Abstract
Aging is a natural process that involves a general decline in many physiological functions, resulting in loss of function and eventually death. Extensive research is being performed in order to elucidate the biology of aging, especially with the advent of newer molecular and genetic methodologies.
The endocrine system plays a major role in orchestrating cellular interactions, metabolism, growth, and senescence. Thus, researchers traditionally used hormones as tools to induce and examine specific biological effects that are associated with aging. Furthermore, because our recent knowledge on hormonal action expanded significantly, downstream pathways and genetic determinants currently prevail in aging research.
In this review, we will summarize the effects of several hormones on human aging and longevity and present recent data from the Longevity Genes Study performed at Albert Einstein College of Medicine, looking at the phenotype and genotype of centenarians and their offspring. We will demonstrate that genetic factors that are associated with human longevity are heritable and may contribute not only to quantitative longevity but also to protection from age-dependent disease and exceptional good health.
Specific genes that encode proteins implicated in endocrine and metabolic pathways may also modulate healthy aging and longevity.
In Which Ways Is the Biology of Aging Different from That of the Young?
The physiological process of aging is associated with progressive loss of function and increased vulnerability to disease, frailty, and disability. Because the incidence of adult diseases increases (in a log scale) with age, a better understanding of the biology of aging could greatly improve our efforts to elucidate the physiopathology of such conditions. Furthermore, targeting specific biological processes that are associated with aging may influence (possibly decrease) the incidence of age-related diseases.
It has been demonstrated that many biological parameters change with aging; however, in most cases it is unclear whether the change reflects a causative factor for, or a result of the aging process. Although all living creatures age at some point and could represent the subject for aging research, our present knowledge on the biology of aging is scarce and insufficient. Only during the last two to three decades, with the development of precise laboratory methodologies, have researchers started looking at particularities of aging biomarkers and correlated them to physiopathological conditions. The fact that the incidence of coronary artery disease and cancer—the leading causes of death in adults—increase with age independently further contributes to the recent immense interest in aging research.
It is a challenge to study the process of aging because many common biomarkers have been found to be shifted from the expected normal limits. First, this finding could represent a contribution to the aging process (e.g. endocrinologists have demonstrated primary and secondary decline in the main endocrine systems with aging). Thus, this theory suggests that manipulating age-related biomarkers can influence the process of aging. Second, these shifts in biomarkers with aging serve as markers of biological or chronological age, and may not contribute greatly to aging within our life span. This concept suggests that there is no role in modifying age-related biomarkers because they represent only an effect of the aging process. And finally, aging-related changes in biomarkers may result from damage and breakdown that occurs with aging, in an effort to buffer, modify, or repair the aging process.
Keeping this complexity in mind, many studies have looked at the consequences of experimental supplementation of hormones (which normally decrease with aging) in elderly subjects. The scientific challenge in such an approach was best demonstrated in the Women’s Health Initiative (WHI). The WHI hypothesized that estrogen is a youthful hormone and will therefore modulate several outcomes related to aging (1). Such a hypothesis is problematic in several ways. First, the numbers of changes with aging are numerous, and the experiment with estrogen is unidimensional. Numerous other hormonal changes occur with aging, for example, and the GH axis also declines. Why would one assume that a single change would have such a drastic effect on the whole aging phenotype? Second, in senescent cells, the secretory profile causes changes in the extracellular environment. For example, many cytokines are secreted from such cells (2), and many cytokine levels are increased with aging (3). This new environment may interact differently with estrogen. There is no doubt that estrogen is a youthful hormone in a young body, but is it youthful in an old body? Lastly, the assumption is that the decline in estrogen is not part of coordinated changes that have protective roles against aging. Because estrogen replacement in elderly women increased the risk of several age-related diseases such as cardiovascular disease, cognitive decline, and breast cancer, the possibility that lower estrogen levels are better has not been ruled out. This example with estrogen supplementation is also valid for several other hormones, as will be discussed further below.
How Can the Approach to the Genetics of Aging be Robust?
Above, we have challenged the notion that there is a cause-and-effect relationship between endocrine changes and aging. However, to determine which of the endocrine changes that occur in the elderly have protective rather than harmful effects, studies looked at the effects of hormone manipulation on the aging process and its related morbidity and mortality (4). A different experimental approach would be genetic studies focusing on old probands and their offspring. Such studies can be performed in a longitudinal aging cohort, following subjects over time to determine specific outcomes and eventually death. Genetic studies genotyping the major functional players in an endocrine pathway in subjects with and without certain outcomes may disclose the specific endocrine pathway (in aging) responsible for, or associated with, the outcome (5). For example, if more women with functional polymorphisms/mutations in the estrogen receptor lived shorter lives and had more age-related disease than those without such a mutation, a stronger cause-and-effect relationship between estrogen axis and aging could have been established (6).
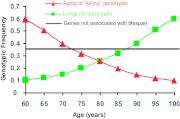
How can such a study design lead to significant discoveries? The premise of this approach, which is different than any genetic study for a specific disease, is based on the fact that most of our population dies by age 100 (only one in 8000 is a centenarian). The average age of death in the United States today is 78, which means that half of the U.S. population will expire at this age. As shown in Fig. 1, although we populate the age axis, the selection is significant. What does it mean for genetic studies? Let’s consider a single nucleotide polymorphism (SNP) that declines across the age axis and is rare in centenarians. Considering the strong selection of survivors, our interpretation would be that this is an aging or “killing” SNP, which allows for very few survivors to age and reach longevity (Fig. 1, in red). The monotonicity of this decline is an important factor in the statistical analysis, excluding false-positive results. A different and more relevant pattern could be a SNP that is monotonically increased across the age axis and is overrepresented in centenarians. Considering the strong selection for centenarians, our interpretation for such a pattern would be that this is a longevity-associated SNP, which allows these subjects to survive (Fig. 1, in green). However, most genotypes are not being associated with longevity (Fig. 1, in black).
Figure 1.
Possibilities for changes in frequency of a functional genotype with age. Green line represents “Longevity genes”—genotype in a gene that contributes to longevity and protection against some age-related diseases. Red line represents “Killing-genes”—the relative prevalence of deleterious genotypes in genes associated with age-related diseases decreases as the population ages, as mortality reduces the proportion of individuals carrying this deleterious genotype. Black line represents the majority of the genes with SNPs whose frequency is not changing with aging in this cross-sectional analysis.
The Longevity Study at Albert Einstein College of Medicine
In line with this theory, we recruited a large cohort of centenarians (average age ∼ 100 yr; range, 95–112 yr) and unrelated subjects (average age ∼ 70 yr; range, 55–94 yr). Those centenarians (7) as well as others across the world (8) have a strong family history of longevity, and this “risk” for longevity (calculated as odds ratio, but depending on the comparison group) ranges from 7- to 18-fold. Those centenarians, as a population, did not interact with the environment as we instruct our patients to do; over 20% were obese in the 1950s (prevalence of obesity then was ∼5%), more than 90% of them smoked (over 20% smoked more than two packs of cigarettes for more than 40 yr), and there was no athlete or vegetarian in the study (9). From a genetic analysis perspective, it is best to study a genetically homogenous population to decrease genetic “noise” or variability. As a classic example, genetic studies on Icelandic and Amish populations were very successful and led eventually to significant genetic discoveries (10). We have chosen Ashkenazi Jews for similar reasons (5). However, because the phenotype at age 100 is deteriorating, we have recruited only subjects whose offspring have agreed to participate. We reason that those offspring are enriched with the phenotype and genotype for longevity, and they are matched by gender and age to the younger unrelated young control group. Interestingly, offspring are healthier than gender- and age-matched controls, and centenarians have the same prevalence of age-related disease as controls 30 yr younger, suggesting that aging was significantly delayed (7,9). An important outstanding fact was the low prevalence of diabetes in centenarians, which is about one third of that of controls approximately 30 yr younger.
When Do We Study Candidate Genes and When Do We Use an Unbiased Approach?
Although aging is such a complex condition, geneticists studying lower organisms have proven the concept that a single alteration in a single gene can lead to exceptional longevity. Variation in single genes can result in substantial life span variations as demonstrated in model organisms (11,12). Genes that are considered part of the insulin/IGF-I (IIS) signaling pathway represent a typical example (13,14). Activation of the DAF-16 (abnormal Dauer Formation-16)/FOXO (Forkhead box) protein by mutations that increase Sir-2 activity or IIS signaling results in prolongation of life span in Caenorhabditis elegans (15). In mammalians, activation of SIRT1 results in modulation of life span, including the cellular response to stress. SIRT1 regulates FOXO transcription factors, a family of proteins that function as sensors in the IIS pathway and influence mammalian longevity (16). Mice with a fat-specific insulin receptor knockout (FIRKO) have reduced fat mass, protected against age-related obesity, and have extended longevity (17). Other mutations in the IIS pathway that may impact longevity in mice include mutations in the IGF-I receptor (IGF-IR) (18), insulin receptor substrate 1 and 2 (19), pregnancy-associated plasma protein-A (20), and the Ames Dwarf mouse mutation (21). A key regulator of this pathway is the transcription factor DAF-16, by inhibiting IIS signaling (21). The human homolog of DAF-16 includes four FOXOs: FOXO1, FOXO3, FOXO4, and FOXO6. A connection between insulin, FOXO, oxidative stress, and human longevity would be particularly interesting because oxidative stress has long been a favorite putative mechanism of aging. Since 1956, the free radical theory of aging has hypothesized that aging results partly from damage to DNA, cells, and tissues from cumulative exposure to reactive oxygen molecules (22), and although not yet universally accepted, supportive evidence has accumulated over the years (23). Thus, FOXO may provide a potential forkhead or bridge between insulin signaling, free radicals, and human aging/longevity. Prior studies demonstrated a link between genes in the IIS pathway and human longevity (24) including a recent report by Suh et al. (25), which links functionally significant IGF-IR mutations to exceptional longevity. Other studies have found links between FOXO genes and other aging phenotypes, including 4-yr survival and stroke risk (26) as well as premature menopause (27). However, many studies in humans have been hampered by small sample sizes, lack of precise phenotyping, and population stratification, among other challenges. A recent report looking at the potential genetic contributions of genes linked to IIS signaling to human longevity demonstrated that genetic variation within the FOXO3A gene is strongly associated with human longevity (28). Long-lived men presented additional phenotypes linked to healthy aging, including lower prevalence of cancer and cardiovascular disease, better self-reported health, and high physical and cognitive function, despite significantly older age. Several of these aging phenotypes were associated with FOXO3A genotype. Long-lived men also exhibited several biological markers indicative of greater insulin sensitivity, and this was associated with homozygosity for the FOXO3A GG genotype (28).
In humans, a phenotype may lead to the candidate approach. It was not uncommon to find high-density lipoprotein (HDL) levels of over 100 mg/dl in our study subjects with longevity and sometimes their HDL was over 140 mg/dl. We have also observed unusual levels of high HDL in families of centenarians, although in prospective studies HDL declines with aging (29). The distribution of plasma HDL in offspring of centenarians (average HDL, 72 mg/dl) was bimodal compared with controls, suggesting two different populations: one that inherited this phenotype, and one that had a normal distribution. Furthermore, HDL and low-density lipoprotein (LDL) particle size were larger in centenarians and their offspring with 0.4–0.7 heritability compared with controls (30). With such a strong phenotype, our initial search was for lipoprotein genotypes that may be responsible for the increased HDL and particle size in centenarians and their offspring.
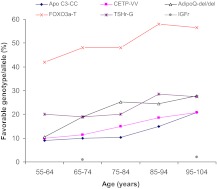
The genotypes that were validated in our study and at least one additional study are shown in Fig. 2. The homozygosity at approximately 60 yr is approximately 8–12%, and at approximately 100 yr, it is 24–32%. Also, the monotonicity increase is obvious and stands the statistical test, although at approximately 59 it seems to slightly decline. Specifically, there was a 2- to 3-fold higher frequency of homozygosity in the favorable genotype for cholesteryl ester transfer protein (CETP) gene codon 405 isoleucine to valine variant (CETP VV), apolipoprotein C-3 (APOC-3) gene codon A (−641) C variant (APOC-3 CC), and genotype of deletion at +2019 in the adiponectin (ADIPOQ) gene. CETP and APOC-3 are candidate genes because of their role in modulating lipoproteins and their particle size. The fat-derived peptide ADIPOQ has been shown in vivo to improve insulin action and decrease arterial wall inflammation (30).
Figure 2.
The basal frequency and trends of favorable longevity genotypes with aging. APOC-3, Genotype apolipoprotein C-3; CETP, homozygosity genotype in cholesterol ester transfer protein; ADIPOQ, del/del deletion homozygosity in the 3″ untranslated region adiponectin gene; FOXO3A, T genotypes in the FOXO 3A gene; TSH-R, genotypes in the TSH receptor gene; and IGF-IR, genotypes in the IGF-IR gene.
More and more genetic studies need to undergo validation in independent populations. In fact, the role of CETP in longevity has been recently validated by other investigators in other population studies. Specifically, Lucchi et al. (31) demonstrated an increase in the VV homozygosity from 7 to 17% in Italians 89 yr of age living around Florence. The VV genotype was associated with lower CETP levels, larger LDL size, and a lower prevalence of disease endpoints. Although Cellini et al. (32) failed to show such an association in Italians, the need for validation is generally reasonable as long as it takes into account the fact that lack of validation does not necessarily indicate a false-positive result. For example, Japanese centenarians do not have the VV polymorphism; however, they carry a significant polymorphism in a different part of the CETP gene that is associated with lower CETP and high HDL levels. Because of this, CETP levels and physiology may be a better marker for longevity than the specific genotype.
Genetic findings in humans also need to eventually be linked to biological functionality (Table 1). This can be done by expressing the genotype in vitro in different systems or, if looking at plasma proteins, by examining the effects of a genotype on their levels. In the cases described above, there were significantly reduced plasma CETP and APOC-3 levels and increased plasma ADIPOQ levels noted in our study population in those who had homozygosity for the longevity genes. Thus, the expression of these genotypes is beneficial. We do not yet have functional analysis of FOXOA3.
Table 1.
Genes, SNPs, and their related molecular pathways (which may be associated with longevity)
| Longevity | Relevant biological action | Chromosomal loci | Ref. | |
|---|---|---|---|---|
| Klotho (KL gene) | + | Insulin sensitivity, modulation of IGF-I and vitamin D | 13q12 | 55 |
| Silent mating type information regulation 2 homolog 1 (SIRT1) | + | Regulate epigenetic gene silencing and suppresses recombination of rDNA, associated with insulin action/sensitivity | 10q21.3 | 56 |
| Catalase (CAT) | + | Antioxidant that protects cells from hydrogen peroxide | 11p13 | 57 |
| Mammalian target of rapamycin (mTOR) | − | Modulates insulin, IGF, and mitogen function | 1P36 | 58 |
| IGF-I/insulin (FOXO) | − | Transcription factors that take part in cell growth and differentiation | 12q23–23 | 59 |
| GH | − | Stimulate growth, production of IGF-I | 17 q22-q24 | 60 |
| TSHβ | + | Production of TSH | 1p13 | 54 |
| Thyrotropin receptor (TSHR) | + | Production of T4 and T3 | 14q31 | 54 |
| CETP | + | Facilitates the transport of cholesteryl esters and triglycerides between the lipoproteins | 16q21 | 61 |
| APOC-3 | + | Inhibits lipoprotein lipase and hepatic lipase | 11q23.1-q23.2 | 29 |
| Adiponectin (AdipoQ) | + | Modulates glucose and fatty acid metabolism | 3q27 | 62 |
+, Favor longevity; −, against longevity.
Another major support for the functional relevance of these longevity polymorphisms is based on intermediate phenotypes. Because CETP and APOC-3 are involved in the regulation of lipoproteins and their particle sizes, we assessed the lipid profile in our entire population and discovered that, in addition to a more favorable lipid profile, subjects with these genotypes have very large lipoprotein particle sizes, specifically larger particle sizes of HDL cholesterol and LDL cholesterol, as well as increased HDL levels (29). Indeed, large lipoprotein size seems to be quite commonly associated with exceptional longevity and may be necessary—although not sufficient—to achieve extreme longevity. These three genotypes may show genetic heterogeneity, i.e. several genes that result in a common lipoprotein phenotype.
Furthermore, the most striking example is that of the favorable CETP genotype and cognitive decline (33). Probands who scored above 25 on the Mini Mental State Exam (consistent with good cognitive function) had a higher frequency of the VV genotype compared with those with abnormal cognitive function (33 vs. 15%; P < 0.01), a finding that was independently validated in the Einstein Aging Study in subjects who developed dementia vs. subjects who did not (34). This was recently extended to show a 70% protection against dementia in subjects with the longevity homozygosity, and not with other longevity genotypes (35). We are currently not able to implicate genes using the end-point of longevity in a prospective manner. However, we conducted a Kaplan-Meier survival analysis that showed that carriers of the CC variant had a statistically significant survival advantage (hazard ratio 1.81, compared with subjects carrying the CA/AA genotype; P = 0.0001). The carriers of the APOC-3 CC genotype lived approximately 4 yr longer than those without, and the effect of the CC genotype remained significant until the age of 105 yr (30).
In addition to a more favorable lipid profile, subjects with these genotypes have very large lipoprotein particle sizes. Moreover, the levels of ADIPOQ, which has antiinflammatory, lipoprotein-modulating, and insulin-sensitizing properties, are increased in centenarians and their offspring, suggesting ADIPOQ as a potential modulator of longevity (30).
Realization of an Unbiased Approach
Considering that there are over 25,000 genes, of which we know reasonably about only several thousand, we are bound to find new genes involved in aging and longevity, as demonstrated in other conditions such as diabetes (36). With the devolvement of newer technologies there are several platforms that populate SNPs and copy variants across the genome, representing most of it. Genome-wide association studies (GWASs) between groups of affected subjects and controls have been emerging for many diseases.
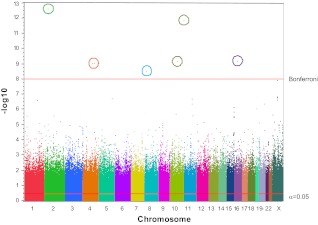
We recently conducted a GWAS of 364 centenarians and 251 controls using Affymetrix 6.0 genome-wide chips (Fig. 3). The chips contain probes for 906,600 SNPs and 946,000 CNVs. The GWAS data on SNPs are visualized in the form of a Manhattan plot, with each dot indicating a different variant (with chromosomes colored). The SNPs of genome-wide significance, forming Bonferroni-corrected peaks with P value < 10−7, have been circled. In GWAS single cohort studies, sample sizes often do not attain the number needed to make meaningful findings, especially in phenotypes as complex as longevity. Hence, to see several genes reaching high significant levels is surprising (30). However, our centenarians are of Ashkenazi Jewish ethnicity and represent a highly homogeneous population. As noted above the huge selection to reach such extreme longevity also helps in getting such significant results. These findings suggest a number of strong modifier genes of more or less equal effect. The top candidates are not in a major endocrine pathway, in fact they are generally genes whose function is yet undetermined. This is a humbling start, and it makes one realize that many great discoveries are ahead, once genes whose function is yet unknown will be assessed.
Figure 3.
Manhattan plot of GWAS of Ashkenazi Jewish centenarians and controls. GWAS of 364 centenarians and 251 controls (about 70 yr of age, from the same geographical area and the same ethnic background), using Affymetrix 6.0 genome-wide chips. The chips contain probes for 906,600 SNPs and 946,000 CNVs. The resulting data set includes (complying with institutional review board specifications) age, gender, SNP, and CNV genotype results as well as results from the statistical analysis. The GWAS data on SNPs are visualized in the form of a Manhattan plot, with each dot indicating a different variant (with chromosomes colored). The SNPs of genome-wide significance form Bonferroni-corrected peaks with P value < 10−7.
Why Is Youthful Not Always Antiaging?
Here we summarize two examples demonstrating that the instinct to replace hormones may in fact be proaging rather than antiaging.
The first example follows the growing evidence to suggest an important role for the GH/IGF system in the regulation of life span and age-related diseases. Disruption of IGF/insulin signaling in lower organisms such as nematodes, yeasts, and flies has been associated with prolongation of life span. In mammalians, the insulin-signaling pathway is considerably more complex and separated from the IGF-axis system that includes IGF-I and IGF-II, six IGF binding proteins (IGFBP-1 to -6), and nine IGFBP-related proteins (37,38). As in lower species, spontaneous genetic alterations to the GH/IGF axis in rodents such as in Snell and Ames mice and heterozygous disruption of the IGF-IR in a genetic model have been shown to prolong life span.
In humans, there is tremendous evidence to suggest that high IGF-I levels are a risk factor for many types of cancers (39). Therefore, since the discovery of the IGF-IR in the 1980s, there has been great interest in developing therapeutic strategies to inhibit IGF-IR signaling, including tyrosine kinase inhibitors and IGF monoclonal antibodies to treat various cancers (40). However, just as high IGF-I levels are associated with cancers (41), low IGF-I has been implicated in the pathogenesis of a wide range of conditions including type 2 diabetes and glucose intolerance (42), osteoporosis (43), poor cognitive function (44,45), and coronary heart disease (25), thus highlighting the complex role of the IGF axis in humans.
Because of the associations of IGF-I on disease risk in humans and its involvement in life span determination of model systems, we recently examined the association of the GH/IGF-I system with human longevity. In a genetic analysis of the IGF-IR in exceptionally long-lived individuals, we identified alterations in the IGF-IR gene that were overrepresented in our female centenarians vs. controls. These mutations were functional, with carriers having a lower maximal height and greater IGF-I levels compared with those without these mutations. Furthermore, a lower number of IGF-IR as well as lower activation of AKT by IGF-I was observed in transformed lymphoblast obtained from these subjects with IGF-IR mutations. Although this suggests a role of this pathway in modulation of human life span and highlights the complex role of the IGF system in various biological functions, it also raises the more relevant clinical question: is GH therapy an effective antiaging therapy?
The second example is the relationship between thyroid levels and aging. Animal studies demonstrate a correlation between extended life span and a decrease in thyroid function (46). Many animal models of exceptional longevity demonstrate significantly decreased or absent thyroid function (47,48,49), suggesting a causative relationship between thyroid function and life span. Furthermore, experimentally induced hypothyroidism in young rats results in extended life span, whereas inducing hyperthyroidism results in significantly shorter life span (50,51). The precise mechanisms responsible for the effects of hypothyroidism on life span have not been clarified, although multiple actions of thyroid hormone (including lowering of the metabolic rate, lowering core body temperature and oxygen consumption, and reducing reactive oxygen species generation and oxidative damage) appear to play an important role in longevity (52).
Elevated plasma TSH levels are increased in human longevity, indicating a possible decline in thyroid function. Researchers at Albert Einstein College of Medicine examined thyroid function in centenarians and their offspring and searched for genetic markers that may be responsible for the specific pattern of thyroid function tests observed in these populations (53). Offspring of centenarians had higher median serum TSH compared with age-matched controls and significant calculated heritability. Allele frequency of two SNPs in the promoter/enhancer region of the TSHR gene, associated with increased serum TSH, was higher in centenarians and their offspring compared with controls (rs10149689 G allele and rs12050077 A allele). Linkage disequilibrium between the two SNPs was high, suggesting interaction between them. Furthermore, GA haplotype frequency was significantly higher among centenarians and offspring compared with controls (54). These findings suggest that a heritable phenotype characterized by raised serum TSH is associated with human longevity. Carriers of rs12050077 and rs10149689 SNPs in the TSHR have higher serum TSH, possibly contributing to decreased thyroid function and longevity. This also raises the more relevant clinical question: is therapy for TSH an effective antiaging therapy?
We conclude that because we age and die at different rates, the selection of survivors allows us to test hypotheses related to aging and the endocrine system with a variety of genetic platforms, in an unbiased fashion. Many endocrine and metabolic pathways are linked genetically with the process of aging and contribute to various phenotypes associated with human aging. A better understanding of the physiology of aging and its associated genetic mechanisms will eventually lead to a better characterization of treatments that are considered “youthful” in elderly people.
Footnotes
This work has been supported by grants from the Paul Beeson Physician Faculty Scholar in Aging Award; the Ellison Medical Foundation Senior Scholar Award; the Glenn Award for Research in Biological Mechanisms of Aging, Resnick Gerontology Center; National Institutes of Health Grants RO1 AG-18728-01A1, RO1 AG024391, PO1 AG027734, and RO1 AG7992; General Clinical Research Center Grant MO1-RR12248; and Diabetes Research and Training Center Grant DK 20541, the Albert Einstein College of Medicine.
Clinical Trial no. NCT00707694.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations: APOC-3, Apolipoprotein C-3; CETP, cholesteryl ester transfer protein; CNV, copy number variant; DAF-16, abnormal Dauer Formation-16; FOXO, Forkhead box; GWAS, genome-wide association study; HDL, high-density lipoprotein; IGFBP, IGF binding protein; IGF-IR, IGF-I receptor; IIS, insulin/IGF-I; LDL, low-density lipoprotein; SNP, single nucleotide polymorphism.
References
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J 2002 Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- Campisi J 1998 The role of cellular senescence in skin aging. J Investig Dermatol Symp Proc 3:1–5 [PubMed] [Google Scholar]
- Campisi J, Yaswen P 2009 Aging and cancer cell biology, 2009. Aging Cell 8:221–225 [DOI] [PubMed] [Google Scholar]
- Roubenoff R 1993 Hormones, cytokines and body composition: can lessons from illness be applied to aging? J Nutr 123:469–473 [DOI] [PubMed] [Google Scholar]
- Bergman A, Atzmon G, Ye K, MacCarthy T, Barzilai N 2007 Buffering mechanisms in aging: a systems approach toward uncovering the genetic component of aging. PLoS Comput Biol 3:e170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman SM 2004 What do hormones have to do with aging? What does aging have to do with hormones? Ann NY Acad Sci 1019:299–308 [DOI] [PubMed] [Google Scholar]
- Atzmon G, Schechter C, Greiner W, Davidson D, Rennert G, Barzilai N 2004 Clinical phenotype of families with longevity. J Am Geriatr Soc 52:274–277 [DOI] [PubMed] [Google Scholar]
- Perls T, Kohler IV, Andersen S, Schoenhofen E, Pennington J, Young R, Terry D, Elo IT 2007 Survival of parents and siblings of supercentenarians. J Gerontol A Biol Sci Med Sci 62:1028–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzmon G, Rincon M, Rabizadeh P, Barzilai N 2005 Biological evidence for inheritance of exceptional longevity. Mech Ageing Dev 126:341–345 [DOI] [PubMed] [Google Scholar]
- Gudmundsson A 2004 Research on aging in Iceland: future potentials. Mech Ageing Dev 125:133–135 [DOI] [PubMed] [Google Scholar]
- Shmookler Reis RJ, Bharill P, Tazearslan C, Ayyadevara S 2009 Extreme-longevity mutations orchestrate silencing of multiple signaling pathways. Biochim Biophys Acta 1790:1075–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan SD, Yen K, Tissenbaum HA 2009 Converging pathways in lifespan regulation. Curr Biol 19:R657–R666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaconstantinou J 2009 Insulin/IGF-1 and ROS signaling pathway cross-talk in aging and longevity determination. Mol Cell Endocrinol 299:89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman DE, Christiansen JS, Johannsson G, Thorner MO, Kopchick JJ 2008 Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res 18:455–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panowski SH, Dillin A 2009 Signals of youth: endocrine regulation of aging in Caenorhabditis elegans. Trends Endocrinol Metab 20:259–264 [DOI] [PubMed] [Google Scholar]
- Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I 2005 Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell 16:4623–4635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM 2006 Longevity in mice: is stress resistance a common factor? Age (Dordr) 28:145–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzenberger M 2004 The GH/IGF-I axis and longevity. Eur J Endocrinol 151(Suppl 1):S23–S27 [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Dominici FP, Arum O, Rocha JS, Al Regaiey KA, Westbrook R, Spong A, Panici J, Masternak MM, Kopchick JJ, Bartke A 2009 Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One 4:e4567 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Austad S 2008 Advances in vertebrate aging research 2007. Aging Cell 7:119–124 [DOI] [PubMed] [Google Scholar]
- Swindell WR 2007 Gene expression profiling of long-lived dwarf mice: longevity-associated genes and relationships with diet, gender and aging. BMC Genomics 8:353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokov A, Chaudhuri A, Richardson A 2004 The role of oxidative damage and stress in aging. Mech Ageing Dev 125:811–826 [DOI] [PubMed] [Google Scholar]
- Knight JA 2000 The biochemistry of aging. Adv Clin Chem 35:1–62 [DOI] [PubMed] [Google Scholar]
- Longo VD 2009 Linking sirtuins, IGF-I signaling, and starvation. Exp Gerontol 44:70–74 [DOI] [PubMed] [Google Scholar]
- Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P 2008 Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA 105:3438–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC 2007 “Sly as a FOXO”: new paths with Forkhead signaling in the brain. Curr Neurovasc Res 4:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA 2003 Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 301:215–218 [DOI] [PubMed] [Google Scholar]
- Anselmi CV, Malovini A, Roncarati R, Novelli V, Villa F, Condorelli G, Bellazzi R, Puca AA 2009 Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res 12:95–104 [DOI] [PubMed] [Google Scholar]
- Barzilai N, Atzmon G, Schechter C, Schaefer EJ, Cupples AL, Lipton R, Cheng S, Shuldiner AR 2003 Unique lipoprotein phenotype and genotype associated with exceptional longevity. JAMA 290:2030–2040 [DOI] [PubMed] [Google Scholar]
- Atzmon G, Rincon M, Schechter CB, Shuldiner AR, Lipton RB, Bergman A, Barzilai N 2006 Lipoprotein genotype and conserved pathway for exceptional longevity in humans. PLoS Biol 4:e113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchi T, Arosio B, Caloni M, Ceconi I, Calabresi C, Scurati S, Vergani C 2005 [I405V polymorphism of the cholesteryl ester transfer protein gene in young and very old individuals]. Ann Ital Med Int 20:45–50 [PubMed] [Google Scholar]
- Cellini E, Nacmias B, Olivieri F, Ortenzi L, Tedde A, Bagnoli S, Petruzzi C, Franceschi C, Sorbi S 2005 Cholesteryl ester transfer protein (CETP) I405V polymorphism and longevity in Italian centenarians. Mech Ageing Dev 126:826–828 [DOI] [PubMed] [Google Scholar]
- Barzilai N, Atzmon G, Derby CA, Bauman JM, Lipton RB 2006 A genotype of exceptional longevity is associated with preservation of cognitive function. Neurology 67:2170–2175 [DOI] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, Verghese J 2009 Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology 73:356–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AE, Wang C, Katz M, Derby CA, Barzilai N, Ozelius L, Lipton RB 2010 Association of a functional polymorphism in the cholesteryl ester transfer protein (CETP) gene with memory decline and incidence of dementia. JAMA 303:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafar-Mohammadi B, McCarthy MI 2008 Genetics of type 2 diabetes mellitus and obesity—a review. Ann Med 40:2–10 [DOI] [PubMed] [Google Scholar]
- Le Roith D 1997 Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med 336:633–640 [DOI] [PubMed] [Google Scholar]
- Rosenfeld RG, Hwa V, Wilson E, Plymate SR, Oh Y 2000 The insulin-like growth factor-binding protein superfamily. Growth Horm IGF Res 10(Suppl A):S16–S17 [DOI] [PubMed] [Google Scholar]
- Rodon J, DeSantos V, Ferry Jr RJ, Kurzrock R 2008 Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol Cancer Ther 7:2575–2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins PJ, Bustin SA 2004 Evidence for a link between IGF-I and cancer. Eur J Endocrinol 151(Suppl 1):S17–S22 [DOI] [PubMed] [Google Scholar]
- Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ 2002 Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet 359:1740–1745 [DOI] [PubMed] [Google Scholar]
- Zofková I 2003 Pathophysiological and clinical importance of insulin-like growth factor-I with respect to bone metabolism. Physiol Res 52:657–679 [PubMed] [Google Scholar]
- Trejo JL, Piriz J, Llorens-Martin MV, Fernandez AM, Bolós M, LeRoith D, Nuñez A, Torres-Aleman I 2007 Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol Psychiatry 12:1118–1128 [DOI] [PubMed] [Google Scholar]
- Juul A, Scheike T, Davidsen M, Gyllenborg J, Jørgensen T 2002 Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation 106:939–944 [DOI] [PubMed] [Google Scholar]
- Janssen JA, Lamberts SW 2002 The role of IGF-I in the development of cardiovascular disease in type 2 diabetes mellitus: is prevention possible? Eur J Endocrinol 146:467–477 [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Pinto M 2009 Endocrine function in naturally long-living small mammals. Mol Cell Endocrinol 299:101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffenstein R 2005 The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci 60:1369–1377 [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A 1996 Dwarf mice and the ageing process. Nature 384:33 [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA 2007 Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev 87:1175–1213 [DOI] [PubMed] [Google Scholar]
- Ooka H, Shinkai T 1986 Effects of chronic hyperthyroidism on the lifespan of the rat. Mech Ageing Dev 33:275–282 [DOI] [PubMed] [Google Scholar]
- Ooka H, Fujita S, Yoshimoto E 1983 Pituitary-thyroid activity and longevity in neonatally thyroxine-treated rats. Mech Ageing Dev 22:113–120 [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Else PL 2000 Mechanisms underlying the cost of living in animals. Annu Rev Physiol 62:207–235 [DOI] [PubMed] [Google Scholar]
- Atzmon G, Barzilai N, Hollowell JG, Surks MI, Gabriely I 2009 Extreme longevity is associated with increased serum thyrotropin. J Clin Endocrinol Metab 94:1251–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzmon G, Barzilai N, Surks MI, Gabriely I 2009 Genetic predisposition to elevated serum thyrotropin is associated with exceptional longevity. J Clin Endocrinol Metab 94:4768–4775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y 1998 Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 242:626–630 [DOI] [PubMed] [Google Scholar]
- Voelter-Mahlknecht S, Mahlknecht U 2006 Cloning, chromosomal characterization and mapping of the NAD-dependent histone deacetylases gene sirtuin 1. Int J Mol Med 17:59–67 [PubMed] [Google Scholar]
- Junien C, Turleau C, de Grouchy J, Saïd R, Rethoré MO L, Tenconi R, Dufier JL 1980 Regional assignment of catalase (CAT) gene to band 11p13. Association with the aniridia-Wilms’ tumor-Gonadoblastoma (WAGR) complex. Ann Genet 23:165–168 [PubMed] [Google Scholar]
- Kaeberlein M 2010 Lessons on longevity from budding yeast. Nature 464:513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissenden JE, Ullrich A, Francke U 1984 Human chromosomal mapping of genes for insulin-like growth factors I and II and epidermal growth factor. Nature 310:781–784 [DOI] [PubMed] [Google Scholar]
- Laron Z 2005 Do deficiencies in growth hormone and insulin-like growth factor-1 (IGF-1) shorten or prolong longevity? Mech Ageing Dev 126:305–307 [DOI] [PubMed] [Google Scholar]
- Humphries SE, Berglund L, Isasi CR, Otvos JD, Kaluski D, Deckelbaum RJ, Shea S, Talmud PJ 2002 Loci for CETP, LPL, LIPC, and APOC3 affect plasma lipoprotein size and sub-population distribution in Hispanic and non-Hispanic white subjects: the Columbia University BioMarkers Study. Nutr Metab Cardiovasc Dis 12:163–172 [PubMed] [Google Scholar]
- Atzmon G, Pollin TI, Crandall J, Tanner K, Schechter CB, Scherer PE, Rincon M, Siegel G, Katz M, Lipton RB, Shuldiner AR, Barzilai N 2008 Adiponectin levels and genotype: a potential regulator of life span in humans. J Gerontol A Biol Sci Med Sci 63:447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]