Abstract
Polychlorinated biphenyls (PCBs) are environmental neurotoxicants known to affect the brain dopaminergic (DA) system. This project investigated whether developmental exposure to PCBs would alter the discriminative stimulus effects of psychostimulant drugs known to act on the DA system. Female Long-Evans rats were orally exposed to 0, 3, or 6 mg/kg/day of an environmentally relevant PCB mixture from four weeks prior to breeding through weaning of their litters on PND 21. When they reached adulthood one male and female/litter were trained to discriminate cocaine (10.0 mg/kg, IP) from saline by repeatedly pairing cocaine injections with reinforcement on one operant response lever, and saline injections with reinforcement on the other lever. After response training, generalization tests to four lower doses of cocaine (7.5, 5.0, 2.5, and 1.25 mg/kg, IP) and to amphetamine (1.0, 0.5, 0.25, and 0.125 mg/kg, IP) were given two days/week, with additional training dose days in-between. Percent responding of the PCB-exposed rats on the cocaine-paired lever was significantly higher than that of controls for the highest generalization dose of cocaine, and lower than that of controls for the highest dose of amphetamine. Response rate and percent responding on the cocaine lever did not differ among the exposure groups on the days when the training dose of cocaine was given, suggesting the generalization test results were not due to pre-existing differences in discrimination ability or rate of responding. These findings suggest developmental PCB exposure can alter the interoceptive cues of psychostimulants.
Keywords: drug discrimination, polychlorinated biphenyls, dopamine pharmacology, behavioral toxicology
1. Introduction
Polychlorinated biphenyls (PCBs) are ubiquitous environmental contaminants that have been used for a number of industrial purposes. They were used as lubricants, flame retardants, and as dielectric fluids in transformers and capacitors until they were banned in the 1970s [8]. Despite their discontinuation, PCBs remain in our environment because of their resistance to chemical and biological degradation. Because of their chemical and physical stability, as well as their lipophilicity, PCBs can readily enter the food chain and bioaccumulate in humans and wildlife [7,10]. PCBs cross the placental barrier and are present in breast milk, thus making early development a vulnerable period for exposure [15,18].
1.1. Developmental PCB Exposure – Behavioral impairments
Rats and monkeys developmentally exposed to PCBs have displayed behavioral deficits across many cognitive tasks, providing evidence that PCBs are neurotoxicants. For instance, rats and monkeys developmentally exposed to PCBs show impaired performance on tasks that assess executive function including cognitive flexibility, working memory, and inhibitory control [reviewed in 46]. The evidence from human studies supports that obtained using animal models. Boucher et al. [3] reviewed a number of human birth cohort studies that examined the neurological, behavioral and cognitive effects of PCB exposure in neonates and young children. Children with higher umbilical cord blood PCB concentrations had problems with inhibitory control, including increased errors of commission on continuous performance tests [e.g., 19,59,60] and increased responding on a differential reinforcement of low response rate (DRL) task [61] similar to the DRL tasks used in rats [45] and monkeys [38] developmentally exposed to PCBs. PCB-exposed children also exhibited deficits on tasks that assess cognitive flexibility and working memory [19] that were similar to those reported using animal models of developmental PCB exposure [reviewed in 46].
1.2 In Vivo Exposure to PCBs and Neurotransmitter System Dysfunction
Substantial research has focused on the underlying neurobiological/neurochemical mechanisms associated with PCB-related behavioral deficits. Numerous in vivo studies have reported reductions in brain dopamine (DA) levels following both perinatal and adult exposure to individual non-coplanar PCB congeners or mixtures of mostly non-coplanar PCB congeners which represent the bulk of human exposure [15]. For example, adult exposure has resulted in reductions of brain dopamine (DA) in both rats [50,57] and non-human primates [49,53,54]. Exposure to similar congeners or mixtures during early development (via both gestational and lactational exposure) has also been reported to produce a decrease in brain DA levels in weanling rats [4]. The reduction in DA does not appear to be region specific. While most studies have focused on the effects of PCBs on DA in the striatum, one study found that gestational and lactational exposure to the non-coplanar congener PCB 47 caused reductions in frontal cortical DA that persisted into adulthood [52].
Inhibition of the dopamine transporter (DAT) which is responsible for the re-uptake of dopamine into the nerve terminal, and the vesicular monoamine transporter (VMAT2) which is responsible for packaging cytosolic DA into vesicles for later release, appear to contribute to the alterations in brain DA concentration [2,11,12,26]. One theory is that DA reduction may be a compensatory response to an increase in cytosolic DA due to VMAT2 inhibition and/or to elevated DA in the nerve terminal due to DAT inhibition [2]. Reduced expression of the DAT and VMAT2 has been demonstrated after PCB exposure in both animals [2,5,12,27,39] and humans [56].
Alterations in concentrations of other brain neurotransmitters including norepinephrine [NE; 55], serotonin [5-HT; 4,16,22,28,51], glutamate [28], and GABA [28] have also been reported following in vivo PCB exposure. However, effects on the DA system have been the most consistently studied and replicated. Based on several studies, it also appears that DA function is more sensitive to disruption by PCB exposure than some of these other neurotransmitter systems [28]. For example, as mentioned above, adult exposure to commercial PCB mixtures Aroclors 1016 or 1260 in non-human primates resulted in a reduction in DA concentration in several brain regions, while similar exposure to Aroclor 1016 did not produce alterations in brain NE, epinephrine, or 5-HT concentrations [53].
1.4 Rationale/Hypothesis of Current Study
Recent results have demonstrated that responding of rats developmentally exposed to PCBs on a DRL task was disrupted to a lesser degree by administration of amphetamine than responding of non-exposed controls [45]. Several potential explanations for these results exist, including the possibility that the PCB-exposed animals were less sensitive to the pharmacological effects of amphetamine due to alterations in DA pharmacology caused by PCB exposure. To evaluate this more closely, the current study examined whether perinatal PCB exposure would alter the sensitivity of rats to the discriminative stimulus properties of cocaine and amphetamine, psychostimulants that are known to act on the DA system. Specifically, the drug discrimination paradigm was used to determine whether there were differences in the ability of PCB-exposed and control rats to discriminate the stimulus effects of varying doses of cocaine and amphetamine from those of saline. It was hypothesized that the interoceptive effects of cocaine and amphetamine would differ between perinatally PCB-exposed rats and non-exposed controls.
2. Methods
2.1 Animals
Thirty-one nulliparous female Long-Evans rats, approximately 60 days of age, were purchased in two cohorts from Harlan (Madison, WI). Animals used in this study were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). The females were individually housed in standard plastic shoebox cages with corn-cob bedding, in a temperature- and humidity-controlled room (22°C, 40-55% humidity) maintained on a 12-hour reverse light-dark cycle (lights off at 0830 h). Standard rat chow and water were available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign and were in accordance with the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals [33] and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research [34].
2.2 Exposure
There was a one week adaptation period before dosing began. Females were assigned to 3 exposure groups (balanced for body weight): control, 3 mg/kg PCB, or 6 mg/kg PCB. The doses chosen were physiologically relevant because offspring born to dams given these doses appear phenotypically similar to children born to mothers with moderate to high PCB body burdens. In particular, at these doses offspring have been shown to weigh less than control pups at birth and at weaning [24] and to show deficits in inhibitory control [45]. Subtle reductions in birth weight and postnatal growth as well as deficits in inhibitory control have also been reported in children born to PCB-exposed mothers [9,19,20,61].
Dams were dosed daily beginning 28 days prior to mating and continuing until their pups were weaned on postnatal day (PND) 21. The PCB mixture was formulated to mimic the congener profile found in walleye (a popular sport-caught fish), taken from the Fox River in northeast Wisconsin, thereby closely mimicking human PCB exposure from fish. The mixture consisted of 35% Aroclor 1242 (Monsanto Lot KB 05-415), 35% Aroclor 1248 (AccuStandards Lot F-110), 15% Aroclor 1254 (Monsanto Lot KB 05-612), and 15% Aroclor 1260 (AccuStandards Lot 021-020). The mixture was found to have relatively low aryl hydrocarbon receptor (AhR) activity, but high ryanodine receptor (RyR) activity [24].
The PCBs were dissolved in corn oil to yield the dosing solutions. Dams were weighed daily and the volume of administered dosing solution adjusted to account for weight gain. For dosing, the appropriate amount of the PCB dosing solution (or corn oil for the controls) was pipetted onto one-half of a small vanilla cookie (Golden Vanilla Wafers, Keebler®) and fed to the dams. Each dam was observed to ensure that the entire cookie was consumed.
2.3 Breeding, Pregnancy, and Weaning
At approximately 100 days of age (4 weeks after the initiation of PCB exposure), females were paired with an unexposed male in a hanging wire cage for eight consecutive days except for a brief period each day when they were weighed and returned to their home cages for dosing. The breeding cages contained standard rat chow and water ad libitum. Females were monitored twice daily for the presence of a sperm plug. On the day of parturition (PND 0), the pups were examined for gross abnormalities, weighed and sexed. On PND 2, the litters were culled to 10 pups (5 males and 5 females, when possible). Extra pups from litters with more than 10 were cross-fostered as needed within treatment groups and to litters of the same age to insure all litters had 8-10 pups. Cross-fostered pups were marked by ear clip and were not used for behavioral testing. In some cases, pups of the same age from the same treatment group were not available for fostering. Litters consisting of less than 7 pups that could not be increased by fostering were not included in the study. Of the 36 females that started the study, 1, 1, and 2 females did not get pregnant and 2, 1, and 0 litters were too small to use from the 0, 3, and 6 mg/kg groups, respectively.
Pups were weighed on PND 7 and 14, and at weaning on PND 21. On the day of weaning, dams from each litter were euthanized and their liver weight and number of uterine implantation sites were recorded. Two male and two female pups from each litter were randomly selected and maintained for behavioral testing – one male and female for this study and the other male and female for a study examining amphetamine behavioral sensitization [36]. The unused pups in each litter were euthanized, and organ weights (brain, liver, thymus) were obtained from one male and one female per litter.
Pups retained for behavioral testing were identified by ear punch and pair-housed in a ventilated rack in plastic rodent cages according to sex and litter. Rats were weighed weekly between PND 21 and 90; food and water were available ad libitum during this time. Beginning on PND 90, rats were weighed daily and access to food was restricted to 85% of the rats' free-feeding weight in order to keep the animals motivated to work for food rewards in the operant chambers. Food restriction has been routinely used in our lab and there is no evidence that it confounds PCB-mediated effects.
2.4 Apparatus
Behavioral testing was conducted in 4 automated operant chambers (Med Associates; St. Albans, VT) housed in sound attenuated cubicles, each ventilated by a fan. All operant chambers contained a stimulus cue lamp above each of the two retractable response levers, which were located symmetrically on both sides of the pellet trough approximately 5.5 cm above the floor. A white-noise generator masked extraneous sounds, and a sonalert speaker was used to signal reinforcement. The experimental contingencies were programmed using Medstate Notation behavioral programming language (Med Associates; St. Albans, VT).
2.5 Procedure
2.5.1 Autoshaping
Operant testing occurred six days per week. Beginning around PND 100, all animals began an autoshaping program to shape them to press the response levers. At the beginning of the session, both levers were extended. The illumination of the cuelight above the right response lever was programmed according to a fixed-time 3-min (FT-3) schedule. Thus, the cue light was illuminated over the right lever every 3 minutes for 15 seconds, after which a food pellet was dispensed. Presses on either lever (cued or uncued) were reinforced. If the rat pressed either lever while the cue light was illuminated, a food pellet was dispensed and the cue light extinguished. Each reinforcer was a single food pellet paired with a 40-ms tone. The FT-3 minute cue illumination schedule continued until a total of 10 lever presses occurred on either response lever, at which point the illumination of the right stimulus lamp according to the FT-3 minute schedule was inactivated, and delivery of reinforcers became contingent on lever presses only. Autoshaping sessions terminated after 60 minutes had elapsed or 100 reinforcers were delivered, whichever occurred first. Once the animal made 100 lever presses within a single session, it was then placed on a fixed ratio 1 (FR1) contingency where every lever press resulted in the delivery of a food pellet.
2.5.2 Fixed Ratio Training
Fixed ratio training programs – including FR1, FR2, FR4, FR7, and FR10 – were used to gradually increase the response requirement. As the animals progressed through these programs, they had to make more lever presses in order to earn a single food reinforcer. The reinforcement lever alternated every 5th trial so the rats would not learn to associate one lever with reinforcement. For each phase, animals had to earn all of the 100 available reinforcers across two consecutive sessions in order to move to the next fixed ratio phase.
2.5.3 Drug Discrimination Training
Once 100 reinforcers were earned on two consecutive sessions under the FR10 response contingency, drug discrimination training began. On training days, each rat received an IP injection of either 1 ml/kg of saline or 10 mg/kg of cocaine in an equivalent amount of saline 10 minutes before the start of the session. During the session, both levers were extended but the rat was only reinforced when the FR10 response requirement was met on the lever paired with the injection type. One lever was active following administration of cocaine, while the other lever was active following administration of saline. The lever paired with cocaine was counterbalanced (right or left) within all treatment groups. The order of training sessions (cocaine or saline) was based on a double alternation sequence (i.e., cocaine-cocaine-saline-saline-cocaine-cocaine⋯). Each session concluded after 200 responses were made on the correct lever. Discrimination training sessions were conducted daily, 6 days per week. Each rat had to meet 2 criteria in the discrimination training sessions before moving to the test phase: the rat was required to have 8 of 10 consecutive sessions with ≥ 80% correct, and the first completed FR10 in each session had to occur on the correct lever (i.e., 10 presses on the saline lever when injected with saline).
2.5.4 Generalization/Cross-Generalization Tests
Once the criterion for discrimination between saline and the training dose of cocaine was met, generalization tests were conducted following IP administration of lower doses of cocaine (7.5, 5.0, 2.5, and 1.25 mg/kg) or of amphetamine (1.0, 0.5, 0.25, and 0.125 mg/kg) in order to determine how well rats generalized the discriminative properties of these drug doses to the training dose of cocaine. Considering only body surface area, the highest test doses of cocaine and amphetamine would be equivalent to human doses of approximately 1.2 and 0.16 mg/kg, respectively [37], for a 60 kg person. Thus, the doses in the current study are quite low, as the typical human daily cocaine [63] and amphetamine [25] doses are usually higher. A cross-generalization test, using a single dose of pentobarbital (5.0 mg/kg), was also done in order to determine whether generalization would occur to a drug that has very different reinforcing properties than those of cocaine or amphetamine. Generalization and cross-generalization test sessions used a discrete-trials procedure. Only one IP injection and post-injection interval was assessed in each test session and only one test session was completed in a day. On test sessions, both levers were active until animals made 10 consecutive responses on either lever at which point a single reinforcement pellet was dispensed and the program terminated. If 15 minutes elapsed before 10 consecutive responses occurred on either lever, the session terminated. The order of administration of the generalization and cross-generalization drug doses was determined using a balanced Latin square design and each test dose was given three times. Test days were Tuesdays and Fridays with regular training days on Mondays, Wednesdays, Thursdays, and Saturdays.
Three rats died during training and four others failed to progress through the training phases and were subsequently dropped. Of those that were dropped, 0, 1, and 3 (2 from same litter) were in the 0, 3, and 6 mg/kg/day PCB groups, respectively. If data were lost due to the death or dropping of an opposite-sex littermate, mean-substitution was used in place of the missing data, rather than dropping the litter altogether which involved dropping usable data from the surviving littermate. The data were graphed both with and without the litters in question and careful visual inspection of the data revealed no obvious differences in the pattern of experimental results.
2.6 Data Analysis
All statistical analyses were conducted using SPSS for MS Windows (version 15.0, SPSS Inc.; Chicago, IL) with statistical significance for the omnibus analyses set at p<0.05. In the case of some repeated measures factors, a sphericity violation was noted. In such cases, a Greenhouse-Geisser correction was used to reduce the risk of a Type I error if ε was < 0.75 and a Huynh-Feldt correction was used when ε was > 0.75 [44]. Analyses requiring such correction are reported in the results using the appropriate adjusted degrees of freedom. In the interest of brevity, only significant exposure-related or sex-related main effects and interactions are reported. Additional post hoc analyses within each drug dose were conducted as appropriate to determine the nature of significant effects that were detected via the initial omnibus analyses.
2.6.1 Reproductive/Developmental endpoints
Reproductive data analyzed included litter size, percent male births, percent live births, percent gestational weight gain, and percent lactational weight gain. Percent gestational weight gain was determined by calculating [(GD 21 weight – conception weight)/conception weight] × 100. Percent lactational weight gain was determined by calculating [(highest lactational weight − parturition weight)/parturition weight] × 100. The ratio of liver:body weight and number of uterine implantation sites in the dam at weaning were also measured. For each dependent variable, a 3 (exposure) × 2 (cohort) between-subjects ANOVA was conducted.
Developmental data analyzed included postnatal weight gain, organ:body weight ratios, and day of eye opening. Postnatal weight gain was determined by collecting body weights on PND 0, 7, 14, and 21. These data were analyzed via a 3 (exposure) × 2 (cohort) × 2 (sex) × 4 (age) mixed ANOVA with sex (nested within litter) and age as repeated measures factors. Organ to body weight ratios for brain, liver, and thymus were measured on the day of weaning and analyzed separately via a 3 (exposure) × 2 (cohort) × 2 (sex) mixed ANOVA with sex nested within litter.
2.6.2 Acquisition
The number of drug discrimination training sessions required to meet the discrimination criterion were determined and analyzed via a 3 (exposure) × 2 (sex) mixed ANOVA with exposure as a between-subjects variable, and sex nested within litter.
2.6.3 Training Sessions
The percent responding on the cocaine-paired lever and response rate data from the cocaine training sessions that occurred between generalization/cross-generalization test days were averaged across all days and analyzed via a 3 (exposure) × 2 (sex) mixed ANOVA with exposure as a between-subjects variable, and sex nested within litter.
2.6.4 Generalization/Cross-generalization response data
The percent of responses on the cocaine lever was determined for each generalization/cross-generalization trial and averaged across all usable trials for each drug dose. For the cocaine and amphetamine trials, the data for each dependent measure were analyzed separately using a 3 (exposure) × 2 (sex) × 2 (drug) × 4 (dose) mixed ANOVA with exposure as a between-subjects variable and sex (nested within litter), drug, and dose as repeated-measures factors. The data for pentobarbital were analyzed in a similar fashion except that drug and dose were not included as variables because there was only one dose of pentobarbital tested.
3.0 Results
3.1 Reproductive/Developmental endpoints
Effects on reproductive and developmental endpoints were similar to those we have reported in previous studies using the same PCB mixture, exposure period and doses [24] (see supplemental figures). Briefly, there was a significant effect of exposure [F(2,24)=6.19, p=0.007] on the liver:body weight ratio in the dams, with the 6, but not the 3, mg/kg/day PCB group having a significantly higher ratio than the 0 mg/kg/day PCB group (p=0.008). A significant exposure x day interaction on postnatal weight gain of the pups was also found [F(2.502,30.029)=5.36, p=0.007]. The exposure x sex interaction was not significant. Pups (both males and females) exposed to 6 mg/kg/day PCBs weighed significantly less than controls on PND 7 (p=0.023), 14 (p<0.001), and 21 (p=0.020). Pups exposed to 3 mg/kg/day PCBs weighed significantly less on PND 14 (p=0.021).
The exposure x day interaction was also significant for the pup liver:body weight ratio analysis [F(2,24)=4.50, p=0.022]. Post hoc analyses revealed all pups (males and females) exposed to PCBs had a significantly higher liver:body weight ratio (p<0.001) relative to their same-sex control counterparts. Significant main effects of exposure [F(2,24)=22.10, p<0.001] and sex [F(1,24)=7.52, p=0.011] were found for thymus:body weight ratio. Both the 3 and 6 mg/kg/day PCB groups had a significantly lower thymus:body weight ratios than controls (p<0.001), with females having a significantly higher ratio than males.
3.2 Acquisition
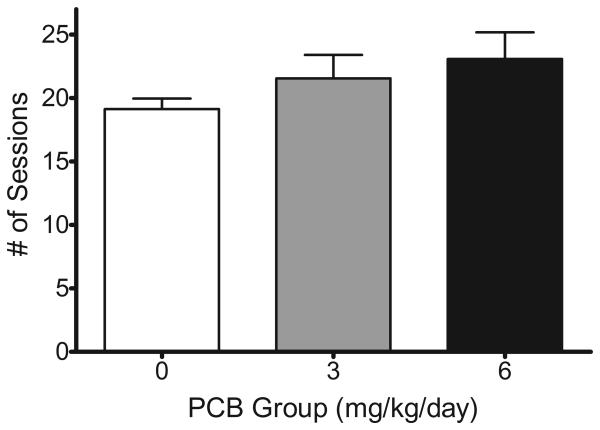
Although increasing PCB dose appeared to increase the mean number of trials required to reach the drug discrimination criterion, rats did learn the task and were able to perform at criterion regardless of exposure group. The mean number of trials required for the exposure groups to reach the drug discrimination criterion is presented in Figure 1. Although there is a trend toward more required trials with increasing PCB dose, the results of the omnibus analysis did not reveal any significant main effects of exposure or sex or a significant exposure x sex interaction.
Figure 1. Acquisition.
Increasing PCB dose appeared to increase the mean number of trials required to reach the discrimination training criterion (+SEM) but no significant differences were found.
3.3 Training Sessions
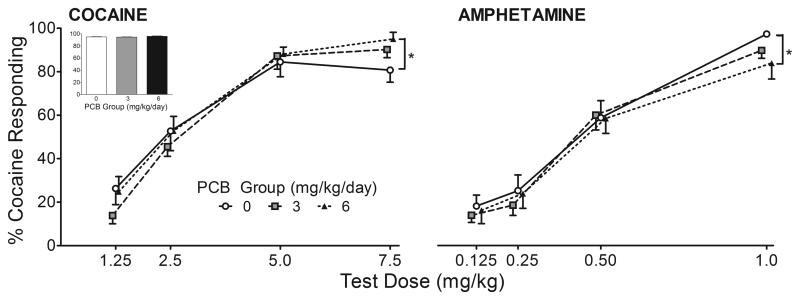
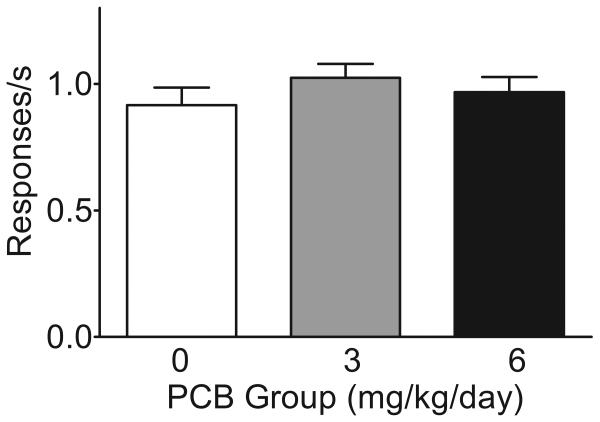
The PCB groups had slightly higher rates of responding on the days in between generalization tests when all rats received the training dose of cocaine. However, analysis of percent responding on the cocaine lever (Figure 2, inset) and response rate (Figure 3) revealed no significant differences between groups.
Figure 2. Percent Responding on Cocaine Lever Following Cocaine or Amphetamine.
There was no significant effect of exposure on percent cocaine responding to the 10 mg/kg training dose of cocaine (inset). During generalization testing, developmental exposure to 6 mg/kg/day PCBs resulted in a significantly higher percentage of responding on the cocaine lever following administration of 7.5 mg/kg cocaine (IP), but a significantly lower percentage following administration of 1.0 mg/kg amphetamine (IP). *p=0.012 and p=0.003 for cocaine and amphetamine, respectively.
Figure 3. Response Rate Following Training Dose of Cocaine.
Analysis of the response rate data averaged across intermittent training sessions that occurred between generalization test sessions revealed no significant effect of PCB exposure.
3.4 Generalization/Cross-generalization response data
Overall, relative to controls, rats exposed to 6.0 mg/kg PCBs showed an increase in percent responding on the cocaine-paired lever at the 7.5 mg/kg dose of cocaine and a decrease in percent responding on the cocaine-paired lever after administration of 1.0 mg/kg amphetamine. This was revealed by a significant exposure x drug x dose interaction [F(6,69)=2.97, p=0.012]. Additional post hoc analysis revealed significant exposure-related effects at the 7.5 mg/kg dose of cocaine (p=0.026) and the 1.0 mg/kg dose of amphetamine (p=0.001). Dunnett analysis at these drug doses revealed a significant difference between the control group and the 6 mg/kg PCB group with the difference being in opposite directions for the two drugs (see Figure 2). There was also a significant exposure x sex x drug interaction [F(2,23)=4.317, p=0.026] on the percent responding on the cocaine-paired lever. For cocaine (averaged across dose), female controls appeared to respond more on the cocaine-paired lever compared to control males, while there were relatively no sex differences in the PCB-exposed groups. For amphetamine, the male and female control groups were similar, while the male 6.0 mg/kg PCB group appeared to respond less on the cocaine-paired lever compared to females in the same exposure group. However, these effects were not found to be significant upon further post-hoc analyses.
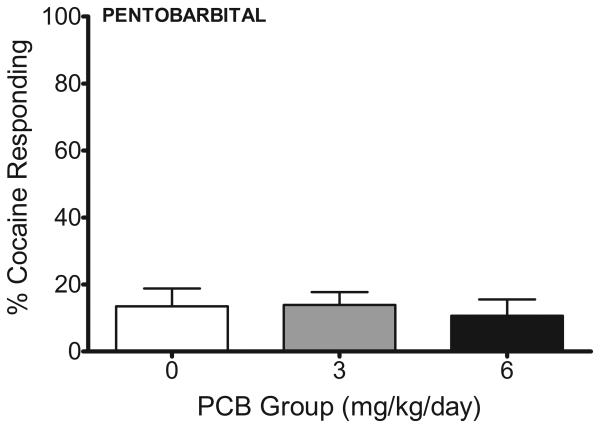
Analysis of percent responding on the cocaine-paired lever following administration of 5.0 mg/kg pentobarbital (IP) did not reveal any significant PCB exposure-related differences. Responding on the cocaine-paired lever following pentobarbital was 3.3% lower than the lowest test dose of amphetamine (0.125 mg/kg), 8.9% lower than the lowest test dose of cocaine (1.250 mg/kg), and 82.6% lower than the training dose of cocaine (10 mg/kg) (Figure 4).
Figure 4. Percent Responding on Cocaine Lever Following Pentobarbital.
Developmental exposure to 3 and 6 mg/kg/day PCBs did not alter the percentage of responding on the cocaine lever following administration of 5.0 mg/kg pentobarbital (IP). Much less responding occurred on the cocaine-paired lever following the test dose of pentobarbital relative to test doses of cocaine and amphetamine.
4.0 Discussion
Overall, the only statistically significant PCB exposure-related differences were seen at the highest test dose of each drug (7.5 mg/kg cocaine and 1.0 mg/kg amphetamine). At these drug doses, the 6 mg/kg PCB group significantly differed from the control group with the difference, unexpectedly, being in opposite directions for the two drugs. The percent responding on the cocaine-paired lever and response rate data from the cocaine training sessions that occurred between generalization/cross-generalization test days was not significantly different. These results indicate that the differences observed for 7.5 mg/kg cocaine and 1.0 mg/kg amphetamine were not due to pre-existing differences between exposure groups, and instead represent real differences in discrimination ability. Likewise, innate differences in discrimination ability are further unlikely to explain the results given that responding on the cocaine-paired lever following 7.5 mg/kg cocaine increased, while responding decreased for 1.0 mg/kg amphetamine. Pentobarbital utilizes reinforcement pharmacology that, unlike cocaine and amphetamine, appears to be non-dopaminergic. Intraperitoneal injections of pentobarbital have been shown to influence DA release in the striatum and nucleus accumbens of rats [1,29]. However, the pentobarbital doses were much higher than used in our study (25-100 mg/kg vs. 5 mg/kg) and actually decreased DA release resulting in an inhibition of dopamine activity – a pharmacological outcome that would not be expected to produce interoceptive cues of reinforcement. The fact that animals did not generalize to the cocaine lever when pentobarbital was the test drug suggests the observed differences for the highest doses of cocaine and amphetamine reflect a change in reinforcement pharmacology unique to these psychostimulants, and not a more global change in overall level of reinforcement experienced.
It is not entirely clear why opposing responses to cocaine and amphetamine were seen, but similar effects have been reported in rats developmentally exposed to lead. In adult animals, previous gestational and lactational exposure to lead produced an increase in the sensitivity to cocaine as exhibited by faster acquisition of self-administration behavior [42], maintenance of self-administration at doses lower than non-exposed controls [32], a higher vulnerability to relapse [30], and an increase in the motor stimulating effects [31]. For methamphetamine, on the other hand, previous gestational and lactational exposure to lead resulted in reduced self-administration acquisition [43], self-administration at doses higher than non-exposed controls [41], and a decreased vulnerability to relapse [43] in adult animals. The motor stimulating effects of methamphetamine were enhanced in adult animals that had perinatal lead exposure, but the effects were only observed during early testing days with controls showing similar levels by later testing days [6].
One reason for the differential effects of cocaine and amphetamine may be related to the different effects these drugs have on the dopamine transporter (DAT). Psychostimulants can be categorized as those that block uptake or those that compete for uptake [21,40]. Cocaine is an uptake blocker, whereas amphetamine is a substrate for the transporter and competes with DA for uptake. Both types of psychostimulants rapidly increase dopaminergic neurotransmission by interfering with proper DAT function. Cocaine directly binds and inhibits the reuptake of DA through the DAT, which leads to increased extracellular DA levels. There is then a compensatory response in which more DATs are recruited to the cell surface. Amphetamine, on the other hand, not only competes with dopamine for uptake, but it also increases extracellular dopamine by reverse transport of dopamine through the DAT [21,40,66]. Amphetamine-induced alterations in DAT trafficking seem to be necessary for this prolonged DA efflux. More specifically, amphetamine reduces the number of cell surface transporters. Thus, cocaine increases dopamine transport capacity (increased DAT membrane expression), whereas amphetamine decreases transport capacity (decreased DAT membrane expression). PCBs have been shown to reduce expression of the DAT [2,5,12,27,39]; therefore, cocaine would be expected to block the reuptake of DA to a greater extent in PCB-exposed animals, thereby enhancing the discriminative stimulus properties of the drug. In contrast, with amphetamine administration, less reverse transport would be occurring in PCB-exposed rats because of reduced cell surface DAT expression; therefore, less DA would be released, and the reinforcing discriminative stimulus effects of the drug would be reduced.
In this study we only saw differences in responding at the highest dose of each drug which may be due to differences in demand for the DAT. At the lower doses of cocaine and amphetamine, the demand on the DAT was not as high, and reduced DAT function in the PCB-exposed animals may not have been sufficient to alter the interoceptive cues at these lower doses. At the highest doses of each drug – where increased DAT activity would be necessary – the limited DAT activity in PCB-exposed animals may not have been able to keep up with demand. This theory is plausible if DAT expression was reduced in the PCB-exposed animals. Future research is planned using Western blot analysis to measure DAT expression in striatum and prefrontal cortex from littermates of the animals tested in this study. This will allow us to determine if a reduction in DAT expression is associated with the PCB exposures used in this study.
The results could also potentially be explained by changes in intracellular calcium signaling following administration of cocaine and/or amphetamine as a result of prior developmental exposure to PCBs. It has been reported that the amphetamine-induced reversal of the DAT which results in DA efflux depends on an increase in the concentration of intracellular calcium stores ([Ca2+]i) [13,14,23]. When the [Ca2+]i was buffered and not allowed to change, DA efflux via reverse transport of the DAT following amphetamine administration did not occur [23]. Interestingly, when amphetamine was not allowed to bind to the DAT due to occupancy by cocaine, the increase in [Ca2+]i also did not occur [14]. These results provide evidence that amphetamine is required to bind to the DAT in order to see the increase in [Ca2+]i that leads to reverse transport, and suggest that cocaine binding to the DAT does not appear to produce a change in [Ca2+]i.
Non-coplanar, ortho-substituted, PCB congeners found in the mixture used in the current study (i.e., the Fox River Mix) have been shown to significantly enhance the activity of ryanodine receptors (RyRs), which are Ca2+-selective ion channels that release endoplasmic reticulum Ca2+ stores when activated [35,65]. Specifically, ortho-substituted PCBs stabilize the high affinity, Ca2+-conducting conformation of the RyR [65], thereby enhancing calcium-induced calcium release (CICR). The prolonged early developmental exposure to PCBs that occurred throughout gestation and lactation may have exacerbated CICR, ultimately resulting in a permanent down-regulation of RyRs – an effect that persisted into adulthood. Altered expression of RyRs in adult animals with a prior history of gestational exposure to PCB 95 has been reported previously [48]. Thus, when given amphetamine later in life, rats with a prior history of PCB exposure may have had more limited ability to release intracellular [Ca2+]i, resulting in less DA efflux with reverse DAT transport. The effect of cocaine, on the other hand, would not be affected by such changes because cocaine increases DA in the synapse by blocking the DAT rather than by reversing DA transport. Future research is planned to examine this possibility.
Lastly, it should also be considered that the results were influenced by other neurotransmitter systems differentially affected by exposure. For example, in addition to DAT, it has been demonstrated that amphetamine also has strong affinity for the norepinephrine transporter (NET), while cocaine exhibits strong affinity to both the serotonin (SERT) and NET transporters [reviewed in 17]. As mentioned previously, in addition to DA, PCBs have also been reported to act on both NE [55] and 5-HT [4,16,22,28,51], so it is possible that alterations in the these other neurotransmitter systems contributed to the discriminative stimulus effects observed.
We have now demonstrated in several paradigms that rats developmentally exposed to PCBs show a reduced behavioral response to amphetamine. As mentioned earlier, a previous study done in our lab found a similar reduced sensitivity to amphetamine in perinatally PCB-exposed rats on drug challenges in a DRL task [45]. Male rats that were given gestational and lactational exposure to PCBs exhibited impaired performance as shown by a lower ratio of reinforced to non-reinforced responses than controls. Amphetamine challenges (0, 0.5, or 1.0 mg/kg IP) administered prior to DRL testing revealed that the responding of the exposed males was less disrupted by the drug challenge. We have also demonstrated behavioral sensitization (an increase in the motor-activating effects of a drug following repeated daily administration) was blunted in PCB-exposed males who were littermates to the animals used in the current study [36]. Behavioral sensitization was assessed using repeated injections of 0.5 mg/kg amphetamine (IP). PCB-exposed males showed greater activation to the initial acute amphetamine injection, but behavioral sensitization occurred later and never increased to the level seen in control males. In this study, amphetamine administration in the males (but not females) exposed to 6 mg/kg PCBs resulted in a trend toward decreased responding on the cocaine-paired lever relative to non-exposed controls. Taken together, the results of these studies indicate males developmentally exposed to PCBs showed less sensitivity to amphetamine compared to non-exposed controls. It should also be mentioned that these effects do not appear to be due to PCB-induced alterations in amphetamine metabolism, because in the behavioral sensitization study analysis of whole brain and serum amphetamine content following a final IP injection of 0.5 mg/kg amphetamine revealed no differences among PCB exposure groups [36].
In all of the previous studies, the reported effects of developmental PCB exposure on the particular behavior of interest were examined following experimenter-administered psychostimulant. Future studies in rats examining self-administration behavior that is subject-initiated are planned to determine if developmental exposure to PCBs contributes to increased and/or escalating voluntary intake of psychostimulants.
5.0 Conclusions
Overall, the results of this study indicate that PCBs can alter the interoceptive cues experienced in response to amphetamine or cocaine administration. Other research in our lab has confirmed that rats (particularly males) developmentally exposed to PCBs show a reduced behavioral response to amphetamine similar to that observed in the current study [36,45]. It has also been demonstrated that rats that are developmentally exposed to PCBs exhibit more inhibitory control deficits than non-exposed controls [45,47,64]. Changes in drug reward sensitivity [58] and deficits in inhibitory control [62] have been reported to be risk-factors for excessive drug-seeking behavior. Taken together, all of these results suggest that developmental exposure to PCBs may increase substance abuse risk.
Supplementary Material
Acknowledgements
This research was supported by NIH/NIEHS grants K99 ES015428, R00 ES015428, R01 ES015687, and T32 ES007326. Special thanks to Mindy Howe for her outstanding technical assistance and to Drs. Paul Kostyniak and Larry Hanson for their assistance in formulating the PCB mixture. We would also like to thank Dr. Joshua Gulley for his advice regarding execution of the drug discrimination task and for his suggestions on earlier versions of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adachi Y, Yamada S, Satomoto M, Watanabe K, Higuchi H, Kazama T, Doi M, Sato S. Pentobarbital inhibits L-DOPA-induced dopamine increases in the rat striatum: An in vivo microdialysis study. Brain Res Bull. 2006;69:593–596. doi: 10.1016/j.brainresbull.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Bemis J, Seegal R. PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicol Sci. 2004;80:288–95. doi: 10.1093/toxsci/kfh153. [DOI] [PubMed] [Google Scholar]
- 3.Boucher O, Muckle G, Bastien C. Prenatal exposure to polychlorinated biphenyls: a neuropsychologic analysis. Environ Health Perspect. 2009;117:7–16. doi: 10.1289/ehp.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castoldi A, Blandini F, Randine G, Samuele A, Manzo L, Coccini T. Brain monoaminergic neurotransmission parameters in weanling rats after perinatal exposure to methylmercury and 2,2′,4,4′,5,5′-hexaclhorobiphenyl (PCB 153) Brain Res. 2006;1112:91–98. doi: 10.1016/j.brainres.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Caudle W, Richardson J, Delea K, Guillot T, Wang M, KD KP, Miller G. Polychlorinated biphenyl-induced reduction of dopamine transporter expression as a precursor to Parkinson's disease-associated dopamine toxicity. Toxicol Sci. 2006;92:490–499. doi: 10.1093/toxsci/kfl018. [DOI] [PubMed] [Google Scholar]
- 6.Clifford P, Hart N, Thompson J, Buckman S, Wellman P, Bratton G, Nation J. Prenatal lead exposure enhances methamphetamine sensitization in rats. Pharmacol Biochem Behav. 2009;93:165–169. doi: 10.1016/j.pbb.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietz R, Riget F, Cleemann M, Aarkrog A, Johansen P, Hansen J. Comparison of contaminants from different trophic levels and ecosystems. Sci Tot Environ. 2000;245:221–231. doi: 10.1016/s0048-9697(99)00447-7. [DOI] [PubMed] [Google Scholar]
- 8.Erickson M. Analytical Chemistry of PCBs. Butterworth Publishers; Boston: 1986. [Google Scholar]
- 9.Fein G, Jacobson J, Jacobson S, Schwartz P, Dowler J. Prenatal exposure to polychlorinated biphenyls: effects on birth size and gestational age. J Pediatr. 1984;105:315–320. doi: 10.1016/s0022-3476(84)80139-0. [DOI] [PubMed] [Google Scholar]
- 10.Fisk A, deWit C, Wayland M, Kuzyk Z, Burgess N, Letcher R, Braune B, Norstrom R, BLum S, Sandau C. An assessment of the toxicological significance of anthropogenic contaminants in Canadian arctic wildlife. Sci Tot Environ. 2005;351-352:57–93. doi: 10.1016/j.scitotenv.2005.01.051. others. [DOI] [PubMed] [Google Scholar]
- 11.Fonnum F, Mariussen E. Mechanisms involved in the neurotoxic effects of environmental toxicants such as polychlorinated biphenyls and brominated flame retardants. J Neurochem. 2009;111:1327–1347. doi: 10.1111/j.1471-4159.2009.06427.x. [DOI] [PubMed] [Google Scholar]
- 12.Fonnum F, Mariussen E, Reistad T. Molecular mechanisms involved in the toxic effects of polychlorinated biphenyls (PCBs) and brominated flame retardants (BFRs) J Toxicol Environ Health A. 2006;69:21–35. doi: 10.1080/15287390500259020. [DOI] [PubMed] [Google Scholar]
- 13.Gnegy M, Khoshbouei H, Berg K, Javitch J, Clarke W, Zhang M, Galli A. Intracellular Ca2+ regulates amphetamine-induced dopamine efflux and currents mediated by the human dopamine transporter. Mol Pharmacol. 2004;66:137–143. doi: 10.1124/mol.66.1.137. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin J, Larson G, Swant J, Sen N, Javitch J, Zahniser N, DeFelice L, Khoshbouei H. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284:2978–2989. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen L. The Ortho Side of PCBs: Occurrence and Disposition. Kluwer Academic Publishers; Norwell, MA: 1999. [Google Scholar]
- 16.Honma T, Suda M, Miyagawa M, Wang R, Kobayashi K, Sekiguchi S. Alteration of brain neurotransmitters in female rat offspring induced by prenatal administration of 16 and 64 mg/kg of 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153) Ind Health. 2009;47:11–21. doi: 10.2486/indhealth.47.11. [DOI] [PubMed] [Google Scholar]
- 17.Howell L, Kimmel H. Monoamine transporters and psychostimulant addiction. Biochem Pharmacol. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson J, Fein G, Jacobson S, Schwartz P, Dowler J. The transfer of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) across the human placenta and into maternal milk. Am J Public Health. 1984;74:378–379. doi: 10.2105/ajph.74.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson J, Jacobson S. Prenatal exposure to polychlorinated biphenyls and attention at school age. J Pediatr. 2003;143:780–788. doi: 10.1067/S0022-3476(03)00577-8. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson J, Jacobson S, Humphrey H. Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. J Pediatr. 1990;116:38–45. doi: 10.1016/s0022-3476(05)81642-7. [DOI] [PubMed] [Google Scholar]
- 21.Kahlig K, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur J Pharmacol. 2003;479:153–158. doi: 10.1016/j.ejphar.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 22.Khan I, Thomas P. Aroclor 1254 inhibits tryptophan hydroxylase activity in rat brain. Arch Toxicol Suppl. 2004;78:316–320. doi: 10.1007/s00204-003-0540-1. [DOI] [PubMed] [Google Scholar]
- 23.Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lung D, Gnegy M, Galli A, Javitch J. N-Terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:e78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostyniak P, Hansen L, Widholm J, Fitzpatrick R, Olson J, Helferich J, Kim K, Sable H, Seegal R, Pessah I. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol Sci. 2005;88:400–411. doi: 10.1093/toxsci/kfi338. others. [DOI] [PubMed] [Google Scholar]
- 25.Lawyer G, Bjerkan PS, Hammarberg A, Jayaram-Lindstrom N, Franck J, Agartz I. Amphetamine dependence and co-morbid alcohol abuse: Associations to brain cortical thickness. BMC Pharmacology. 2010;10:1–10. doi: 10.1186/1471-2210-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyng G, Seegal R. Polychlorinated biphenyl-induced oxidative stress in organotypic co-cultures: experimental dopamine depletion prevents reductions in GABA. Neurotoxicology. 2008;29:301–308. doi: 10.1016/j.neuro.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyng G, Snyder-Keller A, Seegal R. Polychlorinated biphenyl-induced neurotoxicity in organotypic cocultures of developing rat ventral mesencephalon and striatum. Toxicol Sci. 2007;97:128–139. doi: 10.1093/toxsci/kfm027. [DOI] [PubMed] [Google Scholar]
- 28.Mariussen E, Fonnum F. The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology. 2001;159:11–21. doi: 10.1016/s0300-483x(00)00374-7. [DOI] [PubMed] [Google Scholar]
- 29.Masuzawa M, Nakao S, Miyamoto E, Yamada M, Murao K, Nishi K, Shingu K. Pentobarbital inhibits ketamine-induced dopamine release in the rat nucleus accumbens: a microdialysis study. Anesth Analg. 2003;96:148–152. doi: 10.1097/00000539-200301000-00030. [DOI] [PubMed] [Google Scholar]
- 30.Nation J, Cardon A, Heard H, Valles R, Bratton G. Perinatal lead exposure and relapse to drug-seeking behavior in the rat: a cocaine reinstatement study. Psychopharmacology (Berl) 2003;168:236–243. doi: 10.1007/s00213-003-1405-2. [DOI] [PubMed] [Google Scholar]
- 31.Nation J, Heard H, Cardon A, Valles R, Bratton G. Perinatal lead exposure alters the stimulatory properties of cocaine at PND 30 and PND 90 in the rat. Neuropsychopharmacology. 2000;23:444–454. doi: 10.1016/S0893-133X(00)00118-4. [DOI] [PubMed] [Google Scholar]
- 32.Nation J, Smith K, Bratton G. Early developmental lead exposure increases sensitivity to cocaine in a self-administration paradigm. Pharmacol Biochem Behav. 2004;77:127–135. doi: 10.1016/j.pbb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 33.National Institutes of Health . Public Health Service Policy on Humane Care and Use of Laboratory Animals. NIH/Office of Laboratory Animal Welfare; Rockville, MD: 2002. [Google Scholar]
- 34.National Research Council. Institute for Laboratory Animal Research . Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academy Press; Washington, D.C.: 2003. [Google Scholar]
- 35.Pessah I, Cherednichenko G, Lein P. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther. 2010;125:260–285. doi: 10.1016/j.pharmthera.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poon E, Monaikul S, Kostyniak P, Chi L, Schantz S, Sable H. Developmental exposure to polychlorinated biphenyls reduces amphetamine behavioral sensitization in rats (submitted) doi: 10.1016/j.ntt.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Center for Drug Evaluation and Research. U.S. Department of Health and Human Services . Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. 2005. [Google Scholar]
- 38.Rice D. Effects of postnatal exposure of monkeys to a PCB mixture on spatial discrimination reversal and DRL performance. Neurotoxicol Teratol. 1998;20:391–400. doi: 10.1016/s0892-0362(97)00134-7. [DOI] [PubMed] [Google Scholar]
- 39.Richardson J, Miller G. Acute exposure to Aroclor 1016 or 1260 differentially affects dopamine transporter and vesicular monoamine transporter 2 levels. Toxicol Lett. 2004;148:29–40. doi: 10.1016/j.toxlet.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Riddle E, Fleckenstein A, Hanson G. Role of monoamine transporters in mediating psychostimulant effects. AAPS J. 2005;7:E847–E851. doi: 10.1208/aapsj070481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocha A, Valles R, Bratton G, Nation J. Developmental lead exposure alters methamphetamine self-administration in the male rat: acquisition and reinstatement. Drug Alcohol Depend. 2008b;95:23–29. doi: 10.1016/j.drugalcdep.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocha A, Valles R, Cardon A, Bratton G, Nation J. Enhanced acquisition of cocaine self-administration in rats developmentally exposed to lead. Neuropsychopharmacology. 2005;30:2058–2064. doi: 10.1038/sj.npp.1300729. [DOI] [PubMed] [Google Scholar]
- 43.Rocha A, Valles R, Hart N, Bratton G, Nation J. Developmental lead exposure attenuates methamphetamine dose-effect self-administration performance and progressive ratio responding in the male rat. Pharmacol Biochem Behav. 2008a;89:508–514. doi: 10.1016/j.pbb.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogan J, Keselman H, Mendoza J. Analysis of repeated measurements. Brit J Math Stat Psych. 1979;32:269–286. [Google Scholar]
- 45.Sable H, Eubig P, Powers B, Wang V, Schantz S. Developmental exposure to PCBs and/or MeHg: effects on a differential reinforcement of low rates (DRL) operant task before and after amphetamine drug challenge. Neurotoxicol Teratol. 2009;31:149–158. doi: 10.1016/j.ntt.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sable H, Schantz S. Animal Models of Cognitive Impairment (Frontiers in Neuroscience) CRC Press; New York: 2006. Executive function following developmental exposure to polychlorinated biphenyls (PCBs): What animal models have told us, in: E.D. Levin, J.J. Buccafusco (Eds.) pp. 147–167. [PubMed] [Google Scholar]
- 47.Sable HJK, Powers BE, Wang VC, Widholm JJ, Schantz SL. Alterations in DRH and DRL performance in rats developmentally exposed to an environmental PCB mixture. Neurotoxicol Teratol. 2006;28:548–556. doi: 10.1016/j.ntt.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Schantz S, Seo B, Wong P, Pessah I. Long-term effects of developmental exposure to 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) on locomotor activity, spatial learning and memory and brain ryanodine binding. Neurotoxiology. 1997;18:457–467. [PubMed] [Google Scholar]
- 49.Seegal R. Epidemiological and laboratory evidence of PCB-induced neurotoxicity. Crit Rev Toxicol. 1996;26:709–737. doi: 10.3109/10408449609037481. [DOI] [PubMed] [Google Scholar]
- 50.Seegal R. The neurochemical effects of PCB exposure are age-dependent. Arch Toxicol Suppl. 1994;16:128–137. doi: 10.1007/978-3-642-78640-2_15. [DOI] [PubMed] [Google Scholar]
- 51.Seegal R, Brosch K, Bush B. Regional alterations in serotonin metabolism induced by oral exposure of rats to polychlorinated biphenyls. Neurotoxicology. 1986;7:155–65. [PubMed] [Google Scholar]
- 52.Seegal R, Brosch K, Okoniewski R. Effects of in utero and lactational exposure of the laboratory rat to 2,4,2′,4′- and 3,4,3′,4′-tetrachlorobiphenyl on dopamine function. Toxicol Appl Pharmacol. 1997;146:95–103. doi: 10.1006/taap.1997.8226. [DOI] [PubMed] [Google Scholar]
- 53.Seegal R, Bush B, Brosch K. Comparison of effects of Aroclors 1016 and 1260 on non-human primate catecholamine function. Toxicology. 1991;66:145–163. doi: 10.1016/0300-483x(91)90215-m. [DOI] [PubMed] [Google Scholar]
- 54.Seegal R, Bush B, Brosch K. Decreases in dopamine concentrations in adult, non-human primate brain persist following removal from polychlorinated biphenyls. Toxicology. 1994;86:71–87. doi: 10.1016/0300-483x(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 55.Seegal R, Bush B, Brosch K. Polychlorinated biphenyls induce regional changes in brain norepinephrine concentrations in adult rats. Neurotoxicology. 1985;6:13–23. [PubMed] [Google Scholar]
- 56.Seegal R, Marek K, Seibyl J, Jennings D, Molho E, Higgins D, Factor S, Fitzgerald E, Hills E, Korrick S. Occupational exposure to PCBs reduces striatal dopamine transporter densities only in women: a beta-CIT imaging study. Neurobiol Dis. 2010;38:219–225. doi: 10.1016/j.nbd.2010.01.009. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seegal R, Okoniewski R, Brosch K, Bemis J. Polychlorinated biphenyls alter extraneuronal but not tissue dopamine concentrations in adult rat striatum: an in vivo microdialysis study. Environ Health Perspect. 2002;110:1113–7. doi: 10.1289/ehp.021101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stephens D, Duka T, Crombag H, Cunningham C, Heilig M, Crabbe J. Reward sensitivity: issues of measurement, and achieving consilience between human and animal phenotypes. Addict Biol. 2010;15:145–168. doi: 10.1111/j.1369-1600.2009.00193.x. [DOI] [PubMed] [Google Scholar]
- 59.Stewart P, Fitzgerald S, Reihman J, Gump B, Lonky E, Darvill T, Pagano J, Hauser P. Prenatal PCB exposure, the corpus callosum, and response inhibition. Environ Health Perspect. 2003;111:1670–1677. doi: 10.1289/ehp.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stewart P, Reihman J, Gump B, Lonky E, Darvill T, Pagano J. Response inhibition at 8 and 9 1/2 years of age in children prenatally exposed to PCBs. Neurotoxicol Teratol. 2005;27:771–780. doi: 10.1016/j.ntt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Stewart P, Sargent D, Reihman J, Gump B, Lonky E, Darvill T, Hicks H, Pagano J. Response inhibition during differential reinforcement of low rates (DRL) schedules may be sensititive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environ Health Perspect. 2006;114:1923–1929. doi: 10.1289/ehp.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volkow N, Fowler J, Wang G, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Welp E, Bosman I, Langendam M, Totté M, Maes R, Ameijden E.v. Amount of self-reported illicit drug use compared to quantitative hair test results in community-recruited young drug users in Amsterdam. Addiction. 2003;98:987–994. doi: 10.1046/j.1360-0443.2003.00421.x. [DOI] [PubMed] [Google Scholar]
- 64.Widholm J, Villareal S, Seegal S, Schantz S. Spatial alternation deficits following developmental exposure to Aroclor 1254 and/or methylmercury in rats. Toxicol Sci. 2004;82:577–589. doi: 10.1093/toxsci/kfh290. [DOI] [PubMed] [Google Scholar]
- 65.Wong P, Brackney W, Pessah I. ortho-Substituted polychlorinated biphenyls alter microsomal calcium transport by direct interaction with ryanodine receptors of mammalian brain. J Biol Chem. 1997;272:15145–15153. doi: 10.1074/jbc.272.24.15145. [DOI] [PubMed] [Google Scholar]
- 66.Zahniser N, Sorkin A. Trafficking of dopamine transporters in psychostimulant actions. Semin Cell Dev Biol. 2009;20:411–417. doi: 10.1016/j.semcdb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.