates PKA signaling. Gβγ also activates GIRK channels leading to inhibition of excitability. Gαq and Gβγ activate PLC and PLD, leading to the activation of PKC. Several downstream PKA and PKC targets are listed at the bottom. Activation of ionotropic glutamate receptors and L-type calcium channels increases intracellular calcium levels, leading to activation of the Ras-Raf-MEK-ERK pathway. Activation of TrkB receptors also activates the ERK pathway, leading to the phosphorylation of transcription factors CREB and Elk-1. Examples of cross-talk between the PKA, PKC, Cdk5, and ERK signaling pathways are illustrated. This diagram is a simplification that does not incorporate differential expression of kinases in different brain regions and cell types.
Downstream targets of ERKs include transcription factors and immediate early genes that play roles in mediating drug-induced synaptic plasticity, including ribosomal S6 kinase (RSK) and mitogen- and stress-stimulated kinase 1 (MSK1). These downstream kinases phosphorylate and activate the cAMP response element binding protein (CREB). ERKs also can directly phosphorylate the Elk-1 transcription factor. Activation of CREB and Elk-1 can lead to the transcription of the immediate early genes c-fos and zif268, which are induced by several drugs of abuse.2
Effects of Addictive Drugs on ERK Signaling
Administration of addictive drugs to rats and mice can activate ERK in many of the brain regions that constitute or are targets of the mesolimbic dopaminergic system, including the VTA, NAc, prefrontal cortex, bed nucleus of the stria terminalis (BNST), and the amygdala.14,15 ERK activation can be measured as a drug-induced increase in phosphorylated ERK protein (p-ERK) that can be blocked by selective MEK inhibitors, such as U0126, SL327, or PD98059, which prevent ERK activation. Acute cocaine treatment transiently increases p-ERK throughout the striatum through a mechanism that involves activation of D1 dopamine and NMDA receptors. This increase in p-ERK can be blocked by systemic injection of SL327.16,17 SL327 also inhibits cocaine-induced c-fos expression and Elk-1 hyperphosphorylation. Acute and repeated administration of cocaine also increases p-ERK in the prefrontal cortex, BNST, and amygdala. In the VTA, cocaine increases levels of p-ERK, but only after repeated administration.18
Unlike cocaine, amphetamine acutely increases p-ERK in the VTA,19 and like cocaine, it increases p-ERK in the dorsal striatum20,21 and NAc.22 Amphetamine also induces striatal expression of preproenkephalin and preprodynorphin through an ERK-dependent mechanism, since this response is attenuated by systemic or intrastriatal injection of MEK inhibitors.20 Nicotine, like cocaine, also increases p-ERK in the prefrontal cortex and amygdala.23
Acute administration of delta (9)-tetrahydrocannabinol (THC), the major active compound in marijuana, also increases ERK phosphorylation in the dorsal striatum, in ways that parallel effects of stimulants. Unlike stimulants, THC increases ERK phosphorylation in the cerebellum as well.24,25 ERK phosphorylation is also increased by acute administration of opiates. Morphine increases p-ERK in the VTA18 and cerebral cortex.26 In general, stimulants, cannabinoids, and opiates increase p-ERK levels in a distinctive pattern that includes the NAc, lateral bed nucleus of the stria terminalis, central amygdala, and deep layers of the prefrontal cortex.14 Inhibition of MEK prevents these drug-induced increases in p-ERK.20,25
In contrast to drugs listed above, ethanol inhibits ERK phosphorylation. Acute administration of alcohol decreases levels of p-ERK in the amygdala, dorsal striatum, cerebellum, cerebral cortex, and hippocampus.27–29 The mechanism by which ethanol reduces ERK phosphorylation is unknown. However, in rats treated chronically with ethanol, hepatic ERK phosphorylation is decreased in association with elevated levels of the lipid peroxidation product 4-hydroxynonenal, which inhibits ERK phosphorylation in vitro.30
ERK activation and the downstream changes in transcription evoked by stimulant drugs and cannabinoids are dependent on the activation of dopamine and NMDA receptors. Dopamine antagonists prevent the increases in ERK phosphorylation that result from cocaine,16 amphetamine,21 methamphetamine,31 or THC administration.32 Dopamine D1 receptor knockout mice fail to display drug-induced increases in p-ERK17,33 or ERK-dependent increases in phosphorylated CREB (p-CREB)33 or c-fos levels.17 Inhibition of NMDA receptors also prevents the ERK phosphorylation that results from treatment with cocaine,33 amphetamine,21 THC,32 or the cannabinoid receptor 1 (CB1) agonist WIN-55212-2.34 The effect of THC on ERK is mediated through Ras. Knockout mice lacking Ras-GRF1 do not show THC-mediated increases in p-ERK in the dorsal striatum or cerebellum.24
Studies with MEK inhibitors indicate that several drugs of abuse regulate the activity of transcription factors and increase gene expression through ERK-dependent mechanisms. For example, cocaine increases c-fos mRNA and phosphorylation of Elk-1, and these effects are inhibited by the MEK inhibitor SL327.16 SL327 also inhibits increases in c-fos and egr-2 mRNA induced by methamphetamine,35 and increases in proenkephalin and preprodynorphin mRNA induced by amphetamine.20 THC increases Elk-1 phosphorylation and levels of zif268 mRNA levels, and these increases are inhibited by SL327 as well.32
Drug-stimulated ERK activity is regulated by dephosphorylation through a network of phosphatases whose activities are regulated by PKA.36 The striatal-enriched protein tyrosine phosphatase (STEP; PTPN5 protein tyrosine phosphatase, nonreceptor type 5 striatum-enriched) inactivates ERK by dephosphorylating a tyrosine residue within its activation loop. The ability of STEP/PTPN5 to dephosphorylate ERK is lost by PKA-mediated phosphorylation of STEP/PTPN5 at a regulatory serine within its ERK-binding domain. This serine can be dephosphorylated by protein phosphatase 1 (PP1), and PKA-phosphorylated DARPP-32 inhibits PP1. Therefore, in the absence of DARPP-32 inhibition, PP1 can dephosphorylate STEP/PTPN5, allowing it to bind, dephosphorylate, and inactivate ERK. In addition to suppressing STEP activity, DARPP-32 also mediates drug-induced increases in ERK activity by promoting the phosphorylation and activation of MEK. These mechanisms explain why in DARPP-32 knockout mice, cocaine, amphetamine, nicotine, THC, or morphine fail to increase ERK phosphorylation in the NAc.21
Calcium/calmodulin-dependent protein kinases (CaMKs) also regulate activation of ERK by drugs of abuse. KN93, which inhibits type II CaMK (CaMKII), prevents nicotine-mediated increases in p-ERK in mouse cortical neurons.37 Inhibition of CaMKI and its upstream activator CaMK kinase prevents NMDA-mediated increases in p-ERK in mouse hippocampal neurons.38 Given the role of NMDA receptors in drug-induced ERK activation, it is possible that CaMKI is also involved in the activation of ERK by drugs of abuse.
ERK in Addiction-Related Behaviors
Sensitization
Depending on the dose, acute exposure to many addictive drugs increases or decreases motor activity. Repeated drug administration in specific patterns can produce psychomotor sensitization, an increase in locomotor activity, or stereotypy that has been observed with stimulants39 and alcohol.40 Psychomotor sensitization is associated with increased drug reward,41 relapse,42 and consumption43 in rodents. Pretreatment of rodents with systemic SL327 prevents the hyperlocomotion induced by acute injections of cocaine,44 amphetamine,20,44 and methamphetamine.35 The development of psychomotor sensitization is prevented by pretreatment with intra-VTA injections of SL327 in rats given repeated injections of cocaine45 and by systemic pretreatment with SL327 in mice given repeated injections of amphetamine.21 These data indicate that ERK activity is necessary for the expression of hyperlocomotion and for the psychostimulant induction of psychomotor sensitization.
Drug Tolerance
Tolerance can occur with repeated drug administration. The development of tolerance to the hedonic effects of drugs may favor escalation of drug consumption and development of addiction.46 Instead of hyperlocomotion, acute cannabinoid treatment produces hypolocomotion in rodents. Pretreatment with systemic SL327 does not prevent the development of hypolocomotion induced by acute THC injections. Such pretreatments do prevent the development of tolerance to the hypolocomotor effects of repeated THC injection in mice.25 In summary, ERK activity appears to be important for the expression of hyperlocomotor activity from acute administration of many addictive drugs, the development of psychomotor sensitization to psychostimulants, and the development of tolerance to the hypolocomotor effects of cannabinoids.
Drug Withdrawal and Relapse
Addiction is associated with a high risk of relapse long after physical signs of withdrawal have ceased. Relapse can occur after years of abstinence. Cues previously associated with a drug may trigger relapse even after a lengthy period of abstinence. In rat models of drug self-administration followed by prolonged withdrawal (30 days), the presentation of cues previously associated with cocaine47 or alcohol48 can increase levels of p-ERK in the amygdala. Administration of an NMDA receptor antagonist decreases p-ERK and drug-seeking behavior, suggesting that p-ERK facilitates cocaine seeking through an NMDA-dependent mechanism.47,49
Incubation of Drug Craving
The “incubation” of drug craving is a phenomenon whereby cue-induced drug-seeking progressively increases over time during withdrawal. After rats undergo repeated self-administration sessions (for instance, 6 h of cocaine self-administration daily for 10 days), during which cocaine is associated with discrete cues, subsequent cue-induced drug-seeking (nonreinforced responding on the previously active lever) increases over time. Responses are significantly higher after 30 days of cocaine withdrawal than after short-term withdrawal (1–7 days).47,50 This time-dependent increase in responding is associated with increased p-ERK in the central amygdala47 and increased levels of BDNF in the VTA, NAc, and amygdala.50 These increases in BDNF and p-ERK appear to facilitate drug seeking. Thus, BDNF infusions into the VTA increase cue-induced drug seeking for up to 30 days after cocaine withdrawal.51 This effect of BDNF is ERK dependent since it is reversed by intra-VTA infusions of the MEK inhibitor U0126.51 Even in the absence of BDNF infusion, U0126 injections into the central amygdala decrease drug seeking during cocaine withdrawal,47 indicating that ERK activity in the central amygdala is required for drug-seeking during cocaine withdrawal.47 This requirement is specific for cue-induced cocaine seeking but not for reinforced responding. Microinjection of U0126 into the amygdala does not reduce self-administration of cocaine or consumption of palatable foods.47
Conditioned Place Preference
Conditioned place preference (CPP) is a conditioning procedure whereby an animal is injected with a drug in one environmental context and with vehicle in another context (the conditioning phase). After conditioning, the animal is then tested for CPP expression by allowing it to choose between drug- and vehicle-paired contexts. If the animal spends more time in the drug-paired context, the drug is thought to be rewarding. Activation of ERK is necessary for the development of drug-induced CPP. Pretreatment with systemic injections of SL327 during the conditioning phase prevents the development of cocaine-,16 methamphetamine-,35 THC-,32 and morphine-induced CPP.44 A different MEK inhibitor, PD98059, infused directly into the NAc, also inhibits the development of methamphetamine-induced CPP.22 Established cocaine CPP increases phosphorylation of ERK, CREB, and Elk-1 in the rat NAc. These changes are attenuated by intra-VTA injections of U0126.52 Thus ERK activity appears to be involved in mediating the rewarding properties of psychostimulants, cannabinoids, and opiates.
Memory Reconsolidation
When long-term memories are retrieved, they are transiently labile and susceptible to modification by environmental stimuli through a process called reconsolidation.53 The memory of an environmental cue associated with an addictive drug is subject to reconsolidation that is dependent on ERK activity. In rats trained to develop cocaine CPP, an injection of U0126 into the NAc core immediately after CPP testing prevents subsequent expression of CPP on retesting.52 The specific retrieval of the long-term memory is crucial for MEK inhibition to affect this memory. Rats treated with U0126 without a prior CPP test that would retrieve the long-term association did not show a deficit in CPP expression in subsequent tests.52 MEK inhibition also affects CPP memory reconsolidation for other addictive drugs. PD98059 injections into the VTA inhibit reconsolidation of methamphetamine CPP.31 Systemic SL327 injections inhibit reconsolidation of morphine CPP44 in rodents. ERK activation ultimately results in increased protein synthesis. Inhibition of protein synthesis using systemic anisomycin during long-term memory retrieval also impairs reconsolidation of cocaine44 and morphine CPP.54 In addition to CPP reconsolidation, ERK activation is crucial for the reconsolidation of other forms of memory. Inhibition of MEK with U0126 injections into the lateral nucleus of the amygdala inhibits reconsolidation of auditory fear conditioning.55 Intracerebroventricular injection of U0126 inhibits reconsolidation of object recognition.56
Differential Effects of ERK1 and ERK2 in Responses to Drugs of Abuse
Current MEK inhibitors do not distinguish between ERK1 and ERK2. However, studies with ERK1 knockout mice suggest that ERK1 and ERK2 play different roles in some responses to addictive drugs. ERK1 knockout mice show increased ERK2-mediated signaling.57 These mice also show enhanced development of cocaine-induced psychomotor sensitization58 and much greater cocaine-58 and morphine-induced CPP than wild-type mice.57 In addition, place conditioning for cocaine increases phosphorylation of ERK2 but not ERK1 in the rat NAc.52 These results suggest that ERK2 might be the more important ERK isoform in mediating drug-induced CPP and sensitization to cocaine.
cAMP-Dependent Protein Kinase
PKA is multifunctional and is involved in many aspects of neuronal function including synaptic plasticity,59,60 exocytosis,61 and gene transcription.62 PKA can also modulate other second-messenger pathways such as the MAPK signaling pathway.60 PKA is expressed throughout the brain, where it is particularly abundant in the cortex, thalamus, and amygdala and moderately abundant in the midbrain, NAc, and VTA.6
PKA is a tetramer comprising two regulatory subunits and two catalytic subunits. There are four regulatory subunit isoforms (RIα, RIβ, RIIα, RIIβ) and three catalytic isoforms (Cα, Cβ, Cγ); all are widely expressed in the brain.59 As a tetramer, PKA is inactive. Cyclic adenosine 3′, 5′-monophosphate (cAMP), produced by adenylyl cyclase, activates PKA by binding to the regulatory subunits in ways that result in the release and nuclear translocation of active catalytic subunits.63 PKA activity can be measured as increased PKA catalytic subunit translocation63 or as increased PKA-mediated phosphorylation of specific substrates such as the α-amino-3-hydroxy-5-methyl-isoxazolepropionic acid (AMPA) receptor GluR1 subunit Ser-84564 or the NMDA receptor NR1 subunit Ser-897.65
Activation of PKA is dependent on cAMP produced by the activation of one or more of the nine isoforms of adenylyl cyclase that are activated or inhibited in complex manners by G-proteins.66 All adenylyl cyclases are activated by Gαs. AC5 is activated by Gαolf ; AC2, AC4, and AC7 are activated by free Gβγ subunits released from Gi/o; and AC1, AC5, AC6, and AC8 are inhibited by Gαi/o.
Addictive drugs acutely increase levels of extracellular dopamine in the NAc,4 activating adenylyl cyclase and PKA via stimulation of Gs-and Golf-coupled D1 receptors.67 Dopamine also activates D2 receptors coupled to Go, leading to inhibition of several adenylyl cyclase isoforms. Dopamine activation of D2 receptors releases Gβγ subunits, which exert multiple effects that include enhancement of AC2 and AC4 activation, stimulation of G protein-regulated inwardly rectifying K+ (GIRK) channels, and inhibition of L-, N-, and P/Q-type calcium channels. In addition to stimulating dopamine release in the striatum, opiates can activate adenylyl cyclase by binding to opioid receptors that couple to Gi/o.68 The net effect of Gβγ on ion channel function is well established and results in decreased neuronal excitability and neurotransmitter release.67 Gβγ-mediated enhancement of adenylyl cyclase activity by Gi/o-coupled receptors has been documented in vitro and may contribute to signaling in the NAc. Increased firing of accumbal neurons upon co-activation of D1 and D2 receptors appears to involve Gβγ stimulation of adenylyl cylcase.69 Finally, adenylyl cyclases can be modulated by protein kinases, including protein kinase C (PKC), which activates AC2, AC4, and AC7.66
One important downstream regulator of dopaminergic signaling is DARPP-32. DARPP-32 is highly expressed in striatal neurons70 and can act as a protein kinase inhibitor or a protein phosphatase inhibitor, depending on the site of DARPP-32 phosphorylation. When PKA phosphorylates DARPP-32 on Thr-34, it serves as an inhibitor of PP-1.71 Activation of dopaminergic signaling by electrical stimulation of mouse striatal slices or by acute systemic injections of cocaine in mice increases PKA-mediated phosphorylation of DARPP-32 on Thr-34, which then inhibits PP-1 mediated dephosphorylation of PKA substrates,72 effectively amplifying dopamine-stimulated phosphorylation by PKA. Cyclin-dependent protein kinase 5 (Cdk5, discussed in the next section) phosphorylates DARPP-32 at a different site. This Thr-75 phosphorylation renders DARPP-32 an inhibitor of PKA73 and antagonizes several effects of dopamine in the striatum.
PKA phosphorylates NR1 subunits,65 which leads to increased NMDA receptor function.74 Activation of dopamine D1 receptors induces PKA-mediated phosphorylation of NR1 subunits and subsequent phosphorylation and activation of the transcription factor CREB in rat striatal neurons.75 D1 receptor-mediated CREB phosphorylation is blocked by the NMDA receptor antagonist MK801 but not by either of the MEK inhibitors PD98059 or U0126.75 However, PKA can indirectly activate ERK by facilitating the activation of B-Raf through the small G-protein Rap1; B-Raf then phosphorylates and activates MEK.60 Thus, activation of α7 nACh receptors by nicotine stimulates the ERK pathway in a PKA-dependent manner in mouse hippocampal neurons.76
PKA can contribute to drug-induced synaptic plasticity. PKA phosphorylates GluR1 subunits of AMPA receptors, which promotes AMPA receptor trafficking to the cell surface.77 Dopamine D1 receptor agonists, SKF81297 and SCH23390, and the PKA activator Sp-cAMPS increase GluR1 cell surface expression in the NAc78 and the hippocampus of rats.79 Drug-induced activation of PKA can thus increase synaptic strength, measured electrophysiologically as an increase in the ratio of AMPA- to NMDA-mediated current. This provides a mechanism for drug-induced synaptic plasticity. In addition to AMPA currents, PKA can also regulate NMDA-stimulated currents. In rat midbrain slices, acute treatment with cocaine produces a delayed increase in NMDA-mediated currents in VTA dopaminergic neurons that is blocked by the PKA inhibitor Rp-cAMPS and by D1/D5 receptor antagonists.80
Effects of Addictive Drugs on PKA Signaling
Acute administration of psychostimulants increases PKA activity and PKA-mediated phosphorylation of downstream targets. In rodents, acute injections of cocaine increase PKA-mediated phosphorylation of DARPP-32, NR1, and GluR1 in the medial prefrontal cortex and NAc,81 as well as phosphorylation of DARPP-32 in the striatum.72 Acute administration of cocaine in mice can increase PKA-mediated activation of protein phosphatase 2A (PP-2A), which then dephosphorylates DARPP-32 at the Cdk5-mediated phosphorylation site (Thr-75), promoting PKA-mediated signaling.72 The effect of chronic cocaine on PKA signaling depends on the species examined. In rats, chronic exposure to cocaine increases the level of Cdk5 in the striatum, attenuating the effects of PKA82 (discussed in the next section). However, chronic treatment of monkeys with cocaine for one year upregulates PKA catalytic subunit mRNA and protein in the NAc,83 suggesting that there may be an overall upregulation of PKA activity in primate NAc by prolonged exposure to cocaine.
In vitro, acute treatment of mouse striatal neurons with nicotine has different effects on PKA activity depending on the nicotine dose. Acute, low doses of nicotine decrease PKA-mediated DARPP-32 phosphorylation, primarily by activating dopamine D2 receptors.84 High doses of nicotine increase PKA-mediated DARPP-32 phosphorylation, mainly by activating D1 receptors.84 In rats treated with nicotine for 14 days, PKA activity is decreased in whole brain homogenates,85 suggesting that chronic nicotine exposure decreases PKA activity in the brain. However, postmortem studies have shown that smokers and former smokers have higher PKA activity and PKA catalytic subunit protein levels in the NAc and ventral midbrain than do nonsmokers.86 These results indicate that prolonged smoking in humans, similar to chronic cocaine treatment in monkeys,83 may upregulate PKA activity in some brain regions that are important for addiction.
Cannabinoids and opiates can increase PKA activity via dopaminergic stimulation of adenylyl cyclase. Acute treatment with CB1 agonists increases PKA activity in mouse striatum87,88 and NAc.88 This increased PKA activity is attenuated by antagonists of the CB187,88 or D1 receptors.88 The effect of chronic cannabinoid treatment is less clear-cut. Chronic treatment with THC in mice increases PKA activity in the cortex89 but can both increase89 and decrease PKA activity in the cerebellum.90 Similar to cannabinoids, acute stimulation of μ-opioid receptors in rat striatal neurons in vitro increases nuclear translocation of the PKA catalytic subunit and increases levels of p-CREB.68 Opiates can increase PKA signaling; this effect can be enhanced during opiate withdrawal.91 Opiates increase PKA-mediated phosphorylation in several brain regions including the NAc,92,93 striatum,92 hippocampus,94,95 and locus coeruleus,96 but do not increase this phosphorylation in the frontal cortex.97 In cultured striatal neurons, morphine activates PKA by activating μ-opioid receptors, leading to the release of Gβγ from Gi3, and subsequent activation of AC2 and AC4.68 In vivo however, morphine-induced activation of PKA in the NAc and dorsal striatum requires activation of D1 receptors coupled to Gαs/olf since it is blocked by the D1 antagonist SCH23390.92
Ethanol can increase PKA activity acutely by inhibiting adenosine reuptake through the type I equilibrative nucleotide transporter, which increases extracellular adenosine levels, activates Golf-coupled adenosine A2a receptors, stimulates adenylyl cyclase, and increases levels of cAMP.98,99 This process leads to nuclear translocation of the PKA catalytic subunit in NG108-15 neuroblastoma × glioma cells in vitro.100 By contrast, chronic ethanol treatment in rats increases PKA activity in the cerebral cortex of rats.101
Adenosine A2a receptors also mediate the synergistic activation of PKA by combined sub-threshold doses of several addictive drugs.102,103 Cannabinoid and opioid receptor agonists can act together or with ethanol to activate PKA at doses that would not normally activate PKA when given alone. Adenosine A2a receptor antagonists block this effect. CB1 receptor agonists acutely increase PKA-dependent DARPP-32 phosphorylation at Thr-34 in mouse dorsal striatum and NAc.88 This increase is blocked by administration of the A2a receptor antagonist KW6002.88
Withdrawal from chronic drug exposure may induce PKA-mediated synaptic plasticity. In rats, prolonged withdrawal from chronic cocaine increases corticotropin releasing factor (CRF)-mediated long-term potentiation (LTP) in the central nucleus of the amygdala. This effect is significantly decreased by the PKA inhibitor H89.104 Chronic opiate treatment depresses LTP in rat hippocampal CA1 neurons and increases PKA activity, which may represent compensatory actions since opioid receptors are coupled to inhibitory Gi/o proteins.95 After withdrawal from chronic opiate treatment, administration of PKA inhibitors or a single injection of morphine can reduce PKA levels and restore LTP.95 These data suggest that PKA is involved in the enhancement of amygdala LTP noted during withdrawal from chronic cocaine and in the depression of hippocampal LTP noted during chronic opiate treatment.
PKA in Behavioral Responses to Drugs of Abuse
Sensitization
PKA contributes to psychostimulant-induced locomotor sensitization. Intra-VTA injection of the PKA inhibitor Rp-cAMPS decreases amphetamine-induced locomotor sensitization. Sp-cAMPS, a PKA activator, increases amphetamine-induced locomotor sensitization in rats.105 Intracerebroventricular injection of forskolin, which increases PKA activity by increasing production of cAMP, enhances cocaine-induced locomotor sensitization in rats.106
Drug Tolerance
Chronic treatment with cannabinoids and opiates results in tolerance to their analgesic effects through a mechanism that is mediated by PKA. Inhibition of PKA can restore analgesic sensitivity in tolerant animals. In mice tolerant to THC analgesia, intracerebroventricular injections of the PKA inhibitor KT5720 restore analgesic sensitivity.107 In rats tolerant to morphine analgesia, intracerebroventricular injection of PKA antisense oligonucleotides blocks the development of tolerance.108 Intracerebroventricular injections of the PKA inhibitors KT-5720 or 4-cyano-3-methylisoquinoline restore analgesic sensitivity.109
Drug Self-Administration
Manipulation of PKA activity can increase or decrease the self-administration of addictive drugs in rodent models. The direction of the effect varies with the type of addictive drug. Decreasing PKA activity decreases psychostimulant self-administration in rodents. In rats trained to self-administer cocaine, intra-accumbal injections of the PKA inhibitor Rp-cAMPS decrease self-administration, whereas injections of the PKA activator Sp-cAMPS increase self-administration.110 No change in self-administration is seen when Rp-cAMPS or Sp-cAMPS are injected into the caudate-putamen, indicating that the effects of PKA on self-administration are selective for the NAc.110 In progressive ratio paradigms that assess the extent of work that an animal is willing to perform for access to drug, intra-accumbal injections of Rp-cAMPS decrease the breaking point at which rats no longer lever press for cocaine, while Sp-cAMPS increases the breaking point.111 This effect persists for at least 4 days after injection of the PKA modulator.111 These data suggest that PKA in the rat NAc regulates motivation to self-administer cocaine.
PKA also modulates ethanol consumption. Activation of adenosine A2a receptors increases PKA activity.66 Systemic injection of the A2a receptor antagonist 3,7-dimethylpro-pargylxanthine, which is predicted to decrease PKA activation mainly in the striatum where A2a receptors are highly expressed,112 reduces ethanol self-administration in rats.113 Mice that express a dominant negative form of PKA in the forebrain and hippocampus, and therefore have lower brain PKA activity in these areas, show reductions in both ethanol consumption and ethanol preference when compared with wild-type mice.114 However, injection of the PKA inhibitor Rp-cAMPS into the rat central amygdala increases ethanol intake and preference,115 in part by increasing anxiety-like behavior.116 The effects of PKA on behavior thus display regional specificity within the brain.
Rats selected for high ethanol preference (ethanol-preferring “P” rats) show increased anxiety-like behavior and lower levels of p-CREB and neuropeptide Y in the central and medial amygdala when compared with ethanol-nonpreferring “NP” rats.116 P rats show reduced anxiety after voluntary ethanol consumption, whereas NP rats do not. Injection of Sp-cAMPS into the central amygdala of P rats drives levels of ethanol intake, anxiety-like behavior, and amygdala p-CREB toward the lower levels found in NP rats.116 Sp-cAMPS injection does not affect anxiety-like behavior in NP rats. Injection of Rp-cAMPS into the central amygdala of NP rats does increase ethanol consumption and anxiety-like behavior.116 These data show that PKA modulation of ethanol drinking depends on interaction with alleles in P and NP rats that may also be responsible for the levels of drinking for which these animals were selected. Such findings underscore the need to consider pharmacogenomics when developing new treatments for addiction.
Drug Withdrawal and Relapse
PKA activity is increased during withdrawal from many different addictive drugs. In rats withdrawn from cocaine, PKA activity is increased in the NAc for 30 days, returning to baseline by 90 days after withdrawal.117 In mice treated chronically with THC, withdrawal precipitated by acute administration of a CB1 antagonist transiently increases PKA activity in the cerebellum and is associated with increased somatic withdrawal signs such as shakes and tremors.90 In rat striatal neurons, morphine withdrawal precipitated by acute administration of naloxone results in enhanced PKA-mediated p-CREB induction.118 PKA activation and opiate or cannabinoid withdrawal may be causally related. Inhibition of PKA activity decreases withdrawal symptoms in rodents. In THC withdrawn mice, application of Rp-cAMPS to the surface of the cerebellar cortex decreases withdrawal signs. Similar application of Sp-cAMPS in naïve mice mimics some of the signs of THC withdrawal.90 In rats, injection of Rp-cAMPS into the locus coeruleus or periaqueductal gray matter attenuates signs of morphine withdrawal that are precipitated by naloxone.91
The role of PKA in ethanol withdrawal is quite different from its roles in cannabinoid or opiate withdrawal. During ethanol withdrawal, CREB phosphorylation is decreased in the rat central and medial amygdala.115 Local microinjection of Sp-cAMPS into the central amygdala normalizes the level pCREB and attenuates the increased anxiety-like behaviors evoked by ethanol withdrawal. Conversely, microinjection of Rp-cAMPS into the central or basolateral amygdala decreases CREB phosphorylation, increases anxiety, and augments ethanol consumption. The decreased amygdala pCREB is associated with decreased expression of the anxiolytic peptide, neuropeptide Y (NPY). Levels of NPY are increased by local injections of Sp-cAMPS.119 Intracerebroventricular infusion of NPY decreases the anxiety induced by ethanol withdrawal in rats.120 In P rats subjected to repeated bouts of ethanol withdrawal, intracerebroventricular infusion of NPY decreases ethanol drinking.121 In addition to NPY, the neurotrophin BDNF (another CREB-regulated protein) reduces anxiety and drinking behavior. Infusion of BDNF antisense oligonucleotides into the central and medial amygdala, but not into the basolateral amygdala, provokes anxiety-like behavior and increases alcohol intake. Both behaviors are rescued by co-infusion of BDNF.122
PKA can modulate reinstatement of drug-seeking behavior in cocaine-withdrawn rats. Injection of Rp-cAMPS into the NAc of cocaine-withdrawn rats increases responding on cocaine-associated levers.110 In addition, intraperitoneal priming doses of the D2 agonists 7-OH-DPAT or quinpirole, which can decrease PKA activity via Gαi/o, increase responding on cocaine-associated levers in cocaine-withdrawn rats. The D1 agonist SKF82958, which activates PKA via Gαs has a minimal effect.123 Therefore, although decreasing PKA activity increases drug seeking in cocaine-withdrawn animals, pharmacological strategies that increase PKA activity are unlikely to be effective in reducing reinstatement of cocaine self-administration.
Conditioned Place Preference
PKA is required for establishing CPP for several drugs of abuse. The acquisition of amphetamine CPP is decreased in a dose-dependent manner when the PKA inhibitor Rp-cAMPS is co-administered with amphetamine into the NAc during CPP conditioning sessions.124 Intracerebroventricular injection of the PKA inhibitor H89 immediately after each CPP conditioning session decreases consolidation of cocaine CPP during training.125 Similarly, microinjections of Rp-cAMPS into the VTA126 or of H89 into the hippocampal CA1 region127 after each conditioning session decrease consolidation of morphine CPP.126,127 Administration of H89 into CA1 hippocampus before each training session or just before the preference test does not inhibit morphine CPP in rats. Hippocampal PKA thus regulates consolidation but not acquisition or expression of morphine CPP.127 However, Rp-cAMPS, administered into the VTA immediately prior to preference tests, blocks the expression of morphine CPP. VTA PKA can thus regulate both consolidation and expression of morphine CPP.126 These results indicate that PKA acts within specific brain regions that mediate drug reward to help mediate acquisition, consolidation, and expression of drug-induced CPP. Acquisition of amphetamine CPP is also decreased by co-injection of the PKA activator Sp-cAMPS into the NAc during conditioning. PKA activation in the NAc that is unrelated to ongoing DA transmission can also disrupt drug reward learning.124,128
Cyclin-Dependent Protein Kinase 5
Cdk5 is a proline-directed kinase that regulates many aspects of neuronal function including neuronal migration, axonal and synaptic development, synaptic plasticity, secretion, and cell adhesion.129 Cdk5 is strongly expressed throughout the cortex, thalamus, and amygdala, and moderately expressed in the midbrain, VTA, and NAc.6 Cdk5 is inactive alone and requires dimerization with a partner protein, p35, p39, or p67, for activity. Due to restricted expression of these partner proteins, Cdk5 activity is primarily localized to synapses of mature neurons and growth cones of developing neurons. Cdk5 activity is induced by growth factors, such as nerve growth factor (NGF)130 and BDNF,131 which increase p35 transcription via ERK-mediated activation of the transcription factor Egr1.130 Repeated activation of dopamine receptors by exposure to addictive drugs results in accumulation of the long-half-life transcription factor deltaFosB, a splice variant of FosB that increases the transcription of Cdk5.132 Induction of hippocampal deltaFosB by electroconvulsive treatments increases expression of hippocampal Cdk5 in rats.133 Cdk5 activity is primarily measured by assessing the phosphorylation of some of the many specific Cdk5 substrates, which include Ser-551 and Ser-553 of synapsin 1,134 multiple KSPXK sequences in the C-terminus of neurofilament-H,135 Thr-75 of DARPP-32,73 and several serine and threonine residues in the N- and C-termini of histone H1.136
Cdk5 and PKA produce opposing actions on DARPP-32, an important modulator of dopaminergic signaling. As discussed earlier, PKA phosphorylates DARPP-32 on Thr-34, which renders DARPP-32 active as an inhibitor of PP-1.71 Dopaminergic signaling increases PKA-mediated phosphorylation of DARPP-32 on Thr-34 and inhibits dephosphorylation by PP-1, amplifying dopamine-mediated PKA phosphorylation of downstream targets.72 Cdk5 negatively regulates dopaminergic signaling by phosphorylating DARPP-32 on Thr-75. This renders DARPP-32 an inhibitor of PKA and reduces PKA phosphorylation of DARPP-32 at Thr-34.73 Inhibition of Cdk5 with roscovitine decreases DARPP-32 phosphorylation of Thr-75, increases PKA-mediated whole-cell calcium currents,73 and potentiates cocaine-stimulated dopamine accumulation in mouse striatal slices.137 Cdk5 also can inhibit the ERK pathway through phosphorylation and inhibition of MEK. It may thus function in a negative feedback loop that prevents excessive ERK activity.138
Cdk5 regulation of microtubules and the actin cytoskeleton may be important for drug-induced synaptic plasticity.139 Rats treated with intraperitoneal (i.p.) injections of cocaine for 4 weeks show increased densities of dendritic spines on NAc medium spiny neurons when compared with control rats. This alteration is inhibited by pretreatment with the Cdk5 inhibitor roscovitine.140 Thus, targeting Cdk5 signaling may be a valuable approach to attenuating drug-induced changes in synaptic plasticity.
Effect of Addictive Drugs on Cdk5 Signaling
Acute administration of cocaine to mice does not affect Cdk5 activity. It does decrease DARPP-32 phosphorylation at Thr-75 via PKA-mediated activation of PP-2A.72 Chronic administration of cocaine increases Cdk5 activity, likely through the induction of deltaFosB141 and increased transcription of Cdk5.82 Rats treated chronically with cocaine show increased Cdk5 and p35 mRNA in the dorsal striatum and NAc.82 Rats that have been chronically self-administering cocaine display higher NAc Cdk5 protein levels than control rats.142 Similarly, rats chronically treated with methamphetamine143 show increased Cdk5 activity in the ventral striatum. Chronic ethanol exposure also increases Cdk5 immunoreactivity in the rat NAc144 and enhances Cdk5 activity in frontal cortex and cerebellum.145 Mice treated chronically with cocaine82 resemble transgenic mice that overexpress p35.146 In both cases, the effects of single cocaine injections on the induction of PKA-mediated DARPP-32 phosphorylation at Thr-34 are attenuated. Thus, the effects of acute cocaine are dampened in animals exposed repeatedly to cocaine due to the increased expression and activity of Cdk5. High Cdk5 activity can also attenuate downstream activation of other cocaine-stimulated signal transduction pathways. When treated acutely with cocaine, mice that overexpress p35 show reduced phosphorylation of ERK and CREB as well as smaller increases in levels of c-fos than do control mice.146
Not all addictive drugs increase Cdk5 activity. Chronic exposure to morphine decreases Cdk5 levels in rat brain.147 Although the effects of in vivo nicotine have not been investigated, in vitro acute nicotine treatments decrease Cdk5-mediated DARPP-32 phosphorylation in mouse striatal slices.148 For psychostimulants and ethanol, Cdk5 appears to serve as a negative regulator of excess dopaminergic activity and may provide a mechanism for dopaminergic homeostasis during repeated exposure to these drugs.
Cdk5 in Behavioral Responses to Drugs of Abuse
Sensitization
Repeated cocaine injections that result in locomotor sensitization increase Cdk5 levels and Cdk5-mediated DARPP-32 phosphorylation, and decrease PKA-mediated DARPP-32 phosphorylation in the rat dorsal striatum and NAc.149 Pretreatment with the Cdk5 inhibitors roscovitine or olomoucine enhances the development of cocaine-induced locomotor sensitization in mice82 and rats.150 In contrast, heterozygous Cdk5 null mice show reduced morphine-induced locomotor sensitization.151 However, conditional Cdk5 knockout mice, in which forebrain deletion of Cdk5 is achieved in the adult through αCaMKII promoter-driven Cre recombinase, show enhanced cocaine-induced locomotor sensitization.152 Therefore, induction of Cdk5 activity by repeated exposure to cocaine appears to act as an adaptive feedback response that decreases the sensitizing effects of the drug, whereas for morphine Cdk5 appears to facilitate drug sensitization. The mechanisms by which Cdk5 regulates sensitization are not understood but may involve both its kinase activity as well as its recently described kinase independent role in regulating glutamate receptor degradation.153
Drug Tolerance
Cdk5 may also regulate the development of tolerance to morphine analgesia. Intrathecal injection of roscovitine prevents the development of tolerance and reverses established tolerance to morphine analgesia.154 Interestingly, this result is similar to the effect of PKA inhibition on morphine tolerance,108,109 indicating that here Cdk5 and PKA show similar, rather than antagonistic, actions.
Motivated Responding for Cocaine
Repeated exposure to cocaine increases Cdk5 levels, and this appears to exert negative feedback on motivated responding for the drug.82 In rats, intra-NAc injections of the Cdk5 inhibitor olomoucine increase the work which an animal is willing to expend to obtain an injection of cocaine in a progressive ratio paradigm.150 The Cdk5 inhibitor roscovitine, injected into the NAc increases conditioned reinforcement induced by cocaine, which can also be considered a measure of incentive motivation.150
Drug Withdrawal
Cocaine self-administration for 10 days followed by withdrawal for 1, 30, or 90 days does not alter Cdk5 levels in the rat NAc, but withdrawal for 1 day does increase Cdk5 protein in the rat VTA.117 Cdk5 does not increase in the VTA of sucrose-withdrawn rats. The effect of withdrawal from other drugs on Cdk5 levels and the effect, if any, of increased Cdk5 in the VTA on behavioral signs of cocaine withdrawal are not known.
Conditioned Place Preference
Intracerebroventricular injections of roscovitine reduce the development of morphine CPP in mice, and heterozygous Cdk5 knockout mice show attenuated development of morphine CPP compared with wild-type controls.151 However, similar to the effects of Cdk5 on locomotor sensitization discussed above, a different result has been observed for cocaine. In conditional Cdk5 knockout mice, cocaine can cause CPP at low doses that do not induce CPP in wild-type mice, suggesting that decreasing Cdk5 activity reduces the threshold for the development of cocaine CPP.152 The findings in conditional knockout mice suggest that Cdk5 reduces cocaine reward and fits well with results from cocaine self-administration studies noted above, in which Cdk5 acts to decrease motivated responding for cocaine.
Protein Kinase C
Protein kinase C (PKC) is a family of serine-threonine kinases comprising nine genes, all of which are expressed in the central nervous system with isozyme-specific patterns of expression.155 PKC isozymes can be classified into three subgroups based on similarities in structure and regulation: the conventional PKCs (cPKC, α, β, and γ), the novel PKCs (nPKC, δ, ε, η, and θ), and the atypical PKCs (aPKC, ζ, and Ι/λ).156 Conventional PKCs are activated by diacylglycerol (DAG) and calcium, novel PKCs are activated by DAG but not calcium, and the atypical PKCs are not activated by DAG or calcium but instead by other lipid second messengers, such as arachidonic and phosphatidic acid.157 The hydrolysis of phosphatidylinositol-4,5-bis-phosphate by phospholipase C (PLC) generates inositol triphosphosphate (IP3) and DAG. IP3 releases intracellular calcium stores to increase intracellular calcium levels, which can synergize with DAG to activate conventional PKCs.
PKCs are involved in many cellular processes, and thus the downstream substrates of PKCs are numerous. They include several neurotransmitter receptors such as the α4nAChreceptor which can be PKC phosphorylated on Ser-550;158 dopamine D2 receptors phosphorylated by PKC at Ser-229, Ser-228, Thr-352, Thr-354, and Ser-355;159 and CB1 receptors phosphorylated by PKC at Ser-317.160 Other PKC substrates include proteins involved in exocytosis (including Ser-187 of SNAP-25)161 as well as proteins important in synaptic plasticity (such as Ser-36 of neurogranin162 and Ser-41 of neuromodulin).163 In addition, PKC phosphorylates Ser-818 of GluR1, facilitating AMPA receptor externalization and LTP.164 Thus, PKCs may play an important role in drug-induced synaptic plasticity.
Addictive drugs can activate PKCs by stimulating dopamine D2, opioid, or cannabinoid receptors, releasing Gβγ from Gi/o, which stimulates PLC to produce DAG and IP3.165 In addition, hetero-oligomers of D1 and D2 dopamine receptors activate Gq,166 which also activates PLC.165 Phospholipase D (PLD) can produce DAG by cleaving phosphatidylcholine into phosphatidic acid (PA) and free choline.167 PA is then converted to DAG by PA phospho-hydrolase. PLD is also activated by many of the same G protein–coupled receptors as PLC, as well as by the small GTPase RhoA and by cPKCs.167
PKCs require phosphorylation by phosphoinositide-dependent kinase 1 (PDK1) and subsequent autophosphorylation in the catalytic domain to be fully active. For conventional PKCs, this is thought to be a constitutive process, whereas for novel and atypical PKCs, there is a pool of unprocessed enzyme present that can be phosphorylated by PDK1 and recruited into an activatable pool.156
Activation of PKC isozymes involves their translocation to specific subcellular compartments where they can interact with substrates. This process is regulated by binding proteins, such as the protein interacting with C kinase 1 (PICK1),168 which localizes PKC at AMPA receptors, and by the Receptors for Activated C Kinase (RACKs), two of which have been cloned: RACK1 which binds activated PKCβII169 and εRACK (β′COP) which binds activated PKCε.170
The function of PKC isozymes can be probed using activators such as tumor-promoting phorbol esters, which activate conventional and novel PKCs, although it is important to note that other proteins besides PKCs can be activated by phorbol esters.171 However, when reversed by inhibitors of PKC catalytic activity, it is possible to ascribe effects of phorbol esters to activation of PKC. The activation of PKCs can be measured as autophosphorylation of PKCs or their translocation to different cellular compartments. Several inhibitors are commonly used, such as bisindolylmaleimides and related compounds that bind to the purine site in the catalytic domain, or other compounds that do not compete with ATP binding such as chelerythrine and calphostin C. It is important to note that these inhibitors do not distinguish well between PKC isozymes, although one, the indolocarbazole Go6976, inhibits conventional PKCs with good selectivity over novel and atypical PKCs.172 Isozyme-specific manipulations have been best achieved through the use of isozyme-selective peptide inhibitors derived from putative RACK binding domains, over-expression of isozymes, expression of dominant negative mutants of specific isozymes, gene targeting or expression of PKC transgenes, antisense RNA, or RNA interference.
Effects of Addictive Drugs on PKC Signaling
Treatment with phorbol esters increases striatal extracellular dopamine levels in rat brain slices,173 and amphetamine-stimulated dopamine accumulation in rat striatal slices is inhibited by the PKC inhibitors chelerythrine, calphostin C, and Ro31-8220.173,174 Nicotinic agonists cause translocation of PKC activity to the particulate fraction of PC12 cells, a rat neuroendocrine cell line.175 In PC12 cells, nicotine also acutely stimulates dopamine release, which is inhibited by chelerythrine.176 Therefore in some in vitro models PKC modulates levels of extracellular dopamine.
Chronic stimulant treatment has differential effects on the levels of individual PKC isozymes. Chronic treatment of PC12 cells with 1.0 μM of cocaine increases levels of PKCα and PKCδ and decreases levels of PKCε and PKCγ.177 Chronic cocaine treatment in rats increases PKCβI in the medial prefrontal cortex,178 PKCα and PKCε in the hippocampus,179 and PKCγ in the amygdala and NAc core but decreases PKCγ in rat striatum.178 Chronic methamphetamine treatment increases levels of phosphorylated conventional PKC isozymes in rat limbic forebrain180 and levels of phosphorylated PKCs in astrocytes.181
PKC mediates several effects of chronic psychostimulant exposure. Chronic treatment with nicotine enhances amphetamine-induced dopamine accumulation in slices of rat prefrontal cortex, and this effect is attenuated by chelerythrine.182 In rat NAc, methamphetamine-induced sensitization of extracellular dopamine accumulation is attenuated by intra-NAc injections of chelerythrine.180 Chronic cocaine treatment results in impairment of type 1 mGluR-mediated induction of long-term depression (LTD) in pyramidal neurons of the rat medial prefrontal cortex, an effect that is attenuated by pretreatment with bisindolylmaleimide I (BIS).183
The role of PKC in chronic effects of opioid or cannabinoid agonists has not been studied. One report184 has shown that chronic morphine treatment increases and decreases specific PKC isoforms in mouse brain, similar to psychostimulant treatment. In mice, repeated morphine injections increase PKCγ protein levels in the limbic forebrain but not in the lower midbrain. There is no effect of chronic morphine treatment on PKCα, PKCβI, PKCβII, or PKCε in either brain region.
Interactions between ethanol and PKC have been more extensively investigated than PKC interactions with other addictive drugs. Similar to stimulants and opioids, ethanol alters the localization and abundance of PKC. Acute ethanol treatment induces PKC translocation in cultured astroglial cells.185 In the neuroblastoma × glioma hybrid cell line NG108-15, chronic ethanol exposure also induces translocation of PKCδ and PKCε;186 this translocation of PKCε is to the cytosol in a complex with εRACK and is mediated by PKA, possibly through PKA phosphorylation of εRACK.187 In vivo ethanol administered i.p. stimulates phosphorylation of cerebellar PKCε at Ser-729, and this increase in phosphorylated PKCε correlates with the development of acute functional tolerance to the motor-impairing effects of ethanol in mice that is PKCε-dependent.188
Chronic ethanol exposure increases PKC activity and PKCδ and PKCε protein levels in PC12 cells and in NG-108-15 cells.189 In vivo, chronic ethanol treatment decreases PKC activity in the cortex and hippocampus of rats,190 decreases PKCα and PKCγ levels in the frontal cortex in mice,191 and increases PKCα and PKCγ protein levels in the limbic forebrain in mice.191
In vitro studies with peptide inhibitors have been informative in elucidating the role of PKC isozymes in mediating effects of ethanol. Chronic exposure of PC12 cells to ethanol upregulates the abundance of N-type calcium channels, an effect that is blocked by a peptide inhibitor of PKCε.192 Chronic ethanol treatment also enhances NGF-induced neurite outgrowth in PC12 cells, and this response is also attenuated by depletion193 or inhibition of PKCε.194 In PC12 cells, chronic ethanol exposure causes an upregulation of L-type calcium channels, which is mediated by PKCδ.195
Knockout mice and isozyme-selective peptide inhibitors have been also useful in identifying roles for PKC isozymes in effects of ethanol in vivo. Thus, ethanol-induced dopamine release in the NAc appears to require PKCε as it is attenuated in PKCε knockout mice.196 Acute ethanol treatment increases glycine currents in rat VTA neurons; a peptide inhibitor of PKCε reduces this effect.197 Acute ethanol exposure also potentiates GABAA receptor-mediated inhibitory postsynaptic currents in hippocampal pyramidal cells, an effect that is increased in neurons from PKCε knockout mice and abolished in neurons from PKCγ knockout mice.198 PKCγ can be co-immunoprecipitated with α1 and α4 GABAA receptor subunits,199 suggesting that PKCγ interacts directly with GABAA receptors to mediate its effect on receptor function. The PKCγ substrates that mediate this response are not known. On the other hand, PKCε regulates GABAA receptor function by phosphorylating γ2 subunits of synaptic receptors at Ser-327, which results in a diminished enhancement of GABA-stimulated current by ethanol.200
Initial activation of nACh receptors by agonists results in a downregulation and desensitization of the receptor, followed by receptor inactivation and a paradoxical upregulation with chronic agonist exposure.201 Postmortem studies show that smokers have increased nicotinic receptor binding in the brain compared with nonsmokers.202 This nACh receptor upregulation may contribute to the reinforcing effects of nicotine.203 PKC appears to play an important role in nACh receptor cycling. The high-affinity α4β2 nACh receptor is upregulated by chronic nicotine treatment in rats,204 and receptors containing the β2 subunit are implicated in the reinforcing properties of nicotine as mice lacking the β2 subunit show attenuated nicotine self-administration.205 Acute nicotine treatment desensitizes α4β2 nACh receptors, which can recover from desensitization if nicotine treatment is stopped.206 Inhibition of PKC with calphostin C slows recovery, whereas activation of PKC with phorbol esters enhances it.206 In addition, the α4 nicotinic subunit contains a PKC phosphorylation site at Ser-366, and mutation of this site produces receptors that show little recovery from desensitization.206 Interestingly, ethanol can also downregulate nACh receptors, an effect that is attenuated by chelerythrine.207 Chronic nicotine treatment inactivates α4β2 nACh receptors, and this inactivation is mimicked by the PKC inhibitor 2,6-diamino-N-([1-(1-oxotridecyl)-2-piperidinyl] methyl)hexanamide (NPC 15437).208 Prolonged nicotine treatment upregulates homomeric α7 nACh receptors expressed in SH-EP1-hα7 cells, and this upregulation is enhanced by PKCα over-expression; however, upregulation of heteromeric nACh receptors containing α3, α5, α7, β2, and β4 expressed in SH-SY5Y cells is not affected by depletion of PKC.209 These data suggest that PKC is important for recovery from receptor desensitization, receptor inhibition resulting from chronic agonist treatment, and upregulation of homomeric α7 NACh receptors resulting from chronic agonist treatment. Which PKC isozymes mediate these processes is not yet known.
PKC in Behavioral Responses to Drugs of Abuse
Sensitization
PKC inhibition attenuates psychostimulant-induced hyperlocomotion. Intra-NAc infusion of the PKC inhibitor Ro31-8220 inhibits hyperlocomotion induced by amphetamine in rats.174 In cocaine-sensitized rats, cocaine-induced hyperlocomotion is also attenuated by intra-NAc injection of the PKC inhibitor BIS.174 Co-administration of the PKC inhibitor NPC 15437 with repeated injections of methamphetamine attenuates the development of locomotor sensitization in mice.181
Drug Tolerance
Similar to PKA and Cdk5, PKC appears to play a role in opioid tolerance. Intracerebroventricular injection of the PKC inhibitor BIS restores analgesic sensitivity in mice tolerant to morphine analgesia.109 However, unlike inhibition of PKA, inhibition of PKC does not restore analgesic sensitivity to THC in mice made tolerant to THC.107
PKC knockout mice have been useful in determining the role of PKC isozymes in ethanol-related behaviors. Administration of high concentrations of ethanol (3.2–4 g/kg) produces hypnosis and anesthesia, which can be measured as the loss of righting reflex (LORR) in rodents. PKCε knockout mice show an increased duration of the ethanol-induced LORR210 that is due to decreased acute functional tolerance to ethanol.188 PKCε knockout mice also show increased duration of LORR induced by pentobarbital, pregnanolone, or benzodiazepines.210–212 Restoration of PKCε activity by conditional gene expression of PKCε in knockout mice reduces ethanol- and pentobarbital-induced LORR to wild-type levels.211 In contrast to PKCε knockout mice, PKCγ knockout mice show a reduced duration of ethanol-induced LORR compared with wild-type mice.213 Unlike with PKCε knockout mice, this change in LORR duration appears specific for ethanol since PKCγ knockout mice show no differences in pentobarbital-induced LORR.213 PKCγ knockout mice also do not develop tolerance to ethanol-induced LORR or hypothermia on some genetic backgrounds.214
PKCε and PKCγ knockout mice show differences in anxiety-like behavior compared with wild-type mice. PKCε knockout mice have decreased anxiety-like behavior and lower levels of stress hormones compared with wild-type mice, which may be due to increased sensitivity of their GABAA receptors to modulation by endogenous neurosteroids.212 PKCγ knockout mice also show decreased anxiety-like behavior compared with wild-type mice215 and are less sensitive to the anxiolytic effects of ethanol.216 However, PKCγ knockout mice show no differences in flunitrazepam-induced anxiolysis compared with wild-type mice,216 suggesting that the effect of PKCγ is specific to ethanol and not a general effect on other GABA modulators, as seen with the PKCε knockout mice. PKCγ knockout mice also show greater impulsivity than wild-type mice.217 Interestingly, genetic polymorphisms in PKCγ were recently associated with behavioral disinhibition, attention-deficit/hyperactivity disorder, and substance experimentation in one human genetic study.218
Drug Self-Administration and Withdrawal
PKCε and PKCγ knockout mice show opposite responses in ethanol self-administration paradigms. PKCε knockout mice self-administer less ethanol196,210 and have decreased preference for ethanol compared with wild-type mice.210 PKCε activity is decreased by mGluR5 antagonists,219 and systemic administration of the mGluR5 antagonist MPEP decreases ethanol consumption in wild-type mice but not in PKCε knockout mice.220 Restoration of neuronal PKCε activity by conditional gene expression of PKCε in knockout mice increases ethanol consumption to wild-type levels indicating that the effect of the PKCε null mutation on drinking is not due to a developmental abnormality but results instead from deficient PKCε signaling in the adult nervous system.211 In contrast to PKCε knockout mice, PKCγ knockout mice consume more ethanol than wild-type littermates.217
Studies with PKCε knockout mice indicate that PKCε regulates sensitivity to opiates. PKCε knockout mice develop self-administration of morphine at a lower dose and show a greater analgesic response to morphine than wild-type mice.221 It is not known whether the magnitude of self-administration is different at higher doses. Therefore, PKCε reduces acute sensitivity to opiates, but it is not yet known if PKCε alters the reinforcing properties of opiate drugs.
Alcohol withdrawal results in handling-induced convulsions in mice.222 PKCε knockout mice show reduced severity of handling-induced convulsions during abstinence from chronic ethanol exposure.223 In addition, chronic ethanol exposure, particularly when administered in a binge fashion interspersed with periods of abstinence, produces a hyperalgesia in rats that can be attenuated by decreasing PKCε activity through local injection of a PKCε inhibitor peptide into the paw being tested224 or by intrathecal administration of antisense oligonucleotides against PKCε.225 These findings indicate that PKCε contributes to some of the peripheral and central nervous system manifestations of ethanol withdrawal.
Conditioned Place Preference
Nonselective PKC inhibitors decrease CPP induced by many addictive drugs. In rats, chelerytrhine reduces cocaine-induced CPP when administered by intracerebroventricular injection125 and inhibits methamphetamine-induced CPP when administered through intra-NAc injection.180 Likewise, intra-NAc injection of NPC 15437 reduces amphetamine-induced CPP.226 It is not known which PKC isozymes regulate psychostimulant-induced CPP. Studies with PKCγ and PKCε knockout mice have revealed different roles for these isozymes in regulating morphine-induced CPP. Morphine CPP is abolished in PKCγ knockout mice,184 whereas PKCε knockout mice show CPP at low doses of morphine and more prolonged expression of morphine CPP when compared with wild-type mice.221 In contrast to morphine, low doses of ethanol do not result in CPP in PKCε knockout mice but instead cause conditioned place aversion.227 Aversion to low doses of ethanol may explain, in part, why PKCε knockout mice self-administer less ethanol than wild-type littermates. Ethanol CPP has not yet been investigated in PKCγ knockout mice.
Calcium/Calmodulin-Dependent Protein Kinase II
The CaMKII family consists of four closely related genes that encode for α, β, γ, and δ isoforms, which associate as homomers or heteromers to form holoenzymes comprised of 12 functional units.228 CaMKIIα is expressed heavily in the cortex and amygdala, moderately in the NAc, and very moderately in the VTA and midbrain.6 CaMKII is involved in many aspects of neuronal function including synaptic plasticity, gene expression, and neurotransmitter synthesis and release.229 Each functional holoenzyme subunit contains a catalytic and a regulatory domain; the regulatory domain includes an auto-inhibition sequence, a calmodulin binding site, and sites for autophosphorylation.228 CaMKIIs are activated by the binding of calcium to calmodulin (which acts as a calcium sensor), resulting in release of auto-inhibition allowing for autophosphorylation within the regulatory domain.
Autophosphorylation on Thr-286 disables the auto-inhibitory domain, increases the affinity for Ca2+/calmodulin by 1000-fold, and allows the holoenzyme to bind to NMDA receptors.228 The CaMKII holoenzyme is activated to differing degrees depending on the magnitude and duration of the intracellular calcium signal, and autophosphorylation allows for increases in CaMKII activity to persist for hours after levels of intracellular calcium fall.230 Activation of CaMKIIs often involves translocation to target areas and binding to target proteins. Thus, activation of CaMKII can be measured by translocation, or more often by an increase in autophosphorylation or phosphorylation of a specific substrate. CaMKII phosphorylates many substrates important in neuronal function and for responses to addictive drugs including synapsin I at Ser-603,231 CREB at Ser 133 and Ser-142,232 and the AMPA GluR1 subunit at Ser-831.233 Activated CaMKII can also bind to the NR2B subunit of NMDA receptors and contribute to synaptic plasticity and LTP.234 Mutation of the αCaMKII autophosphorylation site Thr-286 abolishes stimulus-evoked LTP in rat hippocampal slices and results in spatial memory deficits in the mutant mice.234 In rat hippocampal cultures, the dopamine D1 agonist SKF 81297 increases GluR1 trafficking to the cell surface, which is partially attenuated by the CaMKII inhibitor KN-62.79 Thus CaMKII plays an important role in synaptic plasticity.
Effects of Addictive Drugs on CaMKII Signaling
Acute administration of psychostimulants has variable effects on CaMKII activity depending on the particular stimulant drug and brain region examined. In rats, acute cocaine treatment (15 mg/kg i.p.) increases CaMKII activity in the NAc,235 whereas acute amphetamine treatment (5 mg/kg i.p.) increases CaMKII activity in the striatum236 and produces several downstream CaMKII-dependent events. Inhibition of CaMKII activity with KN93 attenuates acute amphetamine-induced dopamine efflux from mouse striatal neurons, brain slices, and, in vivo, from the dorsal striatum.237 In mouse striatal synaptosomes, acute amphetamine administration decreases dopamine transporter expression at the cell surface, and this effect is attenuated by KN93.238 In contrast to these effects of amphetamine, acute treatment with methamphetamine (5 mg/kg i.p.) decreases CaMKII activity in the rat striatum, parietal cortex, NAc, frontal cortex, and hippocampus.239
There is cross-talk between CaMKII, ERK, and PKA pathways, with CaMKII modulating stimulant-induced activation of ERK and PKA. In vitro, CaMKII inhibitors attenuate acute nicotine-induced ERK phosphorylation in mouse striatal neurons.37 In rat striatal neurons, acute treatment with a dopamine D1 receptor agonist75 and an NMDA agonist240 increases levels of p-CREB, which is attenuated by the CaMKII inhibitor KN62. In vivo in rats, intrastriatal infusion of the CaMKII inhibitor KN62 dose-dependently inhibits increases in p-ERK and p-CREB induced by acute administration of amphetamine.236
Chronic exposure to psychostimulants increases CaMKII activity when given continuously, intermittently, or by self-administration. In rats, chronic amphetamine treatment increases striatal CaMKII activity but not protein levels241 and chronic intermittent amphetamine treatment increases striatal CaMKIIβ mRNA.242 Chronic cocaine treatment in mice increases CaMKII protein in the VTA,243 and chronic cocaine self-administration in rats increases CaMKII mRNA in the VTA.244 In rats treated for 1 week with daily cocaine injections, the enhanced dopamine accumulation in the NAc resulting from acute amphetamine administration is blocked by intra-NAc injections of the CaMKII inhibitor KN93.245
The effect of chronic nicotine on CaMKII activity has not yet been investigated in animal studies or in vitro. In one negative postmortem study, brains from smokers and nonsmokers had similar levels of CaMKII in the ventral midbrain.86
CaMKII is modulated by cannabinoids and opiates. Acute treatment with low doses of THC in rats decreases CaMKII activity in the hippocampus,246 whereas high doses of morphine acutely increase levels of phosphorylated CaMKII in whole mouse brain homogenates.247 Chronic treatment with morphine increases levels of CaMKIIα in rat hippocampal synaptosomes.248
Withdrawal from chronic drug treatment has differential effects on CaMKII activity. In rats, methamphetamine withdrawal does not alter CaMKII activity in the parietal cortex, NAc, or striatum, but a subsequent challenge dose of methamphetamine reduces CaMKII activity in these brain regions to a greater degree in methamphetamine-withdrawn rats compared with drug-naïve rats.239 Naloxone-precipitated morphine withdrawal increases autophosphorylation of CaMKIIα and CaMKIIβ in mouse cingulate cortex.249 Precipitated and unprecipitated withdrawal increase CaMKII activity and CaMKIIβ protein and mRNA, but not CaMKIIα protein or mRNA, in the rat hippocampus.250 Instead unprecipitated morphine withdrawal decreases autophosphorylated CaMKIIα in synaptosomal fractions of rat hippocampus.248
CaMKII activity plays a role in μ-opioid receptor desensitization,251 which may be important for the development of opioid tolerance. CaMKII phosphorylates the μ-opioid receptor on Ser-261 and Ser-266 in the third intracellular loop, and phosphorylation at these sites mediates receptor desensitization, since mutation of these phosphorylation sites results in a receptor that does not show desensitization to repeated agonist administration.252
CaMKII in Behavioral Responses to Drugs of Abuse
Sensitization
CaMKII contributes to drug-induced hyperlocomotion and locomotor sensitization. In rats, repeated intra-VTA treatment with the glutamate receptor agonist t-ACPD enhances cocaine-induced hyperlocomotion, and this effect is attenuated by intra-VTA injection of KN93.253 In rats, repeated cocaine administration induces locomotor sensitization, which is inhibited by intra-VTA injection of KN93243 and partially attenuated by intra-NAc injection of KN93.254 CaMKII knockout mice develop cocaine-induced locomotor sensitization, but the magnitude of the sensitization is significantly less than in wild-type mice.243
Drug Tolerance
In rats, the development of tolerance to morphine analgesia is attenuated by intrahippocampal injections of the CaMKII inhibitors KN62 and KN93, or CaMKII antisense oligonucleotides.255 This is similar to the attenuation of tolerance to morphine analgesia by PKA inhibition with intracerebroventircular injection of PKA antisense oligonucleotides108 or of Cdk5 inhibition by intrathecal injection of roscovitine.154 These parallel results indicate that tolerance to morphine analgesia is mediated by several protein kinases and all are necessary for its expression.
Drug Withdrawal
The effect of CaMKII inhibition on drug withdrawal has not been extensively studied. However, some evidence indicates that CaMKII contributes to opioid dependence and withdrawal. In rats treated chronically with morphine, signs of naloxone-precipitated withdrawal are decreased by intrahippocampal injections of KN62 or KN93255,256 or by CaMKII antisense oligonucleotides.255
Conditioned Place Preference
CaMKII activity in the hippocampus is important for expression of drug-induced CPP. Repeated injections of amphetamine during CPP conditioning increase CaMKII activity in the rat hippocampus.257,258 Expression of amphetamine CPP is attenuated by pretreatment with intrahippocampal injections of KN93 during conditioning257,258 or immediately before the CPP test.257 However, intra-NAc injections of KN93 during the conditioning phase have no effect.258 Expression of morphine CPP in rats is also attenuated by intrahippocampal injections of KN62 or KN93 during conditioning255,256 and immediately before the CPP test.256
Fyn
Fyn is a member of the Src family of non-receptor tyrosine kinases259 and is expressed throughout the brain, with moderate expression in the amygdala, NAc, VTA, and midbrain.6 Fyn is composed of six domains: an Src homology region that contains lipid modification sites, a unique region, SH2 and SH3 domains, a catalytic domain, and a short auto-inhibitory tail. Fyn is constitutively inactive, a state that is maintained by blockade of the catalytic region by the SH2 and SH3 domains and the auto-inhibitory tail. Phosphorylation at Tyr-527 maintains the interaction of the SH2 region with the catalytic domain. Activation of Fyn involves dephosphorylation of Tyr-527 and release of the SH2 and SH3 domains and auto-inhibitory tail from the catalytic domain, as well as autophosphorylation at Tyr-416.259 Fyn activation is commonly measured as an increase in autophosphorylation at Tyr-416.
Fyn plays an important role in synaptic plasticity and LTP. Knockout of the fyn gene, and not other members of Src kinase family, results in abnormal hippocampal development, deficient hippocampal LTP, and impaired spatial learning.260 Reintroduction of Fyn into knockout mice rescues hippocampal development and induction of LTP.260
Fyn interacts with and regulates the function of many neurotransmitter receptors in the brain and mediates effects of neurotrophins.261 For example, Fyn colocalizes with and mediates adenosine-induced transactivation of Trk-A and Trk-B neurotrophin receptors in vitro.262 Fyn also interacts directly with 5-HT6 receptors through its SH3 domain, and this interaction increases Fyn activity via an unknown mechanism.263 In addition, serotonin-dependent activation of ERK is mediated by Fyn,263 indicating cross-talk between Fyn and ERK signal transduction pathways. Fyn directly interacts with the α7 nACh receptor and may contribute to neuroprotection induced by nicotinic receptor stimulation.264 In addition, Fyn can increase NMDA receptor activity by phosphorylating the NR2B subunit of NMDA receptors, which may modulate susceptibility to seizures265 and underlie BDNF-induced increases in NMDA receptor function.266
Effects of Ethanol on Fyn Signaling
Acute ethanol treatment increases Fyn activity in the rat dorsal striatum,267 cerebral cortex, and cerebellum.268 Ethanol also acutely and transiently inhibits NMDA receptor-mediated excitatory postsynaptic potentials (EPSPs) in the hippocampus, which eventually return to baseline, indicating that NMDA receptors develop an acute tolerance to the inhibitory effects of ethanol.269 Fyn is critical for this process since Fyn knockout mice do not show acute tolerance to ethanol.269 Fyn and NMDA NR2B are found in a complex with the scaffolding protein RACK1,267,270,271 and RACK1 inhibits phosphorylation of NR2B by Fyn.270 Ethanol increases NMDA NR2B subunit phosphorylation267,269 by dissociating this complex, enabling Fyn to phosphorylate NR2B and increase NMDA receptor function.271 Overexpression of RACK1 prevents phosphorylation of NR2B by Fyn and prevents the development of tolerance to the inhibitory effects of ethanol on NMDA receptor function in vitro.271 Thus, ethanol itself normalizes NMDA function by stimulating RACK1 dissociation from the complex, thereby enabling Fyn to phosphorylate NR2B and increase NMDA receptor function.
Fyn in Behavioral Responses to Ethanol
Ethanol Intoxication and Acute Tolerance
Fyn knockout mice and mice that overexpress Fyn under control of an αCaMKII promoter in the forebrain, amygdala, and hippocampus (OVF mice) have been used to investigate the role of Fyn in behavioral responses to ethanol. Homozygous Fyn knockout mice show an increase,272,273 and OVF mice show a decrease, in the duration of the ethanol-induced LORR274 when compared with their corresponding wild-type littermates. Homozygous Fyn knockout mice also show an increase in the duration of ethanol-induced LORR when compared with heterozygous Fyn knockout mice269 or wild-type littermates.272 Neither Fyn knockout mice272,273 nor OVF mice274 show differences in ethanol clearance or initial sensitivity to ethanol. Fyn modulation of NMDA receptor activity may contribute to differences in acute sensitivity to the hypnotic effects of ethanol, since pretreatment with the NR2B-containing NMDA receptor antagonist ifendopril increases the duration of ethanol-induced LORR in wild-type mice to levels seen in homozygous Fyn knockout mice.272 Fyn knockout mice show decreased acute functional tolerance to ethanol, which contributes to their enhanced response to hypnotic doses of ethanol.273 They also show a greater anxiolytic effect of ethanol compared with wild-type mice on the elevated plus-maze, possibly due to an increased basal level of anxiety-like behavior.273
Ethanol Self-Administration
Deletion of Fyn does not appear to affect ethanol self-administration in mice. Female Fyn knockout mice show no differences in ethanol self-administration compared with wild-type mice.273 Depending on the laboratory, male Fyn knockout mice show decreased273 or no difference in ethanol preference compared with wild-type mice.272,275 Male Fyn knockout mice also show no change in stress-associated ethanol self-administration275 or ethanol CPP compared with wild-type mice.272 However, micro-injection of the Fyn kinase inhibitor PP2 or the NR2B inhibitor ifendopril into the dorsal striatum reduces ethanol self-administration in wild type animals, suggesting that the absence of phenotypes in the Fyn knockout may be due to compensatory changes in other gene products that mask a role for Fyn in regulating ethanol self-administration and reward.267 In contrast to Fyn knockout mice, OVF mice show decreased ethanol consumption and preference.274,276
Ethanol Withdrawal and Anxiety
OVF mice do not differ from wild-type mice in anxiety-like behavior as measured by the light-dark test.276 However, when withdrawn from chronic alcohol consumption, wild-type mice show increased anxiety-like behavior, while OVF mice do not. NR2B is hyperphosphorylated in OVF mice, and treatment with the NR2B antagonist ifendopril in alcohol-withdrawn OVF mice increases anxiety-like behavior to levels observed in alcohol-withdrawn wild-type mice. In contrast ifendopril only slightly decreases anxiety-like behavior in alcohol-withdrawn wild-type mice. This suggests that increased phosphorylation of NR2B in OVF mice prevents increases in anxiety-like behavior induced by alcohol withdrawal.
Human Fyn Variants and Alcohol Use Disorders
In one human genetic study, a T-to-C change at genomic position 137346 (−93A/G) in the 5 untranslated region of the fyn gene is associated with increased alcohol consumption, a higher number of drinks consumed, and a greater number of self-reported alcohol withdrawal symptoms as defined by DSM-IV criteria compared with control subjects.277 The functional consequences of this polymorphism are not known, but it is likely to affect expression of Fyn.
Cross-Talk in Protein Kinase Signaling Pathways
The individual signaling cascades that activate ERK, PKA, PKC, CaMKII, and Fyn often affect each other, resulting in cross-talk that can influence the activity or downstream effects of other kinases. These interactions can have similar or opposing effects on downstream targets. PKA and ERK signaling pathways converge, resulting in phosphorylation of the same serine residue on CREB. PKA can also indirectly activate ERK by activating the small G-protein Rap1, which then activates B-Raf, leading to activation of MEK and ERK (Fig. 1).60 Thus, PKA cross-talk with ERK can amplify CREB-mediated gene transcription. CaMKII indirectly modulates the stimulantinduced activation of PKA and ERK signaling pathways. CaMKII inhibitors decrease the activation of ERK by nicotine,37 decrease the activation of ERK and CREB by amphetamine,236 and decrease D175 and NMDA agonist induction of CREB.240 Thus, the inhibition of CaMKII activity may have negative effects on the downstream targets of PKA and ERK pathways.
Cdk5 inhibits PKA and ERK signaling pathways in a negative feedback manner. PKA and Cdk5 act on the same downstream target, DARPP-32, resulting in opposing effects on dopaminergic signaling. Dopamine acutely activates PKA phosphorylation of DARPP-32,72 which enhances dopaminergic signaling.71 Repeated stimulation of dopamine receptors activates Cdk5 phosphorylation of DARPP-32,132 which inhibits dopaminergic signaling.73 Thus, the balance between PKA and Cdk5 activity and phosphorylation of DARPP-32 determines the overall level of dopaminergic signaling. NGF130 and BDNF131 activate ERK, which increases p35 transcription and Cdk5 activity.130 Cdk5 inhibits MEK and ERK activity and may act to limit excessive activation of ERK.138
PKA and ERK signaling pathways show cross-talk with PKC and Fyn pathways, respectively, allowing for integration of different effector signals. PKC can be activated by lipid second messengers generated through receptors coupled to phospholipase C and can in turn activate certain adenylyl cyclases (AC2, AC4, and AC7), leading to activation of PKA.66 Since PKA is primarily activated by receptors coupled to AC via Gαs, this PKC–AC interaction allows for integration of intracellular signals arising from PLC- and AC-coupled receptors. Fyn kinase interacts with the 5-HT6 receptor and mediates the serotonin-dependent activation of ERK,263 allowing integration of serotonin signaling with calcium and neurotrophin stimulation of ERK.
These examples of cross-talk between signaling pathways demonstrate the complex interactions between pathways. Drug-induced activation of protein kinase pathways may have numerous effects on other second messengers, culminating in complex modulation of gene transcription and protein synthesis in drug addiction. Cross-talk between signaling pathways increases the difficulty in predicting the result of targeting a particular protein kinase for drug development, as it may effect multiple signaling pathways.
Summary and Drug-Target Development
Addictive drugs primarily alter the function of receptor-gated ion channels (nicotine, alcohol), neurotransmitter transporters (cocaine, amphetamine) or G protein–coupled receptors (opiates, cannabinoids), and these initial events lead to activation of signaling pathways that result in the activation of the downstream protein kinases described in this review. With the exception of alcohol, drugs of abuse do not appear to directly modulate the function of these kinases. In the case of alcohol, there are some data indicating alcohol inhibition of PKC but only in a highly reduced in vitro system using artificial lipid membranes and purified proteins.278 Convincing evidence for in vivo activation by intoxicating doses of ethanol is lacking however.
The protein kinases reviewed here have numerous downstream effects resulting in changes in gene transcription and protein synthesis that contribute to long-term changes in neural networks involved in addiction-related behavior. Several general conclusions may be drawn regarding the role of protein kinases in behavior related to drugs of abuse. Inhibition of ERK and CaMKII may be beneficial in reducing relapse for psychostimulants and opiates. For most drugs of abuse, inhibition of PKA decreases both drug self-administration and signs of drug withdrawal. For ethanol, however, increasing PKA activity in the central amygdala decreases self-administration and withdrawal-induced anxiety, and these responses may be mediated by increased expression of NPY and BDNF in that brain region. In contrast, deletion of PKCε increases sensitivity to ethanol and morphine and markedly decreases ethanol self-administration. Cdk5 appears to negatively regulate responding for cocaine and cocaine reward, suggesting that strategies designed to activate Cdk5 may be useful to treat cocaine addiction. Although Fyn appears to contribute to acute tolerance to the intoxicating effects of ethanol, data on Fyn regulation of ethanol self-administration remain inconclusive. Overall, protein kinase modulation of drug dependence, self-administration, and reward depends on the drug being studied and on the brain region in which the kinase is expressed and modulated.
Given these generalizations, it might seem beneficial to develop inhibitors of ERK, PKA, CaMKII, and PKCε to treat addiction. For PKCε this may be a reasonable approach since PKCε knockout mice are viable and show few phenotypes that raise the specter of serious side effects resulting from use of a PKCε inhibitor. However, ERK, CaMKII, and PKA are multifunctional kinases and the likelihood of inhibitors producing serious side effects is high. An alternative to modulating these kinases directly is to manipulate the signaling pathways in which they function. One approach is to develop drugs that regulate upstream activators or downstream effectors of these kinases. For example, A2a receptors activate PKA, and A2a antagonists decrease alcohol consumption in some models.279 Likewise NPY, whose expression is increased by PKA, diminishes alcohol consumption when overexpressed in transgenic mice.280 Since NPY-Y2 receptors act as autoreceptors that decrease NPY release, the use of NPY-Y2 antagonists has been explored as a strategy to reduce drinking in rodents. In rats the NPY-Y2 antagonist BIIE0246 reduces alcohol consumption, especially in animals with a history of ethanol dependence resulting from exposure to ethanol vapor.281
In addition to manipulating upstream receptors and downstream molecules in kinase signaling cascades, another approach involves identifying direct kinase substrates in the hope that some will be useful targets for pharmaceutical development. Unfortunately, for most kinases this has been a difficult task and progress has been limited. Traditionally, kinase substrates have been identified pharmacologically using kinase activators or inhibitors. The specificity of these drugs can be problematic and the results of such studies often fail to identify a kinase substrate.282 However, Shokat and colleagues283 have promoted a novel chemical-genetics approach to identify kinase substrates that target the structurally conserved purine-binding pocket found in all kinases. The strategy involves generating mutant alleles that can use ATP analogs in addition to ATP to phosphorylate substrate proteins. The mutation creates a “hole” in the pocket by replacing a conserved bulky amino acid with a smaller residue (Fig. 2). ATP analogs (*ATP) possessing a side group “bump” designed to fit into the hole can be used to identify substrates specific to the analog-sensitive (as) kinase mutant. Specificity for the mutant kinase is achieved because wild-type kinases cannot efficiently use the ATP analogs as phosphate donors. To facilitate the identification and purification of substrates, *ATP-γS can be used instead of *ATP to label kinase substrates. This results in the transfer of a thiophosphate group onto the target substrate residue instead of a phosphate group. Thiophosphorylated residues can then be alkylated by para-nitrobenzylmesylate to create an epitope that is recognized by specific antibodies. The tagged substrates can be detected by western blot analysis and isolated by immunoprecipitation or immunoaffinity purification for detection by mass spectrometry. The approach has been successfully used to identify direct substrates of kinases.283 In our view, as-kinases could be extremely useful in isolating distinct kinase substrates phosphorylated in response to drugs of abuse. The hope is that some of these substrates will prove to be targets for the development of useful agents to treat substance abuse in the future.
Figure 2.
The use of analog-sensitive kinases can facilitate substrate identification. The analog-sensitive kinase (as-kinase) is designed to incorporate ATP-γS analogs that have a bulky side group (*ATP-γS). Native kinases can incorporate normal ATP-γS but cannot utilize the *ATP-γS. Substrates phosphorylated by the as-kinase can be identified by alkylation with para-nitrobenzylmesylate (PNBM) to create an epitope that is recognized by antibodies specific for the thiophosphate ester tag. The substrates can be isolated by immunoprecipitation or immunoaffinity purification and analyzed by mass spectrometry. Adapted from Reference 276.
Acknowledgments
This work was supported by U.S. Public Health Service grants AA013588 and AA017072 and contracts WB1XWH-06-1-0159 and W81XWH-07-1-0078 from the U.S. Army, and by funds provided by the State of California for medical research on alcohol and substance abuse through the University of California at San Francisco to R.O.M.
Footnotes
Conflicts of Interest The authors declare no conflicts of interest.
References
- 1.Hyman SE. Addiction: a disease of learning and memory. Am. J. Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 2.Hyman SE, et al. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 3.Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Nestler EJ. Is there a common molecular pathway for addiction? Nat. Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 5.Li CY, et al. Genes and (common) pathways underlying drug addiction. PLoS Comput. Biol. 2008;4:e2. doi: 10.1371/journal.pcbi.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 7.Luscher C, Ungless MA. The mechanistic classification of addictive drugs. PLoS Med. 2006;3:e437. doi: 10.1371/journal.pmed.0030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girault JA, et al. ERK2: a logical AND gate critical for drug-induced plasticity? Curr. Opin. Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat. Rev. Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 10.Hetman M, Gozdz A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur. J. Biochem. 2004;271:2050–2055. doi: 10.1111/j.1432-1033.2004.04133.x. [DOI] [PubMed] [Google Scholar]
- 11.Farnsworth CL, et al. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature. 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- 12.Lu L, et al. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Wang JQ, et al. Regulation of mitogen-activated protein kinases by glutamate receptors. J. Neurochem. 2007;100:1–11. doi: 10.1111/j.1471-4159.2006.04208.x. [DOI] [PubMed] [Google Scholar]
- 14.Valjent E, et al. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur. J. Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- 15.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 16.Valjent E, et al. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J. Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, et al. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J. Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berhow MT, et al. Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J. Neurosci. 1996;16:4707–4715. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajadhyaksha A, et al. L-type Ca2+ channels mediate adaptation of extracellular signal-regulated kinase 1/2 phosphorylation in the ventral tegmental area after chronic amphetamine treatment. J. Neurosci. 2004;24:7464–7476. doi: 10.1523/JNEUROSCI.0612-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi X, McGinty JF. Extracellular signal-regulated mitogen-activated protein kinase inhibitors decrease amphetamine-induced behavior and neuropeptide gene expression in the striatum. Neuroscience. 2006;138:1289–1298. doi: 10.1016/j.neuroscience.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Valjent E, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc. Natl. Acad. Sci. U.S.A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerdjikov TV, et al. Place preference induced by nucleus accumbens amphetamine is impaired by antagonists of ERK or p38 MAP kinases in rats. Behav. Neurosci. 2004;118:740–750. doi: 10.1037/0735-7044.118.4.740. [DOI] [PubMed] [Google Scholar]
- 23.Brunzell DH, et al. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J. Neurochem. 2003;84:1431–1441. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 24.Rubino T, et al. Modulation of extracellular signal-regulated kinases cascade by chronic delta 9-tetrahydrocannabinol treatment. Mol. Cell Neurosci. 2004;25:355–362. doi: 10.1016/j.mcn.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Rubino T, et al. Ras/ERK signalling in cannabinoid tolerance: from behaviour to cellular aspects. J. Neurochem. 2005;93:984–991. doi: 10.1111/j.1471-4159.2005.03101.x. [DOI] [PubMed] [Google Scholar]
- 26.Ferrer-Alcon M, et al. Long-term regulation of signalling components of adenylyl cyclase and mitogen-activated protein kinase in the pre-frontal cortex of human opiate addicts. J. Neurochem. 2004;90:220–230. doi: 10.1111/j.1471-4159.2004.02473.x. [DOI] [PubMed] [Google Scholar]
- 27.Chandler LJ, Sutton G. Acute ethanol inhibits extracellular signal-regulated kinase, protein kinase B, and adenosine 3′:5′-cyclic monophosphate response element binding protein activity in an age- and brain region-specific manner. Alcohol. Clin. Exp. Res. 2005;29:672–682. doi: 10.1097/01.alc.0000158935.53360.5f. [DOI] [PubMed] [Google Scholar]
- 28.Kalluri HS, Ticku MK. Role of GABA(A) receptors in the ethanol-mediated inhibition of extracellular signal-regulated kinase. Eur. J. Pharmacol. 2002;451:51–54. doi: 10.1016/s0014-2999(02)02100-3. [DOI] [PubMed] [Google Scholar]
- 29.Sanna PP, et al. ERK regulation in chronic ethanol exposure and withdrawal. Brain Res. 2002;948:186–191. doi: 10.1016/s0006-8993(02)03191-8. [DOI] [PubMed] [Google Scholar]
- 30.Sampey BP, et al. Ethanol-induced modulation of hepatocellular extracellular signal-regulated kinase-1/2 activity via 4-hydroxynonenal. J. Biol. Chem. 2007;282:1925–1937. doi: 10.1074/jbc.M610602200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizoguchi H, et al. Regulations of methamphetamine reward by extracellular signal-regulated kinase 1/2/ets-like gene-1 signaling pathway via the activation of dopamine receptors. Mol. Pharmacol. 2004;65:1293–1301. doi: 10.1124/mol.65.5.1293. [DOI] [PubMed] [Google Scholar]
- 32.Valjent E, et al. Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur. J. Neurosci. 2001;14:342–352. doi: 10.1046/j.0953-816x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- 33.Jiao H, et al. Dopamine D(1) and D(3) receptors oppositely regulate NMDA- and cocaine-induced MAPK signaling via NMDA receptor phosphorylation. J. Neurochem. 2007;103:840–848. doi: 10.1111/j.1471-4159.2007.04840.x. [DOI] [PubMed] [Google Scholar]
- 34.Moranta D, et al. Acute, chronic and withdrawal effects of the cannabinoid receptor agonist WIN55212-2 on the sequential activation of MAPK/Raf-MEK-ERK signaling in the rat cerebral frontal cortex: short-term regulation by intrinsic and extrinsic pathways. J. Neurosci. Res. 2007;85:656–667. doi: 10.1002/jnr.21140. [DOI] [PubMed] [Google Scholar]
- 35.Salzmann J, et al. Importance of ERK activation in behavioral and biochemical effects induced by MDMA in mice. Br. J. Pharmacol. 2003;140:831–838. doi: 10.1038/sj.bjp.0705506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul S, et al. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat. Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- 37.Steiner RC, et al. Nicotine-induced phosphorylation of ERK in mouse primary cortical neurons: evidence for involvement of glutamatergic signaling and CaMKII. J. Neurochem. 2007;103:666–678. doi: 10.1111/j.1471-4159.2007.04799.x. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt JM, et al. Calmodulin-dependent kinase kinase/calmodulin kinase I activity gates extracellular-regulated kinase-dependent long-term potentiation. J. Neurosci. 2005;25:1281–1290. doi: 10.1523/JNEUROSCI.4086-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoffelmeer AN, et al. Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J. Neurosci. 2002;22:3269–3276. doi: 10.1523/JNEUROSCI.22-08-03269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevenson RA, et al. Comparison of ethanol locomotor sensitization in adolescent and adult DBA/2J mice. Psychopharmacology (Berl.) 2008;197:361–370. doi: 10.1007/s00213-007-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen RM, et al. Low and high locomotor responsiveness to cocaine predicts intravenous cocaine conditioned place preference in male Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2007;86:37–44. doi: 10.1016/j.pbb.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Vries TJ, et al. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur. J. Neurosci. 1998;10:3565–3571. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- 43.Risinger FO, et al. Motivational properties of ethanol in mice selectively bred for ethanol-induced locomotor differences. Psychopharmacology (Berl.) 1994;116:207–216. doi: 10.1007/BF02245064. [DOI] [PubMed] [Google Scholar]
- 44.Valjent E, et al. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc. Natl. Acad. Sci. U.S.A. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierce RC, et al. Neurotrophin-3 contributes to the initiation of behavioral sensitization to cocaine by activating the Ras/Mitogen-activated protein kinase signal transduction cascade. J. Neurosci. 1999;19:8685–8695. doi: 10.1523/JNEUROSCI.19-19-08685.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl.) 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- 47.Lu L, et al. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat. Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 48.Radwanska K, et al. Alcohol relapse induced by discrete cues activates components of AP-1 transcription factor and ERK pathway in the rat basolateral and central amygdala. Neuropsychopharmacology. 2008;33:1835–1846. doi: 10.1038/sj.npp.1301567. [DOI] [PubMed] [Google Scholar]
- 49.Lu L, et al. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol. Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Grimm JW, et al. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J. Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu L, et al. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J. Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Dudai Y. Reconsolidation: the advantage of being refocused. Curr. Opin. Neurobiol. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 54.Milekic MH, et al. Persistent disruption of an established morphine conditioned place preference. J. Neurosci. 2006;26:3010–3020. doi: 10.1523/JNEUROSCI.4818-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duvarci S, et al. Activation of extracellular signal-regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur. J. Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- 56.Kelly A, et al. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J. Neurosci. 2003;23:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazzucchelli C, et al. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34:807–820. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 58.Ferguson SM, et al. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog. Neurobiol. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Waltereit R, Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol. Neurobiol. 2003;27:99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- 61.Evans GJ, Morgan A. Regulation of the exocytotic machinery by cAMP-dependent protein kinase: implications for presynaptic plasticity. Biochem. Soc. Trans. 2003;31:824–827. doi: 10.1042/bst0310824. [DOI] [PubMed] [Google Scholar]
- 62.Sands WA, Palmer TM. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal. 2008;20:460–466. doi: 10.1016/j.cellsig.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Meinkoth JL, et al. Signal transduction through the cAMP-dependent protein kinase. Mol. Cell Biochem. 1993;127–128:179–186. doi: 10.1007/BF01076769. [DOI] [PubMed] [Google Scholar]
- 64.Roche KW, et al. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- 65.Tingley WG, et al. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J. Biol. Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- 66.Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem. J. 2003;375:517–529. doi: 10.1042/BJ20031061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neve KA, et al. Dopamine receptor signaling. J. Recept. Signal Transduct. Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- 68.Yao L, et al. Activator of G protein signaling 3 regulates opiate activation of protein kinase A signaling and relapse of heroin-seeking behavior. Proc. Natl. Acad. Sci. U.S.A. 2005;102:8746–8751. doi: 10.1073/pnas.0503419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hopf FW, et al. Atypical protein kinase C is a novel mediator of dopamine-enhanced firing in nucleus accumbens neurons. J. Neurosci. 2005;25:985–989. doi: 10.1523/JNEUROSCI.3099-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ouimet CC, et al. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J. Neurosci. 1984;4:111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hemmings HCJ, et al. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- 72.Nishi A, et al. Amplification of dopaminergic signaling by a positive feedback loop. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12840–12845. doi: 10.1073/pnas.220410397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bibb JA, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 74.Raman IM, et al. Beta-adrenergic regulation of synaptic NMDA receptors by cAMP-dependent protein kinase. Neuron. 1996;16:415–421. doi: 10.1016/s0896-6273(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 75.Dudman JT, et al. Dopamine D1 receptors mediate CREB phosphorylation via phosphorylation of the NMDA receptor at Ser897-NR1. J. Neurochem. 2003;87:922–934. doi: 10.1046/j.1471-4159.2003.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dajas-Bailador FA, et al. Nicotine activates the extracellular signal-regulated kinase 1/2 via the alpha7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. J. Neurochem. 2002;80:520–530. doi: 10.1046/j.0022-3042.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 77.Man HY, et al. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc. Natl. Acad. Sci. U.S.A. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mangiavacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J. Neurochem. 2004;88:1261–1271. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- 79.Gao C, et al. Activation of D1 dopamine receptors increases surface expression of AMPA receptors and facilitates their synaptic incorporation in cultured hippocampal neurons. J. Neurochem. 2006;98:1664–1677. doi: 10.1111/j.1471-4159.2006.03999.x. [DOI] [PubMed] [Google Scholar]
- 80.Schilstrom B, et al. Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. J. Neurosci. 2006;26:8549–8558. doi: 10.1523/JNEUROSCI.5179-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scheggi S, et al. Behavioral expression of cocaine sensitization in rats is accompanied by a distinct pattern of modifications in the PKA/DARPP-32 signaling pathway. J. Neurochem. 2007;103:1168–1183. doi: 10.1111/j.1471-4159.2007.04818.x. [DOI] [PubMed] [Google Scholar]
- 82.Bibb JA, et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- 83.Freeman WM, et al. Chronic cocaine-mediated changes in non-human primate nucleus accumbens gene expression. J. Neurochem. 2001;77:542–549. doi: 10.1046/j.1471-4159.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- 84.Hamada M, et al. Differential regulation of dopamine D1 and D2 signaling by nicotine in neostriatal neurons. J. Neurochem. 2004;90:1094–1103. doi: 10.1111/j.1471-4159.2004.02574.x. [DOI] [PubMed] [Google Scholar]
- 85.Sun X, et al. Activities of cAMP-dependent protein kinase and protein kinase C are modulated by desensitized nicotinic receptors in the rat brain. Neurosci. Lett. 2004;367:19–22. doi: 10.1016/j.neulet.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 86.Hope BT, et al. Long-term upregulation of protein kinase A and adenylate cyclase levels in human smokers. J. Neurosci. 2007;27:1964–1972. doi: 10.1523/JNEUROSCI.3661-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andersson M, et al. Cannabinoid action depends on phosphorylation of dopamine- and cAMP-regulated phosphoprotein of 32 kDa at the protein kinase A site in striatal projection neurons. J. Neurosci. 2005;25:8432–8438. doi: 10.1523/JNEUROSCI.1289-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Borgkvist A, et al. Regulation of DARPP-32 phosphorylation by Delta(9)-tetrahydrocannabinol. Neuropharmacology. 2008;54:31–35. doi: 10.1016/j.neuropharm.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 89.Rubino T, et al. Chronic delta-9-tetrahydrocannabinol treatment increases cAMP levels and cAMP-dependent protein kinase activity in some rat brain regions. Neuropharmacology. 2000;39:1331–1336. doi: 10.1016/s0028-3908(99)00196-3. [DOI] [PubMed] [Google Scholar]
- 90.Tzavara ET, et al. Cannabinoid withdrawal is dependent upon PKA activation in the cerebellum. Eur. J. Neurosci. 2000;12:1038–1046. doi: 10.1046/j.1460-9568.2000.00971.x. [DOI] [PubMed] [Google Scholar]
- 91.Punch LJ, et al. Opposite modulation of opiate withdrawal behaviors on microinfusion of a protein kinase A inhibitor versus activator into the locus coeruleus or periaqueductal gray. J. Neurosci. 1997;17:8520–8527. doi: 10.1523/JNEUROSCI.17-21-08520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Borgkvist A, et al. Activation of the cAMP/PKA/DARPP-32 signaling pathway is required for morphine psychomotor stimulation but not for morphine reward. Neuropsychopharmacology. 2007;32:1995–2003. doi: 10.1038/sj.npp.1301321. [DOI] [PubMed] [Google Scholar]
- 93.Self DW, et al. Biochemical adaptations in the mesolimbic dopamine system in response to heroin self-administration. Synapse. 1995;21:312–318. doi: 10.1002/syn.890210405. [DOI] [PubMed] [Google Scholar]
- 94.Bao G, et al. Morphine and heroin differentially modulate in vivo hippocampal LTP in opiate-dependent rat. Neuropsychopharmacology. 2007;32:1738–1749. doi: 10.1038/sj.npp.1301308. [DOI] [PubMed] [Google Scholar]
- 95.Pu L, et al. Hippocampal long-term potentiation is reduced by chronic opiate treatment and can be restored by re-exposure to opiates. J. Neurosci. 2002;22:1914–1921. doi: 10.1523/JNEUROSCI.22-05-01914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lane-Ladd SB, et al. CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. J. Neurosci. 1997;17:7890–7901. doi: 10.1523/JNEUROSCI.17-20-07890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garcia-Sevilla JA, et al. Neurofilament proteins and cAMP pathway in brains of mu-, delta- or kappa-opioid receptor gene knock-out mice: effects of chronic morphine administration. Neuropharmacology. 2004;46:519–530. doi: 10.1016/j.neuropharm.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 98.Nagy LE, et al. cAMP-dependent protein kinase regulates inhibition of adenosine transport by ethanol. Mol. Pharmacol. 1991;40:812–817. [PubMed] [Google Scholar]
- 99.Dohrman DP, et al. Ethanol-induced translocation of protein kinase A occurs in two phases: control by different molecular mechanisms. Alcohol. Clin. Exp. Res. 2002;26:407–415. [PubMed] [Google Scholar]
- 100.Dohrman DP, et al. Ethanol causes translocation of cAMP-dependent protein kinase catalytic subunit to the nucleus. Proc. Natl. Acad. Sci. U.S.A. 1996;93:10217–10221. doi: 10.1073/pnas.93.19.10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pandey SC, et al. Effects of chronic ethanol intake and its withdrawal on the expression and phosphorylation of the creb gene transcription factor in rat cortex. J. Pharmacol. Exp. Ther. 2001;296:857–868. [PubMed] [Google Scholar]
- 102.Yao L, et al. Addicting drugs utilize a synergistic molecular mechanism in common requiring adenosine and Gi-beta gamma dimers. Proc. Natl. Acad. Sci. U.S.A. 2003;100:14379–14384. doi: 10.1073/pnas.2336093100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yao L, et al. Adenosine A2a blockade prevents synergy between mu-opiate and cannabinoid CB1 receptors and eliminates heroin-seeking behavior in addicted rats. Proc. Natl. Acad. Sci. U.S.A. 2006;103:7877–7882. doi: 10.1073/pnas.0602661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pollandt S, et al. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur. J. Neurosci. 2006;24:1733–1743. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- 105.Tolliver BK, et al. Necessary role for ventral tegmental area adenylate cyclase and protein kinase A in induction of behavioral sensitization to intra-ventral tegmental area amphetamine. J. Pharmacol. Exp. Ther. 1999;289:38–47. [PubMed] [Google Scholar]
- 106.Schroeder JA, et al. Repeated intracerebroventricular forskolin administration enhances behavioral sensitization to cocaine. Behav. Brain Res. 2004;153:255–260. doi: 10.1016/j.bbr.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 107.Lee MC, et al. The role of several kinases in mice tolerant to delta 9-tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 2003;305:593–599. doi: 10.1124/jpet.102.044446. [DOI] [PubMed] [Google Scholar]
- 108.Shen J, et al. Role of cAMP-dependent protein kinase (PKA) in opioid agonist-induced muopioid receptor downregulation and tolerance in mice. Synapse. 2000;38:322–327. doi: 10.1002/1098-2396(20001201)38:3<322::AID-SYN11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 109.Smith FL, et al. PKC and PKA inhibitors reinstate morphine-induced behaviors in morphine tolerant mice. Pharmacol. Res. 2006;54:474–480. doi: 10.1016/j.phrs.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 110.Self DW, et al. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J. Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lynch WJ, Taylor JR. Persistent changes in motivation to self-administer cocaine following modulation of cyclic AMP-dependent protein kinase A (PKA) activity in the nucleus accumbens. Eur. J. Neurosci. 2005;22:1214–1220. doi: 10.1111/j.1460-9568.2005.04305.x. [DOI] [PubMed] [Google Scholar]
- 112.Fink JS, et al. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res. Mol. Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 113.Thorsell A, et al. Effect of the adenosine A2a receptor antagonist 3,7-dimethyl-propargylxanthine on anxiety-like and depression-like behavior and alcohol consumption in Wistar Rats. Alcohol. Clin. Exp. Res. 2007;31:1302–1307. doi: 10.1111/j.1530-0277.2007.00425.x. [DOI] [PubMed] [Google Scholar]
- 114.Wand G, et al. The cAMP-protein kinase A signal transduction pathway modulates ethanol consumption and sedative effects of ethanol. J. Neurosci. 2001;21:5297–5303. doi: 10.1523/JNEUROSCI.21-14-05297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pandey SC, et al. The decreased phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats. Alcohol. Clin. Exp. Res. 2003;27:396–409. doi: 10.1097/01.ALC.0000056616.81971.49. [DOI] [PubMed] [Google Scholar]
- 116.Pandey SC, et al. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J. Clin. Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu L, et al. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J. Neurochem. 2003;85:1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- 118.Chartoff EH, et al. Dopamine-dependent increases in phosphorylation of cAMP response element binding protein (CREB) during precipitated morphine withdrawal in primary cultures of rat striatum. J. Neurochem. 2003;87:107–118. doi: 10.1046/j.1471-4159.2003.01992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang H, Pandey SC. Effects of PKA modulation on the expression of neuropeptide Y in rat amygdaloid structures during ethanol withdrawal. Peptides. 2003;24:1397–1402. doi: 10.1016/j.peptides.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 120.Woldbye DP, et al. Ethanol withdrawal in rats is attenuated by intracerebroventricular administration of neuropeptide Y. Alcohol. Alcohol. 2002;37:318–321. doi: 10.1093/alcalc/37.4.318. [DOI] [PubMed] [Google Scholar]
- 121.Gilpin NW, et al. Neuropeptide Y reduces oral ethanol intake in alcohol-preferring (P) rats following a period of imposed ethanol abstinence. Alcohol. Clin. Exp. Res. 2003;27:787–794. doi: 10.1097/01.ALC.0000065723.93234.1D. [DOI] [PubMed] [Google Scholar]
- 122.Pandey SC, et al. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J. Neurosci. 2006;26:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Self DW, et al. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- 124.Beninger RJ, et al. cAMP-dependent protein kinase and reward-related learning: intra-accumbens Rp-cAMPS blocks amphetamine-produced place conditioning in rats. Psychopharmacology (Berl.) 2003;170:23–32. doi: 10.1007/s00213-003-1510-2. [DOI] [PubMed] [Google Scholar]
- 125.Cervo L, et al. Protein kinases A and C are involved in the mechanisms underlying consolidation of cocaine place conditioning. Brain Res. 1997;775:30–36. doi: 10.1016/s0006-8993(97)00866-4. [DOI] [PubMed] [Google Scholar]
- 126.Harris GC, et al. Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neuroscience. 2004;129:841–847. doi: 10.1016/j.neuroscience.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 127.Sharifzadeh M, et al. Intra-hippocampal inhibition of protein kinase AII attenuates morphine-induced conditioned place preference. Pharmacol. Biochem. Behav. 2006;85:705–712. doi: 10.1016/j.pbb.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 128.Gerdjikov TV, Beninger RJ. Differential effects of calcineurin inhibition and protein kinase A activation on nucleus accumbens amphetamine-produced conditioned place preference in rats. Eur. J. Neurosci. 2005;22:697–705. doi: 10.1111/j.1460-9568.2005.04256.x. [DOI] [PubMed] [Google Scholar]
- 129.Dhavan R, Tsai LH. A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 130.Harada T, et al. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat. Cell Biol. 2001;3:453–459. doi: 10.1038/35074516. [DOI] [PubMed] [Google Scholar]
- 131.Tokuoka H, et al. Brain-derived neurotrophic factor-induced phosphorylation of neurofilament-H subunit in primary cultures of embryo rat cortical neurons. J. Cell Sci. 2000;113:1059–1068. doi: 10.1242/jcs.113.6.1059. [DOI] [PubMed] [Google Scholar]
- 132.McClung CA, et al. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res. Mol. Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 133.Chen J, et al. Induction of cyclin-dependent kinase 5 in the hippocampus by chronic electroconvulsive seizures: role of [Delta]FosB. J. Neurosci. 2000;20:8965–8971. doi: 10.1523/JNEUROSCI.20-24-08965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Matsubara M, et al. Site-specific phosphorylation of synapsin I by mitogen-activated protein kinase and Cdk5 and its effects on physiological functions. J. Biol. Chem. 1996;271:21108–21113. doi: 10.1074/jbc.271.35.21108. [DOI] [PubMed] [Google Scholar]
- 135.Shetty KT, et al. cdc2-like kinase from rat spinal cord specifically phosphorylates KSPXK motifs in neurofilament proteins: isolation and characterization. Proc. Natl. Acad. Sci. U.S.A. 1993;90:6844–6848. doi: 10.1073/pnas.90.14.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Langan TA, et al. Mammalian growth-associated H1 histone kinase: a homolog of cdc2+/CDC28 protein kinases controlling mitotic entry in yeast and frog cells. Mol. Cell Biol. 1989;9:3860–3868. doi: 10.1128/mcb.9.9.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chergui K, et al. Cyclin-dependent kinase 5 regulates dopaminergic and glutamatergic transmission in the striatum. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2191–2196. doi: 10.1073/pnas.0308652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sharma P, et al. Phosphorylation of MEK1 by cdk5/p35 down-regulates the mitogen-activated protein kinase pathway. J. Biol. Chem. 2002;277:528–534. doi: 10.1074/jbc.M109324200. [DOI] [PubMed] [Google Scholar]
- 139.Benavides DR, Bibb JA. Role of Cdk5 in drug abuse and plasticity. Ann. N. Y. Acad. Sci. 2004;1025:335–344. doi: 10.1196/annals.1316.041. [DOI] [PubMed] [Google Scholar]
- 140.Norrholm SD, et al. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hope BT, et al. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 142.Seiwell AP, et al. Increased accumbens Cdk5 expression in rats after short-access to self-administered cocaine, but not after long-access sessions. Neurosci. Lett. 2007;417:100–105. doi: 10.1016/j.neulet.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen PC, Chen JC. Enhanced Cdk5 activity and p35 translocation in the ventral striatum of acute and chronic methamphetamine-treated rats. Neuropsychopharmacology. 2005;30:538–549. doi: 10.1038/sj.npp.1300604. [DOI] [PubMed] [Google Scholar]
- 144.Camp MC, et al. Neuroadaptations of Cdk5 in cholinergic interneurons of the nucleus accumbens and prefrontal cortex of inbred alcohol-preferring rats following voluntary alcohol drinking. Alcohol. Clin. Exp. Res. 2006;30:1322–1335. doi: 10.1111/j.1530-0277.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- 145.Rajgopal Y, Vemuri MC. Ethanol induced changes in cyclin-dependent kinase-5 activity and its activators, P35, P67 (Munc-18) in rat brain. Neurosci. Lett. 2001;308:173–176. doi: 10.1016/s0304-3940(01)02011-0. [DOI] [PubMed] [Google Scholar]
- 146.Takahashi S, et al. Increased activity of cyclin-dependent kinase 5 leads to attenuation of cocaine-mediated dopamine signaling. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1737–1742. doi: 10.1073/pnas.0409456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ferrer-Alcon M, et al. Downregulation of neuronal cdk5/p35 in opioid addicts and opiate-treated rats: relation to neurofilament phosphorylation. Neuropsychopharmacology. 2003;28:947–955. doi: 10.1038/sj.npp.1300095. [DOI] [PubMed] [Google Scholar]
- 148.Hamada M, et al. Nicotine regulates DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of 32 kDa) phosphorylation at multiple sites in neostriatal neurons. J. Pharmacol. Exp. Ther. 2005;315:872–878. doi: 10.1124/jpet.105.090852. [DOI] [PubMed] [Google Scholar]
- 149.Scheggi S, et al. Dopamine and cyclic AMP-regulated phosphoprotein-32 phosphorylation pattern in cocaine and morphine-sensitized rats. J. Neurochem. 2004;90:792–799. doi: 10.1111/j.1471-4159.2004.02510.x. [DOI] [PubMed] [Google Scholar]
- 150.Taylor JR, et al. Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. Proc. Natl. Acad. Sci. U.S.A. 2007;104:4147–4152. doi: 10.1073/pnas.0610288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Narita M, et al. Implication of cyclin-dependent kinase 5 in the development of psychological dependence on and behavioral sensitization to morphine. J. Neurochem. 2005;93:1463–1468. doi: 10.1111/j.1471-4159.2005.03136.x. [DOI] [PubMed] [Google Scholar]
- 152.Benavides DR, et al. Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability. J. Neurosci. 2007;27:12967–12976. doi: 10.1523/JNEUROSCI.4061-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hawasli AH, Bibb JA. Alternative roles for Cdk5 in learning and synaptic plasticity. Biotechnol. J. 2007;2:941–948. doi: 10.1002/biot.200700093. [DOI] [PubMed] [Google Scholar]
- 154.Parkitna JR, et al. Effects of glycogen synthase kinase 3beta and cyclin-dependent kinase 5 inhibitors on morphine-induced analgesia and tolerance in rats. J. Pharmacol. Exp. Ther. 2006;319:832–839. doi: 10.1124/jpet.106.107581. [DOI] [PubMed] [Google Scholar]
- 155.Naik MU, et al. Distribution of protein kinase Mzeta and the complete protein kinase C isoform family in rat brain. J. Comp. Neurol. 2000;426:243–258. doi: 10.1002/1096-9861(20001016)426:2<243::aid-cne6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 156.Olive MF, Messing RO. Protein kinase C isozymes and addiction. Mol. Neurobiol. 2004;29:139–154. doi: 10.1385/mn:29:2:139. [DOI] [PubMed] [Google Scholar]
- 157.Hirai T, Chida K. Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J. Biochem. (Tokyo) 2003;133:1–7. doi: 10.1093/jb/mvg017. [DOI] [PubMed] [Google Scholar]
- 158.Pollock VV, et al. Cyclic AMP-dependent protein kinase (PKA) phosphorylates Ser362 and 467 and protein kinase C phosphorylates Ser550 within the M3/M4 cytoplasmic domain of human nicotinic receptor alpha4 subunits. J. Neurochem. 2007;103:456–466. doi: 10.1111/j.1471-4159.2007.04853.x. [DOI] [PubMed] [Google Scholar]
- 159.Namkung Y, Sibley DR. Protein kinase C mediates phosphorylation, desensitization, and trafficking of the D2 dopamine receptor. J. Biol. Chem. 2004;279:49533–49541. doi: 10.1074/jbc.M408319200. [DOI] [PubMed] [Google Scholar]
- 160.Garcia DE, et al. Protein kinase C disrupts cannabinoid actions by phosphorylation of the CB1 cannabinoid receptor. J. Neurosci. 1998;18:2834–2841. doi: 10.1523/JNEUROSCI.18-08-02834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nagy G, et al. Protein kinase C-dependent phosphorylation of synaptosome-associated protein of 25 kDa at Ser187 potentiates vesicle recruitment. J. Neurosci. 2002;22:9278–9286. doi: 10.1523/JNEUROSCI.22-21-09278.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang KP, et al. Characterization of a 7.5-kDa protein kinase C substrate (RC3 protein, neurogranin) from rat brain. Arch. Biochem. Biophys. 1993;305:570–580. doi: 10.1006/abbi.1993.1463. [DOI] [PubMed] [Google Scholar]
- 163.Oehrlein SA, et al. Phosphorylation of GAP-43 (growth-associated protein of 43 kDa) by conventional, novel and atypical isotypes of the protein kinase C gene family: differences between oligopeptide and polypeptide phosphorylation. Biochem. J. 1996;317:219–224. doi: 10.1042/bj3170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Boehm J, et al. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 165.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J. Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 166.George SR, O’Dowd BF. A novel dopamine receptor signaling unit in brain: heterooligomers of D1 and D2 dopamine receptors. ScientificWorld Journal. 2007;7:58–63. doi: 10.1100/tsw.2007.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Becker KP, Hannun YA. Protein kinase C and phospholipase D: intimate interactions in intracellular signaling. Cell. Mol. Life Sci. 2005;62:1448–1461. doi: 10.1007/s00018-005-4531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gardner SM, et al. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 169.Ron D, et al. Coordinated movement of RACK1 with activated betaIIPKC. J. Biol. Chem. 1999;274:27039–27046. doi: 10.1074/jbc.274.38.27039. [DOI] [PubMed] [Google Scholar]
- 170.Csukai M, et al. The coatomer protein beta’-COP, a selective binding protein (RACK) for protein kinase Cepsilon. J. Biol. Chem. 1997;272:29200–29206. doi: 10.1074/jbc.272.46.29200. [DOI] [PubMed] [Google Scholar]
- 171.Brose N, Rosenmund C. Move over protein kinase C, you’ve got company: alternative cellular effectors of diacylglycerol and phorbol esters. J. Cell Sci. 2002;115:4399–4411. doi: 10.1242/jcs.00122. [DOI] [PubMed] [Google Scholar]
- 172.Martiny-Baron G, et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 173.Kantor L, Gnegy ME. Protein kinase C inhibitors block amphetamine-mediated dopamine release in rat striatal slices. J. Pharmacol. Exp. Ther. 1998;284:592–598. [PubMed] [Google Scholar]
- 174.Browman KE, et al. Injection of the protein kinase C inhibitor Ro31–8220 into the nucleus accumbens attenuates the acute response to amphetamine: tissue and behavioral studies. Brain Res. 1998;814:112–119. doi: 10.1016/s0006-8993(98)01040-3. [DOI] [PubMed] [Google Scholar]
- 175.Messing RO, et al. Nicotinic and muscarinic agonists stimulate rapid protein kinase C translocation in PC12 cells. J. Neurosci. 1989;9:507–512. doi: 10.1523/JNEUROSCI.09-02-00507.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Funk CK, Dohrman DP. Chronic ethanol exposure inhibits dopamine release via effects on the presynaptic actin cytoskeleton in PC12 cells. Brain Res. 2007;1185:86–94. doi: 10.1016/j.brainres.2007.09.069. [DOI] [PubMed] [Google Scholar]
- 177.Onaivi ES, et al. In vivo ibogaine blockade and in vitro PKC action of cocaine. Ann. N. Y. Acad. Sci. 1998;844:227–244. doi: 10.1111/j.1749-6632.1998.tb08238.x. [DOI] [PubMed] [Google Scholar]
- 178.Steketee JD, et al. The effects of acute and repeated cocaine injections on protein kinase C activity and isoform levels in dopaminergic brain regions. Neuropharmacology. 1998;37:339–347. doi: 10.1016/s0028-3908(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 179.Freeman WM, et al. Cocaine-responsive gene expression changes in rat hippocampus. Neuroscience. 2001;108:371–380. doi: 10.1016/s0306-4522(01)00432-8. [DOI] [PubMed] [Google Scholar]
- 180.Narita M, et al. Implications of protein kinase C in the nucleus accumbens in the development of sensitization to methamphetamine in rats. Neuroscience. 2004;127:941–948. doi: 10.1016/j.neuroscience.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 181.Narita M, et al. Long-lasting change in brain dynamics induced by methamphetamine: enhancement of protein kinase C-dependent astrocytic response and behavioral sensitization. J. Neurochem. 2005;93:1383–1392. doi: 10.1111/j.1471-4159.2005.03097.x. [DOI] [PubMed] [Google Scholar]
- 182.Drew AE, Werling LL. Nicotinic receptor-mediated regulation of the dopamine transporter in rat prefrontocortical slices following chronic in vivo administration of nicotine. Schizophr. Res. 2003;65:47–55. doi: 10.1016/s0920-9964(02)00500-5. [DOI] [PubMed] [Google Scholar]
- 183.Huang CC, et al. Repeated cocaine administration impairs group II metabotropic glutamate receptor-mediated long-term depression in rat medial prefrontal cortex. J. Neurosci. 2007;27:2958–2968. doi: 10.1523/JNEUROSCI.4247-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Narita M, et al. Involvement of protein kinase Cgamma isoform in morphine-induced reinforcing effects. Neuroscience. 2001;103:309–314. doi: 10.1016/s0306-4522(00)00572-8. [DOI] [PubMed] [Google Scholar]
- 185.Skwish S, Shain W. Ethanol and diolein stimulate PKC translocation in astroglial cells. Life Sci. 1990;47:1037–1042. doi: 10.1016/0024-3205(90)90476-8. [DOI] [PubMed] [Google Scholar]
- 186.Gordon AS, et al. Ethanol alters the subcellular localization of delta- and epsilon protein kinase C in NG108-15 cells. Mol. Pharmacol. 1997;52:554–559. doi: 10.1124/mol.52.4.554. [DOI] [PubMed] [Google Scholar]
- 187.Yao L, et al. Dopamine and ethanol cause translocation of {epsilon}PKC associated with {epsilon}RACK: cross-talk between PKA and PKC signaling pathways. Mol. Pharmacol. 2008 doi: 10.1124/mol.107.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wallace MJ, et al. Acute functional tolerance to ethanol mediated by protein kinase Cepsilon. Neuropsychopharmacology. 2007;32:127–136. doi: 10.1038/sj.npp.1301059. [DOI] [PubMed] [Google Scholar]
- 189.Messing RO, et al. Chronic ethanol exposure increases levels of protein kinase C delta and epsilon and protein kinase C-mediated phosphorylation in cultured neural cells. J. Biol. Chem. 1991;266:23428–23432. [PubMed] [Google Scholar]
- 190.Pascale A, et al. Chronic low doses of ethanol affect brain protein kinase C and ultrasonic calls in rats. Alcohol. 1997;14:557–561. doi: 10.1016/s0741-8329(97)00047-5. [DOI] [PubMed] [Google Scholar]
- 191.Narita M, et al. Changes in Ca2+-dependent protein kinase C isoforms induced by chronic ethanol treatment in mice. Neurosci. Lett. 2001;307:85–88. doi: 10.1016/s0304-3940(01)01939-5. [DOI] [PubMed] [Google Scholar]
- 192.McMahon T, et al. Protein kinase C epsilon mediates up-regulation of N-type calcium channels by ethanol. Mol. Pharmacol. 2000;57:53–58. [PubMed] [Google Scholar]
- 193.Roivainen R, et al. Protein kinase C isozymes that mediate enhancement of neurite outgrowth by ethanol and phorbol esters in PC12 cells. Brain Res. 1993;624:85–93. doi: 10.1016/0006-8993(93)90063-s. [DOI] [PubMed] [Google Scholar]
- 194.Hundle B, et al. An inhibitory fragment derived from protein kinase Cepsilon prevents enhancement of nerve growth factor responses by ethanol and phorbol esters. J. Biol. Chem. 1997;272:15028–15035. doi: 10.1074/jbc.272.23.15028. [DOI] [PubMed] [Google Scholar]
- 195.Gerstin EHJ, et al. Protein kinase Cdelta mediates ethanol-induced up-regulation of L-type calcium channels. J. Biol. Chem. 1998;273:16409–16414. doi: 10.1074/jbc.273.26.16409. [DOI] [PubMed] [Google Scholar]
- 196.Olive MF, et al. Reduced operant ethanol self-administration and in vivo mesolimbic dopamine responses to ethanol in PKCepsilon-deficient mice. Eur. J. Neurosci. 2000;12:4131–4140. doi: 10.1046/j.1460-9568.2000.00297.x. [DOI] [PubMed] [Google Scholar]
- 197.Jiang ZL, Ye JH. Protein kinase C epsilon is involved in ethanol potentiation of glycine-gated Cl(−) current in rat neurons of ventral tegmental area. Neuropharmacology. 2003;44:493–502. doi: 10.1016/s0028-3908(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 198.Proctor WR, et al. Ethanol differentially enhances hippocampal GABA A receptor-mediated responses in protein kinase C gamma (PKC gamma) and PKC epsilon null mice. J. Pharmacol. Exp. Ther. 2003;305:264–270. doi: 10.1124/jpet.102.045450. [DOI] [PubMed] [Google Scholar]
- 199.Kumar S, et al. Association of protein kinase C with GABA(A) receptors containing alpha1 and alpha4 subunits in the cerebral cortex: selective effects of chronic ethanol consumption. J. Neurochem. 2002;82:110–117. doi: 10.1046/j.1471-4159.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- 200.Qi ZH, et al. Protein kinase C epsilon regulates gamma-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phosphorylation of gamma2 subunits. J. Biol. Chem. 2007;282:33052–33063. doi: 10.1074/jbc.M707233200. [DOI] [PubMed] [Google Scholar]
- 201.Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr. Drug Targets CNS Neurol. Disord. 2002;1:359–385. doi: 10.2174/1568007023339184. [DOI] [PubMed] [Google Scholar]
- 202.Perry DC, et al. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J. Pharmacol. Exp. Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- 203.Buisson B, Bertrand D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol. Sci. 2002;23:130–136. doi: 10.1016/S0165-6147(00)01979-9. [DOI] [PubMed] [Google Scholar]
- 204.Flores CM, et al. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol. Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- 205.Picciotto MR, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 206.Fenster CP, et al. Regulation of alpha4beta2 nicotinic receptor desensitization by calcium and protein kinase C. Mol. Pharmacol. 1999;55:432–443. [PubMed] [Google Scholar]
- 207.Dohrman DP, Reiter CK. Ethanol modulates nicotine-induced upregulation of nAChRs. Brain Res. 2003;975:90–98. doi: 10.1016/s0006-8993(03)02593-9. [DOI] [PubMed] [Google Scholar]
- 208.Eilers H, et al. Functional deactivation of the major neuronal nicotinic receptor caused by nicotine and a protein kinase C-dependent mechanism. Mol. Pharmacol. 1997;52:1105–1112. [PubMed] [Google Scholar]
- 209.Nuutinen S, et al. Nicotine-induced upregulation of human neuronal nicotinic alpha7-receptors is potentiated by modulation of cAMP and PKC in SH-EP1-halpha7 cells. Eur. J. Pharmacol. 2006;544:21–30. doi: 10.1016/j.ejphar.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 210.Hodge CW, et al. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat. Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- 211.Choi DS, et al. Conditional rescue of protein kinase C epsilon regulates ethanol preference and hypnotic sensitivity in adult mice. J. Neurosci. 2002;22:9905–9911. doi: 10.1523/JNEUROSCI.22-22-09905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hodge CW, et al. Decreased anxiety-like behavior, reduced stress hormones, and neurosteroid supersensitivity in mice lacking protein kinase Cepsilon. J. Clin. Invest. 2002;110:1003–1010. doi: 10.1172/JCI15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Harris RA, et al. Mutant mice lacking the gamma isoform of protein kinase C show decreased behavioral actions of ethanol and altered function of gamma-aminobutyrate type A receptors. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3658–3662. doi: 10.1073/pnas.92.9.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bowers BJ, et al. Decreased ethanol sensitivity and tolerance development in gamma-protein kinase C null mutant mice is dependent on genetic background. Alcohol. Clin. Exp. Res. 1999;23:387–397. [PubMed] [Google Scholar]
- 215.Bowers BJ, et al. Mice lacking PKC gamma exhibit decreased anxiety. Behav. Genet. 2000;30:111–121. doi: 10.1023/a:1001951104208. [DOI] [PubMed] [Google Scholar]
- 216.Bowers BJ, et al. Differential sensitivity to the anxiolytic effects of ethanol and flunitrazepam in PKCgamma null mutant mice. Pharmacol. Biochem. Behav. 2001;69:99–110. doi: 10.1016/s0091-3057(01)00510-x. [DOI] [PubMed] [Google Scholar]
- 217.Bowers BJ, Wehner JM. Ethanol consumption and behavioral impulsivity are increased in protein kinase Cgamma null mutant mice. J. Neurosci. 2001;21:RC180. doi: 10.1523/JNEUROSCI.21-21-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schlaepfer IR, et al. The human protein kinase C gamma gene (PRKCG) as a susceptibility locus for behavioral disinhibition. Addict. Biol. 2007;12:200–209. doi: 10.1111/j.1369-1600.2007.00063.x. [DOI] [PubMed] [Google Scholar]
- 219.Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem. J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Olive MF, et al. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol. Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- 221.Newton PM, et al. Increased response to morphine in mice lacking protein kinase C epsilon. Genes Brain Behav. 2007;6:329–338. doi: 10.1111/j.1601-183X.2006.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Becker HC, et al. Exacerbation of ethanol withdrawal seizures in mice with a history of multiple withdrawal experience. Pharmacol. Biochem. Behav. 1997;57:179–183. doi: 10.1016/s0091-3057(96)00303-6. [DOI] [PubMed] [Google Scholar]
- 223.Olive MF, et al. Reduced ethanol withdrawal severity and altered withdrawal-induced c-fos expression in various brain regions of mice lacking protein kinase C-epsilon. Neuroscience. 2001;103:171–179. doi: 10.1016/s0306-4522(00)00566-2. [DOI] [PubMed] [Google Scholar]
- 224.Dina OA, et al. Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. J. Neurosci. 2000;20:8614–8619. doi: 10.1523/JNEUROSCI.20-22-08614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Dina OA, et al. Ethanol withdrawal induces hyperalgesia mediated by PKCepsilon. Eur. J. Neurosci. 2006;24:197–204. doi: 10.1111/j.1460-9568.2006.04886.x. [DOI] [PubMed] [Google Scholar]
- 226.Aujla H, Beninger RJ. Intra-accumbens protein kinase C inhibitor NPC 15437 blocks amphetamine-produced conditioned place preference in rats. Behav. Brain Res. 2003;147:41–48. doi: 10.1016/s0166-4328(03)00136-0. [DOI] [PubMed] [Google Scholar]
- 227.Newton PM, Messing RO. Increased sensitivity to the aversive effects of ethanol in PKCepsilon null mice revealed by place conditioning. Behav. Neurosci. 2007;121:439–442. doi: 10.1037/0735-7044.121.2.439. [DOI] [PubMed] [Google Scholar]
- 228.Hudmon A, Schulman H. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu. Rev. Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- 229.Yamauchi T. Neuronal Ca2+/calmodulin-dependent protein kinase II—discovery, progress in a quarter of a century, and perspective: implication for learning and memory. Biol. Pharm. Bull. 2005;28:1342–1354. doi: 10.1248/bpb.28.1342. [DOI] [PubMed] [Google Scholar]
- 230.Ahmed T, Frey JU. Plasticity-specific phosphorylation of CaMKII, MAP-kinases and CREB during late-LTP in rat hippocampal slices in vitro. Neuropharmacology. 2005;49:477–492. doi: 10.1016/j.neuropharm.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 231.Czernik AJ, et al. Amino acid sequences surrounding the cAMP-dependent and calcium/calmodulin-dependent phosphorylation sites in rat and bovine synapsin I. Proc. Natl. Acad. Sci. U.S.A. 1987;84:7518–7522. doi: 10.1073/pnas.84.21.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sun P, et al. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- 233.Mammen AL, et al. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J. Biol. Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- 234.Lisman J, et al. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 235.Mattson BJ, et al. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J. Neurochem. 2005;95:1481–1494. doi: 10.1111/j.1471-4159.2005.03500.x. [DOI] [PubMed] [Google Scholar]
- 236.Choe ES, Wang JQ. CaMKII regulates amphetamine-induced ERK1/2 phosphorylation in striatal neurons. Neuroreport. 2002;13:1013–1016. doi: 10.1097/00001756-200206120-00006. [DOI] [PubMed] [Google Scholar]
- 237.Fog JU, et al. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51:417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 238.Wei Y, et al. Dopamine transporter activity mediates amphetamine-induced inhibition of Akt through a Ca2+/calmodulin-dependent kinase II-dependent mechanism. Mol. Pharmacol. 2007;71:835–842. doi: 10.1124/mol.106.026351. [DOI] [PubMed] [Google Scholar]
- 239.Akiyama K, Suemaru J. Effect of acute and chronic administration of methamphetamine on calcium-calmodulin dependent protein kinase II activity in the rat brain. Ann. N. Y. Acad. Sci. 2000;914:263–274. doi: 10.1111/j.1749-6632.2000.tb05201.x. [DOI] [PubMed] [Google Scholar]
- 240.Das S, et al. NMDA and D1 receptors regulate the phosphorylation of CREB and the induction of c-fos in striatal neurons in primary culture. Synapse. 1997;25:227–233. doi: 10.1002/(SICI)1098-2396(199703)25:3<227::AID-SYN1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 241.Iwata SI, et al. Enhanced dopamine release and phosphorylation of synapsin I and neuromodulin in striatal synaptosomes after repeated amphetamine. J. Pharmacol. Exp. Ther. 1997;283:1445–1452. [PubMed] [Google Scholar]
- 242.Greenstein R, et al. Amphetamine sensitization elevates CaMKIIbeta mRNA. Synapse. 2007;61:827–834. doi: 10.1002/syn.20429. [DOI] [PubMed] [Google Scholar]
- 243.Licata SC, et al. Suppressing calcium/calmodulin-dependent protein kinase II activity in the ventral tegmental area enhances the acute behavioural response to cocaine but attenuates the initiation of cocaine-induced behavioural sensitization in rats. Eur. J. Neurosci. 2004;19:405–414. doi: 10.1111/j.0953-816x.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- 244.Backes E, Hemby SE. Discrete cell gene profiling of ventral tegmental dopamine neurons after acute and chronic cocaine self-administration. J. Pharmacol. Exp. Ther. 2003;307:450–459. doi: 10.1124/jpet.103.054965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pierce RC, Kalivas PW. Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J. Neurosci. 1997;17:3254–3261. doi: 10.1523/JNEUROSCI.17-09-03254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Rubino T, et al. Cellular mechanisms underlying the anxiolytic effect of low doses of peripheral Delta9-tetrahydrocannabinol in rats. Neuropsychopharmacology. 2007;32:2036–2045. doi: 10.1038/sj.npp.1301330. [DOI] [PubMed] [Google Scholar]
- 247.Shukla PK, et al. Phosphorylation of neurogranin, protein kinase C, and Ca2+/calmodulin dependent protein kinase II in opioid tolerance and dependence. Neurosci. Lett. 2006;404:266–269. doi: 10.1016/j.neulet.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 248.Zhong W, et al. Opiate withdrawal induces dynamic expressions of AMPA receptors and its regulatory molecule CaMKIIalpha in hippocampal synapses. Life Sci. 2006;79:861–869. doi: 10.1016/j.lfs.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 249.Hamdy MM, et al. Molecular mechanisms in dizocilpine-induced attenuation of development of morphine dependence: an association with cortical Ca2+/calmodulin-dependent signal cascade. Behav. Brain Res. 2004;152:263–270. doi: 10.1016/j.bbr.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 250.Lou L, et al. Modulation of Ca2+/calmodulin-dependent protein kinase II activity by acute and chronic morphine administration in rat hippocampus: differential regulation of alpha and beta isoforms. Mol. Pharmacol. 1999;55:557–563. [PubMed] [Google Scholar]
- 251.Mestek A, et al. The human mu opioid receptor: modulation of functional desensitization by calcium/calmodulin-dependent protein kinase and protein kinase C. J. Neurosci. 1995;15:2396–2406. doi: 10.1523/JNEUROSCI.15-03-02396.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Koch T, et al. Site mutation in the rat muopioid receptor demonstrates the involvement of calcium/calmodulin-dependent protein kinase II in agonist-mediated desensitization. J. Neurochem. 1997;69:1767–1770. doi: 10.1046/j.1471-4159.1997.69041767.x. [DOI] [PubMed] [Google Scholar]
- 253.Dunn JM, et al. Repeated administration of AMPA or a metabotropic glutamate receptor agonist into the rat ventral tegmental area augments the subsequent behavioral hyperactivity induced by cocaine. Psychopharmacology (Berl.) 2005;179:172–180. doi: 10.1007/s00213-004-2054-9. [DOI] [PubMed] [Google Scholar]
- 254.Pierce RC, et al. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J. Pharmacol. Exp. Ther. 1998;286:1171–1176. [PubMed] [Google Scholar]
- 255.Fan GH, et al. Inhibition of calcium/calmodulin-dependent protein kinase II in rat hippocampus attenuates morphine tolerance and dependence. Mol. Pharmacol. 1999;56:39–45. doi: 10.1124/mol.56.1.39. [DOI] [PubMed] [Google Scholar]
- 256.Lu L, et al. Inhibition of the amygdala and hippocampal calcium/calmodulin-dependent protein kinase II attenuates the dependence and relapse to morphine differently in rats. Neurosci. Lett. 2000;291:191–195. doi: 10.1016/s0304-3940(00)01352-5. [DOI] [PubMed] [Google Scholar]
- 257.Sakurai S, et al. Roles of hippocampal N-methyl-D-aspartate receptors and calcium/calmodulin-dependent protein kinase II in amphetamine-produced conditioned place preference in rats. Behav. Pharmacol. 2007;18:497–506. doi: 10.1097/FBP.0b013e3282ee7b62. [DOI] [PubMed] [Google Scholar]
- 258.Tan SE. Impairing the amphetamine conditioning in rats through the inhibition of hippocampal calcium/calmodulin-dependent protein kinase II activity. Neuropharmacology. 2002;42:540–547. doi: 10.1016/s0028-3908(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 259.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu. Rev. Cell. Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 260.Grant SG, et al. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 261.Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci. 2003;91:267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- 262.Rajagopal R, Chao MV. A role for Fyn in Trk receptor transactivation by G-protein-coupled receptor signaling. Mol. Cell Neurosci. 2006;33:36–46. doi: 10.1016/j.mcn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 263.McGarrigle D, Huang XY. GPCRs signaling directly through Src-family kinases. Sci STKE. 2007;2007:e35. doi: 10.1126/stke.3922007pe35. [DOI] [PubMed] [Google Scholar]
- 264.Kihara T, et al. alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity. J. Biol. Chem. 2001;276:13541–13546. doi: 10.1074/jbc.M008035200. [DOI] [PubMed] [Google Scholar]
- 265.Kojima N, et al. Higher seizure susceptibility and enhanced tyrosine phosphorylation of N-methyl-D-aspartate receptor subunit 2B in fyn transgenic mice. Learn Mem. 1998;5:429–445. [PMC free article] [PubMed] [Google Scholar]
- 266.Xu F, et al. Brain-derived neurotrophic factor rapidly increases NMDA receptor channel activity through Fyn-mediated phosphorylation. Brain Res. 2006;1121:22–34. doi: 10.1016/j.brainres.2006.08.129. [DOI] [PubMed] [Google Scholar]
- 267.Wang J, et al. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J. Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Nishio H, Suzuki K. Ethanol-induced Cas tyrosine phosphorylation and Fyn kinase activation in rat brain. Alcohol. Clin. Exp. Res. 2002;26:38S–43S. doi: 10.1097/01.ALC.0000026832.06336.A1. [DOI] [PubMed] [Google Scholar]
- 269.Miyakawa T, et al. Fyn-kinase as a determinant of ethanol sensitivity: relation to NMDA-receptor function. Science. 1997;278:698–701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- 270.Yaka R, et al. NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5710–5715. doi: 10.1073/pnas.062046299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yaka R, et al. Scaffolding of Fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. J. Neurosci. 2003;23:3623–3632. doi: 10.1523/JNEUROSCI.23-09-03623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yaka R, et al. Fyn kinase and NR2B-containing NMDA receptors regulate acute ethanol sensitivity but not ethanol intake or conditioned reward. Alcohol. Clin. Exp. Res. 2003;27:1736–1742. doi: 10.1097/01.ALC.0000095924.87729.D8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Boehm S.L.n., et al. Deletion of the fyn-kinase gene alters behavioral sensitivity to ethanol. Alcohol. Clin. Exp. Res. 2003;27:1033–1040. doi: 10.1097/01.ALC.0000075822.80583.71. [DOI] [PubMed] [Google Scholar]
- 274.Boehm S.L.n., et al. Over-expression of the fyn-kinase gene reduces hypnotic sensitivity to ethanol in mice. Neurosci. Lett. 2004;372:6–11. doi: 10.1016/j.neulet.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 275.Cowen MS, et al. Role of Fyn tyrosine kinase in ethanol consumption by mice. Alcohol. Clin. Exp. Res. 2003;27:1213–1219. doi: 10.1097/01.ALC.0000081630.14159.02. [DOI] [PubMed] [Google Scholar]
- 276.Stork O, et al. Resistance to alcohol withdrawal-induced behaviour in Fyn transgenic mice and its reversal by ifenprodil. Brain Res. Mol. Brain Res. 2002;105:126–135. doi: 10.1016/s0169-328x(02)00400-x. [DOI] [PubMed] [Google Scholar]
- 277.Schumann G, et al. Analysis of genetic variations of protein tyrosine kinase fyn and their association with alcohol dependence in two independent cohorts. Biol. Psychiatry. 2003;54:1422–1426. doi: 10.1016/s0006-3223(03)00635-8. [DOI] [PubMed] [Google Scholar]
- 278.Slater SJ, et al. Inhibition of protein kinase C by alcohols and anaesthetics. Nature. 1993;364:82–84. doi: 10.1038/364082a0. [DOI] [PubMed] [Google Scholar]
- 279.Arolfo MP, et al. Ethanol operant self-administration in rats is regulated by adenosine A2 receptors. Alcohol. Clin. Exp. Res. 2004;28:1308–1316. doi: 10.1097/01.alc.0000139821.38167.20. [DOI] [PubMed] [Google Scholar]
- 280.Thiele TE, et al. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- 281.Rimondini R, et al. Suppression of ethanol self-administration by the neuropeptide Y (NPY) Y2 receptor antagonist BIIE0246: evidence for sensitization in rats with a history of dependence. Neurosci. Lett. 2005;375:129–133. doi: 10.1016/j.neulet.2004.10.084. [DOI] [PubMed] [Google Scholar]
- 282.Bain J, et al. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Allen JJ, et al. A semisynthetic epitope for kinase substrates. Nat. Methods. 2007;4:511–516. doi: 10.1038/nmeth1048. [DOI] [PMC free article] [PubMed] [Google Scholar]