Abstract
Background
Abuse of gamma-hydroxybutyrate (GHB) poses a public health concern. In previous studies, intravenous (IV) self-administration of GHB doses up to 10 mg/kg was not maintained in non-human primates under limited-access conditions, which was inconsistent with the usual good correspondence between drugs abused by humans and those self-injected by laboratory animals.
Methods
Self-administration of GHB was studied in 10 baboons using procedures standard for our laboratory to assess drug abuse liability. Each self-injection depended on completion of 120 or 160 lever responses. Sessions ran continuously; a 3-h timeout limited the number of injections per 24 h to 8. Self-injection was established at 6–8 injections/day with cocaine (0.32 mg/kg/injection) prior to substitution of each GHB dose (3.2–178 mg/kg/injection) or vehicle for 15 days. Food pellets were available 24 h/day.
Results
GHB maintained significantly greater numbers of injections when compared to vehicle in 6 of the 9 baboons that completed GHB evaluations that included 32 mg/kg/injection or higher. The baboons that self-administered GHB at high rates were ones for which GHB was the first drug each had tested under the 24-hr/day cocaine baseline procedure. Self-injection of the highest doses of GHB decreased food-maintained responding.
Conclusions
High-dose GHB can function as a reinforcer in non-human primates under 24-h access, but self-administration history may be important. The findings are consistent with the demonstrated abuse liability of GHB in humans, and remove GHB as an exception to the typical good correspondence between those drugs abused by humans and those self-administered by nonhuman primates.
Keywords: Xyrem®, Gamma-hydroxybuturate (GHB), Cocaine, Self-administration, Operant behavior, Abuse liability
1.0 Introduction
Gamma-hydroxybutyrate (GHB) is an endogenous substance found in the brain in μmol concentrations (Wong et al., 2004). GHB was first synthesized as a compound for use as a sedative and anesthetic agent in the 1960s (Laborit, 1973; Wedin et al., 2006) and is currently used for the treatment of narcolepsy (Wedin et al., 2006). During clinical trials, GHB (50–150 mg/kg) was shown to be effective in reducing cataplexy, daytime sleepiness, and hypnogogic hallucinations in narcoleptic patients (Broughton and Mamelak, 1979; Mamelak et al., 1986; Scharf et al., 1985; Scrima et al., 1990); and long-term efficacy for the treatment of cataplexy associated with narcolepsy has been demonstrated (U.S.Xyrem® Multicenter Study Group, 2004). GHB has also been investigated as a possible treatment for alcohol dependence, and is currently prescribed for this purpose outside of the United States (Addolorato et al., 2009). GHB, however, is also a drug of abuse used recreationally for euphoric, intoxicating, and relaxing effects (Galloway et al., 2000b; Miotto et al., 2001). Because of both the therapeutic use and reported abuse, GHB received a unique dual classification under the Controlled Substances Act (DEA/ODE, 2000). For medical use, the marketed product (Xyrem®) is classified as Schedule III, whereas unapproved forms are classified as Schedule I.
The typical therapeutic dose of GHB for the treatment of narcolepsy is 4.5 g/night divided into two doses of 2.25 g taken at bedtime and then 2–4 hr later (Carter et al., 2009b). Reported euphoric doses of GHB in humans vary but are thought generally to fall between 20–30 mg/kg (Gonzalez and Nutt, 2005; Nicholson and Balster, 2001) though doses as high as 75 mg/kg have been reported (Abanades et al., 2006). The actual doses taken recreationally are difficult to determine as GHB is often from an illicit source of unknown purity. In addition, some users take doses at regular 2–3 hr intervals for ‘round the clock’ use resulting in total daily doses that exceed therapeutic levels (Galloway et al., 1997). Likewise, some alcohol dependent patients treated with GHB took up to 6–7 times the prescribed dose and reported “craving” for GHB (Addolorato et al., 1999; Addolorato et al., 1996). The reported effects of GHB include euphoria, relaxation, drowsiness, disinhibition, and a heightened sense of touch and sexuality, anxiety, dizziness, agitation, and with overdose as such adverse effects as nausea, vomiting, and respiratory depression leading to coma and sometimes death (Degenhardt et al., 2002; Galloway et al., 2000a; Miotto et al., 2001). There are numerous case reports indicating that abuse of high doses of GHB leads to physical dependence, and symptoms of withdrawal can include severe agitation, hallucinations, tachycardia, insomnia, increased blood pressure, decreased food intake, and tremor (Miotto et al., 2001). In some case studies, GHB users reported a progressive escalation in dose to avoid withdrawal symptoms or to achieve the desired effect of GHB (Craig et al., 2000; Galloway et al., 1997; Hutto et al., 2000). The physical dependence potential of GHB has been confirmed in laboratory studies in baboons; GHB produced signs of withdrawal when drug was discontinued following chronic administration, and the withdrawal syndrome was similar to that reported in humans (Weerts et al., 2005).
Studies in humans have concluded GHB shares some psychomotor, subjective and cognitive effects of the benzodiazepine triazolam and the barbiturate pentobarbital, though the abuse potential of GHB was characterized as being higher than triazolam, and the likelihood of negative consequences associated with an accidental overdose appears to be greater for GHB when compared to triazolam (Carter et al., 2009a; Carter et al., 2006; Griffiths and Johnson, 2005). Case reports and DEA encounters have provided strong evidence of GHB abuse; however, self-administration studies in laboratory animals have yielded mixed results. The use of self-administration procedures in laboratory animals is the gold standard for investigations of abuse liability and reinforcer efficacy, in part because these procedures have excellent internal and predictive validity (Ator and Griffiths, 2003; Carter and Griffiths, 2009). In mice and rats, GHB has been reported to maintain intravenous (IV) (Fattore et al., 2000a; Martellotta et al., 1998) and oral self-administration (Colombo et al., 1995; Colombo et al., 1998), as well as to produce a conditioned place preference (Fattore et al., 2000b; Itzhak and Ali, 2002; Martellotta et al., 1997; Watson et al., 2009). GHB injected directly into the ventral tegmental area of rats produced conditioned place preference, but IV self-administration was observed at only one dose (0.01 mg/kg/infusion) and was variable across days (Watson et al., 2009). Under limited-access (1–2 h) conditions in rhesus monkeys, in which GHB was substituted for either phencyclidine or methohexital, GHB did not maintain reliable IV self-administration (Beardsley et al., 1996; Woolverton et al., 1999). These results were surprising given the generally good correspondence between drugs that humans abuse and those that are self-administered by laboratory animals (Griffiths et al., 1979; Johanson and Balster, 1978).
In our laboratory, we have assessed the reinforcing effects of a wide range of drugs, including sedatives, by use of a standardized self-administration paradigm in baboons (Ator, 2000; Ator et al., 2010; Griffiths et al., 1979; Griffiths et al., 1991; Griffiths et al., 1992; Weerts et al., 1999). As in the procedures with rhesus monkeys cited above, the test drug is substituted for a compound used to maintain a self-injection baseline. In our procedure, each test dose is substituted for cocaine for at least 15 days during sessions than run continuously, 24 hr/day. Drug accumulation is limited by a 3-h timeout after each self-injection. In the context of the emerging evidence for GHB abuse in the early ‘90’s, we studied IV self-injection of GHB in baboons using this procedure. Our initial results, in 3 baboons, were consistent with those for rhesus monkeys in that IV GHB failed to function as a reinforcer, and were not published. Given continuing reports of increased GHB abuse and findings from later work in our laboratory on GHB physical dependence in the baboon (Weerts et al., 2005), we decided to reexamine IV GHB self-injection in a larger number of subjects and investigate a higher dose range than we had studied earlier. When our data contrasted with the earlier findings for some animals, but not others, we extended the investigation ultimately to include 10 baboons. Thus, the present manuscript describes self-administration data from both the initial series of studies in 3 baboons (Group A) and new data in 7 baboons (Group B) using a different vehicle (sterile water vs. saline) and greater injection volume to increase drug solubility and allow the study of high doses (100–178 mg/kg) GHB doses.
2.0 Materials and Methods
2.1 Subjects
Subjects were adult male baboons (Papio hamadryas anubis, olive baboons) weighing between 21.8 – 41.5 kg (see table 1). Each was surgically prepared with a chronically indwelling silastic catheter implanted in either a femoral or jugular vein (using procedures described in Lukas et al., 1982). The catheters were protected by a vest and tether system to which baboons were habituated for 2–4 weeks prior to surgery.
Table 1.
Mean baboon weights, and order of study of vehicle and GHB doses (mg/kg/injection) substituted for cocaine (0.32 mg/kg/injection) for each baboon.
| Baboon | Average BW (kg) | Order of GHB (mg/kg) Conditions | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| AC | 26.5 | S | 3.2 | 10 | 32 | 18^ | 100 | - | - |
| DI | 24.0 | S | 3.2 | - | - | - | - | - | - |
| PE | 25.7 | S | 10 | 32 | 56 | 100 | - | - | - |
| BO | 39.2 | W | 56 | 130v | 178v | 100v | 32 | W | 10 |
| CR | 38.3 | W | 32 | 100 | 56 | 78^ | 78 | 10 | - |
| DS | 36.0 | W | 32 | 100v | 130v | W | 56v | - | - |
| SI | 32.2 | 56 | W^ | 100 | - | - | - | - | - |
| JN | 38.8 | 32 | 56 | 100v | 130 | W | - | - | - |
| KR | 22.9 | 32 | 56 | W | 10 | 100v | 130v | W | - |
| XA | 22.3 | 32 | 56 | W | 56 | 100v | 130^ | - | - |
Indicates dose availability was less than 15 days
S indicates saline vehicle was used
W indicates sterile water vehicle was used
indicates the GHB dose condition was followed by vehicle substitution to evaluate extinction before returning to cocaine baseline
Baboons differed in both their behavioral and pharmacological histories as well as the timeframe during which they served as subjects. Group A included the three baboons mentioned that were studied first. These baboons (identified as AC, DI, PE) had extensive and exclusive histories of IV drug self-administration under the same 24-hr cocaine baseline procedure used in the present study. The additional commonalities in their experimental histories were that all three baboons had self-administered triazolam, zolpidem, 4 -methyl-aminorex, and mazindol (Griffiths et al., 1991; Griffiths et al., 1992; Kaminski et al., 1996). AC and PE also had self-administered propylhexedrine (Kaminski et al., 1996), methcathinone (Kaminski and Griffiths, 1994), and alpha-ethyl-amphetamine (unpublished). Group B consisted of seven baboons (identified as BO, CR, DS, JN, KR, SI, XA) for which study of GHB began approximately 10 years after that of Group A. Only two of those baboons (CR and SI) had experience with IV drug self-administration under the 24-hr cocaine baseline procedure with 3-h timeouts, and both had been studied in that procedure with triazolam, bretazenil, and THIP (Ator et al., 2000; and unpublished). Baboon CR also had self-administered zaleplon, zolpidem lorazepam, midazolam, pentazocine, CP-615,003, and CP-730,330 (Ator, 2000; and unpublished). Baboons BO, CR, and DS had experience in one or two studies with chronic intragastric drug delivery for assessment of physical dependence (Weerts et al, 1998; Ator et al, 2000). Baboons BO and DS did not, however, have any experience with drug self-administration prior to training under the 24-hr procedure for the present study. Baboons JN, KR, XA had extensive experience under a limited access 2-hr self-administration procedure with IV cocaine and acute and sub-chronic pre-treatment with GABAergic drugs (Weerts et al., 2007b; Weerts et al., 2005), but they had no experience with IV drug self-administration under the 24-hr cocaine baseline. Thus, for baboons BO, DS, JN, KR, and XA, GHB represented the first drug they had experience self-administering other than cocaine.
Tap water from a drinking spout located on the front of the cage was continuously available, and water intake was recorded daily. Baboons had ad libitum 24-hr/day access to food pellets under conditions described below, and were given two pieces of fresh produce and a multivitamin at approximately 11:00 h each day. The baboons had constant visual and auditory contact with other male baboons; and toys for environmental enrichment. The room ceiling lights were brightly illuminated for 13 h/day (6:00–19:00h) and were dimly illuminated for the remaining 11 h/day. Windows in the housing area also provided natural light. Baboons were anesthetized every 2–3 weeks with ketamine HCl (preceded by atropine SO4 to control secretions) to permit cage washing, weighing, physical examinations, and treatment of the catheter exit site. Housing and care of the animals were consistent with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health.
2.2 Apparatus
Baboons were housed in standard stainless steel primate cages that also served as experimental chambers. Cages were equipped with a bench that ran along one of the side walls and had an aluminum intelligence panel that was mounted on the rear wall. A speaker for delivery of white noise and tones was mounted behind the panel. The intelligence panel contained a pull-and-release lever (Lindsley operandum from Gerbrands, Arlington, MA or model ENV-122 from Med Associates, St. Albans, VT) for drug self-injection and a vertically-operated lever (custom made or Med-Associates model ENV-121) for food pellet delivery, both of which were mounted in the lower left quadrant of the panel within easy reach of the baboon when seated on the bench. A distinctively colored jewel light (1.5 cm diameter) was mounted above each lever (e.g., green over the self-injection lever and blue over the pellet lever). Food pellets (1-g banana-flavored pellets, Bio-Serv, Inc., Frenchtown, NJ) were delivered from a pellet feeder into a recessed hopper located in the center of the panel. The food hopper contained a light panel (5 × 5 cm) that flashed during each pellet delivery. A second light panel (5 × 5 cm), or “baylight,” which could be transilluminated by a green bulb, was located in the upper left quadrant of the intelligence panel.
The IV catheters were protected by a tether/harness/vest system that allowed virtually unrestricted movement within the cage (Lukas et al., 1982). If necessary to protect the catheter for an individual baboon, a custom-made shirt (Lomir, Malone, NY) was worn over the harness. The catheter was attached to a custom infusion system that delivered fluid via three separate peristaltic pumps (Model 1201 or Model 66 Harvard Apparatus, Natick, MA or Watson-Marlow, Model 403U/R1). Drug was injected into the catheter with the first pump (see drug section for volumes) and then flushed into the vein with 5 ml of saline from the second pump. To maintain catheter patency, a third pump continuously infused heparinized saline (5–10 units/ml) for a total of 200–250 ml/24 h. The peristaltic pumps, infusion systems, drug solutions, pellet feeder, and drinking water bottles were located on a metal grating that ran above the cage.
Experimental control and data collection were accomplished using personal computers with MED Associates Inc. (St. Albans, VT) software and instrumentation.
2.3 Drugs
Cocaine HCl was obtained from Research Triangle Institute (RTI, Research Triangle, NC) via the National Institute on Drug Abuse drug supply program. GHB sodium salt was obtained from RTI and Sigma-Aldrich (St. Louis, MO). Cocaine (0.32 mg/kg) was dissolved in 0.9% saline at a volume of 5 ml per injection. For Group A, GHB doses of 3.2, 10.0, 18.0, 32.0 and 56.0 mg/kg were dissolved in 0.9% saline at a volume of 5 ml per injection. Saline self-injection was also assessed at this volume. The 100 mg/kg dose was dissolved in saline at a volume of 10 ml per injection. Due to concern over solubility issues, higher doses of GHB were not examined in that group. For Group B, GHB self-injection was determined using a different vehicle (sterile water for injection) and greater injection volumes to increase drug solubility and allow the study of a higher dose range (32–178 mg/kg) GHB doses. Specifically, injection volumes for Group B were 10 ml for GHB doses of 10, 32, 56, and 78 mg/kg; and were 15 ml for 100 and 130 mg/kg; except that volume of injection was 20 ml for baboon BO at 100 mg/kg. Only baboon BO was studied at 178 mg/kg, and volume of injection was 20 ml. For sterile water self-injection, volumes of 10 and/or 15 ml were assessed; and 20 ml was assessed also in baboon BO. All drug doses were calculated based on the salt. Drug solutions were sterilized by filtration (22 mm Millipore Corp., Bedford MA).
2.4 Experimental Procedures
2.4.1 Drug Self-injection procedure
Experimental sessions were continuous (i.e., 24 h/day). All drug changes and data collection were conducted between 08:00 h and 08:30 h, and the “start time” for each 24 h session was operationally defined as 08:30 h. Each injection was contingent upon completion of a fixed number of responses on the pull-and-release lever (i.e., a fixed-ratio, FR, reinforcement schedule). The requirement was 120 for baboon PE and 160 for all other baboons. There was no time limit for completion of the response requirement. The availability of an injection was indicated by onset of white noise, a 5-s tone, and illumination of the jewel light over the pull-and-release lever. Each release produced a 0.1 s feedback tone. Upon completion of the FR response requirement, the jewel light and white noise were extinguished, the drug injection was initiated at a rate of approximately 5 ml/90 s, followed by a 5-ml saline flush. Completion of the response requirement was also followed by a 3-h timeout, during the first hour of which the green baylight was illuminated; the timeout limited the total number of injections that could be obtained per day to 8. Responses on the drug lever during the timeout were recorded but had no programmed consequence. At the same time, the food pellets were continuously available according to an FR-30 requirement on the vertically-operated lever; the jewel light over that lever was continuously illuminated. Each pellet delivery was accompanied by a 100-ms flash of the light panel in the food hopper. When a baboon worked for less than 100 pellets for three consecutive days, he received a 100-g supplement of standard primate biscuits.
2.4.2 Cocaine baseline procedure
Baboons in all groups had been trained to self-inject cocaine (0.32 mg/kg/injection) under a procedure in which the response requirement was gradually increased across injections and days until the terminal FR value was reached and the baboon was self-administering the criterion level of 6–8 injections per day. Because this criterion was not reliably achieved at FR 160 for baboon PE, the FR value of 120 was used for that baboon. Criterion performance under the cocaine baseline was defined as 6–8 self-injections per day for 3 consecutive days. When the cocaine baseline criterion performance was met, a test dose of GHB (3.2 – 178.0 mg/kg/injection) or its vehicle was substituted for cocaine for a minimum of 15 days. Table 1 shows the order in which GHB doses and vehicle were substituted for cocaine for each baboon. The period of substitution was extended beyond 15 days when equipment problems occurred (e.g., malfunctions of catheter/infusion systems or the computer) or to further characterize self-injection. Cocaine self-injection was re-established, and criterion performance met before each dose evaluation. The reinstatement of this cocaine baseline condition thus established maximal self-injection performance prior to each substitution and assured initial exposure to each test dose. For baboons for which GHB (56–130 mg/kg) served as a reinforcer, the vehicle was substituted directly for GHB for 15 or more days to evaluate extinction of self-injection responses (see footnotes in Table 1)
2.5 Data analysis
A single-subject design was used in which each baboon served as his own control. Data reported for cocaine are the grand mean from the last 3 days of the cocaine baseline periods that preceded each test dose. Under the cocaine baseline procedure, the substitution of each test dose typically results in a period of transition from the cocaine self-injection baseline for the first days, followed by stabilization of responding maintained by the test dose. The last 5 days of the period of substitution are viewed as representative of the rate of self-injection maintained by the test dose per se. Thus, the mean number of injections/24 hours over the last 5 days of a test condition was used to represent the level of self-injection of each dose and vehicle. Reinforcement by a GHB dose for an individual baboon was concluded if the mean rate of self-administration was greater than two standard deviations (SD) of the mean for vehicle (defined as the critical value); this is analogous to a one-tailed test. Under the present procedure, rates of self-injection that are greater than those maintained by vehicle but below 4 injections/day are characterized as low; those that are 4 or 5 injections per day as moderate, and those of 6–8 injections/day as high.
3.0 Results
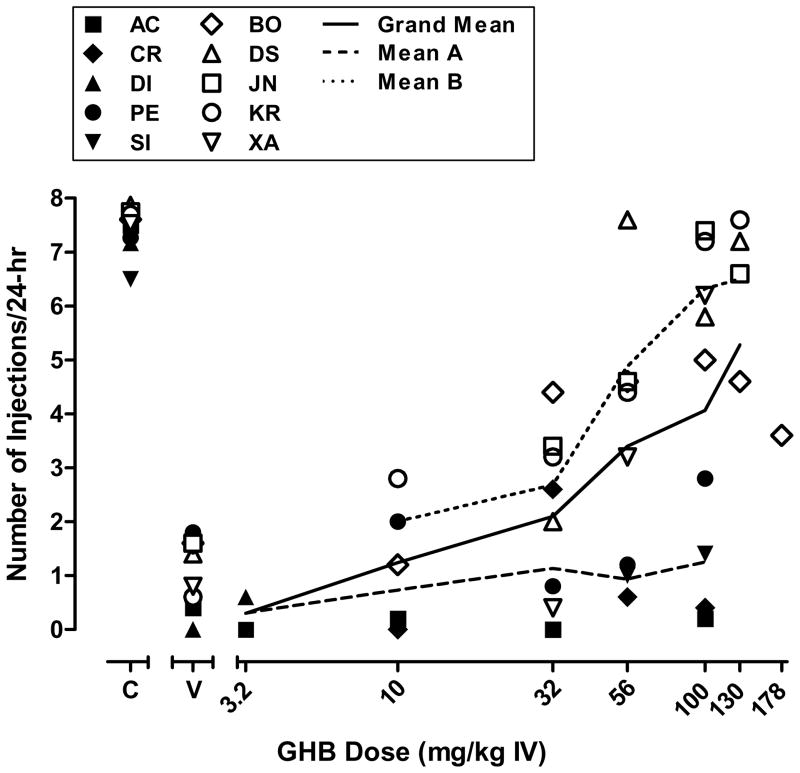
Figure 1 shows the mean number of self-injections obtained during the last 5 days of periods in which vehicle and each dose of GHB was substituted for 0.32 mg/kg/injection cocaine for all baboons studied. During evaluation of GHB, the mean number of injections per day was 7–8 for cocaine and was less than 2 per day for vehicle for all baboons. The critical values for characterizing the number of GHB injections as significantly greater than under vehicle conditions were as follows: AC (2.19), BO (4.28), CR (2.38), DI (0), DS (3.18), JN (3.88), KR (2.39), PE (3.48), and XA (2.47) (see Table 1 footnote for SI). As shown in figure 1, GHB self-injection was variable across baboons. One baboon (DI) completed only one GHB dose condition (3.2 mg/kg), and did not self-inject that dose greater than vehicle. Of the 9 baboons that were studied at doses of 10 mg/kg or higher, GHB reinforcement was demonstrated in 6 baboons (BO, CR, DS, JN, KR, XA). GHB doses of 32 mg/kg/injection and higher maintained rates of self-injection significantly greater than vehicle in those baboons, and except for CR, the rate of self-injection was a generally ascending function of dose. Low to moderate rates of self-injection were obtained in baboon CR and only at 32 mg/kg GHB. High mean rates of self-injection were obtained at 56 mg/kg (DS), 100 mg/kg (JN, KR, XA) and 130 mg/kg (DS, JN, KR), while only moderate rates of self-injection were obtained at these doses in baboon BO. Thus, baboon BO was evaluated at 178 mg/kg/injection GHB. Self-injection at that dose was lower than at 130, resulting in GHB self-injection being an inverted-U shaped function of dose in that baboon. Three baboons (AC, PE, SI) completed dose evaluations up to 100 mg/kg GHB, but did not reliably self-inject any GHB dose greater than vehicle.
Figure 1.
Mean number of self-injections of GHB. Data points shown are the individual means of the last 5 days of dose substitution or vehicle (“V”) availability. Doses were studied in the order shown in Table 1 for each baboon. Data for cocaine (“C”) are the grand means from the last 3 days of the cocaine baseline periods that preceded each test dose. Subjects with a history of self-administration of a variety of drugs using the 24 hr procedure (Mean A) are shown using filled symbols and subjects lacking an extensive history of self-administration (Mean B) are shown using clear symbols. The grand mean is illustrated with a solid line.
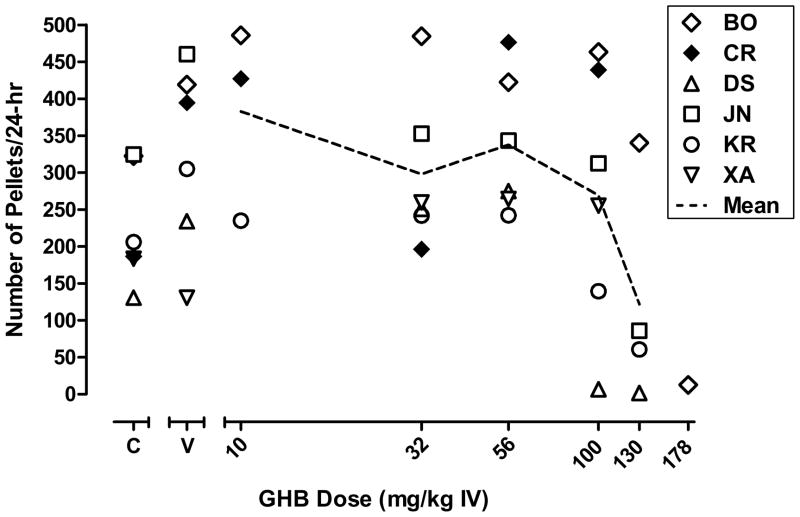
There was considerable variability in the number of pellets delivered per day, depending on the baboon (figure 2). During self-injection of vehicle, the mean number of pellets earned per day ranged from 57 to 572 across baboons for which at least one dose of GHB served as a reinforcer (BO, CR, DS, JN, KR, XA). When self-injected, high doses of GHB (100–178 mg/kg) decreased mean pellets per day.
Figure 2.
Mean number of pellets earned during self-injection of vehicle, cocaine, and GHB for baboons that self-administered at least one dose of GHB significantly above vehicle. Data points shown are the individual means of the last 5 days of dose substitution or vehicle (“V”) availability. Data for cocaine (“C”) are the grand means from the last 3 days of the cocaine baseline periods that preceded each test dose. Other details same as in figure 1.
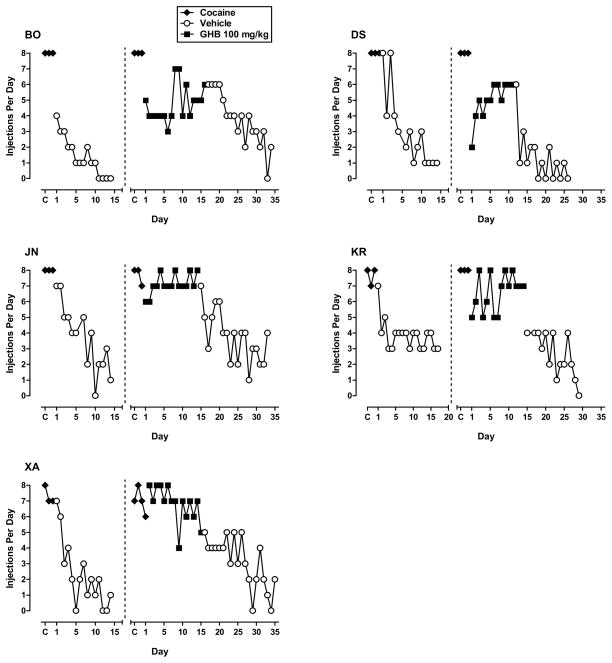
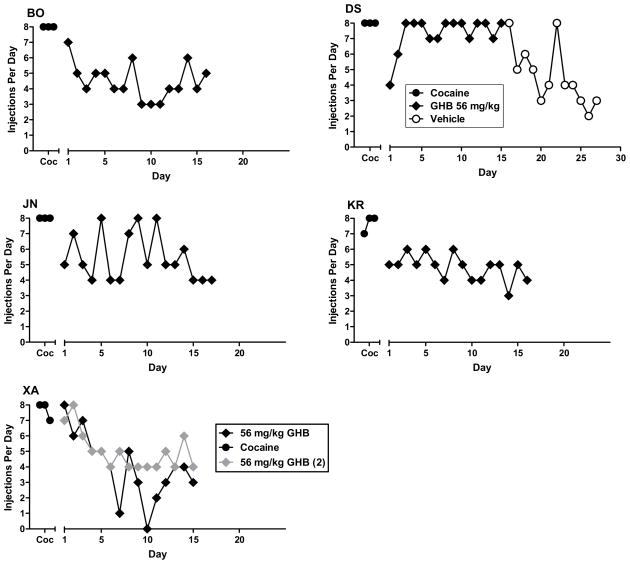
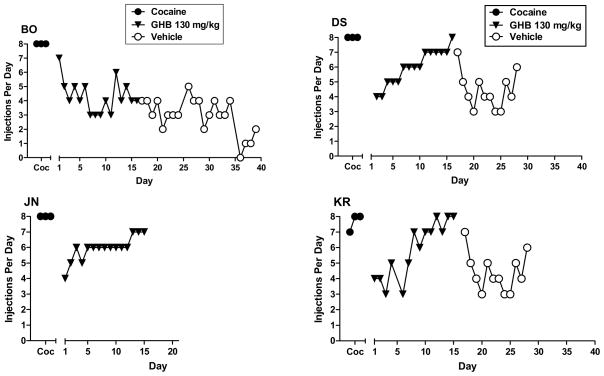
For the baboons for which GHB ultimately maintained high rates of responding, the pattern of day-to-day self injection differed depending on the GHB dose available for injection. As shown in Figure 3, the number of injections/day progressively decreased and stabilized when vehicle was substituted for cocaine. GHB 56–100 mg/kg maintained moderate to high rates of self-injection by at least the second day of availability in all those all those baboons (figs. 3, 4, 5). The exception was some days with zero or low self-injection for baboon XA at 56 mg/kg from days 5–10; but when the dose was studied a second time, rate of self-injection stabilized in the moderate range by the fourth day. For three of the four baboons studied at 130 mg/kg, rate of self-injection increased across the period of substitution, perhaps reflecting tolerance to initial sedating effects of this dose (fig. 5).
Figure 3.
Number of self-injections per day for the 3-day baseline period for cocaine (C), for consecutive days of vehicle or 100 mg/kg GHB availability, when substituted for cocaine, and for consecutive days of vehicle when substituted for GHB (extinction). Data panels shown are for the five baboons (designated BO, DS, JN, KR and XA) in which 100 mg/kg GHB maintained self-administration greater than vehicle.
Figure 4.
Number of self-injections per day for the 3-day baseline period for cocaine (Coc) and consecutive days of 56 mg/kg GHB availability, when substituted for cocaine. Data panels shown are for the five baboons (designated BO, DS, JN, KR and XA) in which 56 mg/kg GHB maintained self-administration greater than vehicle. For baboon DS, self-injection of vehicle substituted for GHB to evaluate extinction of responding is also shown.
Figure 5.
Number of self-injections per day for the 3-day baseline period for cocaine (Coc), for consecutive days of 130 mg/kg GHB availability, when substituted for cocaine, and for consecutive days of vehicle when substituted for GHB (extinction). Other details as in Figure 4.
When vehicle was substituted for GHB, injections/day decreased compared to the rate of self-injection being maintained by the GHB dose, providing further evidence for GHB reinforcement (figs. 3, 4, 5). Note that although rate of decline in self-injection when vehicle was substituted for GHB 100 mg/kg could be seen as generally comparable to that when vehicle was substituted for cocaine, rate of self-injection when vehicle was substituted for 130 mg/kg GHB did not show the same pattern of decrease as it had for the lower doses for the same baboon. Rather rate of vehicle self-injection remained rather stably in the moderate range for the 3 baboons studied, and only decreased to the low range by 18 days after vehicle substitution for baboon BO (fig. 5).
4.0 Discussion
The present study extensively investigated IV GHB self-administration in a total of 10 baboons. The initial findings with the first group of 3 animals were consistent with those of previous researchers who failed to find reliable GHB self-administration in rhesus macaques and concluded that GHB abuse potential had not been demonstrated (Beardsley et al., 1996; Woolverton et al., 1999). In the context of later work in our laboratory on GHB physical dependence, we revisited the question of GHB self-administration with the aim of studying higher doses. We changed the vehicle and injection volume in order to increase drug solubility of GHB. We found robust GHB self-administration in the majority of baboons tested. For the 6 baboons for which GHB served as a reinforcer, rate of self-injection was a generally ascending function of dose up to 130 mg/kg. Assessment of the rate and patterning of GHB self-administration in individual animals across multi-week periods of availability revealed that high doses were self-administered at moderate to high rates continuously across the 15-day period of GHB availability. In the baboons that self-injected doses of 100 mg/kg or greater, food-maintained responding was decreased. This effect is consistent with those previously reported for intragastric doses of GHB in baboons (Goodwin et al., 2005; Weerts et al., 2005); and suppression of food intake has also been reported following administration of GHB in rodents (Carter et al., 2004; Colombo et al., 1998; Itzhak and Ali, 2002) and pigeons (Koek et al., 2004).
Due to the individual differences in GHB self-administration, we evaluated different factors that may have influenced results across baboons such as the total mg GHB received per dose given the 17 kg spread in average weights, volume of injection, type of vehicle, and experimental history). A review of the detailed experimental history of each baboon revealed the one variable that generally differentiated the baboons for which GHB served as a reinforcer from those in which it did not was participation in studies with the substitution of an array of drugs. That is, baboons for which GHB was the first drug tested after training under our cocaine substitution procedure showed not only GHB reinforcement, but also self-injected GHB at high rates. For the one baboon (CR) that had an extensive self-administration history and also met the criterion for GHB reinforcement, performance was very different from the other baboons in that the mean peak rate of self-injection was in the low range and occurred at only one intermediate dose. One commonality in the self-administration experience of this baboon and the four that did not show GHB reinforcement is that all had experience self-administering GABAergic agonists (see Methods), although only baboon SI’s previous experience was exclusively GABAergic agonists. Thus, further research is needed to determine whether this correlational finding can be confirmed by experimental manipulation. Pharmacological history and self-administration experience have been shown to affect responses to psychoactive drugs in a number of studies (Fantegrossi et al., 2004; Griffiths and Weerts, 1997; Weerts et al., 2007a).
The behavioral effects of GHB are often compared to benzodiazepines and barbiturates. Carter and colleagues compared the cognitive, psychomotor, and subjective effects of GHB to traizolam in healthy human subjects. They reported that GHB was less disruptive on psychomotor and cognitive tasks when compared to triazolam but that both compounds produced similar participant ratings on the subjective effects measures (Carter et al., 2009a). In an earlier study, Carter and colleagues reported that when rated on measures of likelihood of abuse, GHB fell between pentobarbital (greatest likelihood) and triazolam (Carter et al., 2006; also see review by Griffiths and Johnson, 2005). Using the same procedures as described in the present study, we have reported that both benzodiazepines and barbiturates maintain levels of self-injection significantly above vehicle in baboons (Ator, 2000; Griffiths et al., 1991; Griffiths and Wolf, 1990; Weerts et al., 1999; Weerts and Griffiths, 1999). The high rates of self-injection, coupled with the primarily ascending dose-effect function, in the baboons that self-administered GHB is more similar to self-injection maintained under these procedures by potent hypnotic drugs (e.g., barbiturates zolpidem, zaleplon and flunitrazepam), than to most benzodiazepines (Griffiths et al., 1981, 1991, 1992; Ator, 2000; Ator et al., 2005).
Two studies have examined the IV reinforcing effects of GHB in rhesus monkeys (Beardsley et al., 1996; Woolverton et al., 1999) using similar procedures. Each drug injection was available during 1–2 h sessions under an FR 10 schedule of lever responding and each injection was followed by a brief (e.g. 10-s) time-out. Similar to the current study, both used a standard drug baseline procedure. GHB (0.3–7.5 mg/kg/infusion) did not generally maintain self-injection above vehicle levels when substituted for PCP, but did so in one of the four monkeys at one dose (Beardsley et al., 1996). When substituted for the barbiturate methohexital), GHB (0.01–10.0 mg/kg per injection) met the criterion for reinforcement compared to vehicle control levels in two of the three monkeys tested, but did so only at one dose for each monkey (3.2 or 10.0 mg/kg) (Woolverton et al., 1999), results that are reminiscent of the findings with the one baboon in the present study that had a history of testing self-administration of other drugs but showed reinforcement, albeit with a low rate of GHB self-injection and at only one dose.
Thus, the finding that GHB was self-administered in the current study is not inconsistent with the previous IV self-administration studies in monkeys (Beardsley et al., 1996; Woolverton et al., 1999) although the earlier studies concluded lack of significant GHB reinforcement, and ours concludes robust GHB reinforcement in 5 baboons. One differential procedural variable is the lower dose range studied in the rhesus monkeys; but given the short timeouts (10 s), this may not have been limiting because multiple injections of the higher doses could have been taken in rapid succession. However, GHB possesses potent CNS depressant effects that can last for hours, and under the limited access conditions with short timeouts, drug-induced behavioral suppression may have interfered with self-injection responding. In the current study, there was a 3-h timeout following injections, allowing recovery from the sedative effects prior to availability of the next injection. In addition, in the earlier studies each GHB dose was available for only one (Woolverton et al., 1999) or four (Beardsley et al., 1996) sessions, whereas in the present study, each dose was available for 15 or more days. Such longer periods may have allowed development of tolerance to the motor impairing effects of GHB as suggested in the day-by-day patterning in the 130 mg/kg GHB condition in the present study. Interestingly, the procedure used in the present study, which allows 24-hour access and imposes 3-hour intervals between injections, more closely resembles the pattern of human GHB abusers, whom use every 2–3 hours around the clock (Dyer et al., 2001), and thus may account for the differences in the results across studies.
The present results indicate that GHB can function as a reinforcer, maintaining high rates of self-administration in non-human primates under conditions of long-term drug availability and access throughout the day. These data are particularly important because they support the generally good correspondence between drugs abused by humans and those self-administered by laboratory animals and extends this finding to an abused drug with a relatively novel mechanism of action. Because abuse liability assessments of novel compounds depend significantly upon pre-clinical self-administration data, continuing validation of these procedures is critical to their use in drug development and risk assessment (Ator and Griffiths, 2003; Panlillo and Goldberg, 2007; Carter and Griffiths, 2009). Given continuing interest in development of GHB as a treatment for alcoholism, and GHB’s unique classification under the Controlled Substances Act in both Schedule I (denoting the highest potential for abuse) and Schedule III (due its efficacy in narcolepsy treatment), GHB is of continuing interest to multiple populations of scientists, clinicians and regulatory agencies. The present data should contribute to discussions of future drug development and clinical GHB use, as well as future regulatory issues surrounding this drug.
Acknowledgments
Role of Funding Source
Funding for this study was provided initially by NIH/NIDA contract N01 DA 8-7071 and subsequently by NIH/NIDA grant R01 DA 014919. Dr. Goodwin’s effort was partially supported by NIH/NIDA grant F32 DA019294; the NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors would like to acknowledge the excellent technical assistance of Samuel Womack and Kelly Lane in the conduct of these studies. We also thank the Research Triangle Institute (RTI) and the NIDA drug supply program for providing the cocaine and GHB used in these studies.
Footnotes
Contributors
Authors Goodwin, Weerts, Kaminski, Griffiths and Ator designed the study. Authors Weerts and Ator wrote the protocol. Authors Goodwin and Kaminski managed the literature searches and summaries of previous related work. Authors Goodwin and Weerts undertook the statistical analysis, and author Goodwin wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abanades S, Farre M, Segura M, Pichini S, Barral D, Pacifici R, Pellegrini M, Fonseca F, Langohr K, De la Torre R. Gamma-hydroxybutyrate (GHB) in humans: Pharmacodynamics and pharmacokinetics. Ann N Y Acad Sci. 2006;1074:559–576. doi: 10.1196/annals.1369.065. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Capristo E, Gessa GL, Caputo F, Stefanini GF, Gasbarrini G. Long-term administration of GHB does not affect muscular mass in alcoholics. Life Sci. 1999;65:L191–196. doi: 10.1016/s0024-3205(99)00395-1. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Castelli E, Stefanini GF, Casella G, Caputo F, Marsigli L, Bernardi M, Gasbarrini G. An open multicentric study evaluating 4-hydroxybutyric acid sodium salt in the medium-term treatment of 179 alcohol dependent subjects. GHB Study Group. Alcohol Alcoholism. 1996;31:341–345. doi: 10.1093/oxfordjournals.alcalc.a008160. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Ferrulli A, Caputo F, Gasbarrini A. The therapeutic potential of gamma-hydroxybutyric acid for alcohol dependence: balancing the risks and benefits. A focus on clinical data. Expert Opin Investig Drugs. 2009;18:675–686. doi: 10.1517/13543780902905855. [DOI] [PubMed] [Google Scholar]
- Ator NA. Zaleplon and triazolam: drug discrimination, plasma levels and self-administration in baboons. Drug Alcohol Depend. 2000;61:55–68. doi: 10.1016/s0376-8716(00)00123-x. [DOI] [PubMed] [Google Scholar]
- Ator NA, Atack JR, Hargreaves RJ, Burns HD, Dawson GR. Reducing abuse liability of GABAA/benzodiazepine ligands via selective partial agonist efficacy at alpha1 and alpha2/3 subtypes. J Pharmacol Exp Ther. 2010;332:4–16. doi: 10.1124/jpet.109.158303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70:S55–S72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Ator NA, Weerts EM, Kaminski BJ, Kautz MA, Griffiths RR. Zaleplon and triazolam physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. Drug Alcohol Depend. 2000;61:69–84. doi: 10.1016/s0376-8716(00)00122-8. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL, Harris LS. Evaluation of the discriminative stimulus and reinforcing effects of gammahydroxybutyrate (GHB) Psychopharmacology (Berl) 1996;127:315–322. doi: 10.1007/s002130050092. [DOI] [PubMed] [Google Scholar]
- Broughton R, Mamelak M. The treatment of narcolepsy-cataplexy with nocturnal gamma- hydroxybutyrate. Can J Neurol Sci. 1979;6:1–6. doi: 10.1017/s0317167100119304. [DOI] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend. 2009;105(Suppl 1):S14–25. doi: 10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Griffiths RR, Mintzer MZ. Cognitive, psychomotor, and subjective effects of sodium oxybate and triazolam in healthy volunteers. Psychopharmacology (Berl) 2009a;206:141–154. doi: 10.1007/s00213-009-1589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Pardi D, Gorsline J, Griffiths RR. Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem): differences in characteristics and misuse. Drug Alcohol Depend. 2009b;104:1–10. doi: 10.1016/j.drugalcdep.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Richards BD, Mintzer MZ, Griffiths RR. Relative abuse liability of GHB in humans: A comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of traizolam, pentobarbital, and GHB. Neuropsychopharmacology. 2006:1–15. doi: 10.1038/sj.npp.1301146. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Carter LP, Wu H, Chen W, Cruz CM, Lamb RJ, Koek W, Coop A, France CP. Effects of gamma-hydroxybutyrate (GHB) on schedule-controlled responding in rats: role of GHB and GABA-B receptors. J Pharmacol Exp Ther. 2004;308:182–188. doi: 10.1124/jpet.103.058909. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Balaklievskaia N, Diaz G, Lobina C, Reali R, Gessa G. Oral self-administration of gamma-hydroxybutyric acid in the rat. Eur J Pharmacol. 1995;285:103–107. doi: 10.1016/0014-2999(95)00493-5. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Fa M, Lobina C, Reali R, Gessa G. Gamma-hydroxybutyric acid intake in ethanol-preferring sP and -nonpreferring sNP rats. Physiol Behav. 1998;64:197–202. doi: 10.1016/s0031-9384(98)00033-x. [DOI] [PubMed] [Google Scholar]
- Craig K, Gomez H, McManus J, Bania T. Severe gamma-hydroxybutyrate withdrawal: a case report and literature review. J Emerg Med. 2000;18:65–70. doi: 10.1016/s0736-4679(99)00163-8. [DOI] [PubMed] [Google Scholar]
- DEA/ODE. Gamma Hydroxybutyric acid (GHB, liquid X, Goop, Georgia Home Boy) Drug Enforcement Ageny, Office of Diversion Control; Washington, DC: 2000. [Google Scholar]
- Degenhardt L, Darke S, Dillon P. GHB use among Australians: characteristics, use patterns and associated harm. Drug Alcohol Depend. 2002;67:89–94. doi: 10.1016/s0376-8716(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Dyer J, Roth B, Hyma B. Gamma-hydroxybutyrate withdrawal syndrome. Ann Emerg Med. 2001;37:147–153. doi: 10.1067/mem.2001.112985. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Woods JH, Winger G. Transient reinforcing effects of phenylisopropylamine and indolealkylamine hallucinogens in rhesus monkeys. Behav Pharmacol. 2004;15:149–157. doi: 10.1097/00008877-200403000-00007. [DOI] [PubMed] [Google Scholar]
- Fattore L, Martellotta M, Cossu G, Fratta W. Gamma-hydroxybutyric acid: an evaluation of its rewards properties in rats and mice. Alcohol. 2000a;20:247–256. doi: 10.1016/s0741-8329(99)00088-9. [DOI] [PubMed] [Google Scholar]
- Fattore L, Martellotta MC, Cossu G, Fratta W. Gamma-hydroxybutyric acid: an evaluation of its rewarding properties in rats and mice. Alcohol. 2000b;20:247–256. doi: 10.1016/s0741-8329(99)00088-9. [DOI] [PubMed] [Google Scholar]
- Galloway G, Frederick-Osborne S, Seymour R, Contini S, Smith D. Abuse and therapeutic potential of gamma-hydroxybutyric acid. Alcohol. 2000a;20:263–269. doi: 10.1016/s0741-8329(99)00090-7. [DOI] [PubMed] [Google Scholar]
- Galloway G, Frederick S, Staggers F, Gonzales M, Stalcup S, Smith D. Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence. Addiction. 1997;92:89–96. [PubMed] [Google Scholar]
- Galloway GP, Frederick-Osborne SL, Seymour R, Contini SE, Smith DE. Abuse and therapeutic potential of gamma-hydroxybutyric acid. Alcohol. 2000b;20:263–269. doi: 10.1016/s0741-8329(99)00090-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Nutt DJ. Gamma hydroxy butyrate abuse and dependency. Journal of Psychopharamacology. 2005;19:195–204. doi: 10.1177/0269881105049041. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Froestl W, Weerts EM. Involvement of gamma-hydroxybutyrate (GHB) and GABA-B receptors in the acute behavioral effects of GHB in Baboons. Psychopharmacolgy. 2005;180:342–351. doi: 10.1007/s00213-005-2165-y. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Brady JV, Bradford LD. Predicting the abuse liability of drugs with animal drug self-administration prodcedures: Psychomotor stimulants and hallucinogens. In: Thompson T, Dews PB, editors. Advances in Behavioral Pharmacology. Academic Press; New York: 1979. pp. 163–208. [Google Scholar]
- Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66(Suppl 9):31–41. [PubMed] [Google Scholar]
- Griffiths RR, Lamb RJ, Sannerud CA, Ator NA, Brady JV. Self-injection of barbiturates, benzodiazepines and other sedative- anxiolytics in baboons. Psychopharmacology. 1991;103:154–161. doi: 10.1007/BF02244196. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Sannerud CA, Ator NA, Brady JV. Zolpidem behavioral pharmacology in baboons: self-injection, discrimination, tolerance and withdrawal. J Pharmacol Exp Ther. 1992;260:1199–1208. [PubMed] [Google Scholar]
- Griffiths RR, Weerts EM. Benzodiazepine self-administration in humans and laboratory animals-- implications for problems of long-term use and abuse. Psychopharmacology (Berl) 1997;134:1–37. doi: 10.1007/s002130050422. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Wolf B. Relative abuse liability of different benzodiazepines in drug abusers. J Clin Psychopharmacol. 1990;10:237–243. [PubMed] [Google Scholar]
- Hutto B, Fairchild A, Bright R. gamma-Hydroxybutyrate withdrawal and chloral hydrate. Am J Psychiatry. 2000;157:1706. doi: 10.1176/appi.ajp.157.10.1706. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ali S. Repeated administration of gamma-hydroxybutyric acid (GHB) to mice: assessment of the sedative and rewarding effects of GHB. Ann N Y Acad Sci. 2002;965:451–460. doi: 10.1111/j.1749-6632.2002.tb04186.x. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Balster RL. A summary of the results of a drug self-administration study using substitution procedures in rhesus monkeys. Bull Narc. 1978;30:43–54. [PubMed] [Google Scholar]
- Kaminski BJ, Griffiths RR. Intravenous self-injection of methcathinone in the baboon. Pharmacol Biochem Behav. 1994;47:981–983. doi: 10.1016/0091-3057(94)90307-7. [DOI] [PubMed] [Google Scholar]
- Kaminski BJ, Sannerud CA, Griffiths RR. Intravenous self-injection of psychomotor stimulant-anorectics in the baboons. Exp Clin Psychopharmacol. 1996;4:141–150. [Google Scholar]
- Koek W, Flores LR, Carter LP, Lamb RJ, Chen W, Wu H, Coop A, France CP. Discriminative Stimulus Effects of {gamma}-Hydroxybutyrate in Pigeons: Role of Diazepam-Sensitive and -Insensitive GABAA and GABAB Receptors. J Pharmacol Exp Ther. 2004;308:904–911. doi: 10.1124/jpet.103.056093. [DOI] [PubMed] [Google Scholar]
- Laborit H. Gamma-hydroxybutyrate, succinic semialdehyde and sleep. Prog Neurobiol. 1973;1:257–274. [Google Scholar]
- Lukas S, Griffiths R, Bradford L, JVB, Daley L. A tethering system for intravenous and intragastric drug administration in the baboon. Pharmacol Biochem Behav. 1982;17:823–829. doi: 10.1016/0091-3057(82)90366-5. [DOI] [PubMed] [Google Scholar]
- Mamelak M, Scharf M, Woods M. Treatment of narcolepsy with gamma-hydroxybutyrate: a review of clinical and sleep laboratory findings. Sleep. 1986;9:285–289. doi: 10.1093/sleep/9.1.285. [DOI] [PubMed] [Google Scholar]
- Martellotta M, Cossu G, Fattore L, Gessa G, Fratta W. Intravenous self-administration of gamma-hydroxybutyric acid in drug-naive mice. Eur Neuropsychopharmacol. 1998;8:293–296. doi: 10.1016/s0924-977x(97)00087-4. [DOI] [PubMed] [Google Scholar]
- Martellotta MC, Fattore L, Cossu G, Fratta W. Rewarding properties of gamma-hydroxybutyric acid: an evaluation through place preference paradigm. Psychopharmacology (Berl) 1997;132:1–5. doi: 10.1007/s002130050312. [DOI] [PubMed] [Google Scholar]
- Miotto K, Darakjian J, Basch J, Murray S, Zogg J, Rawson R. Gamma-hydroxybutyric acid: patterns of use, effects and withdrawal. Am J Addict. 2001;10:232–241. doi: 10.1080/105504901750532111. [DOI] [PubMed] [Google Scholar]
- Nicholson KL, Balster RL. GHB: a new and novel drug of abuse. Drug Alcohol Depend. 2001;63:1–22. doi: 10.1016/s0376-8716(00)00191-5. [DOI] [PubMed] [Google Scholar]
- Scharf M, Brown D, Woods M, Brown L, Hirschowitz J. The effects and effectiveness of gamma-hydroxybutyrate in patients with narcolepsy. J Clin Psych. 1985;46:222–225. [PubMed] [Google Scholar]
- Scrima L, Hartman P, Johnson F, Thomas E, Hiller F. The effects of gamma-hydroxybutyrate on the sleep of narcolepsy patients: a double-blind study. Sleep. 1990;13:479–490. doi: 10.1093/sleep/13.6.479. [DOI] [PubMed] [Google Scholar]
- U.S. Xyrem® Multicenter Study Group. Sodium oxybate demonstrates long-term efficacy for the treatment of cataplexy in patients with narcolepsy. Sleep Med. 2004:119–123. doi: 10.1016/j.sleep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Watson J, Guzzetti S, Franchi C, Di Clemente A, Burbassi S, Emri Z, Leresche N, Parri HR, Crunelli V, Cervo L. Gamma-hydroxybutyrate does not maintain self-administration but induces conditioned place preference when injected in the ventral tegmental area. Int J Neuropsychopharmacol. 2009:1–11. doi: 10.1017/S1461145709990186. [DOI] [PubMed] [Google Scholar]
- Wedin G, Hornfeldt C, Ylitalo L. The clinical development of gamma-hydroxybyrate (GHB) Current Drug Safety. 2006;1:99–106. doi: 10.2174/157488606775252647. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Ator NA, Griffiths RR. Comparison of the intravenous reinforcing effects of propofol and methohexital in baboons. Drug Alcohol Depend. 1999;57:51–60. doi: 10.1016/s0376-8716(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, Goodwin AK. The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol. 2007a;15:309–327. doi: 10.1037/1064-1297.15.4.309. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Froestl W, Kaminski BJ, Griffiths RR. Attenuation of cocaine-seeking by GABA B receptor agonists baclofen and CGP44532 but not the GABA reuptake inhibitor tiagabine in baboons. Drug Alcohol Depend. 2007b;89:206–213. doi: 10.1016/j.drugalcdep.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Griffiths RR, Brown PR, Froestl W, Jakobs C, Gibson KM. Spontaneous and precipitated withdrawal after chronic intragastric administration of gamma-hydroxybutyrate (GHB) in baboons. Psychopharmacology. 2005;179:678–687. doi: 10.1007/s00213-004-2079-0. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Griffiths RR. Evaluation of the intravenous reinforcing effects of clonidine in baboons. Drug Alcohol Depend. 1999;53:207–214. doi: 10.1016/s0376-8716(98)00130-6. [DOI] [PubMed] [Google Scholar]
- Wong CG, Gibson KM, Snead OC., 3rd From the street to the brain: neurobiology of the recreational drug gamma-hydroxybutyric acid. Trends Pharmacol Sci. 2004;25:29–34. doi: 10.1016/j.tips.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Rowlett JK, Winger G, Woods JH, Gerak LR, France CP. Evaluation of the reinforcing and discriminative stimulus effects of gamma-hydroxybutyrate in rhesus monkeys. Drug Alcohol Depend. 1999;54:137–143. doi: 10.1016/s0376-8716(98)00153-7. [DOI] [PubMed] [Google Scholar]