Abstract
Background
Patients with hypomorphic nuclear factor-κB essential modulator (NEMO) mutations have extensive phenotypic variability that can include atypical infectious susceptibility.
Objective
This study may provide important insight into immunologic mechanisms of host defense.
Methods
Immunologic evaluation, including studies of Toll-like receptor (TLR) function, was performed in a 6-month-old boy with normal ectodermal development who was diagnosed with Pneumocystis pneumonia and cytomegalovirus sepsis.
Results
Genomic and cDNA sequencing demonstrated a novel NEMO missense mutation, 337G->A, predicted to cause a D113N (aspartic acid to asparagine) substitution in the first coiled-coil region of the NEMO protein. Quantitative serum immunoglobulins, lymphocyte subset numbers, and mitogeninduced lymphocyte proliferation were essentially normal. The PBMC responses to TLR ligands were also surprisingly normal, whereas natural killer cell cytolytic activity, T-cell proliferative responses to specific antigens, and T-cell receptor–induced NF-κB activation were diminished.
Conclusion
Unlike the unique NEMO mutation described here, the most commonly reported mutations are clustered at the 3′ end in the tenth exon, which encodes a zinc finger domain. Because specific hypomorphic variants of NEMO are associated with distinctive phenotypes, this particular NEMO mutation highlights a dispensability of the region including amino acid 113 for TLR signaling and ectodysplasin A receptor function. This region is required for certain immunoreceptor functions as demonstrated by his susceptibility to infections as well as natural killer cell and T-cell defects.
Keywords: NEMO, Toll-like receptors
Mutations in the nuclear factor-κB essential modulator (NEMO) gene (also referred to as IKBKG) on the X chromosome have been associated with anhidrotic ectodermal dysplasia as well as immunodeficiency in male subjects.1 Complete deficiency of NEMO activity is incompatible with male survival, because at least some NEMO function is required for fetal development. Women who possess 1 completely nonfunctional copy of NEMO have incontinentia pigmenti characterized by dermal scarring and abnormal pigmentation.2 As a result, the NEMO mutants compatible with male survival are hypomorphic and still allow some critical NEMO functions to occur, whereas others fail. The majority of male patients with NEMO hypomorphisms reported to date have alterations affecting the c-terminus of the protein containing a zinc-finger domain.3
These patients typically have ectodermal dysplasia and immunodeficiency characterized by impaired B-cell function and susceptibility to severe infections.4 Some patients with mutations affecting other regions of NEMO have distinct phenotypes, including normal ectodermal development,5 lymphedema, and osteopetrosis6 as well as less severe impairment of B-cell function.5 One defect that has been relatively pervasive in male patients with NEMO hypomorphisms described thus far has been an impairment of Toll-like receptor (TLR) function,7 accounting for their susceptibility to mycobacteria and other pathogens.
Nuclear factor-κB essential modulator is a scaffold protein of 519 amino acids and is an integral part of the inhibitor of nuclear factor-κB (IκB) kinase (IKK) complex. NEMO and the IKK are critical links in facilitating the nuclear translocation of nuclear factor-κB (NF-κB) transcription factors. The classic IKK complex consists of NEMO and at least 2 kinase subunits, IKK-α and IKK-β. When assembled and activated after the ligation of a relevant receptor, IKK can target IκB in the cell cytoplasm to affect its phosphorylation, ubiquitination, and degradation. Because IκB is bound to NF-κB and prevents it from translocating into the nucleus, the degradation of IκB frees NF-κB to move into the nucleus and subsequently promote gene transcription. NF-κB is required for the signal transduction of a number of surface and cytoplasmic receptors including T-cell receptors (TCRs), B-cell receptors, IL-1 receptor and TNF receptor superfamilies, and the TLRs. NEMO is therefore an essential regulator of NF-κB signaling, and mutations have the potential to result in broad immune dysfunction.
We report a case which presented with Pneumocystis jiroveci pneumonia, no signs of ectodermal dysplasia, and a novel NEMO mutation associated with defective T-cell and natural killer (NK) cell function as well as defective TCR-induced NF-κB activation, but having intact TLR-induced TNF responses.
METHODS
Case report
The male patient was born at 31 weeks of gestation from a pregnancy complicated by pre-eclampsia. His parents are white, unrelated, with no family history of incontinentia pigmenti, immunodeficiency, miscarriages, or pediatric male deaths. He developed normally until 6 months of age. At that time he developed acute respiratory distress, cyanosis, and hypoxia requiring hospitalization. A chest x-ray revealed bilateral infiltrates, and a bronchoscopy demonstrated Pneumocystis jirovecii. He was treated with high-dose trimethoprim/sulfa. Two weeks later, he developed an elevated temperature and was diagnosed with cytomegalovirus viremia by PCR. He also had Rotavirus detected in his stool. He responded to therapy with ganciclovir and was discharged. Since this admission, he has been treated with prophylactic trimethoprim/sulfa, intravenous immunoglobulin, and cytomegalovirus-specific IgG. He had his first tooth eruption at 12 months, does not have oligodontia, and has been observed to perspire normally.
NEMO sequencing
Nuclear factor-κB essential modulator sequencing was performed by using a series of primer sets directed at amplifying each of the individual NEMO exons as described8 (primers available on request). To confirm that the identified NEMO mutation was in the functional NEMO gene and not the NEMO pseudogene, cDNA was prepared, and the sequence corresponding to the expressed NEMO message was amplified and sequenced as described.8
Flow cytometry
Lymphocyte immunophenotyping was performed on heparinized whole blood, and percentages of CD3+αδ/β+ and CD3+γ/δ+ T cells were determined. Lymphocytes were gated on the basis of bright CD45 expression and low side light scatter (CellQuest Pro; BD Bioscience, San Jose, Calif).
In vitro assays
After separation, PBMCs were washed and then used in in vitro assays including lymphocyte proliferation, TLR ligand–induced TNF production, and NKcell cytotoxicity. Lymphocyte proliferation was determined after 3 days of mitogen (phytohemagglutinin, concanavalin A, or pokeweed) stimulation or 6 days stimulation with antigen (tetanus toxoid or Candida albicans) by using 3H-thymidine incorporation as described.9 TLR ligand–induced TNF responses were measured by ELISA 24 hours after stimulation as described.7 NK cell cytotoxicity against K562 erythroleukemia target cells was measured by using 51Cr-release assay according to previously published methods10 with PBMCs obtained during periods of relative wellness and >6 months after the initial presentation.
IκB degradation
The degradation of IκB was evaluated by Western blotting for IκB. Briefly, PBMC were unstimulated or stimulated with flagellin using the same concentration as in the TLR assays for 30 minutes at 37°C. In these assays, conditions that resulted in the near maximal decrease in cellular IκB levels as determined in time course experiments in control PBMCs (data not shown) were used. After stimulation, cells were immediately lysed by using NuPAGE LDS sample buffer (Invitrogen, Carlsbad, Calif) and boiled for 5 minutes. Proteins in solution were separated on 4% to 12% Novex Bis-Tris gels and transferred to PVDF membranes (Invitrogen). Membranes were blocked in 3% BSA, incubated with rabbit polyclonal anti-IκBα C-21 (Santa Cruz Biotechnology, Santa Cruz, Calif), washed extensively, and incubated with horseradish peroxidase–conjugated donkey antirabbit secondary antibody (Santa Cruz Biotechnology). Bound antibody was detected by using an enhanced chemiluminescent detection system (Amersham Pharmacia, Piscataway, NJ). To ensure adequate loading, membranes were stripped by using 0.2 mol/L glycine pH 2.5, 0.05% Tween-20, and 140 mmol/L NaCl in TRIS-buffered saline at 50°C for 30 minutes and then reprobed for α-tubulin or actin by using polyclonal antibody. Where indicated, Western blot band intensities were quantified by using Image J software (NIH Shareware).
T-cell enrichment and TCR stimulation
T cells were isolated by using RosetteSep Human T Cell Enrichment Cocktail (StemCell Technologies, Seattle, Wash) from heparinized whole blood according to the manufacturer’s recommendations. Blood was obtained during periods of clinical stability and more than 6 months after the initial clinical presentation. After separation, cells were washed twice in PBS and resuspended in RPMI 1640 media. The purity of isolated T cells was evaluated by fluorescence-activated cell sorting and demonstrated to be >90% CD3+ cells. Enriched T cells were then either media control–treated or stimulated with mAb anti-TCR clone C30511 hybridoma supernatant for 30 minutes. After treatment, cells were immediately lysed by using NuPAGE LDS sample buffer (Invitrogen), boiled for 5 minutes, and evaluated for IκB degradation as described.
RESULTS
Adaptive immunity
Because the patient had symptomatic infection with Pneumocystis and cytomegalovirus at 6 months of age, we initially evaluated his adaptive immunity. The studies reported were obtained 1 week after admission. Serum IgM and IgG levels were normal for his age, thus demonstrating effective immunoglobulin production and class switching to IgG (Table I). Further, he produced specific antibody to diphtheria, tetanus, and Hemophilus influenza B. Although he received a single Prevnar vaccination (Wyeth, Collegeville, Pa), all titers to the 7 serotypes were <200 ng antibody nitrogen N/mL. This is in contrast to the hyper-IgM phenotype that is often seen in NEMO, where there is hypogammaglobulinemia, absent specific antibody, and occasionally elevated IgM.12 This suggests at least some degree of effective CD40-mediated class differentiation and B-cell development.
TABLE I.
Serum immunoglobulin values and B-cell and T-cell enumeration
| Serum Ig levels | Serum antibody titers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgM | IgA | Hemophilus influenzae B | Diphtheria | Tetanus | Pneumococcal (all) | |||||
| 467 | 41 | <10 | 1.47 ug/mL | 0.048 IU/mL | 0.86 IU/mL | <200 ngAb N/mL | |||||
| (215–704) | (35–102) | (8.1–68) | (0.05–0.35) | (>0.01) | (>0.1) | (>200) | |||||
| Lymphocyte Subset Distribution | |||||||||||
| CD3% | CD3abs | CD4% | CD4abs | CD8% | CD8abs | CD4/CD8 | CD19% | CD19abs |
CD16/ 56% |
CD16/ 56abs |
CD3+/γδ+ |
| 51% | 4293 | 29% | 2425 | 21% | 1758 | 1.4 | 46% | 3872 | 0.7%,* 2%† | 75,* 169† | 3% |
| (50% to 77%) | (2400–6900) | (33% to 58%) | (1400–5100) | (13% to 26%) | (600–2200) | (1.6–3.8) | (11% to 45%) | (350–2500) | (2% to 14%) | (170–830) | (0.5% to 6%) |
Ig values are mg/dL (normal ranges).
At time of diagnosis.
At time of NK cytotoxicity analysis.
Quantitative evaluation of lymphocyte subsets in peripheral blood demonstrated normal numbers and distribution of CD19+ and CD3+ cells. There was also a normal number of both CD3+ CD4+ and CD3+ CD8+ T cells. More than 96% of the CD3+ T cells stained positively for α/β TCR and 3% for γ/δ. NK cell numbers at presentation were low but increased to within the normal age-specific range subsequently.13 Lymphocyte proliferative function was measured using a 3-day exposure to the nonspecific mitogens phytohemagglutinin, concanavalin A, or pokeweed mitogen and was within the normal range (Table II). The ability of PBMCs to uptake, present, and respond to specific antigen was measured by the proliferative response to a 7-day exposure to either tetanus toxoid or Candida albicans, although these tests are not entirely reliable in a 6-month-old. There was no proliferative response to either antigen. These results demonstrate that T lymphocytes were present in normal numbers and distribution and capable of proliferative function, but may be impaired in their ability to proliferate specific antigens. This paradigm of abnormal antigen-induced but normal mitogen-induced lymphocyte proliferation has been previously identified in patients with NEMO mutations, even in ones who have maintained the ability to generate specific antibody in vivo.4 Although this potential abnormality could explain a susceptibility to Pneumocystis and cytomegalovirus, the deficit was disproportionate in light of the severity of the clinical presentation.
TABLE II.
Lymphocyte stimulation studies
| Mitogens | |||
|---|---|---|---|
| Patient average CPM |
Control average CPM |
Reference CPM value |
|
| Media (ug/mL culture) | 308 | 234 | |
| PHA (10.0) | 118,697 | 197,760 | <45,000 |
| PHA (5.0) | 95,383 | 197,375 | >25,000 |
| PHA (2.5) | 65,454 | 158,621 | >17,000 |
| ConA (5.0) | 35,322 | 42,183 | >4500 |
| ConA (1.7) | 4242 | 7484 | >1400 |
| ConA (0.6) | 610 | 2243 | >350 |
| Pokeweed (25) | 18,477 | 36,035 | >3800 |
| Pokeweed (8.3) | 15,820 | 36,636 | >3500 |
| Pokeweed (2.8) | 14,697 | 37,851 | >3000 |
| Antigens | |||
|
Patient average CPM |
Control average CPM |
Reference SI value |
|
| Media | 214 | 607 | |
| CAND (6.5K PNU) | 242 | 32,687 | >5 |
| TET (0.2 LFU) | 254 | 13,827 | >5 |
| TET (0.07 LFU) | 531 | 16,468 | >5 |
| TET (0.02 LFU) | 390 | 15,651 | >5 |
CPM, Counts per minute; ConA, concanavalin A; PHA, phytohemagglutinin; SI, stimulation index; CAND, Candida; PNU, protein nitrogen units; TET, tetanus; LFU, level of flocculation units/mL.
Identification of a novel NEMO mutation
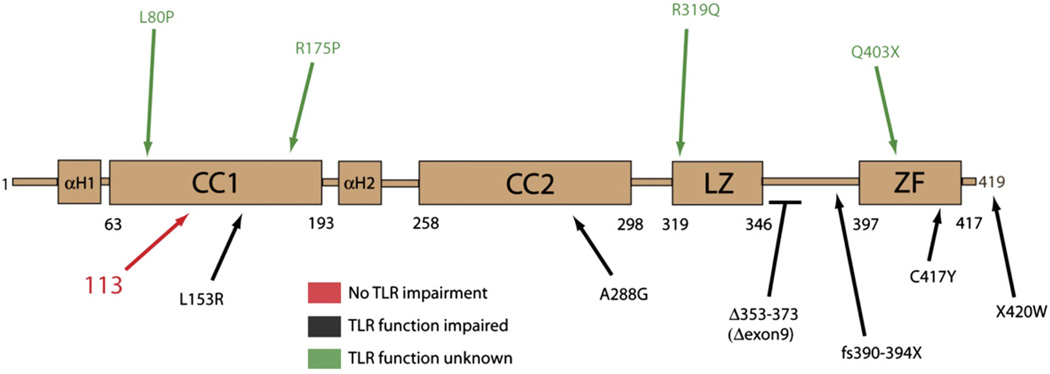
Susceptibility to Pneumocystis and cytomegalovirus with normal serum immunoglobulin levels, normal T-cell and B-cell numbers, and mitogen-induced T-cell proliferation is an unusual phenotype for a primary immunodeficiency. One disease that has been described to share some of these findings is a deficiency in NEMO function.4,6 Although this patient did not have ectodermal dysplasia, the absence of ectodermal dysplasia is an increasingly common feature of patients identified as having particular NEMO mutations.5,14 The NEMO gene was sequenced first using primers against individual exons from genomic DNA and then from cDNA prepared from PBMCs. Both tests were performed because of the presence of a NEMO pseudogene that can contain an alteration and interfere with the genomic analyses of the transcribed NEMO exons. Using this 2-tiered approach, the patient demonstrated a novel missense mutation in the IKBKG gene with a substitution of guanine for adenine at position 337. This would be predicted to result in an amino acid alteration at position 113 of aspartic acid to asparagine (D113N). The patient’s mother was heterozygous for this mutation. The NEMO alteration predicted by this mutation would affect the first coiled-coil domain and could result in substantial charge variation because of the loss of the aspartic acid residue. This mutation is unusual in its position within NEMO and is unlike others previously reported (Fig 1).
FIG 1.
Map demonstrating the NEMO hypomorphism (red) in the patient relative to other related and major hypomorphisms associated with immunodeficiency. The ability of these alterations to impair TLR signaling is noted.
NK cell function
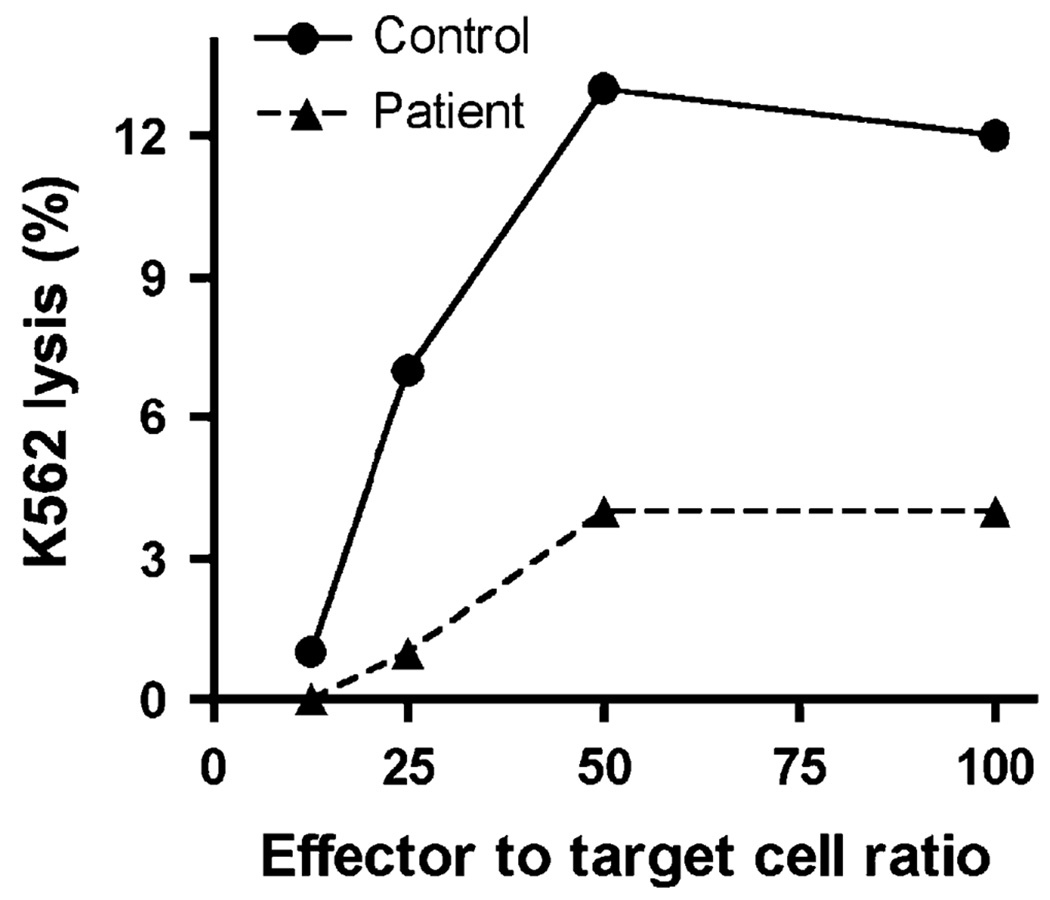
In addition to Pneumocystis, the patient also had persistent cytomegalovirus viremia. Cytomegalovirus infection has been previously reported in patients with a NEMO mutation.6,10 Although atypical susceptibility to and unusual consequences of cytomegalovirus infection can be associated with T-cell impairment, appropriate defense against cytomegalovirus also requires NK cell function.15 Because deficiencies of NK cells have been identified in patients with a NEMO mutation,5,10 NK cell cytotoxicity was assessed to determine whether a deficiency in NK cell defenses could have contributed to his infectious phenotype. NK cell cytotoxicity was measured in PBMCs using a 4-hour 51Cr-release assay against K562 target cells and was depressed (Fig 2). At the time of testing, the percentage of NK cells in the patient was within normal range as determined by fluorescence-activated cell sorting (Table I), and thus the reduced activity is less likely to be a feature of NK cell numbers in this assay. The combination of reduced NK cell function together with decreased antigen-specific lymphocyte responses resulting from this patient’s novel NEMO mutation likely contributed to the susceptibility to infection.
FIG 2.
NK cell cytotoxicity measured by the lysis of K562 target cells at differing effector to target cell ratios using a 51Cr-release assay. Each data point was repeated in triplicate with a variation of <10%, and the experiment is representative of 2 independent experiments.
TCR signaling to NF-κB
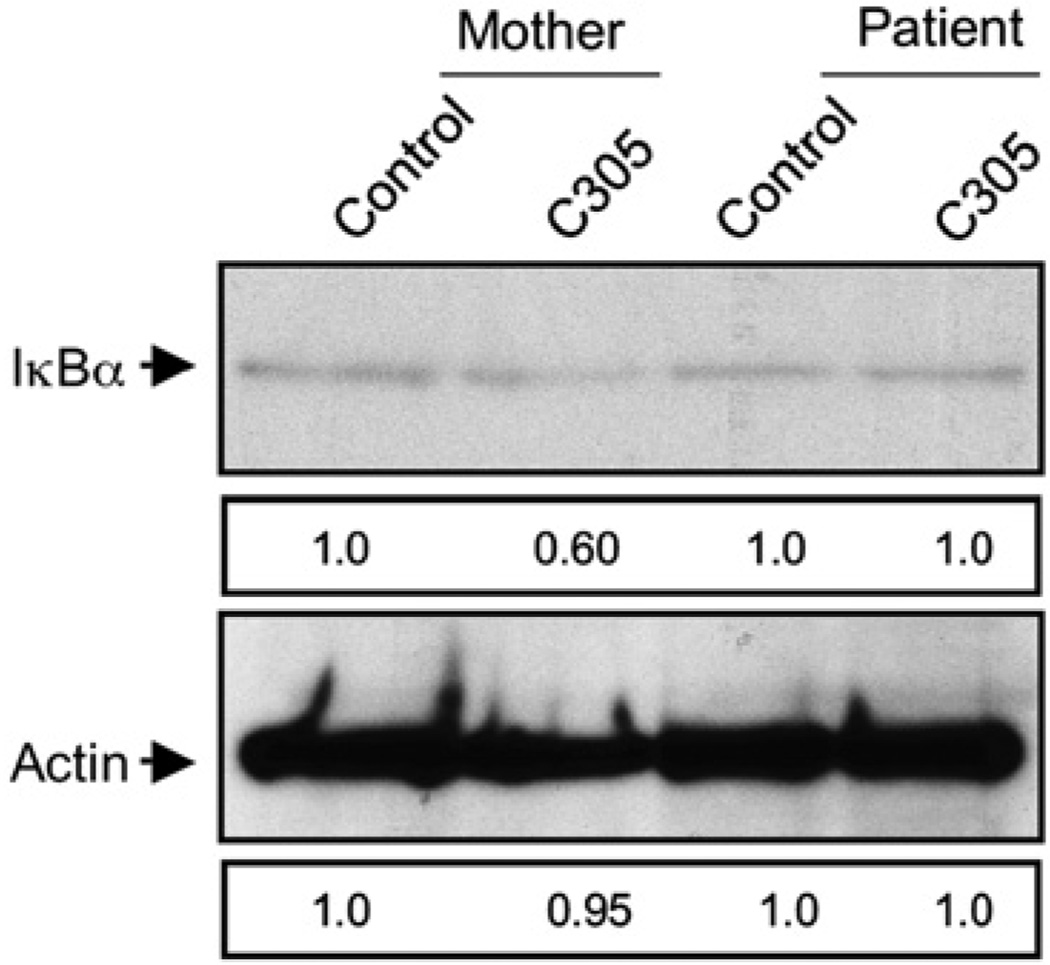
Because defects of NK cell and T-cell function could have resulted in susceptibility to Pneumocystis or cytomegalovirus, the effect of specific cell stimulation on NF-κB activation was evaluated. Because ligation of the TCR has been previously linked to activation of the NF-κB pathway,16 TCR-induced signaling was studied in patient T cells. CD3+ T cells were enriched from the patient and his mother and were activated in vitro for 30 minutes by using the multivalent mAb C305 directed against the TCR. The quantity of IκB among total cell lysate was determined by Western blot and was decreased after C305 stimulation of T cells from the patient’s mother, but not from the patient (Fig 3). This demonstrates that T cells possessing the NEMO 337 G>A mutation have a reduced ability to access the NF-κB pathway after TCR ligation.
FIG 3.
Impaired TCR-induced IκB degradation in patient T cells. Highly enriched maternal (left) and patient (right) CD3+ T cells were control-treated or activated for 30 minutes with C305 anti-TCR mAb and whole cell lysates evaluated for the presence of IκB (top) or actin (bottom) by Western blot. Numbers depict band intensity relative to control, and blots are representative of 2 experiments.
TLR function and signaling
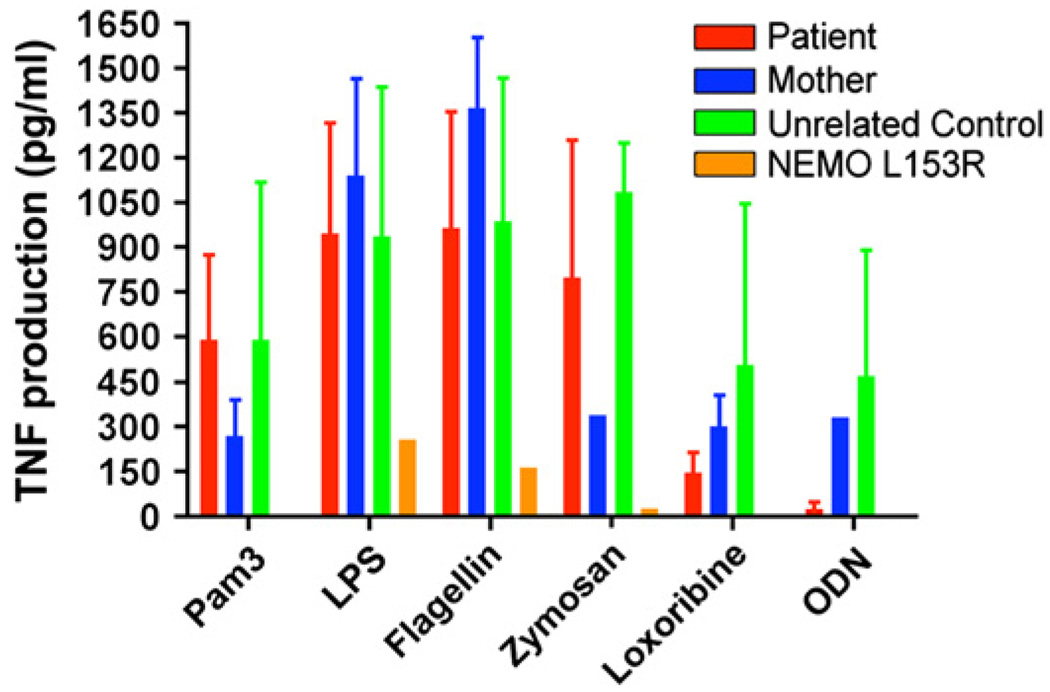
The TLR system can also actively participate in generation of an immune response against DNA viruses including cytomegalovirus17 and is impaired in all NEMO patients studied to date7 (Fig 1). Thus, we evaluated TLR responses in this patient because they might have contributed to his susceptibility to infection. Patient PBMCs were stimulated with TLR ligands according to an established protocol,7 and TNF production was measured. Interestingly, he had normal TLR ligand-induced TNF responses in multiple evaluations over time (Fig 4). TNF responses of PBMCs from this patient with NEMO D113N in response to ligand for TLR 1/2, 2/6, 3, 4, 5, 7/8, and 9 were statistically indistinguishable from control PBMCs. In comparison, TLR ligand-induced TNF responses of PBMCs from a patient with a predicted NEMO L153R alteration previously reported to have depressed responses were clearly distinguished from this patient and the control donors (Fig 4).
FIG 4.
Intact TLR-induced TNF responses. Patient PBMCs (red), maternal PBMCs (blue), unrelated control donor PBMCs (green), or PBMCs from a patient with a previously described NEMO L153R hypomorphism (gold) were incubated for 18 hours with TLR ligands. Supernatants were assayed for the presence of TNF by ELISA.
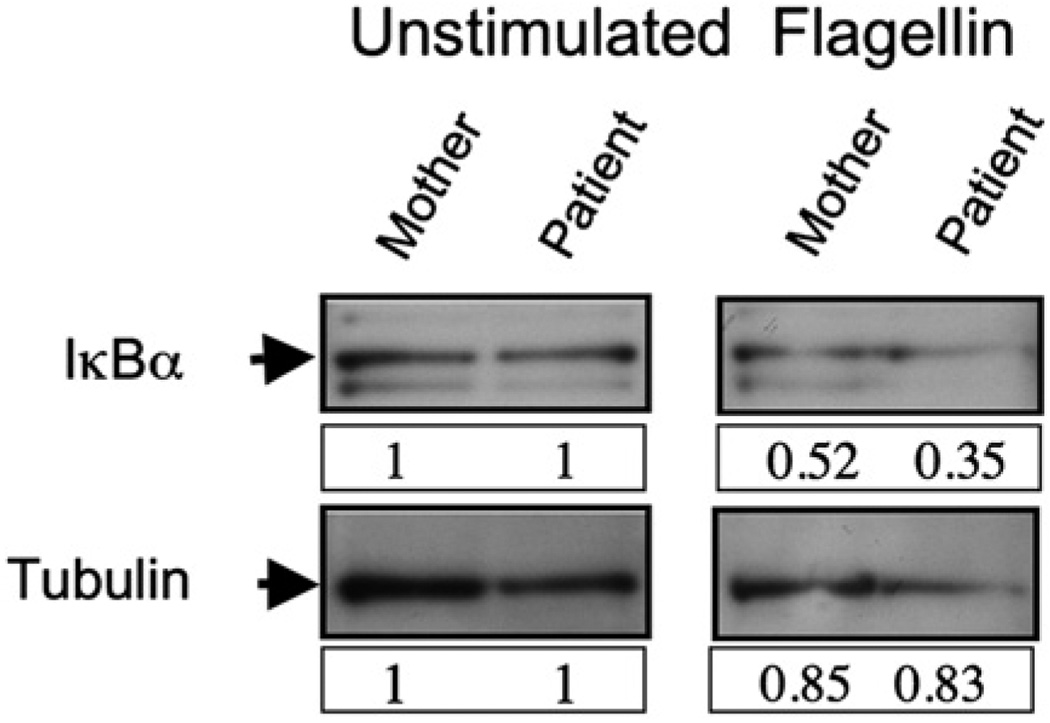
To determine that TLR ligation was indeed inducing NEMO function, we evaluated TLR ligand-induced IκB degradation. Rapid TLR signaling depends on the canonical pathway of NF-κB activation, and thus NEMO is an essential component of the kinase complex required for IκB phosphorylation and degradation. 18 After 30 minutes of stimulation of PBMCs with the TLR5 ligand flagellin, the quantity of cellular IκB was reduced (Fig 5). Given the rapid time frame of this activation, this indicates intact TLR ligand-induced NEMO function in the patient.
FIG 5.
Normal IκB degradation after exposure of patient cells to the TLR5 ligand flagellin. Whole-cell lysate IκBα Western blot (top) of unstimulated (left) or TLR5 ligand-stimulated (right) PBMCs. Membranes were stripped and reprobed for tubulin (bottom) as a loading control. Lysates from maternal PBMCs (left lanes) were compared with patient PBMCs (right lanes). Numbers depict band intensity relative to control, and blots are representative of 3 independent experiments.
DISCUSSION
This patient presented with a unique NEMO mutation and phenotype, identified primarily because of the presenting infection with Pneumocystis. For most male patients presenting with Pneumocystis, a diagnosis of severe combined immunodeficiency disease or X-linked hyper-IgM syndrome is considered. As a rule, patients with severe combined immunodeficiency disease have severe T-cell lymphopenia, hypogammaglobulinemia, and impaired proliferative responses to mitogens and specific antigens, unlike this patient. Patients with X-linked hyper-IgM syndrome almost uniformly have hypogammaglobulinemia, and not a single patient in the US registry series of 79 patients presented with an IgG level greater than 300 mg/dL.19 Although reported as a pathogen in patients with NEMO,6,20–22 Pneumocystis has been less common in the earlier reports. There are too few NEMO patients who have had Pneumocystis infection to determine whether susceptibility is associated with specific NEMO genotypes. Therefore, until more patient natural histories are collected, Pneumocystis infection should be considered in patients who have NEMO deficiency. Conversely, NEMO deficiency should be considered in patients with otherwise unexplained Pneumocystis infection.
Unlike many reportedNEMOcases, this patient had essentially normal B-cell numbers and serum immunoglobulin levels, in keeping with a normal capacity for class switching as well as the capacity to make specific antibody. This distinguishes him from the usual patient described with NEMO deficiency or any of the forms of hyper-IgM syndrome. The antigen-induced maturation, survival, and activation of B cells through the B-cell receptor is known to use NF-κB in the signaling cascade; however, this patient’s NEMO mutation did not seem to affect the numbers and distribution of B cells or immunoglobulin isotypes. The functional interaction between CD40 and CD40L, which is essential for class switching and B-cell proliferation, requires NF-κB activity. B-cell function, however, has been reported in patients with a NEMO mutation,5 which is likely to be a feature of either the specific NEMO hypomorphism or contributions from alternative costimulatory mechanisms. Regarding the former, there is a clear association with a complete lack of class switch recombination in patients with extreme c-terminal NEMO hypomorphisms.3 In terms of the latter, it is speculated that B-lymphocyte stimulators/B cell–activating factor-induced and a B-cell proliferation–inducing ligand-induced costimulation, which can bypass canonical NF-κB signaling, may be relevant.23 This may also explain why certain NEMO patients who have a more typical hyper-IgM phenotype can have elevated levels of IgA.4 In our patient, class switching may have occurred through these pathways, but more likely indicates that the NEMO D113N hypomorphism does not impair CD40 and B-cell receptor–induced signals.
Most patients with hypomorphic mutations of NEMO experience pyogenic bacterial or mycobacterial infections,3 but the patient described here presented with Pneumocystis and chronic cytomegalovirus viremia. These infections are typically found in patients with a profound T-cell defect. This patient did have normal T-cell numbers and distribution of subsets but lacked antigen-specific lymphocyte proliferation. Although this may have been a result of his relatively young age, it is most likely an indication of a defect of antigen-specific T-cell function. This was determined directly by evaluating IκB degradation after TCR stimulation (Fig 3). Patient T cells had decreased TCR-induced activation of NF-κB and thus demonstrate the effect of this NEMO mutation. In experimental systems, it has been demonstrated that after ligation, TCR signals are transmitted to NF-κB through CARMA proteins Bcl-10 and Malt1.16 It is likely that the ability of this upstream complex to interact with NEMO in the IKK complex is affected by the D113N hypomorphism. The resulting disturbance of antigen-specific T-cell function is potentially informative about how TCR signaling links to NEMO and is likely related to the patient’s presenting infection. Interestingly, the NEMO region between residues 50 and 100 has been defined as critical in interacting with CARMA1-Bcl10 complexes.24 The CARMA1-Bcl10-ubiquitin-conjugating enzyme 13 complex affects NEMO ubiquitination at the NEMO zinc finger domain. NEMO ubiquitination is necessary for the full activation of the IKK complex in response to relevant signals.25,26 This patient’s mutation is in the first coiled-coil domain. The first coiled-coil domain is near the site where NEMO binds to IKKβ, and given the proximity of this critical region to the patient’s mutation, it is possible that the significant charge27 alteration resulting from D113N may disrupt NEMO binding to the CARMA-containing, TCR-induced complex, and/or IKK kinase activity in response to upstream stimuli.
A defect in antigen-specific TCR function could also explain the patient’s unusual susceptibility to cytomegalovirus. Susceptibility to cytomegalovirus has been previously described in NEMO patients10,19 and in at least 1 case was associated with some normal cytomegalovirus-specific adaptive immune responses. 10 Thus, a number of innate responses against cytomegalovirus could be defective in NEMO deficiency and contribute to susceptibility to infection. Two hypomorphic mutations that have been previously defined as defective in patients with NEMO impair TLR responses and NK cell cytotoxicity. The latter has been reported in a patient with a NEMO hypomorphism and severe cytomegalovirus infection.10 Both NK cells and TLR responses are believed to be important in defense against cytomegalovirus.17,28 NK cells are valuable in defense against cytomegalovirus, in part because they are capable of recognizing cells with downregulated MHCclass I—a specific evasion mechanism used by the virus. As a result, many human NK cell deficiency states are associated with susceptibility to cytomegalovirus disease.15 NK cells were present in the patient, but cytotoxicity was decreased. This pattern has been found in patients with a variety of NEMO hypomorphisms, one of which was a missense mutation of the N-terminal portion of NEMO and thus more similar to our patient.4,10 Thus, it is possible that the patient’s persistent cytomegalovirus infection, rather than being secondary to compromised TLR or adaptive immune function, may be related to reduced NK cytotoxicity. At a minimum, the combination of decreased NK cell cytotoxicity and impaired antigen-specific T-cell responses may have created an immunologic deficiency resulting in cytomegalovirus susceptibility.
Recently, Fusco et al29 described the NEMO D113N as a common polymorphism seen in 1 of 120 normal control alleles. However, this finding could not be confirmed by Aradhya et al,30 who did not identify any single nucleotide polymorphisms in the NEMO coding sequence in their analysis of 700 normal chromosomes. In an author’s laboratory, NEMO sequencing is offered for diagnostic purposes, and they have also not observed a polymorphism in the NEMO coding region in a panel of normal controls (200 chromosomes; A. Jain, unpublished data). Taken together, these findings highlight the importance of an undisrupted NEMO gene sequence. They also compared the NEMO sequence in several species. The nucleotide in question (337G) is 100% conserved among Bos taurus, Canis familiaris, Gallus gallus, Macaca mulatta, Monodelphis domestica, Mus musculus, Ornithorhynchus anatinus, and Pan troglodytes. Moreover, codon 113 is highly conserved among species in that it encodes either an aspartate (D) or a glutamate (E); both are negatively charged acidic amino acids. The D113N mutation is an acidic to an uncharged amino acid substitution. Such a mutation is not biochemically neutral and would be expected to have a functional consequence. In keeping with this hypothesis, we demonstrated abnormalities in functional assays such as NK cell cytotoxicity, T-cell proliferation, and impaired NF-κB activation after ligation of the TCR. Nevertheless, we were unable to assess directly the effect of the NEMO D113N mutation with complementation experiments due to poorly characterized in vitro experimental models. Despite the lack of accessible human complementation systems to validate the effect of NEMO D113N mutation in the conserved domain of NEMO, the lack of sequence variation at this location in a multispecies comparison as well as a large panel of normal human controls implicates a major contribution for NEMO in this patient’s phenotype.
Because the TLR system can recognize herpes viruses through TLR917 and has been described as impaired in all NEMO patients reported to date, a TLR function defect could have also contributed to the patient’s infectious susceptibility. Repeated evaluations of the patient’s PBMCs, however, demonstrated intact TLR function, and therefore this case is contrary to all previous reported cases of NEMO. TLR signaling, with the exception of TLR3 and TLR4, occurs through the MyD88-dependent pathway and NF-κB. The normal TLR function in this patient most likely signifies the dispensability of the region including NEMO residue 113 in TLR signaling.
Because the infectious, immunologic, and ectodermal characteristics of NEMO vary significantly, it is likely that particular NEMO hypomorphisms will provide instructive insights into how NEMO participates in particular signaling pathways and contribute to host defense. Another instructive finding in this patient is his lack of ectodermal dysplasia. Ectodermal dysplasia results from impaired NF-κB activation during development in response to ligation of the ectodysplasin A receptor, a TNF superfamily receptor, by its ligand ectodysplasin A. Patients with ectodermal dysplasia have impaired development of ectodermderived tissues with hypohidrosis (caused by lack of eccrine sweat glands), conical teeth, delayed or incomplete dentition, and sparse hair. Although a lack of ectodermal dysplasia is now reported in an increasing number of patients with NEMO hypomorphisms, this patient’s NEMO D113N alterations appear to have a common theme of not impairing TNF superfamily receptor function. Mechanistic biochemical experiments to evaluate the utility of this region of NEMO signaling pathways exclusive to non-TNF superfamily and non-TLR receptors that activate NF-κB will likely be informative and represent an important lead provided by this patient.
In summary, this patient presents with a novel and informative NEMO mutation. He demonstrated essentially normal activation of cells after TLR ligation, further distinguishing his defect from other NEMO defects that have been evaluated. He did manifest defects in antigen-specific T-cell function, and TCR-induced NF-κB activation, as well as decreased NK cell cytotoxicity, which most likely explains an immune defect underlying his susceptibility to Pneumocystis and cytomegalovirus. These results suggest the dispensability of amino acid 113 for some immunoreceptors, but not for signaling downstream of NK cell activation receptors and the TCR, or the defense against opportunistic infection.
Clinical implications
Patients with NEMO mutations demonstrate phenotypic and immunologic heterogeneity. A novel mutation in the coiled-coil region of the NEMO protein is described that retains normal TLR function but abnormal TCR activation and infection susceptibility.
Acknowledgments
Supported in part by grants from the US Immunodeficiency Network and Philadelphia Department of Health (J.S.O.), and by the Jeffrey Model Diagnostic Center at the Children’s Hospital of Philadelphia.
Abbreviations used
- IκB
Inhibitor of nuclear factor-κB
- IKK
Inhibitor of nuclear factor-κB kinase
- NEMO
Nuclear factor-κB essential modulator
- NF-κB
Nuclear factor-κB
- NK
Natural killer
- TCR
T-cell receptor
- TLR
Toll-like receptor
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
REFERENCES
- 1.Zonana J, Elder ME, Schneider LC, Orlow SJ, Moss C, Golabi M, et al. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO) Am J Hum Genet. 2000;67:1555–1562. doi: 10.1086/316914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smahi A, Courtois G, Rabia SH, Doffinger R, Bodemer C, Munnich A, et al. The NF-kappaB signaling pathway in human diseases: from incontinentia pigmenti to ectodermal dysplasias and immune-deficiency syndromes. Hum Mol Genet. 2002;11:2371–2375. doi: 10.1093/hmg/11.20.2371. [DOI] [PubMed] [Google Scholar]
- 3.Orange JS, Levy O, Geha RS. Human disease resulting from gene mutations that interfere with appropriate nuclear factor-kappaB activation. Immunol Rev. 2005;203:21–37. doi: 10.1111/j.0105-2896.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 4.Orange JS, Jain A, Ballas ZK, Schneider LC, Geha RS, Bonilla FA. The presentation and natural history of immunodeficiency caused by nuclear factor kappa B essential modulator mutation. J Allergy Clin Immunol. 2004;113:725–733. doi: 10.1016/j.jaci.2004.01.762. [DOI] [PubMed] [Google Scholar]
- 5.Orange JS, Levy O, Brodeur SR, Krzewski K, Roy RM, Niemela JE, et al. Human nuclear factor kappa B essential modulator mutation can result in immunodeficiency without ectodermal dysplasia. J Allergy Clin Immunol. 2004;114:650–656. doi: 10.1016/j.jaci.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 6.Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27:277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 7.Deering RP, Orange JS. Development of a clinical assay to evaluate toll-like receptor function. Clin Vaccine Immunol. 2006;13:68–76. doi: 10.1128/CVI.13.1.68-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain A, Ma CA, Liu S, Brown M, Cohen J, Strober W. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nature Immunol. 2001;2:223–228. doi: 10.1038/85277. [DOI] [PubMed] [Google Scholar]
- 9.Harbeck RJ, Giclas PG. Manual of methods and procedures in clinical laboratory immunology. New York: Raven Press; 1991. pp. 211–219. [Google Scholar]
- 10.Orange JS, Brodeur SR, Jain A, Bonilla FA, Schneider LC, Kretschmer R, et al. Deficient natural killer cell cytotoxicity in patients with IKK-gamma/NEMO mutations. J Clin Invest. 2002;109:1501–1509. doi: 10.1172/JCI14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss A, Stobo JD. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984;160:1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain A, Ma CA, Lopez-Granados E, Means G, Brady W, Orange JS, et al. Specific NEMO mutations impair CD40-mediated c-Rel activation and B cell terminal differentiation. J Clin Invest. 2004;114:1593–1602. doi: 10.1172/JCI21345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comans-Bitter WM, de Groot R, van den BR, Neijens HJ, Hop WC, Groeneveld K, et al. Immunophenotyping of blood lymphocytes in childhood: reference values for lymphocyte subpopulations. J Pediatr. 1997;130:388–393. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]
- 14.Niehues T, Reichenbach J, Neubert J, Gudowius S, Puel A, Horneff G, et al. Nuclear factor κB essential modulator-deficient child with immunodeficiency yet without anhidrotic ectodermal dysplasia. J Allergy Clin Immunol. 2004;114:1456–1462. doi: 10.1016/j.jaci.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 15.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4:1545–1558. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 16.Thome M. CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat Rev Immunol. 2004;4:348–359. doi: 10.1038/nri1352. [DOI] [PubMed] [Google Scholar]
- 17.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 19.Winkelstein JA, Marino MC, Ochs H, Fuleihan R, Scholl PR, Geha R, et al. The X-linked hyper-IgM syndrome. Medicine. 2003;82:373–384. doi: 10.1097/01.md.0000100046.06009.b0. [DOI] [PubMed] [Google Scholar]
- 20.Orstavik KH, Kristiansen M, Knudsen GP, Storhaug K, Vege A, Eiklid K, et al. Novel splicing mutation in the NEMO (IKK-gamma) gene with severe immunodeficiency and heterogeneity of X-chromosome inactivation. Am J Med Gen. 2006;140:31–39. doi: 10.1002/ajmg.a.31026. [DOI] [PubMed] [Google Scholar]
- 21.Dupuis-Girod S, Corradini N, Hadj-Rabia S, Fournet JC, Faivre L, Le Deist F, et al. Osteopetrosis, lymphedema, anhidrotic ectodermal dysplasia, and immunodeficiency in a boy and incontinentia pigmenti in his mother. Pediatrics. 2002;109:e97. doi: 10.1542/peds.109.6.e97. [DOI] [PubMed] [Google Scholar]
- 22.Schmid JMP, Junge SA, Hossle JP, Schneider EM, Roosnek E, Seger RA, et al. Transient hemophagocytosis with deficient cellular cytotoxicity, monoclonal immunoglobulin M gammopathy, increased T-cell numbers, and hypomorphic NEMO mutation. Pediatrics. 2006;117:e1049–e1056. doi: 10.1542/peds.2005-2062. [DOI] [PubMed] [Google Scholar]
- 23.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BlyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stilo R, Liguoro D, Di Jeso B, Formisano S, Consiglio E, Leonardi A, et al. Physical and functional interaction of CARMA1 and CARMA3 with Ikappa kinase gamma-NFkappaB essential modulator. J Biol Chem. 2004;279:34323–34331. doi: 10.1074/jbc.M402244200. [DOI] [PubMed] [Google Scholar]
- 25.Zhou H, Wertz I, O’Rourke K, Ultsch M, Seshagiri S, Eby M, et al. Bcl10 activates the NF-kappaB pathway through ubiquitination of NEMO. Nature. 2004;427:167–171. doi: 10.1038/nature02273. [DOI] [PubMed] [Google Scholar]
- 26.Tang ED, Inohara N, Wang CY, Nunez G, Guan KL. Roles for homotypic interactions and transautophosphorylation in IkappaB kinase beta IKKbeta) activation. J Biol Chem. 2003;278:38566–38570. doi: 10.1074/jbc.M304374200. [DOI] [PubMed] [Google Scholar]
- 27.May MJ, D’Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 28.Welsh RM, Brubaker JO, Vargas-Cortes M, O’Donnell CL. Natural killer (NK) cell response to virus infections in mice with severe combined immunodeficiency: the stimulation of NK cells and the NK cell-dependent control of virus infections occur independently of T and B cell function. J Exp Med. 1991;173:1053–1063. doi: 10.1084/jem.173.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fusco F, Bardaro T, Fimiani G, Mercadente V, Miano MG, Falco G, et al. Molecular analysis of the genetic defect in a large cohort of IP patients and identification of novel NEMO mutations interfering with NF-κB activation. Hum Mol Genet. 2004;13:1763–1773. doi: 10.1093/hmg/ddh192. [DOI] [PubMed] [Google Scholar]
- 30.Aradhya S, Woffendin H, Jakins T, Bardara T, Esposito T, Smahi A, et al. A recurrent deletion in the ubiquitously expressed NEMO (IKK-gamma) gene accounts for the vast majority of incontinentia pigmenti mutations. Hum Mol Genet. 2001;10:2171–2179. doi: 10.1093/hmg/10.19.2171. [DOI] [PubMed] [Google Scholar]