Abstract
Background
Recurrent DVT risk factors include a first idiopathic DVT, strongly suggesting the existence of undiagnosed and/or unidentified prothrombotic abnormalities.
Objectives
To evaluate the impact of locally increased RBC aggregation on DVT pathogenesis in a rabbit model.
Methods
DVT presence, flow and aggregation were measured in-situ with ultrasound. Greatly enhanced aggregation was achieved by covalent linkage of Pluronic F98 to the RBC surface; coating with Pluronic F68, which very mildly enhances aggregation, was used as a coating control. On day 1, endothelial damage and a partial stenosis were surgically created on the left femoral vein while the right femoral vein was not manipulated.
Results
A thrombus was formed within 30 minutes in 6 of 7 left femoral veins of animals receiving a 30% volume blood exchange with F98-coated RBC, whereas a thrombus occurred in only 1 of 7 veins in F68-transfused controls. In vivo imaging using quantitative ultrasound confirmed increased aggregation in the thrombosed veins of the F98 group compared with the F68 group and the contralateral vessel. For each group, 5 animals were followed for 2 weeks before sacrifice. In F98-transfused animals, lysis of clots occurred and the presence of chronic thrombi totally occluding the vein in 3 of 5 animals was confirmed by histology. Conversely, in the F68 group, a single disorganized blood clot was observed in 1 of 5 animals.
Conclusions
A marked increase in RBC aggregation promotes thrombosis in rabbit femoral veins, confirming a pathophysiological role of locally altered hemorheology in the onset of DVT.
Keywords: hemorheology, Pluronics, rabbit model, thrombosis, ultrasound tissue characterization, quantitative ultrasound imaging
Introduction
Clinical conditions associated with DVT are classified as transient/provoked (e.g., surgery, trauma, immobilization) or persistent (e.g., cancer, paralysis, sex, age, hormone intake), with the latter group at higher risk of recurrence [1;2]. Interestingly, a first DVT episode of idiopathic origin, representing nearly half of DVT cases [1;3], increases the risk of recurrent DVT by a ratio of 1.9 compared to provoked first DVT episode [4;5]. The role of molecular thrombophilia and anticoagulant deficiencies have been investigated in large scale observational studies [1;4], yet have not been shown to be predictive of recurrent DVT. These findings suggest that other factors, perhaps on the local scale, remain undiagnosed and/or unidentified in DVT recurrence risk profiling.
Despite implications arising from several correlation studies [6–9], hyperviscosity and red blood cell hyperaggregation are classic prothrombotic abnormalities that are often overlooked in first time and recurrent DVT patients. Of particular interest, one recent large case-control study found that hyperfibrinogenemia, but not thrombophilia, hypercysteinemia or anticoagulation deficiencies, increased the DVT recurrence hazard ratio confidence interval above unity [4]. Plasma fibrinogen concentration is a primary determinant of red blood cell (RBC) aggregation and hence of the non-Newtonian flow behavior of blood: aggregation at low shear or flow rates greatly increases blood viscosity, while at high rates, aggregates are dispersed and viscosity decreases [10]. In another study based on a one-year follow-up, patients with persistent DVT risk factors had higher plasma fibrinogen, RBC aggregation and plasma viscosity than patients with transient risk factors [2], suggesting that these abnormal rheological parameters could contribute to maintaining DVT risk.
Unfortunately, a complete understanding of the role of altered hemorheology in DVT is hampered by conflicting reports in the literature. For example, hyperfibrinogenemia, which has long been identified as a potential risk factor of DVT [7], has recently been shown to increase DVT occurrence with a risk ratio of 4.2 for patients aged above 45 [11]. However, a similar risk ratio could not be established in younger patients [11] nor in individuals presenting with genetically elevated fibrinogen [12]. These differences are possibly be due to whether systemic or local conditions are considered: local regions of slow flow and hence elevated aggregation and viscosity may be more relevant than estimates of general flow dynamics based solely on fibrinogen levels.
In this study, we tested the general hypothesis that locally increased RBC aggregation could tilt the haemostatic equilibrium beyond its prothrombotic threshold and trigger thrombosis in venous flow. Ultrasound imaging, which can measure flow and is sensitive to in vivo and in vitro RBC aggregation [13], was used to detail local flow and RBC aggregation. Our findings in an animal model support the hypothesis that local characterisation of aggregation could be of predictive value in patients at risk for DVT.
Materials and Methods
Animals and groups
Twelve New Zealand white rabbits weighting 3.3 to 3.5 kg were used, randomly assigned to either the hyperaggregating F98 group (n = 6) or to the control F68 group (n = 6). One animal in each group was studied to examine the acute response following lumen area reduction and endothelial damage of both left (LFV) and right (RFV) femoral veins. The other five animals per group underwent vessel stenosis and endothelial damage only on the LFV with the contralateral RFV used as a paired control for aggregation measurements. Within each group of five animals, longitudinal follow-up was performed for two weeks at five time points: pre-intervention (d0), 30 minutes post-intervention (d1), and on days 4 (d4), 9 (d9) and 14 (d14). All procedures were performed aseptically and were approved by the animal care committee of the Centre de Recherche du Centre Hospitalier de l'Université de Montréal in accordance with the Canadian Council on Animal Care guidelines and also conformed with the guide for the care and use of laboratory animals of the USA National Institutes of Health (assurance number A5377-01).
Blood exchange transfusion and DVT model
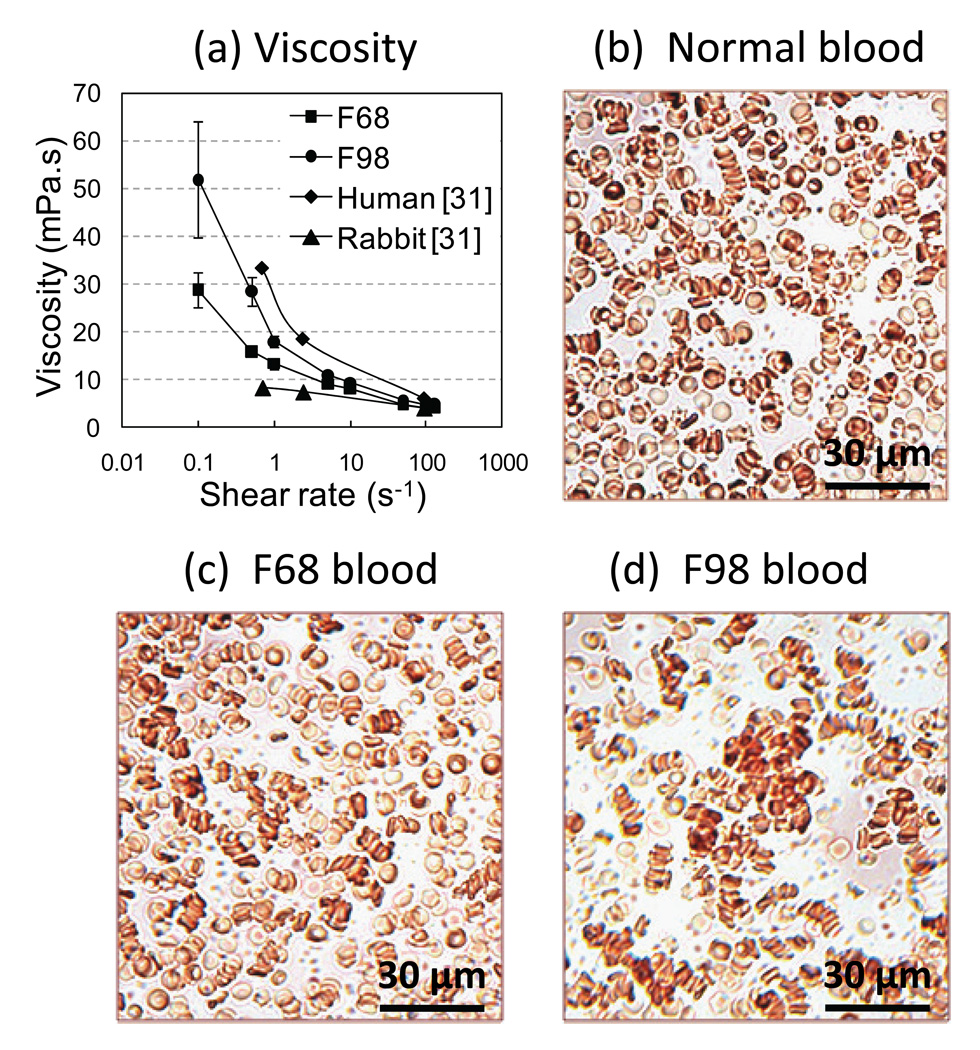
Blood collection from donor animals and preparation of coated RBC were performed the day prior to the surgery; the cells were stored at 4°C overnight. Blood transfusions were performed the next day. The covalent attachment of Pluronic copolymers F68 and F98 to the RBC surface was employed, as described previously [14–16]. Briefly, Pluronics are block copolymers comprised of a central poly(propylene glycol) -PPG- chain flanked by two poly(ethylene glycol) -PEG- chains. In aqueous solution, these copolymers micellize (self-aggregate) at a critical temperature primarily dependent on the molecular weight of the PPG core. By utilizing the known micellization behavior of Pluronics, following covalent attachment of its derivatized form to the RBC surface, it is possible to create RBC with defined aggregation tendencies. In this study, the Pluronic F98 coating (0.75 mg/mL) at a 30% volume concentration when mixed with whole rabbit blood indeed induced a strong increase in low shear blood viscosity (Figure 1a – LS300, Prorheo Inc, Hanover, Germany) whereas the Pluronic F68 polymer (0.75 mg/mL) only induced a very mild change in viscosity. The aggregation level attained in the F98 group was comparable to human physiological level but remained higher compared to non-coated or F68-coated rabbit blood (Figure 1a). Pluronic coatings did not affect RBC shape or deformability but induced different aggregation levels (Figures 1b, c and d).
Figure 1.
(a) Low shear viscosity as a function of the shear rate for 30% Pluronic F68-coated RBC, 30% Pluronic F98-coated RBC, and normal values for uncoated rabbit and human bloods taken from [31]. Microscopic images of diluted rabbit RBC (H~5%) suspended in native plasma at 37°C for: (b) normal rabbit blood; (c) 30% Pluronic F68-coated RBC showing RBC aggregates comparable to normal rabbit blood; and (d) 30% Pluronic F98-coated RBC showing enhanced aggregation.
Rabbits were anesthetized using 2% isoflurane in 100% oxygen. Heart rate, oxygen saturation and body temperature were continuously monitored with a multi-parameter physiologic monitor (LW6000, Digicare Biomedical Technology, Boynton Beach, FL), with body temperature maintained at 37 ± 1 °C. Normal saline (i.e., 0.9% NaCl) was continuously infused at a rate of 10 ml/kg/hour. The left jugular vein and contralateral common carotid artery were exposed and canulated with 22 G (0.95 mm diameter) catheters (Smiths Medical ASD, Southington, CT). Thirty percent of total blood volume, estimated at 6% of body weight, was exchanged with coated blood warmed to 37 °C by simultaneous venous injection and arterial withdrawal using a Harvard double syringe pump (PHD2000, Harvard Apparatus, Holliston, MA) at a flow rate of 20 ml/min. The left jugular vein and common carotid artery catheters were then removed, these vessels ligatured and the surgical opening sutured.
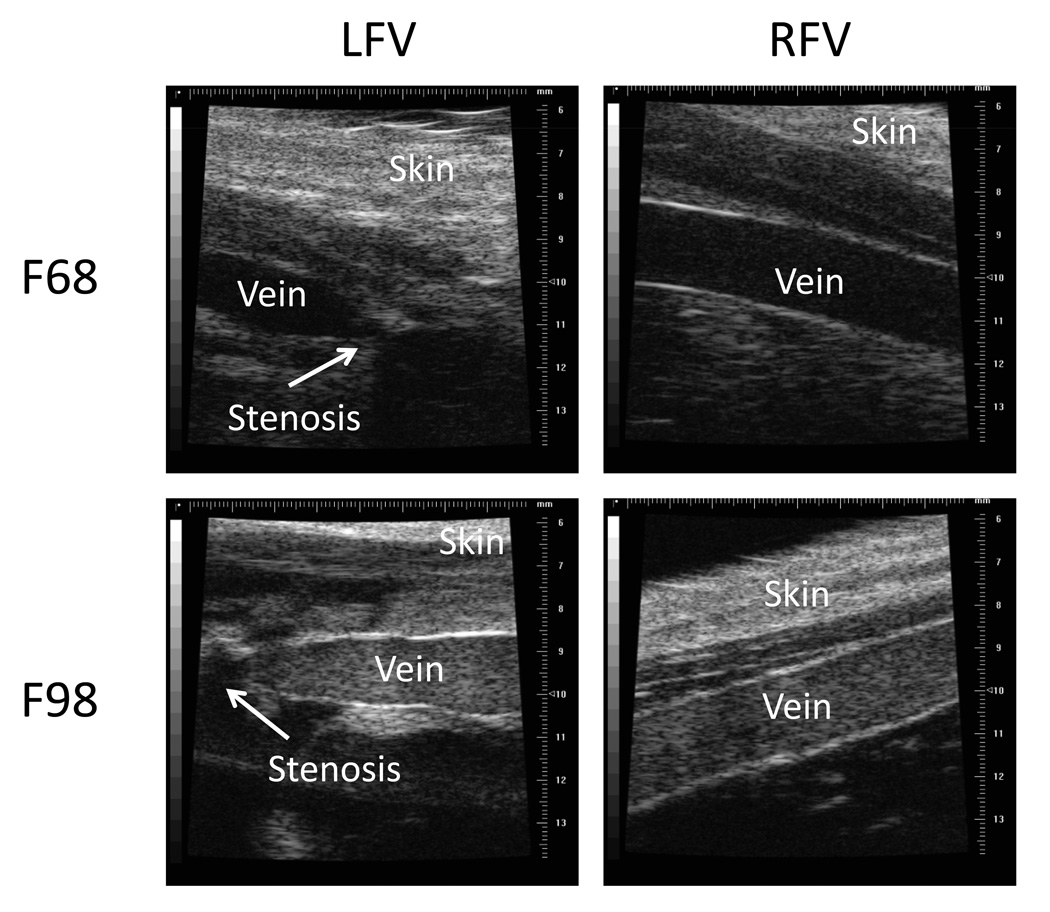
The DVT model used herein involved venous flow reduction and endothelial damage. Flow reduction was achieved by creating a 0.7 mm diameter stenosis in the femoral vein (Figure 2). The intervention consisted in: 1) isolating the LFV 1.5 cm proximal to the saphenous-popliteal bifurcation (vein diameter about 2 mm); 2) placing a 24 G (0.7 mm diameter) catheter (Smiths Medical ASD) along the vein; 3) tying both vein and catheter together using a silk suture; 4) gently pulling the catheter completely away from the vein. This procedure thus created an approximately 88 % cross-sectional surface stenosis and a reduction of flow upstream of the suture. To further promote thrombosis, two separate endothelial injuries, 2 mm apart, were created by clamping the femoral vein with forceps upstream of the suture. To standardize the protocol, the forceps were closed to the first locked position for 2 seconds followed by immediate release, and the procedure was repeated at the second position; hemorrhage from the vessel was never observed. The surgical opening was then closed. For one animal in each group concomitant partial stenosis and endothelial damage were performed on both LFV and RFV to examine the acute response.
Figure 2.
B-mode ultrasound images of the prothrombotic left vein (LFV) and the contralateral control right vein (RFV) for F68 and F98 transfused rabbits. The partial stenosis is indicated by an arrow on the LFV panels. The strong venous echogenicity in F98 transfused animals is indicative of RBC aggregation.
In vivo monitoring using ultrasound
An ultrasound biomicroscope (Vevo660, Visualsonics, Toronto, ON, Canada) equipped with a 35 MHz center frequency probe (RMV703) was used to image venous flow. The B-mode, pulse-wave Doppler-mode and a tissue characterization mode, based on the structure factor size and attenuation estimator (SFSAE) [17;18], respectively were employed to characterize the presence of thrombi, flow velocity and RBC aggregation. The SFSAE utilizes a spectral model that allows extracting attenuation compensated mean aggregate diameter D, from the analysis of radiofrequency echoes backscattered by blood. D is the ratio of the diameter of a fractal isotropic aggregate to the diameter of one RBC and is smaller than 1 for disaggregated RBC [17]. SFSAE images were computed slightly upstream of the stenosis. Ultrasound measurements were performed at each time point: pre-intervention (d0), 30 minutes post-intervention (d1), days 4 (d4), 9 (d9) and 14 (d14). For the acute study, ultrasound measurements were performed at pre-intervention (d0) and 30 minutes post-intervention (d1) for both LFV and RFV.
Laboratory blood tests
White cell, RBC and platelet counts, hemoglobin, hematocrit, prothrombin time and fibrinogen concentration were measured using standard hematology and clinical laboratory methods on samples obtained by venipuncture of the central ear artery on days d0, d1, d9 and d14.
Histology
On d14, a 2 cm section of tissue including the silk suture and the distal portion of the vein of interest was isolated and fixed in formalin for 48 hours. The tissues were then washed, embedded in wax, serially sliced at 5 µm thickness in the axial plane at intervals of 50 µm along the vessel length and then stained with hematoxylin and eosin. Bright field microscopy examination of these sections by three independent experts listed in Acknowledgements confirmed thrombus presence and general composition.
Statistical analyses
The design included double controls: an inter-individual control group (F98 versus F68) and an intra-individual control side (LFV versus RFV). All data are means ± standard errors. Aggregation data were analyzed with ANOVA computed using the generalized linear model in SPSS (v.17, SPSS Inc, Chicago, IL), with time and side as repeated measures and group as the independent factor. When interactions were present, local analyses were performed by successively isolating interacting factors. The threshold of significance was set at 5%.
Results
Blood velocity in veins and thrombus formation
Pre-intervention Doppler velocities were 10.0 ± 0.3 cm/s in the left and right femoral veins. In the two acute animals, on d1, a thrombus that stopped flow formed in both veins of the F98 animal but no thrombus was observed for the F68 animal, for which the velocities were reduced to 4 cm/s by the presence of the stenosis. For the chronic F68 control group, the RFV velocity on d1 was similar to pre-intervention values (9.4 ± 0.4 cm/s, p = 0.73) and was significantly reduced to 1.6 ± 0.7 cm/s (p < 0.001) in the prothrombotic LFV upstream of the stenosis; one animal had a LFV thrombus on d1. After two weeks (d14), the initial LFV thrombus was still present in that animal but no thrombi were found in the other 4 rabbits. Reverse flow was observed for another animal but no thrombus was detected on d1 or at d4, d9 and d14. For the chronic F98 group on d1, the RFV velocity was unchanged compared to d0 (8.2 ± 0.9 cm/s, p = 0.10) whereas at the same time point complete stasis and thrombosis in the prothrombotic LFV were observed in 4 out of 5 animals. Two of these thrombi were recanalized on d9; for the F98 animal without thrombosis post-intervention, the LFV flow was reduced to 1 cm/s on d1 and a thrombus had formed by d9.
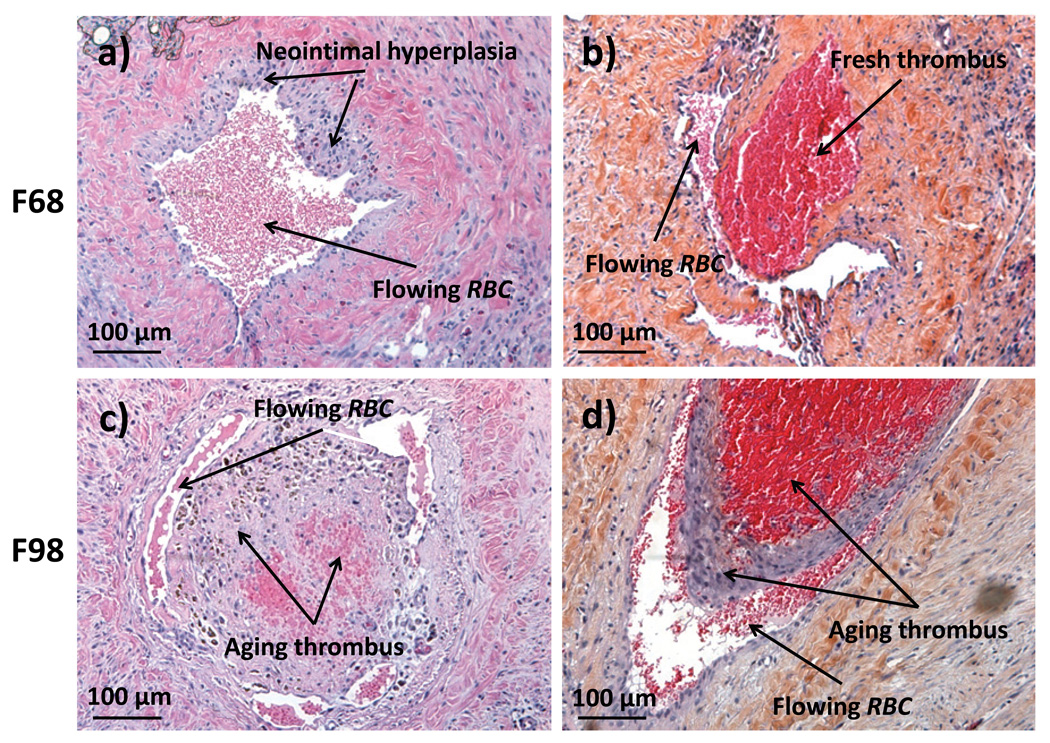
When all 7 prothrombotic veins in the F98 transfused group are considered, 6 developed a thrombus on d1, whereas for the F68 group only 1 out of 7 prothrombotic veins had a thrombus at the same time point (Table 1a). These proportions are statistically significant (Fisher exact test, p = 0.03), whereas the proportions of thrombi on d14 were not significantly different (Table 1b, Fisher exact test, p = 0.52). As also indicated in Table 1c, the presence of occluding thrombi detected with ultrasound was confirmed by histology with haematoxilin / eosin staining: 1) For the F68 group, 4 out of 5 prothrombotic LFV were normal or showed slight neointimal hyperplasia with neoendothelialization that remained confined close to the vessel wall (Figure 3a); 2) In the single F68-treated animal with thrombosis, a young disorganized blood clot was attached to the wall (Figure 3b); 3) In the F98 group, prothrombotic LFV cross-sections were almost completely obstructed by partially organized thrombi with neointima in 3 out of 5 rabbits (examples in Figures 3c and 3d).
Table 1.
Presence of occluding thrombus (Thr) in LFV: (a) 30 minutes post-intervention (i.e., d1) based on ultrasound assessment; (b) at day 14 (d14) pre-harvest based on ultrasound assessments; and (c) after tissue harvest and histology analysis. Proportions were only significantly different between groups on d1 based on the Fisher exact test.
| (a) | (b) | (b) | ||||||
|---|---|---|---|---|---|---|---|---|
| d1* | Thr | No Thr | d14 | Thr | No Thr | Histology | Thr | No Thr |
| F68 | 1 | 6 | F68 | 1 | 4 | F68 | 1 | 4 |
| F98 | 6 | 1 | F98 | 3 | 2 | F98 | 3 | 2 |
p = 0.03
Figure 3.
Bright field microscopy of H&E stained cross sections of LFV at day 14 upstream of the silk suture producing the partial stenosis showing: a) neointimal hypersplasia found in the F68 group LFV; b) a disorganized young thrombus that occurred in only 1 of 5 F68 animals; c) and d) completely obstructed lumen by partially organized thrombi in a F98 group rabbit.
Blood tests
Laboratory measurements for the F98 and F68 groups on d0, d1, d9 and d14 are summarized in Table 2. Baseline values were found to be within normal range for New Zealand White rabbits [19]. Two-way ANOVA with time as the repeated factor revealed no statistical differences between F98 and F68 groups for all parameters except the white cell count and fibrinogen level. White cell counts were higher in the F68 group on d1 and lower on d9 but only different from the d0 level on d9. Fibrinogen was higher in the F68 group on d1 and different from the d0 level and was also increased in the F98 group on d9 and different from the d0 level. Time following intervention influenced all parameters except prothrombin time: hemoglobin, hematocrit, white and red cell counts significantly decreased after d1, platelets decreased on d1 only in F98 and increased on d9 in F68.
Table 2.
Blood analysis pre-intervention (d0), 30 minutes post-intervention (d1) and on days 9 (d9) and 14 (d14).
| Group | d0 | d1 | d9 | d14 | ||||
|---|---|---|---|---|---|---|---|---|
| White cell count | F98 | 6.4 ± 0.7 | 5.2 ± 0.8 |
|
4.4 ± 0.9 |
|
2.0 ± 0.5° | |
| [× 109/L] | F68 | 4.9 ± 0.6 | 7.3 ± 0.8 | 1.7 ± 0.5° | 1.1 ± 0.3° | |||
| Red cell count | F98 |
|
5.9 ± 0.2 | 5.5 ± 0.1 | 4.5 ± 0.1° | 4.0 ± 0.3° | ||
| [× 1012/L] | F68 | 6.0 ± 0.1 | 5.2 ± 0.5 | 4.8 ± 0.4° | 4.5 ± 0.3° | |||
| Hemoglobin | F98 |
|
128 ± 1 | 118 ± 4 | 101 ± 2° | 91 ± 6° | ||
| [g/L] | F68 | 128 ± 2 | 113 ± 12 | 105 ± 7° | 98± 7° | |||
| Hematocrit | F98 |
|
0.37 ± 0.01 | 0.34 ± 0.01 | 0.30 ± 0.01° | 0.27 ± 0.02° | ||
| [V/V] | F68 | 0.37 ± 0.02 | 0.33 ± 0.03 | 0.31 ± 0.02 | 0.29 ± 0.02° | |||
| Platelets | F98 |
|
345 ± 93 | 225 ± 23° | 460 ± 45 | 429 ± 40 | ||
| [× 109/L] | F68 | 332 ± 34 | 304 ± 32 | 466 ± 25° | 353 ± 36 | |||
| Prothrombin time | F98 |
|
8.1 ± 0.1 | 8.1 ± 0.2 | 7.7 ± 0.1 | 7.8 ± 0.1 | ||
| [s] | F68 | 8.2 ± 0.2 | 7.7 ± 0.3 | 7.9 ± 0.1 | 8.0 ± 0.1 | |||
| Fibrinogen | F98 | 2.5 ± 0.1 | 2.7 ± 0.3 |
|
4.0 ± 0.5° |
|
3.1 ± 0.3 | |
| [g/L] | F68 | 2.4 ± 0.1 | 4.6 ± 0.7° | 2.7 ± 0.2 | 2.4 ± 0.1 | |||
indicates significant difference (p < 0.05) from value at d0.
indicates significant difference (p < 0.05) between F68 and F98 groups.
N.S.: not significant. When interactions were present, local analyses were performed using Tukey multiple comparison procedures. Values are mean ± standard error, n = 5 / group.
In-vivo RBC aggregation monitoring
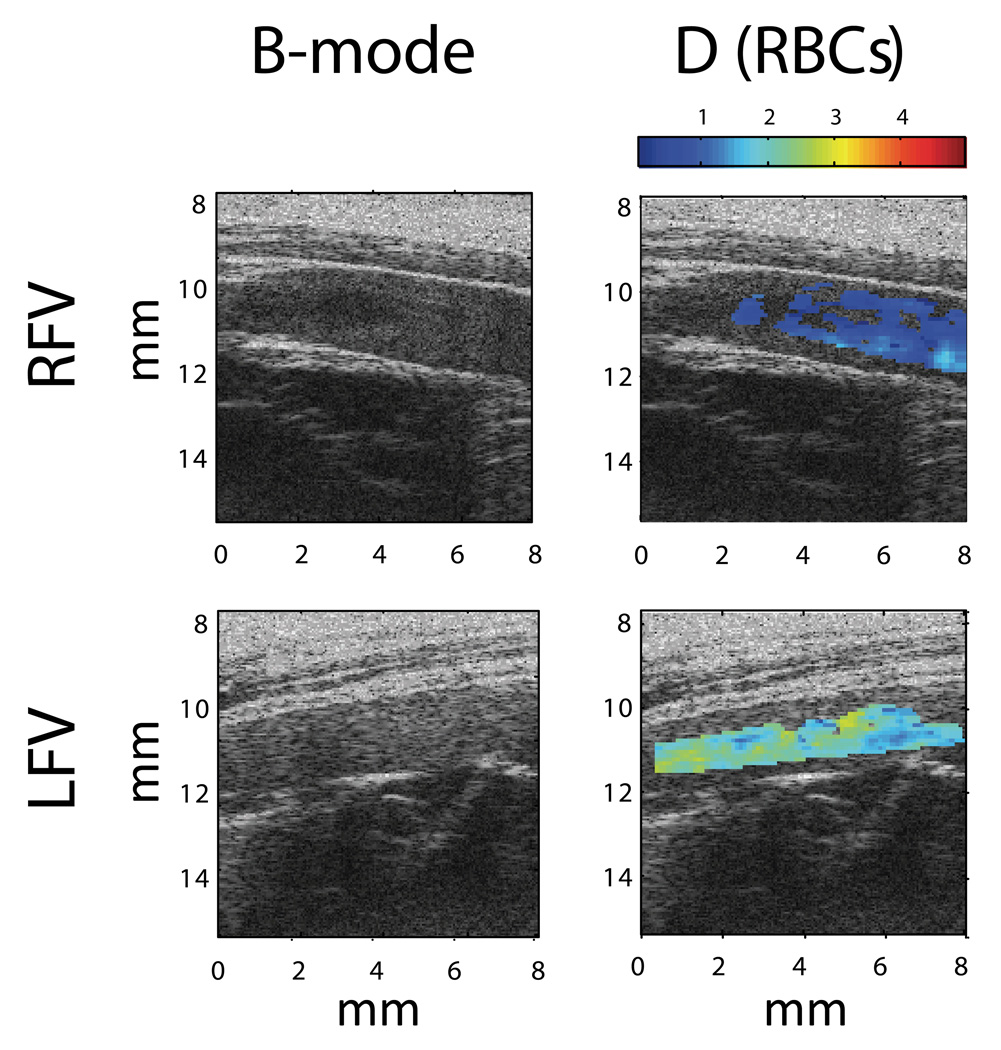
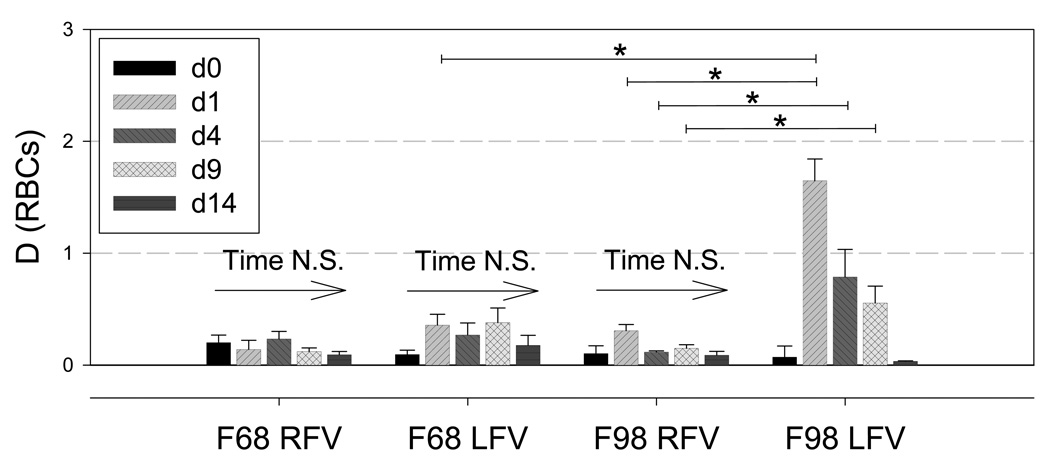
Figure 4 shows examples of ultrasound SFSAE images of the parameter D in the control RFV and upstream of the prothrombotic LFV of a F98 transfused rabbit. Spatially averaged values of D for all animals are reported in Figure 5. In the RFV, group and time had no effect on D, whereas in the LFV D values were significantly higher in F98 versus F68 only on d1. In the F98 group, D was significantly higher in the LFV compared with the RFV on d1, d4 and d9 but not at pre-intervention (i.e., d0) or d14. The D parameter for LFV in F98 transfused animals was significantly higher on d1 compared to all other measurement times. To summarize, the SFSAE could differentiate the in vivo level of aggregation through the skin in situ: 1) between groups with different aggregation tendencies; 2) for different flow conditions (i.e., LFV versus RFV); 3) at various time points following infusion of RBC with enhanced aggregation.
Figure 4.
B-mode images and superimposed parametrical images of in situ RBC aggregation level determined by the structure parameter D (SFSAE) for RFV and LFV in one F98 rabbit. The D parameter is the mean aggregate diameter in terms of number of RBC [17].
Figure 5.
Aggregate size parameter D (defined as the ratio of the diameter of a fractal isotropic aggregate to the diameter of one RBC, that is smaller than 1 for disaggregated RBC [17], at d0, d1, d4, d9 and d14 for the control F68 and hyperaggregating F98 groups. Significant differences (*p < 0.05) were found for D in the F98 group between LFV and RFV on d1, d4 and d9, and between d1 and all other time points within LFV in F98. On d1, significantly different values of D were found between the F68 and F98 groups.
Discussion
Aggregation and acute DVT
In this study, a local increase of RBC aggregation was the dominant differential factor triggering occlusive DVT in the LFV of the F98 group and not in the F68 group. Our results demonstrate that a local blood viscosity increase, caused by RBC aggregation, reduced flow in the stenosed LFV in the F98 group beyond the thrombotic threshold, thus triggering the formation of thrombosis [20]. Conversely, in the control F68 group, the prothrombotic threshold for thrombosis was not met despite identical flow reducing and endothelial damaging interventions. These local hemorheological differences could be monitored using ultrasound.
A calibrated flow reduction approach was chosen over a totally occlusive Wessler DVT model to better mimic the complex dynamic microenvironnent of a thrombogenic region at risk for DVT (e.g., a low flow recirculation zone behind a venous valve). Interestingly, on d1, the increase in aggregation was observed only in the LFV, which supports that an abnormal systemic aggregation tendency (F98 group) can be offset in normal flow conditions (RFV) by flow shear forces. We emphasize that an increase in aggregation and associated hyperviscosity are local parameters that depend on flow conditions.
Hematological profiles
As noted in Table 2, no differences were found in hematological parameters between groups on d1 except for fibrinogen level and white cell count that were significantly higher in F68 rabbits; the fibrinogen level also increased from d0 to d1 in the F68 group. As noted below, an increase of one or both of these parameters might be expected to enhance thrombosis yet thrombus formation was greater in the F98 animals.
A mild surgery-related inflammatory state causing increased fibrinogen in both groups, together with an concomitant increase of fibrinogen consumption during thrombus formation only in the F98 animals, could explain the fibrinogen differences [21]. Similarly, an inflammatory-related greater white cell count at d1 in the F68 group compared to the F98 group would be consistent with higher leukocyte recruitment and extravasation in F98 transfused rabbits. Greater leukocyte margination (i.e., displacement toward the wall) due to enhanced RBC aggregation [22] has been shown to promote DVT through the accumulation of leukocytes on platelet thrombi [23] and increased tissue factor expression by activated leukocytes [24]. The platelet count for the F98 group but not the F68 animals decreased at d1 (Table 2). This decrease is consistent with enhanced platelet margination related to enhanced red cell aggregation [25;26] that promotes collisions, wall adhesion, activation of platelets and thrombus formation. Our results thus suggest a possible synergistic role of blood hyperviscosity on increased leukocyte and platelet margination contributing to DVT formation at the cellular level in our model.
Pluronic-coated RBC transfusions as an hyper-aggregation animal model
In our experiment, we specifically enhanced aggregation by transfusing F98 Pluronic-coated RBC, thereby affecting only the intrinsic tendency of RBC to aggregate. Transfusion of Pluronic-coated red cells have been previously and successfully used to modulate in vivo aggregation [15;16], and has major advantages in that: 1) the F98-coating only modifies red cell aggregation without affecting plasma composition (e.g., infusion of high molecular weight dextrans) or RBC mechanical properties; 2) any artifacts associated with F98 polymer coating can be eliminated by comparison to coating with a Pluronic that does not alter aggregation (e.g. F68) [14]. Pluronics are non-toxic and have found numerous applications in areas of biomaterials, drug delivery and cardiovascular therapeutics [27].
In the present study, the long term effects of infusing F98 coated RBC on thrombus evolution were monitored for two weeks. During this period the effects of this infusion became less evident, with 2 out of 5 thrombi from the F98 group having retracted at d9. However, it has been previous shown that F98 coated rat RBC are progressively removed from the circulation following infusion [15], and thus we are presently unable to determine whether increased aggregation is involved in maintaining thrombosis over the time period utilized in this study.
A new cellular imaging method for in-situ characterization of RBC aggregation
This study is the first in vivo demonstration that quantitative ultrasound (i.e., SFSAE) can characterize RBC aggregation with sufficient sensitivity to monitor its variations over time within different vascular beds (Figures 4 and 5). In situ measurements are a major improvement over laboratory blood tests which overlook confounding variables such as flow conditions, vascular tone regulation or the presence of collateral vascular beds. It is expected that local assessment of aggregation using ultrasound could shed some light on the abovementioned discrepancies regarding the effects of fibrinogen on DVT [7;11]. Our results infer that it is not fibrinogen per se but rather its effect on RBC aggregation that is one mechanism triggering DVT in humans. Indeed, zones of reduced flow in the elderly caused by impaired flow mediated, endothelium dependent dilation [28] could initiate a vicious cycle of increased aggregation, increased viscosity and a further reduction of flow thereby leading to an increased risk of DVT. In younger patients, the viscosity increase could be compensated by an active vascular dilation reserve [29]. Similarly, locally reduced flow conditions, favorable to a first thrombus episode, could also plausibly explain why fibrinogen is a risk factor for DVT recurrence (i.e., same reduced flow conditions exist after thrombus lysis or recanalization) but not consistently for an initial DVT since local flow conditions can vary from patient to patient.
Clinical implications and conclusions
The fact that a first idiopathic DVT accounts for up to 47% of DVT recurrence indicates that the etiology of DVT is not well understood. Determining and understanding other mechanisms implicated in DVT, such as altered local hemorheological parameters, should aid in preventing thrombus formation and optimizing prophylaxis strategies. Our results indicate that locally increased RBC aggregation is not merely a secondary surrogate marker of inflammation [30], but can contribute to trigger thrombus formation. Hemorheological parameters should be screened more routinely in DVT recurrence risk profiling.
Acknowledgements
The authors thank Dr. H. Héon, Dr. S. Qi, N. Beauchemin and B. Chayer for helping with the animal procedures, A. Major, Dr. I. Salazkin and Dr. L. Gaboury with histological analyses and M. Chagnon with the statistical analyses.
Sources of funding
This work was supported by the Canadian Institutes of Health Research (MOP-84358 and CMI-72323), the Heart and Stroke Foundation of Canada (PG-05-0313), the National Institutes of Health of USA (HL01572, HL078655 and HL090511) and the Natural Sciences and Engineering Research Council of Canada (#ES D3-317051 - 2005)
Reference List
- 1.Agnelli G, Becattini C. Treatment of DVT: how long is enough and how do you predict recurrence. J Thromb Thrombolysis. 2008;25:37–44. doi: 10.1007/s11239-007-0103-z. [DOI] [PubMed] [Google Scholar]
- 2.Alt E, Banyai S, Banyai M, Koppensteiner R. Blood rheology in deep venous thrombosis--relation to persistent and transient risk factors. Thromb Res. 2002;107:101–107. doi: 10.1016/s0049-3848(02)00302-x. [DOI] [PubMed] [Google Scholar]
- 3.Vaya A, Mira Y, Martinez M, Villa P, Ferrando F, Estelles A, Corella D, Aznar J. Biological risk factors for deep vein trombosis. Clin Hemorheol Microcirc. 2002;26:41–53. [PubMed] [Google Scholar]
- 4.Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293:2352–2361. doi: 10.1001/jama.293.19.2352. [DOI] [PubMed] [Google Scholar]
- 5.Schulman S, Ogren M. New concepts in optimal management of anticoagulant therapy for extended treatment of venous thromboembolism. Thromb Haemost. 2006;96:258–266. doi: 10.1160/TH06-05-0235. [DOI] [PubMed] [Google Scholar]
- 6.Lowe GD. Etiopathogenesis of cardiovascular disease: hemostasis, thrombosis, and vascular medicine. Ann Periodontol. 1998;3:121–126. doi: 10.1902/annals.1998.3.1.121. [DOI] [PubMed] [Google Scholar]
- 7.Dormandy JA, Edelman JB. High blood viscosity: an aetiological factor in venous thrombosis. Br J Surg. 1973;60:187–190. doi: 10.1002/bjs.1800600306. [DOI] [PubMed] [Google Scholar]
- 8.Zuccarelli F, Taccoen A, Razavian M, Chabanel A. Increasing erythrocyte aggregability with the progressive grades of chronic venous insufficiency: importance and mechanisms. J Cardiovasc Surg. 1995;36:387–391. [PubMed] [Google Scholar]
- 9.Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Hansen JB. Hematocrit and risk of venous thromboembolism in a general population. The Tromso study. Haematologica. 2010;95:270–275. doi: 10.3324/haematol.2009.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chien S. Blood viscosity: Influence of erythrocyte aggregation. Science. 1967;157:829–831. doi: 10.1126/science.157.3790.829. [DOI] [PubMed] [Google Scholar]
- 11.Van Hylckama V, Rosendaal FR. High levels of fibrinogen are associated with the risk of deep venous thrombosis mainly in the elderly. J Thromb Haemost. 2003;1:2677–2678. doi: 10.1111/j.1538-7836.2003.0543b.x. [DOI] [PubMed] [Google Scholar]
- 12.Renner W, Cichocki L, Forjanics A, Koppel H, Gasser R, Pilger E. G-455A polymorphism of the fibrinogen beta gene and deep vein thrombosis. Eur J Clin Invest. 2002;32:755–758. doi: 10.1046/j.1365-2362.2002.01070.x. [DOI] [PubMed] [Google Scholar]
- 13.Shung KK, Sigelmann RA, Reid JM. Scattering of ultrasound by blood. IEEE Trans Biomed Eng. 1976;BME-23:460–467. doi: 10.1109/tbme.1976.324604. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong JK, Meiselman HJ, Wenby RB, Fisher TC. Modulation of red blood cell aggregation and blood viscosity by the covalent attachment of Pluronic copolymers. Biorheology. 2001;38:239–247. [PubMed] [Google Scholar]
- 15.Baskurt OK, Yalcin O, Ozdem S, Armstrong JK, Meiselman HJ. Modulation of endothelial nitric oxide synthase expression by red blood cell aggregation. Am J Physiol. 2004;286:H222–H229. doi: 10.1152/ajpheart.00532.2003. [DOI] [PubMed] [Google Scholar]
- 16.Yalcin O, Uyuklu M, Armstrong JK, Meiselman HJ, Baskurt OK. Graded alterations of RBC aggregation influence in vivo blood flow resistance. Am J Physiol. 2004;287:H2644–H2650. doi: 10.1152/ajpheart.00534.2004. [DOI] [PubMed] [Google Scholar]
- 17.Yu FTH, Cloutier G. Experimental ultrasound characterization of red blood cell aggregation using the structure factor size estimator. J Acoust Soc Am. 2007;122:645–656. doi: 10.1121/1.2735805. [DOI] [PubMed] [Google Scholar]
- 18.Franceschini E, Yu FT, Destrempes F, Cloutier G. Ultrasound characterization of red blood cell aggregation with intervening attenuating tissue-mimicking phantoms. J Acoust Soc Am. 2010;127:1104–1115. doi: 10.1121/1.3277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hewitt CD, Innes DJ, Savory J, Wills MR. Normal biochemical and hematological values in New Zealand white rabbits. Clin Chem. 1989;35:1777–1779. [PubMed] [Google Scholar]
- 20.Hathcock JJ. Flow effects on coagulation and thrombosis. Arterioscler Thromb Vasc Biol. 2006;26:1729–1737. doi: 10.1161/01.ATV.0000229658.76797.30. [DOI] [PubMed] [Google Scholar]
- 21.Monkhouse FC, Milojevic S. Changes in fibrinogen level after infusion of thrombin and thromboplastin. Am J Physiol. 1960;199:1165–1168. doi: 10.1152/ajplegacy.1960.199.6.1165. [DOI] [PubMed] [Google Scholar]
- 22.Pearson MJ, Lipowsky HH. Influence of erythrocyte aggregation on leukocyte margination in postcapillary venules of rat mesentery. Am J Physiol. 2000;279:H1460–H1471. doi: 10.1152/ajpheart.2000.279.4.H1460. [DOI] [PubMed] [Google Scholar]
- 23.Kirchhofer D, Riederer MA, Baumgartner HR. Specific accumulation of circulating monocytes and polymorphonuclear leukocytes on platelet thrombi in a vascular injury model. Blood. 1997;89:1270–1278. [PubMed] [Google Scholar]
- 24.Niemetz J. Tissue-factor-endowed leukocytes do cause thrombosis. Blood. 2005;106:1506–1507. doi: 10.1182/blood-2005-03-1163. [DOI] [PubMed] [Google Scholar]
- 25.Nash GB, Watts T, Thornton C, Barigou M. Red cell aggregation as a factor influencing margination and adhesion of leukocytes and platelets. Clin Hemorheol Microcirc. 2008;39:303–310. [PubMed] [Google Scholar]
- 26.Goldsmith HL, Bell DN, Spain S, McIntosh FA. Effect of red blood cells and their aggregates on platelets and white cells in flowing blood. Biorheology. 1999;36:461–468. [PubMed] [Google Scholar]
- 27.Singh-Joy SD, McLain VC. Safety assessment of poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183, 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, poloxamer 105 benzoate, and poloxamer 182 dibenzoate as used in cosmetics. Int J Toxicol. 2008;27 Suppl 2:93–128. doi: 10.1080/10915810802244595. [DOI] [PubMed] [Google Scholar]
- 28.Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol. 2006;291:H3043–H3049. doi: 10.1152/ajpheart.00190.2006. [DOI] [PubMed] [Google Scholar]
- 29.Meiselman HJ, Baskurt OK. Hemorheology and hemodynamics: Dove andare? Clin Hemorheol Microcirc. 2006;35(IP - 1–2):37–43. [PubMed] [Google Scholar]
- 30.Berliner S, Rogowski O, Aharonov S, Mardi T, Tolshinsky T, Rozenblat M, Justo D, Deutsch V, Serov J, Shapira I, Zeltzer D. Erythrocyte adhesiveness/aggregation: a novel biomarker for the detection of low-grade internal inflammation in individuals with atherothrombotic risk factors and proven vascular disease. Am Heart J. 2005;149:260–267. doi: 10.1016/j.ahj.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 31.Windberger U, Bartholovitsch A, Plasenzotti R, Korak KJ, Heinze G. Whole blood viscosity, plasma viscosity and erythrocyte aggregation in nine mammalian species: reference values and comparison of data. Exp Physiol. 2003;88:431–440. doi: 10.1113/eph8802496. [DOI] [PubMed] [Google Scholar]