Abstract
Dynamic stimuli are ubiquitous in natural viewing conditions implying that grouping operations need to operate, not only in space, but also jointly in space and time. Moreover, in natural viewing, attention plays an important role in controlling how resources are allocated. We investigated how attention interacts with spatiotemporal perceptual grouping by using a bistable stimulus, called the Ternus-Pikler display. Ternus-Pikler displays can give rise to two different motion percepts, called Element Motion (EM) and Group Motion (GM), the former dominating at short Inter-Stimulus Intervals (ISIs) and the latter at long ISIs. Our results indicate that GM grouping requires more attentional resources than EM grouping. Different theoretical accounts of perceptual grouping and attention are discussed and evaluated in the light of the current results.
Keywords: Ternus-Pikler display, Perceptual grouping, Attention
1. Introduction
1.1. Attitude, attention, and perceptual organization
Gestalt psychologists defined attitude as a force that provides directedness to perception, such as the expectation of a particular organization or outcome (Koffka, 1935). Attention was defined as a special type of attitude representing an unspecific directedness, i.e., without a specific expectation of a particular organization or outcome, towards an object. Given the central role that perceptual organization plays in Gestalt theory, an important question was the relationship between attention and perceptual organization. Gestalt psychologists viewed these two processes as distinct but functionally interdependent (Koffka, 1922, p. 561). They proposed that attention can influence perceptual organization. However, this influence is in general limited because the “force” provided by attention may not be sufficient to overcome the strength of perceptual organization. To show how attention can influence perceptual grouping, one can consider stimuli, such as ambiguous figures, where the stability of perceptual organization is relatively weak. The well-known Rubin's vase shown in Fig. 1a is ambiguous and bistable in that it can be perceived either as a vase or two faces. Shifting attention from the white part to the dark part of the figure can lead to switches between these two percepts, illustrating how attention can influence perceptual organization. To demonstrate cases where attitude, attention, or learning are unable to overcome perceptual organization, Koffka (1935) provided examples from Gottschaldt's (1926, 1929) experiments. One example is illustrated in Fig. 1b and 1c. Figure 1c contains as a sub-part the hexagon shown in Fig. 1b. Regardless of how much attention or training is provided, the line segments making up the hexagon fail to group as a distinct figure within the larger and more complex stimulus.
Figure 1.
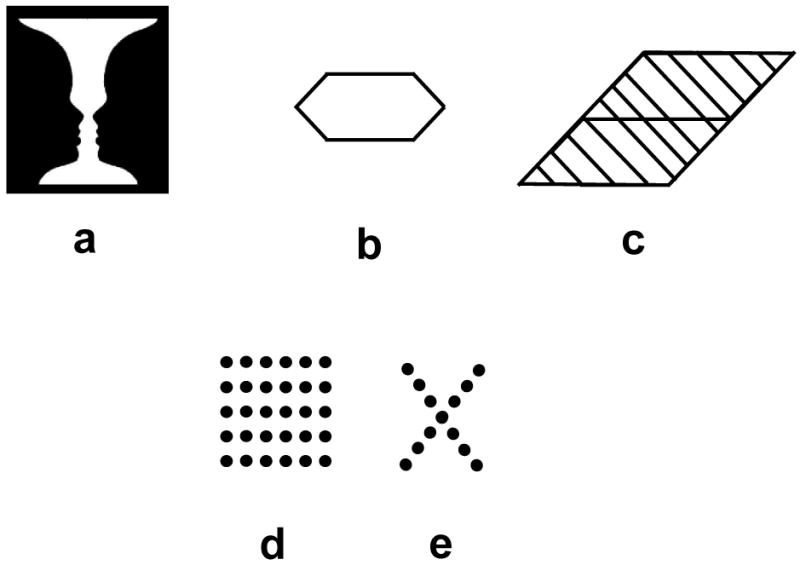
Various stimulus configurations used to investigate the role of attention on perceptual grouping.
The dot-square pattern shown in Fig. 1d is another example of whole-part relation in perceptual grouping. At a large scale of organization, the ensemble of dots can be grouped into a square. Yet, at a finer scale, based on horizontal versus vertical proximity between the dots, they can be also grouped and perceived as an array of horizontal lines. Unlike the example of Fig. 1c, here learning and attention can facilitate the perception of horizontal lines. The configuration in Fig. 1d was used in the earliest studies examining the relationship between attitudes, attention, and perceptual grouping. Based on his experiments with rats (Krechevski, 1938), Krech viewed perceptual organization as a process that can create a spectrum of outcomes ranging from simpler homogeneous structures (e.g., dots in Fig. 1d grouped into a global homogeneous square pattern without column row differentiation) to more complex differentiated structures (e.g., dots grouped in an array of horizontal lines). According to his hypothesis, which organization will prevail depends on attitudes or motivational factors; the organism is predicted to proceed from homogeneous to more differentiated forms until its goal is satisfied. To test this hypothesis with humans, Krech and Calvin (1953) presented observers briefly with the two patterns shown in Fig. 1d and 1e. In a given trial, the location of each pattern, left or right, was selected randomly. The observers were asked to decide which of the two is the “correct symbol”. Observers did not know the criterion for correctness and therefore had to start by guessing. Feedback was given after each trial and trials continued until observers discovered the underlying rule. The criterion for correctness was not based on shape information (square versus X) but rather on location (alternating left, right, left, right in the succession of trials). After each trial, the observer was also asked to reproduce each symbol as accurately as possible by placing beads on a board. The task of finding the “correct symbol” was a decoy task and the real interest was whether the observers would reproduce the horizontal groupings in the dot arrays based on the horizontal proximity. Krech's prediction was that during learning trials, observers' perception of the dot stimuli will progress from a homogeneous square pattern to a differentiated line array pattern. Accordingly, during the first trials observers are expected to place beads in equidistant holes on the reproduction board. As they try to solve the problem, their perception will progress into more differentiated forms leading the placement of beads according to horizontal proximity relation. The results supported their hypothesis by showing that the percentage of observers reporting linear groupings increased from 14.2% in the first trial to 46.4% in the ninth trial. Köhler and Adams (1958) followed up this study with a similar experimental design where the decoy task was to make aesthetic judgments about small cardboard figures that were positioned on a background similar to that of Fig. 1d. After six trials, observers were given a surprise question asking them to describe the background patterns. Their results indicated that the proximity of dots needed for the perception of horizontal groups was much higher when attention was drawn by the decoy task compared to the case when there was no decoy task. Taken together, these studies show that, under conditions of “incidental perception” (Köhler & Adams, 1958) or “inattention” (Mack et al., 1992; Rock et al., 1992), observers seem to be aware of simpler homogeneous organizations but not detailed ones and that attitudes and attention can modulate perceptual grouping by enabling the articulation of more detailed perceptual organizations.
In ensuing years, as research focused on the details of Gestalt “laws” of grouping, significant differences between various types of groupings emerged. For example, in “similarity grouping”, the propensity of grouping depends on which similarity dimension is considered. In order to elucidate similarity dimensions that are critical for grouping, Beck (1966a, 1966b, 1967) used simple geometric shapes such as L, +, T, U, X, and showed that both the slopes of component lines of figures (e.g., 0 and 90 degree components in L and 45 degree components in X) and the configuration of these components (e.g., T vs +) play a major role in similarity-based grouping. Beck (1972) generalized these findings by proposing that grouping occurs for those figural properties that the visual system responds strongly before focal attention is deployed. This view of grouping occurring prior to the focusing of attention has been supported by other researchers (e.g., Caelli & Julesz, 1979; Julesz, 1991). Treisman and colleagues proposed the Feature Integration Theory where grouping within a feature dimension, such as color, can occur without focal attention while any grouping across feature dimensions, such as color and orientation, requires focal attention (Treisman & Gelade, 1980; Treisman, 1982).
While research from 1960s to 1980s used techniques such as visual search to distinguish between distributed versus focal attention and to investigate the role of focal attention in perceptual grouping, Mack, Rock, and colleagues returned to the methods used in 1950s to investigate whether grouping occurs in the “complete absence” of (as opposed to distributed) attention (Mack et al., 1992). In a search paradigm, observers look actively for a pre-defined or an odd target. In Gestalt terminology, this would represent a pre-established attitude, a general form of attention (in current literature it is termed distributed attention). Typical methods to minimize the effects of attitudes or distributed attention consist of giving “decoy” or “cover” tasks to the observer and have them report their perception indirectly (e.g., Krech & Calvin, 1953) or by surprise questions (e.g., Köhler & Adams, 1958; Mack et al., 1992; Rock et al., 1992). Using the latter methodology, Mack et al. reported that texture segregation, and similarity grouping by lightness and proximity do not occur in the absence of attention. Their findings are in agreement with the earlier reports that attributed the lack of grouping under conditions of “inattention” to the tendency of larger organizations to suppress more articulated organizations (Köhler & Adams, 1958) or to a preference of homogenous configurations over more differentiated ones (Krech & Calvin, 1953). A study using a dual-task methodology also provided support for the finding that perceptual grouping by proximity and similarity requires attentional resources (Barchilon Ben-av et al., 1992).
One shortcoming of methods using decoy/cover tasks is that, since observers are given surprise questions after the presentation of the stimulus, they have to rely on memory to report their perception. As a result, it is not clear to which extent their reports are influenced and/or limited by memory mechanisms (Rock et al., 1992; Moore & Egeth, 1997). To circumvent this problem, Moore & Egeth (1997) used an implicit-measure approach where the task of the observers was to judge the length of line segments. These line segments were presented along with background elements which were irrelevant to the task according to task description given to the observers. However, background elements were arranged so that, if perceptually grouped, they could create length illusions (Müller-Lyer or Ponzo illusions). Thus, observers' length judgments would reflect whether or not unattended background elements were perceptually grouped. Using this method, Moore and Egeth provided evidence that grouping occurs without attention and suggested that grouping failures reported in earlier studies may be due to failures of memory. Subsequent studies supported these findings (Chan & Chua, 2003; Russell & Driver, 2005; Lamy et al., 2006; Shomstein et al., 2010).
Based on the review above, findings about whether grouping occurs before or after attention may appear contradictory. However, this apparent contradiction ceases to be valid if we note that explicit and implicit measures may be tapping at different levels and processes. In fact, a similar situation exists in visual masking where a mask stimulus can render a target stimulus “invisible” as measured by direct methods. Yet, it can be shown by indirect methods that this invisible target stimulus, which fails to reach the observer's awareness, can nevertheless prime other stimuli (e.g., Ansorge et al., 1998; Breitmeyer, et al., 2004, 2005; Klotz & Wolff, 1995; Vorberg et al., 2003). These findings have been explained by a theoretical view according to which stimuli are processed in parallel and interacting pathways at multiple levels of complexity (e.g., Breitmeyer et al., 2004, 2005). The failure to reach awareness does not imply that the stimulus has not been processed elsewhere in the visual system. In fact, in the context of grouping, it has been shown that, even when using implicit measures, not all groupings occur automatically in the absence of attention; instead the complexity of the grouping may dictate the attentional resources required (Kimchi & Razpurker-Apfeld, 2004).
In our study, we consider a bistable stimulus where two grouping organizations compete. In this stimulus, the percept is not the presence or absence of grouping but rather one of two possible groupings. Thus, we are not addressing whether grouping takes place before or after attention but instead whether and how attention interacts with grouping.
1.2. Spatiotemporal grouping
The focus of aforementioned studies has been grouping in space. However, natural environment is composed of both static and dynamic stimuli and the latter require simultaneous grouping in space and time. To investigate this question, the Gestalt psychologist Joseph Ternus (1926) adapted and modified a stimulus paradigm that was used previously by Pikler (1917). Fig. 2 illustrates a typical “Ternus-Pikler display” consisting of two frames each containing three elements. The three elements in the first frame are shifted to the right in the second frame by one inter-element spacing so that the two rightmost elements of the first frame spatially overlap with the two leftmost elements of the second frame. The stimulus is designed so as to create an ambiguity: As shown in Fig. 2 left panel, one possible grouping can be based on spatial position such that two of the elements may be grouped together by virtue of their spatial position that remains invariant over time. The remaining elements (the leftmost element in Frame 1 and the rightmost element in Frame 2) can be grouped together by apparent motion, a percept called element motion (EM). Another possible grouping can be based on a larger scale grouping of the three disks as a single Gestalt, resulting in a percept where the three disks move in group, as shown in Fig. 2 right panel. This latter percept is called group motion (GM).
Figure 2.
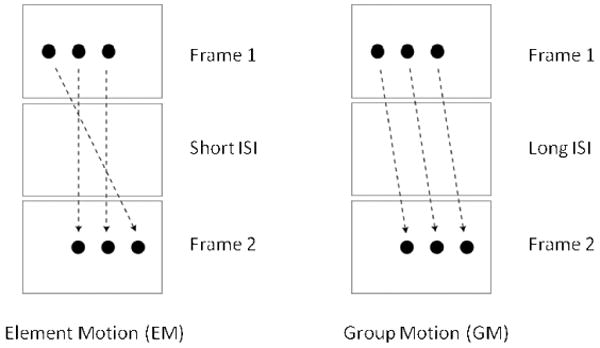
The Ternus-Pikler display. Two possible motion percepts: Element Motion (EM) at short ISIs and Group Motion (GM) at long ISIs.
Several studies showed that both EM and GM are possible percepts and which one will prevail depends on stimulus parameters, such as inter-element separation, element size, spatial frequency, contrast, inter-stimulus interval (ISI), luminance, frame duration, and eccentricity (Alais & Lorenceau, 2002; Breitmeyer et al.,1988; Breitmeyer & Ritter, 1986a, 1986b; Casco, 1990; Casco & Spinelli, 1988; Dawson et al.,1994; Ma-Wyatt, Clifford, & Wenderoth, 2005; Pantle & Petersik, 1980; Pantle & Picciano, 1976; Petersik & Grassmuck, 1981; Petersik & Pantle, 1979; Petersik et al., 2003; Ritter & Breitmeyer, 1989; Rutherford, 2003).
We chose the Ternus-Pikler display to investigate interactions between grouping and attention because (i) this display allows us to examine spatiotemporal grouping, (ii) being a bistable display, we expect it to be easily amenable to attentional modulation much like the static ambiguous figures discussed earlier, and because (iii) the transition between the two possible groupings can be easily controlled by a single parameter, the ISI. Our approach was to use a dual-task paradigm to control attentional resources devoted to the Ternus-Pikler display.
Determining how attention modulates EM and GM percepts also allows the assessment of several theoretical accounts of the bistable nature of the Ternus-Pikler display (rev. Petersik & Rice, 2006). Petersik and Pantle noticed that stimulus parameters such as frame duration, ISI, luminance contrast, viewing conditions (binocular or dichoptic) that favored EM (GM) percept were also those favoring short-range (long-range) motion and attributed EM percepts to short-range motion process and the GM percepts to long-range motion process (Braddick, 1980; Braddick & Adlard, 1978; Pantle & Picciano, 1976; Petersik, 1989; Petersik & Pantle, 1979). It was also suggested that short-range motion is a pre-attentive process, but the long-range motion is attentive (Dick et al., 1987; Ivry & Cohen, 1990; Nakayama & Silverman, 1986). Thus, this theory of Ternus-Pikler phenomenon predicts fewer GM reports when the attentional load by a dual task increases.
Another theory has attributed EM and GM percepts to sustained and transient channels, respectively (with neural correlates identified as parvocellular and magnocellular systems) (Breitmeyer & Ritter, 1986b; Cestnick & Coltheart, 1999; Petersik & Pantle, 1979). Activities attributed to sustained (transient) channels exhibit long (short) persistence (rev. Breitmeyer & Öğmen, 2006, pp. 141-164). When visible persistence is long and ISI is relatively short, activities generated by the spatially overlapping elements in the two frames will merge in time and this temporally integrated activity will signal a static element at that particular location. This explains why the two spatially overlapping elements appear stationary for short ISIs (Fig. 2, left panel, EM). Since there is no spatially overlapping stimulus for the leftmost element in Frame 1 and the rightmost element in Frame 2, motion will be perceived between these two elements the same way apparent motion is perceived between two spatiotemporally displaced stimuli (Fig. 2, left panel, EM). The theory suggests that when visible persistence can no longer bridge and integrate the activities of spatially overlapping elements, all three elements will undergo apparent motion to their nearest neighbors (Fig. 2, right panel, GM). In addition, it was shown that spatial attention favors temporal integration over longer ISIs (Visser & Enns, 2001; Yeshurun et al., 2002) and parvocellular over magnocellular functioning (Yeshurun, 2004; Yeshurun & Carrasco, 1999; Yeshurun & Levy, 2003). Based on these results, this theory predicts fewer EM reports when the attentional load by a dual task increases.
More recently, it has been proposed that the percept experienced in the Ternus-Pikler display is the result of a competition between two grouping processes: temporal grouping and spatial grouping (Alais & Lorenceau, 2002; Gepshtein & Kubovy, 2000; He & Ooi, 1999; Kramer & Rudd, 1999; Kramer & Yantis, 1997; Wallace & Scott-Samuel, 2007). According to this spatiotemporal grouping hypothesis, EM and GM percepts depend on how the elements are grouped in space (i.e., within-frame grouping) and in time (i.e., across-frame grouping). If the stimulus parameters favor spatial grouping, the three elements in the first frame will be grouped into a single Gestalt and they will move in tandem to produce GM. On the other hand, if stimulus parameters favor temporal grouping, the overlapping elements will be grouped across the two frames producing EM. These theories do not make predictions about the role of attention on grouping because, a priori, it is not clear whether attention would favor spatial or temporal grouping.
We can also apply Krech's and Köhler's hypotheses to Ternus-Pikler displays. Both of these hypotheses predict that attention will favor the more detailed and articulated groupings. Although the definition of “more articulated” in the Ternus-Pikler displays is not as straightforward as for the configuration in Fig. 1d, it may be reasonable to assume that GM represents a more homogeneous and simple configuration because a single group undergoes common motion. In contrast, in EM, the percept consists of two configurations, a group of static disks and a moving disk. If this interpretation is correct, then these theories will predict fewer EM reports when the attentional load by a dual task increases.
Thus, establishing whether attention plays a role in modulating the different percepts found in the Ternus-Pikler display can lead to tests and improvements of various theoretical accounts proposed for perceptual grouping operations that take place during motion perception.
2. General Methods
2.1. Apparatus
Stimuli were generated via the visual stimulus generator card (VSG 2/5; Cambridge Research Systems). Stimuli were displayed on a 22-in. color monitor set at a resolution of 800 × 500 with a refresh rate of 160 Hz. The distance between the monitor and the observer was 97 cm at which the screen covered a 28 deg by 18 deg visual area. The room, in which the experiments were conducted, was dimly illuminated by the light coming from the image on the screen. A head-chin rest was used to aid the observer to keep his/her head still while fixating his/her eyes on the fixation point displayed at the center of the monitor. Behavioral responses were recorded for offline analysis via a joystick connected to the computer which drives the VSG card.
2.2. Observers
Participants were one of the authors (MA) and two volunteers who were unaware of the purpose of the experiments. The age of the participants ranged from 25 to 27 years. All participants had normal or corrected-to-normal vision. The experiments were undertaken with the permission of The University of Houston Committee for the Protection of Human Subjects and informed consent was obtained from the participants before the experiments started.
3. Experiment 1: The Ternus-Pikler Display in the Periphery
Our main experiment requires the placement of the Ternus-Pikler display in the peripheral visual field. Previous research showed that, in general, one obtains more GM responses for parafoveal stimuli; however, there are also interactions between eccentricity and other relevant variables such as element size and ISI (Breitmeyer & Ritter, 1986b; Rutherford, 2003). Our goal in the first experiment was to quantify EM and GM percepts for different stimulus positions in the visual field for the stimulus parameters that will be used in the main experiment investigating interactions of attention with grouping.
3.1. Methods
The stimulus was a classical Ternus-Pikler display which consisted of two frames each having three filled rectangles1. Each element was 8.5 arcmin wide and 42.7 arcmin high. The three elements in the first frame were shifted randomly to the right or left (or, up or down, see Fig. 3) in the second frame by an inter-element separation of 55.5 arcmin. The luminance of the elements was 4 cd/m2 on a background luminance of 40 cd/m2. Each frame lasted for 70 ms and the two frames were separated by a variable ISI: 0, 6, 13, 19, 25, 31, 38, 44, or 50 ms. A pilot study showed that for ISIs greater than 50 ms, GM was perceived exclusively. The Ternus-Pikler display was presented for these two frames and the ISI only. The task of the observer was to report the perceived motion: EM or GM. The percentage of GM reports was plotted as a function of the ISI. There were five conditions depending on the visual field in which the Ternus-Pikler display was presented: left, right, upper, lower visual fields and fovea (Fig. 3).
Figure 3.
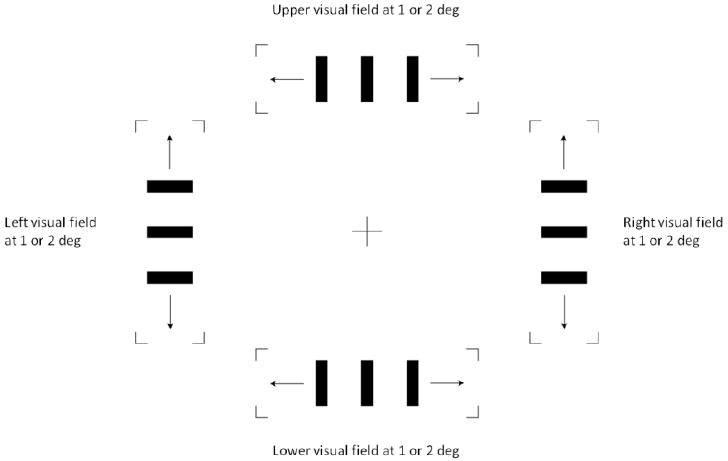
Possible locations of the Ternus-Pikler display in Experiment 1. The Ternus-Pikler display was presented at 1 or 2 deg of visual eccentricity at one out of four visual quadrants. There was only one Ternus-Pikler display on the screen at a given time. In the figure, the four Ternus-Pikler stimuli are shown for display purposes only. The arrows denote the direction of motion of the Ternus-Pikler display. Two Fovea conditions (rotated vertical and unrotated horizontal versions of the Ternus-Pikler display) were also used (not shown in the Figure to avoid clutter). In each condition, the task was to report the dominant motion percept: EM or GM.
Different eccentricity and visual field conditions were run in separate sessions. There were 10 trials per ISI in a given session yielding a total of 90 trials per session. Each observer participated in two sessions for each condition.
3.2. Results and Discussion
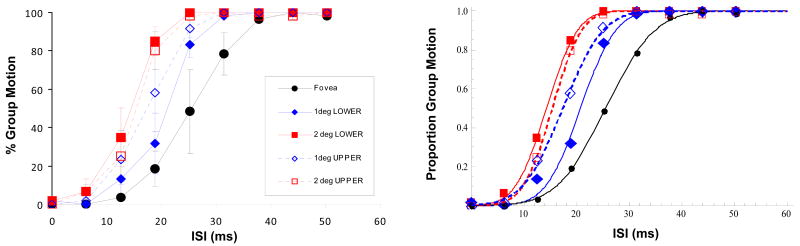
The left panel of Fig. 4 shows the average results for the Upper and Lower visual field conditions along with the baseline Fovea condition (unrotated version). The right panel shows the same data with cumulative Gaussian fits. The main effect of the ISI was significant [F(8,16) = 78.227, p < 0.001] such that GM responses increased with the ISI, replicating previous findings (e.g., Pantle & Picciano, 1976; Petersik & Pantle, 1979). The experimental condition was also significant [F(4,8) = 36.920, p < 0.001].
Figure 4.
Left panel: The results of Experiment 1 for the Upper and Lower visual field conditions along with the baseline Fovea condition (unrotated version). Error bars represent ±1 SEM. Right panel: The same data with cumulative Gaussian fits.
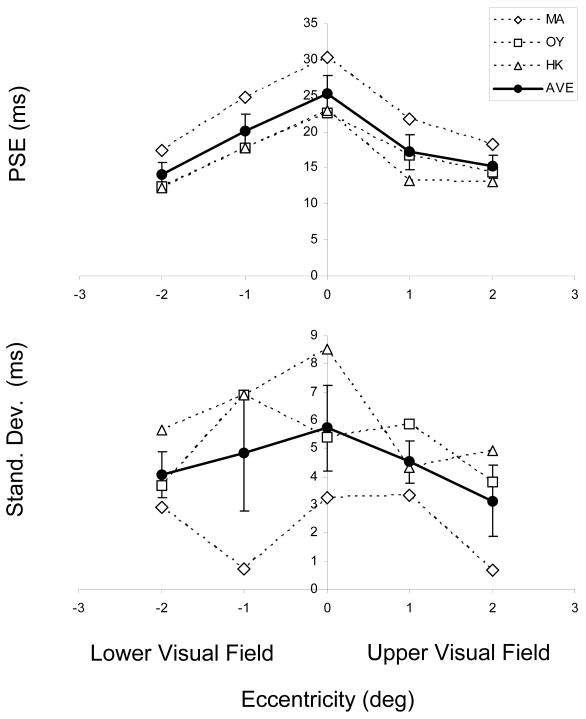
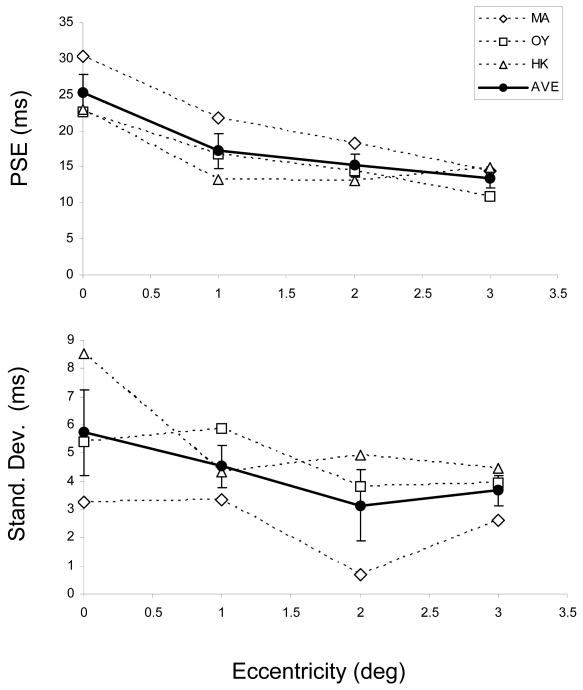
We have also analyzed the data by fitting cumulative Gaussian functions to individual observer data thereby estimating the Point of Subjective Equality (PSE) as well as the standard deviation (SD) of the Gaussian for each condition. Fig. 5 shows the individual values as well as the group average. The average of individually estimated PSEs is virtually identical to those estimated from combined data (Fig 4, right panel). These PSEs show a systematic decrease with increasing eccentricity indicating an increase in GM percepts. The effect of eccentricity is significant [F(4,10)=4.358, p=0.027]. On the other hand, individual observer SD values do not show any systematic dependence on eccentricity; although the average data suggest a decrease as a function of eccentricity, this is not significant [F(4,10)=0.483, p=0.748].
Figure 5.
Top panel: The Point of Subjective Equality (PSE) for each observer (estimated by the 50% point of the cumulative Gaussian fitted to the individual observer data) as a function of vertical eccentricity. Bottom panel: The standard deviation values of these Gaussians as a function of vertical eccentricity. Also plotted are the averages ±1 SEM of the three observers' data.
Taken together, these results show that the percentage of GM responses increases with eccentricity or equivalently the PSE values shift to lower ISIs with eccentricity. Our results are in agreement with those of Breitmeyer and Ritter (1986b) who also showed that GM responses increase and PSEs shift to lower values with eccentricity in the upper visual field. They attributed the effect of eccentricity to shorter visible persistence at more peripheral locations. According to their theory, EM percepts are generated if the elements in the two frames of the Ternus-Pikler display are “bridged” by visible persistence. Since visible persistence decreases with eccentricity (Di Lollo & Hogben, 1985; Mezrich, 1984), more GM responses are expected in peripheral presentations. However, it should be noted that this theory has been questioned by more recent studies, for general reasons (e.g., Kramer & Rudd, 1999; Kramer & Yantis, 1997).
It is also well documented that performance for stimuli displayed in the upper and lower visual fields may differ (e.g., Cameron, 2005; Carrasco et al., 2001; Danckert & Goodale, 2003; Levine & McAnany, 2005; Skrandies, 1987). However, in the case of Ternus-Pikler displays, we did not observe such a difference. The percentage of GM responses was not significantly different for the upper and lower visual fields either at 1 deg eccentricity [F(1,2) = 3.424, p = 0.205] or at 2 deg eccentricity [F(1,2) = 10.562, p = 0.083]. Similarly the PSEs were not significantly different for the upper and lower visual fields either at 1 deg eccentricity [F(1,4) = 0.7, p = 0.45] or at 2 deg eccentricity [F(1,4) = 0.282, p = 0.624].
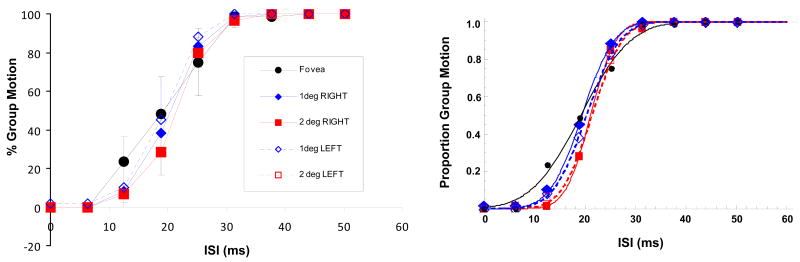
The left panel of Fig. 6 shows the average results for the Right and Left visual field conditions along with the baseline Fovea condition (rotated version)2. The right panel shows the same data with cumulative Gaussian fits. The main effect of the ISI was again significant [F(8,16) = 79.958, p < 0.001] such that GM responses increased with the ISI. However, the experimental condition was not significant [F(4,8) = 0.646, p = 0.645] suggesting that there was no effect of eccentricity and visual field on GM responses.
Figure 6.
Left panel: The results of Experiment 1 for the Right and Left visual field conditions along with the baseline Fovea condition (rotated version). Error bars represent ±1 SEM. Right panel: The same data with cumulative Gaussian fits.
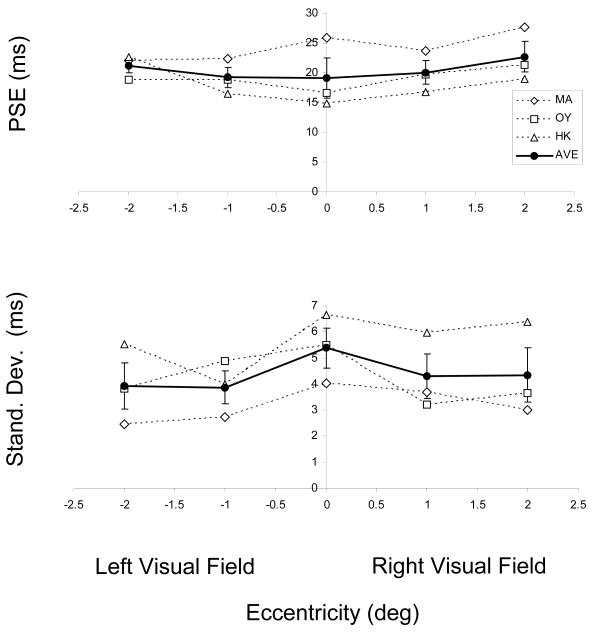
Fig. 7 shows the individual PSE and SD values as well as the group averages. As expected, there is no effect of eccentricity on PSE values [F(4,10)=0.415, p=0.794]. The same holds for SD values [F(4,10)=0.513, p=0.728].
Figure 7.
Top panel: The Point of Subjective Equality (PSE) for each observer (estimated by the 50% point of the cumulative Gaussian fitted to the individual observer data) as a function of horizontal eccentricity. Bottom panel: The standard deviation values of these Gaussians as a function of horizontal eccentricity. Also plotted are the averages ±1 SEM of the three observers' data.
Previously, Casco and Spinelli (1988) found a left vs. right visual field asymmetry in GM responses at 4 deg eccentricity such that more GM percepts were reported in the left (right) visual field of right (left) handed subjects. Moreover, GM responses for foveal responses, instead of being consistently lower, were between left and right field responses. However, Rutherford (2003) reported no significant effect of visual field for stimuli presented foveally and 5.5 deg in the left and right visual fields, in agreement with our results. She also showed that changing the task to EM vs not EM (from EM vs GM) and increasing the size of elements made the main effect of visual field significant, with more “not EM” responses for the right visual field compared to left visual field. However, “not EM” responses for peripheral and foveal stimuli were still not significantly different from each other.
Taken together, with the parameters and the task used in our study, our results show an increase in GM responses and a decrease in PSE values for an increase in eccentricity in upper and lower visual fields but no difference in GM responses and PSE values for the left and right visual fields when compared to foveal stimuli. These results are in agreement with those of Breitmeyer & Ritter (1986b) and Rutherford (2003).
4. Experiment 2: Effect of Attention on the Ternus-Pikler Display
In experiment 2, we presented the Ternus-Pikler display in the upper visual field at 3deg of eccentricity to maximize the percentage of GM responses in order to study the effects of attention on GM.
4.1. Methods
There were three experimental conditions.
In the attention task (Attention-Only condition), squares or disks, were foveally presented in close succession at the center of the screen. Per trial, 10 squares and disks were presented in total. The number and the presentation order of the squares and the disks were determined randomly for each trial. A side of the square was 21.3 long and the diameter of the disk was 23.5 arcmin leading to approximately the same area for both elements. Elements had a luminance value of 4 cd/m2 on a background luminance of 40 cd/m2 and were presented for 150 ms. Three difficulty levels were employed by varying the ISI between elements (Lavie, 1995; Lavie & Tsal, 1994). An ISI of 265 ms for the Easy condition, 190 ms for the Medium condition, and 100 ms for the Hard condition. The task of the observer was to report whether an even or odd number of squares has appeared within one block of presentations. Each observer participated in three sessions for each difficulty level. Each session comprised 56 trials and the three difficult levels were run in separate sessions.
In the Ternus-Pikler-Only condition, the Ternus-Pikler display was presented at 3 deg eccentricity in the upper visual field. The display consisted of two frames each having three circular elements. Each element had a diameter of 21.3 arcmin. The horizontal center-to-center distance between the elements was 55.5 arcmin. The luminance of the elements was 4 cd/m2 on a background luminance of 40 cd/m2. Each frame lasted for 70 ms and the two frames were separated by a variable ISI: 0, 6, 13, 19, 25, 31, or 38 ms. The direction of motion of the Ternus-Pikler display (right or left) was randomized from trial to trial. The task of the observer was to report the motion percept: EM vs. GM. The percentage of GM responses was plotted as a function of the ISI. Each observer participated in three sessions. There were eight trials for each ISI in a given session yielding a total of 56 trials per session.
In the Dual-Task condition, the Attention-Only and the Ternus-Pikler-Only conditions were combined. The primary task was attention task. Only the correct trials in the attention task were considered for analysis. Each observer participated in at least four sessions for each difficulty level of the attention task. Since the performance was in general poorer in the Hard condition of the attention task, more sessions were run for the Hard condition than the Medium or Easy conditions to achieve similar number of correct trials. The three difficulty levels were run in separate sessions. There were eight trials for each ISI in a given session yielding a total of 56 trials per session.
4.2. Results and Discussion
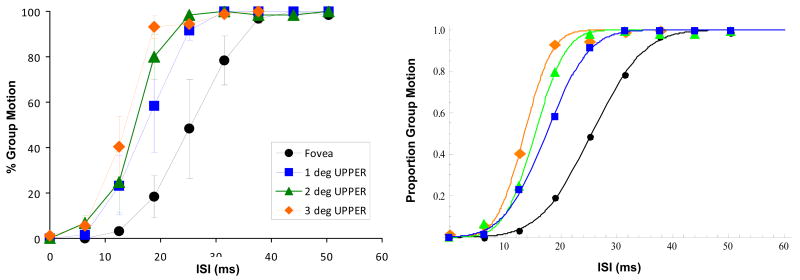
In Fig. 8, the results of the Ternus-Pikler-Only condition are plotted along with the two upper visual field eccentricity conditions of Experiment 1. More GM percepts were reported at 3 deg eccentricity than at 1 or 2 deg eccentricities; however, the effect is not significant, F(2,4) = 1.633, p = 0.303]. GM percepts were weakest at the fovea. Figure 9 shows the PSEs and SDs estimated by fitting cumulative Gaussian functions to individual observers' data. The effect of eccentricity is significant for PSE [F(3,8)=6.706, p=0.014] but not for SD [F(3,8)=1.065, p=0.416].
Figure 8.
Left panel: GM responses at 3 deg eccentricity in the upper visual field of Experiment 2 compared to those at 1 and 2 deg eccentricities in the upper visual field of Experiment 1. The baseline Fovea condition (unrotated version) of Experiment 1 is also shown. Error bars represent ±1 SEM. Right panel: The same data with cumulative Gaussian fits.
Figure 9.
Top panel: The Point of Subjective Equality (PSE) for each observer (estimated by the 50% point of the cumulative Gaussian fitted to the individual observer data) as a function of vertical eccentricity. Bottom panel: The standard deviation values of these Gaussians as a function of vertical eccentricity. Also plotted are the averages ±1 SEM of the three observers' data.
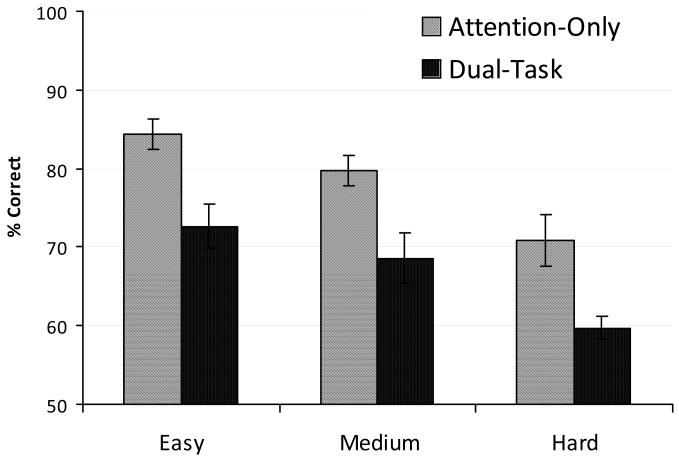
Fig. 10 shows performance for the Attention-Only and the Dual-Task condition. Repeated measures ANOVA revealed a significant effect of the experimental condition [F(1,2) = 372.721, p = 0.003]. Performance decreased in the Dual-Task condition compared to the Attention-Only condition. There was also a significant main effect of the difficulty level [F(2,4) = 25.803, p = 0.005]. Performance was highest for the Easy condition and lowest for the Hard condition. Finally, there was no significant interaction between the experimental condition and the difficulty level [F(2,4) = 0.039, p = 0.962] suggesting that the performance drop in the Dual-Task condition with respect to the Attention- Only condition was similar for all three difficulty levels. This result suggests that the Ternus-Pikler display draws approximately the same amount of attentional resources under the three levels of difficulty of the primary task. Given the similar amount of attentional resources, we expect grouping data to be similar under these three levels of difficulty.
Figure 10.
Performance in the Attention-Only and divided attention (Dual-Task) condition for the three levels of difficulty. Error bars represent ±1 SEM.
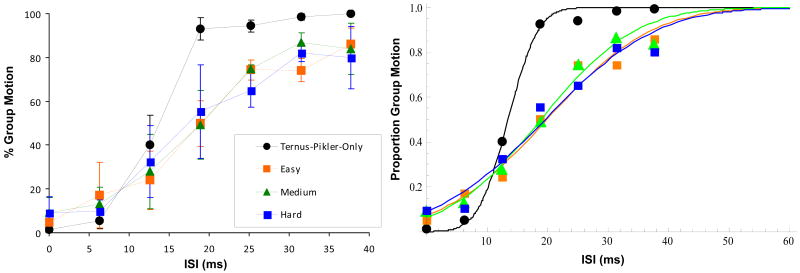
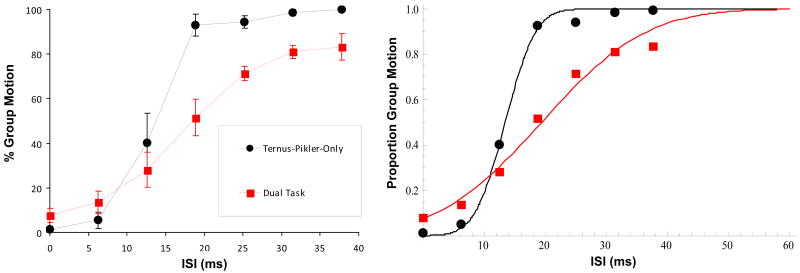
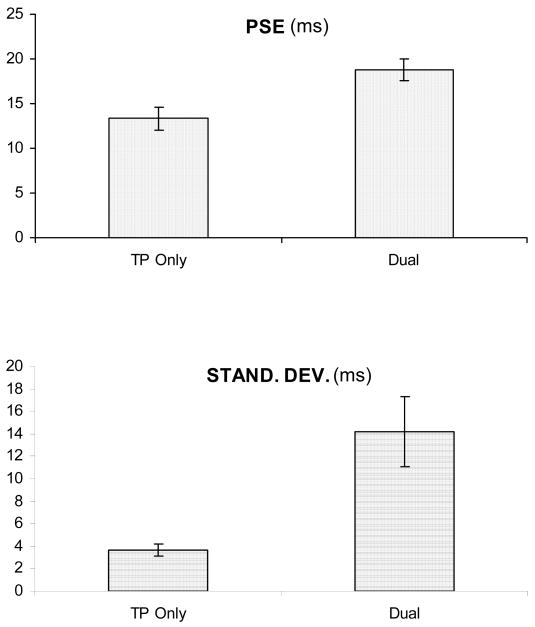
Fig. 11 shows the performance in the Ternus-Pikler task for the Ternus-Pikler-Only condition and the Dual-Task condition averaged over three observers. The main effect of the ISI was significant [F(6,12) = 20.428, p < 0.001] such that GM responses increased with the ISI. The experimental condition was also significant [F(3,6) = 5.615, p = 0.035]. Additionally, post-hoc pairwise comparisons with Bonferroni adjustment revealed no significant difference between the three difficulty levels. In agreement with this, the PSE and SD values computed from individual observers data were not significantly different across the three difficulty levels [F(2,6) = 0.054, p = 0.948 and F(2,6) = 0.008, p = 0.992, respectively]. This confirms the expectation based on similar amount of attentional resources allocated to the Ternus-Pikler task at all three difficulty levels (see Fig. 10). For the rest of the analysis, we combined the Ternus-Pikler Dual-Task data from the three levels of difficulty. The Ternus-Pikler-Only condition was compared to the average of the three difficulty levels (Fig. 12) in the ISI range of 13-38 ms. The result of this analysis showed that fewer GM responses were reported in the divided attention conditions than in the Ternus-Pikler-Only condition [F(1,2) = 53.976, p = 0.018]. Hence, our results show that GM responses decrease in divided attention conditions. Comparison of PSE values (Fig. 13 top panel) between Ternus-Pikler-Only and Dual-Task conditions indicate a significant difference [t(10)=2.43, p=0.035]. The data also suggest a difference in SD values (Fig 12 right panel, Fig. 13, lower panel); however, the difference was not statistically significant [t(10)=1.91, p=0.085].
Figure 11.
Left panel. The percentage of GM responses in the Ternus-Pikler display under full attention (Ternus-Pikler-Only) and divided attention (Easy, Medium, Hard) conditions. Error bars represent ±1 SEM. Right panel. The same data with cumulative Gaussian fits.
Figure 12.
Same as Fig. 11 but the results for the three difficulty levels are averaged.
Figure 13.
PSE (top) and SD (bottom) values averaged across the three observers for Ternus-Pikler-Only and Dual-Task conditions.
An alternative analysis of this data would be to consider the fact that, with the withdrawal of attention, the probability of GM over EM percepts may be limited by an upper-bound, i.e. GM may not reach 100% even for large ISIs. Indeed data hints at such a possibility (Fig. 12). To assess this possibility, we fitted individual observer's data with a cumulative
Gaussian whose gain could vary to produce different upper saturation values. According to this analysis, none of the parameters in the Dual-Task condition were significantly different from the Ternus-Pikler-Only condition (t(10)=0.379, p=0.712; t(10)=1.491, p=0.166; t(10)=2.054, p=0.066, for PSE, SD, and gain parameters, respectively). Given that the prior analysis produced significantly different number of GM responses and a significant shift in the PSE value, we suggest that the effect of attention is to shift the balance of competition to higher PSE values thereby producing fewer GM responses with the range of ISIs used in this study.
5. General Discussion
Dynamic stimuli are ubiquitous in natural viewing conditions implying that grouping operations need to operate, not only in space, but also jointly in space and time. Moreover, in natural viewing, attention plays an important role in controlling how resources are allocated. The goal of our study was to investigate how attention interacts with spatiotemporal perceptual grouping by using a bistable stimulus. Ternus-Pikler displays can give rise to EM or GM percepts, the former dominating at short ISIs and the latter at long ISIs. Our results indicate that GM grouping requires more attentional resources than EM grouping. In the following, we discuss theoretical implications of this finding.
According to one theory of spatiotemporal grouping in Ternus-Pikler displays, the EM and GM percepts are related to the parvo- and magno-cellular systems, respectively. This theory was initially cast in terms of the psychophysically defined transient and sustained systems (Breitmeyer & Ritter, 1986b; Petersik & Pantle, 1979). Breitmeyer and Ritter (1986a, 1986b) proposed that the shift from EM to GM percept is related to visible persistence. If the ISI is short, the two central elements of the Ternus-Pikler display are perceived stationary because of visible persistence. Hence, no GM but EM perception prevails. Insofar as stronger persistence relies on strong sustained channel responses (Breitmeyer, 1980; Breitmeyer & Öğmen, 2006), one would expect more EM responses when the sustained channels are more active. Conversely, one would expect GM responses to increase as transient activity becomes increasingly dominant. However, our results do not support this account. It was shown that attention increases the duration of visible persistence (Visser & Enns, 2001; Yeshurun, et al., 2002). Hence, it is expected that when attention is withdrawn from the Ternus-Pikler display via a dual-task procedure, the visible persistence of the elements should decrease. Shorter visible persistence predicts more GM responses. To the contrary, we found fewer GM responses when attention was withdrawn from the Ternus-Pikler display.
Later, the same theory was recast in terms of anatomically defined magnocellular and parvocellular systems mediating GM and EM percepts, respectively (Cestnick & Coltheart, 1999). This might have been partly stimulated by the observation that dyslexic readers, who are assumed to have an impairment in the magnocellular system (Livingstone et al., 1991; Lovegrove, 1996; Stein & Walsh, 1997), report fewer GM responses than normal readers (Cestnick & Coltheart, 1999; Davis, et al., 2001). However, our results do not support this account either. It was shown that spatial attention favors parvocellular over magnocellular functioning (Yeshurun, 2004; Yeshurun & Carrasco, 1999; Yeshurun & Levy, 2003). More specifically, Yeshurun and colleagues showed that spatial attention improves spatial resolution, which is thought to be mediated by the parvocellular system, and impairs temporal resolution, which is thought to be mediated by the magnocellular system. Based on these results, it is expected that, when attention is withdrawn from the Ternus-Pikler task, the dominance of the parvocellular functioning over the magnocellular one should decrease which, in turn, should increase GM responses. To the contrary, we found fewer GM responses when attention was withdrawn from the Ternus-Pikler display.
Another theory attributed EM and GM percepts to a hypothetical dichotomy in the human visual motion system: a short-range motion process mediating EM percepts and a long-range motion process mediating GM percepts (Braddick, 1980; Braddick & Adlard, 1978; Grossberg & Rudd, 1992; Pantle & Picciano, 1976; Petersik, 1989; Petersik & Pantle, 1979). This distinction was inspired by the observation that the stimulus parameters that characterize EM and GM percepts are also the characteristics of the short- and long-range motion processes, respectively (reviews: Anstis, 1980; Braddick, 1980; Petersik, 1989). It was also suggested that short-range motion is a pre-attentive process whereas the long-range motion is attentive (Dick et al., 1987; Ivry & Cohen, 1990; Nakayama & Silverman, 1986). For example, Ivry and Cohen (1990) used a visual search paradigm to investigate apparent motion perception. They asked observers to detect a horizontally moving target in a background of vertically moving distractor objects. The movement conditions of the target and distractor objects were manipulated to fall within the criteria for either short- or long-range motion processes. They found that when observers were detecting short-range motion, the reaction time (RT) curve for target-present trials was flat as the display size increased. Flat RT curves are assumed to be an indicator of a pre-attentive process (Pashler, 1998). On the other hand, they also found that the RT curve for detecting the long-range motion increased with the display size, a characteristic of an attention demanding process. Hence, according to the short- vs. long-range motion dichotomy, it is expected that when attention is withdrawn from the Ternus-Pikler task, the reports of GM perception, which is thought to be mediated by the attention demanding long-range motion process, should decrease. Our results support this prediction. However, it should be noted that although this dichotomy can explain the specific effect of attention on EM vs. GM responses, the validity of this theory has been called into question by other lines of research (Cavanagh, 1991; Cavanagh & Mather, 1989; Dodd et al., 2005; Odic & Pratt, 2008; Patterson et al., 1991; Scott-Samuel & Hess, 2001).
Recently, the bistable nature of the Ternus-Pikler display has been discussed in a more descriptive framework wherein two grouping processes compete against each other to drive the resulting percept: temporal grouping mediating EM percepts and spatial grouping mediating GM percepts (Alais & Lorenceau, 2002; Gepshtein & Kubovy, 2000; He & Ooi, 1999; Kramer & Rudd, 1999; Kramer & Yantis, 1997; Wallace & Scott-Samuel, 2007). According to this spatiotemporal grouping hypothesis, EM and GM percepts depend on how the elements are grouped in space (i.e., within-frame grouping) and in time (i.e., across-frame grouping). For example, He and Ooi (1999) investigated the effects of various stimulus factors on the percentage of GM responses, such as form similarity, 3-D proximity, depth, common surface, occlusion, and priming, all of which can affect the perceptual organization of a given display. They found that the stimulus factors which favor the spatial or within-frame grouping of the elements yield more GM responses and vice versa. A priori, it is not clear whether attention would favor spatial or temporal grouping and thus these theories do not make any prediction about our data.
Within the context of grouping theories, as we mentioned in the Introduction, Krech's and Köhler's hypotheses predict that attention will favor the more detailed and articulated groupings. Among the various correspondence matches, it may be reasonable to assume that GM represents a more homogeneous and simple configuration, because a single group of dots undergoes common motion. Accordingly, these theories predict fewer EM reports when the attentional load increases by a dual-task. Our results do not support this prediction. However, instead of assessing complexity of groupings based on phenomenal appearance of stimuli, one can also consider the constraints of the visual system. It is well known that early visual system has a retinotopic organization. The logic of the Ternus-Pikler display is to pit retinotopic matches against more global grouping matches. The two spatially overlapping elements in the two frames are presented at the same retinotopic location. By virtue of the retinotopic organization in the early visual system, one may expect these elements to be grouped together yielding the EM percept. To generate GM, the visual system needs to “remap” the elements according to their global configuration so as to pair overlapping elements not with their retinotopic matches but with their figural matches (e.g., central element in the group of three dots) (Öğmen et al., 2006). From this perspective, EM may be viewed as the simpler configuration depending mainly on retinotopy which is hard wired in the early visual system. The prediction of this hypothesis would be fewer GM responses in dual-task condition, which is in agreement with our findings.
Ternus-Pikler display has been used as a probe to understand how features are non-retinotopically attributed over space and time for moving objects (Öğmen et al., 2006). Results suggest that the attribution of features from one frame to another follows motion-induced grouping (see also Otto et al., 2006, 2008). More specifically, the results show that a Vernier offset present in the first frame of the Ternus-Pikler display can be attributed to a spatially non-overlapping element in the second frame only if these two elements are perceptually grouped via motion. Recently, this finding has been generalized to show that spatiotemporal grouping in Ternus-Pikler stimuli can serve as a reference frame, not only for form perception, but also for motion perception and visual search (Boi et al., 2009). Here, we showed that group motion percept suffers under divided attention conditions. This result predicts that, under divided attention conditions, the feature attribution or non-retinotopic computation in the Ternus-Pikler display should also become weaker, because it is the GM percept which establishes non-retinotopic feature attribution. In agreement with the original observations of Gestalt psychologists, we suggest that perceptual organization can be modulated by attention, particularly in the case of weak configurations as found in ambiguous bistable stimuli. However this influence is in general limited because the “force” provided by attention may not be sufficient to overcome the strength of perceptual organization. Indeed, this is what we found when we studied feature attribution and integration in unambiguous motion streams where attention failed to modulate feature attribution and integration (Otto et al., in press). Taken together, these observations support the view that grouping and attention are parallel and interacting processes. Attention can have a significant effect on weak (i.e., ambiguous, bistable, etc.) groupings however no effect on “strong” groupings. From this perspective, grouping may be considered “pre-attentive” for the latter but not for the former cases.
Acknowledgments
This work was supported in part by award number R01 EY018165 from NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Footnotes
In pilot studies, circular and single-pixel line elements were also used. The results were not different.
It should be noted that significantly more GM percepts were reported in the Fovea rotated condition than in the Fovea unrotated condition [F(1,2) = 42.857, p = 0.023].
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alais D, Lorenceau J. Perceptual grouping in the Ternus display: Evidence for an ‘association field’ in apparent motion. Vision Research. 2002;42:1005–1016. doi: 10.1016/s0042-6989(02)00021-4. [DOI] [PubMed] [Google Scholar]
- Ansorge U, Klotz W, Neumann O. Manual and verbal responses to completely masked (unreportable) stimuli: Exploring some conditions for the metacontrast dissociation. Perception. 1998;27:1177–1189. doi: 10.1068/p271177. [DOI] [PubMed] [Google Scholar]
- Anstis SM. The perception of apparent movement. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 1980;290:153–168. doi: 10.1098/rstb.1980.0088. [DOI] [PubMed] [Google Scholar]
- Beck J. Slant and shape variables in perceptual grouping. Science. 1966a;154:538–540. doi: 10.1126/science.154.3748.538. [DOI] [PubMed] [Google Scholar]
- Beck J. Effect of orientation and of shape similarity on perceptual grouping. Perception & Psychophysics. 1966b;1:300–302. [Google Scholar]
- Beck J. Perceptual grouping produced by line figures. Perception & Psychophysics. 1967;2:491–495. [Google Scholar]
- Beck J. Similarity grouping and peripheral discriminability under uncertainty. American Journal of Psychology. 1972;85:1–19. [PubMed] [Google Scholar]
- Ben-Av MB, Sagi D, Braun J. Visual attention and perceptual grouping. Perception & Psychophysics. 1992;52:277–294. doi: 10.3758/bf03209145. [DOI] [PubMed] [Google Scholar]
- Boi M, Öğmen H, Krummenacher J, Otto TU, Herzog MH. A (fascinating) litmus test for human retino- vs. non-retinotopic processing. Journal of Vision. 2009;9(13):5, 1–11. doi: 10.1167/9.13.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddick OJ. Low-level and high-level processes in apparent motion. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 1980;290:137–151. doi: 10.1098/rstb.1980.0087. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, Adlard A. Apparent motion and the motion detector. In: Armington J, Krauskopf J, Wooten BR, editors. Visual Psychophysics and Physiology. New York: Academic Press; 1978. pp. 417–426. [Google Scholar]
- Breitmeyer BG. Unmasking visual masking: A look at the “why” behind the veil of the “how”. Psychological Review. 1980;87:52–69. [PubMed] [Google Scholar]
- Breitmeyer BG, May JG, Williams MC. Spatial frequency and contrast effects on percepts of bistable stroboscopic motion. Perception & Psychophysics. 1988;44:525–531. doi: 10.3758/bf03207486. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG, Öğmen H. Visual masking: Time slices through conscious and unconscious vision. 2nd. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- Breitmeyer BG, Öğmen H, Chen J. Unconscious priming by color and form: Different processes and levels. Consciousness and Cognition. 2004;13:138–157. doi: 10.1016/j.concog.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG, Öğmen H, Ramon J, Chen J. Unconscious priming by form and their parts. Visual Cognition. 2005;12:720–736. [Google Scholar]
- Breitmeyer BG, Ritter A. The role of visual pattern persistence in bistable stroboscopic motion. Vision Research. 1986a;26:1801–1806. doi: 10.1016/0042-6989(86)90131-8. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG, Ritter A. Visual persistence and the effect of eccentric viewing, element size, and frame duration on bistable stroboscopic motion percepts. Perception & Psychophysics. 1986b;39:275–280. doi: 10.3758/bf03204935. [DOI] [PubMed] [Google Scholar]
- Caelli T, Julesz B. Psychophysical evidence for global feature processing in visual texture discrimination. J Opt Soc Am. 1979;69:675–678. doi: 10.1364/josa.69.000675. [DOI] [PubMed] [Google Scholar]
- Cameron EL. Perceptual inhomogeneities in the upper visual field. Journal of Vision. 2005;5(8):176, 176a. doi: 10.1167/5.8.176. Abstract. http://journalofvision.org/5/8/176/ [DOI]
- Carrasco M, Talgar CP, Cameron EL. Characterizing visual performance fields: Effects of transient cover attention, spatial frequency, eccentricity, task and set size. Spatial Vision. 2001;15:61–75. doi: 10.1163/15685680152692015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casco C. The relationship between visual persistence and event perception in bistable motion display. Perception. 1990;19:437–445. doi: 10.1068/p190437. [DOI] [PubMed] [Google Scholar]
- Casco C, Spinelli D. Left-right visual field asymmetry in bistable motion perception. Perception. 1988;17:721–727. doi: 10.1068/p170721. [DOI] [PubMed] [Google Scholar]
- Cavanagh P. Short-range vs. long-range motion: Not a valid distinction. Spatial Vision. 1991;5:303–309. doi: 10.1163/156856891x00065. [DOI] [PubMed] [Google Scholar]
- Cavanagh P, Mather G. Motion: The long and short of it. Spatial Vision. 1989;4:103–129. doi: 10.1163/156856889x00077. [DOI] [PubMed] [Google Scholar]
- Cestnick L, Coltheart M. The relationship between language-processing and visual-processing deficits in developmental dyslexia. Cognition. 1999;71:231–255. doi: 10.1016/s0010-0277(99)00023-2. [DOI] [PubMed] [Google Scholar]
- Chan WY, Chua FK. Grouping with and without attention. Psychonomic Bulletin & Review. 2003;10:932–938. doi: 10.3758/bf03196554. [DOI] [PubMed] [Google Scholar]
- Danckert JA, Goodale MA. Ups and downs in the visual control of action. In: Johnson SH, editor. From intentions to movement: A cognitive neuroscience approach to the problem of realizing actions. Cambridge, MA: MIT Press; 2003. pp. 29–64. [Google Scholar]
- Davis C, Castles A, McAnally K, Gray J. Lapses of concentration and dyslexic performance on the Ternus task. Cognition. 2001;81:B21–B31. doi: 10.1016/s0010-0277(01)00129-9. [DOI] [PubMed] [Google Scholar]
- Dawson MRW, Nevin-Meadows N, Wright RD. Polarity matching in the Ternus configuration. Vision Research. 1994;34:3347–3359. doi: 10.1016/0042-6989(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Hogben JH. Suppression of visible persistence. Journal of Experimental Psychology: Human Perception and Performance. 1985;11:304–316. doi: 10.1037/0096-1523.11.3.304. [DOI] [PubMed] [Google Scholar]
- Dick M, Ullman S, Sagi D. Parallel and serial processes in motion detection. Science. 1987;237:400–402. doi: 10.1126/science.3603025. [DOI] [PubMed] [Google Scholar]
- Dodd MD, McAuley T, Pratt J. An illusion of 3-D motion with the Ternus display. Vision Research. 2005;45:969–973. doi: 10.1016/j.visres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Gepshtein S, Kubovy M. The emergence of visual objects in space-time. Proceedings of the Natural Academy of Science USA. 2000;97:8186–8191. doi: 10.1073/pnas.97.14.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschaldt K. Über den Einfluss der Erfahrung auf die Wahrnehmung von Figuren. I. Psychologische Forschung. 1926;8:261–317. [Google Scholar]
- Gottschaldt K. Über den Einfluss der Erfahrung auf die Wahrnehmung von Figuren. II. Psychologische Forschung. 1929;12:1–87. [Google Scholar]
- Grossberg S, Rudd ME. Cortical dynamics of visual motion perception: Short-range and long-range apparent motion. Psychological Review. 1992;99:78–121. doi: 10.1037/0033-295x.99.1.78. [DOI] [PubMed] [Google Scholar]
- He ZJ, Ooi TL. Perceptual organization of apparent motion in the Ternus display. Perception. 1999;28:877–892. doi: 10.1068/p2941. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Cohen A. Dissociation of short- and long-range apparent motion in visual search. Journal of Experimental Psychology: Human Perception and Performance. 1990;16:317–331. doi: 10.1037//0096-1523.16.2.317. [DOI] [PubMed] [Google Scholar]
- Julesz B. Early vision and focal attention. Reviews of Modern Physics. 1991;63:735–767. [Google Scholar]
- Kimchi R, Razpurker-Apfeld I. Perceptual grouping and attention: Not all groupings are equal. Psychonomic Bulletin & Review. 2004;11:687–696. doi: 10.3758/bf03196621. [DOI] [PubMed] [Google Scholar]
- Klotz W, Wolff P. The effect of a masked stimulus on the response to the masking stimulus. Psychological Research. 1995;58:92–101. doi: 10.1007/BF00571098. [DOI] [PubMed] [Google Scholar]
- Koffka K. Perception: An introduction to the Gestalt-theorie. Psychological Bulletin. 1922;19:531–585. [Google Scholar]
- Koffka K. Principles of Gestalt Psychology. New York: Harcourt Brace, & World; 1935. [Google Scholar]
- Köhler W, Adams PA. Perception and attention. American Journal of Psychology. 1958;71:489–503. [PubMed] [Google Scholar]
- Kramer P, Rudd M. Visible persistence and form correspondence in Ternus apparent motion. Perception & Psychophysics. 1999;61:952–962. doi: 10.3758/bf03206909. [DOI] [PubMed] [Google Scholar]
- Kramer P, Yantis S. Perceptual grouping in space and time: Evidence from the Ternus display. Perception & Psychophysics. 1997;59:87–99. doi: 10.3758/bf03206851. [DOI] [PubMed] [Google Scholar]
- Krech D, Calvin A. Levels of perceptual organization and cognition. Journal of Abnormal and Social Psychology. 1953;48:394–400. doi: 10.1037/h0061276. [DOI] [PubMed] [Google Scholar]
- Krechevsky I. An experimental investigation of the principle of proximity in the visual perception of the rat. Journal of Experimental Psychology. 1938;22:497–523. [Google Scholar]
- Lamy D, Segal H, Ruderman L. Grouping does not require attention. Perception & Psychophysics. 2006;68:17–31. doi: 10.3758/bf03193652. [DOI] [PubMed] [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology: Human Perception and Performance. 1995;26:1387–1400. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Lavie N, Tsal Y. Perceptual load as a major determinant of the locus of selection in visual attention. Perception & Psychophysics. 1994;56:183–197. doi: 10.3758/bf03213897. [DOI] [PubMed] [Google Scholar]
- Levine MW, McAnany JJ. The relative capabilities of the upper and lower visual hemifields. Vision Research. 2005;45:2820–2830. doi: 10.1016/j.visres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Livingstone M, Drislane F, Rosen G, Galaburda A. Physiological evidence for a magnocellular deficit in developmental dyslexia. Proceedings of the New York Academy of Science. 1991;88:7943–7947. doi: 10.1073/pnas.88.18.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovegrove WJ. Dyslexia and a transient/magnocellular pathway deficit: The current situation and future directions. Australian Journal of Psychology. 1996;48:167–171. [Google Scholar]
- Ma-Wyatt A, Clifford CWG, Wenderoth P. Contrast configuration influences grouping in apparent motion. Perception. 2005;34:669–685. doi: 10.1068/p3444. [DOI] [PubMed] [Google Scholar]
- Mack A, Tang B, Tuma R, Kahn S, Rock I. Perceptual organization and attention. Cognitive Psychology. 1992;24:475–501. doi: 10.1016/0010-0285(92)90016-u. [DOI] [PubMed] [Google Scholar]
- Mezrich JJ. The duration of visual persistence. Vision Research. 1984;24:631–632. doi: 10.1016/0042-6989(84)90118-4. [DOI] [PubMed] [Google Scholar]
- Moore CM, Egeth H. Perception without attention: Evidence of grouping under conditions of inattention. Journal of Experimental Psychology: Human Perception and Performance. 1997;23:339–352. doi: 10.1037//0096-1523.23.2.339. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Silverman GH. Serial and parallel processing of visual feature conjunctions. Nature. 1986;320:264–265. doi: 10.1038/320264a0. [DOI] [PubMed] [Google Scholar]
- Odic D, Pratt J. Solving the correspondence problem within the Ternus display: The differential-activation theory. Perception. 2008;37:1790–1804. doi: 10.1068/p5670. [DOI] [PubMed] [Google Scholar]
- Öğmen H, Otto TU, Herzog MH. Perceptual grouping induces non-retinotopic feature attribution in human vision. Vision Research. 2006;46:3234–3242. doi: 10.1016/j.visres.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Otto TU, Öğmen H, Herzog MH. The flight path of the phoenix—The visible trace of invisible elements in human vision. Journal of Vision. 2006;6(10):1079–1086. doi: 10.1167/6.10.7. 7. http://journalofvision.org/6/10/7/ [DOI] [PubMed]
- Otto TU, Öğmen H, Herzog MH. Assessing the microstructure of motion correspondences with non-retinotopic feature attribution. Journal of Vision. 2008;8(7):1–15. doi: 10.1167/8.7.16. 16. http://journalofvision.org/8/7/16/ [DOI] [PubMed]
- Otto TU, Öğmen H, Herzog MH. Attention and non-Retinotopic feature integration. Journal of Vision. doi: 10.1167/10.12.8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantle AJ, Petersik JT. Effects of spatial parameters on the perceptual organization of a bistable motion display. Perception & Psychophysics. 1980;27:307–312. doi: 10.3758/bf03206119. [DOI] [PubMed] [Google Scholar]
- Pantle A, Picciano L. A multistable movement display: Evidence for two separate motion systems in human vision. Science. 1976;193:500–502. doi: 10.1126/science.941023. [DOI] [PubMed] [Google Scholar]
- Pashler HE. Attention. Hove: Psychology Press; 1998. [Google Scholar]
- Patterson R, Hart P, Nowak D. The cyclopean Ternus display and the perception of element versus group movement. Vision Research. 1991;31:2085–2092. doi: 10.1016/0042-6989(91)90166-3. [DOI] [PubMed] [Google Scholar]
- Petersik JT. The two-process distinction in apparent motion. Psychological Bulletin. 1989;106:107–127. doi: 10.1037/0033-2909.106.1.107. [DOI] [PubMed] [Google Scholar]
- Petersik JT, Grassmuck J. High fundamental spatial frequencies and edges have different perceptual consequences in the ‘group/end-to-end’ movement phenomenon. Perception. 1981;10:375–382. doi: 10.1068/p100375. [DOI] [PubMed] [Google Scholar]
- Petersik JT, Pantle A. Factors controlling the competing sensations produced by a bistable stroboscopic motion display. Vision Research. 1979;19:143–154. doi: 10.1016/0042-6989(79)90044-0. [DOI] [PubMed] [Google Scholar]
- Petersik JT, Rice CM. Spatial correspondence and relation correspondence: Grouping factors that influence perception of the Ternus display. Perception. 2008;37:725–739. doi: 10.1068/p5900. [DOI] [PubMed] [Google Scholar]
- Petersik JT, Schellinger AR, Geiger SL. Do variables that affect similar bistable apparent-movement displays result in similar changes in perception? Spatial Vision. 2003;16:105–123. doi: 10.1163/15685680360511636. [DOI] [PubMed] [Google Scholar]
- Pikler J. Sinnesphysiologische Untersuchungen. Leipzig: Barth; 1917. [Google Scholar]
- Ritter AD, Breitmeyer BG. The effects of dichoptic and binocular viewing on bistable motion percepts. Vision Research. 1989;29:1215–1219. doi: 10.1016/0042-6989(89)90067-9. [DOI] [PubMed] [Google Scholar]
- Rock I, Linnett CM, Grant P, Mack A. Perception without attention: Results of a new method. Cognitive Psychology. 1992;24:502–534. doi: 10.1016/0010-0285(92)90017-v. [DOI] [PubMed] [Google Scholar]
- Russell C, Driver J. New indirect measures of “inattentive” visual grouping in a change-detection task. Perception & Psychophysics. 2005;67:606–623. doi: 10.3758/bf03193518. [DOI] [PubMed] [Google Scholar]
- Rutherford B. Laterality and pattern persistence in bistable motion perception. Brain and Cognition. 2003;53:335–341. doi: 10.1016/s0278-2626(03)00138-6. [DOI] [PubMed] [Google Scholar]
- Scott-Samuel NE, Hess RF. What does the Ternus display tell us about motion processing in human vision? Perception. 2001;30:1179–1188. doi: 10.1068/p3247. [DOI] [PubMed] [Google Scholar]
- Shomstein S, Kimchi R, Hammer M, Behrmann M. Perceptual grouping operates independently of attentional selection: Evidence from hemispatial neglect. Attention, Perception, & Psychophysics. 2010;72:607–618. doi: 10.3758/APP.72.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrandies W. Progress in Sensory Physiology. Vol. 8. Germany: Springer-Verlag; 1987. The upper and lower visual field of man: Electrophysiological and functional differences; pp. 162–189. [Google Scholar]
- Stein J, Walsh V. To see but not to read; the magnocellular theory of dyslexia. Trends in Neurosciences. 1997;20:147–152. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- Ternus J. Experimentelle Untersuchungen über phänomenale Identität. Psychologische Forschung. 1926;7:81–136. [Google Scholar]; Ellis WD, translator and editor. A Sourcebook of Gestalt Psychology. New York: Humanities Press; 1950. [Google Scholar]
- Treisman A. Perceptual grouping and attention in visual search for features and for objects. Journal of Experimental Psychology: Human Perception and Performance. 1982;8:194–214. doi: 10.1037//0096-1523.8.2.194. [DOI] [PubMed] [Google Scholar]
- Treisman A, Gelade G. A feature integration theory of attention. Cognitive Psychology. 1980;12:97–36. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Visser TAW, Enns JT. The role of attention in temporal integration. Perception. 2001;30:135–145. doi: 10.1068/p3089. [DOI] [PubMed] [Google Scholar]
- Vorberg D, Mattler U, Heinecke A, Schmidt T, Schwarzbach J. Different time courses for visual perception and action priming. Proceedings of the Natural Academy of Science USA. 2003;100:6275–6280. doi: 10.1073/pnas.0931489100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JM, Scott-Samuel NE. Spatial versus temporal grouping in a modified Ternus display. Vision Research. 2007;47:2353–2366. doi: 10.1016/j.visres.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y. Isoluminant stimuli and red background attenuate the effects of transient spatial attention on temporal resolution. Vision Research. 2004;44:1375–1387. doi: 10.1016/j.visres.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y, Carrasco M. Spatial attention improves performance in spatial resolution tasks. Vision Research. 1999;39:293–306. doi: 10.1016/s0042-6989(98)00114-x. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y, Levy L. Transient spatial attention degrades temporal resolution. Psychological Science. 2003;14:225–231. doi: 10.1111/1467-9280.02436. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y, Levy L, Marom G. Spatial attention and visual temporal processes. Journal of Vision. 2002;2(7):591, 591a. doi: 10.1167/2.7.591. Abstract. http://journalofvision.org/2/7/591/ [DOI]