Abstract
IL-12 is a dimeric cytokine that is produced primarily by APCs. In this study we examined the role that the p38 MAPKs (MAPK/p38) play in regulating IL-12 production. We show that inhibition of p38 dramatically increased IL-12 production upon stimulation, while decreasing TNF-α. This reciprocal effect on these two cytokines following MAPK/p38 inhibition occurred in many different APCs, following a variety of different stimuli. IL-12 production was also increased in macrophages treated with small interfering RNA to limit p38α expression, and in macrophages deficient in MKK3, a kinase upstream of p38. The increase in IL-12 production following MAPK/p38 inhibition appears to be due to enhanced IL-12 (p40) mRNA stability. We show that MAPK/p38 inhibition can promote Th1 immune responses and thereby enhance vaccine efficacy against leishmaniasis. In a mouse model of Leishmania major infection, vaccination with heat-killed L. major plus CpG and SB203580 elicited complete protection against infection compared with heat-killed L. major plus CpG without SB203580. Thus, this work suggests that MAPK/p38 inhibitors may be applied as adjuvants to bias immune responses and improve vaccinations against intracellular pathogens.
Interleukin-12 is a 70-kDa cytokine and the “founding member” of a small number of heterodimeric cytokines. IL-12p70 is composed of two covalently linked subunits, p35 and p40, which are encoded by two separated genes. APCs, particularly dendritic cells (DCs) and activated macrophages, are the main producers of IL-12 (1). IL-23, another heterodimeric cytokine with overlapping but distinct biological function from IL-12, consists of a p19 subunit paired with the common p40 subunit. Both of these cytokines can induce IFN-γ production from T cells (2). Both IL-12 and IL-23 production are controlled mainly at the level of p40 gene expression, despite the fact that this subunit is made in vast excess of the other subunits. The p40 subunit can associate with either p35 or p19 to form a heterodimer, or it can remain in solution as a monomer. p40 subunits have been reported to form homodimers that can bind to the murine IL-12R and antagonize IL-12p70 binding/signaling in vitro (3); however, the physiological relevance of these homodimers, particularly in human immune responses, is questionable (1, 4). The IFN-γ that is produced in response to IL-12 or IL-23 is largely responsible for the proinflammatory activity of these cytokines. Therefore, the proper regulation of p40-containing cytokines is critical to maintaining effective immunity but also preventing autoimmune pathology. Mice deficient in IL-12p40 show deficient Th1 development with reduced delayed-type hypersensitivity responses and NK cell responses (1).
The MAPKs play important roles in many cellular processes, including growth, differentiation, apoptosis, and the immune response (5, 6). Four major MAPK pathways have been identified in mammalian cells, ERK, p38, JNK, and ERK5. The p38 pathway is associated with cytokine production, inflammation, cell growth and differentiation, and cell death. This pathway is strongly activated by inflammatory cytokines such as IL-1 and TNF-α and also by environmental stress. The p38 pathway consists of several MAPKKKs, including MKKKs 1–4, two MAPKKs, MKK3 and MKK6, and the four p38 isoforms, α, β, γ, and δ. Both p38α and p38β are ubiquitously expressed, whereas p38γ is expressed in skeletal muscle, and p38δ gene expression is found primarily in the lung, kidney, testis, pancreas, and small intestine. MKK3 and MKK6 exhibit high enzymatic specificity toward the p38 MAPK. MKK3 preferentially targets the p38α and p38β, whereas MKK6 can activate all p38 isoforms. Downstream substrates for p38 kinases include nuclear kinases MAPKAPK-2 (MK2), MSK1/2, and MNK, as well as transcription factors ATF-1/2, CHOP, MEF2, Elk-1, NF-κB, and p53 (6). Some studies suggest that p38 is required for both Th1 and Th2 differentiation as well as IFN-γ production (7). The p38 kinases can regulate cytokine production at the level of transcription (IFN-γ) or by stabilizing mRNAs (TNF-α) (8, 9).
Leishmaniasis is a worldwide disease with an estimated 12 million people infected throughout 88 countries (10). Effective primary immunity against Leishmania spp. requires IL-12–dependent production of IFN-γ from T cells. In animal studies, mice deficient in IL-12 are more susceptible to leishmaniasis (11), and the administration of rIL-12 to mice enables them to resolve Leishmania infection (12). The role of the MAPK/p38 pathway on the regulation of Leishmania infection has not been fully explored (13). In the present study, we investigated the role of p38 activation by different TLR agonists on IL-12 production in bone marrow-derived macrophages (BMMφs) and DCs. Our results demonstrate that the inhibition of p38 activation resulted in enhanced IL-12p40 and IL-12p70 production. The increase in IL-12p40 gene expression following p38 inhibition was primarily controlled at the level of mRNA stability. We further demonstrate that inhibition of p38 activation in APCs preferentially induced Th1 responses, and thereby enhanced vaccination against Leishmania major. Our results suggest that the activation of MAPK/p38 in APCs can limit Th1 immune responses, and that this pathway can be targeted to enhance cell-mediated immunity.
Materials and Methods
Mice, BMMφs, and bone marrow-derived DCs
BALB/c and C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). IL-10 knockout mice on the BALB/c background, MKK3 knockout mice on the C57BL/6 background, and DO11.10 mice on the BALB/c background were purchased from the The Jackson Laboratory (Bar Harbor, ME). All mice were maintained in high efficiency particle air-filtered Thoren units (Thoren Caging Systems, Hazleton, PA) at the University of Maryland. All animal studies were reviewed and approved by the University of Maryland Institutional Animal Care and Use Committee. BMMφs were prepared as previously described (14, 15). Briefly, bone marrow was flushed from the femurs and tibias of mice at 6–10 wk of age. The cells were plated in petri dishes in DMEM/F12 supplemented with 10% FBS, glutamine, penicillin/streptomycin, and 10% conditioned medium from L-929 cells. Cells were fed on days 2 and 5. On day 7, macrophages were removed from petri dishes and cultured on tissue culture dishes in complete medium without L cell-conditioned medium. Cells were used the next day. Bone marrow-derived DCs (BMDCs) were generated as previously described (16) with minor modifications. Briefly, bone marrow was prepared as described above. The cells were plated in petri dishes in RPMI 1640 complete medium and 10% conditioned medium from GM-CSF–producing J588L cells. On days 3 and 6, half of the medium was removed and replaced with fresh conditioned medium. On day 8, the suspension cells were harvested for experiments.
Reagents
TLR ligands were obtained from InvivoGen (San Diego, CA). The p38 inhibitors, SB203580, SB239063, and the structurally related control compound SB202474 were purchased from Calbiochem/EMD Biosciences (San Diego, CA). The effect of p38 inhibitors alone on cell survival was examined using CellTiter 96 AQueous assay provided by Promega (Madison, WI), and no cytotoxic effect was observed at the concentration up to 20 μM for 24 h of cell culture (data not shown). Anti-p38 (total and phospho-Thr180/Tyr182) Abs and other Abs unless specified were obtained from Cell Signaling Technology (Beverly, MA). TRIzol reagent was purchased from Invitrogen (Carlsbad, CA). RNase-free DNase I was obtained from Roche Diagnostics (Indianapolis, IN).
Measurement of cytokine and NO production
Approximately 2 × 105 cells were plated per well overnight in a 48-well plate in DMEM/F12 medium with 10% FBS. Cells were then washed and activated with 10 ng/ml LPS. Supernatants were harvested at different times. Cytokines (IL-12/IL-23p40, IL-10, TNFα, IL-12p70, IFN-γ, IL-4, and IL-5) were measured by a sandwich ELISA using Ab pairs provided by BD Biosciences (San Diego, CA), according to the manufacturer’s instructions. NO production was assayed by measuring the accumulation of NO2– in the medium of macrophages 24 h after stimulation using the Griess reagent as described previously (17).
Generation of small interfering RNA and cell transfections
A SignalSilence p38 MAPK small interfering RNA (siRNA) kit (Cell Signaling Technology) was used to reduce endogenous p38 protein expression. For cell transfections, 5 × 106 primary BMMφs were transfected on day 6 with 100 nM siRNA using Nucleofection technology from Amaxa (Lonza Walkersville, Walkersville, MD) and then stimulated 48 h later. Gene silencing was confirmed by Western blotting and quantitative real-time RT-PCR.
Western blotting
A total of 2 × 106 BMMφs per well were plated overnight in 6-well plates. Cells were treated with 10 ng/ml LPS in a final volume of 1 ml of DMEM/F12. Cells were then lysed in ice-cold lysis buffer (100 mM Tris [pH 8], 2 mM EDTA, 100 mM NaCl, 1% Triton X-100 containing complete EDTA-free protease inhibitors from Roche Diagnostics, which included 5 mM sodium vanadate, 10 mM sodium fluoride, 10 mM β-glycerophosphate sodium, and 5 mM sodium pyrophosphate. Equal amounts of protein were loaded onto 10% SDS-polyacrylamide gels and then transferred to polyvinylidene difluoride membranes. Membranes were incubated with primary Abs overnight at 4°C, washed, and incubated with secondary Ab with HRP conjugates. The specific protein bands were visualized by using a Lumi-LightPLUS chemiluminescent substrate (Roche Diagnostics).
RNA isolation and RT-PCR
TRIzol reagent was used to extract RNA from BMMfs (3–4 × 106 cells/reaction). Homogenization was conducted to facilitate RNA extraction from footpads and lymph nodes. RNase-free DNase I was used to remove contaminating genomic DNA. ThermoScript RT-PCR system (Invitrogen) was used to generate cDNA from DNA-free RNA by using random hexamers or oligo(dT)20. Quantitative real-time PCR was used to measure both mature and premature il12p40 mRNA levels. Premature il12p40 mRNA was analyzed by using random hexamer-generated cDNA and the primer pairs: sense, 5′-TCTGAGCCACTCACATCTGCT-3′ (intronic primer) and antisense, 5′-GGCCAATGAGAGTTCCTGTT-3′; and GAPDH primer pairs: sense, 5′-TGTTCCTACCCCCAATGTGT-3′ and antisense 5′-TCCCAAGTCACTGTCACACC-3′ (intronic primer). Mature IL-12p40 mRNA was amplified by using oligo(dT)20-generated cDNA and the primer pairs: sense, 5′-GGAGGTCAGCTGGGAGTACC-3′ and antisense, 5′-AGGAACGCACCTTTCTGGTT-3′; and gapdh primer pairs: sense, 5′-TGCAGTGCCAGCCTCGTG-3′ and antisense, 5′-TTGATGGCAACAATCTCCACTT-3′. The primers used to amplify other genes were: tnf-α primer pairs: sense, 5′-AAAGGGATGAGAAGTTCCCAAAT-3′ and antisense, 5′-GTCTTTGAGATCCATGCGGTTG-3′; p38α (mapk14) primer: forward primer, 5′-AAGACTCGTTGGAACCCCAG-3′ and reverse primer, 5′-TCCAGTAGGTCGACAGCCAG-3′; p38β (mapk11) primer: forward primer, 5′-AAGCCCAGTGTCCCTCCTAA-3′ and reverse primer, 5′-CCACAGGCAACCACAAATCT-3′; p38δ (mapk13) primer: forward primer, 5′-GCTCACCCCTTCTTTGAACC-3′ and reverse primer, 5′-TTCGTCCACGCTGAGTTTCT-3′; p38γ (mapk12) primer: forward primer, 5′-AGCCCTCAGGCTGTGAATCT-3′ and reverse primer, 5′-CATATTTCTGGGCCTTGGGT-3′.
Ag priming, challenge studies, and parasite quantitation
BMMφs (0.5 × 106/well) derived from BALB/c mice were incubated with OVA protein (100μg/ml), OVA plus CpG (10 ng/ml) with SB202474 (5 μM), and OVA plus CpG (10 ng/ml) with SB203580 (5 μM) overnight. Supernatants were collected to detect cytokine production, and cells were washed with PBS twice. The treated macrophages were then incubated with the purified CD3+ T cells that were obtained from splenocytes of DO10.11 mice by a negative selection kit according to the instruction supplied by the manufacture (R&D Systems, Minneapolis, MN). Supernatants were collected 72 h later to detect cytokine production. For vivo studies, OVA (25 μg) and CpG (0.5 μg) were mixed with either 20 μM SB203580 (MAPK/p38 inhibitor) or SB202474 (control compound) in a final volume of 25 μl and injected twice (7 d apart) into the hind footpad of DO10.11 mice. On the 10th day after the first footpad priming, mice were injected i.p. with OVA (50 μg) and serum was collected for cytokine detection. In similar studies using C57BL/6 mice, heat-killed L. major (HKLM; 25 μg) was used in place of OVA protein.
For challenge experiments, L. major Friedlin strain, clone V1 (MHOM/IL/80/Friedlin) was used. Parasites were maintained as previously described (15). Footpad-derived amastigotes were obtained by centrifugation at 1000 × g for 10 min. Stationary-phase promastigotes were obtained by growing parasites in Schneider’s complete medium with 20% FBS at 25°C. Mice were inoculated in the right hind footpad with 1 × 105 L. major promastigotes, as indicated in the figure legends. Lesion size was measured with a digital thickness gauge (Chicago Brand Industrial, Fremont, CA) and expressed as the difference in thickness between the infected and the contralateral (noninfected) footpad, as previously described (15). Parasite burdens were determined by a limiting dilution assay, as previously described (15). Briefly, the cell suspensions were serially diluted in Schneider’s complete medium and observed 7 d later for the growth of promastigotes. Parasite burdens were expressed as the negative log10 dilution in which parasite growth was visible.
Quantitative-real time PCR and data analysis
Real-time PCR was conducted with the Roche LightCycler 480 sequence detection system (Roche Diagnostics) using iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA) following the manufacturers’ instructions. The relative differences among samples were determined using the ΔΔCT methods as previously described (14, 15). The CT value for GAPDH gene was used to normalize loading in the RT-PCRs. A ΔΔCT value was then obtained by subtracting control ΔCT values from the corresponding experimental ΔCT.The ΔΔCT values were converted to fold difference compared with the control by raising 2 to the ΔΔCT power. An unpaired Student t test was used for statistical analysis. The p values < 0.05 were considered to be statistically significant.
Results
MAPK/p38 inhibition has reciprocal effects on the production of IL-12 and TNF-α
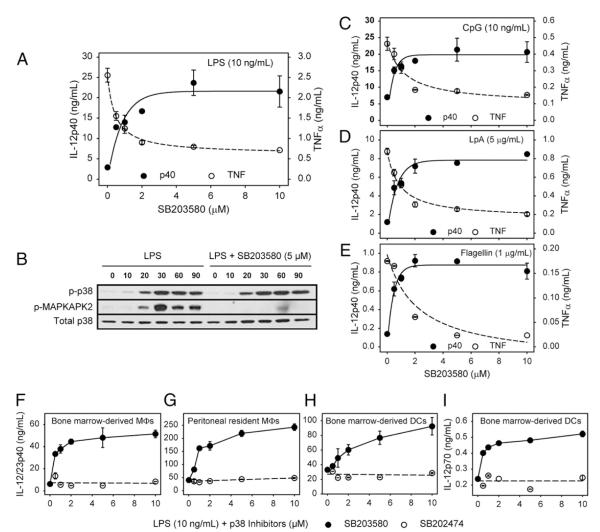
The ligation of TLRs on macrophages can promote the production of proinflammatory cytokines, including IL-12p40 and TNF-α. It has previously been shown that the MAPK p38 plays a positive role in promoting TNF-α production by stimulated macrophages (18). We examined the effect that MAPK/p38 activation has on IL-12p40 production. Macrophages were stimulated with the TLR4 ligand LPS (10 ng/ml) in the presence or absence of the MAPK/p38 inhibitor SB203580. As expected, LPS induced the production of both TNF-α and IL-12p40 from BMMφs. Inhibition of MAPK/p38 by SB203580 resulted in a dose-dependent increase in IL-12p40 production (Fig. 1A). TNF-α production, in contrast, was decreased in a similar dose-dependent manner (Fig. 1A). Similar results were found when a second-generation MAPK/p38 inhibitor designated SB239063 was used (data not shown). The inhibition of MAPK/p38 activation by SB203580 was examined by Western blot analysis (Fig. 1B). LPS stimulation of macrophages resulted in the phosphorylation of both MAPK/p38 and its downstream substrate MK2 (Fig. 1B, left). Both p38 and MK2 activation were detected as early as 10 min after stimulation, reaching maximum levels at 30 min, and persisting for 90 min. Treatment of cells with SB203580 did not affect the levels of phosphorylated p38, as expected, but it inhibited the enzymatic activity of p38 and blocked the phosphorylation of its downstream kinase MK2 (Fig. 1B).
FIGURE 1.
MAPK/p38 inhibition increases IL-12 production by stimulated macrophages and DCs. A, Macrophages (3 × 105 cells) were pretreated with increasing concentrations of SB203580 for 1 h and then stimulated with LPS (10 ng/ml) overnight. Supernatants were harvested, and IL-12p40 and TNF-α production were determined by ELISA. B, Macrophages (2 × 106 cells) were pretreated with SB203580 (5 μM) for 1 h. Cells were then stimulated with LPS (10 ng/ml) for 0, 10, 20, 30, 60, and 90 min. Cell lysates were prepared for Western blot analysis to detect p-p38, p-MAPKAPK2, and total p38 protein. C–E, Macrophages (3 × 105 cells) were pretreated with increasing concentrations of SB203580 for 1 h and stimulated with CpG (10 ng/ml) (C), lipoprotein A(5 μg/ml) (D), or flagellin (1 μg/ml) (E) overnight. IL-12p40 and TNF-α production were determined as described above. F–I, BMMφs (F), peritoneal macrophages (G), and BMDCs (H, I) were pretreated with increasing concentrations of SB203580 (●), or inactive SB202474 (엯) for 1 h and then stimulated with LPS (10 ng/ml) overnight. Supernatants were harvested for ELISA analysis of IL-12p40 (F–H) or IL-12p70 production (I). Values represent the mean ± SD of triplicate determinations, and the data represent one of three independent experiments.
To test whether MAPK/p38 inhibition-induced IL-12p40 production was unique to TLR4 stimulation, we stimulated macrophages with a variety of stimuli, including CpG (Fig. 1C), lipoprotein A (Fig. 1D), or flagellin (Fig. 1E), which are ligands for TLR9, TLR2/TLR6, and TLR5, respectively. All of the TLR agonists tested were able to induce TNF-α and IL-12p40 production from macrophages, and in all cases SB203580 displayed similar effects on cytokine production, increasing IL-12p40 in a dose-dependent manner. The total amount of cytokine induced by these different TLR agonists varied, but the extent of MAPK/p38-mediated IL-12 enhancement and TNF inhibition was comparable. Thus, these data indicate that MAPK/p38 activation can influence IL-12p40 in response to a variety of stimuli.
To determine whether this phenomenon was specific to BMMφs, we carried out similar experiments using resident peritoneal macrophages and BMDCs. Following stimulation with LPS, SB203580 increased IL-12p40 production from BMMφs (Fig. 1F), resident peritoneal macrophages (Fig. 1G), and BMDCs (Fig. 1H). In BMDCs, MAPK/p38 inhibition also enhanced LPS-induced IL-12p70 production (Fig. 1I). For all of these studies, the control compound SB202474 (open circles) failed to influence cytokine production. A similar increase in IL-12p70 production occurred in IFN-γ–primed LPS-stimulated macrophages treated with SB203580 (data not shown). Therefore, these data show that MAPK/p38 inhibition enhanced IL-12 production, and they suggest that activation of the MAPK/p38 pathway plays a negative regulatory role in IL-12 production by APCs.
MAPK/p38 inhibition increases IL-12 production due to enhanced p40 mRNA stability
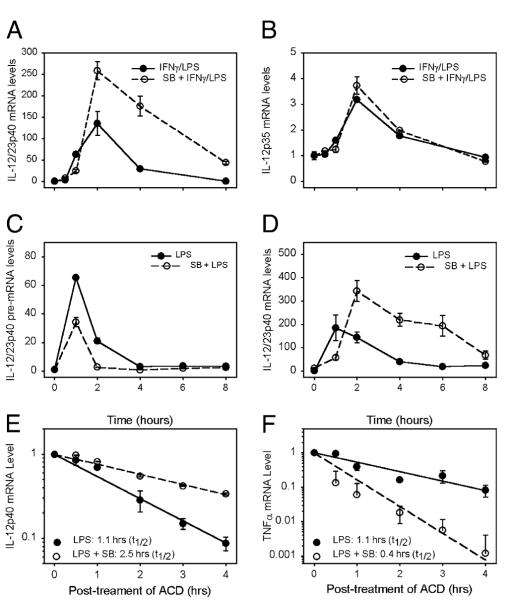
To determine the cytokine subunit affected by MAPK/p38 inhibition, we examined p35 as well as p40 mRNA production in IFN-γ–primed LPS-stimulated macrophages. IL-12p40 mRNA was increased in the presence of SB203580 (Fig. 2A), whereas the p35 subunit remained essentially unchanged (Fig. 2B). We also examined mRNA encoding the p19 subunit of IL-23, which remained essentially unchanged by the addition of SB203580 (data not shown). We therefore focused on the regulation of the p40 subunit. To gain further insight into the molecular mechanisms of il12p40 gene expression by MAPK/p38, we investigated changes in il12 transcription. Nuclear prespliced mRNA (pre-mRNA) and cytoplasmic mature mRNA were isolated following stimulation of macrophages with LPS and measured by real-time PCR as previously described (14). Pre-mRNA expression quickly reached maximal levels at 1 h after LPS stimulation and returned to basal levels by 4 h (Fig. 2C, solid line). IL-12p40 mature mRNA induced by LPS peaked between 1 and 2 h after stimulation and returned to basal level between 4 and 6 h (Fig. 2D, solid line). Interestingly, p38 inhibition by SB203580 inhibited initial il12p40 transcription (Fig. 2C, dashed line) but enhanced mature p40 mRNA formation (Fig. 2D, dashed line). As p38 inhibition had a positive effect on IL-12p40 mRNA accumulation but not on transcription, we studied the kinetics of IL-12p40 mRNA degradation in detail (Fig. 2E). Macrophages were treated with SB203580 (open circles, dashed line) or its drug vehicle (closed circles, solid line) and then stimulated with LPS for 2 h, at which time the transcription inhibitor actinomycin D (10 μg/ml) was added. IL-12p40 mRNA degradation was measured at different times during the next 4 h. In the presence of SB203580, IL-12p40 mRNA was more stable. Its half-life increased by ~2-fold (Fig. 2E). In contrast, TNF-α mRNA was less stable when p38 was inhibited, and its half-life was reduced >2-fold (Fig. 2F). These data indicate that the inhibition of MAPK/p38 resulted in increased IL-12p40 production due at least in part to enhanced mRNA stability.
FIGURE 2.
MAPK/p38 inhibition increases IL-12p40 production via an accumulation of IL-12p40 mRNA. A, and B, BMMφs (3 × 105 cells) were pretreated with SB203580 (엯, dashed line), or saline (●, solid lines) for 1 h and then stimulated with IFN-γ (100 U/ml) and LPS (10 ng/ml). Cytoplasmic RNAs were isolated at the designated times, and real-time PCR was performed to detect the presence of IL-12p40 mRNA (A) and IL-12p35 mRNA (B). Data are expressed as fold increase relative to unstimulated cells. C and D, Macrophages (4 × 106 cells) were pretreated with SB203580 (5 μM) (dashed line, 엯) or with saline (solid line, ●) and then stimulated with LPS (10 ng/ml). Cytoplasmic and nuclear RNA were isolated at different times as indicated. Real-time PCR was performed to detect the presence of IL-12p40 pre-mRNA (C) and IL-12p40 mature mRNA (D). Data are expressed as fold increase relative to unstimulated cells. E and F, Macrophages were pretreated with drug vehicle (solid line with ●) or SB203580 (5 μM) (dashed line with 엯) for 1 h and stimulated with LPS (10 ng/ml) for 2 h. Actinomycin D (10 μg/ml) was added and RNA was isolated at the indicated times. Quantitative real-time PCR was performed to analyze the mRNA stability of IL-12p40 (E) and TNF-α (F), and data are presented in arbitrary units on a log scale. Data represent one of three independent experiments (mean ± SD of triplicate determinations).
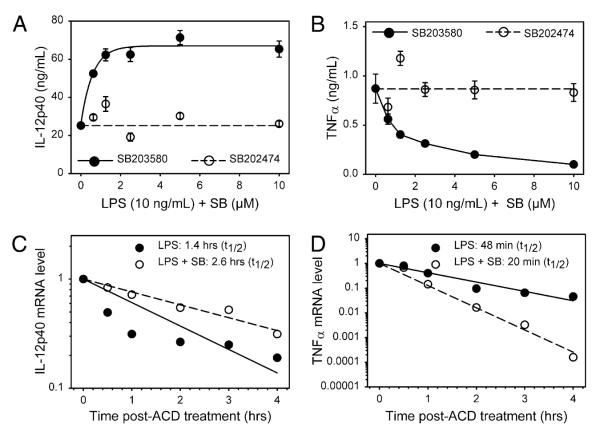
It is well known that IL-10 can inhibit the transcription and translation of IL-12. Therefore, macrophages derived from IL-10 knockout mice were used to determine whether IL-12 enhancement caused by the inhibition of MAPK/p38 was due to a modulation of IL-10. MAPK/p38 inhibition by SB203580 increased IL-12p40 production by ~3-fold in IL-10–/– macrophages (Fig. 3A), whereas TNF-α production was significantly reduced by this inhibitor (Fig. 3B). This increase in IL-12 production occurred despite the fact that basal LPS-induced IL-12 production was much higher in IL-10–/– macrophages as compared with the cells derived from control littermates. Similar to our observations in wild-type cells, LPS-induced IL-12p40 mRNA was more stable in the presence of the p38 inhibitor (Fig. 3C), whereas TNF-α mRNA was less stable in treated cells (Fig. 3D). These data indicate that the enhanced production of IL-12 following p38 inhibition occurs independently of IL-10.
FIGURE 3.
The effect of MAPK/p38 on IL-12 is independent of IL-10. IL-10–/– macrophages were pretreated with increasing concentrations of SB203580 for 1 h and then stimulated with LPS (10 ng/ml) overnight. Supernatants were harvested, and IL-12p40 (A) and TNF-α (B) production were determined by ELISA. Data represent one of three independent experiments (mean ± SD of triplicates). C and D, IL-10–/– macrophages were pretreated with SB203580 (5 μM) (dashed line, 엯) or with saline (solid line, ◯) for 1 , and stimulated with LPS (10 ng/ml) for 2 h. Actinomycin D (10 μg/ml) was added and RNA was isolated at the indicated times. Quantitative real-time PCR was performed to analyze the stability of cytokine mRNA (IL-12p40 [C] and TNF-α [D]). Data represent one of three independent experiments (mean ± SD of triplicates).
IL-12 production is increased in macrophages deficient in p38 or MKK3
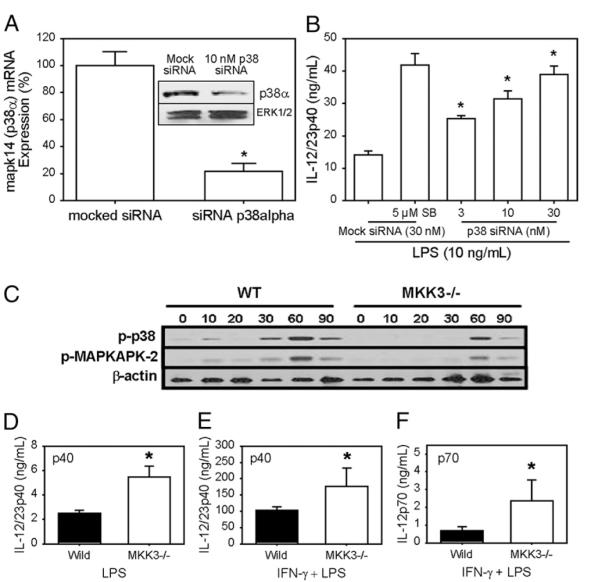
Four isoforms of MAPK/p38, designated α, β, γ, and δ, have been identified. Our Western blot analysis could only detect the presence of the α form in BMMφs (data not shown). By real-time PCR analysis, p38α mRNA was the dominant mRNA species, exceeding p38β levels by >10-fold. MAPK/p38d and γ mRNA expression were undetectable (data not shown). To examine the role of p38 in IL-12 induction, siRNA specific for p38α were introduced into BMMφs 48 h before cells were stimulated with LPS. The addition of siRNA specific for p38α decreased p38α mRNA expression by 80% by quantitative real-time PCR (Fig. 4A) and protein expression by 60% (Western blotting; Fig. 4A, inset). The knockdown of p38 in primary macrophages resulted in a dose-dependent increase in IL-12 p40 production in response to LPS stimulation (Fig. 4B). At 30 nM siRNA, IL-12p40 production was comparable to cells treated with the SB203580 inhibitor (Fig. 4B).
FIGURE 4.
MAPK/p38 knockdown or MKK3 deletion increases IL-12 production. A, siRNA (10 nM) to p38α or mock siRNA was transfected into BMMφs by nucleofection, and BMMφs were cultured for 48 h. Total RNA was isolated and real-time PCR was performed to analyze p38α mRNA. Insert, Cell lysates from siRNA-transfected macrophages were prepared for Western blotting analysis using Ab to p38α. Total ERK was used as a loading control. B, BMMφs were transfected with mock siRNA for 48 h and treated with saline or SB203580 for 1 h. Parallel BMMφs were transfected with 3, 10, or 30 nM siRNA specific for p38α for 48 h and then stimulated with LPS (10 ng/ml) overnight. Supernatants were harvested to detect IL-12p40 protein by ELISA. C, BMMφs from MKK3–/– mice and control littermates were stimulated with LPS (10 ng/ml) for the indicated times. Cell lysates were prepared for Western blotting analysis using Abs to p-p38, p-MK2, and β-actin as a loading control. D–F, BMMφs from control littermates (filled bars) and MKK3 knockout mice (open bars) were primed with IFN-γ (100 U/ml) and then stimulated with LPS (10 ng/ml) overnight. ELISA was performed to detect cytokine production. Data represent one of three independent experiments (mean ± SD of triplicates for ELISA data). The p values were determined by a Student t test. *p < 0.05.
We also examined IL-12 production in mice genetically deficient in the gene encoding MKK3, one of the upstream kinases that activates MAPK/p38 (5). These mice are defective in p38 activation, and therefore the extent of LPS-induced phosphorylation of p38 and MK2 was reduced, compared with the wild-type cells (Fig. 4C). Stimulation of macrophages from MKK3–/– mice with LPS resulted in higher levels of IL-12p40 production (Fig. 4D). Furthermore, when these macrophages were primed with IFN-γ and then stimulated with LPS macrophages from MKK3–/–, mice produced more IL-12p40 (Fig. 4E) and p70 (Fig. 4F) than did cells from control littermate mice.
Inhibition of MAPK/p38 activation favors a Th1 immune response
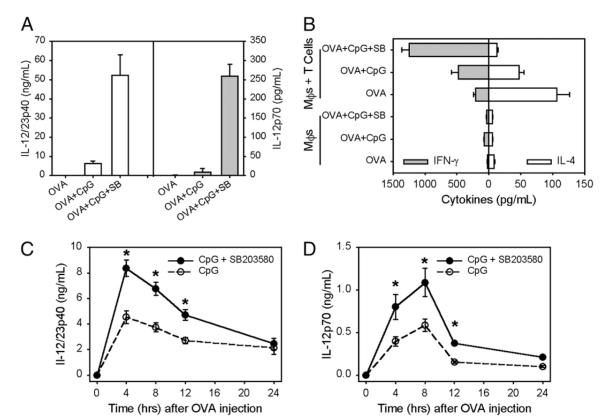
Because we observed that MAPK/p38 inhibition could increase IL-12 production in vitro, we investigated whether such inhibition could promote Th1 immune responses. CpG has been widely used as an adjuvant for protein-based vaccinations (19-21). Therefore, we stimulated macrophages in vitro with OVA protein and CpG in the presence of the MAPK/p38 inhibitor SB203580. OVA itself induced no detectable IL-12, as expected. The addition of CpG with OVA induced nanogram levels of IL-12p40 production by macrophages (Fig. 5A, open bars). It also induced a small but measurable amount of IL-12p70 (Fig. 5A, gray bars). The inhibition of p38 activation by SB203580 strongly increased CpG-induced IL-12 production from macrophages. IL-12p40 (Fig. 5A, open bars) was increased by 10-fold and IL-12p70 (Fig. 5A, gray bars) was even more dramatically increased. T cells derived from D011.10 mice were cocultured with stimulated macrophages for 3 d, and supernatants were collected to look for Th1 or Th2 skewing. Macrophages that had been incubated with OVA plus CpG in the presence of SB203580 elicited more IFN-γ (Fig. 5B, gray bars) and less IL-4 (Fig. 5B, open bars) from DO11.10 T cells than did macrophages that had been incubated with OVA and CpG without the inhibitor, indicating enhanced Th1 priming.
FIGURE 5.
Inhibition of MAPK/p38 activity favors a Th1 response. A, BMMφs were stimulated with OVA (100 μg/ml), OVA plus CpG (10 ng/ml) with and without SB203580 (5 μM) overnight. Supernatants were collected to detect IL-12p40 and IL-12p70. B, BMMφs were treated as described in A and cocultured with or without D011.10 T cells for 3 d. Supernatants were harvested to detect IFN-γ and IL-4 production by ELISA. C and D, D011.10 BALB/c mice were injected in the hind footpad with a 25-μl mixture containing OVA (25 μg) and CpG (0.5 μg) plus SB203580 (20 μM) (◯) or SB202474 (엯) on day 0 and again on day 7. On day 10, the mice received an injection of OVA (50 μg) i.p. Periorbital blood samples (~0.2 ml) were taken from mice at each time interval indicated in the figure legend. An ELISA was performed to detect IL-12p40 (C) and IL-12p70 (D). Values represent the mean ± SD (n = 4 mice/group). The p values were determined by a Student t test. *p < 0.05.
To determine whether the in vitro enhancement of Th1 polarization could also be observed in vivo, studies were undertaken in which DO1110 mice were primed with OVA plus CpG in the presence or absence of SB203580. Ten days after priming, mice were injected with OVA Ag i.p. and IL-12 production was measured during the next 12 h (Fig. 5C, 5D). Mice primed with Ag plus CpG in the presence of SB203580 produced more IL-12p40 (Fig. 5C) and IL-12p70 (Fig. 5D) relative to mice receiving Ag plus CpG alone. These results suggest that the inhibition of MAPK/p38 can promote Th1 responses.
The inhibition of MAPK/p38 enhances CpG adjuvanticity and improves vaccine efficacy
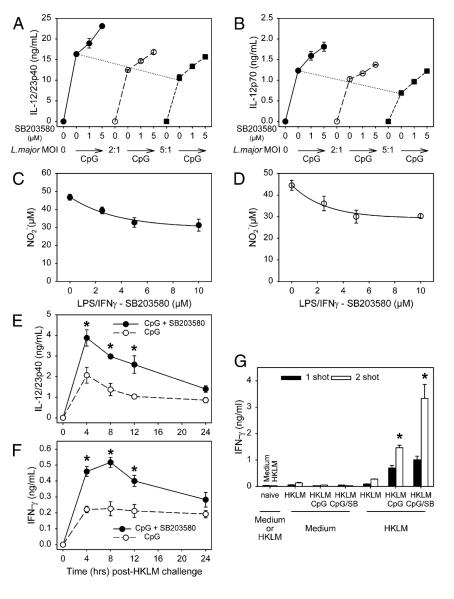
We examined whether the inhibition of MAPK/p38 activation would improve vaccine efficacy and enhance protection against L. major, an intracellular parasite whose clearance is linked to Th1 immunity (21). First, we examined whether macrophages or DCs infected with parasites would respond to SB203580 treatment similarly to uninfected cells. BMMφs (Fig. 6A) or BMDCs (Fig. 6B) were treated with increasing concentrations of SB203580 and then infected with increasing multiplicities of infection (MOIs) of L. major parasites in the presence or absence of CpG. In the absence of CpG neither macrophages (Fig. 6A) nor DCs (Fig. 6B) produced IL-12 in response to L. major infection. The addition of CpG to macrophages and DCs induced IL-12p40 and p70 production, respectively. Infection with increasing amounts of L. major parasites caused a slight decrease in CpG-induced IL-12 production (Fig. 6A, 6B, dashed lines), but in all cases the inhibition of MAPKp38 by SB203580 resulted in a dose-dependent increase in CpG-induced IL-12p40 production from macrophages (Fig. 6A) and IL-12p70 production from DCs (Fig. 6B). To determine whether SB203580 treatment interfered with the microbicidal activity of activated macrophages, NO production by activated macrophages was measured. Macrophages were primed in vitro with IFN-γ and then stimulated with LPS in the presence or absence of SB203580. The production of NO by uninfected (Fig. 6C)and L. major-infected (Fig. 6D) macrophages was measured 24 h after LPS stimulation. The inhibition of MAPK/p38 caused only a modest decrease in NO production by activated macrophages, and infection with L. major had no effect on the degree of inhibition. In both infected and uninfected macrophages, treatment with high doses of SB203580 (10 μM) resulted in a modest decrease (<30%) in NO production.
FIGURE 6.
SB203580 enhances CpG effects to induce a Th1 response. A, BMMφs or (B) BMDCs derived from C57BL/6 mice were treated without or with increasing doses of SB203580 for 1 h and then infected without or with L. major parasites at MOIs of 0 (◯), 2:1 (엯), or 5:1 (■) in the absence or presence of CpG for 16 h. Supernatants were harvested and subjected to ELISA to detect IL-12p40 (A) and IL-12p70 (B) production. C and D, BMMφs derived from C57BL/6 mice were primed with IFN-γ (100 U/ml) for 2 h and then infected without (C) or with L. major parasites at MOIs of 5:1 in the presence of SB203580 at increasing dosages for 1 h. The cells were then stimulated with LPS for 24 h and the supernatants were collected. Equal volumes of supernatants were mixed with Griess reagent for 10 min, and NO2– accumulation was measured. E and F, C57BL/6 mice were injected in the hind footpad with a 25-μl mixture containing HKLM (25 μg) and CpG (0.5 μg) plus SB203580 (20 μM) (◯) or the inactive SB202474 (엯). This was repeated 7 d later. On day 10, mice received an injection of HKLM (50μg) i.p. A periorbital blood sample (~0.2 ml) was taken from each mouse at the indicated time intervals and an ELISA was performed to detect IL-12p40 (E) and IFN-γ (F). Values represent the mean ± SD (n = 4 mice/group). G, C57BL/6 mice were injected in the hind footpad with two shots (day 0 and day 10, n = 4) or one shot (day 7, n = 4) of HKLM (25 μg) and CpG (0.5 mg) plus SB203580 (20 μM) (◯) or inactive SB202474 (엯). On day 17, splenocytes from naive or injected mice were harvested and incubated with or without HKLM for 72 h. Supernatants were collected and IFN-γ was measured by ELISA. Values represent the mean ± SD (n = 4 mice/group). The p values were determined by a Student t test. *p < 0.05.
We then examined whether SB203580 had an effect on recall responses of mice that were primed with HKLM and CpG and then re-exposed to HKLM in vivo. Upon recall, mice primed with HKLM and CpG in the presence of SB203580 had significantly higher levels of IL-12p40 (Fig. 6E) and IFN-γ (Fig. 6F) in their serum than did mice primed in the absence of SB203580. Ex vivo IFN-γ production from splenocytes of mice primed in vivo with HKLM and CpG in the presence or absence of SB203580 was also examined (Fig. 6G). Splenocytes from mice that were primed in the presence of SB203580 produced higher levels of IFN-γ upon ex vivo restimulation with HKLM than did splenocytes from mice that were primed in the absence of SB203580. The effect of SB203580 treatment was especially pronounced in mice that had been given two injections with HKLM and CpG plus inhibitor (Fig. 6G, open bars). Taken together, these data suggest that inhibition of MAPK/p38 at the time of Ag priming increases IL-12p40 production and leads to higher amounts of IFN-γ production, indicative of enhanced Th1 responses.
Finally, the capacity of SB203580 to enhance CpG adjuvanticity was tested in a murine model of leishmaniasis (Fig. 7). BALB/c mice were vaccinated twice (1 wk apart) with HKLM and CpG, with or without SB203580. Mice were then infected with live L. major, and lesion progression was monitored over an 8-wk period. As shown in Fig. 7A, control naive BALB/c mice (filled circles) or mice vaccinated with HKLM and a control (nonstimulatory) CpG (open circles) or SB203580 (filled inverted triangles) developed lesions within 5 wk of infection that became progressively larger until the experiment was terminated. Mice vaccinated with HKLM plus CpG were partially protected and developed significantly smaller lesions after 5 wk of infection (Fig. 7A, open inverted triangles). However, the inhibition of MAPK/p38 with SB203580 at the time of vaccination significantly enhanced the level of protection as indicated by a further reduction in lesion size as compared with HKLM plus CpG (Fig. 7A, filled squares, weeks 6–8). In addition to lesion size, parasite burdens at the site of infection were quantitated. Mice vaccinated with HKLM and CpG in the presence of SB203580 had significantly fewer parasites than did untreated naive mice or mice treated with HKLM and CpG alone (Fig. 7B). With regard to the immune response, cytokine production by draining lymph node cells was analyzed ex vivo on day 56 postinfection. Lymph node suspensions from mice vaccinated with HKLM and CpG with SB203580 produced more IFN-γ and less IL-4 than did cells from unvaccinated mice or mice vaccinated with HKLM and CpG without MAPK/p38 inhibition or mice vaccinated with HKLM and MAPKp38 inhibition but without CpG (Fig. 7C). Thus, in this model, inhibition of MAPK/p38 activation at the time of vaccination enhanced the adjuvanticity of CpG and promoted a greater Th1 immune response, leading to an increased level of protection against L. major challenge.
FIGURE 7.
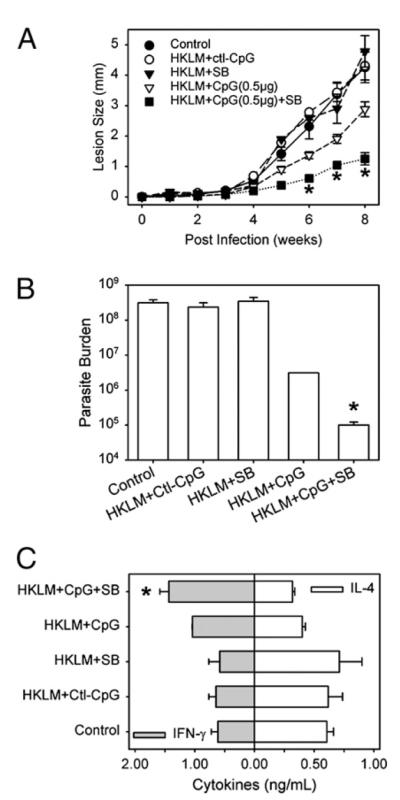
The inhibition of MAPK/p38 enhances CpG adjuvant effects to vaccinate against L. major in mice. A, BALB/c mice were injected in the left hind footpad with PBS (◯) or HKLM (50 μg) with control CpG oligomers (0.5 μg/ml) (엯) or HKLM (50 μg) with SB203580 (20 μM) (▼) or with active CpG (0.5 μg/ml) (Δ). Some mice received HKLM, CpG oligomers, and SB203580 (20 μM) (▼). Mice were injected twice on day 0 and day 7. On day 30, mice were challenged with 1 × 105 L. major metacyclic promastigotes in their right footpad. Footpad lesions were monitored weekly. B, Parasite burdens in infected footpads were determined by limiting dilution assays. One representative experiment of three is shown. C, Cytokine production by lymph node T cells from infected mice. Lymph nodes were removed on day 56 and stimulated with anti-CD3 and anti-CD28 for 48 h. Cells were stimulated with PMA for 5 h, and supernatants were harvested and assayed for IFN-γ and IL-4 by ELISA. Data represent mean ± SD. The p values were determined by a Student t test. *p < 0.05.
Discussion
In this paper, we provide evidence to support the idea that the manipulation of MAPK activation levels can exert a profound effect on APC cytokine production. Specifically, we show that the inhibition of MAPK/p38 activation can result in the hyperproduction of IL-12 by stimulated macrophages and DCs and effect the character of an ensuing immune response. We used two MAPK/p38 inhibitors with different structures, SB203580 and SB239063, to enhance IL-12 production. We also demonstrated that limiting the expression of MAPK/p38 protein by specific siRNAs and using mice with targeted deletions of the activating kinase (MKK3) upstream of p38 had similar stimulatory effects on IL-12 production. APCs pretreated with MAPK/p38 inhibitors skewed Ag-specific T cells to produce more IFN-γ and less IL-4. Thus, this work suggests that MAPK/p38 inhibitors may be applied along with adjuvants to improve vaccines against intracellular pathogens.
Previous studies have indicated that MAPK/p38 can promote inflammation by targeting NF-κB to the promoters of inflammatory genes (22) and by stabilizing inflammatory gene transcripts (18). However, the role of MAPK/p38 activation on IL-12 activity has remained somewhat controversial. In an early report, Salmon et al. (23) reported that SB203580 could enhance LPS-initiated IL-12 production by peritoneal exudate macrophages. Kim et al. (24) recently observed that the deletion of p38a resulted in increased expression of several proinflammatory cytokines, including IL-12p40. However, Kang et al. (25) showed that in macrophages the deletion of p38α had a negative effect on IL-12 production. Furthermore, deletions in MK2, the kinase directly downstream of MAPK/p38α/β (5, 26), resulted in more IL-12 production from LPS-stimulated macrophages (27). MKK3 is the dominant upstream kinase that controls activation of MAPK/p38 kinases (5, 26). Lu et al. (28) reported that IL-12 production was reduced in the “elicited” peritoneal macrophages from MKK3-deficient mice. In the present study, we showed that MAPK/p38 inhibition by SB203580 enhanced IL-12p40 production from macrophages and IL-12p70 production from DCs. Our results are in agreement with the results from a report on MK2–/– macrophages (27) and a more recent study in which Plasmodium falciparum glycosylphosphatidylinositols or LPS-induced IL-12p40 production was enhanced in MK2–/– macrophages or SB203580-treated macrophages (29).
The role of MAPK/p38 activation in T cells has been extensively studied (9, 30, 31). Murphy and colleagues (31) reported that CD4+ T cells without p38α could differentiate into Th1 cells that produce normal levels of IFN-γ. However, they were defective in their response to IL-12 and IL-18 stimulation. The TCR complex-associated kinase Zap70 can phosphorylate p38 to induce the production of IFN-γ. This is controlled by the members of growth arrest and DNA damage-inducible genes family (32). Resting T cells from growth arrest and DNA damage-inducible gene 45α-deficient mice undergo spontaneous activation of p38 without activation of the upstream MAPK kinase (32), whereas DCs from these mice have a diminished p38 activity in response to TLR11 and TLR4 ligands (33).
In the present work, we demonstrate that MAPK/p38 inhibition results in an increased half-life of IL-12p40 mRNA. MK2, a major downstream target of MAPK/p38α, has been implicated in the regulation of cytokine mRNA stability and translation possibly through the modification of mRNA-binding proteins by phosphorylation (18). TNF-α is one of the cytokines whose mRNA is stabilized by the activation of the MAPK/p38–MK2 pathway (8). In the present study, MK2 activation was inhibited by the MAPK/p38 inhibitor, and this resulted in a decrease in TNF-α mRNA half-life, consistent with these previous observations. However, this same treatment resulted in more than a doubling of the half-life of IL-12p40 mRNA. The mechanism for this increased mRNA stability is not known. Tristetraprolin has been identified as a target of MK2 (18, 27, 34). Phosphorylated tristetraprolin binds to the AU-rich region of 3′-mRNA of TNF-α to prevent its degradation (34). A typical AU-rich element has not been definitively identified in the 3′-untranslated region of IL-12p40 mRNA, and thus tristetraprolin may not function on IL-12p40 mRNA as it does with TNF-α or other mRNAs containing AU-rich elements. Akira and colleagues (35) recently reported Zc3h12a accelerates IL-6 mRNA degradation and other genes, including IL-12/23p40 and calcitonin receptor. How the inactivation of MK2 or the inhibition of MAPK/p38 enhances LPS-induced IL-12p40 gene expression via mRNA stabilization will be addressed in future experiments. The MAPK/p38–MK2 pathway can also regulate targets other than tristetraprolin. In the study of P. falciparum glycosylphosphatidylinositols-induced IL-12p40 in MK2–/– macrophages, the enhanced binding of NF-κB to IL-12p40 promoter region and the reduction in the expression of transcriptional repressors GAP-12 and c-Maf were attributed to increased IL-12p40 gene expression (29). In our study, IL-12p40 transcription was moderately decreased in the macrophages treated with MAPK/p38 inhibitors, as determined by the production of nuclear pre-mRNA. Furthermore, p38 inhibition actually reduced il12p40 promoter activity in a transient transfection of il12p40 promoter reporter experiments (data not shown). Thus, it is unlikely that the inhibition of these transcriptional repressors accounts for the increased IL-12 production in MAPK/p38 inhibited macrophages.
Several earlier studies have suggested that infection of macrophages or DCs by some strains of Leishmania spp. can induce IL-12 production (36-38), suggesting that the inhibition of MAPK/p38 might favor parasite survival through a mechanism that involves reduced IL-12 production by infected cells (13, 39, 40). Our present studies and those of others indicate that the parasites by themselves were unable to induce IL-12 production from macrophages or DCs. In our hands, L. major-infected cells are less responsive to CpG stimulation and produce reduced amounts of IL-12 (Fig. 6) (15); however, they are no less responsive to p38 inhibition. These infected cells efficiently upregulate IL-12 production in response to treatment with SB203580. We also demonstrate that MAPK/p38 inhibition only minimally affects NO production by activated macrophages, so the improvements seen in vaccinated mice are not due to a direct effect on parasite killing by SB203580-treated macrophages but rather to increased IL-12 production. IL-12 functions as a Th1-skewing cytokine to induce IFN-γ (1, 9). Thus, MAPK/p38 inhibition would be a potential strategy to modulate the host immunity to vaccines. Indeed, macrophages primed with Ag together with the MAPK/p38 inhibitor skewed T cells to produce more IFN-γ. Thus, p38 inhibitors could potentially be used as coadjuvants to boost cell-mediated immunity and improve vaccines against a variety of intracellular pathogens. In our hands, the inhibition of MAPK/p38 resulted in an enhancement of CpG-induced production of both IL-12p40 and IL-12p70 from macrophages and DCs, respectively. Furthermore, when SB203580 was combined with low-dose CpG, IL-12 production was enhanced and there was an increase in the production of IFN-μ production. This increase appeared to be Ag-specific because a second administration of Ag (Fig. 6E) resulted in higher levels of IFN-γ.
In summary, we examined the influence of MAPK/p38 activation on the production of IL-12 production in stimulated macrophages. We show that cells deficient in p38 produce more IL-12 as a result of an increase in the half-life of IL-12 p40 mRNA. We demonstrate that this increased IL-12 production can influence adaptive immune responses and suggest that manipulating the MAPKs may be a feasible approach to improving vaccines against intracellular pathogens. These studies suggest that inhibitors of MAPK/p38 may also prove effective in atopic diseases where uncontrolled Th2 responses predominate. Different generations of MAPK/p38 inhibitors with more potency and better specificity are being developed (26). Our data suggest that these inhibitors may have several unanticipated applications. We propose that the targeting of MAPK/p38 activation cannot only control unwanted inflammatory response, such as TNF overproduction, but may also improve vaccinations against intracellular microorganisms by increasing IL-12 production.
Acknowledgments
This work was supported in part by National Institutes of Health Grant AI55576.
Abbreviations used in this paper
- BMDC
bone marrow-derived dendritic cell
- BMMφ
bone marrow-derived macrophage
- DC
dendritic cell
- HKLM
heat-killed Leishmania major
- MOI
multiplicity of infection
- pre-mRNA
nuclear prespliced mRNA
- siRNA
small interfering RNA
Footnotes
Disclosures The authors have no financial conflicts of interest.
References
- 1.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 2.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 3.Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28:33–38. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 5.Gaestel M. MAPKAP kinases—MKs—two’s company, three’s a crowd. Nat. Rev. Mol. Cell Biol. 2006;7:120–130. doi: 10.1038/nrm1834. [DOI] [PubMed] [Google Scholar]
- 6.Dunn KL, Espino PS, Drobic B, He S, Davie JR. The Ras-MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem. Cell Biol. 2005;83:1–14. doi: 10.1139/o04-121. [DOI] [PubMed] [Google Scholar]
- 7.Rincón M, Pedraza-Alva G. JNK and p38 MAP kinases in CD4+ and CD8+ T cells. Immunol. Rev. 2003;192:131–142. doi: 10.1034/j.1600-065x.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 8.Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, Gaestel M. MAPKAP kinase 2 is essential for LPS-induced TNF-α biosynthesis. Nat. Cell Biol. 1999;1:94–97. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- 9.Rincón M, Enslen H, Raingeaud J, Recht M, Zapton T, Su MS, Penix LA, Davis RJ, Flavell RA. Interferon-γ expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO J. 1998;17:2817–2829. doi: 10.1093/emboj/17.10.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotez PJ, Remme JH, Buss P, Alleyne G, Morel C, Breman JG. Combating tropical infectious diseases: report of the Disease Control Priorities in Developing Countries Project. Clin. Infect. Dis. 2004;38:871–878. doi: 10.1086/382077. [DOI] [PubMed] [Google Scholar]
- 11.Mattner F, Magram J, Ferrante J, Launois P, Di Padova K, Behin R, Gately MK, Louis JA, Alber G. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur. J. Immunol. 1996;26:1553–1559. doi: 10.1002/eji.1830260722. [DOI] [PubMed] [Google Scholar]
- 12.Heinzel FP, Schoenhaut DS, Rerko RM, Rosser LE, Gately MK. Recombinant interleukin 12 cures mice infected with Leishmania major. J. Exp. Med. 1993;177:1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Junghae M, Raynes JG. Activation of p38 mitogen-activated protein kinase attenuates Leishmania donovani infection in macrophages. Infect. Immun. 2002;70:5026–5035. doi: 10.1128/IAI.70.9.5026-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Edwards JP, Mosser DM. Dynamic and transient remodeling of the macrophage IL-10 promoter during transcription. J. Immunol. 2006;177:1282–1288. doi: 10.4049/jimmunol.177.2.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Z, Mosser DM, Zhang X. Activation of the MAPK, ERK, following Leishmania amazonensis infection of macrophages. J. Immunol. 2007;178:1077–1085. doi: 10.4049/jimmunol.178.2.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 17.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahtani KR, Brook M, Dean JL, Sully G, Saklatvala J, Clark AR. Mitogen-activated protein kinase p38 controls the expression and post-translational modification of tristetraprolin, a regulator of tumor necrosis factor α mRNA stability. Mol. Cell. Biol. 2001;21:6461–6469. doi: 10.1128/MCB.21.9.6461-6469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCluskie MJ, Krieg AM. Enhancement of infectious disease vaccines through TLR9-dependent recognition of CpG DNA. Curr. Top. Microbiol. Immunol. 2006;311:155–178. doi: 10.1007/3-540-32636-7_6. [DOI] [PubMed] [Google Scholar]
- 20.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 21.Rhee EG, Mendez S, Shah JA, Wu CY, Kirman JR, Turon TN, Davey DF, Davis H, Klinman DM, Coler RN, et al. Vaccination with heat-killed leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against Leishmania major infection. J. Exp. Med. 2002;195:1565–1573. doi: 10.1084/jem.20020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-κB recruitment. Nat. Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 23.Salmon RA, Guo X, Teh HS, Schrader JW. The p38 mitogen-activated protein kinases can have opposing roles in the antigen-dependent or endotoxin-stimulated production of IL-12 and IFN-γ. Eur. J. Immunol. 2001;31:3218–3227. doi: 10.1002/1521-4141(200111)31:11<3218::aid-immu3218>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Kim C, Sano Y, Todorova K, Carlson BA, Arpa L, Celada A, Lawrence T, Otsu K, Brissette JL, Arthur JS, Park JM. The kinase p38α serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat. Immunol. 2008;9:1019–1027. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang YJ, Chen J, Otsuka M, Mols J, Ren S, Wang Y, Han J. Macrophage deletion of p38α partially impairs lipopolysaccharide-induced cellular activation. J. Immunol. 2008;180:5075–5082. doi: 10.4049/jimmunol.180.7.5075. [DOI] [PubMed] [Google Scholar]
- 26.Gaestel M, Mengel A, Bothe U, Asadullah K. Protein kinases as small molecule inhibitor targets in inflammation. Curr. Med. Chem. 2007;14:2214–2234. doi: 10.2174/092986707781696636. [DOI] [PubMed] [Google Scholar]
- 27.Kotlyarov A, Gaestel M. Is MK2 (mitogen-activated protein kinase-activated protein kinase 2) the key for understanding post-transcriptional regulation of gene expression? Biochem. Soc. Trans. 2002;30:959–963. doi: 10.1042/bst0300959. [DOI] [PubMed] [Google Scholar]
- 28.Lu HT, Yang DD, Wysk M, Gatti E, Mellman I, Davis RJ, Flavell RA. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 1999;18:1845–1857. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu J, Wu X, Goel S, Gowda NM, Kumar S, Krishnegowda G, Mishra G, Weinberg R, Li G, Gaestel M, et al. MAPK-activated protein kinase 2 differentially regulates Plasmodium falciparum glycosylphosphatidylinositol-induced production of tumor necrosis factor-α and interleukin-12 in macrophages. J. Biol. Chem. 2009;284:15750–15761. doi: 10.1074/jbc.M901111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu JJ, Tripp CS, Russell JH. Regulation and phenotype of an innate Th1 cell: role of cytokines and the p38 kinase pathway. J. Immunol. 2003;171:6112–6118. doi: 10.4049/jimmunol.171.11.6112. [DOI] [PubMed] [Google Scholar]
- 31.Berenson LS, Yang J, Sleckman BP, Murphy TL, Murphy KM. Selective requirement of p38α MAPK in cytokine-dependent, but not antigen receptor-dependent, Th1 responses. J. Immunol. 2006;176:4616–4621. doi: 10.4049/jimmunol.176.8.4616. [DOI] [PubMed] [Google Scholar]
- 32.Salvador JM, Mittelstadt PR, Belova GI, Fornace AJ, Jr., Ashwell JD. The autoimmune suppressor Gadd45α inhibits the T cell alternative p38 activation pathway. Nat. Immunol. 2005;6:396–402. doi: 10.1038/ni1176. [DOI] [PubMed] [Google Scholar]
- 33.Jirmanova L, Jankovic D, Fornace AJ, Jr., Ashwell JD. Gadd45α regulates p38-dependent dendritic cell cytokine production and Th1 differentiation. J. Immunol. 2007;178:4153–4158. doi: 10.4049/jimmunol.178.7.4153. [DOI] [PubMed] [Google Scholar]
- 34.Sun L, Stoecklin G, Van Way S, Hinkovska-Galcheva V, Guo RF, Anderson P, Shanley TP. Tristetraprolin (TTP)-14-3-3 complex formation protects TTP from dephosphorylation by protein phosphatase 2a and stabilizes tumor necrosis factor-α mRNA. J. Biol. Chem. 2007;282:3766–3777. doi: 10.1074/jbc.M607347200. [DOI] [PubMed] [Google Scholar]
- 35.Matsushita K, Takeuchi O, Standley DM, Kumagai Y, Kawagoe T, Miyake T, Satoh T, Kato H, Tsujimura T, Nakamura H, Akira S. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature. 2009;458:1185–1190. doi: 10.1038/nature07924. [DOI] [PubMed] [Google Scholar]
- 36.Konecny P, Stagg AJ, Jebbari H, English N, Davidson RN, Knight SC. Murine dendritic cells internalize Leishmania major promastigotes, produce IL-12 p40 and stimulate primary T cell proliferation in vitro. Eur. J. Immunol. 1999;29:1803–1811. doi: 10.1002/(SICI)1521-4141(199906)29:06<1803::AID-IMMU1803>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 37.von Stebut E, Belkaid Y, Jakob T, Sacks DL, Udey MC. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J. Exp. Med. 1998;188:1547–1552. doi: 10.1084/jem.188.8.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doherty TM, Coffman RL. Ability of macrophage subsets to transfer resistance to murine leishmaniasis is dependent on IL-12 production. Eur. J. Immunol. 1999;29:522–529. doi: 10.1002/(SICI)1521-4141(199902)29:02<522::AID-IMMU522>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Wang L, Zhao Y, Wang Y, Wang Z, Qiao Z. Testosterone attenuates p38 MAPK pathway during Leishmania donovani infection of macrophages. Parasitol. Res. 2006;99:189–193. doi: 10.1007/s00436-006-0168-1. [DOI] [PubMed] [Google Scholar]
- 40.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat. Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]