Abstract
Context: Inactivating mutations of the calcium-sensing receptor (CaSR) cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Most mutations are clustered in the N-terminal and Cys-rich regions of the extracellular domain (ECD) and seven-transmembrane domain. Disease-causing mutations are uncommon in the C terminus of ECD.
Objective: The aim of the study was to characterize the CaSR mutations causing neonatal severe hyperparathyroidism in a consanguineous family.
Methods: Parathyroid glands from the index patient were stained for CaSR protein. The CaSR gene was sequenced, mutations were recreated in CaSR cDNA, and HEK293 cells were transfected with the CaSR mutant expression vector. Cellular CaSR targeting was detected by immunoblotting and immunocytochemistry; CaSR activity was assayed by inositol phosphate accumulation, MAPK activation, and single-cell microfluorimetry.
Results: Immunocytochemistry showed reduced intracellular CaSR in patient parathyroids. An in-frame homozygous deletion/insertion mutation, c.1031 > 1034 (delACAAinsT), replaced His344-Asn345 with a single Leu in CaSR loop III. The mutant reduced cell surface expression of CaSR in transfected HEK293 cells. Inositol phosphate accumulation, MAPK activation, and single-cell microfluorimetry revealed blunted signaling responses of the mutant receptor to changes in extracellular Ca2+ concentration.
Conclusion: Deletion of His344-Asn345 in the ECD loop III region affects cell surface targeting of CaSR in transfected cells and in affected parathyroid glands. Absence of conserved Asn345 may interfere with CaSR folding or glycosylation, leading to poor protein targeting to the cell membrane. This loss-of-function mutant indicates that the ECD loop III is required for CaSR activity.
A novel loss-of-function mutation from hypercalcemic patients establishes the significance of the extracellular domain Loop III region in the calcium-sensing receptor.
The G protein-coupled calcium-sensing receptor (CaSR) detects minute changes in serum calcium required for extracellular calcium homeostasis by modulating calcium reabsorption in the kidney and the secretion of PTH (1,2,3). The CaSR, along with metabotropic glutamate receptors (4), sweet and “umami” taste receptors (5,6), form Family C of the G protein-coupled receptor (GPCR) superfamily, having one of the largest known extracellular domains (ECDs) [>600 amino acids (a.a.)] among all GPCRs. Based on similarities to the sequences of the N-terminal ECD of Family C GPCRs and the family of bacterial periplasmic amino acid binding proteins, CaSR is modeled as a bilobed “venus-flytrap,” shifting between open and closed states; binding Ca2+ stabilizes the closed conformation (7,8,9). However, four segments in the human CaSR ECD and rat metabotropic glutamate receptor-1 do not align with those bacterial proteins, forming protruding loops (I-IV) within lobe I. The function of these loops in human CaSR was assessed by expressing a series of deletion mutants of these four loops in vitro and analyzing their ability to form fully processed CaSR and response to extracellular calcium stimuli (9). As expected, deletion of portions of either loop I (a.a. 50–59) or loop IV (a.a. 438–445) did not reduce expression on the cell surface but significantly reduced calcium activation. Deletion of the entire loop II (a.a. 117–137) abolished receptor expression and function. Up to 21 residues (a.a. 365–385) of loop III (a.a. 337–415) could be deleted without impairing receptor expression or activation (9,10).
Since the initial cloning of bovine (1) and human (11) CASR, about 250 human mutations have been identified (12,13). CaSR mutations cause three distinct diseases: heterozygous inactivating mutations cause recessive familial hypocalciuric hypercalcemia (FHH), homozygous inactivating mutations cause neonatal severe hyperparathyroidism (NSHPT), and activating mutations cause autosomal dominant hypocalcemia (14,15,16). The correlations between genotype and phenotype of CaSR mutations have helped to elucidate the structure and function of the human CaSR and the GPCR superfamily (17).
Most disease-causing mutations occur in the first 300 amino acids, in an 84-a.a. cysteine-rich region (a.a. 528–606) next to the venus-flytrap, and in the seven-transmembrane domains (a.a. 607–851) (12). About 90% are missense mutations; only four deletion mutations, six insertion mutations, and one deletion/insertion mutation have been found (http://www.casrdb.mcgill.ca). Based on the lack of disease-causing mutations in the C-terminal region of the ECD (a.a. 300–520) (13) and the fact that a large portion of loop III (a.a. 365–385) could be deleted without impairing receptor function (9), it has been speculated that loop III is functionally unimportant (13,17). Although two missense mutations (Cys395Arg and Gly397Arg) and one nonsense mutation (Trp352X) associated with hypercalcemia have been found in this region (16,18), these mutations have not been studied functionally to establish the activity of the mutant CaSR or the correlation between the mutation and the clinical phenotype. Thus, the functional importance of the loop III region remains uncertain.
We identified the novel in-frame insertion/deletion mutation (His344-Asn345>Leu) in loop III of the ECD in a kindred with multiple family members suffering from NSHPT and FHH. This mutation dramatically reduced CaSR activity as assessed by three different endpoints in its signaling pathway. Loss of CaSR signaling through the mutant protein correlates with the decreased targeting of mutant CaSR to the cell surface. The lack of cell surface expression of the mutant in vitro matches the intracellular retention of the CaSR revealed in the parathyroid glands of the index patient with NSHPT. This novel mutant establishes the functional importance of the loop III region of ECD in CaSR, furthering our understanding of the structure and function of Family C GPCRs.
Patients and Methods
Patients
A 17-month-old female was evaluated in 1980 for severe hypotonia, microcephaly, developmental delay, and severe hypercalcemia. Total serum Ca ranged between 4.4 and 5.1 mmol/liter (normal, 2.2–2.7), and her ionized serum Ca2+ was 1.90–2.25 mmol/liter (normal, 1.0–1.31). Serum phosphate was 1.3–2.3 mmol/liter (normal, 1.0–1.5); magnesium, 0.78–1.1 mmol/liter (normal, 0.8–1.3); serum PTH was not documented before surgical removal of the parathyroids. Total parathyroidectomy together with vitamin D and calcium supplements normalized her serum Ca2+, but she remained severely mentally disabled. When the sister of the index patient was born 10 yr later, her total serum Ca was 3.3 mmol/liter at birth and reached 4.7 mmol/liter within 3 d. Serum phosphate was 1.5–2.0 mmol/liter; magnesium, 0.7–1.2 mmol/liter; and ionized serum Ca2+, 2.03–2.47 mmol/liter. Immunoassays for serum intact PTH ranged from 934 to >2000 pg/ml (normal, 11–54), and she underwent total parathyroidectomy on d 6 of life. She has subsequently done well and has had normal mental development. A younger brother, currently age 14, developed normally. His total serum Ca was 2.9–3.0 mmol/liter; phosphate, 2.0–2.1 mmol/liter; and urine calcium, less than 0.05 mmol/liter. The consanguineous parents, their paternal uncles, and their paternal grandfather all have hypercalcemia, suggesting FHH. The family pedigree was drawn by collecting historical information and by obtaining total serum Ca levels from individual members (Fig. 1A). The parathyroidectomies were performed based on the clinical indications. All patient studies were performed according to a protocol approved by the University of California, San Francisco (UCSF), Committee on Human Research.
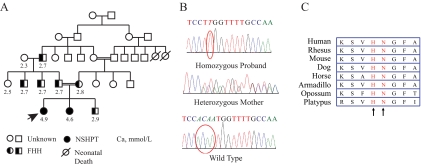
Figure 1.
Genetics of CaSR. A, Pedigree of the studied family. Representative total serum calcium values (mmol/liter) are shown below the family member’s symbol. DNA was available for study only in the index patient (indicated by arrow), her sister, and their mother. B, CaSR sequencing from the affected individuals. The proband is homozygous for a four-nucleotide deletion with a thymidine insertion (circled); the mother is heterozygous. A normal control is shown with nucleotides 1031–1034 circled. C, Amino acid alignment of the loop III region of CaSR from eight mammals, showing conservation of His344 and Asn345.
Preparation of genomic DNA and sequencing of CASR
With informed consent, we obtained blood from the index patient, her sister, and their mother. DNA was extracted using a QiaAmp kit (QIAGEN, Valencia, CA). DNA was sequenced using ABI BigDye v3.1 dye terminator sequencing chemistry and an ABI PRISM 3730xl capillary DNA analyzer (Applied Biosystems, Foster City, CA). Fourteen pairs of oligonucleotides (Supplemental Table 1, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org) were used to cover all seven exons and 27–240 base pairs of their intron-exon junctions.
Constructs and site-directed mutagenesis of the human CASR
The human CASR cDNA construct in pCR3.1 was described previously (19). The His344-Asn345>Leu mutant was made with the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) using the complimentary 33-base primers (forward, 5′-CCAGGAAGTCTGTCCTTGGTTTTGCCAAGGAGT-3′; and reverse primer, 5′-ACTCCTTGGCAAAACCAAGGACAGACTTCCTGG-3′). The accuracy of the mutagenesis was confirmed by direct DNA sequencing. The constructs were confirmed by DNA sequencing before plasmids were used for transfection.
Cell culture and transfection
Human embryonic kidney (HEK293) cells were cultured in DMEM containing 10% heat-inactivated fetal bovine serum, 100 μg/ml streptomycin, and 100 U/ml penicillin at 37 C in 5% CO2. Cells were grown to about 80% confluence before transfection. cDNA constructs were transfected using FuGENE 6 (Roche Applied Science, Indianapolis, IN) with DNA to transfectant ratio of 1 μg per 1 μl. Cells were used for experimentation 36–48 h after transfection.
Immunoprecipitation and Western blotting of CaSR
Confluent 100-mm plates of HEK293 cells transfected with pCR3.1 expressing wild-type (WT) or mutant CaSR or empty vector were washed with ice-cold PBS, scraped, and centrifuged at 5000 × g for 10 min. Cell pellets were resuspended in 0.4 ml of lysis buffer (50 mm Tris-HCl, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, with protease inhibitor cocktail from Roche) and shaken gently at 4 C for 30 min, and particulate matter was removed by centrifugation at 10,000 × g for 15 min. Protein concentrations were determined using a BCA assay (Bio-Rad Laboratories, Hercules, CA). Cell lysates were prepared in Laemmli sample buffer (Bio-Rad Laboratories) with 100 μm dithiothreitol added before loading onto 7.5% SDS-PAGE. After gel electrophoresis, the proteins were transferred to nylon membrane (0.2 μm), probed with anti-CaSR monoclonal antibody ADD (raised against a synthetic peptide corresponding to residues 214–235 of human CaSR) (19,20) in 3% milk/PBS at 4 C overnight, and subsequently probed with horseradish peroxidase-conjugated secondary antibody. Blots were washed with PBS and developed by ECL Plus (GE Health Science) reagents, and the signals were captured on x-ray film.
Biotinylation of cell surface proteins
Transfected HEK293 cells were washed with ice-cold PBS three times and incubated with EZ-Link sulfo-NHS-LC-Biotin (sulfosuccinimidyl-6-[biotin-amido] hexanoate; Pierce, Rockford, IL) per the manufacturer’s protocol. This water-soluble, membrane-impermeable biotin reagent (1 mg/ml) was added to the cells at 4 C for 30 min with gentle shaking; unbound biotin and byproducts were quenched and removed by three washes with 100 mm glycine in PBS for 30 min and twice in PBS for 10 min each. Cells were lysed as described above, and biotinylated proteins were precipitated with NeutrAvidin agarose resins (Thermo Scientific, Boston, MA) (200 μl of protein in 200 μl of binding buffer). The biotinylated proteins were subsequently eluted, electrophoresed, and immunoblotted with anti-CaSR ADD antibody.
Immunostaining and confocal microscopy
Transfected HEK293 cells grown on coverslips were fixed with 4% paraformaldehyde for 20 min and permeabilized with 80% methanol. After overnight incubation at 4 C with rabbit antibovine CaSR (500 nm), cells were washed and incubated with fluorescein and Texas Red-conjugated anti-IgG antibodies for 60 min at room temperature (21). Fluorescent images were obtained by using Gel Mount and a Leica TCS confocal microscope (22).
MAPK pathway activation
Transfected HEK293 cells seeded in 12-well plates were serum-starved for 8 h to inactivate ERK1/2 before the cells were challenged with various concentrations of Ca2+ for 5 min. Cells were immediately placed on ice and lysed. Activation of ERK1/2 was probed with anti-phospho-p44/42 MAPK (catalog no. 9101; Cell Signaling Technology, Beverly, MA), and anti-total ERK (sc-1647; Santa Cruz Biotechnology, Santa Cruz, CA) as primary antibodies.
Immunohistochemistry of parathyroid tissue
Fixed paraffin-embedded parathyroid tissue obtained from the index patient at surgery was retrieved from the archives of the UCSF Department of Pathology and compared with parathyroid tissues obtained at autopsy from subjects with normal calcium metabolism. The paraffin blocks were cut in 5-μm sections, deparaffinized, and rehydrated by standard methods. Adjacent sections were stained with hematoxylin and eosin. For immunolocalization, the hydrated sections on slides were washed in PBS, incubated in 3% hydrogen peroxide in water for 15 min, and washed twice. After antigen retrieval in citrate buffer, the sections were blocked with serum buffer in 0.3% Triton X-100 for 1–2 h and drained. The sections then were incubated in polyclonal rabbit antihuman CaSR antibody (LifeSpan Biosciences, Seattle, WA) at 1:250 overnight at 4 C. The sections were washed in PBS/Triton X-100/serum three times for 10 min each, then incubated in biotinylated goat antirabbit secondary antibody (Vector Labs, Burlingame, CA) for 1 h, and washed three times in 50 mm PBS for 5 min each. Each section was incubated in avidin-biotin complexes and developed with the peroxidase reaction using diaminobenzidine as chromogen. After rinsing in water, the sections were counterstained, dehydrated, and coverslipped. CaSR staining was captured using AxioVision 3.1 software with a Zeiss camera at high power (×400).
[3H]Polyphosphoinositol hydrolysis
Total inositol phosphate (IP) production was assessed in triplicate as described (22). HEK293 cells were transfected for 24 h, then labeled with [3H]myo-inositol (2 μCi/ml) for 24 h, and split into six-well plates. The cells were stimulated with 0.5 to 30 mm CaCl2 in the presence of 10 mm LiCl for 1 h; total IP was extracted with 20 mm formic acid, neutralized with 1 mm NH4OH, and applied to an anion exchange column (Dowex AG1-x8; Bio-Rad Laboratories). Total IPs were eluted with 2 m ammonium formate and 100 mm formic acid and quantified by scintillation counting. Calcium-stimulated accumulation of IP in different conditions was analyzed in five independent experiments.
Quantitation of intracellular Ca2+ concentration by single cell fluorometry
Intracellular Ca2+ concentrations were measured using fluorescent ratio imaging with MetaFluor Imaging software (Universal Imaging Corp., West Chester, PA) as described (23). HEK293 cells cultured on glass coverslips coated with polylysine-D were loaded sequentially by incubation in 5 μm fura-2-acetoxymethyl ester (Invitrogen, San Diego, CA) (24) for 20 min at 37 C in loading solution [20 mm HEPES (pH 7.4), containing 125 mm NaCl, 3 mm KCl, 1.25 mm CaCl2, 1.0 mm MgSO4, 1 mm KH2PO4 (pH 7.6), and 0.1% dextrose supplemented with fresh 0.1% BSA]. The cells were then washed twice in bathing solution [20 mm HEPES (pH 7.4), containing 125 mm NaCl, 4 mm KCl, 0.5 mm CaCl2, 0.5 mm MgCl2, and 0.1% dextrose supplemented with fresh 0.1% BSA] for 10 min each (25). Each coverslip was placed in a temperature-controlled modified Sykes-Moore chamber mounted on a Nikon TE2000 inverted fluorescence microscope (26). Cells were stimulated with either bathing solution or raising Ca2+ concentrations in bathing solution. Fura-2-acetoxymethyl ester fluorescence was measured every 10 sec for 10 min at excitation settings of 340 and 380 nm. Approximately 60–70 cells were imaged per coverslip, and more than three coverslips were studied in each of three independent experiments with separate transfections with empty vector, WT CaSR, or mutant CaSR cDNAs. The intracellular Ca2+ concentration was estimated from the ratio of fura-2-acetoxymethyl ester emission and comparison with fura-2-acetoxymethyl ester standards. Data were plotted in raster plots using Transform (Fortner Software, Sterling, VA). The mean Ca2+ concentration before and after calcium stimulation of individual cells was measured and normalized for the length of the sampling period, giving a mean value of the intracellular Ca2+ concentration.
Results
Genetics
Sequencing of the CASR gene in the index patient and her sister with NSHPT showed that both had a homozygous deletion of four nucleotides and an insertion of one nucleotide, (c.1031 > 1034 delACAAinsT), thereby replacing His344 and Asn345 with a single Leu and maintaining the reading frame (Fig. 1B). The mother is heterozygous for the mutation. This mutation resides in the loop III region of the ECD of the CaSR. These two amino acids are evolutionarily conserved among mammals, from platypus to human (Fig. 1C).
Cellular location of WT and mutant CaSRs
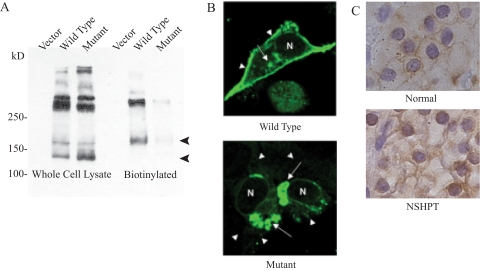
To determine the subcellular localization of the mutant receptor in vitro, the His344-Asn345>Leu mutant was recreated in a human CaSR expression vector by site-directed mutagenesis and transiently transfected into HEK293 cells. Cells were transfected in parallel with the WT cDNA, mutant cDNA, or empty vector in each experiment. Western blotting showed that total levels of both WT and mutant CaSR were comparable: in whole cell lysates, both the 140- and 160-kD monomeric forms of CaSR were detected, along with the multimers migrating above 250 kD (Fig. 2A). However, very little of the mutant receptor reached the cell surface, indicated by a greater than 90% reduction in biotinylated mutant CaSR compared with biotinylated WT receptor. Confocal microscopy of cells expressing the receptors clearly showed nuclear localization of the mutant CaSR, whereas the WT resided on the cell surface as expected (Fig. 2B).
Figure 2.
Cellular location of CaSR. A, Western blotting of the CaSR in whole cell lysates (left) and biotinylated cell surface proteins (right) from HEK293 cells transfected with vector and cDNA expression vectors for WT and mutant CaSR. The bottom two bands, corresponding to 140 and 160 kD, are the monomeric forms of CaSR; the upper bands are multimers or aggregates of the protein under reducing conditions. B, Dual fluorescence confocal microscopy. HEK293 cells were transfected with vectors expressing WT (top panel) or mutant (bottom panel) CaSR. Most WT CaSR is targeted to the cell surface (arrowheads), with little remaining intracellularly (arrows), whereas most of the mutant CaSR remains intracellular. C, Immunohistochemical staining of CaSR in parathyroid tissue. Top, Normal adult control shows anti-CaSR staining mainly along the surface of the chief cells; bottom, parathyroid tissue from the index patient shows perinuclear staining with absence of cell surface staining.
CaSR immunocytochemistry of parathyroid tissue from the index patient suggested reduced expression of CaSR on the cell surface (Fig. 2C). Two staining patterns were seen in the normal controls: a distinct rim-like staining along cell surface in chief cells, consistent with previous findings (27); and a reticular pattern of staining inside a smaller number of the chief cells. In addition, the oxyphil cells clearly had CaSR immunoreactivity on the cell surface. In a control adult gland, about 30–40% of cells were stained with anti-CaSR; the remainder of the cells was negative. In a control newborn, 80% of the cells stained for anti-CaSR with a pattern that looked similar to the adult control. In the index patient’s parathyroid tissue, there was no cell surface staining for CaSR, but some staining was seen near the nucleus. Thus, both the confocal microscopy results and the pathological specimen indicate that the mutant CaSR was not targeted to the cell surface.
Functional activity of the mutant CaSR
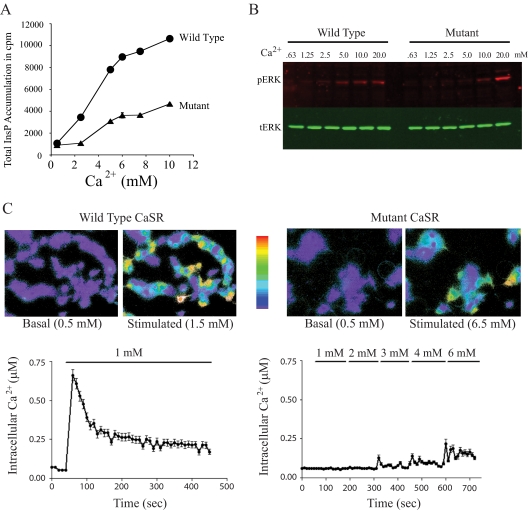
To assess the potential activity of the loop III missense mutation found in this family, we first expressed the mutant CaSR in HEK293 cells and measured accumulation of IPs in response to elevated extracellular Ca2+ concentrations. In comparison to the WT CaSR, the mutant CaSR elicited the production of less than half as much total IPs (Fig. 3A). Stimulating the cells with strontium elicited a similar pattern (data not shown).
Figure 3.
Functional assays of the mutant CaSR. A, Phospholipase C activation in response to raising extracellular Ca2+ concentration in HEK293 cells transfected with either the WT or mutant CaSR. Total accumulated [3H]IP in response to rising extracellular Ca2+ concentrations is shown as means and sd of triplicate determinations in one representative experiment (of five). B, Western blot showing activation of the MAPK pathway in response to varying the extracellular Ca2+ concentration. Left, WT CaSR; right, mutant CaSR. pERK level was detected in red; tERK was detected in green. A representative experiment (of four) is shown. C, Fluorometry study showing release of intracellular calcium in response to stimulation of transfected HEK293 cells with extracellular calcium. The cells were loaded with Fura2, and the images were scaled to reflect intracellular Ca concentrations, ranging from 0–1 μm (scale bar; blue, no calcium; red, 1 μm Ca2+). The data show the intracellular Ca2+ concentration of an average 60–70 cells in a single experiment. Left, cells expressing the WT CaSR stimulated by 1 mm Ca2+; right, cells expressing the mutant CaSR stimulated by 0.5 to 6.5 mm extracellular Ca2+.
Activation of the CaSR normally stimulates the MAPK pathway (28,29). Calcium-dependent ERK phosphorylation through the mutant CaSR was assessed by Western blotting. Transfected HEK293 cells were stimulated with rising extracellular Ca2+ concentrations from 0.63 to 20 mm for 5 min. In cells expressing the WT CaSR, as little as 1.25 mm Ca2+ induced ERK phosphorylation, whereas 5–10 mm Ca2+ was needed to activate ERK in the cells expressing the mutant CaSR (Fig. 3B). At the superphysiological concentration of 20 mm Ca2+, the phosphorylation signal appeared slightly stronger. Similarly, the results of IP experiments were variable with 20 and 30 mm Ca2+ (data not shown).
Binding of extracellular calcium to the CaSR directly increases intracellular Ca2+ release (21). Using single cell microfluorimetry with the intracellular fluorescent Ca2+ indicator, HEK293 cells expressing either WT or mutant CaSR were loaded with fura-2. This live cell imaging was used to measure intracellular Ca2+ concentrations in response to extracellular Ca2+ (Fig. 3C). Whereas cells expressing the WT receptor respond rapidly to 1 mm extracellular Ca2+ with a rise in intracellular Ca2+ to approximately 0.7 μm followed by a sustained level of approximately 0.25 mm, cells expressing the mutant showed no response to 1 mm extracellular Ca2+ and a minimal response even when the extracellular Ca2+ concentration was raised to 6.5 mm. This reduced signaling response is compatible with decreased CaSR targeting to the cell membrane. The temporal responses of the cells expressing WT or mutant CaSR in response to the stepwise increases in extracellular Ca2+ were also recorded live by capturing images of the changes in the fura-2 color of the cells at 0.1-sec intervals and “stacking” the images to make a movie. The cells expressing the WT CaSR release stored intracellular Ca2+ within seconds, whereas the cells expressing the mutant CaSR had a prolonged delay and did not release calcium until the extracellular Ca2+ reached 6.5 mm (Supplemental Movie).
Discussion
Unlike most GPCRs, many naturally occurring CaSR mutations causing both gain-of-function and loss-of-function have been identified, facilitating the elucidation of structure/function relationships (12,13). Our index patient and her sister were homozygous for the loss-of-function mutation His344-Asn345>Leu, causing NSHPT, whereas their mother was heterozygous and had FHH. Inactivating CaSR mutations cause either FHH or NSHPT by several mechanisms. Most CaSR inactivating mutations prevent receptor targeting to the cell surface, resulting in the gene dosage effect seen in our patients, but some mutants act as dominant-negatives (30,31,32). Missense mutations of critical cysteines or the introduction of new cysteine residues in the ECD may inactivate the receptor by disrupting the formation of disulfide bonds. Some inactivating mutations impair Ca2+ binding or agonist-induced conformational changes necessary for proper signaling; mutations in the intracellular loops may prevent G protein coupling.
Our data show that the His344-Asn345>Leu mutation is poorly targeted to the cell membrane, but the mechanism by which these residues participate in targeting the CaSR to the cell surface is not obvious. Glycosylation facilitates protein folding, quality control, sorting, degradation, trafficking, and targeting to the cell surface (33,34).
Most GPCRs in Family A have a relatively short ECD with one or two glycosylation sites; glycosylation of at least one site is usually required for efficient trafficking to the cell surface (35,36,37). The ECD of the CaSR has 11 potential N-linked glycosylation sites having the sequence motif N-X-S or N-X-T (38). Eight of these 11 sites can be glycosylated in vitro; disruption of as few as three of these sites impairs proper processing and cell surface expression of the receptor (38). In human CaSR, N(345)-G-F is not a potential glycosylation site based on the consensus recognition motif, but absence of this sequence does not completely exclude the possibility of glycosylation because a new consensus sequence (N-X-C) has been identified using liquid chromatography-mass spectrometry technology (39). Our Western blotting data show that comparable amounts of WT and mutant CaSR were expressed in HEK293 cells; less of the presumptive mature (160 kDa) glycosylated form was found on the cell surface in cells expressing the mutant CaSR. This finding suggests that decreased cell surface targeting of the mutant receptor may be due to defective glycosylation of the receptor. In addition, shortening of the hinge region of the loop III by one amino acid may interfere with proper protein folding and protein targeting. It is also possible that the mutant receptor is potentially functional if it were to reach the cell membrane, and that only the trafficking is disordered. However, in the absence of a direct Ca2+ binding assay, these two possibilities cannot be distinguished.
The perinuclear location of the mutant CaSR in transfected HEK293 cells seen by confocal microscopy correlates with the intracellular retention of CaSR seen in the parathyroid tissue from the index patient, and both studies correlate with decreased CaSR activity. Immunohistochemical staining of the CaSR has been reported in parathyroid tissues from adult patients with parathyroid adenomas or hyperplasia (27), but immunostaining of CaSR has not been reported in parathyroid glands from patients with NSHPT. The disease is rare, these patients are usually very young, and the very small amounts of tissue usually obtained are typically required to confirm the diagnosis. Because the index patient was older at the time of diagnosis and surgery, sufficient tissue was available for study. In the normal parathyroid tissue, both chief cells and oxyphil cells expressed CaSR. The chief cells synthesize PTH and release it in response to changes in extracellular Ca2+ concentration detected by the CaSR. The presence of CaSR on oxyphil cells is compatible with the clinical observation that oxyphil adenomas can cause primary hyperparathyroidism (40). However, the biological function of parathyroid oxyphil cells and their role in calcium homeostasis remains unknown.
No Ca2+ binding assay is available to assess CaSR ligand binding pharmacology directly; hence, its function is assessed by the Ca2+-induced responsiveness of signaling pathways in transfected HEK293 cells. Assays of IP accumulation, cell surface targeting, and visualization of intracellular calcium release, measuring different endpoints of the CaSR signaling pathway, showed that the novel mutant we identified had profoundly decreased activity. Our identification and characterization of the novel His344-Asn345>Leu mutant thus establishes the importance of the loop III region of the ECD in CaSR signaling.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health (NIH) Grant K08 DK064626 (to Q.D.); a Veterans Affairs Merit Review (to D.S.); NIH/National Institute on Aging Grant R01AG21353-6 and National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01AR055588 (to W.C. and D.S.); and NIH Grant R01 DK37922 (to W.L.M.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 14, 2010
Abbreviations: a.a., Amino acids; CaSR, calcium-sensing receptor; ECD, extracellular domain; FHH, familial hypocalciuric hypercalcemia; GPCR, G protein-coupled receptor; HEK, human embryonic kidney; IP, inositol phosphate; NSHPT, neonatal severe hyperparathyroidism; WT, wild-type.
References
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC 1993 Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366:575–580 [DOI] [PubMed] [Google Scholar]
- Brown EM, MacLeod RJ 2001 Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81:239–297 [DOI] [PubMed] [Google Scholar]
- Brown EM 2007 Clinical lessons from the calcium-sensing receptor. Nat Clin Pract Endocrinol Metab 3:122–133 [DOI] [PubMed] [Google Scholar]
- Okamoto N, Hori S, Akazawa C, Hayashi Y, Shigemoto R, Mizuno N, Nakanishi S 1994 Molecular characterization of a new metabotropic glutamate receptor mGluR7 coupled to inhibitory cyclic AMP signal transduction. J Biol Chem 269:1231–1236 [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ 2003 Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112:293–301 [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF 2003 Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301:850–853 [DOI] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K 2000 Structure basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407:971–977 [DOI] [PubMed] [Google Scholar]
- O'Hara PJ, Sheppard PO, Thøgersen H, Venezia D, Haldeman BA, McGrane V, Houamed KM, Thomsen C, Gilbert TL, Mulvihill ER 1993 The ligand-binding domain in metabotropic glutamate receptors is related to bacterial periplasmic binding proteins. Neuron 11:41–52 [DOI] [PubMed] [Google Scholar]
- Reyes-Cruz G, Hu J, Goldsmith PK, Steinbach PJ, Spiegel AM 2001 Human Ca(2+) receptor extracellular domain. Analysis of function of lobe I loop deletion mutants. J Biol Chem 276:32145–32151 [DOI] [PubMed] [Google Scholar]
- Ray K, Hauschild BC, Steinbach PJ, Goldsmith PK, Hauache O, Spiegel AM 1999 Identification of the cysteine residues in the amino-terminal extracellular domain of the human Ca(2+) receptor critical for dimerization. Implications for function of monomeric Ca(2+) receptor. J Biol Chem 274:27642–27650 [DOI] [PubMed] [Google Scholar]
- Garrett JE, Capuano IV, Hammerland LG, Hung BC, Brown EM, Hebert SC, Nemeth EF, Fuller F 1995 Molecular cloning and functional expression of human parathyroid calcium receptor cDNAs. J Biol Chem 270:12919–12925 [DOI] [PubMed] [Google Scholar]
- Hu J, Spiegel AM 2003 Naturally occurring mutations of the extracellular Ca2+-sensing receptor: implications for its structure and function. Trends Endocrinol Metab 14:282–288 [DOI] [PubMed] [Google Scholar]
- Hendy GN, D'Souza-Li L, Yang B, Canaff L, Cole DE 2000 Mutations of the calcium-sensing receptor (CASR) in familial hypocalciuric hypercalcemia, neonatal severe hyperparathyroidism, and autosomal dominant hypocalcemia. Hum Mutat 16:281–296 [DOI] [PubMed] [Google Scholar]
- Brown EM, Pollak M, Hebert SC 1998 The extracellular calcium-sensing receptor: its role in health and disease. Annu Rev Med 49:15–29 [DOI] [PubMed] [Google Scholar]
- Waller S, Kurzawinski T, Spitz L, Thakker R, Cranston T, Pearce S, Cheetham T, van't Hoff WG 2004 Neonatal severe hyperparathyroidism: genotype/phenotype correlation and the use of pamidronate as rescue therapy. Eur J Pediatr 163:589–594 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Tanaka H, Tsuzuki K, Tsuyuki M, Igaki H, Ichinose Y, Aya K, Nishioka N, Seino Y 1997 Two novel missense mutations in calcium-sensing receptor gene associated with neonatal severe hyperparathyroidism. J Clin Endocrinol Metab 82:2716–2719 [DOI] [PubMed] [Google Scholar]
- Hu J, Spiegel AM 2007 Structure and function of the human calcium-sensing receptor: insights from natural and engineered mutations and allosteric modulators. J Cell Mol Med 11:908–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigouroux C, Bourut C, Guerci B, Ziegler O, Magré J, Capeau J, Meyer L 2000 A new missense mutation in the calcium-sensing receptor in familial benign hypercalcaemia associated with partial lipoatrophy and insulin resistant diabetes. Clin Endocrinol (Oxf) 53:393–398 [DOI] [PubMed] [Google Scholar]
- Ray K, Fan GF, Goldsmith PK, Spiegel AM 1997 The carboxyl terminus of the human calcium receptor. Requirements for cell-surface expression and signal transduction. J Biol Chem 272:31355–31361 [DOI] [PubMed] [Google Scholar]
- Goldsmith PK, Fan GF, Ray K, Shiloach J, McPhie P, Rogers KV, Spiegel AM 1999 Expression, purification, and biochemical characterization of the amino-terminal extracellular domain of the human calcium receptor. J Biol Chem 274:11303–11309 [DOI] [PubMed] [Google Scholar]
- Chang W, Pratt S, Chen TH, Nemeth E, Huang Z, Shoback D 1998 Coupling of calcium receptors to inositol phosphate and cyclic AMP generation in mammalian cells and Xenopus laevis oocytes and immunodetection of receptor protein by region-specific antipeptide antisera. J Bone Miner Res 13:570–580 [DOI] [PubMed] [Google Scholar]
- Chang W, Tu C, Cheng Z, Rodriguez L, Chen TH, Gassmann M, Bettler B, Margeta M, Jan LY, Shoback D 2007 Complex formation with the type B γ-aminobutyric acid receptor affects the expression and signal transduction of the extracellular calcium-sensing receptor. Studies with HEK-293 cells and neurons. J Biol Chem 282:25030–25040 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Paruthiyil S, Butler P, Weiner RI 2004 Role of cAMP signaling in the mediation of dopamine-induced stimulation of GnRH secretion via D1 dopamine receptors in GT1–7 cells. Neuroendocrinology 80:2–10 [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY 1985 A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450 [PubMed] [Google Scholar]
- Bai M, Quinn S, Trivedi S, Kifor O, Pearce SH, Pollak MR, Krapcho K, Hebert SC, Brown EM 1996 Expression and characterization of inactivating and activating mutations in the human Ca2+o-sensing receptor. J Biol Chem 271:19537–19545 [DOI] [PubMed] [Google Scholar]
- Blackman BE, Yoshida H, Paruthiyil S, Weiner RI 2007 Frequency of intrinsic pulsatile gonadotropin-releasing hormone secretion is regulated by the expression of cyclic nucleotide-gated channels in GT1 cells. Endocrinology 148:3299–3306 [DOI] [PubMed] [Google Scholar]
- Kifor O, Moore Jr FD, Wang P, Goldstein M, Vassilev P, Kifor I, Hebert SC, Brown EM 1996 Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab 81:1598–1606 [DOI] [PubMed] [Google Scholar]
- Kifor O, MacLeod RJ, Diaz R, Bai M, Yamaguchi T, Yao T, Kifor I, Brown EM 2001 Regulation of MAP kinase by calcium-sensing receptor in bovine parathyroid and CaR-transfected HEK293 cells. Am J Physiol Renal Physiol 280:F291–F302 [DOI] [PubMed] [Google Scholar]
- Loretz CA, Pollina C, Hyodo S, Takei Y, Chang W, Shoback D 2004 cDNA cloning and functional expression of a Ca2+-sensing receptor with truncated carboxyterminal tail from the Mozambique tilapia (Oreochromis mossambicus). J Biol Chem 279:53288–53297 [DOI] [PubMed] [Google Scholar]
- Pearce SH, Bai M, Quinn SJ, Kifor O, Brown EM, Thakker RV 1996 Functional characterization of calcium-sensing receptor mutations expressed in human embryonic kidney cells. J Clin Invest 98:1860–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M, Pearce SH, Kifor O, Trivedi S, Stauffer UG, Thakker RV, Brown EM, Steinmann B 1997 In vivo and in vitro characterization of neonatal hyperparathyroidism resulting from a de novo, heterozygous mutation in the Ca2+-sensing receptor gene: normal maternal calcium homeostasis as a cause of secondary hyperparathyroidism in familial benign hypocalciuric hypercalcemia. J Clin Invest 99:88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcocci C, Borsari S, Pardi E, Dipollina G, Giacomelli T, Pinchera A, Cetani F 2003 Familial hypocalciuric hypercalcemia in a woman with metastatic breast cancer: a case report of mistaken identity. J Clin Endocrinol Metab 88:5132–5136 [DOI] [PubMed] [Google Scholar]
- Helenius A 1994 How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol Biol Cell 5:253–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A, Aebi M 2004 Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem 73:1019–1049 [DOI] [PubMed] [Google Scholar]
- Kaushal S, Ridge KD, Khorana HG 1994 Structure and function in rhodopsin: the role of asparagine-linked glycosylation. Proc Natl Acad Sci USA 91:4024–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding DX, Vera JC, Heaney ML, Golde DW 1995 N-Glycosylation of the human granulocyte-macrophage colony-stimulating factor receptor α subunit is essential for ligand binding and signal transduction. J Biol Chem 270:24580–24584 [DOI] [PubMed] [Google Scholar]
- García Rodríguez C, Cundell DR, Tuomanen EI, Kolakowski Jr LF, Gerard C, Gerard NP 1995 The role of N-glycosylation for functional expression of the human platelet-activating factor receptor. Glycosylation is required for efficient membrane trafficking. J Biol Chem 270:25178–25184 [DOI] [PubMed] [Google Scholar]
- Ray K, Clapp P, Goldsmith PK, Spiegel AM 1998 Identification of the sites of N-linked glycosylation on the human calcium receptor and assessment of their role in cell surface expression and signal transduction. J Biol Chem 273:34558–34567 [DOI] [PubMed] [Google Scholar]
- Kaji H, Saito H, Yamauchi Y, Shinkawa T, Taoka M, Hirabayashi J, Kasai K, Takahashi N, Isobe T 2003 Lectin affinity capture, isotope-tagged, mass spectrometry to identify N-linked glycoproteins. Nat Biotechnol 21:667–672 [DOI] [PubMed] [Google Scholar]
- McGregor DH, Lotuaco LG, Rao MS, Chu LL 1978 Functioning oxyphil adenoma of parathyroid gland (an ultrastructural and biochemical study). Am J Pathol 92:691–711 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.