Abstract
Hydrogels that solidify in response to a dual, physical and chemical, mechanism upon temperature increase were fabricated and characterized. The hydrogels were based on N-isopropylacrylamide, which renders them thermoresponsive, and contained covalently crosslinkable moieties in the macromers. The effects of the macromer end group, namely acrylate or methacrylate, and the fabrication conditions were investigated on the degradative and swelling properties of the hydrogels. The hydrogels exhibited higher swelling below their lower critical solution temperature (LCST). When immersed in cell culture media at physiological temperature, which was above their LCST, hydrogels showed constant swelling and no degradation over eight weeks, with methacrylated hydrogels having higher swelling than their acrylated analogs. In addition, hydrogels immersed in cell culture media under the same conditions showed lower swelling as compared to phosphate buffered saline. The interplay between chemical crosslinking and thermally induced phase separation affected the swelling characteristics of hydrogels in different media. Mesenchymal stem cells encapsulated in the hydrogels in vitro were viable over three weeks and markers of osteogenic differentiation were detected when the cells were cultured with osteogenic supplements. Hydrogel mineralization in the absence of cells was observed in cell culture medium with the addition of fetal bovine serum and β-glycerol phosphate. The results suggest that these hydrogels may be suitable as carriers for cell delivery in tissue engineering.
Keywords: hydrogel, thermoresponsive, N-isopropylacrylamide, covalent crosslinking, cell encapsulation
1. Introduction
Thermally responsive hydrogels have attracted much research interest over the past years for their use in biomedical applications [1–3]. They have been reported in drug delivery [4, 5], cell sheet tissue engineering [6], biosensors [7], myocardial injection therapy [8] and cell delivery [9–11]. Thermoresponsive systems use temperature as a stimulus to allow for solidification and changes in swelling. This is beneficial for the above mentioned functions, as the temperature change from ambient to physiological can be employed. Advantages of thermoresponsive hydrogels include a fast gelation once the transition temperature is reached, and a mild solidification process without the need for a chemical initiator system. Many natural and synthetic polymers exhibit thermoresponsive hydrogel characteristics, and among those, polymers based on poly(N-isopropylacrylamide) (pNiPAAm) belong to some of the most widely investigated systems. The homopolymer pNiPAAm has a transition temperature around 32°C, which can be tuned when copolymerized with more hydrophilic or hydrophobic monomers [12] and allows for a transition temperature that can be close to physiological. A problem associated with linear pNiPAAm is the extensive collapse of the gel at 37°C. One strategy to improve the stability and mechanical properties of pNiPAAm-based hydrogels is the modification of the polymer with functional groups that enable covalent crosslinking. Systems that gel with temperature increase to physiological and can form chemical crosslinks upon a Michael-addition reaction have been recently proposed [13-16].
Our group has previously reported the synthesis and characterization of macromers for the fabrication of hydrogels that can form in situ with two independent, physical and chemical, solidification mechanisms [17]. The thermoresponsive properties of the macromers are imparted by N isopropylacrylamide, and additional comonomers include pentaerythritol diacrylate monostearate, acrylamide and hydroxyethyl acrylate. The latter monomer contains hydroxyl groups that can be modified towards acrylate or methacrylate moieties. These moieties can be covalently crosslinked with the addition of a water-soluble, thermal free-radical initiator system. Increased stability and higher viscosity values could be achieved when the hydrogels were physically and chemically gelled as opposed to only thermally gelled controls. The in vitro cytocompatibility of the macromers has been previously evaluated with favorable results [18]. Parameters, such as crosslinking time, that allow for high viability of encapsulated cells during hydrogel formation have been established.
The objective of the present study is the fabrication and characterization of thermally responsive and chemically crosslinkable hydrogels that form upon temperature increase to physiological. The ultimate goal of this work is the application of hydrogels for cell delivery in craniofacial bone tissue engineering. The potential of these hydrogels towards this aim in terms of hydrogel properties, cell encapsulation and mineralization are investigated. More specifically, we examine the effect of macromer end group (acrylate or methacrylate) on the swelling and degradation of the hydrogels. The resulting effects of these properties on encapsulated mesenchymal stem cell viability and osteogenic differentiation are evaluated.
2. Materials and Methods
2.1. Macromer synthesis and characterization
Thermally gelling macromers were synthesized from pentaerythritol diacrylate monostearate (PEDAS), N-isopropylacrylamide (NiPAAm), acrylamide (AAm) and 2-hydroxyethyl acrylate (HEA) in a 1:14:3:3 molar ratio via free-radical polymerization reaction as previously described [17]. This monomer ratio had been determined during previous studies to yield macromers with optimal gelation and stability properties as well as a sufficient number of hydroxyl groups available for chemical modification. Subsequently, the free hydroxyls of the HEA were modified with an acrylate or methacrylate group through a reaction with acryloyl or methacryloyl chloride, respectively, and characterization followed with established methods [17]. The highest modification with methacrylate groups achieved was 1.3 groups per chain as determined by 1H-NMR. Formulations that contained 1.3 acrylate or methacrylate groups per chain were used for these studies.
2.2. Hydrogel fabrication
Methacrylated macromer solutions were prepared in phosphate buffered saline (PBS, pH=7.4, Gibco Life, Grand Island, NY) in a concentration of 10% (w/v). Ammonium persulfate (APS) and N,N,N’,N’-tetramethylethylenediamine (TEMED) (Sigma-Aldrich, St. Louis, MO) were used as free-radical initiators for the chemical crosslinking of the macromers. Stock solutions of the initiator system in double distilled (dd) water were added to the macromer solution in different concentrations resulting in final APS/TEMED concentrations of 10, 15 and 20 mM. The mixture was gently agitated and quickly pipeted into Teflon molds (6 mm in diameter, 3 mm in height). The hydrogels were then formed at 37°C inside a cell culture incubator over 10, 20 or 30 min. After fabrication, hydrogels were blotted dry, weighed (Wf ) and placed in dd water at 37°C for one day. Gels were removed from the water, carefully blotted and the wet mass was obtained (Ws). The hydrogels were dried overnight in a lyophilizer and weighed (Wd). Sol fraction is calculated as (Wf − Wd)/ Wf. Swelling ratio is calculated as (Ws − Wd)/ Wd.
2.3. Hydrogel swelling below and above the LCST
Hydrogels were fabricated using APS/TEMED in a 20 mM concentration and 30-min incubation at 37°C. Hydrogels were weighed after fabrication, swollen in dd water at 4°C for 20h, blotted and weighed. Subsequently, they were immersed in dd water at 37°C for 20h. The wet mass was obtained after careful blotting and gels were finally dried and weighed. Swelling ratio was calculated as outlined above.
2.4. Hydrogel degradation and swelling in cell culture medium and PBS
Hydrogel samples were prepared as described below in paragraph 2.6 for cell encapsulation but with the addition of PBS instead of cell suspension. The gels were immersed in complete osteogenic media or PBS in an incubator at 37°C over 8 weeks. Samples were removed at 1h, 1, 7, 14, 21, 28 and 56d and sol fraction and swelling ratio were calculated. Additionally, samples immersed in media were prepared and saved as controls for the biochemical assays as follows below.
2.5. Rat mesenchymal stem cell (MSC) isolation and preculture
Cells were harvested from the marrow of the femora and tibiae of male 6–8 week old Fischer 344 rats (Charles River Laboratories, Wilmington, MA) as previously described [19]. The protocol followed in these studies was approved by the Rice University Institutional Animal Care and Use Committee. The cells were cultured in minimum essential medium Eagle-alpha modification (Sigma) supplemented with 10% v/v fetal bovine serum (FBS, Cambrex BioScience, Charles City, IA), 10 mM β-glycerol-2-phosphate, 50 μg/mL ascorbic acid, 50 μg/mL gentamicin, 100 μg/mL ampicillin and 1.25 μg/mL fungizone (Sigma). The medium was changed after 24h to remove non-adherent cells and every 2–3 days thereafter until confluence was reached.
2.6. Cell encapsulation and culture
Vacuum-dried macromers were sterilized with UV irradiation for 3h [18]. 10% w/v solutions were prepared in sterile PBS and stock solutions of APS and TEMED in dd water were sterile-filtered. Complete osteogenic medium was prepared as the medium used for MSC preculture but with the addition of 10 nM dexamethasone, an osteogenic supplement. Prior to encapsulation, cells were counted in a hemocytometer with trypan blue to confirm cell viability. Macromer solutions were mixed with APS and TEMED (final concentration 20 mM) on ice. Subsequently, cells were added in a final concentration of 10 million cells/mL, the mixture was gently shaken and pipeted into sterile Teflon molds. After 30 min in an incubator, the gels were transferred to 12-well plates and 2.5 mL complete osteogenic medium was added. The medium was changed after 1h to remove hydrogel leachables and every 2–3 days during the culture duration. After 1h, 1, 7, 14 and 21d of culture, hydrogels were removed from the medium and immersed in PBS at 37°C for 30 min, then carefully blotted and weighed. Samples were prepared for biochemical assays (n=3–5), confocal fluorescence microscopy (n=3) and histology (n=2).
2.7. Biochemical assays
Samples reserved for biochemical assays were homogenized with a pellet grinder in 0.5 mL of sterile dd water and stored at −20°C. Prior to analyses, samples underwent three freeze-thaw-sonication cycles, with sonication performed on ice for 30 min.
The hydrogel homogenates were analyzed for DNA content, alkaline phosphatase (ALP) activity, collagen and calcium content. The assays are described in more detail elsewhere [19–21]. Prior to DNA and ALP analyses, samples were centrifuged at 4000 rpm for 5 min. Double-stranded DNA content was evaluated using the PicoGreen assay (Invitrogen, Eugene, OR) according to the manufacturer’s instructions. Alkaline phosphatase (ALP) activity was evaluated with phosphatase substrate capsules in an alkaline buffer solution compared to serial dilutions of p-nitrophenol standards (all from Sigma) and absorbance was measured. After the DNA and ALP assays were performed, a volume of proteinase K solution equal to the remaining volume of the hydrogel homogenates was added and samples were incubated at 56°C for 16h. The proteinase K solution was made of 1 mg/mL proteinase K, 0.01 mg/mL pepstatin A and 0.185 mg/mL iodoacetamide in a tris-EDTA buffer (6.055 mg/mL tris(hydroxymethylaminomethane) and 0.372 mg/mL EDTA, pH=7.6). An aliquot of the digested sample was analyzed for hydroxyproline content as a measure of total collagen as previously described [22]. For calcium analysis, a solution of acetic acid was added to the homogenates resulting in a final concentration of 0.5 N and samples were placed on a shaker at 120 rpm overnight to dissolve deposited salts. The assay was performed with a commercially available kit (Genzyme Diagnostics, Cambridge, MA) according to the manufacturer’s instructions. For all assays, blank hydrogels from the same time points were used as controls to subtract the fluorescence or absorbance of the material from the sample readings.
2.8. Confocal fluorescence microscopy
Sections of 0.7 mm thickness were cut from the hydrogels in the transverse direction and incubated with 2 μM calcein AM and 4 μM ethidium homodimer-1 according to the manufacturer’s instructions (Live/Dead viability/cytoxicity kit, Invitrogen, Eugene, OR). As a dead control, hydrogel sections were treated with 70% ethanol for 10 min prior to staining with Live/Dead. Cell-free hydrogels were used as additional controls. Cellular viability and distribution were evaluated with a confocal fluorescence microscope (LSM 510 META, Carl Zeiss, Germany) using a 10x objective. Argon and helium-neon lasers were used for excitation at 488 and 543 nm, respectively, and emission filters at 505–526 and 612–644 nm were employed.
2.9. Histology
Hydrogels were embedded in freezing medium (Histo-Prep, Fisher Scientific, Fair Lawn, NJ) and frozen at −20°C. As controls, blank hydrogels were processed and stained in the same manner. Frozen longitudinal sections of 8 μm thickness were cut with a cryostat (Leica CM3050S). Sections were fixed in 10% formalin (Formalde-Fresh, Fisher), stained with Mayer hematoxylin and counterstained with Eosin-Y (Sigma). A second set of sections was stained with von Kossa reagent. Samples were immersed in 1% silver nitrate solution (Sigma) under a UV lamp at 365 nm for 30 min to visualize mineralization and counterstained with Eosin-Y. Sections were imaged with a light microscope (Eclipse E600, Nikon) and camera (3CCD Color Video Camera, Sony).
2.10. Cell-free hydrogel mineralization
Hydrogels were fabricated as outlined above and were soaked in complete osteogenic medium, osteogenic medium but without the addition of FBS, or simulated body fluid (SBF) which was prepared according to Kokubo and Takadama [23]. Samples were incubated for 7, 14, 21 and 28d at 37°C, with medium changes every 2–3 days. A set of hydrogels was saved after fabrication and not immersed in any medium, serving as control. At time points, hydrogels were rinsed by immersing in dd water at 37°C for 30 min. Gels were lyophilized, weighed, homogenized in 0.5 N acetic acid and analyzed for calcium as described above.
2.11. Statistical analysis
Data from the studies were analyzed using single-factor analysis of variance (ANOVA) with a 95% confidence interval and Tukey’s post hoc test. The global effects of the conditions in the swelling study in medium and PBS were evaluated with a two-factor ANOVA and a 95% confidence interval. Data are reported as mean ± standard deviation. In the case of a sample size of n=2, data denote average ± range.
3. Results
3.1. Effect of initiator concentration and fabrication interval on hydrogel swelling
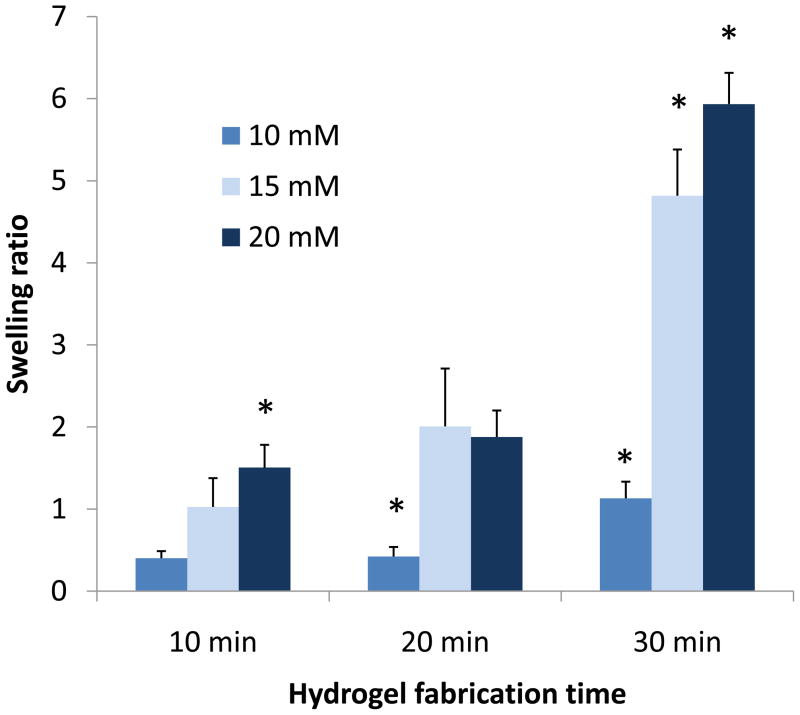
Higher initiator concentrations and fabrication intervals resulted in increased hydrogel swelling (Figure 1). The hydrogels fabricated with 20 mM APS/TEMED and incubated for 30 min at 37°C showed a higher swelling ratio than all other groups (5.9±0.4).
Figure 1.
Swelling behavior of hydrogels with methacrylate end groups fabricated with different concentrations of APS/TEMED initiator system (10–20 mM) and 10-30 min fabrication intervals at 37°C. Hydrogels were swollen in double distilled water for 24h. Data are reported as mean ± standard deviation for a sample size of n=3–4. Asterisk denotes statistical significance between groups from the same fabrication interval (p<0.05).
3.2. Hydrogel swelling below and above the LCST
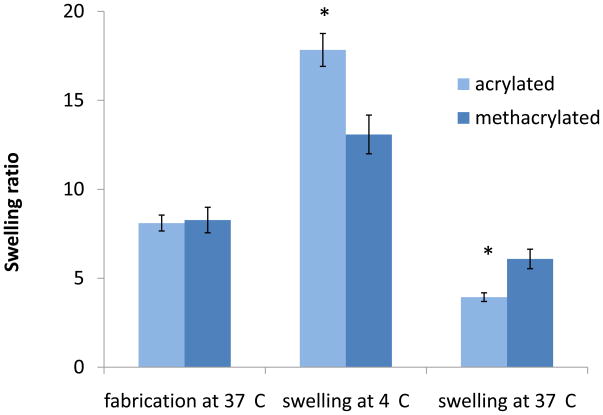
The lower critical transition temperature (LCST) of the acrylated macromers was 26.5±0.5°C and that of the methacrylated macromers was 12.7±0.4°C as determined rheologically [17, 18]. The hydrogels swelled significantly when placed in water at 4°C after fabrication (Figure 2). The acrylated hydrogels showed a swelling ratio of 17.8±0.9 whereas the methacrylated hydrogels had a ratio of 13.1±1.1. When the hydrogels were incubated in water at 37°C, the extent of swelling decreased, with the methacrylated hydrogels exhibiting significantly higher swelling than their acrylated analogs (6.1±0.6 and 3.9±0.2, respectively).
Figure 2.
Swelling behavior of hydrogels with acrylate (n=4) and methacrylate (n=5) end groups at different temperatures. Hydrogels were weighed after fabrication, swollen in double-distilled (dd) water at 4° C (below their LCST) for 20h, weighed and swollen in dd water at 37°C (above their LCST) for 20h. Data are reported as mean ± standard deviation. Asterisk denotes statistically significant differences in swelling between formulations at each temperature (p<0.05).
3.3. Hydrogel degradation and swelling in cell culture medium and PBS
Osteogenic cell culture medium
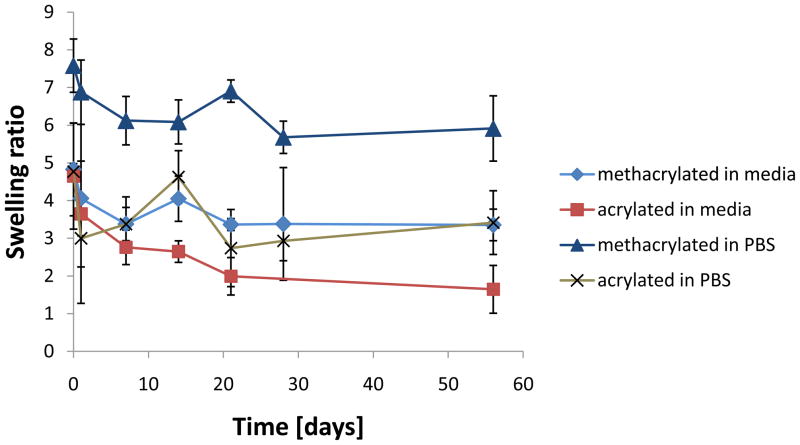
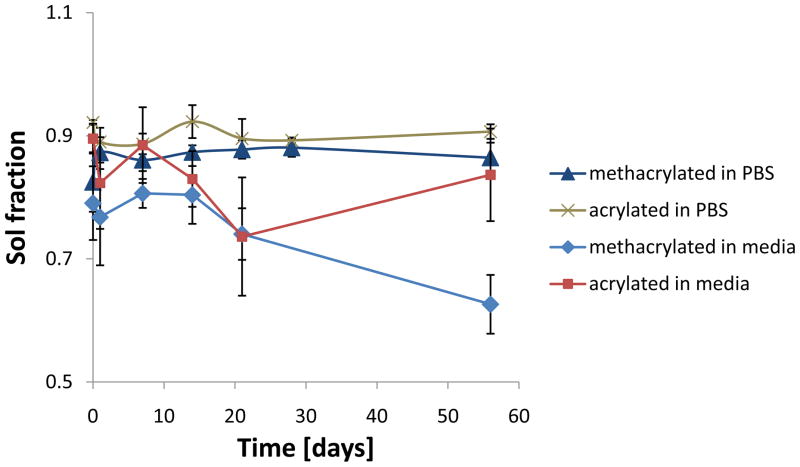
The swelling ratio of the acrylated hydrogels at 1h was significantly higher than at 21 and 56 days (Figure 3). For the methacrylated hydrogels, no significant changes were observed. As for the sol fraction, no increase that would indicate mass loss was noted. On the contrary, the sol fraction of the methacrylated hydrogels at 56d was significantly lower than that measured at the time points up to 14d (Figure 4).
Figure 3.
Swelling behavior of hydrogels incubated in cell culture media or PBS at 37°C over 8 weeks (n=2–5). Data are reported as mean ± standard deviation or as average ± range in the case of n=2 (n=2 was obtained for acrylated gels in PBS at 1h and methacrylated gels in media at 28d). After equilibrium was reached at 1d, there were no significant differences in swelling between time points within each formulation. Acrylated hydrogels showed statistically significantly (p<0.05) lower swelling than their methacrylated analogs at all time points when incubated in PBS, whereas this effect was observed after 14d in media. Both acrylated and methacrylated hydrogels showed significantly (p<0.05) lower swelling in cell culture media compared to PBS.
Figure 4.
Sol fraction of hydrogels incubated in cell culture media or PBS at 37° C over 8 weeks (n=2–5). Data are reported as mean ± standard deviation or as average ± range in the case of n=2 (n=2 was obtained for acrylated gels in PBS at 1h and methacrylated gels in media at 28d). A statistically significant (p<0.05) decrease in sol fraction was observed for the methacrylated hydrogels immersed in media, whereas no differences were noted for all other groups.
PBS
After 1h, no significant differences in swelling were found for the acrylated hydrogels. The swelling at 1h was higher also for the methacrylated formulation as compared to the 28d time point. Acrylated hydrogels swelled less than the methacrylated counterparts at all time points. The sol fraction remained constant for both formulations over the course of 8 weeks.
Overall, the global effect of the incubation medium was statistically significant for both acrylated and methacrylated formulations, which each exhibited lower swelling in osteogenic cell culture medium compared to PBS.
3.4. MSC encapsulation in hydrogels
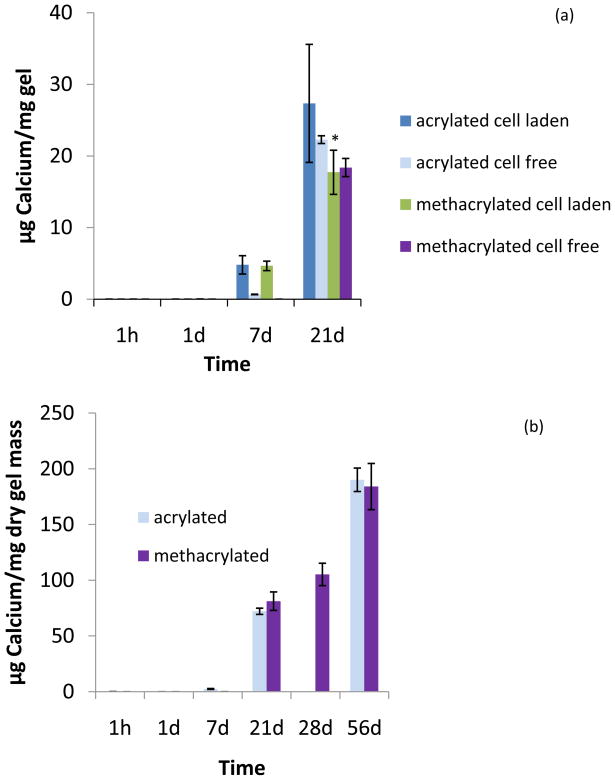
The biochemical assays revealed a drop in DNA content after the first week for both formulations (Supplemental Figure I). Hematoxylin and eosin stained sections showed uniform cellular distribution throughout the hydrogels as seen in sections taken from the edge as well as from the center of the gels (Figure 5). Cell viability was confirmed with Live/Dead staining and confocal microscopy. Live cells could be observed throughout the hydrogel over the whole period of culture (Figure 6). Due to the strong autofluorescence of the hydrogels at the wavelength used for the ethidium homodimer-1 dye, imaging of the dead cells was not possible. Alkaline phosphatase was detected after 1h in culture, peaked at 1d for both formulations and decreased at later time points (Supplemental Figure II). The hydroxyproline assay did not show any collagen production for either formulation over the duration of the study. As for mineralization, calcium accumulation could be found for the cell-encapsulating hydrogels by day 7 (Figure 7). By day 21, however, cell-free hydrogels had also accumulated calcium at the same amount as the cell-laden hydrogels. Von Kossa staining confirmed the presence of calcium salts throughout the cell-laden hydrogels at 21d (Supplemental Figure III).
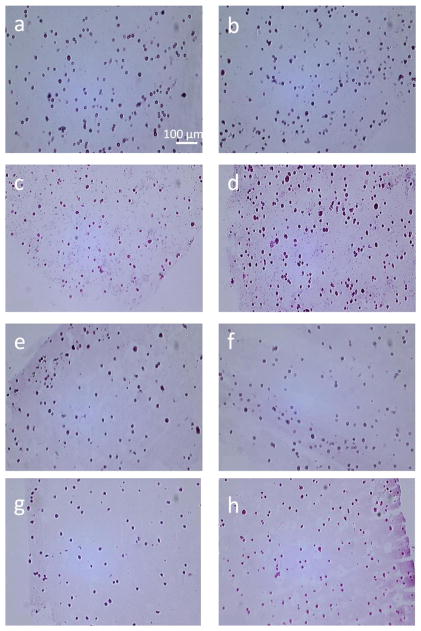
Figure 5.
Hematoxylin and eosin stained longitudinal sections from the edge (left column, images a, c, e and g) and the center of MSC-laden hydrogels (right column, images b, d, f and h). Images a-d correspond to acrylated hydrogels after 1 (a-b) and 21 (c-d) days of culture. Images e-h correspond to methacrylated hydrogels after 1 (e-f) and 21 (g-h) days of culture. Cellular distribution could be observed throughout the hydrogels at both time points. Scale bar indicates 100 μm.
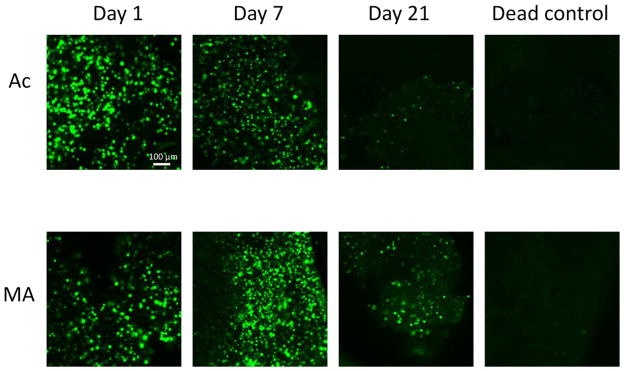
Figure 6.
Fluorescence microscopy images of sections taken from the center of the hydrogels (thickness=0.7 mm) stained with Live/Dead reagent at various time points. Live cells fluoresce green. Strong autofluorescence of the hydrogels at the wavelengths used for the dead dye component was observed and the images are therefore not shown. Included is a dead control treated with 70% ethanol for 10 min in which no live cells are observed. Acrylated hydrogels are denoted as Ac, whereas MA denotes methacrylated hydrogels. Scale bar indicates 100 μm.
Figure 7.
Calcium content of hydrogels after culture in osteogenic media (n=3–5). (a) Calcium content per mg of “wet” hydrogel for mesenchymal stem cell-laden and cell-free hydrogels. (b) Calcium content per dry mass for cell-free hydrogels over 8 weeks. Due to experimental difficulties, the assay could not be performed on acrylated hydrogels on d28. Data are reported as mean ± standard deviation. Statistical significance between cell-free formulations at time points is marked with an asterisk (p<0.05).
3.5. Cell-free hydrogel mineralization in different media
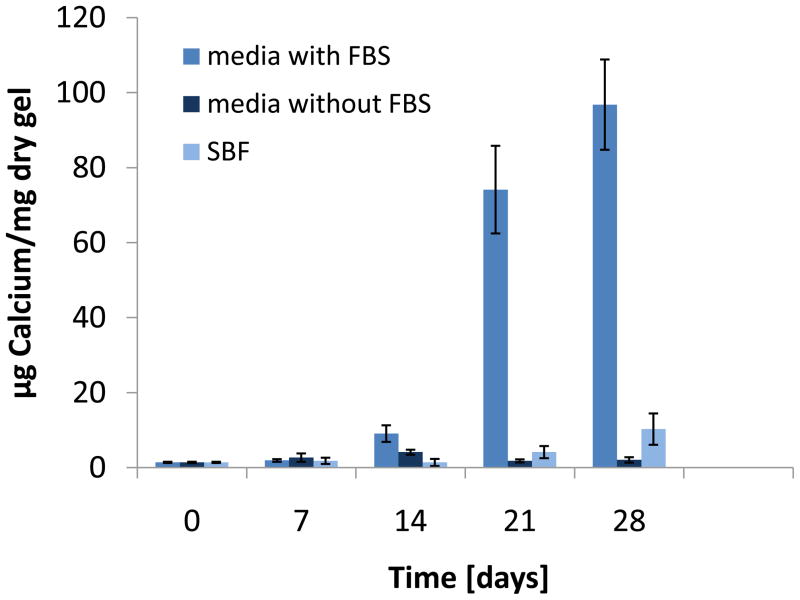
Analysis for calcium showed significant accumulation in samples incubated with complete osteogenic medium over four weeks (Figure 8). Hydrogels immersed in osteogenic medium without FBS as well as simulated body fluid did not yield mineralization. Scanning electron microscopy (SEM) coupled with an energy dispersive spectrometer (EDS) detector confirmed the presence of calcium and phosphorus in the hydrogels incubated with the FBS-containing medium over 28 days but not in the two other groups (Supplemental Figure IV).
Figure 8.
Calcium content of cell-free hydrogels immersed in complete osteogenic media, osteogenic media without fetal bovine serum (FBS) and simulated body fluid (SBF) at 37°C over 4 weeks (n=4–6). Data are reported as mean ± standard deviation.
4. Discussion
The fabrication of hydrogels via a dual gelation mechanism was the objective of this study. The macromers used as building blocks for the hydrogels were designed to employ a thermoresponsive domain and chemically crosslinkable (meth)acrylate end groups. A physical gelation mechanism imparted by the thermoresponsive pNiPAAm segment in the macromer takes place once the incubation temperature reaches the macromers’ LCST, and covalent crosslinking of the macromer chains at the (meth)acrylate groups is triggered by the addition of a thermal free-radical initiator system. The effect of the macromer structure (acrylate or methacrylate functional groups) on the properties of the resulting hydrogels was investigated. The swelling and degradation characteristics of the hydrogels were evaluated and these properties were examined with respect to mesenchymal stem cell viability and osteogenic potential upon encapsulation.
4.1. Hydrogel swelling and degradation
Hydrogel systems based on N-isopropylacrylamide show a temperature-responsive swelling behavior. Among other factors, the swelling of these hydrogels is dependent on the hydrophilicity of the building blocks [10, 17], the temperature [10, 15, 16] and in certain cases the pH [8, 24, 25] of the medium. Moreover, pNiPAAm, which has an LCST around 32°C [12], has been shown to fully phase separate and precipitate at 37°C when it is in its linear, uncrosslinked state.
The first study reported in this manuscript showed increased hydrogel swelling with longer fabrication times and increased initiator concentrations (Figure 1). When the thermal initiator system was added in a final concentration of 20 mM and the hydrogels were formed for 30 minutes at 37°C, higher swelling ratios were observed as opposed to hydrogels fabricated for shorter intervals and/or lower initiator concentrations. The phenomenon taking place at the incubation temperature of 37°C is a complex interplay between two kinetically independent mechanisms. Covalent crosslinking of the macromolecules takes place at the same time as those chains undergo a thermally-induced phase transition and collapse. The results suggest that longer fabrication intervals and higher initiator concentrations decreased the degree of collapse of the hydrogels during fabrication and also upon immersion in water at 37°C, possibly by the formation of a more crosslinked network. Without the network formed by the covalent chain crosslinking, it is likely that the macromer chains would have further collapsed, leading to lower swelling ratios.
The effect of temperature on the swelling of the hydrogels in water was investigated at temperatures below (4°C) and above (37°C) the LCST of the macromers (Figure 2). The phenomenon of increased swelling below the transition temperature has been reported for NiPAAm-based systems and the water content has been shown to decrease as the temperature approaches and surpasses the LCST [10, 15, 16]. In our studies, the swelling ratio for both hydrogel formulations was significantly higher at 4°C than at 37°C and both hydrogel types expelled water when incubated at 37°C. An interesting observation was the higher temperature-responsiveness exhibited for the acrylated as opposed to the methacrylated formulation. Acrylated hydrogels swelled more than methacrylated at 4°C but exhibited higher shrinkage at 37°C. This may be attributed to the higher flexibility of the polymer chains having an acrylate end group. The additional methyl group of the methacrylated formulations may hinder the chains from fully undergoing the pNiPAAm-associated, temperature driven collapse above the LCST, and also from their complete hydration below the LCST. It is also likely that due to the higher hydrophobicity of the methacrylated chains a “skin layer” is formed which does not allow for water to be fully expelled from the interior of the hydrogel once the temperature increases [26]. Factors that have been demonstrated to control the swelling behavior is the crosslinking density for hydrophilic, crosslinked hydrogel systems [27] or the difference between the incubation temperature and the LCST for uncrosslinked, pNiPAAm-based hydrogels [17]. In the present system, there is an interrelation between the hydrophobic, pNiPAAm-driven chain aggregation and the presence of chemical crosslinks but the covalent crosslink density and temperature difference from LCST do not seem to be the governing forces directly determining the hydrogel swelling behavior.
Further swelling and degradation studies were performed over eight weeks in complete osteogenic media and PBS. Lower swelling was observed for samples immersed in cell culture media as compared to PBS. The presence of solutes such as monosaccharides, amino acids and serum proteins in the complete osteogenic media may have decreased the affinity of the polymer chains for water, leading to higher shrinking. Additionally, the experimental temperature was above the hydrogels’ LCST, which renders them more hydrophobic. This may facilitate protein adsorption from the serum on the hydrogels which can enhance hydrophobic interactions and lead to lower swelling. In both immersing media, the swelling at 1h was generally different from subsequent time points suggesting that equilibrium had not yet been reached (Figure 3). After this time point, there were no notable differences in swelling for each formulation, which is also consistent with the fact that no hydrogel degradation was observed (Figure 4). Hydrogel chain degradation translates in an altered mesh structure and allows for changes in hydrogel swelling [13, 28]. The degradation of this hydrogel system is expected to occur via hydrolysis of the ester bonds on pentaerythritol diacrylate monostearate and hydroxyethyl acrylate and its (meth)acrylated end groups. The present study showed that the time interval of eight weeks in vitro at 37°C was not sufficient for chain hydrolysis. As mentioned previously, the incubation temperature is above the LCST and the chains are partially dehydrated which likely shields esters from hydrolytic degradation. No mass loss was noted in the hydrogels incubated in PBS, as expressed with a constant sol fraction and dry mass. In addition, solid state NMR analysis (data not shown) did not detect any acidic compounds which would have resulted from ester hydrolysis. Further analysis with longer time frames could establish the degradation rate of the hydrogels.
4.2. Mesenchymal stem cell encapsulation
One advantage of in situ forming hydrogels is the three-dimensional incorporation of cells and the potential control over their spatial distribution. In our system, homogeneous cellular distribution could be observed throughout the hydrogels for both formulations over three weeks of culture (Figure 5). Hydrogel sections were taken both from the center and the edge of the hydrogels and stained with calcein AM and ethidium homodimer-1, markers for live and dead cells, respectively. Cells retained their viability over the entire period of the culture (Figure 6). The cytocompatibility of the macromers for various times of crosslinking was evaluated in a previous study [18]. In this case, the reaction rate of macromer crosslinking was fast enough as not to cause any significant adverse effects to encapsulated cells. The hydrogel systems presented in this study have relatively lower swelling ratios compared to purely hydrophilic systems. However, the water retained by both hydrogel types seemed to facilitate nutrient and oxygen diffusion into the hydrogel as well as waste product removal, a fact evidenced by viable cells. Even though there are no quantitative data for the Live/Dead assay, there seems to be a decrease in cell viability at 21 days, particularly for the acrylated formulation. As will be discussed below, there was protein and mineral accumulation in the hydrogels over time. It is possible that at this time point in culture, the diffusion through the hydrogels was lower, especially for the acrylated hydrogels which exhibited an overall lower swelling than the methacrylated hydrogels.
One problem encountered in these studies was the incomplete DNA extraction from the cells and/or the gels. Double-stranded DNA could be detected until the first day of culture, but a significant drop occurred at later time points. The presence of live cells however was confirmed with the Live/Dead staining. Possible interactions of the material and matrix components with DNA as well as material autofluorescence were accounted for. Moreover, the presence of single- and double-stranded DNA was evaluated with the Oli-Green assay kit [29] with minimal detection. Therefore, quantitative DNA assay results (included as Supplemental Figure I) may not accurately reflect the total number of live cells.
Mesenchymal stem cells can undergo osteogenic differentiation when cultured in the presence of supplements such as dexamethasone [30]. The osteogenic marker alkaline phosphatase (ALP) was detected when the cell-laden gels were cultured in complete osteogenic media (Supplemental Figure II). Calcium, another marker for osteogenic differentiation, was present in the cell-laden gels as early as after one week of culture. There did not seem to be an effect of (meth)acrylated end group on mineralization. At 14 days, cell-free hydrogels had started to accumulate calcium, but at a significantly lower amount than cell-laden gels. At 21 days however, the calcium content of cell-laden and cell-free hydrogels was not statistically different (Figure 7). Gene expression analysis and immunochemical assays for markers such as osteonectin and osteopontin could be employed to additionally confirm the osteogenic differentiation of the cells in future studies. Mineralized extracellular matrix has been shown to enhance osteogenic differentiation of mesenchymal stem cells [22]. Future in vivo studies could determine whether the mineralization of the hydrogels is a parameter that could further promote cell osteogenic differentiation in this system.
4.3. Cell–free hydrogel mineralization
The mechanism of hydrogel mineralization was further evaluated with cell-free hydrogels immersed in three different mineralizing media, namely complete osteogenic medium, the same osteogenic medium but without the addition of fetal bovine serum (FBS) and simulated body fluid (SBF). SBF has a slightly higher concentration of calcium ions (2.5 mM) than the complete osteogenic medium (1.8 mM). Both contain inorganic phosphate (1 mM), whereas the latter also contains organic phosphate in the form of β-glycerol phosphate (10 mM). Hydrogel mineralization in the absence of cells generally occurs through the addition of anionic, calcium-binding functional groups on the polymer chains, the incorporation of inorganic particles into the matrix or the formation of nucleation sites by methods that mimic the physiological mineralization process [31].
The results from the present studies indicate that the hydrogel mineralization here is mediated by the fetal bovine serum (Figure 8). Fetal bovine serum can be extremely variable in composition [32] and often contains salts of calcium and phosphate [33, 34]. It has been reported that certain serum proteins act as calcification factors in the presence of adequate amounts of phosphate salts [35, 36]. There is also evidence in the literature that proteins found in serum exhibit endogenous alkaline phosphatase activity and can therefore hydrolyze the organic β-glycerol phosphate [37, 38]. These reports are in agreement with our studies, which showed that serum in the presence of inorganic calcium and phosphate salts as well as high amounts of β-glycerol phosphate initiated mineralization. Since the incubation temperature of 37°C is higher than the LCST, the hydrogels are in a hydrophobic state, which likely favors protein adsorption from the serum. This fact is evidenced also by the lower swelling observed in hydrogels immersed in complete osteogenic cell culture medium, which contains proteins, as opposed to samples immersed in PBS as discussed in Section 4.1. Inorganic salts alone, as in SBF, or organic phosphate in the absence of serum, as in FBS-free media, did not result in hydrogel mineralization.
5. Conclusions
These studies demonstrated the fabrication of hydrogels with a dual solidification mechanism, namely lower critical solution behavior and covalent crosslinking. Parameters that control the swelling behavior of the hydrogels are the chemistry of the macromer end group (acrylate or methacrylate), the concentration of the initiator system used, the fabrication interval as well as the incubation temperature and medium. These factors exhibit their effects on swelling by affecting the chemical crosslinking as well as the chain aggregation upon thermal transition. The hydrogels exhibited constant swelling and no degradation over an eight-week incubation period in vitro at 37°C. Encapsulated mesenchymal stem cells showed uniform distribution and viability throughout the hydrogels over a culture period of 21 days. Markers for cell osteogenic differentiation, alkaline phosphatase and calcium, were detected when the hydrogels were cultured in the presence of osteogenic supplements. Additionally, the hydrogels showed mineralization in the absence of cells but with addition of osteogenic cell culture medium supplemented with fetal bovine serum and βglycerol phosphate.
Supplementary Material
(I) and (II): Biochemical assays performed on cell-laden hydrogels cultured in osteogenic media over 21d (n=3–5). Assay readings were corrected for with blank hydrogels. (I) DNA content as determined with PicoGreen reagent. (II) Alkaline phosphatase activity. (III) Histological sections of cell-laden hydrogels stained with von Kossa reagent. Acrylated and methacrylated hydrogel sections after one-day and 21-day culture are presented. (IV) Energy dispersive spectra of cell-free hydrogels immersed for 28 days in (a) complete osteogenic media containing FBS, (b) FBS-free media, (c) SBF. Samples were sputter-coated in gold prior to SEM-EDS analysis. The signals for carbon and nitrogen derive from the polymer. Calcium and phosphorus are only detected for the samples in FBS-containing media. The calcium to phosphorus ratio for these samples was 1.10±0.23 and was determined for n=3.
Acknowledgments
This work was supported by the National Institutes of Health (R01 DE17441). The authors would like to thank Dr. Brendan Lee and Ms. Ming-Ming Jiang for use and assistance at the Bone Histology/Immunohistochemistry Core. Dr. Lawrence Alemany and Mr. Richard Thibault are acknowledged for assistance with solid state NMR and SEM-EDS respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Advanced drug delivery reviews. 2002;54:37–51. doi: 10.1016/s0169-409x(01)00242-3. [DOI] [PubMed] [Google Scholar]
- 2.Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm. 2008;68:34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruel-Gariepy E, Leroux JC. In situ-forming hydrogels--review of temperature-sensitive systems. Eur J Pharm Biopharm. 2004;58:409–426. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Fundueanu G, Constantin M, Stanciu C, Theodoridis G, Ascenzi P. pH- and temperature-sensitive polymeric microspheres for drug delivery: the dissolution of copolymers modulates drug release. Journal of materials science. 2009;20:2465–2475. doi: 10.1007/s10856-009-3807-0. [DOI] [PubMed] [Google Scholar]
- 5.Misra GP, Singh RS, Aleman TS, Jacobson SG, Gardner TW, Lowe TL. Subconjunctivally implantable hydrogels with degradable and thermoresponsive properties for sustained release of insulin to the retina. Biomaterials. 2009;30:6541–6547. doi: 10.1016/j.biomaterials.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Z, Akiyama Y, Yamato M, Okano T. Comb-type grafted poly(N-isopropylacrylamide) gel modified surfaces for rapid detachment of cell sheet. Biomaterials. 31:7435–7443. doi: 10.1016/j.biomaterials.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 7.Gant RM, Abraham AA, Hou Y, Cummins BM, Grunlan MA, Cote GL. Design of a self-cleaning thermoresponsive nanocomposite hydrogel membrane for implantable biosensors. Acta biomaterialia. 6:2903–2910. doi: 10.1016/j.actbio.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto KL, Ma Z, Nelson DM, Hashizume R, Guan J, Tobita K, Wagner WR. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials. 2009;30:4357–4368. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan J, Hong Y, Ma Z, Wagner WR. Protein-reactive, thermoresponsive copolymers with high flexibility and biodegradability. Biomacromolecules. 2008;9:1283–1292. doi: 10.1021/bm701265j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stile RA, Burghardt WR, Healy KE. Synthesis and Characterization of Injectable Poly(N-isopropylacrylamide)-Based Hydrogels That Support Tissue Formation in Vitro. Macromolecules. 1999;32:7370–7379. [Google Scholar]
- 11.Vermonden T, Fedorovich NE, van Geemen D, Alblas J, van Nostrum CF, Dhert WJ, Hennink WE. Photopolymerized thermosensitive hydrogels: synthesis, degradation, and cytocompatibility. Biomacromolecules. 2008;9:919–926. doi: 10.1021/bm7013075. [DOI] [PubMed] [Google Scholar]
- 12.Schild HG. Poly(N-isopropylacrylamide): Experiment, Theory and Application. Prog Polym Sci. 1992;17:163–249. [Google Scholar]
- 13.Censi R, Fieten PJ, di Martino P, Hennink WE, Vermonden T. In Situ Forming Hydrogels by Tandem Thermal Gelling and Michael Addition Reaction between Thermosensitive Triblock Copolymers and Thiolated Hyaluronan. Macromolecules. 2010;43:5771–5778. doi: 10.1016/j.jconrel.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 14.Cheng V, Lee BH, Pauken C, Vernon BL. Poly(N-isopropylacrylamide-co-Poly(ethylene glycol))-Acrylate Simultaneously Physically and Chemically Gelling Polymer Systems. Journal of Applied Polymer Science. 2007;106:1201–1207. [Google Scholar]
- 15.Lee BH, West B, McLemore R, Pauken C, Vernon BL. In-situ injectable physically and chemically gelling NIPAAm-based copolymer system for embolization. Biomacromolecules. 2006;7:2059–2064. doi: 10.1021/bm060211h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robb SA, Lee BH, McLemore R, Vernon BL. Simultaneously physically and chemically gelling polymer system utilizing a poly(NIPAAm-co-cysteamine)-based copolymer. Biomacromolecules. 2007;8:2294–2300. doi: 10.1021/bm070267r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hacker MC, Klouda L, Ma BB, Kretlow JD, Mikos AG. Synthesis and characterization of injectable, thermally and chemically gelable, amphiphilic poly(N-isopropylacrylamide)-based macromers. Biomacromolecules. 2008;9:1558–1570. doi: 10.1021/bm8000414. [DOI] [PubMed] [Google Scholar]
- 18.Klouda L, Hacker MC, Kretlow JD, Mikos AG. Cytocompatibility evaluation of amphiphilic, thermally responsive and chemically crosslinkable macromers for in situ forming hydrogels. Biomaterials. 2009;30:4558–4566. doi: 10.1016/j.biomaterials.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thibault RA, Scott Baggett L, Mikos AG, Kasper FK. Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements. Tissue engineering. 16:431–440. doi: 10.1089/ten.tea.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo X, et al. In vitro generation of an osteochondral construct using injectable hydrogel composites encapsulating rabbit marrow mesenchymal stem cells. Biomaterials. 2009;30:2741–2752. doi: 10.1016/j.biomaterials.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao J, Guo X, Nelson D, Kasper FK, Mikos AG. Modulation of osteogenic properties of biodegradable polymer/extracellular matrix scaffolds generated with a flow perfusion bioreactor. Acta biomaterialia. 6:2386–2393. doi: 10.1016/j.actbio.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Brazel CS, Peppas NA. Synthesis and Characterization of Thermo-and Chemomechanically Responsive Poly(N-isopropylacrylamide-co-methacrylic acid) Hydrogels. Macromolecules. 1995;28:8016–8020. [Google Scholar]
- 25.Xu FJ, Kang ET, Neoh KG. pH- and temperature-responsive hydrogels from crosslinked triblock copolymers prepared via consecutive atom transfer radical polymerizations. Biomaterials. 2006;27:2787–2797. doi: 10.1016/j.biomaterials.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida R, Uchida K, Kaneko Y, Sakai K, Kikuchi A, Sakurai Y, Okano T. Comb-type grafted hydrogels with rapid de-swelling response to temperature changes. Nature. 1995;374:240–242. [Google Scholar]
- 27.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 28.Temenoff JS, Park H, Jabbari E, Conway DE, Sheffield TL, Ambrose CG, Mikos AG. Thermally Cross-linked Oligo(poly(ethylene glycol) fumarate) Hydrogels Support Osteogenic Differentiation of Encapsulated Marrow Stromal Cells in Vitro. Biomacromolecules. 2004;5:5–10. doi: 10.1021/bm030067p. [DOI] [PubMed] [Google Scholar]
- 29.Kasper FK, Seidlits SK, Tang A, Crowther RS, Carney DH, Barry MA, Mikos AG. In vitro release of plasmid DNA from oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2005;104:521–539. doi: 10.1016/j.jconrel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Jager M, Feser T, Denck H, Krauspe R. Proliferation and osteogenic differentiation of mesenchymal stem cells cultured onto three different polymers in vitro. Annals of biomedical engineering. 2005;33:1319–1332. doi: 10.1007/s10439-005-5889-2. [DOI] [PubMed] [Google Scholar]
- 31.Gkioni C, Leeuwenburgh S, Douglas T, Mikos AG, Jansen J. Mineralization of Hydrogels for Bone Regeneration. Tissue Eng Part B Rev. doi: 10.1089/ten.TEB.2010.0462. [DOI] [PubMed] [Google Scholar]
- 32.Honn KV, Singley JA, Chavin W. Fetal bovine serum: a multivariate standard. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY. 1975;149:344–347. doi: 10.3181/00379727-149-38804. [DOI] [PubMed] [Google Scholar]
- 33.Boskey AL, Roy R. Cell culture systems for studies of bone and tooth mineralization. Chemical reviews. 2008;108:4716–4733. doi: 10.1021/cr0782473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedraza CE, Chien YC, McKee MD. Calcium oxalate crystals in fetal bovine serum: implications for cell culture, phagocytosis and biomineralization studies in vitro. Journal of cellular biochemistry. 2008;103:1379–1393. doi: 10.1002/jcb.21515. [DOI] [PubMed] [Google Scholar]
- 35.Price PA, Chan WS, Jolson DM, Williamson MK. The elastic lamellae of devitalized arteries calcify when incubated in serum: evidence for a serum calcification factor. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1079–1085. doi: 10.1161/01.ATV.0000216406.44762.7c. [DOI] [PubMed] [Google Scholar]
- 36.Price PA, June HH, Hamlin NJ, Williamson MK. Evidence for a serum factor that initiates the re-calcification of demineralized bone. The Journal of biological chemistry. 2004;279:19169–19180. doi: 10.1074/jbc.M307880200. [DOI] [PubMed] [Google Scholar]
- 37.Hamlin NJ, Price PA. Mineralization of decalcified bone occurs under cell culture conditions and requires bovine serum but not cells. Calcified tissue international. 2004;75:231–242. doi: 10.1007/s00223-004-0190-1. [DOI] [PubMed] [Google Scholar]
- 38.Khouja HI, Bevington A, Kemp GJ, Russell RG. Calcium and orthophosphate deposits in vitro do not imply osteoblast-mediated mineralization: mineralization by betaglycerophosphate in the absence of osteoblasts. Bone. 1990;11:385–391. doi: 10.1016/8756-3282(90)90131-h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(I) and (II): Biochemical assays performed on cell-laden hydrogels cultured in osteogenic media over 21d (n=3–5). Assay readings were corrected for with blank hydrogels. (I) DNA content as determined with PicoGreen reagent. (II) Alkaline phosphatase activity. (III) Histological sections of cell-laden hydrogels stained with von Kossa reagent. Acrylated and methacrylated hydrogel sections after one-day and 21-day culture are presented. (IV) Energy dispersive spectra of cell-free hydrogels immersed for 28 days in (a) complete osteogenic media containing FBS, (b) FBS-free media, (c) SBF. Samples were sputter-coated in gold prior to SEM-EDS analysis. The signals for carbon and nitrogen derive from the polymer. Calcium and phosphorus are only detected for the samples in FBS-containing media. The calcium to phosphorus ratio for these samples was 1.10±0.23 and was determined for n=3.