Abstract
The RNA recognition motif (RRM) is a prevalent class of RNA binding domain. Although a number of RRM/RNA structures have been determined, thermodynamic analyses are relatively few. Here, we use isothermal titration calorimetry to characterize single-stranded (ss)RNA binding by four representative RRM-containing proteins: (i) U2AF65, (ii) SXL, (iii) TIA-1, and (iv) PAB. In all cases, ssRNA binding is accompanied by remarkably large favorable enthalpy changes (−30 to −60 kcal mol−1) and unfavorable entropy changes. Alterations of key RRM residues and binding sites indicate that under the nearly physiological conditions of these studies, large thermodynamic changes represent a signature of specific ssRNA recognition by RRMs.
The RNA recognition motif (RRM) is an abundant domain among proteins with central roles in post-transcriptional gene regulation (1) (Figure 1). Multiple RRMs often occur per polypeptide. For example, tandem RRMs of the splicing factors U2AF65, TIA-1 and SXL recognize U-rich pre-mRNA sites (2, 3). The poly(A) binding protein (PAB) enhances translation following RRM-mediated recognition of the mRNA tail (4). Beyond the well-established role of canonical RRMs to bind RNA, RRM variants such as the U2AF Homology Motif (UHM) are dedicated to protein-protein interactions (5). This breadth of functions illustrates the importance of elucidating the structural and thermodynamic forces responsible for RRM interactions.
Figure 1.
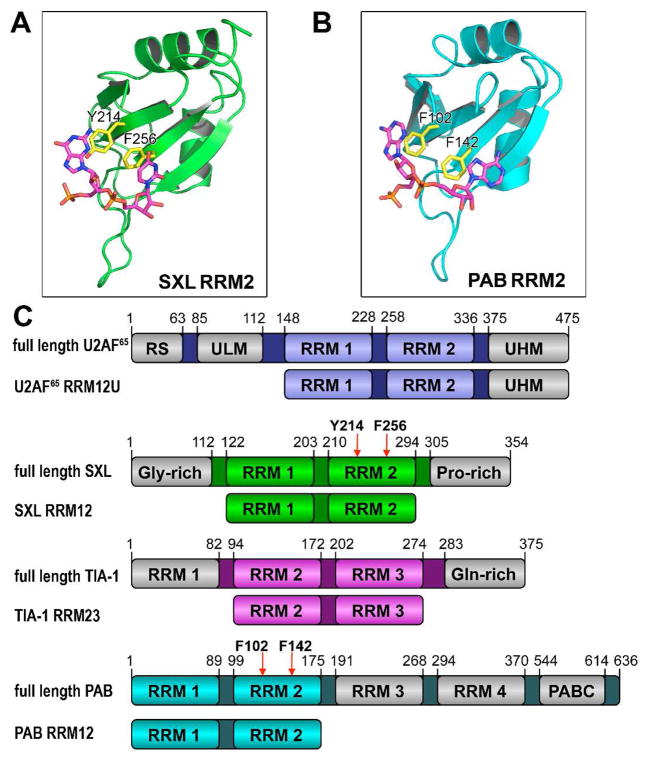
Structures of the second RRMs of (A) SXL (PDB ID 1B7F) or (B) PAB (PDB ID 1CVJ). RNP residues altered by mutagenesis are colored yellow. (C) U2AF65, TIA-1, SXL, and PAB domain organization and constructs used in this study. RRM, RNA recognition motif; RS, arginine-serine rich domain; ULM, UHM ligand motif; UHM, U2AF homology motif; Gly-rich, glycine-rich domain; Pro-rich, proline-rich domain; Gln-rich, glutamine-rich domain; PABC, peptide-binding domain.
Despite extensive structural and functional investigations of RRMs, full thermodynamic characterizations of the enthalpy and entropy changes during RRM/RNA binding are scarce. A thorough characterization of RNA binding by an RRM has been completed for the U1A splicing factor (6). However, this rare example focuses on a single RRM binding a single-stranded (ss)RNA site within a stem loop, which differs from the prevalent systems of multiple RRMs recognizing unstructured ssRNAs. Enthalpy and entropy changes of ssRNA binding have been determined for a number of unrelated domains, including Hfq, GLD-1, trp attenuation protein (TRAP), tristetraprolin, and T4 translational regulatory protein (7–11). Given the diversity of these domain classes, it is unsurprising that no common thermodynamic themes of ssRNA binding have emerged to date. In contrast, protein/protein, protein/ligand, and protein/DNA interactions have been investigated in depth.
Previously, we found a large favorable enthalpy change (ΔH) and a large unfavorable entropy change (−TΔS) for poly(U) recognition by the tandem RRMs of U2AF65 (ΔH −70 kcal mol−1, −TΔS 61 kcal mol−1) (12). Notably, these enthalpy and entropy changes were similar in magnitude to those observed for the single other example of RRM/RNA binding (U1A RRM/RNA stem loop, ΔH −68 kcal mol−1, −TΔS 58 kcal mol−1) (6). To explore the generality of these observations for other RRM-containing proteins, we used isothermal titration calorimetry (ITC) to determine the enthalpy and entropy changes of ssRNA binding by four RRM-containing proteins: U2AF65, SXL, TIA-1, and PAB. Constructs composed of two consecutive RRMs represented the major sites of RNA recognition for each protein (U2AF65 R12U, SXL RRM12, TIA-1 RRM23, and PAB RRM12) (2, 3, 14) (Figure 1C). To enable future comparison with higher order complexes, the U2AF65 construct includes a C-terminal UHM that lacks detectable RNA affinity (5).
The ability of U2AF65, SXL, and TIA-1 to recognize comparable U-rich sequences offered the opportunity to compare various RRM-containing proteins binding identical RNA sites (3, 15). We preferred a poly(U) binding site for ITC, since this sequence is expected to lack (i) intra- or inter-molecular secondary structures or (ii) multiple nonidentical binding sites that would complicate analyses. The optimal, poly(U) site of U2AF65 is well-established (2, 16). We verified the poly(U) preferences of SXL and TIA-1 by fluorescence anisotropy (Figure S1). Both splicing factors bound a 20-uridine (U20) RNA with higher affinity than their natural splice sites (SXL RRM12 KD: 7 ± 3 nM for U20 vs. 47 ± 10 nM for tra splice site; TIA-1 RRM23 KD: 33 ± 6 nM for U20 vs. 118 ± 16 nM for fas splice site). Likewise, a 20-adenosine RNA (A20) is similar in length to the repeating units of PAB/poly(A) mRNA tail complexes (17), and represents a specific PAB binding site for direct comparison with U20 RNAs. We subsequently focused on the U20 and A20 RNA binding sites.
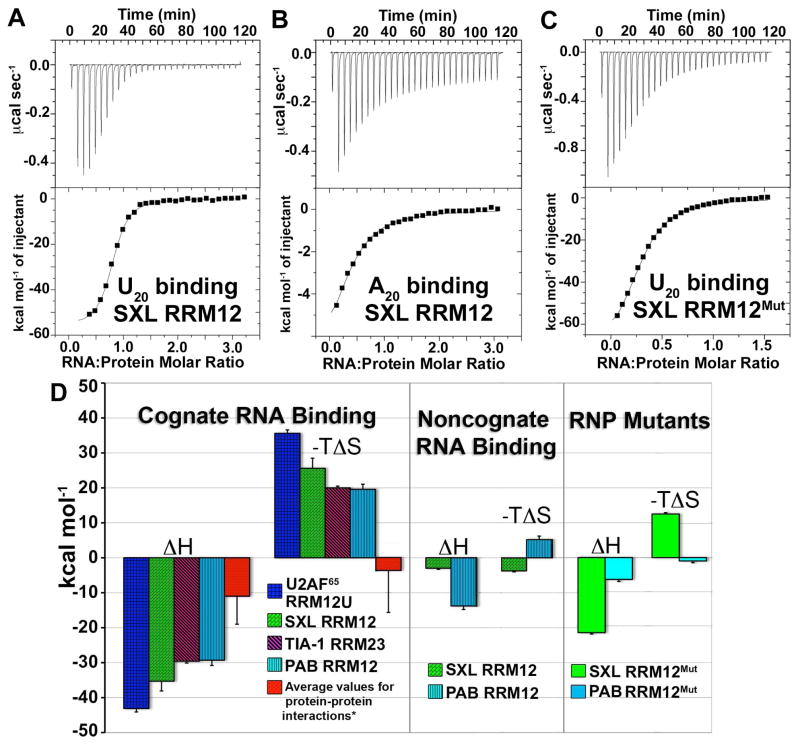
Since the HEPES buffer used previously was replaced with BES here (to take advantage of a lower heat capacity change of ionization), we repeated the U2AF65 RRM12U/U20 titrations in BES for comparison (Figure S2A). Consistent with a mere 1 kcal mol−1 difference in the enthalpies of buffer ionization (18), the results agreed within error (ΔH −61.3 ± 10.8 kcal mol−1, −TΔS of 53.0 ± 10.7 kcal mol−1) (Table S1). Large enthalpy and entropy changes also resulted for specific RNA recognition by SXL, TIA-1, and PAB (SXL RRM12/U20: ΔH −35.3 ± 2.9 kcal mol−1, −TΔS = 25.6 ± 2.8 kcal mol−1; TIA-1 RRM23/U20: ΔH −29.6 ± 0.5 kcal mol−1, −TΔS 20.0 ± 0.5 kcal mol−1; PAB RRM12/A20: ΔH −29.3 ± 1.5 kcal mol−1, −TΔS 19.6 ± 1.5 kcal mol−1) (Figures 2, S2, Table S1). These results indicated that, considering the U1A RRM, five unrelated RRM-containing proteins with <25% sequence identity exhibited large favorable enthalpy changes and unfavorable entropy changes for recognition of diverse RNA sites (Figure 2).
Figure 2.
Representative isotherms for titration of (A) U20 RNA into SXL RRM12; (B) A20 RNA into SXL RRM12; (C) U20 RNA into SXL RRM12Mut in 100 mM NaCl, 25 mM BES pH 7.4 at 303 K. All other isotherms are represented in the Supplementary Data. (D) Bar graph representations of thermodynamic changes. Left panel, specific RNA binding by wild-type RRM-containing proteins: U2AF65RRM12U/U20, SXL RRM12/U20, TIA-1 RRM23/U20, and PAB RRM12/A20. (*)Averaged nonredundant enthalpy (www.bioinfodatabase.com/pint/) and entropy (13) changes for protein-protein interactions. Central panel, noncognate RNA binding: SXL RRM12/A20 and PAB RRM12/U20. Right panel, RNP mutant proteins: SXL RRM12Mut/U20 and PAB RRM12Mut/A20.
Control titrations of U20 or A20 RNAs into buffer demonstrated that the heats of RNA dilution were not responsible for the apparent enthalpy and entropy changes (Figure S3A,B). To further ensure that the observed values did not depend on the configuration of the experiment, ‘reverse’ titrations of SXL in the syringe into U20 RNA in the sample cell showed similar enthalpy and entropy changes (ΔH −43.6 ± 1.5 kcal mol−1, −TΔS 33.9 ± 1.6 kcal mol−1) as the ‘forward’ titrations of U20 RNA into SXL (Figure S3C, Table S1).
To the best of our knowledge, comparably large enthalpy and entropy changes for ssRNA recognition have been documented for only two cases besides RRMs: the Sm-like fold of Hfq (ΔH −41 kcal mol−1, −TΔS 30 kcal mol−1 for poly(A)) (7) and a CCCH-zinc finger of tristetraprolin (ΔH −46 kcal mol−1, −TΔS 35 kcal mol−1 for an AU-rich element) (10). Large, exothermic enthalpy changes also are observed for the well-studied case of ssDNA binding by SSB (ΔH −70 kcal mol−1 for poly(T)) (19). In contrast, other characterized motifs bind ssRNA with moderate enthalpy and entropy changes (8, 11) and can be entropically driven (9).
We next investigated the possible influence of proton exchange with the surrounding medium during RRM/RNA complex formation. To avoid possible competition between phosphate buffer and binding sites for the phosphodiester RNA backbone, we took advantage of the different ionization enthalpies of Tris compared with BES buffers (ΔHi, Tris 11.7 kcal mol−1; ΔHi, BES 6.0 kcal mol−1; ΔΔH 5.7 kcal mol−1 per proton transfer) (18, 20). Enthalpy changes for the representative SXL RRM12/U20 system were the same within error when measured in Tris compared with BES buffers (ΔHTris −39.4 ± 0.2 kcal mol−1) (Figure S4, Table S1). This result demonstrated that proton exchange was not responsible for the large observed magnitudes of the enthalpy and entropy changes.
We then examined whether the enthalpy and entropy changes depended on formation of specific RRM/RNA contacts (Figures 2, S5). We focused on SXL and PAB for these experiments, since the affinities of these proteins for nonspecific RNAs were sufficiently high to remain within the reliable range for ITC measurements, and structures of the RNA complexes were known. For SXL RRM12, the magnitude of the enthalpy changes became nearly 90% less favorable for nonspecific A20 binding (ΔH −3.1 ± 0.3 kcal mol−1). Indeed, the SXL RRM12/A20 binding reaction became entropically favorable (−TΔS −3.7 ± 0.3 kcal mol−1). The magnitudes of the enthalpy and entropy changes also decreased for nonspecific RNA binding by PAB RRM12 (ΔH −13.8 ± 0.8 kcal mol−1, −TΔS 5.3 ± 0.5 kcal mol−1). Hence, the large enthalpy and entropy changes appeared to depend on specific RNA contacts by the RRM fold. Since nonspecific RNA binding often depends on favorable electrostatic interactions, this result further suggested that counterion release by complex formation was unlikely to dominate the observed enthalpy and entropy changes. Accordingly, relatively few RRM/RNA salt bridges are evident among the proteins studied here (21–23) (approximately four compared with 23 for a representative salt-dependent complex - IHF/dsDNA (24)).
To further investigate the role of specific RNA contacts, we mutated conserved aromatic residues in the characteristic ribonucleoprotein motifs (RNP1 and RNP2) (Figure 1). These residues stack with RNA bases in the majority of canonical RRM structures, including SXL and PAB (22, 23). Double mutations of F256A/Y214A in SXL RRM2 (SXL RRM12Mut) and F102A/F142A in PAB RRM2 (PAB RRM12Mut) decreased the enthalpy and entropy changes of target RNA binding relative to the wild-type counterparts, and converted the PAB RRM12Mut/A20 into an entropically favorable interaction (SXL RRM12Mut/U20: ΔH −22.3 ± 0.4 kcal mol−1, −TΔS 14.5 ± 0.4 kcal mol−1; PAB RRM12Mut/A20: ΔH −6.4 ± 0.6 kcal mol−1, −TΔS −1.0 ± 0.5 kcal mol−1) (Figure 2, S6, Table S1).
Based on our results for the SXL and PAB mutants, base stacking by conserved RNP residues contributes to the large thermodynamic changes for RRM/RNA interactions. In support of this conclusion, π stacking interactions are often enthalpically favorable (25, 26). Notably, Hfq and tristetraprolin, which exhibit large enthalpy and entropy changes for ssRNA binding, like the RRM-containing proteins engage in extensive π stacking between aromatic side chains and the RNA bases (27, 28). Similarly, aromatic SSB side chains stack with bound ssDNA bases (29), and site-directed mutagenesis of these residues reduces the enthalpy and entropy changes for nucleic acid binding (30). In contrast, GLD-1 and TRAP, which lack large favorable enthalpy and unfavorable entropy changes for ssRNA binding, also lack extensive contacts between aromatic side chains and nucleic acid bases (31, 32). Thus, the conservation of the RNP motifs provides a rationale for the shared thermodynamic signature of large favorable enthalpy changes among RRM/RNA complexes characterized to date. Future studies will tell whether this theme continues to hold for the many diverse members of the RRM family, and for other nucleic acid-binding domains.
Supplementary Material
Acknowledgments
We thank D. Turner, B. Miller and V. Frasca for insightful discussions.
Footnotes
This work was supported by the National Institutes of Health (Grant GM070503 to C.L.K.).
SUPPORTING INFORMATION AVAILABLE
Table S1, Figures S1–S5, and Supplementary Methods. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Maris C, Dominguez C, Allain FH. FEBS J. 2005;272:2118–2131. doi: 10.1111/j.1742-4658.2005.04653.x. [DOI] [PubMed] [Google Scholar]
- 2.Singh R, Banerjee H, Green MR. RNA. 2000;6:901–911. doi: 10.1017/s1355838200000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dember LM, Kim ND, Liu KQ, Anderson P. J Biol Chem. 1996;271:2783–2788. doi: 10.1074/jbc.271.5.2783. [DOI] [PubMed] [Google Scholar]
- 4.Mangus DA, Evans MC, Jacobson A. Genome Biol. 2003;4:223. doi: 10.1186/gb-2003-4-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kielkopf CL, Lücke S, Green MR. Genes Dev. 2004;18:1513–1526. doi: 10.1101/gad.1206204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams DJ, Hall KB. J Mol Biol. 1996;257:265–275. doi: 10.1006/jmbi.1996.0161. [DOI] [PubMed] [Google Scholar]
- 7.Mikulecky PJ, Kaw MK, Brescia CC, Takach JC, Sledjeski DD, Feig AL. Nat Struct Mol Biol. 2004;11:1206–1214. doi: 10.1038/nsmb858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryder SP, Frater LA, Abramovitz DL, Goodwin EB, Williamson JR. Nat Struct Mol Biol. 2004;11:20–28. doi: 10.1038/nsmb706. [DOI] [PubMed] [Google Scholar]
- 9.Baumann C, Otridge J, Gollnick P. J Biol Chem. 1996;271:12269–12274. doi: 10.1074/jbc.271.21.12269. [DOI] [PubMed] [Google Scholar]
- 10.Brewer BY, Malicka J, Blackshear PJ, Wilson GM. J Biol Chem. 2004;279:27870–27877. doi: 10.1074/jbc.M402551200. [DOI] [PubMed] [Google Scholar]
- 11.Webster KR, Spicer EK. J Biol Chem. 1990;265:19007–19014. [PubMed] [Google Scholar]
- 12.Jenkins JL, Shen H, Green MR, Kielkopf CL. J Biol Chem. 2008;283:33641–33649. doi: 10.1074/jbc.M806297200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stites WE. Chem Rev. 1997;97:1233–1250. doi: 10.1021/cr960387h. [DOI] [PubMed] [Google Scholar]
- 14.Burd CG, Matunis EL, Dreyfuss G. Mol Cell Biol. 1991;11:3419–3424. doi: 10.1128/mcb.11.7.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh R, Valcarcel J, Green MR. Science. 1995;268:1173–1176. doi: 10.1126/science.7761834. [DOI] [PubMed] [Google Scholar]
- 16.Coolidge CJ, Seely RJ, Patton JG. Nucleic Acids Res. 1997;25:888–896. doi: 10.1093/nar/25.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baer BW, Kornberg RD. Proc Natl Acad Sci U S A. 1980;77:1890–1892. doi: 10.1073/pnas.77.4.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukada H, Takahashi K. Proteins. 1998;33:159–166. [PubMed] [Google Scholar]
- 19.Ferrari ME, Lohman TM. Biochemistry. 1994;33:12896–12910. doi: 10.1021/bi00209a022. [DOI] [PubMed] [Google Scholar]
- 20.Bernhard SA. J Biol Chem. 1956;218:961–969. [PubMed] [Google Scholar]
- 21.Sickmier EA, Frato KE, Shen H, Paranawithana SR, Green MR, Kielkopf CL. Mol Cell. 2006;23:49–59. doi: 10.1016/j.molcel.2006.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handa N, Nureki O, Kurimoto K, Kim I, Sakamoto H, Shimura Y, Muto Y, Yokoyama S. Nature. 1999;398:579–585. doi: 10.1038/19242. [DOI] [PubMed] [Google Scholar]
- 23.Deo RC, Bonanno JB, Sonenberg N, Burley SK. Cell. 1999;98:835–845. doi: 10.1016/s0092-8674(00)81517-2. [DOI] [PubMed] [Google Scholar]
- 24.Holbrook JA, Tsodikov OV, Saecker RM, Record MT., Jr J Mol Biol. 2001;310:379–401. doi: 10.1006/jmbi.2001.4768. [DOI] [PubMed] [Google Scholar]
- 25.Mascotti DP, Lohman TM. Biochemistry. 1992;31:8932–8946. doi: 10.1021/bi00152a033. [DOI] [PubMed] [Google Scholar]
- 26.Makhatadze GI, Privalov PL. Biophys Chem. 1994;50:285–291. doi: 10.1016/0301-4622(93)e0096-n. [DOI] [PubMed] [Google Scholar]
- 27.Schumacher MA, Pearson RF, Moller T, Valentin-Hansen P, Brennan RG. EMBO J. 2002;21:3546–3556. doi: 10.1093/emboj/cdf322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudson BP, Martinez-Yamout MA, Dyson HJ, Wright PE. Nat Struct Mol Biol. 2004;11:257–264. doi: 10.1038/nsmb738. [DOI] [PubMed] [Google Scholar]
- 29.Raghunathan S, Kozlov AG, Lohman TM, Waksman G. Nat Struct Biol. 2000;7:648–652. doi: 10.1038/77943. [DOI] [PubMed] [Google Scholar]
- 30.Kerr ID, Wadsworth RI, Cubeddu L, Blankenfeldt W, Naismith JH, White MF. EMBO J. 2003;22:2561–2570. doi: 10.1093/emboj/cdg272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valverde R, Edwards L, Regan L. FEBS J. 2008;275:2712–2726. doi: 10.1111/j.1742-4658.2008.06411.x. [DOI] [PubMed] [Google Scholar]
- 32.Antson AA, Dodson EJ, Dodson G, Greaves RB, Chen X, Gollnick P. Nature. 1999;401:235–242. doi: 10.1038/45730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.