Synopsis
Previous studies showed that apolipoprotein E (apoE) expression in macrophages suppresses inflammatory response. Whether the endogenously synthesized apoE acts intracellularly or after its secretion in suppressing macrophage inflammation remains unclear. The current study used the murine macrophage cell line RAW 264.7 to examine the influence of exogenous apoE on macrophage inflammatory responses induced by toll-like receptor (TLR)-4 and TLR-3 agonists lipopolysaccharide (LPS) and poly(I-C), respectively. Results showed that exogenously added apoE suppressed LPS- and poly(I-C)-induction of interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α secretion by RAW 264.7 cells. The mechanism was related to apoE suppression of TLR agonist-induced phosphorylation of c-Jun N-terminal kinase (JNK) and c-Jun. A peptide containing tandem repeat sequence of the receptor binding domain of apoE, apoE(141–155)2, was similarly effective in inhibiting LPS- and poly(I-C)-induced macrophage inflammatory response. Reductive methylation of lysine residues in apoE, which abolished its receptor binding capability without affecting its ability to interact with heparin-sulfate proteoglycans (HSPG), inhibited the ability of apoE to suppress macrophage response to LPS but had no effect on apoE suppression of poly(I-C)-induced macrophage activation. The ability of apoE to suppress poly(I-C)-induced pro-inflammatory cytokine production was abolished by heparinase treatment of RAW 264.7 cells to remove cell surface HSPG. Taken together, these results indicate that exogenous apoE inhibits macrophage inflammatory response to TLR4- and TLR-3 agonists through distinct mechanisms related to receptor and HSPG binding, respectively, and that these inhibitory effects converged on suppression of JNK and c-Jun activation that are necessary for macrophage activation.
Keywords: Apolipoprotein E, Inflammation, Lipoprotein Receptors, Signal Transduction, c-Jun N-terminal Kinase
INTRODUCTION
Atherosclerosis is a chronic inflammatory disease of the vessel wall. A prominent event associated with the development and progression of atherosclerosis is the recruitment of monocytes to site of vascular injury, their differentiation into macrophages, and macrophage activation to produce pro-inflammatory cytokines (reviewed in refs. [1, 2]). Recent data suggest that inflammation of adipose tissue with differentiation and activation of tissue macrophages also contributes to obesity-associated metabolic disorders [2, 3]. The processes associated with vascular and adipose inflammation share similar characteristics with resident cells in each tissue (smooth muscle cells in the vasculature and adipocytes in adipose tissues), expressing monocyte chemotactic protein-1 to recruit circulating monocytes and secreting lipid metabolites and inflammatory cytokines to promote macrophage activation.
Another protein that is synthesized and secreted by adipocytes and smooth muscle cells with functional implications in metabolic regulation is apolipoprotein E (apoE) [4–7]. This is a multi-functional protein that circulates in plasma in association with lipoproteins. A major function of apoE is its role in modulating cellular cholesterol efflux and serving as the ligand for receptor-mediated cellular uptake and plasma clearance of atherogenic lipoproteins. Accordingly, apoE exerts cardiovascular benefits by preventing hypercholesterolemia and reducing lipid deposition in the vessel wall. Additionally, increasing evidence has suggested that apoE may have lipid transport-independent functions in protection against cardiovascular disease [8–10]. These include the ability of apoE to inhibit lipid oxidation and protect against cellular oxidative damage [11], suppress adhesion molecule expression on endothelial cell surface [12], and inhibit smooth muscle cell activation [13–15]. These cell regulatory properties of apoE are likely mediated via cell signaling pathways as a consequence of its interaction with cell surface receptors, particularly those in the LDL receptor family [16–18]. ApoE secreted by astrocytes in the brain also interacts with LDL receptor family proteins in neuronal cells in modulating cell signal events that are important for neurological health [19].
The physiological functions of apoE expressed in adipose tissue and smooth muscle cells have not been completely elucidated. Recent studies showed that apoE recruited from plasma circulation as well as synthesized endogenously in smooth muscle cells regulates inducible nitric oxide synthase expression in the vessel wall in inhibiting vascular occlusive diseases [7, 20]. In contrast, apoE synthesized in adipocytes is required for triglyceride storage and adipocyte differentiation [5], but exogenously-derived apoE failed to induce cellular triglyceride accumulation and adipocyte differentiation in apoE−/− mice [5]. However, apoE synthesized in adipocytes is not only present intracellularly but is also secreted in a manner inducible by peroxisome proliferator-activated receptor γ agonists and inhibited by tumor necrosis factor-α (TNF-α) [21]. In view of the well established anti-inflammatory properties of apoE in other cell types [8–10], these results suggest that exogenous apoE may act as a paracrine factor in regulating the functions of tissue macrophages. This study was undertaken to evaluate the impact of exogenous apoE on macrophage activation and determine if apoE binding to lipoprotein receptors is involved.
MATERIALS AND METHODS
Materials
Lipopolysaccharide (LPS) from E. coli, poly(I-C), and heparinase III were obtained from Sigma. Dulbecco’s modified Eagles’ medium (DMEM) and fetal bovine serum were purchased from Life Technologies, Inc. Immunoassay kit for IL-1β quantification was obtained from R & D Systems and ELISA kits for IL-6 and TNF-α measurements were purchased from B & D Biosciences. Antibodies against JNK, phospho-JNK, c-Jun, and phospho-c-Jun (ser63) were purchased from Cell Signaling, Inc. Horseradish peroxidase (HRP)-conjugated secondary antibodies and reagents for chemiluminescence detection of Western blots were obtained from Amersham Biosciences. Receptor associated protein (RAP) was isolated as glutathione S-transferase (GST)-conjugated fusion protein by glutathione-agarose affinity chromatography of medium collected from E coli harboring the GST-RAP cDNA (generous gift from Dr. Dudley Strickland, University of Maryland) [22].
Human ApoE and ApoE Peptide
Recombinant human apoE was obtained from the culture media of human apoE3 cDNA transfected HEK293 cells (generous gift from Drs. Godfrey S. Getz and Catherine A. Reardon, University of Chicago) [23] and then purified by heparin-Sepharose affinity column chromatography before use. The purity of the apoE was assessed by SDS-PAGE, and samples containing only a single band with Mr = 34,000 were used. Purified apoE was dialyzed against ammonium bicarbonate, lyophilized, and stored at −80 ºC until use. The lyophilized apoE was resuspended in PBS and added directly to the culture medium without reconstitution with lipids. In selected experiments, lysine residues in apoE were modified by reductive methylation according to the procedure of Weisgraber et al. [24]. The reductively methylated apoE was also dialyzed against PBS prior to addition to cell culture medium for experiments.
A peptide containing tandem repeat of the apoE sequence from residue 141–155, designated as apoE(141–155)2, was synthesized chemically by the Synpep Co. (Hopkinton, MA). The sequence of the peptide was verified by mass spectrometry. The lyophilized peptide was reconstituted with PBS and stored at −20 ºC until use.
Cell Culture
The murine monocyte-macrophage cell line RAW 264.7 was obtained from ATCC (Manassas, VA) and maintained in Dulbecco’s modified Eagles Medium (DMEM) supplemented with 10% fetal bovine serum at 37 ºC in 5% CO2 environment. The cells were pre-incubated for 30 min with or without apoE prior to the addition of LPS or poly(I-C) at the concentrations as indicated for each experiment. Cell culture medium was collected after 8 h. Cytokines secreted into the medium were measured using commercially available immunoassay and ELISA kits.
Western Blot Analysis
Total cellular protein was isolated after the experimental period by sonicating the cells in ice-cold RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.5% sodium deoxycholate, 1% igepal, 0.1% SDS, and 1 mM EDTA) plus 1X protease inhibitor cocktail (Sigma) and 1X phosphatase inhibitor cocktails I and II (Sigma). For analysis, 100 μg of protein was resolved by SDS-PAGE and transferred to PVDF paper. Nonspecific sites on the membranes were blocked with buffer containing 5% nonfat milk, and then probed with antibodies against total JNK, phospho-JNK, total c-Jun, and phospho-c-Jun(ser63). Immunoreactive bands were detected by incubation with HRP-conjugated second antibodies and then visualized by chemiluminescence.
Statistical Analysis
All values are expressed as means ± S.D. of triplicate determinations from 2 separate experiments. An ANOVA was used to analyze statistical significant differences. When significance was reached, a post hoc analysis using the Bonferroni/Dunn test was performed.
RESULTS
Previous studies using macrophages isolated from wild type and apoE−/− mice as well as monocyte-derived macrophage cell lines with or without apoE expression have established an autocrine function of endogenously-derived apoE in limiting macrophage pro-inflammatory response [25, 26]. The current study used the RAW 264.7 monocyte-macrophage cell line, which does not express apoE endogenously [27], to assess the influence of exogenous apoE on macrophage inflammatory response. This cell line did not secrete detectable levels of pro-inflammatory cytokines such as interleukin (IL)-6, IL-1β, and tumor necrosis factor-α (TNF-α) into the culture media when incubated under basal conditions. In contrast, these cytokines were detected in the cultured media when RAW 264.7 cells were stimulated by incubation with 25 ng/ml of the toll-like receptor (TLR)-4 ligand LPS or with 25 μg/ml of the TLR-3 ligand poly(I-C). The inclusion of apoE in the incubation media showed a concentration-dependent reduction of IL-6, IL-1β, and TNF-α secretion stimulated by both LPS and poly(I-C) (Fig. 1). These results documented the ability of exogenous apoE to suppress TLR-4 and TLR-3 mediated macrophage inflammatory response.
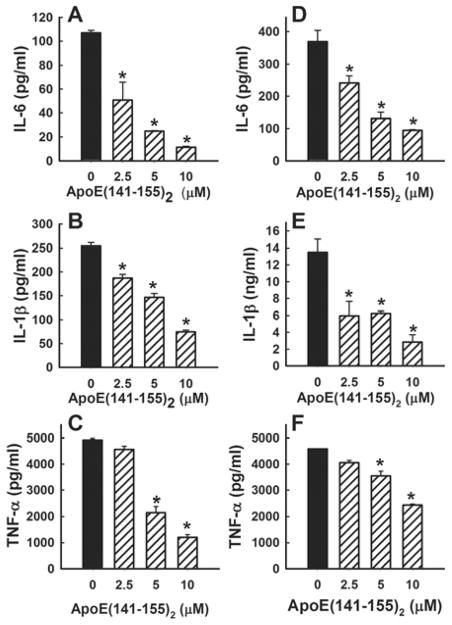
Figure 1. Apolipoprotein E inhibition of LPS and poly(I-C) stimulation of pro-inflammatory cytokine production by RAW 264.7 cells.
Murine RAW 264.7 cells were pre-incubated with apoE for 30 min at the concentrations indicated prior to the addition of 25 ng/ml LPS (panels A–C) or 25 μg/ml poly(I-C) (panels D–F). The incubation was continued at 37 ºC for 8 h and the culture medium was collected. The concentration of IL-6 (panels A, D), IL-1β (panels B, E), and TNF-α (panels C, F) in the culture medium was detected by immunoassay or ELISA kits. The data represent means ±S.D. from 2 separate experiments, each performed in triplicates. * indicates significant difference from samples incubated without apoE at P<0.01.
Apolipoprotein E is a cholesterol transport protein with the capability of interacting with cell surface receptors and heparin sulfate proteoglycans (HSPG) to modulate cell signaling events [10]. Hence, apoE inhibition of LPS and poly(I-C) activation of macrophages may be due to its modulation of cell membrane microenvironment, thereby affecting TLR-mediated cell signaling events, or alternatively may be a direct result of apoE binding to cell surface proteins in mediating cell signaling. Therefore, the next set of experiments used the apoE(144–155)2 peptide, which contains a tandem repeat sequence of the receptor binding domain but lacks the lipid binding domain of apoE [28], to distinguish between these possibilities. Results, as depicted in Fig. 2, showed that the apoE(141–155)2 peptide also inhibited LPS and p(I-C) induction of IL-6, IL-1β, and TNF-α secretion in a concentration-dependent manner.
Figure 2. Inhibition of LPS and poly(I-C) stimulated pro-inflammatory cytokine production by apoE(141–155)2 peptide.
Murine RAW 264.7 cells were pre-incubated with the apoE(141–155)2 peptide for 30 min at the concentrations indicated prior to the addition of 25 ng/ml LPS (panels A–C) or 25 μg/ml Poly(I-C) (panels D–F). The incubation was continued at 37 ºC for 8 h and the culture medium was collected. The concentration of IL-6 (panels A, D), IL-1β (panels B, E), and TNF-α (panels C, F) in the culture medium was detected by immunoassay or ELISA kits. The data represent means ±S.D. from 2 separate experiments, each performed in triplicates. * indicates significant difference from samples incubated without apoE at P<0.01.
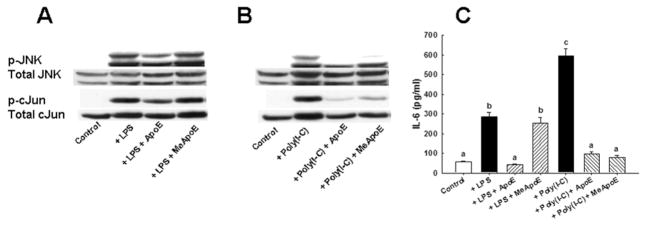
Lipopolysaccharide-induced IL-6, IL-1β, and TNF-α synthesis in monocyte-macrophages requires the activation of Jun N-terminal kinase (JNK) and c-Jun [29, 30]. In our experiments, incubation of RAW 264.7 cells with LPS also resulted in time-dependent increase of JNK and c-Jun phosphorylation (Fig. 3A and B). Interestingly, poly(I-C) also increased JNK and c-Jun phosphorylation in RAW 264.7 cells in a time-dependent manner (Fig. 3A and B). Pre-incubation of cells with apoE or the apoE(141–155)2 peptide significantly reduced JNK and c-Jun phosphorylation in response to both LPS and poly(I-C) (Fig. 3C and D). Taken together, these results indicated that apoE, via its receptor binding domain interaction with cell surface receptors and/or HSPG, inhibits pro-inflammatory cytokine production by suppressing TLR-3 and TLR-4 mediated JNK activation.
Figure 3. Apolipoprotein E and apoE(141–155)2 peptide inhibit LPS and poly(I-C) stimulation of JNK and cJun phosphorylation.
Murine RAW 264.7 cells were pre-incubated with or without 50 μg/ml apoE or 0–10 μM of the apoE(141–155)2 peptide prior to the addition of 25 ng/ml LPS or 25 μg/ml poly(I-C). Panel A shows LPS and poly(I-C) induction of JNK phosphorylation in a time-dependent manner. Induction of both JNK1 and JNK2 bands are indicated from the blots. Panel B shows LPS and poly(I-C) induction of c-Jun phosphorylation in a time-dependent manner. Panels C and D show apoE and apoE(141–155)2 peptide, respectively, inhibited LPS induced JNK and c-Jun phosphorylation 60 min after the addition of the TLR agonists. The data are derived from samples pooled from triplicate plates and are representatives of 2 separate experiments.
The difference between receptor versus HSPG binding properties of apoE in suppressing TLR-mediated JNK activation and inflammatory cytokine production was assessed by examining the influence of reductively methylated apoE on RAW 264.7 activation by LPS and poly(I-C). Previous studies have shown that reductive methylation of lysine residues in apoE abolished its ability to bind receptors without interfering with its heparin binding properties [24, 31]. The methylated apoE has also been used successfully in previous experiments to identify distinct pathways for apoE inhibition of smooth muscle cell migration and proliferation [17]. In the current experiments, reductive methylation was shown to abolish the ability of apoE to inhibit LPS-induced JNK and c-Jun phosphorylation (Fig. 4A). In contrast, the methylated apoE remained effective in inhibiting JNK and c-Jun phosphorylation induced by poly(I-C) (Fig. 4B). The different effectiveness of methylated apoE in inhibiting LPS- and poly(I-C)-stimulated JNK and c-Jun phosphorylation was reflected by its ability to suppress pro-inflammatory cytokine secretion in response to these TLR ligands. As shown in Fig. 4C, the methylated apoE was effective in inhibiting IL-6 secretion in response to poly(I-C) stimulation but was incapable of suppressing IL-6 secretion in response to LPS.
Figure 4. Reductive methylation abolished apoE inhibition of LPS induced macrophage activation but had no effect on apoE inhibition of poly(I-C)-induced events.
Murine RAW 264.7 macrophages were pre-incubated with 50 μg/ml of apoE or methylated apoE (MeApoE) prior to the addition of 25 ng/ml LPS or 25 μg/ml poly(I-C). Cell lysates were prepared after 60 min to detect JNK and cJun phosphorylation induced by LPS (panel A) or poly(I-C) (panel B). Panel C compares the influence of apoE versus MeApoE on IL-6 production after 8 h stimulation with LPS or poly(I-C). The data are means ± S.D. from triplicate plates in 2 separate experiments. Bars with different letters indicate significant difference at P<0.01.
The ability of apoE, but not methylated apoE, to inhibit LPS-induced pro-inflammatory cytokine production by RAW 264.7 cells suggested that apoE binding to cell surface receptors, probably members of the LDL receptor family proteins, is required for inhibition of macrophage activation induced through the TLR-4 pathway. The involvement of LDL receptor family proteins in apoE mediated cell signaling events was typically explored in previous experiments with the receptor associated protein (RAP), which competitively inhibits ligand binding to this family of receptor proteins [32]. However, this approach cannot be used for the current study as RAP binding to cell surface receptors also resulted in a concentration-dependent inhibition of pro-inflammatory cytokine production in response to LPS (Fig. 5A). Nevertheless, these results illustrated that binding of exogenous ligands to LDL receptor family proteins on macrophage cell surface is sufficient to suppress LPS stimulation. In contrast, the removal of cell surface HSPG by heparinase treatment had no effect on apoE inhibition of LPS-induced cytokine production (Fig. 5B), indicating that apoE binding to HSPG was not involved. Interestingly, heparinase treatment abolished the inhibitory effects of apoE on macrophage activation induced by poly(I-C) (Fig. 5C). The latter observation indicated that apoE inhibition of poly(I-C)-induced macrophage activation is mediated via its interaction with HSPG on the cell surface.
Figure 5. Influence of lipoprotein receptor associated protein (RAP) and heparinase treatment on LPS- and poly(I-C)-induced pro-inflammatory cytokine production.
Panel A shows the influence of 30 min RAP pre-incubation on LPS-stimulated IL-6 production by murine RAW 264.7 cells. Panels B and C show the influence of pre-incubating RAW 264.7 cells for 30 min with 1 unit/ml of heparinase III on the ability of apoE to inhibit LPS- and poly(I-C)-induced IL-6 production. The data represent means ± S.D. from triplicate plates in 2 separate experiments. Bars with different letters in each graph indicate significant difference at P<0.01.
DISCUSSION
Earlier studies comparing inflammatory response between wild type and apoE-deficient mice revealed elevated levels of IL-6, IL-1β, and TNF-α in apoE−/− mice in response to LPS or other TLR agonists [25, 33]. The exaggerated inflammatory response of apoE−/− mice can be reversed by apoE infusion or reconstituted expression of apoE in the liver [25]. The protective effect of apoE against LPS-induced inflammatory response was reportedly due to LPS binding to apoE-enriched lipoproteins resulting in its rapid clearance from circulation [33]. Subsequent studies comparing macrophages with and without apoE expression showed that endogenously expressed apoE also suppresses agonist-induced pro-inflammatory cytokine production [26, 34]. Whether the endogenously synthesized apoE acts intracellularly or after its secretion in suppressing macrophage inflammatory response remains unclear. In the current study, we showed that exogenous apoE suppresses macrophage response to both TLR-3 and TLR-4 agonists. Thus, apoE secreted by macrophages can act in a paracrine and/or autocrine manner in limiting inflammatory cytokine secretion. Moreover, these results indicate that apoE synthesized by tissue resident cells and/or recruited from plasma circulation may also act in a paracrine manner in suppressing tissue inflammatory response.
Apolipoprotein E is a lipid transport protein capable of promoting cholesterol efflux from cells [35]. Therefore, it is possible that exogenous apoE may inhibit macrophage activation by promoting cholesterol efflux, thereby perturbing membrane microenvironment and disrupting TLR signaling pathways that are required for activation of cytokine production. The current study showed that a tandem repeat peptide containing the receptor binding domain of apoE but lacking in the lipid binding domain required to promote cholesterol efflux [35] is also capable of inhibiting LPS- and poly(I-C)-induced inflammatory cytokine production in macrophages. These studies illustrated that apoE can inhibit macrophage inflammatory response in a mechanism independent of lipid transport. Our data revealed a mechanism involving apoE binding to cell surface receptors and HSPG and the consequential inhibition of JNK and c-Jun phosphorylation that is required for IL-6, IL-1β, and TNF-α secretion.
It is important to note that the apoE(141–155)2 peptide appeared to be more effective than intact apoE, at the concentrations tested in our experiments, in inhibiting JNK phosphorylation even though their effectiveness in inhibiting IL-6 and TNF-α secretion was similar. The inhibition of IL-1β secretion also appeared to be more effective with apoE than with the apoE(141–155)2 peptide. Taken together, these results indicate that additional mechanism(s) may be necessary for complete apoE inhibition of TLR agonist-induced inflammatory responses. Although the current study did not identify this additional mechanism, previous studies have shown that the C-terminal domain in apoE is necessary to promote cholesterol efflux via ABCA1 [35], and that ABCA1-mediated cholesterol efflux also suppresses inflammatory response [36]. Therefore, it is likely that the maximal anti-inflammatory properties of apoE are due to the additive effects of its two independent functions: by promoting cholesterol efflux via ABCA1 and by regulation of cell signal transduction via interaction with LDL receptor family proteins and HSPG.
The current study also showed that reductive methylation of apoE abolished its ability to suppress LPS-induced JNK and c-Jun phosphorylation. As a result, LPS-induced pro-inflammatory cytokine production was not blocked by methylated apoE. Since lysine residues in apoE do not participate in cholesterol efflux [35], and reductive methylation abolished apoE binding to LDL receptor family proteins but not HSPG [24, 31], these observations provided additional evidence to support the conclusion that cholesterol efflux is not sufficient but receptor binding is necessary for apoE inhibition of LPS-induced macrophage activation. Surprisingly, reductive methylation of lysine residues in apoE did not abolish its ability to inhibit poly(I-C)-induced macrophage activation. Moreover, apoE failed to suppress poly(I-C)-induced inflammatory cytokine production in macrophages depleted of cell surface HSPG with heparinase treatment. In contrast, apoE remained effective in suppressing LPS-induced inflammatory response in heparinase treated RAW 264.7 cells. Taken together, these latter two observations revealed that apoE inhibition of poly(I-C)-induced macrophage activation is mediated by a process via its interaction with HSPG and is distinct from the mechanism by which it suppresses LPS-induced macrophage response.
The importance of LDL receptor-related proteins (LRP) in mediating apoE inhibition of LPS-induced macrophage activation is supported by additional experiments showing another LRP ligand RAP was also effective in reducing LPS-induced pro-inflammatory cytokine production. These observations appeared to contradict most previous studies using RAP to explore the functional significance of apoE-LRP interaction, with results typically showing RAP inhibits instead of mimicking the apoE effects [32]. However, it is important to note that most previous studies were performed by prolonged pre-incubation of cells with RAP prior to testing its influence on apoE modulation of cell functions. This manipulation resulted in down-regulation of LRP expression on the cell surface to abolish the apoE effects [32]. In the current study, we took advantage of the observation that RAP added to cell culture medium also serves as a ligand and competitively inhibits apoE binding to LRP [37] and showed exogenous RAP resembled apoE in inhibiting LPS-induced macrophage activation. Thus, regardless of the exogenous ligand used, LRP binding is sufficient to trigger signaling events that inhibit the LPS-induced macrophage pro-inflammatory response.
The importance of LDL receptor family proteins and their interaction with apoE in modulating cell functions has attracted considerable attention in recent years. Impetus for this new direction was prompted by the discovery that the C-terminal cytoplasmic domains of these receptors contain phosphorylation sites for protein kinases and interact with adaptor proteins important for cell signal transduction [38, 39]. Interestingly, the LRP mediated signaling events appeared to differ among various cell types. For example, LRP has been shown to be an important modulator of platelet derived growth factor (PDGF) and transforming growth factor-β (TGF-β) signaling events in smooth muscle cells [40]. The absence of LRP in smooth muscle cells results in exuberant atherosclerosis and neointimal hyperplasia due to the constitutive activation of PDGF and TGF-β signaling events [41, 42]. The binding of apoE to LRP on smooth muscle cells inhibits cell migration in a process that is mediated by protein kinase A activation [18]. The binding of apoE to LRP also inhibits the tyrosine kinase Src activity in fibroblasts [38, 39]. In the adipose cell lineage, LRP in pre-adipocyte fibroblasts modulates Wnt signaling cascade to regulate adipocyte differentiation [43]. The lack of LRP expression in mature adipocytes results in hypotrophic cells that are defective in lipid uptake and storage [43, 44]. Finally, in neuronal cells, apoE binding to LRP and other members of the same gene family decreases JNK and c-Jun activation in a mechanism related to receptor interaction with the JNK interacting protein JIP [32, 45]. In view of previous studies documenting that src kinase-mediated tyrosine phosphorylation and JIP interaction with TLR4 are important events associated with LPS-induced JNK phosphorylation and pro-inflammatory cytokine secretion [46, 47], apoE inhibition of LPS-induced macrophage activation is likely due to inhibition of one or both of these pathways. Since a number of LDL receptor family proteins contain motifs capable of JIP interaction and modulation of tyrosine kinase activity, we are currently designing experiments to identify the specific receptor involved.
The current study also showed that apoE suppression of poly(I-C)-induced JNK and c-Jun activation and cytokine production in macrophages is mediated via its interaction with HSPG instead of LDL receptor family proteins. This observation is consistent with the previous suggestion that different toll-like receptor agonists induce distinct macrophage responses [48]. The fact that both LPS and poly(I-C) induced JNK and c-Jun phosphorylation increase inflammatory cytokine production indicates that the TLR4 and TLR3 pathways converge at or prior to the JNK activation step. Moreover, the ability of apoE to inhibit LPS- and poly(I-C)-induced JNK and c-Jun activation via distinct mechanisms, by binding to either cell surface receptors or HSPG respectively, suggests that apoE inhibits distinct upstream events leading to JNK activation. As discussed above, it is likely that apoE binding to LRP and/or its related receptors inhibits tyrosine kinase activity that leads to inhibition of LPS-induced JNK activation. The mechanism by which apoE binding to HSPG results in inhibition of poly(I-C)-induced activation of TLR-3, an intracellular receptor, remains to be determined. Our results indicated that apoE binding to HSPG on the cell surface directly stimulates mechanisms that interfere with TLR-3 activation. A recent report showing that the ability of a similar apoE mimetic peptide to inhibit IκB degradation and prevent nuclear factor-κB translocation [49], a process required for TLR activation [50], is consistent with this possibility. Regardless, results of the current study add to the literature documenting the specific influence of apoE binding to cell receptors versus HSPG in modulating distinct cell functions and cell signaling responses [17].
Acknowledgments
FUNDING
This work was supported by a grant from the National Institutes of Health (RO1 DK74932).
Abbreviations used
- apoE
apolipoprotein E
- DMEM
Dulbecco’s modified Eagles medium
- HRP
horseradish peroxidase
- HSPG
heparin sulfate proteoglycans
- IL
interleukin
- JNK
c-Jun N-terminal kinase
- JIP
JNK interacting protein
- LPS
lipopolysaccharide
- LRP
LDL receptor related protein
- PDGF
platelet-derived growth factor
- TGF-β
transforming growth factor-β
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-α
References
- 1.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 3.de Ferranti S, Mozaffarian D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 4.Zechner R, Moser R, Newman TC, Fried SK, Breslow JL. Apolipoprotein E gene expression in mouse 3T3L1 adipocytes and human adipose tissue and its regulation by differentiation and lipid content. J Biol Chem. 1991;266:10583–10588. [PubMed] [Google Scholar]
- 5.Huang ZH, Reardon CA, Mazzone T. Endogenous apoE expression modulates adipocyte triglyceride content and turnover. Diabetes. 2006;55:3394–3402. doi: 10.2337/db06-0354. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber BM, Jones HV, Franzblau C. Apolipoprotein E expression in aortic smooth muscle cells: the effect of B-VLDL. J Lipid Res. 1994;35:1177–1186. [PubMed] [Google Scholar]
- 7.Moore ZWQ, Zhu B, Kuhel DG, Hui DY. Vascular apolipoprotein E expression and recruitment from circulation to modulate smooth muscle cell response to endothelial denudation. Am J Pathol. 2004;164:2109–2116. doi: 10.1016/S0002-9440(10)63769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtiss LK, Boisvert WA. Apolipoprotein E and atherosclerosis. Curr Opin Lipidol. 2000;11:243–251. doi: 10.1097/00041433-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Curtiss LK. ApoE in atherosclerosis. A protein with multiple hats. Arterioscler Thromb Vasc Biol. 2000;20:1852–1853. doi: 10.1161/01.atv.20.8.1852. [DOI] [PubMed] [Google Scholar]
- 10.Swertfeger DK, Hui DY. Apolipoprotein E: A cholesterol transport protein with lipid transport-independent cell signaling properties. Front Biosci. 2001;6:d526–d535. doi: 10.2741/swertfeg. [DOI] [PubMed] [Google Scholar]
- 11.Miyata M, Smith JD. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat Genet. 1996;14:55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- 12.Stannard AK, Riddell DR, Sacre SM, Tagalakis AD, Langer C, von Eckardstein A, Cullen P, Athanasopoulos T, Dickson G, Owen JS. Cell-derived apolipoprotein E (ApoE) particles Inhibit vascular cell adhesion molecule-1 (VCAM-1) expression in human endothelial cells. J Biol Chem. 2001;276:46011–46016. doi: 10.1074/jbc.M104812200. [DOI] [PubMed] [Google Scholar]
- 13.Ishigami M, Swertfeger DK, Hui MS, Granholm NA, Hui DY. Apolipoprotein E inhibition of vascular smooth muscle cell proliferation but not the inhibition of migration is mediated through activation of inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2000;20:1020–1026. doi: 10.1161/01.atv.20.4.1020. [DOI] [PubMed] [Google Scholar]
- 14.Ishigami M, Swertfeger DK, Granholm NA, Hui DY. Apolipoprotein E inhibits platelet-derived growth factor-induced vascular smooth muscle cell migration and proliferation by suppressing signal transduction and preventing cell entry to G1 phase. J Biol Chem. 1998;273:20156–20161. doi: 10.1074/jbc.273.32.20156. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura A, Baitsch D, Telgmann R, Feuerborn R, Weissen-Plenz G, Hagedorn C, Saku K, Brand-Herrmann SM, von Eckardstein A, Assmann G, Nofer JR. Apolipoprotein E interrupts interleukin-1β signaling in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1610–1617. doi: 10.1161/ATVBAHA.106.129957. [DOI] [PubMed] [Google Scholar]
- 16.Sacre SM, Stannard AK, Owen JS. Apolipoprotein E (apoE) isoforms differentially induce nitric oxide production in endothelial cells. FEBS Letters. 2003;540:181–187. doi: 10.1016/s0014-5793(03)00261-8. [DOI] [PubMed] [Google Scholar]
- 17.Swertfeger DK, Hui DY. Apolipoprotein E receptor binding versus heparan sulfate proteoglycan binding in Its regulation of smooth muscle cell migration and proliferation. J Biol Chem. 2001;276:25043–25048. doi: 10.1074/jbc.M102357200. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Hui DY. Apolipoprotein E binding to low density lipoprotein receptor-related protein-1 inhibits cell migration via activation of cAMP-dependent protein kinase A. J Biol Chem. 2003;278:36257–36263. doi: 10.1074/jbc.M303171200. [DOI] [PubMed] [Google Scholar]
- 19.Herz J. The LDL receptor gene family: (Un)expected signal transducers in the brain. Neuron. 2001;29:571–581. doi: 10.1016/s0896-6273(01)00234-3. [DOI] [PubMed] [Google Scholar]
- 20.Moore ZWQ, Hui DY. Apolipoprotein E inhibition of vascular hyperplasia and neointima formation requires inducible nitric oxide synthase. J Lipid Res. 2005;46:2083–2090. doi: 10.1194/jlr.M500177-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yue L, Rasouli N, Ranganathan G, Kern PA, Mazzone T. Divergent effects of peroxisome proliferator-activated receptor γ agonists and tumor necrosis factor α on adipocyte ApoE expression. J Biol Chem. 2004;279:47626–47632. doi: 10.1074/jbc.M408461200. [DOI] [PubMed] [Google Scholar]
- 22.Williams SE, Ashcom JD, Argraves WS, Strickland DK. A novel mechanism for controlling the activity of α2-macroglobulin receptor/low density lipoprotein receptor-related protein. Multiple regulatory sites for 39-kDa receptor-associated protein. J Biol Chem. 1992;267:9035–9040. [PubMed] [Google Scholar]
- 23.LaDu M, Falduto M, Manelli A, Reardon C, Getz G, Frail D. Isoform-specific binding of apolipoprotein E to β-amyloid. J Biol Chem. 1994;269:23403–23406. [PubMed] [Google Scholar]
- 24.Weisgraber KH, Innerarity TL, Mahley RW. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978;253:9053–9062. [PubMed] [Google Scholar]
- 25.Ali K, Middleton M, Pure E, Rader DJ. Apolipoprotein E suppresses the Type I inflammatory response in vivo. Circ Res. 2005;97:922–927. doi: 10.1161/01.RES.0000187467.67684.43. [DOI] [PubMed] [Google Scholar]
- 26.Tsoi LM, Wong KY, Liu YM, Ho YY. Apoprotein E isoform-dependent expression and secretion of pro-inflammatory cytokines TNF-α and IL-6 in macrophages. Arch Biochem Biophys. 2007;460:33–40. doi: 10.1016/j.abb.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Huang ZH, Fitzgerald ML, Mazzone T. Distinct cellular loci for the ABCA1-dependent and ABCA1-independent lipid efflux mediated by endogenous apolipoprotein E expression. Arterioscler Thromb Vasc Biol. 2006;26:157–162. doi: 10.1161/01.ATV.0000193627.12516.1d. [DOI] [PubMed] [Google Scholar]
- 28.Dyer CA, Smith RS, Curtiss LK. Only multimers of a synthetic peptide of human apolipoprotein E are biologically active. J Biol Chem. 1991;266:15009–15015. [PubMed] [Google Scholar]
- 29.Swantek JL, Cobb MH, Geppert TD. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-alpha) translation: glucocorticoids inhibit TNF-alpha translation by blocking JNK/SAPK. Mol Cell Biol. 1997;17:6274–6282. doi: 10.1128/mcb.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuyt LML, Dokter WHA, Birkenkamp K, Koopmans SB, Lummen C, Kruijer W, Vellenga E. Extracellular-Regulated Kinase 1/2, Jun N-Terminal Kinase, and c-Jun Are Involved in NF-κB-Dependent IL-6 Expression in Human Monocytes. J Immunol. 1999;162:4893–4902. [PubMed] [Google Scholar]
- 31.Mahley RW, Weisgraber KH, Innerarity TL. Interaction of plasma lipoproteins containing apolipoproteins B and E with heparin and cell surface receptors. Biochim Biophys Acta. 1979;575:81–91. doi: 10.1016/0005-2760(79)90133-4. [DOI] [PubMed] [Google Scholar]
- 32.Hoe HS, Harris DC, Rebeck GW. Multiple pathways of apolipoprotein E signaling in primary neurons. J Neurochem. 2005;93:145–155. doi: 10.1111/j.1471-4159.2004.03007.x. [DOI] [PubMed] [Google Scholar]
- 33.Van Oosten M, Rensen PCN, Van Amersfoort ES, Van Eck M, Van Dam AM, Breve JJP, Vogel T, Panet A, Van Berkel TJC, Kuiper J. Apolipoprotein E protects against bacterial lipopolysaccharide-induced lethality. A new therapeutic approach to treat gram-negative sepsis. J Biol Chem. 2001;276:8820–8824. doi: 10.1074/jbc.M009915200. [DOI] [PubMed] [Google Scholar]
- 34.Jofre-Monseny L, Loboda A, Wagner AE, Huebbe P, Boesch-Saadatmandi C, Jozkowicz A, Minihane AM, Dulak J, Rimbach G. Effects of apoE genotype on macrophage inflammation and heme oxygenase-1 expression. Biochem Biophys Res Commun. 2007;357:319–324. doi: 10.1016/j.bbrc.2007.03.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vedhachalam C, Narayanaswami V, Neto N, Forte TM, Phillips MC, Lund-Katz S, Bielicki JK. The C-terminal lipid-binding domain of Apolipoprotein E is a highly efficient mediator of ABCA1-dependent cholesterol efflux that promotes the assembly of high-density lipoproteins. Biochemistry. 2007;46:2583–2593. doi: 10.1021/bi602407r. [DOI] [PubMed] [Google Scholar]
- 36.Tang C, Liu Y, Kessler PS, Vaughan AM, Oram JF. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J Biol Chem. 2009;284:32336–32343. doi: 10.1074/jbc.M109.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herz J, Goldstein JL, Strickland DK, Ho YK, Brown MS. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/α-2 macroglobuin receptor. J Biol Chem. 1991;266:21232–21338. [PubMed] [Google Scholar]
- 38.Boucher P, Liu P, Gotthardt M, Hiesberger T, Anderson RGW, Herz J. Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low density lipoprotein receptor-related protein in caveolae. J Biol Chem. 2002;277:15507–15513. doi: 10.1074/jbc.M200428200. [DOI] [PubMed] [Google Scholar]
- 39.Loukinova E, Ranganathan S, Kuznetsov S, Gorlatova N, Migliorini MM, Loukinov D, Ulery PG, Mikhailenko I, Lawrence DA, Strickland DK. Platelet-derived growth factor (PDGF)-induced tyrosine phosphorylation of the low density lipoprotein receptor-related protein (LRP). Evidence for integrated co-receptor function between LRP and the PDGF. J Biol Chem. 2002;277:15499–15506. doi: 10.1074/jbc.M200427200. [DOI] [PubMed] [Google Scholar]
- 40.Boucher P, Li WP, Matz RL, Takayama Y, Auwerx J, Anderson RGW, Herz J. LRP1 functions as an atheroprotective integrator of TGFβ and PDGF signals in the vascular wall: implications for marfan syndrome. PLoS ONE. 2007;2(5):e448. doi: 10.1371/journal.pone.0000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boucher P, Gotthardt M, Li WP, Anderson RGW, Herz J. LRP: Role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 42.Basford JE, Moore ZWQ, Zhou L, Herz J, Hui DY. Smooth muscle LDL receptor-related protein-1 inactivation reduces vascular reactivity and promotes injury-induced neointima formation Arterioscler. Thromb Vasc Biol. 2009;29:1772–1778. doi: 10.1161/ATVBAHA.109.194357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terrand J, Bruban V, Zhou L, Gong W, El Asmar Z, May P, Zurhove K, Hafner P, Philippe C, Woldt E, Matz RL, Gracia C, Metzger D, Auwerx J, Herz J, Boucher P. LRP1 controls intracellular cholesterol storage and fatty acid synthesis through modulation of Wnt signaling. J Biol Chem. 2009;284:381–388. doi: 10.1074/jbc.M806538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofmann SM, Zhou L, Perez-Tilve D, Greer T, Grant E, Wancata l, Thomas A, Pfluger PT, Basford JE, Gilham D, Herz J, Tschöp MH, Hui DY. Adipocyte LDL receptor-related protein-1 expression modulates postprandial lipid transport and glucose homeostasis in mice. J Clin Invest. 2007;117:3271–3282. doi: 10.1172/JCI31929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000;275:25616–25624. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- 46.Aki D, Mashima R, Saeki K, Minoda Y, Yamauchi M, Yoshimura A. Modulation of TLR signalling by the C-terminal Src kinase (Csk) in macrophages. Genes Cells. 2005;10:357–368. doi: 10.1111/j.1365-2443.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 47.Matsuguchi T, Masuda A, Sugimoto K, Nagai Y, Yoshikai Y. JNK-interacting protein 3 associates with Toll-like receptor 4 and is involved in LPS-mediated JNK activation. EMBO J. 2003;22:4455–4464. doi: 10.1093/emboj/cdg438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones BW, Means TK, Heldwein KA, Keen MA, Hill PJ, Belisle JT, Fenton MJ. Different Toll-like receptor agonists induce distinct macrophage responses. J Leukoc Biol. 2001;69:1036–1044. [PubMed] [Google Scholar]
- 49.Singh K, Chaturvedi R, Asim M, Barry DP, Lewis ND, Vitek MP, Wilson KT. The apolipoprotein E-mimetic peptide COG112 inhibits the inflammatory response to Citrobacter rodentium in colonic epithelial cells by preventing NF-κB activation. J Biol Chem. 2008;283:16752–16761. doi: 10.1074/jbc.M710530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mäkelä SM, Strengell M, Pietila TE, Österlund P, Julkunen I. Multiple signaling pathways contribute to synergistic TLR ligand-dependent cytokine gene expression in human monocyte-derived macrophages and dendritic cells. J Leukoc Biol. 2009;85:664–672. doi: 10.1189/jlb.0808503. [DOI] [PubMed] [Google Scholar]