Abstract
This study used eye-tracking to examine how 20-month old toddlers with autism spectrum disorder (ASD) (N=28), typical development (TD) (N=34), and non-autistic developmental delays (DD) (N=16) monitored the activities occurring in a context of an adult-child play interaction. Toddlers with ASD, in comparison to control groups, showed less attention to the activities of others and focused more on background objects (e.g. toys). In addition, while all groups spent the same time overall looking at people, toddlers with ASD looked less at people's heads and more at their bodies. In ASD, these patterns were associated with cognitive deficits and greater autism severity. These results suggest that the monitoring of the social activities of others is disrupted early in the developmental progression of autism, limiting future avenues for observational learning.
Keywords: activity monitoring, autism, eye-tracking, joint attention, social learning, observational learning
1. Introduction
Social and communicative difficulties, stereotyped behaviors, and restricted interests lie at the core of autism spectrum disorders (ASDs) (American Psychiatric Association, 2000). As our understanding of autism has increased, however, so too has our appreciation of its heterogeneity (Happé et al., 2006; Szatmari, 1999; Trikalinos et al., 2005). Some have argued that, in order to understand the complex genetic and epigenetic relationships in ASD, it is necessary to consider autism not as a reflection of singular deviations in specific functional cognitive or social modules, but as the emergent and recurrent property of atypical preferences, percepts, learning, and experience (Jones and Klin, 2009; Karmiloff-Smith, 2007; Klin et al., 2003; Johnson et al., 2005). In this study we examine how toddlers with ASD perceive and monitor people engaged in a shared activity. This simple act of activity monitoring is an expression not only of a person's experience-dependent understanding of the scene, but also provides access to new experiences as actions unfold. Thus activity monitoring may be related to both cause and consequence of atypical social and cognitive development in individuals with ASD.
In typical development, the ability to understand intentional and goal directed actions of others arises early in infancy (e.g. Baldwin et al., 2001; Biro and Leslie, 2007; Falck-Ytter et al., 2006; Woodward, 1998, 1999; for reviews see Aschersleben, 2006; Tomasello et al., 2005). However, less attention has been paid towards the relative salience of actions as compared to other salient constructs in ecological contexts. Amongst the exceptions is the work of Bahrick and colleagues (2000; 2008) who showed that when 5 ½ month old infants are presented with videos of people performing everyday tasks, such as brushing their teeth, actions are prioritized for memory over both the identities of the people and the objects those people employ. Furthermore, it is only when the presentation time of the scenes is extended, or the infants are older, that memory for faces and actions is achieved simultaneously (Bahrick and Newell, 2008). These results imply that when the attentional resources are constrained, even faces, one of the most privileged socially-relevant objects (Cohen Kadosh and Johnson, 2007; Farah et al., 1998; Haan et al., 2002; Halit et al., 2003; Hershler and Hochstein, 2005; Valenza et al., 1996), ultimately lose to actions.
Attention to the actions and activities of others is also a critical component of the learning and development of cognitive and social skills. For example, attention to others and their actions facilitates learning about affordances (Gibson, 1988; Huang and Charman, 2005; Loveland, 1991; Meltzoff, 1995), is a requisite for imitation and emulation (Abravanel et al., 1976; Carpenter, 2006; Heyes, 2001; Meltzoff and Moore, 1977; Tomasello, 1996; Want and Harris, 2002), and is crucial to the development of higher-level cognitive skills such as joint attention, social play, and the comprehension of intentions, goals, and motivations (Bakeman and Adamson, 1984; Carpenter et al., 1998; Moore and Dunham, 1995). The fact that skills such as affordance learning, imitation, and joint attention emerge in a regular fashion (e.g. see Carpenter et al., 1998; Trevarthen & Aitken, 2001), together with their relationships with later development of language and theory of mind skills (e.g. see Charman et al., 2000), argue for mutual interdependencies and suggest that common requirements, such as activity monitoring, may evolve together with the skills themselves.
Many of the skills outlined above have been found to be impaired in autism spectrum disorders. Children with ASD have been shown to use objects in an atypical manner, for example by spinning coins, shaking toy cars, or using a sock as a container (Bruckner and Yoder, 2007; Ozonoff et al., 2007). These unusual object manipulations may indicate self-stimulatory or regulatory behavior (Turner, 1997, 1999; Whitman, 2004); however, as noted by Loveland (1991), such behaviors might also indicate that they have not discovered the culturally appropriate affordances of objects via typical observation of adults and peers. In this case, attending to the behaviors of others would be a requisite to learning about those socially agreed upon conventions. Studies have also found deficits in imitation in ASD (Charman et al., 1997; Colombi et al., 2009; Rogers, Hepburn, Stackhouse, & Wehner, 2003; Vivanti, Nadig, Ozonoff, & Rogers, 2008; for reviews see Williams, Whiten, & Singh, 2004; Rogers & Williams, 2006). For example, Vivanti and colleagues (2008) showed that high-functioning children with autism were less precise in imitation than controls. Furthermore, greater attention to actions in children with autism corresponded to better imitation of certain types of gestures. Through eye-tracking, the authors were able to differentiate between attention to the actor, background, and the act itself, bringing into focus the possibility that a seemingly similar overall engagement in an experimental task may be comprised of very different internal patterns of selective attention. Finally, systematic deficits observed in joint attention suggest that reduced attention to the attentional focus of others may be a particularly striking characteristic of ASD (Bono et al., 2004; Bruinsma et al., 2004; Charman, 2003; Charman et al., 1997; Dawson et al., 2004; Hecke et al., 2007; Leekam et al., 2000; Leekam and Ramsden, 2006; Mundy and Vaughan, 2002; Mundy et al., 1990; Sullivan et al., 2007). Taken as a whole, these studies suggest that activity monitoring, a component of all these skills, may be affected by the developmental progression of the autistic syndrome.
In this study we examine to what extent toddlers with ASD attend to the activities of others as compared to chronologically matched typically developing (TD) toddlers and chronologically and mental-age matched toddlers with developmental delays (DD). Traditionally, in studies of phenomena such as joint attention and imitation, the child is explicitly included as an active participant in the ongoing social exchange. By contrast, in this study, we examine the gaze response of children to the activities of others during natural viewing. Also in contrast to other studies, there is no attempt to actively engage the child's attention socially at the onset of the experiment (e.g. through infant-directed motherese or direct gaze), there are no predefined instructions to the subjects, and the study is conducted via presentation of a naturalistic play interaction. The study targets toddlers at 20 months of age, the earliest age at which a stable diagnosis of ASD can be obtained (Chawarska et al., 2007), employing an ecologically valid paradigm in terms of what children may naturally encounter at any age.
Based on the extant literature, we hypothesize that that toddlers with ASD will spend less time attending to the actors of the scene and the area of shared activity. Instead, we expect they will spend more time looking at toys and objects in the background. Finally, given hypothesized relationships between social functioning and visual scanning patterns in ASD (Anderson et al., 2006; Jones et al., 2008; Klin et al., 2002; Speer et al., 2007; Chawarska and Shic, 2009), we expect that deviations from prototypical scanning behavior will correlate with measures of social deficits and impaired cognitive functioning in toddlers with ASD.
2. Results
To examine if the groups included into the study differed in their overall level of attention, we compared the toddlers on the total time spent looking at the movie. There were no between group differences: on average, toddlers with ASD viewed the scene for 23.5 s (SD = 5.6), the DD group for 24.6 s (SD = 5.1), and the TD group for 26.2 s (SD = 5.0) (p>.13 for all pairwise group comparisons).
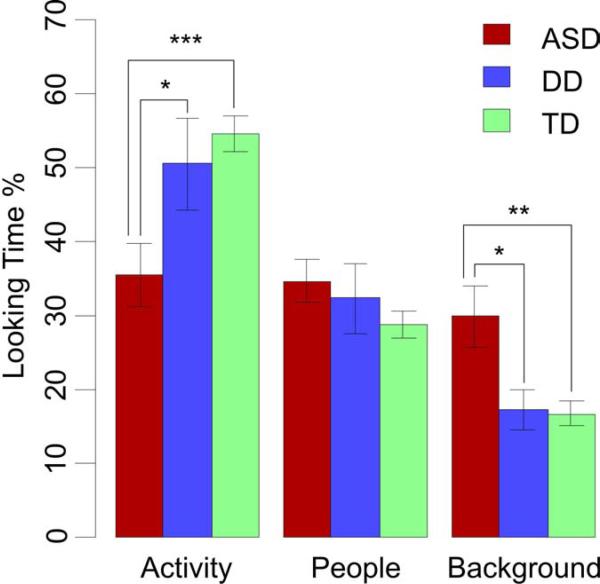
To examine overall differences in scanning patterns between groups, a between-group ANOVA was performed on the proportion of time spent looking at each ROI (Table 2). This analysis indicated group differences for Activity and Background but not for People (Figure 2). Planned contrasts showed that toddlers with ASD attended less to the Activity area and more to Background areas than DD or TD controls.
Table 2.
Percentage of Time Spent Looking at Regions of Interest (ROIs) in 20-month old Toddlers.
| Looking Time, Mean (SD), % of Total | Pairwise p valuea | |||||||
|---|---|---|---|---|---|---|---|---|
| Region | ASD | TD | DD | F2,77 | p value | ASD vs TD | ASD vs DD | TD vs DD |
| Activity | 35.5 (21.8) | 54.6 (13.8) | 50.5 (24.7) | 7.79 | .001 | .001*** | .032* | .486 |
| Background | 29.8 (20.9) | 16.7 (9.6) | 17.2 (10.6) | 6.90 | .002 | .003** | .016* | .911 |
| People | 34.7 (14.7) | 28.7 (10.2) | 32.3 (18.8) | 1.44 | .243 | .290 | .786 | .786 |
| Looking Time, Mean (SD) % of People | ||||||||
|---|---|---|---|---|---|---|---|---|
| Head | 49.4 (25.6) | 64.1 (22.6) | 65.2 (15.5) | 4.04 | .021 | .036* | .056~ | .871 |
| Body | 50.6 (25.6) | 35.9 (22.6) | 34.8 (15.5) | “ | “ | “ | “ | “ |
| Looking Time, Mean (SD) % of People | ||||||||
|---|---|---|---|---|---|---|---|---|
| Child | 46.0 (20.3) | 45.9 (19.2) | 56.7 (18.9) | 1.94 | .151 | .980 | .214 | .214 |
| Adult | 54.0 (20.3) | 54.1 (19.2) | 43.3 (18.9) | “ | “ | “ | “ | “ |
Holm-Bonferroni correction for 3 comparisons
p<.001
p<.01
p<.05
p<.10
Figure 2.
Proportion of time spent examining the Activity, People, and Background regions of interest for ASD, DD, and TD children. * p < .05; ** p < .01. Error bars are ±1 SE.
We also considered a more fine-grained examination of looking at People. A between-group ANOVA followed by planned contrasts showed that although the overall level of attention to People was not different between groups, when looking at People, the ASD group attended more to Body areas and less to the Head of characters as compared to TD toddlers. We also compared whether the proportion of attention to the People region varied by person identity. However, no between-group differences were observed in attention towards the Adult or the Child in the scene.
To better understand the relationships between activity monitoring and clinical features of toddlers with ASD, we examined correlations between dependent measures obtained via eye-tracking and measures of social disability (ADOS-G) and cognitive performance (MSEL) (Table 3). Greater difficulties with Social Affect were associated with increased attention to the Background and decreased attention to the Activity, Heads, and the scene as a whole (decreased total time looking at the scene). Lower levels of both verbal and nonverbal mental age (VMA and NVMA, respectively) were associated with increased attention to People and decreased attention to the Activity. A multiple regression on attention to the Activity and People was conducted to disentangle the effects of Social Affect and NVMA and indicated that for attention to the Activity both Social Affect (β=-.39, p<.05) and NVMA (β =.54, p<.001) contributed, but that attention towards People was primarily driven by NVMA (β=-.58, p<.01) as compared to Social Affect (β=.03). An examination of correlations in the DD group indicated that attention towards the Activity was likewise modulated by NVMA (r=.53, p<.05) but not VMA (r=.21) or Social Affect (r=-.10); attention towards People was modulated by NVMA (r=-.60, p<. 05).
Table 3.
Correlations between dependent measures of scene scanning and social and cognitive functioning in ASD
| Social Disability1 | Cognitive Functioning2 | |||
|---|---|---|---|---|
| Eye-tracking Measure | SA | SRB | NVMA | VMA |
| Total time | -.480** | -.328 | .226 | .049 |
| Activity | -.403* | -.230 | .557** | .570** |
| Background | .391* | .357 | -.174 | -.212 |
| People | .041 | -.168 | -.581** | -.545** |
| Heads | -.432* | -.009 | .153 | .234 |
Autism Diagnostic Observation schedule – Generic (ADOS-G) Module 1 (Lord et al., 2002), N=28
Mullen Scales of Early Learning (MSEL) (Mullen, 1995), N=27
SA: Social Affect, SRB: Stereotyped & Repetitive Behavior; NVMA: Non-verbal Mental Age; VMA: Verbal Mental Age
p<.05
p<.01
3. Discussion
While a vast majority of studies of social perception in young children with autism have focused on attention to faces and facial cues, our study examined the ability of these children to attend to the shared activities of others. This is important because attending to what others do is the critical first step in understanding what they do: a deficit at this stage limits further learning, potentially reducing the relevance of others’ activities to the observer and consequently depressing the salience of those activities in the future. The results show that 20 month-old toddlers with ASD attend less to the activities of others than typically developing or developmentally delayed toddlers, diverting their attention to elements of the background. This phenomenon appears to be specific to toddlers with ASD, as matched for CA and MA toddlers with developmental delays showed patters of attention similar to that observed in typically developing toddlers.
While in toddlers with ASD spent a similar amount of time looking at People as their comparison groups, a closer examination of the constituents of People-looking suggest that, similarly as in the Klin et al. (2002) study, toddlers with ASD attended more to Bodies and less to Heads. Diminished looking at Heads was also linked to increased difficulties in social-communicative function in ASD, suggesting that while overall attention to People as a whole was not different between groups, specific internal patterns of scanning the characters of the scene were predictive of social function. Of particular interest is whether looking at Heads could be further decomposed into looking at Eyes versus looking at Mouths. Previous work has suggested that toddlers with ASD attend more to mouths than eyes in comparison to control groups when viewing dynamic videos of actresses emulating dyadic interactions (Jones et al., 2008; though see Merin et al., 2007 and Young et al., 2009 for additional perspectives). In the current study, eye-looking versus mouth-looking was not a hypothesis and the face region was fairly small (approximately 1.5 × 2.5 visual degrees), and thus the breakdown of eye versus mouth was not attempted. Given the fundamental importance of internal face scanning strategies to the processing and recognition of faces (Chawarska and Shic, 2009), however, future studies will be conducted with this analysis in mind. Nonetheless, the overall lack of differences between groups in looking at People highlights the complex and often subtle interactions between different competitors for visual attention. It is possible that, in the absence of the activity in the scene, between-group differences for looking at People would be more pronounced, as would specific differences in looking at Head and Body regions.
Several, not necessarily exclusive, hypotheses could be advanced to explain decreased activity monitoring in toddlers with ASD. First, this deficit might be associated with atypical processing of perceptual aspects of the scene such as contrast and motion. A number of studies in older individuals suggest that low-level perception may be altered in ASD including enhanced sensitivity to spatial contrast (Bertone et al., 2005; McCleery et al., 2007; Sanchez-Marin and Padilla-Medina, 2008; Shic et al., 2007; though see Koh et al., 2010; for reviews see Mottron et al., 2006; Simmons et al., 2009). In this context, the decreased attention to activities may be secondary to increased preference for objects with certain perceptual characteristics within the scene (e.g. see Sasson, Turner-Brown, Holtzclaw, Lam, & Bodfish, 2008; Zwaigenbaum et al., 2005). Moreover, decreased sensitivity to and/or preference for biological motion has been documented both in toddlers (Klin et al., 2009) and older individuals with ASD (Bertone et al., 2003; Blake et al., 2003; Milne et al., 2002; Shic et al., 2007). A deficit in this area could help to explain the unequal distribution between groups to background elements as compared to activity area. This type of deficit could be detrimental to the child's development on several levels. Cues derived from biological motion are essential for regulating attention in early development. In fact, studies of typical development suggest that parents often demonstrate actions to infants and toddlers using exaggerated motions termed “motionese” (Brand et al., 2002; Brand and Shallcross, 2008). An early limited sensitivity to such biological kinetic cues could make it more difficult to process this didactic form of scaffolding and communication. The effects of atypical salience of certain features of the visual field could be further compounded by difficulties in executive functioning in ASD. Executive control functions are subserved by a distributed neural system (Newman et al., 2003; for a review, see Collette et al., 2006) which appear to be atypically activated and functionally underconnected in ASD (e.g. see Just et al., 2007; Kana et al., 2007; Shafritz et al., 2008). Deficits in this area may be at least partially responsible for inability to inhibit the draw of highly perceptually salient but irrelevant to a task at hand elements of the visual scene.
The second set of hypotheses draws upon a potential association between activity monitoring and the ability to understand various aspects of the scene under consideration. According to the moderate discrepancy hypothesis (McCall and McGhee, 1977) children will attend to those aspects of the environment that are only slightly outside their ability to comprehend, i.e. a child will preferentially attend to stimuli which are neither too simple, given his or her capabilities, nor too complex. The decreased attention to activities exhibited by toddlers with ASD with greater cognitive deficits and the similarity of these associations with toddlers with DD suggests that the salience of the shared activity might indeed be affected by the ability of the individuals with ASD to comprehend the significance of this type of interaction. However, it is unlikely that cognitive level of functioning alone would explain the phenomenon, as toddlers with ASD monitor the activity of others less frequently than MA- and CA- matched toddlers with developmental delays. As the primary characteristic differentiating ASD and DD groups is the level of autism symptomatology, most likely the activity monitoring in ASD is gated by social functioning, i.e. attending to the activities of others is a reflection of the salience assigned towards social aspects of a visual scene. This view is supported by the inverse relationship found between social deficits and the overall amount of time spent looking at the scene: the toddlers who exhibit the greatest degree of social deficits also look least at the scene as a whole, and, when they do, look least at the activity. Furthermore, those ASD toddlers who were excluded from analysis due to poor attention to the task were more impaired socially than the included toddlers, suggesting that the effects observed might represent the “tip of the iceberg” in relationship to the most severely impaired toddlers with ASD.
Finally, the observed deficit might be a manifestation of disturbed social development early in infancy. By 18 months of age TD infants show reliable coordinated joint attention with caregivers and accurate decoding of the target of gaze (Bakeman and Adamson, 1984; Butterworth and Jarrett, 1991; Carpenter et al., 1998). It is possible that in TD toddlers this sensitivity to the attentional focus of others bolsters the saliency of the shared activity area and, by contrast, limited joint attention skills result in toddlers with ASD having a limited appreciation for the significance of the shared focus of others. In typical development, age-related changes in joint attention behavior are supported by neurodevelopmental mechanisms that evolve throughout infancy (e.g. see Hoehl et al., 2008; Mundy et al., 2000, 2003; Striano et al., 2006; for reviews see Grossmann and Johnson, 2007; Hari and Kujala, 2009; Itier and Batty, 2009; Nummenmaa and Calder, 2009). By contrast, in individuals with ASD, brain activity in response to gaze appears abnormal or delayed (e.g. see Grice et al., 2005; Kylliäinen and Hietanen, 2006; Senju et al., 2005; for a review see Senju and Johnson, 2009). Thus, it is plausible that a combination of atypical neural activation by social stimuli coupled with the altered development of perceptual and cognitive sensitivity to actions leads to widespread depression of a network involved in processing, understanding, and attributing relevance to the activities of others. Though our study was not designed to directly disambiguate amongst these possibilities, future modifications of our paradigm, in conjunction with neuroimaging and electrophysiological techniques, will help clarify the contributors to our observed trends.
The current study has several limitations. First, the developmentally delayed group is small relative to the other two groups, limiting the statistical power of comparisons between DD and ASD groups. Second, the stimulus shown represents only a subset of possible shared activities, and further studies will need to consider the specific impact of particular content and context on the scanning patterns of toddlers. Third, the relationship of our results to eventual outcome is not yet known, and this information will be crucial in placing activity monitoring into the larger ecology of eye-tracking work on infants and toddlers with ASD.
This study demonstrates that prototypic attention towards shared activities is disrupted in ASD by 20 months of age. Though likely a reflection of an ongoing atypical developmental process, it is important to realize that this disruption, at an age where rapid development and skill acquisition is occurring in TD toddlers, may critically impact the content of their social experiences, specifically, and learning via observation, in general. A simple scene, such as a play activity between two individuals, necessarily entails a complex dynamic of turn-taking, synchronized verbal and non-verbal communication, and shared attention. To a typically developing infant, these ebbs and flows of the social milieu are fundamental, and, one might argue, instinctual—for the next step after a period of observing others play, after the rules have been comprehended, and the actors understood, is to join in on the exchange. This cycle of passive observation and active participation builds a foundation by which later social skills may be acquired, assembled, comprehended, and interpreted. The cycle also builds a common history, a point of unification by which the attention of typically developing toddlers may be directed. By comparison, it appears that toddlers with ASD are not engaged with activities to the same extent; they thus are limited in their exposure to this common experience, and this may impact the later scaffolding of their social development. It is an open question to what extent this atypical trajectory can be changed; however, enhancing attention early in development not only to other people but also to their activities may open new avenues for intervening and fostering the development of key cognitive and social skills.
4. Experimental Procedure
Participants
Three groups of 20-month old toddlers (N=78) were recruited for this study: toddlers with autism spectrum disorder (ASD) (N = 28), typically developing (TD) toddlers (N = 34), and toddlers displaying developmental delays (DD) but who did not meet criteria for ASD (N=16) (Table 1). Classification of developmental status was determined by clinicians on the basis of a review of medical and developmental history, diagnostic tests (Autism Diagnostic Observation schedule – Generic (ADOS-G) Module 1) (Lord et al., 2002), and developmental tests (Mullen Scales of Early Learning (MSEL)) (Mullen, 1995). Previous work in a similar setting and age range has shown the stability of the broadly-defined diagnosis of ASD to be excellent (Chawarska et al., 2007, 2009). The ASD group was comprised of 20 toddlers diagnosed with autistic disorder and 8 toddlers diagnosed with pervasive developmental disorder - not otherwise specified (PDD-NOS) (American Psychiatric Association, 2000). The DD group included subjects who presented with global delays or language impairments based on clinical judgment and performance profiles on the same battery of tests as used in the ASD sample. Four subjects in the DD group were siblings of children previously diagnosed with ASD who exhibited language delays but no ASD features; their inclusion did not alter the significance of any of the reported findings. The status of TD toddlers was confirmed by direct observation of play and interaction skills, assessment of nonverbal cognitive skills, and medical and developmental history. None of the TD toddlers had a history of ASD in 1st or 2nd degree relatives. All toddlers in the study were born after 32 weeks gestation, suffered no major prenatal or perinatal insults, had no known visual or auditory abnormalities, and had no history of medical conditions associated with autism (e.g. tuberous sclerosis or fragile × syndrome) or any other identified genetic disorder.
Table 1.
Sample Characterization (means and standard deviations)
| Measure | ASD | DD | TD |
|---|---|---|---|
| N | 28 | 16 | 34 |
| Chronological age [months] | 20.7 (3.0) | 19.3 (2.6) | 19.6 (2.8) |
| Male : Female | 22:6 | 10:6 | 22:12 |
| Nonverbal MA (NVMA) [months]1 | 18.4 (4.1)a | 18.0 (4.2)a | 21.0 (2.9)b |
| Nonverbal Developmental Quotient (NVDQ)1 | 89.9 (17.3)a | 93.7 (19.4)a | 107.5 (10.8)b |
| Verbal MA (VMA) [months]1,2 | 12.1 (5.9)a | 12.5 (4.4)a | 21.0 (4.4)b |
| Verbal Developmental Quotient (VDQ)1,2 | 59.7 (28.5)a | 64.9 (22.1)a | 106.7 (18.9)b |
| Social affect (SA)3 | 13.3 (4.3)a | 7.9 (3.6)b | - |
| Stereotyped & repetitive behaviors (SRB)3 | 4.1 (2.1)a | 1.6 (1.3)b | - |
| ADOS Total3 | 17.4 (5.6)a | 9.6 (4.1)b | - |
One child in the ASD group was not administered the MSEL
Two children in the TD group were not administered verbal components of the MSEL
Two children in the DD group were not administered the ADOS
Different superscripts indicate significantly different groups, p < .05
Different superscripts indicate significantly different groups, p < .05
All three groups were matched on chronological age (see Table 1 for sample characterization). ASD and DD groups were also matched on nonverbal and verbal mental age (NVMA and VMA, respectively) and developmental quotient (NVDQ and VDQ); as expected, the TD group had higher verbal and nonverbal skills. The ASD group exhibited greater deficits than the DD group on the ADOS-G in both Social Affect and Stereotyped and Repetitive Behaviors domains.
An additional 20 subjects were tested but not included in this study. One child's data (ASD: n=1) was not recorded due to technical problems. The remaining 19 toddlers were excluded due to non-optimal arousal state (upset or falling asleep; ASD: n=2, DD: n=2, TD: n=2), inattention (ASD: n=5, TD: n=3), or a high activity level resulting in failed calibration (ASD: n=1, DD: n=1, TD: n=3). Toddlers with ASD excluded for reasons other than technical problems (n=8), as compared to included toddlers with ASD (n=28), exhibited more severe autism symptoms (excluded group's ADOS total: M=21.9, SD=4.1; included: M=17.4, SD=5.6; t (34) = 2.1, p < .05) but did not differ in terms of mental age.
Apparatus and Stimuli
Apparatus
Gaze patterns were recorded with a SMI iView X™ RED dark-pupil 60Hz eye-tracking system (Sensomotoric Instruments, 2005). Data were processed using custom software written in MATLAB™ (MathWorks, 2009), which provided standard processing of eye-tracking data including blink detection, outlier detection, eye-tracking calibration and recalibration, measurements of experimental error, and region-of-interest analysis (Duchowski, 2003; Shic, 2008). Statistical analyses were accomplished through software written in Perl (ActiveState, 2009), SPSS (SPSS, Inc., 2006), and R (R Development Core Team, 2009).
Stimuli
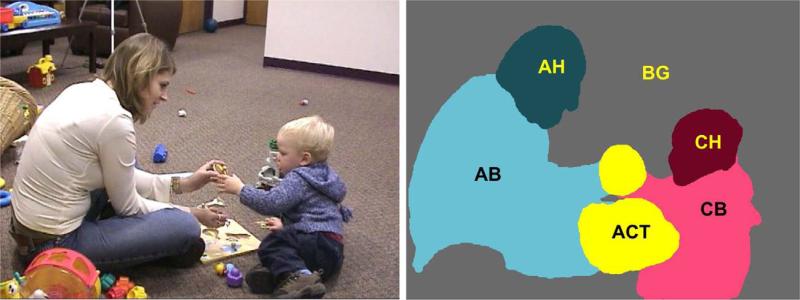
The stimulus used in this study was a 30-second video of a female adult and a male toddler playing with an inset puzzle (Figure 1). The scene was extracted from an unscripted observational video and thus included natural referencing (e.g. the adult pointing to a slot on the puzzle) and vocalizations (e.g., the adult saying “Good!”). Toys were strewn about in the background; furniture, walls, and the doors were clearly evident. The displayed video was 800 × 600 pixels in size and displayed at a screen resolution of 1280 × 800 pixels, occupying an area 24.5 × 18.4 visual degrees at the center of a 24” wide-screen LCD presentation monitor when viewed from a distance of 75 cm. Sound for the video was emitted via a stereo sound bar attached to the monitor. The experimental task was programmed and displayed using the software Presentation® (Neurobehavioral Systems, 2006).
Figure 1.
An example frame from the start of the video stimulus (left) and the corresponding regions of interest (ROIs) (right). Regions are Activity (ACT; area of characters’ shared focus), Adult Head (AH), Adult Body (AB), Child Head (CH), Child Body (CB), and Background (BG; comprised of toys and room elements).
Procedure
Toddlers were seated in a car seat in a dark and soundproof room 75 cm in front of a 24” widescreen LCD monitor positioned so that their eyes were aligned with the center of the monitor. Except for the monitor and eye-tracking cameras, the room was covered in dark cloth, thus providing little or no visual distractions to the toddlers. The toddlers's parent sat 6 feet behind the child and the experimenter operating the experiment and eye-tracker was separated from the child by a curtain.
Experiments began with the presentation of children's videos to help put the toddlers at ease and to provide an opportunity for optimization of data acquisition by the eye-tracker. This was followed by a 5-point eye-tracking calibration procedure with targets consisting of small animated figures (radius 1 visual degree) presented together with contingent sound. Subsequent to calibration, the target activity monitoring video was presented for 30s.
Data Reduction
Standard region-of-interest (ROI) analysis techniques were adapted for the analysis of gaze patterns (Figure 1). The ROIs examined in the primary analysis were Background areas (toys and room elements of the scene such as walls, furniture, and the floor), People (the adult and child), and the Activity area (the focus of shared attention by the adult and child). In a secondary analysis, attention towards People was further decomposed into attention towards the Head and Body as well as the Child and Adult. Regions were overdrawn by 0.5 visual degrees in order to compensate for calibration drift. The video stimulus was designed such that no major movements of ROIs occurred. However, to accommodate the relatively minor motions that did occur (e.g. movements of hands while arranging puzzle pieces, head turns towards a person to respond to or initiate a verbal exchange), ROIs were adjusted every second.
Analytic Strategy
. In the primary analysis, we considered patterns of scanning over the entire scene. Dependent variables in this analysis were the total time spent looking at the scene and the percentage of total time spent looking at specific ROIs (Activity, Background, and People). In a secondary analysis, we considered patterns of scanning specific to looking at People. Dependent variables in this analysis were the time spent looking at Head and Body areas as well as the Child and the Adult as a percentage of time spent looking at People. Hypotheses regarding between-group differences were tested using an analysis of variance approach with a Holm-Bonferroni correction for multiple comparisons (Aickin and Gensler, 1996; Hochberg, 1988; Holm, 1979). Relationships between scanning patterns and cognitive and social functioning were tested using Pearson product-moment correlation analysis.
Acknowledgements
The study was supported by NIMH grant T32 MH18268 (to FS), P50 MH 081756 (Autism Centers of Excellence) project 2 (PI: KC), NICHD P01 HD 003008 Project 1 (PI: KC), Autism Speaks and the NAAR foundation (to KC), and the National Science Foundation CDI award #0835767 (PIs: KC & BS). We would like to thank Suzanne Macari for her insights regarding the subjects and details of this work; Warren Jones for his help in initial conceptualization of experiments from which this project grew; Amanda Mossman and Tina Goldsmith for their contribution to subject characterization; Marika Coffman, Mairin Melvedt, Jessica Reed, Brittany Butler, Rebecca Doggett, Paula Ogston, and Joslin Latz for obtaining data of the highest quality; Lindsey Szauter for her assistance in analysis; and the children and families, without whom this study and others would not be possible.
A preliminary version of this study was presented at the 8th Annual International Meeting for Autism Research (IMFAR) in Chicago, Illinois, USA, May, 2009.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Abravanel E, Levan-Goldschmidt E, Stevenson MB. Action Imitation: The Early Phase of Infancy. Child Development. 1976;47:1032–1044. [PubMed] [Google Scholar]
- ActiveState ActivePerl (Version 5.10) [Computer Software] 2009 Available at: http://www.activestate.com/activeperl/
- Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. American Journal of Public Health. 1996;86:726–728. doi: 10.2105/ajph.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth ed., text revision American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Anderson CJ, Colombo J, Shaddy DJ. Visual Scanning and Pupillary Responses in Young Children with Autism Spectrum Disorder. Journal of Clinical and Experimental Neuropsychology. 2006;28:1238–1256. doi: 10.1080/13803390500376790. [DOI] [PubMed] [Google Scholar]
- Aschersleben G. Early development of action control. Psychology Science. 2006;48:405. [Google Scholar]
- Bahrick LE, Lickliter R. Intersensory redundancy guides attentional selectivity and perceptual learning in infancy. Developmental Psychology. 2000;36:190–201. doi: 10.1037//0012-1649.36.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrick LE, Newell LC. Infant discrimination of faces in naturalistic events: actions are more salient than faces. Dev Psychol. 2008;44:983–96. doi: 10.1037/0012-1649.44.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakeman R, Adamson LB. Coordinating Attention to People and Objects in Mother-Infant and Peer-Infant Interaction. Child Development. 1984;55:1278–1289. [PubMed] [Google Scholar]
- Baldwin DA, Baird JA, Saylor MM, Clark MA. Infants parse dynamic action. Child Development. 2001:708–717. doi: 10.1111/1467-8624.00310. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain. 2005;128:2430–2441. doi: 10.1093/brain/awh561. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Motion Perception in Autism: A “Complex” Issue. Journal of Cognitive Neuroscience. 2003;15:218–225. doi: 10.1162/089892903321208150. [DOI] [PubMed] [Google Scholar]
- Biro S, Leslie AM. Infants’ perception of goal-directed actions: development through cue-based bootstrapping. Developmental Science. 2007;10:379–398. doi: 10.1111/j.1467-7687.2006.00544.x. [DOI] [PubMed] [Google Scholar]
- Blake R, Turner LM, Smoski MJ, Pozdol SL, Stone WL. Visual Recognition of Biological Motion Is Impaired in Children with Autism. Psychological Science. 2003;14:151–157. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- Bono MA, Daley T, Sigman M. Relations Among Joint Attention, Amount of Intervention and Language Gain in Autism. Journal of Autism and Developmental Disorders. 2004;34:495–505. doi: 10.1007/s10803-004-2545-x. [DOI] [PubMed] [Google Scholar]
- Brand RJ, Baldwin DA, Ashburn LA. Evidence for ‘motionese’: modifications in mothers’ infant-directed action. Developmental Science. 2002;5:72–83. [Google Scholar]
- Brand RJ, Shallcross WL. Infants prefer motionese to adult-directed action. Developmental Science. 2008;11:853–861. doi: 10.1111/j.1467-7687.2008.00734.x. [DOI] [PubMed] [Google Scholar]
- Bruckner CT, Yoder P. Restricted object use in young children with autism: Definition and construct validity. Autism. 2007;11:161–171. doi: 10.1177/1362361307075709. [DOI] [PubMed] [Google Scholar]
- Bruinsma Y, Koegel Robert L., Koegel Lynn Kern. Joint attention and children with autism: A review of the literature. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:169–175. doi: 10.1002/mrdd.20036. [DOI] [PubMed] [Google Scholar]
- Butterworth G, Jarrett N. What minds have in common is space: Spatial mechanisms serving joint visual attention in infancy. British Journal of Developmental Psychology. 1991;9:55–72. [Google Scholar]
- Carpenter M. Instrumental, Social, and Shared Goals and Intentions in Imitation. In: Rogers SJ, Williams JHG, editors. Imitation and the social mind: Autism and typical development. Guilford Press; New York: 2006. pp. 48–70. [Google Scholar]
- Carpenter M, Nagell K, Tomasello M. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monogr Soc Res Child Dev. 1998;63:i–vi. 1–143. [PubMed] [Google Scholar]
- Charman T. Why is joint attention a pivotal skill in autism? Philosophical Transactions: Biological Sciences. 2003;358:315–324. doi: 10.1098/rstb.2002.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman T, Baron-Cohen S, Swettenham J, Baird G, Cox A, Drew A. Testing joint attention, imitation, and play as infancy precursors to language and theory of mind. Cognitive Development. 2000;15:481–498. [Google Scholar]
- Charman T, Swettenham J, Baron-Cohen S, Cox A, Baird G, Drew A. Infants With Autism: An Investigation of Empathy, Pretend Play, Joint Attention, and Imitation. Developmental Psychology. 1997;33:781–789. doi: 10.1037//0012-1649.33.5.781. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Macari S, Volkmar F. A prospective study of toddlers with ASD: short-term diagnostic and cognitive outcomes. Journal of Child Psychology and Psychiatry. 2009;50:1235–1245. doi: 10.1111/j.1469-7610.2009.02101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Volkmar F. Autism spectrum disorder in the second year: stability and change in syndrome expression. Journal of Child Psychology and Psychiatry. 2007;48:128–138. doi: 10.1111/j.1469-7610.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Shic F. Looking But Not Seeing: Atypical Visual Scanning and Recognition of Faces in 2 and 4-Year-Old Children with Autism Spectrum Disorder. JADD. 2009;39:1663–1672. doi: 10.1007/s10803-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K, Johnson MH. Developing a cortex specialized for face perception. Trends in Cognitive Sciences. 2007;11:367–369. doi: 10.1016/j.tics.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Collette F, Hogge M, Salmon E, Van der Linden M. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139:209–221. doi: 10.1016/j.neuroscience.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Colombi C, Liebal K, Tomasello M, Young G, Warneken F, Rogers SJ. Examining correlates of cooperation in autism: Imitation, joint attention, and understanding intentions. Autism. 2009;13:143–163. doi: 10.1177/1362361308098514. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40:271–282. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Duchowski AT. Eye Tracking Methodology: Theory and Practice. 1st ed. Springer; 2003. [Google Scholar]
- Falck-Ytter T, Gredeback G, von Hofsten C. Infants predict other people's action goals. Nat Neurosci. 2006;9:878–879. doi: 10.1038/nn1729. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Drain M, Tanaka JN. What Is “Special” About Face Perception? PSYCHOLOGICAL REVIEW-NEW YORK- 1998;105:482–498. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- Gibson EJ. Exploratory behavior in the development of perceiving, acting, and the acquiring of knowledge. Annual review of psychology. 1988;39:1–42. [Google Scholar]
- Grice SJ, Halit H, Farroni T, Baron-Cohen S, Bolton P, Johnson MH. Neural correlates of eye-gaze detection in young children with autism. Cortex. 2005;41:342–353. doi: 10.1016/s0010-9452(08)70271-5. [DOI] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH. The development of the social brain in human infancy. European Journal of Neuroscience. 2007;25:909–919. doi: 10.1111/j.1460-9568.2007.05379.x. [DOI] [PubMed] [Google Scholar]
- Haan M, Pascalis O, Johnson MH. Specialization of Neural Mechanisms Underlying Face Recognition in Human Infants. Journal of Cognitive Neuroscience. 2002;14:199–209. doi: 10.1162/089892902317236849. [DOI] [PubMed] [Google Scholar]
- Halit H, de Haan M, Johnson MH. Cortical specialisation for face processing: face-sensitive event-related potential components in 3-and 12-month-old infants. Neuroimage. 2003;19:1180–1193. doi: 10.1016/s1053-8119(03)00076-4. [DOI] [PubMed] [Google Scholar]
- Happé F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nature neuroscience. 2006;9:1218. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- Hari R, Kujala MV. Brain basis of human social interaction: From concepts to brain imaging. Physiological reviews. 2009;89:453. doi: 10.1152/physrev.00041.2007. [DOI] [PubMed] [Google Scholar]
- Hecke AVV, Mundy Peter C., Acra C. Françoise., Block Jessica J., Delgado Christine E. F., Parlade Meaghan V., Meyer Jessica A., Neal A. Rebecca, Pomares Yuly B. Infant Joint Attention, Temperament, and Social Competence in Preschool Children. Child Development. 2007;78:53–69. doi: 10.1111/j.1467-8624.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershler O, Hochstein S. At first sight: A high-level pop out effect for faces. Vision Research. 2005;45:1707–1724. doi: 10.1016/j.visres.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Heyes C. Causes and consequences of imitation. Trends in Cognitive Sciences. 2001;5:253–261. doi: 10.1016/s1364-6613(00)01661-2. [DOI] [PubMed] [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Vol. 75. Biometrika Trust; 1988. [Google Scholar]
- Hoehl S, Reid V, Mooney J, Striano T. What are you looking at? Infants’ neural processing of an adult's object-directed eye gaze. Developmental Science. 2008;11:10–16. doi: 10.1111/j.1467-7687.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979:65–70. [Google Scholar]
- Huang CT, Charman T. Gradations of emulation learning in infants’ imitation of actions on objects. Journal of Experimental Child Psychology. 2005;92:276–302. doi: 10.1016/j.jecp.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Batty M. Neural bases of eye and gaze processing: the core of social cognition. Neuroscience & Biobehavioral Reviews. 2009;33:843–863. doi: 10.1016/j.neubiorev.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Griffin R, Csibra G, Halit H, Farroni T, de Haan M, Tucker LA, Baron-Cohen S, Richards J. The emergence of the social brain network: Evidence from typical and atypical development. Development and Psychopathology. 2005;17:599–619. doi: 10.1017/S0954579405050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Carr K, Klin A. Absence of Preferential Looking to the Eyes of Approaching Adults Predicts Level of Social Disability in 2-Year-Old Toddlers With Autism Spectrum Disorder. Arch Gen Psychiatry. 2008;65:946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- Jones W, Klin A. Heterogeneity and Homogeneity Across the Autism Spectrum: The Role of Development. Journal of Amer Academy of Child & Adolescent Psychiatry. 2009;48:471. doi: 10.1097/CHI.0b013e31819f6c0d. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17:951. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory Control in High-Functioning Autism: Decreased Activation and Underconnectivity in Inhibition Networks. Biological Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Atypical epigenesis. Developmental Science. 2007;10:84–88. doi: 10.1111/j.1467-7687.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F. The Enactive Mind, or from Actions to Cognition: Lessons from Autism. Philosophical Transactions: Biological Sciences. 2003;358:345–360. doi: 10.1098/rstb.2002.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual Fixation Patterns During Viewing of Naturalistic Social Situations as Predictors of Social Competence in Individuals With Autism. Archives of General Psychiatry. 2002;59:809. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Klin A, Lin D, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh H, Milne E, Dobkins K. Spatial Contrast Sensitivity in Adolescents with Autism Spectrum Disorders. [July 13, 2010];Journal of Autism and Developmental Disorders. 2010 doi: 10.1007/s10803-010-0953-7. Available at: http://dx.doi.org/10.1007/s10803-010-0953-7. [DOI] [PubMed]
- Kylliäinen A, Hietanen J. Skin Conductance Responses to Another Person's Gaze in Children with Autism. Journal of Autism and Developmental Disorders. 2006;36:517–525. doi: 10.1007/s10803-006-0091-4. [DOI] [PubMed] [Google Scholar]
- Leekam SR, Lopez B, Moore C. Attention and Joint Attention in Preschool Children with Autism. Developmental Psychology. 2000;36:261–73. doi: 10.1037//0012-1649.36.2.261. [DOI] [PubMed] [Google Scholar]
- Leekam S, Ramsden C. Dyadic Orienting and Joint Attention in Preschool Children with Autism. Journal of Autism and Developmental Disorders. 2006;36:185–197. doi: 10.1007/s10803-005-0054-1. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule: ADOS: Manual. Western Psychological Services. 2002 [Google Scholar]
- Loveland KA. Social affordances and interaction II: Autism and the affordances of the human environment. Ecological Psychology. 1991;3:99–119. [Google Scholar]
- MathWorks [August 16, 2009];MATLAB 7.8 (Release R2009a) [Computer Software] 2009 Available at: http://www.mathworks.com.
- McCall RB, McGhee PE. The discrepancy hypothesis of attention and affect in infants. The structuring of experience. 1977:179–210. [Google Scholar]
- McCleery JP, Allman E, Carver LJ, Dobkins KR. Abnormal Magnocellular Pathway Visual Processing in Infants at Risk for Autism. Biological Psychiatry. 2007;62:1007–1014. doi: 10.1016/j.biopsych.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN. Understanding the intentions of others: Re-enactment of intended acts by 18-month-old children. Developmental Psychology. 1995;31:838–850. doi: 10.1037/0012-1649.31.5.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff A, Moore M. Imitation of Facial and Manual Gestures by Human Neonates. Science. 1977;198:75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- Merin N, Young G, Ozonoff S, Rogers SJ. Visual Fixation Patterns during Reciprocal Social Interaction Distinguish a Subgroup of 6-Month-Old Infants At-Risk for Autism from Comparison Infants. Journal of Autism and Developmental Disorders. 2007;37:108–121. doi: 10.1007/s10803-006-0342-4. [DOI] [PubMed] [Google Scholar]
- Milne E, Swettenham J, Hansen P, Campbell R, Jeffries H, Plaisted K. High Motion Coherence Thresholds in Children with Autism. Journal of Child Psychology and Psychiatry. 2002;43:255–263. doi: 10.1111/1469-7610.00018. [DOI] [PubMed] [Google Scholar]
- Moore C, Dunham PJ, editors. Joint Attention: Its Origins and Role in Development. 1st ed. Lawrence Erlbaum; 1995. [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. American Guidance Service; Circle Pines, MN: 1995. [Google Scholar]
- Mundy P, Vaughan A. Joint Attention and Its Role in the Diagnostic Assessment of Children with Autism. Assessment for Effective Intervention. 2002;27:57–60. [Google Scholar]
- Mundy P, Card J, Fox N. EEG correlates of the development of infant joint attention skills. Developmental Psychobiology. 2000;36:325–338. [PubMed] [Google Scholar]
- Mundy P, Fox N, Card J. EEG coherence, joint attention and language development in the second year. Developmental Science. 2003;6:48–54. [Google Scholar]
- Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. Journal of Autism and Developmental Disorders. 1990;20:115–128. doi: 10.1007/BF02206861. [DOI] [PubMed] [Google Scholar]
- Neurobehavioral Systems [September 7, 2006];Presentation (Version 10.1) [Computer Software] 2006 Available at: http://www.neurobs.com.
- Newman SD, Carpenter PA, Varma S, Just MA. Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia. 2003;41:1668–1682. doi: 10.1016/s0028-3932(03)00091-5. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Calder AJ. Neural mechanisms of social attention. Trends in Cognitive Sciences. 2009;13:135–143. doi: 10.1016/j.tics.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Ozonoff SJ, Goldring S, Young GS, Greiss-Hess L, Steele J, Macari S, Herrera A, Rogers SJ. Motor Development and Early Identification of Autism.. 6th Annual International Meeting for Autism Research.; Seattle, Washington. 2007. [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing (Version 2.9.2) [Computer Software] R Foundation for Statistical Computing; Vienna, Austria: 2009. [August 24, 2009]. Available at: http://www.R-project.org. [Google Scholar]
- Rogers SJ, Hepburn SL, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2003;44:763. doi: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Williams JHG. Imitation in autism: Findings and controversies. Imitation and the social mind: Autism and typical development. 2006:277–309. [Google Scholar]
- Sanchez-Marin F, Padilla-Medina J. A Psychophysical Test of the Visual Pathway of Children with Autism. Journal of Autism and Developmental Disorders. 2008;38:1270–1277. doi: 10.1007/s10803-007-0507-9. [DOI] [PubMed] [Google Scholar]
- Sasson NJ, Turner-Brown LM, Holtzclaw TN, Lam KS, Bodfish JW. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research. 2008;1:31–42. doi: 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A, Johnson MH. Atypical eye contact in autism: Models, mechanisms and development. Neuroscience & Biobehavioral Reviews. 2009;33:1204–1214. doi: 10.1016/j.neubiorev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Senju A, Tojo Y, Yaguchi K, Hasegawa T. Deviant gaze processing in children with autism: An ERP study. Neuropsychologia. 2005;43:1297–1306. doi: 10.1016/j.neuropsychologia.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Sensomotoric Instruments iView X (TM) RED. 2005.
- Shafritz KM, Dichter GS, Baranek GT, Belger A. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biological psychiatry. 2008;63:974. doi: 10.1016/j.biopsych.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shic F. Computational Methods for Eye-Tracking Analysis: Applications to Autism. 2008.
- Shic F, Chawarska K, Lin D, Scassellati B. Measuring context: The gaze patterns of children with autism evaluated from the bottom-up. In Development and Learning, 2007.; ICDL IEEE 6th International Conference on; 2007. pp. 70–75. [Google Scholar]
- Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Research. 2009;49:2705–2739. doi: 10.1016/j.visres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Speer LL, Cook AE, McMahon WM, Clark E. Face processing in children with autism: Effects of stimulus contents and type. Autism. 2007;11:265–277. doi: 10.1177/1362361307076925. [DOI] [PubMed] [Google Scholar]
- SPSS, Inc. SPSS (Version 15.0) [Computer Software] 2006 Available at: http://www.spss.com.
- Striano T, Reid VM, Hoehl S. Neural mechanisms of joint attention in infancy. European Journal of Neuroscience. 2006;23:2819–2823. doi: 10.1111/j.1460-9568.2006.04822.x. [DOI] [PubMed] [Google Scholar]
- Sullivan M, Finelli J, Marvin A, Garrett-Mayer E, Bauman M, Landa R. Response to Joint Attention in Toddlers at Risk for Autism Spectrum Disorder: A Prospective Study. Journal of Autism and Developmental Disorders. 2007;37:37–48. doi: 10.1007/s10803-006-0335-3. [DOI] [PubMed] [Google Scholar]
- Szatmari P. Heterogeneity and the genetics of autism. Journal of Psychiatry and Neuroscience. 1999;24:159. [PMC free article] [PubMed] [Google Scholar]
- Tomasello M. Do apes ape? In: Heyes CM, Galef BG, editors. Social learning in animals: the roots of culture. Academic Press; 1996. [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: The origins of cultural cognition. Behavioral and Brain Sciences. 2005;28:675–691. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- Trevarthen C, Aitken KJ. Infant Intersubjectivity: Research, Theory, and Clinical Applications. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42:3–48. [PubMed] [Google Scholar]
- Trikalinos TA, Karvouni A, Zintzaras E, Ylisaukko-Oja T, Peltonen L, Järvelä I, Ioannidis JPA. A heterogeneity-based genome search meta-analysis for autism-spectrum disorders. Molecular Psychiatry. 2005;11:29–36. doi: 10.1038/sj.mp.4001750. [DOI] [PubMed] [Google Scholar]
- Turner M. 3 Towards an executive dysfunction account of repetitive behaviour in autism. Autism as an executive disorder. 1997:57. [Google Scholar]
- Turner M. Annotation: Repetitive Behaviour in Autism: A Review of Psychological Research. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 1999;40:839–849. [PubMed] [Google Scholar]
- Valenza E, Simion F, Cassia VM, Umilta C. Face preference at birth. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:892–903. doi: 10.1037//0096-1523.22.4.892. [DOI] [PubMed] [Google Scholar]
- Vivanti G, Nadig A, Ozonoff S, Rogers SJ. What do children with autism attend to during imitation tasks? Journal of Experimental Child Psychology. 2008;101:186–205. doi: 10.1016/j.jecp.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Want SC, Harris PL. How do children ape? Applying concepts from the study of non-human primates to the developmental study of ‘imitation’ in children. Developmental Science. 2002;5:1. [Google Scholar]
- Whitman TL. The development of autism: a self-regulatory perspective. Jessica Kingsley Publishers; 2004. [Google Scholar]
- Williams J, Whiten A, Singh T. A Systematic Review of Action Imitation in Autistic Spectrum Disorder. Journal of Autism and Developmental Disorders. 2004;34:285–299. doi: 10.1023/b:jadd.0000029551.56735.3a. [DOI] [PubMed] [Google Scholar]
- Woodward AL. Infants selectively encode the goal object of an actor's reach. Cognition. 1998;69:1–34. doi: 10.1016/s0010-0277(98)00058-4. [DOI] [PubMed] [Google Scholar]
- Woodward AL. Infants’ ability to distinguish between purposeful and non-purposeful behaviors. Infant Behavior and Development. 1999;22:145–160. [Google Scholar]
- Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behavior and affect at 6 months: predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science. 2009;9999 doi: 10.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]