Abstract
Background
Adherence is a strong determinant of viral suppression with antiretroviral therapy (ART), but measuring it is challenging. Medication delivery can be measured accurately in settings with computerized prescription databases. We studied the association between Medication Possession Ratio (MPR), virologic suppression, and resistance to ART in Côte d’Ivoire.
Methods
We conducted a prospective cohort study of HIV-1 infected adults initiating ART in three clinics using computerized monitoring systems. Patients had viral load (VL) tests at month 6 (M6) and month 12 (M12) after ART initiation, and genotype tests if VL was detectable (≥300 copies/ml). MPR was defined as the number of daily doses of antiretroviral drug actually provided divided by the total number of follow-up days since ART initiation.
Results
Overall, 1,573 patients started ART with stavudine/zidovudine plus lamivudine plus nevirapine/efavirenz. At M6 and M12, 996 and 942 patients were in active follow-up; 20% (M6) and 25% (M12) of patients had detectable VL, including 7% (M6) and 11% (M12) with ≥1 resistance mutation. Among patients with MPR ≥95%, 80–94%, 65–79%, 50–64% and <50% at M12, the proportion with detectable VL [resistance] was 9% [4%], 17% [7%], 45% [24%], 67% [31%], and 85% [37%]. Among patients with ≥1 mutation at M12, 86% were resistant to lamivudine/emtricitabine and/or nevirapine/efavirenz but not to other drugs.
Conclusion
MPR was strongly associated with virologic outcomes. Half of those with detectable VL at M12 had no resistance mutations. MPR should be used at M6 to identify patients who might benefit from early interventions to reinforce adherence.
INTRODUCTION
Adherence to antiretroviral therapy (ART) has been shown to be a strong determinant of virologic suppression in HIV-infected patients 1, 2. Adherence is the most important predictor of success on ART and prevents the development of resistance in the long term 3–5. HIV care providers worldwide face the challenges of identifying those patients who do not take their medications regularly in the first few months after ART initiation and improving their adherence 6, 7.
There is no easily measurable gold standard for detecting poor adherence 8, 9. However, estimating adherence is particularly difficult in resource-limited countries, for two reasons.
First, plasma HIV-1 RNA tests, which can identify patients who do not take their medication early in treatment, are not routinely available in many settings. In contrast, in high-income countries HIV-1 RNA is measured on average every three months and patients who have detectable HIV-1 RNA in the first months of ART are targeted for adherence interventions 10.
Second, even when patients are willing to take their medications regularly, they may not receive them on time. In some settings, external obstacles to drug delivery can be as common as the intrinsic factors associated with non-adherence 2, 6, 11–13. These logistical obstacles make it more complicated to monitor adherence, both because they cannot always be distinguished from patients’ reluctance to pick up their drugs, and because focusing on adherence may not be productive when the real problem lies with the drug delivery system.
In this context, a method for determining the amount of medications a patient has received can serve to simultaneously measure the health care system’s ability to provide medications as well as some aspects of adherence. In contrast to pill intake, medication delivery can be measured accurately, even in low resource settings, where an increasing number of HIV care centers use computerized prescription databases 14–20. These databases can be used to measure pill possession on an individual basis, provided they are prospectively managed through standardized procedures 21.
In 2006, we launched a prospective cohort study of HIV-infected adults who initiated ART at three HIV care centers equipped with computerized prescription databases in Abidjan, the economic capital of Côte d’Ivoire, West Africa. Our objective was to describe the association between medication possession and virologic outcomes in the first year of ART.
METHODS
The VOLTART cohort
We conducted a prospective cohort study of long-term virologic outcomes on ART (VOLTART cohort). HIV-infected adults who started ART between February 2006 and May 2007 at one of three HIV outpatient clinics in Abidjan and showed up for their six-month visit were eligible for the study. The three clinics were the Centre de Prise en Charge et de Formation (CePReF) 22, the Yopougon Attiè clinic, and the HIV care center affiliated with the National Center for Blood Transfusion (CNTS) 23. Study subjects received the same standard care and treatment as other HIV-infected patients on ART at their respective clinics. In addition, they received free plasma HIV-1 RNA and genotype tests every six months. In each study center, a research coordinator prospectively managed and monitored the prescription database.
Standard care and treatment
The standard of HIV care for HIV-infected adults on ART in Côte d’Ivoire has been described elsewhere 24. During the study period, all patients followed in the three study clinics initiated ART according to the 2003–2006 World Health Organization (WHO) criteria: WHO clinical stage 4 regardless of CD4 count, CD4 count ≤200/mm3 regardless of WHO stage, or WHO stage 3 and CD4 count 200–350/mm3 25. Chemistry exams (serum creatinine and transaminases) were performed before ART initiation. When patients were HIV-1-infected, first-line ART consisted of two nucleoside reverse transcriptase inhibitors (NRTI) and one non-nucleoside reverse transcriptase inhibitor (NNRTI). When patients were HIV-2 or HIV-1 and HIV-2 infected, first-line ART consisted of two NRTIs and one protease inhibitor (PI). CD4 counts and complete blood counts were measured every six months. Patients paid a fixed rate of US$2 per month for antiretroviral drugs and laboratory tests until August 2008, when the national HIV program made them available for free. All patients with CD4 counts ≤500/mm3 were also given cotrimoxazole prophylaxis. Isoniazid (INH) prophylaxis was not recommended as it is not part of the national treatment guidelines. Support groups were organized to encourage patients to adhere to therapy, and a community-based team made telephone calls or home visits when patients did not show up for clinic visits or to pick up antiretroviral drugs 26.
Monitoring and tracking system
All three study centers used the same standardized forms to record the following variables at routine visits: (i) initial visit: date, sex, date of birth (or age), height, weight, HIV type (HIV-1, HIV-2, or both); follow-up visit: date, weight; (iii) ART initiation visit: date, WHO clinical stage, weight; (iv) drug prescription (antiretroviral or other): date, name and quantity of drugs delivered; (v) CD4 count and complete blood count measurement: date, CD4 count, CD4 percentage, hemoglobin level, and platelet, granulocyte and leukocyte counts; (v) telephone call and home visit: dates at which patients were contacted, and vital status on that date; (vi) patients known to have died: date of death.
Additional procedures
The care provided to patients who agreed to participate in the study differed from that provided to other patients at the same clinic in three ways: (i) plasma HIV-1 RNA tests were performed every six months (ANRS real-time PCR; Biocentric, Bandol, France; threshold for detection, 300 copies/ml)27; (ii) if HIV RNA exceeded 300 copies/ml, we used the ANRS consensus technique 28 to perform an automated population full-sequence analysis of the reverse transcriptase and protease genes and interpreted genotype results using the French resistance algorithm (www.hivfrenchresistance.org); (iii) a research coordinator was devoted to monitoring and managing the cohort data and helping track patients by telephone and/or home visit. Genotype tests were performed in the virology laboratory of Necker hospital in Paris, France. This laboratory undergoes an annual external quality assurance evaluation 29.
Statistical analysis
Patients were defined as lost to follow up if: (i) their last contact with study team was <month 12; (ii) they were not known to be dead or transferred out before month 12; (iii) no further information on their vital status could be obtained within the 6 months following study endpoint (ie: between month 12 and month 18).
The medication possession ratio (MPR) was defined as the number of daily doses of antiretroviral drugs dispensed by the pharmacy to each patient, divided by that patient’s total follow-up time in days since ART initiation. We used generalized logistic regression analysis to estimate the association between MPR from baseline to month 12 and: (i) virologic failure and wild type HIV-1, defined as HIV-1 RNA ≥300 copies/ml at month 12 and no resistance mutations, or (ii) virologic failure and resistance, defined as HIV-1 RNA ≥300 copies/ml at month 12 and at least one resistance mutation. Results were adjusted for sex and baseline CD4 count. Analyses were performed with SAS® software, version 9.1 (SAS institute Inc. Cary, North Caroline, USA).
RESULTS
Patients
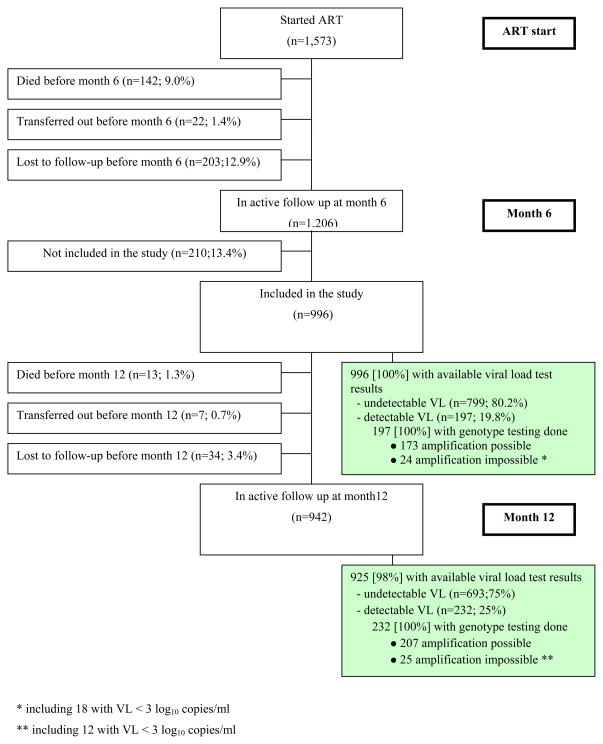
Overall, 1,573 adults started ART at the three study centers between February 2006 and May 2007. At month 6, 1,206 patients were still alive and in care. Of these, 996 gave written informed consent to participate in the cohort. Median age was 36 years (interquartile range [IQR], 30–43) and 747 patients (75%) were women. At month 6 and month 12, 100% and 98% of study subjects had an HIV-1 RNA test done (Figure 1).
Figure 1.
Flow chart of the study
- lost to follow up before month 6: (i) last contact with study team <month-6; (ii) not dead or transferred out before month-12; (iii) no further information on vital status being obtained within the 6 months following study endpoint (ie: between month-12 and month-18;
- lost to follow up before month 12: (i) last contact with study team <month-12; (ii) not dead or transferred out before month-12; (iii) no further information on vital status being be obtained within the 6 months following study endpoint (ie: between month-12 and month-18.
Table 1 presents the main characteristics of the study participants at ART initiation, month 6 and month 12. The 210 patients who were in care at month 6 but refused to participate in the study differed significantly from the 996 patients who enrolled with respect to several baseline and follow-up characteristics. At baseline, patients not included were more likely than patients who enrolled to be men (32% vs. 25%, p=0.05), less likely to receive d4T-3TC-NVP (48% vs. 58%) and more likely to receive ZDV-3TC-EFV (29% vs. 26%), d4T-3TC-EFV (10% vs. 8%), or other regimens (13% vs. 9%)(p=0.02). We did not find a significant difference in clinical stage (p=0.20), CD4 count (p=0.43), age (p=0.29), or body mass index (p=0.62) distributions. During follow-up, patients not included were more likely to die (6.2% vs. 1.3%, p<0.001), transfer out (3.3% vs. 0.7%, p<0.005), or be lost to follow-up (18.1% vs. 3.3%, p<0.001) before month 12 than patients who enrolled.
Table 1.
Summary of key characteristics at ART initiation, month 6 and month 12
| ART initiation (M0) n=996 |
Month 6 (M6) n=996 ** |
Month 12 (M12) n=942 ** |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Undetectable VL. | Detectable VL | Undetectable VL | Detectable VL | |||||||
| N=799 | N=197 | N=693 | N=232 | |||||||
| Regimen, n (%) | ||||||||||
| d4T 3TC NVP | 580 | (58%) | 413 | (52%) | 84 | (43%) | 328 | (47%) | 103 | (44%) |
| d4T 3TC EFV | 76 | (8%) | 123 | (15%) | 27 | (14%) | 104 | (15%) | 31 | (13%) |
| ZDV 3TC EFV | 254 | (26%) | 199 | (25%) | 56 | (28%) | 203 | (29%) | 63 | (27%) |
| Others | 86 | (9%) | 64 | (8%) | 30 | (15%) | 58 | (8%) | 35 | (15%) |
| Treatment modification, n (%) | ||||||||||
| One drug only | - | - | 137 | (17%) | 44 | (22%) | 161 | (23%) | 67 | (29%) |
| Entire regimen | - | - | 11 | (1%) | 6 | (3%) | 15 | (2%) | 4 | (2%) |
| CD4 count*, cells/mm3 | 148 | (68; 229) | 285 | (184; 407) | 215 | (131; 345) | 315 | (220; 450) | 232 | (128; 335) |
| CD4 change since M0* | - | - | +133 | (+71; +206) | +86 | (+26; +177) | +171 | (+98; +260) | +94 | (+22; +173) |
| CD4 change since M6* | - | - | - | - | - | - | +37 | (−17; +105) | −19 | (−84; +55) |
| BMI* | 19.8 | (17.8; 22.1) | 22.1 | (19.9; 24.5) | 21.5 | (19.6; 23.6) | 22.9 | (20.6; 25.4) | 22.2 | (20.0; 24.3) |
| BMI change since M0* | - | - | +1.9 | (+0.7; +3.7) | +1.6 | (+0.4; +3.6) | +2.8 | (+1.0; +4.6) | +2.4 | (+0.8; +4.5) |
| BMI change since M6* | - | - | - | - | - | - | +0.8 | (−0.3; +1.8) | +0.4 | (−0.9; +1.5) |
| Medication Possession Ratio * | - | - | 0.96 | (0.86; 1.00) | 0.76 | (0.57; 0.93) | 0.94 | (0.86;0.99) | 0.72 | (0.55; 0.87) |
| Getoype tests, n (%) ‡ | ||||||||||
| At least 1 mutation (any drug) ‡‡ | - | - | 69 | (35%) | - | - | 106 | (51%) | ||
| At least 1 mutation (NNRTI) | - | - | 64 | (32%) | - | - | 97 | (47%) | ||
| At least 1 mutation (NRTI) | - | - | 40 | (20%) | - | - | 75 | (36%) | ||
ART: antiretroviral therapy; NRTI: nucleoside reverse transcriptase inhibitor; NNRTI: non nucleoside reverse transcriptase inhibitor; d4T: stavudine; 3TC: lamivudine; FTC: emtricitabine; EFV: efavirenz; NVP: nevirapine; ZDV: zidovudine.
BMI: body mass index.
VL: plasma HIV-1 RNA Viral load
Median (interquartile range [IQR])
Plasma HIV-1 RNA was available for 996/996 (100%) patients at month 6, and 925/942 (98%) patients at month 12
Genotype tests: among the 197 patients with detectable VL at month 6, 24 had genotype testing but amplification was impossible, and 173 had genotype testing with amplification possible; among the 232 patients with detetectable VL at month 12, 25 had genotype testing but amplification was impossible, and 207 had genotype testing with amplification possible.
Resistance mutations are detailed in Appendix, Table A1, A2 and A3.
Other Missing values:
- At M0 (among 996 included patients) : Weight n=3 (0.3%)
- At M6 (among 996 included patients): CD4 n=5 (0.5%)
- At M12 (among 942 patients who were neither dead nor lost to follow-up before month 12): CD4 n=24 (2.5%), Weight n=21 (2.2%)
Plasma HIV-1 RNA
We obtained HIV-1 RNA test results for 996 patients at month 6 and 925 patients at month 12.
At month 6, 80% of patients had undetectable viral load and 20% had HIV-1 RNA ≥300 copies/ml, including 14% with HIV-1 RNA ≥1000 copies/ml. At month 12, 75% of patients had undetectable viral load and 25% had HIV-1 RNA ≥300 copies/ml, including 21 % with HIV-1 RNA ≥1000 copies/ml (Table 1).
Of the 168 patients who had HIV-1 RNA ≥300 copies/ml at month 6 and HIV-1 RNA test results at month 12, 44% had undetectable viral load at month 12. Of the 757 patients who had undetectable viral load at month 6 and had HIV-1 RNA test results at month 12, 18% had HIV-1 RNA ≥300 copies/ml at month 12. Of the 232 patients who had HIV-1 RNA ≥300 copies/ml at month 12, 40% already had HIV-1 RNA ≥300 copies/ml at month 6 and thus remained on a failed ART regimen for more than 6 months. The other 60% patients had undetectable viral load at month 6 and thus remained on failed ART for fewer than 6 months (Table 2).
Table 2.
Virologic status at month 6 and month 12 (n= 996)
| Month 6 | Month 12 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viral load |
Genotype |
|||||||||||
| NA | Undetectable | Detectable | NA | No resistance | Any resistance* | |||||||
| N | (Col%) | N | N | (Row%) | N | (Row%) | N | N | (Row%) | N | (Row%) | |
| Overall | 996 | - | 71 | 693 | (75%) | 232 | (25%) | 25 | 101 | (49%) | 106 | (51%) |
| VL undetectable | 799 | (80%) | 42 | 619 | (82%) | 138 | (18%) | 18 | 72 | (60%) | 48 | (40%) |
| VL detectable | 197 | (20%) | 29 | 74 | (44%) | 94 | (56%) | 7 | 29 | (33%) | 58 | (67%) |
| Genotype | ||||||||||||
| NA | 24 | - | 7 | 15 | (88%) | 2 | (12%) | 0 | 1 | (50%) | 1 | (50%) |
| No resistance | 104 | (60%) | 14 | 49 | (54%) | 41 | (46%) | 4 | 22 | (59%) | 15 | (41%) |
| Any resistance* | 69 | (40%) | 8 | 10 | (16%) | 51 | (84%) | 3 | 6 | (13%) | 42 | (88%) |
N: number of patients
NA: non available (patients dead, lost to follow-up or transferred out)
Col%: percentages of patients with detectable and undetectable patients at Month-5 are among those with available viral load (N=996); percentages of patients with wild strains and resistant strains at Month-12 are among those with available genotype results (N=173)
Row%: percentages of patients with detectable and undetectable patients at Month-12 are among those with available viral load (N=925); percentages of patients with no resistance and resistance at Month-12 are among those with available genotype results (N=207)
Resistance mutations are detailed in Appendix, Table A1, A2 and A3.
Resistance to antiretroviral medications
At month 6, 69 patients were infected with HIV-1 strains that had at least one resistance mutation. These patients represented 7% of the 996 patients study subjects, 35% of the 197 patients who had detectable HIV-1 RNA, and 40% of the 173 patients who had available genotype test results. Of the 69 resistant virus, all but one (99%) were resistant to lamivudine/emtricitabine and/or nevirapine/efavirenz (lamivudine/emtricitabine only, n=4, nevirapine/efavirenz only, n=28, both n=36). Only one of these strains had thymidine analogue mutations (TAMs), accounting for 0.1% of the 996 study subjects and for 1% of the 69 patients harbouring a virus with at least one mutation (appendix, Table A1).
At month 12, 106 patients were infected with HIV strains that had at least one resistance mutation. These patients represented 11% of the 925 study subjects who were still in active follow-up, 46% of the 232 patients who had detectable HIV RNA, and 51% of the 207 patients who had available genotype test results. Of the 106 resistant virus, all but one (99%) were resistant to lamivudine/emtricitabine and/or nevirapine/efavirenz (lamivudine/emtricitabine only, n=8, nevirapine/efavirenz only, n= 33, both n= 64). Eight patients had a virus with TAMs, accounting for 0.9% of the 925 patients included in the study and for 7.5% of the 106 patients harbouring a virus with at least one mutation (appendix, Table A2). No virus was found resistant to the new generation of NNRTIs (etravirine) either at month 6 or at month 12.
Of the 11% of patients (n=106/925) who were found to have at least one resistance mutation at month 12, 4.5% (n=42/925) already had at least one resistance mutation at month 6, 1.7% (n=15/925) had detectable HIV-1 RNA but no mutations at month 6, and 5.2% (n=48/925) had undetectable HIV-1 RNA at month 6 (Table 2). Finally, among the eight patients who had TAMs at month 12, one (12.5%) had undetectable HIV-1 RNA at month 6.
Medication Possession Ratio
Median MPR was 95% (IQR, 81%–99%; range, 0%–161%) from ART initiation to month 6 and 90% (IQR, 78%–98%; range, 0%–139%) from ART initiation to month 12. As MPR increased, the proportion of patients with HIV-1 RNA ≥300 copies/ml regardless of resistance decreased.
At month 6, 62% of patients whose MPR was <50% had detectable HIV-1 RNA, of whom 20% had at least one resistance mutation. These proportions decreased to 9% and 2% when MPRs were >95% (Figure 2A). At month 12, 85% of patients whose MPR was <50% had detectable HIV-1 RNA, of whom 37% had at least one resistance mutation. These percentages were 9% and 4% when MPRs were >95% (Figure 2B).
Figure 2.
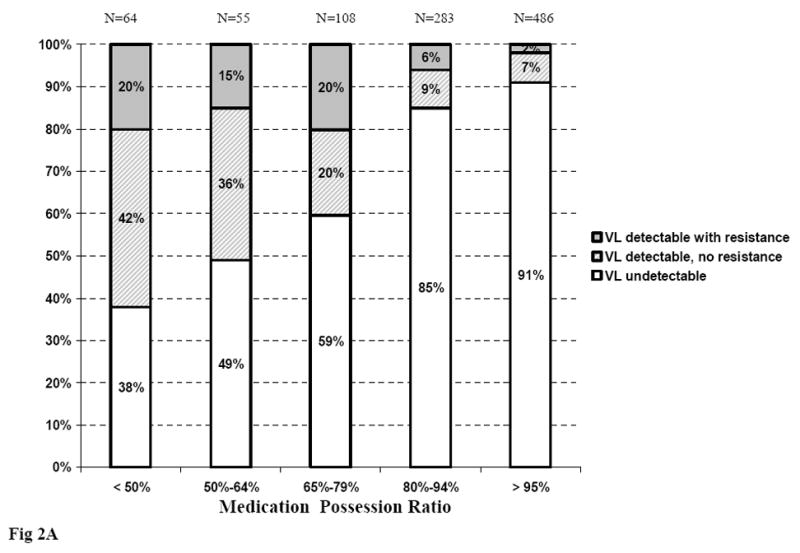
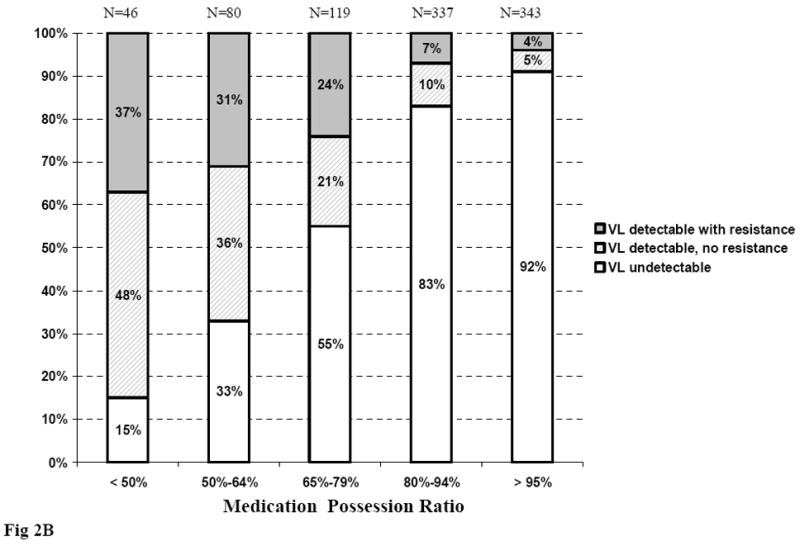
Virological status at month 6 and month 12, according to Medication Possession Ratio
Figure 2A. Virological status at month 6, according to Medication Possession Ratio between ART initiation and month 6
Figure 2B. Virological status at month 12, according to Medication Possession Ratio between ART initiation and month 12
ART: Antiretroviral treatment; VL: viral load (plasma HIV-1 RNA)
At month 6, the median MPR among patients with undetectable HIV-1 RNA was 96% (IQR, 86%–100%), and was significantly higher than for patients with detectable HIV-1 RNA and no resistance mutations (median, 77%; IQR, 53%–96%; p<0.0001) as well as for patients with both detectable HIV-1 RNA and resistance mutations (median, 75%; IQR, 61%–86%; p<0.0001). Among patients with detectable HIV-1 RNA, there was no significant difference in MPR distribution between patients with and without resistance (Figure 3A). In patients with detectable viral load at month 6, the median MPR increased with decreasing levels of viral load (median MPR 0.91, 0.77, 0.73, and 0.64 in patients with HIV-1 RNA <3 log10 copies/ml, 3–3.9 log10 copies/ml, 4–4.9 log10 copies/ml and ≥5 log10 copies/ml, respectively; p<0.0001).
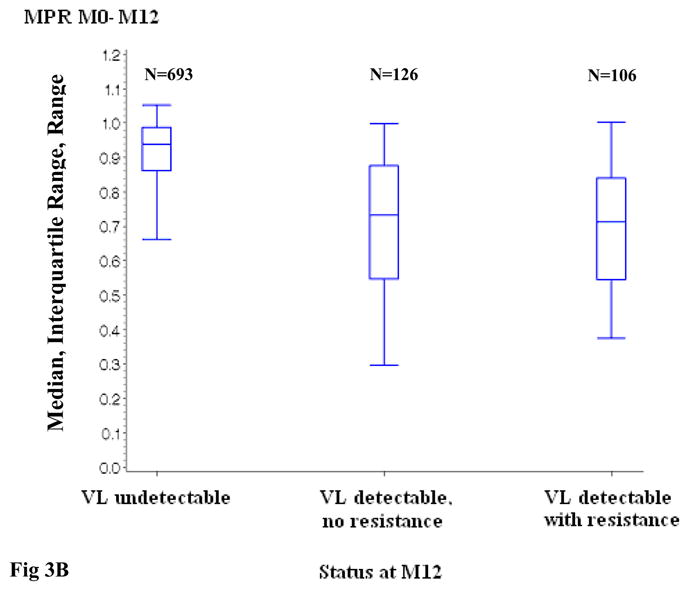
Figure 3.
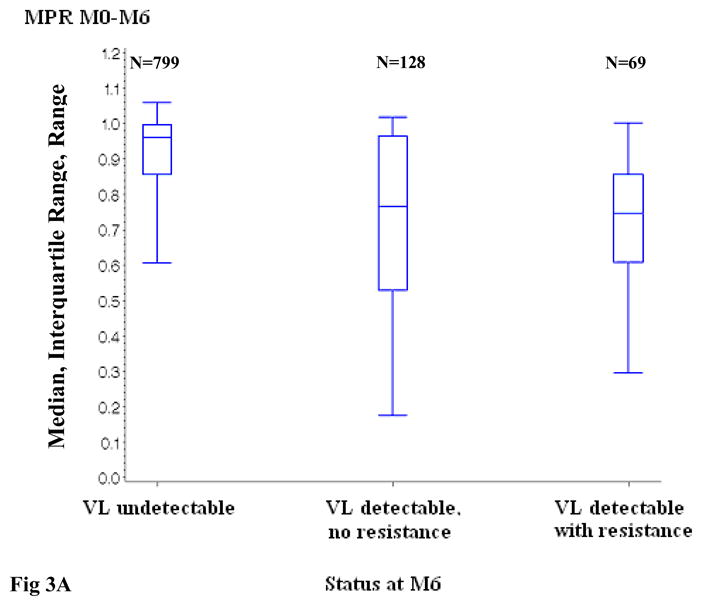
Distribution of the Medication Possession Ratio, according to virologic status at month 6 and month 12
Figure 3A. Distribution of the Medication Possession Ratio between ART initiation and month 6, according to virologic status at month 6
Figure 3B. Distribution of the Medication Possession Ratio between ART initiation and month 12, according to virologic status at month 12
ART: Antiretroviral treatment; VL: viral load (plasma HIV-1 RNA)
MPR: Medication Possession Ratio
M0: ART initiation; M6: month 6; M12: month 12
VL detectable, no resistance versus VL undetectable : p <0.0001 at month 6 and month 12
VL detectable with resistance versus VL undetectable : p <0.0001 at month 6 and month 12
VL detectable with resistance versus VL detectable, no resistance: p= 0.64 at month 6 and 0.76 at month 12
At month 12, the median MPR for patients with undetectable HIV-1 RNA was 94% (IQR, 86%–99%) and again was significantly higher than for patients with detectable HIV-1 RNA and no resistance mutations (median, 73%; IQR, 55%–88%; p<0.0001) as well as for patients with both detectable HIV-1 RNA and resistance mutations (median, 71%; IQR, 55%–84%; p<0.0001). Among patients with detectable HIV-1 RNA at month 12, there was no significant difference between patients with and without resistance (Figure 3B). In patients with detectable viral load at month 12, the median MPR increased with decreasing levels of viral load (median MPR 0.89, 0.69, 0.74, and 0.65 in patients with HIV-1 RNA <3 log10 copies/ml, 3–3.9 log10 copies/ml, 4–4.9 log10 copies/ml and ≥5 log10 copies/ml, respectively; p<0.0001).
In multivariate analysis, MPR was associated with both detectable HIV-1 RNA with wild type virus and detectable HIV-1 RNA with at least one resistance mutation at month 12, after adjusting for baseline CD4 count and sex (Table 3). There was no interaction between regimen and MPR for the association with virologic status (p=0.98).
Table 3.
Factors associated with virologic outcomes at month 12
| Virologic outcomes, vs. undetectable VL | Univariate analaysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | ||
| History of pMTCT with NVP, Yes vs. No | 0.53 | ||||||
| Detec VL, no resistance | 0.4 | (0.1–3.0) | 0.36 | - | - | - | |
| Detec VL, resistance | 0.5 | (0.1–3.6) | 0.46 | - | - | - | |
| First line regimen, vs d4T 3TC EFV | 0.61 | ||||||
| ZDV 3TC EFV | Detec VL, no resistance | 0.7 | (0.3–1.5) | 0.34 | - | - | - |
| Detec VL, resistance | 1.7 | (0.6–4.7) | 0.28 | - | - | - | |
| d4T 3TC NVP | Detec VL, no resistance | 0.8 | (0.4–1.5) | 0.44 | - | - | - |
| Detec VL, resistance | 1.4 | (0.5–3.7) | 0.48 | - | - | - | |
| Baseline BMI, vs. < 18.5 kg/m2 | 0.33 | ||||||
| >22.5 | Detec VL, no resistance | 0.6 | (0.4–1.0) | 0.06 | - | - | - |
| Detec VL, resistance | 0.7 | (0.4–1.3) | 0.24 | - | - | - | |
| 18.5–22.5 | Detec VL, no resistance | 0.8 | (0.5–1.2) | 0.19 | - | - | - |
| Detec VL, resistance | 0.8 | (0.5–1.3) | 0.45 | ||||
| MPR, vs. >95% | <0.0001 | <0.0001 | |||||
| < 80% | Detec VL, no resistance | 15.1 | (8.4–27.0) | <0.0001 | 15.7 | (8.7–28.3) | <0.0001 |
| Detec VL, resistance | 17.1 | (9.1–32.2) | <0.0001 | 18.4 | (9.7–35.0) | <0.0001 | |
| 80–95% | Detec VL, no resistance | 2.4 | (1.3–4.4) | 0.006 | 2.4 | (1.3–4.4) | 0.006 |
| Detec VL, resistance | 2.0 | (1.0–4.0) | 0.05 | 2.0 | (1.0 – 4.0) | 0.06 | |
| Pre-ART CD4 count, vs. <100/mm3 | 0.005 | 0.002 | |||||
| > 250/mm3 | Detec VL, no resistance | 1.1 | (0.7–1.8) | 0.75 | 1.0 | (0.6 – 1.7) | 0.97 |
| Detec VL, resistance | 0.4 | (0.2–0.8) | 0.007 | 0.4 | (0.2 – 0.7) | 0.002 | |
| 100–250/mm3 | Detec VL, no resistance | 0.8 | (0.5–1.2) | 0.29 | 0.7 | (0.5 – 1.2) | 0.21 |
| Detec VL, resistance | 0.5 | (0.3–0.8) | 0.002 | 0.4 | (0.3 – 0.7) | 0.001 | |
| Sex, Male vs.Female | 0.15 | 0.08 | |||||
| Detec VL, no resistance | 1.5 | (1.0–2.3) | 0.05 | 1.7 | (1.1 – 2.7) | 0.03 | |
| Detec VL, resistance | 1.1 | (0.7–1.7) | 0.82 | 1.0 | (0.6 – 1.8) | 0.90 | |
Vs: Versus
pMTCT : prevention of mother to child transmission of HIV-1
NVP: nevirapine; ZDV: zidovudine; EFV: efavirenz; 3TC: lamivudine; d4T: stavudine
MPR: medication possession ratio
VL: plasma HIV-1 RNA
Detec: detectable (>300 copies/ml)
OR: Odds Ratio
CI: Confidence Interval
Discussion
We conducted a prospective cohort study nested in three large HIV care programs in Abidjan, Côte d’Ivoire. The study participants received the same care and treatment as non-participants at the same HIV care centers. In addition to providing regular follow-up, they also had HIV-1 RNA done every six months, with genotype resistance tests when HIV-1 RNA was detectable. We recorded virologic and genotype data on these patients in their first year of ART. We had four main findings.
First, 20% and 25% of patients had plasma HIV-1 RNA ≥300 copies at month 6 and month 12, respectively. The percentage of patients infected with resistant HIV-1 strains was 7% at month 6 and 11% at month 12. These rates are at the lower bound of estimates reported in others studies on patients on ART in sub-Saharan Africa 30–37.
Second, we found that viral suppression was a dynamic process. In other words, virologic success at month 6 did not guarantee virologic success at month 12. Among patients who had detectable HIV-1 RNA at month 12, 60% had undetectable viral load at month 6. It is likely that these patients did not adhere to their medications well enough to remain undetectable beyond month 6.
Third, 49% of the participants who had detectable HIV-1 RNA and available genotype test results at month 12 had no resistance mutations. Preventing resistance from developing is clearly one important objective in the early phases of HIV treatment. This very large proportion of patients with measurable HIV-1 RNA levels, but no resistance, suggests that challenges for adherence persist through month 12. A routine HIV-1 RNA test at month 12 could thus help identify many patients who might benefit from an intervention to improve adherence and prevent resistance. However, it also indicates that half of those patients with detectable HIV-1 RNA at month 12 already had resistant HIV strains and that HIV-1 RNA testing in the absence of genotype testing, therefore, cannot differentiate between patients who should switch to second-line ART due to resistance and patients who could still benefit from improved adherence to first-line ART.
Fourth, as expected, most of the resistance mutations that developed in the first year of NNRTI-based first-line ART were to lamivudine/emtricitabine and/or to nevirapine/efavirenz. TAMs were very rare at month 6, but their prevalence increased to 7.5% of all resistant strains and 0.9% of the overall study population at month 12. This trend is important, because it illustrates the difficulty of targeting patients who are resistant to lamivudine/emtricitabine and/or nevirapine/efavirenz and then switching them to a second-line regimen before they develop resistance to other NRTIs. This goal is not achievable in settings where genotype tests are not available, and even less so when HIV-1 RNA tests are not available for routine care. Furthermore, one patient in our study who had TAMs at month 12 had undetectable HIV RNA at month 6, suggesting that even if genotype tests were available a genotype test six months after ART initiation would not be sufficient. Of note, very few patients had a virus resistant to abacavir, ddI or tenofovir at month 12, and none had resistance to etravirine.
This study has several limitations. First we did not have genotype tests results prior to ART initiation. Virologic resistance at 6 months may in part be due to primary resistance 32, 38. However, the prevalence of resistance mutations in adults with recent HIV infection in Côte d’Ivoire was recently estimated to be lower than 5% 39. Second, some data are missing, because 13% of the patients who were in active follow-up at month 6 refused to enroll in the study, 2% of the patients who enrolled at month 6 did not receive HIV RNA measurements at month 12, and 11% of patients who had detectable HIV RNA did not have available genotype test results. As compared to patients who enrolled, those who refused to enroll were less likely to receive d4T-3TC-NVP and had higher rates of death and loss to follow-up between month-6 and month-12. Third, the threshold of HIV RNA detectability was ≥300 copies/ml, which may have led us to underestimate the proportion of patients who were not completely virologically suppressed, and thus the proportion of patients with resistance. Fourth, nearly one third of the patients started on ART in these programs either died, transferred out or were lost to follow-up before month 6. However, their outcomes were likely worse than those of patients who enrolled in the study, and the association we found between MPR and virologic outcomes in patients who enrolled is likely to also exist in patients who did not.
In conclusion, in these patients still alive and in care after 6 months of ART, MPR was strongly associated with virologic outcomes during the first year on ART, and half of patients with detectable plasma HIV-1 RNA at month 12 had no resistance mutations. In the absence of HIV RNA tests, MPR should be used at month 6 to identify patients who might benefit from early interventions to reinforce adherence.
Acknowledgments
Funding: Agence Nationale de Recherches sur le SIDA et les hepatitis virales (ANRS 12136, ANRS 12212), National Institute of Allergy and Infectious Diseases (R01 AI058736, K24 AI062476) and Institute of International Education Fulbright (Fellowship CS)
References
- 1.Chene G, Sterne JA, May M, Costagliola D, Ledergerber B, Phillips AN, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003;362:679–86. doi: 10.1016/s0140-6736(03)14229-8. [DOI] [PubMed] [Google Scholar]
- 2.Fielding KL, Charalambous S, Stenson AL, Pemba LF, Martin DJ, Wood R, et al. Risk factors for poor virological outcome at 12 months in a workplace-based antiretroviral therapy programme in South Africa: a cohort study. BMC Infect Dis. 2008;8:93. doi: 10.1186/1471-2334-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanson DL, Adje-Toure C, Talla-Nzussouo N, Eby P, Borget MY, Kouadio LY, et al. HIV type 1 drug resistance in adults receiving highly active antiretroviral therapy in Abidjan, Cote d’Ivoire. AIDS Res Hum Retroviruses. 2009;25:489–95. doi: 10.1089/aid.2008.0273. [DOI] [PubMed] [Google Scholar]
- 4.Bangsberg DR. Preventing HIV antiretroviral resistance through better monitoring of treatment adherence. J Infect Dis. 2008;197 (Suppl 3):S272–8. doi: 10.1086/533415. [DOI] [PubMed] [Google Scholar]
- 5.Danel C, Gabillard D, Inwoley A, Chaix ML, Toni TD, Moh R, et al. Medium-term probability of success of antiretroviral treatment after early warning signs of treatment failure in West African adults. AIDS Res Hum Retroviruses. 2009;25:783–93. doi: 10.1089/aid.2009.0018. [DOI] [PubMed] [Google Scholar]
- 6.Carrieri P, Cailleton V, Le Moing V, Spire B, Dellamonica P, Bouvet E, et al. The dynamic of adherence to highly active antiretroviral therapy: results from the French National APROCO cohort. J Acquir Immune Defic Syndr. 2001;28:232–9. doi: 10.1097/00042560-200111010-00005. [DOI] [PubMed] [Google Scholar]
- 7.Rueda S, Park-Wyllie LY, Bayoumi AM, Tynan AM, Antoniou TA, Rourke SB, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev. 2006;3:CD001442. doi: 10.1002/14651858.CD001442.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller LG, Hays RD. Adherence to combination antiretroviral therapy: synthesis of the literature and clinical implications. AIDS Read. 2000;10:177–85. [PubMed] [Google Scholar]
- 9.Kouanfack C, Laurent C, Peytavin G, Ciaffi L, Ngolle M, Nkene YM, et al. Adherence to antiretroviral therapy assessed by drug level monitoring and self-report in cameroon. J Acquir Immune Defic Syndr. 2008;48:216–9. doi: 10.1097/QAI.0b013e3181743955. [DOI] [PubMed] [Google Scholar]
- 10.Kredo T, Van der Walt JS, Siegfried N, Cohen K. Therapeutic drug monitoring of antiretrovirals for people with HIV. Cochrane Database Syst Rev. 2009:CD007268. doi: 10.1002/14651858.CD007268.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Diabate S, Alary M, Koffi CK. Determinants of adherence to highly active antiretroviral therapy among HIV-1-infected patients in Cote d’Ivoire. Aids. 2007;21:1799–803. doi: 10.1097/QAD.0b013e3282a5667b. [DOI] [PubMed] [Google Scholar]
- 12.Ramadhani HO, Thielman NM, Landman KZ, Ndosi EM, Gao F, Kirchherr JL, et al. Predictors of incomplete adherence, virologic failure, and antiviral drug resistance among HIV-infected adults receiving antiretroviral therapy in Tanzania. Clin Infect Dis. 2007;45:1492–8. doi: 10.1086/522991. [DOI] [PubMed] [Google Scholar]
- 13.Eholie SP, Tanon A, Polneau S, Ouiminga M, Djadji A, Kangah-Koffi C, et al. Field adherence to highly active antiretroviral therapy in HIV-infected adults in Abidjan, Cote d’Ivoire. J Acquir Immune Defic Syndr. 2007;45:355–8. doi: 10.1097/QAI.0b013e31805d8ad0. [DOI] [PubMed] [Google Scholar]
- 14.Bisson GP, Gross R, Bellamy S, Chittams J, Hislop M, Regensberg L, et al. Pharmacy refill adherence compared with CD4 count changes for monitoring HIV-infected adults on antiretroviral therapy. PLoS Med. 2008;5:e109. doi: 10.1371/journal.pmed.0050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitahata MM, Reed SD, Dillingham PW, Van Rompaey SE, Young AA, Harrington RD, et al. Pharmacy-based assessment of adherence to HAART predicts virologic and immunologic treatment response and clinical progression to AIDS and death. Int J STD AIDS. 2004;15:803–10. doi: 10.1258/0956462042563666. [DOI] [PubMed] [Google Scholar]
- 16.Nachega JB, Hislop M, Dowdy DW, Lo M, Omer SB, Regensberg L, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006;43:78–84. doi: 10.1097/01.qai.0000225015.43266.46. [DOI] [PubMed] [Google Scholar]
- 17.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 18.Goldman JD, Cantrell RA, Mulenga LB, Tambatamba BC, Reid SE, Levy JW, et al. Simple adherence assessments to predict virologic failure among HIV-infected adults with discordant immunologic and clinical responses to antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:1031–5. doi: 10.1089/aid.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146:564–73. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 20.Gross R, Yip B, Lo Re V, 3rd, Wood E, Alexander CS, Harrigan PR, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis. 2006;194:1108–14. doi: 10.1086/507680. [DOI] [PubMed] [Google Scholar]
- 21.Acri T, Tenhave TR, Chapman JC, Bogner HR, Gross R. Lack of Association between Retrospectively Collected Pharmacy Refill Data and Electronic Drug Monitoring of Antiretroviral Adherence. AIDS Behav. 2008 doi: 10.1007/s10461-008-9502-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Messou E, Anglaret X, Duvignac J, Konan-N’dri E, Komena E, Gnokoro J, et al. Antiretroviral treatment changes in adults from Cote d’Ivoire: the roles of tuberculosis and pregnancy. AIDS. 24:93–9. doi: 10.1097/QAD.0b013e32832ec1c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minga A, Danel C, Abo Y, Dohoun L, Bonard D, Coulibaly A, et al. Progression to WHO criteria for antiretroviral therapy in a 7-year cohort of adult HIV-1 seroconverters in Abidjan, Cote d’Ivoire. Bull World Health Organ. 2007;85:116–23. doi: 10.2471/BLT.06.032292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messou E, Anglaret X, Duvignac J, Konan-N’dri E, Komena E, Gnokoro J, et al. Antiretroviral treatment changes in adults from Cote d’Ivoire: the roles of tuberculosis and pregnancy. Aids. 2010;24:93–9. doi: 10.1097/QAD.0b013e32832ec1c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. [Accessed January 8 2009];2006 revision. 2006 http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. [PubMed]
- 26.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d’Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–82. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rouet F, Ekouevi DK, Chaix ML, Burgard M, Inwoley A, Tony TD, et al. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited setting. J Clin Microbiol. 2005;43:2709–17. doi: 10.1128/JCM.43.6.2709-2717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasquier C, Millot N, Njouom R, Sandres K, Cazabat M, Puel J, et al. HIV-1 subtyping using phylogenetic analysis of pol gene sequences. J Virol Methods. 2001;94:45–54. doi: 10.1016/s0166-0934(01)00272-5. [DOI] [PubMed] [Google Scholar]
- 29.Descamps D, Delaugerre C, Masquelier B, Ruffault A, Marcelin AG, Izopet J, et al. Repeated HIV-1 resistance genotyping external quality assessments improve virology laboratory performance. J Med Virol. 2006;78:153–60. doi: 10.1002/jmv.20522. [DOI] [PubMed] [Google Scholar]
- 30.Hamers RL, Derdelinckx I, van Vugt M, Stevens W, de Wit TFR, Schuurman R. The status of HIV-1 resistance to antiretroviral drugs in sub-Saharan Africa. Antiviral Therapy. 2008;13 (5):625–39. [PubMed] [Google Scholar]
- 31.Vergne L, Kane CT, Laurent C, Diakhate N, Gueye NF, Gueye PM, et al. Low rate of genotypic HIV-1 drug-resistant strains in the Senegalese government initiative of access to antiretroviral therapy. Aids. 2003;17 (Suppl 3):S31–8. doi: 10.1097/00002030-200317003-00005. [DOI] [PubMed] [Google Scholar]
- 32.Adje-Toure C, Celestin B, Hanson D, Roels TH, Hertogs K, Larder B, et al. Prevalence of genotypic and phenotypic HIV-1 drug-resistant strains among patients who have rebound in viral load while receiving antiretroviral therapy in the UNAIDS-Drug Access Initiative in Abidjan, Cote d’Ivoire. Aids. 2003;17 (Suppl 3):S23–9. doi: 10.1097/00002030-200317003-00004. [DOI] [PubMed] [Google Scholar]
- 33.Adje C, Cheingsong R, Roels TH, Maurice C, Djomand G, Verbiest W, et al. High prevalence of genotypic and phenotypic HIV-1 drug-resistant strains among patients receiving antiretroviral therapy in Abidjan, Cote d’Ivoire. J Acquir Immune Defic Syndr. 2001;26:501–6. doi: 10.1097/00126334-200104150-00018. [DOI] [PubMed] [Google Scholar]
- 34.Barth RE, van der Loeff MF, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 10:155–66. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 35.Bile EC, Adje-Toure C, Borget MY, Kalou M, Diomande F, Chorba T, et al. Performance of drug-resistance genotypic assays among HIV-1 infected patients with predominantly CRF02_AG strains of HIV-1 in Abidjan, Cote d’Ivoire. J Clin Virol. 2005;32:60–6. doi: 10.1016/j.jcv.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Johannessen A, Naman E, Kivuyo SL, Kasubi MJ, Holberg-Petersen M, Matee MI, et al. Virological efficacy and emergence of drug resistance in adults on antiretroviral treatment in rural Tanzania. BMC Infect Dis. 2009;9:108. doi: 10.1186/1471-2334-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seyler C, Adje-Toure C, Messou E, Dakoury-Dogbo N, Rouet F, Gabillard D, et al. Impact of genotypic drug resistance mutations on clinical and immunological outcomes in HIV-infected adults on HAART in West Africa. Aids. 2007;21:1157–64. doi: 10.1097/QAD.0b013e3281c615da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toni T, Masquelier B, Minga A, Anglaret X, Danel C, Coulibaly A, et al. HIV-1 antiretroviral drug resistance in recently infected patients in Abidjan, Cote d’Ivoire: A 4-year survey, 2002–2006. AIDS Res Hum Retroviruses. 2007;23:1155–60. doi: 10.1089/aid.2007.0072. [DOI] [PubMed] [Google Scholar]
- 39.Ayouba A, Lien TT, Nouhin J, Vergne L, Aghokeng AF, Ngo-Giang-Huong N, et al. Low prevalence of HIV type 1 drug resistance mutations in untreated, recently infected patients from Burkina Faso, Cote d’Ivoire, Senegal, Thailand, and Vietnam: the ANRS 12134 study. AIDS Res Hum Retroviruses. 2009;25:1193–6. doi: 10.1089/aid.2009.0142. [DOI] [PubMed] [Google Scholar]