Abstract
Background: Despite considerable racial and geographical differences in human phenotypes and in the incidence of diseases that may be associated with sex steroid action, there are few data concerning variation in sex steroid levels among populations. We designed an international study to determine the degree to which geography and race influence sex steroid levels in older men.
Methods: Using mass spectrometry, concentrations of serum androgens, estrogens, and sex steroid precursors/metabolites were measured in 5003 older men from five countries. SHBG levels were assessed using radioimmunoassay.
Results: There was substantial geographical variation in the levels of sex steroids, precursors, and metabolites, as well as SHBG. For instance, Asian men in Hong Kong and Japan, but not in the United States, had levels of total testosterone approximately 20% higher than in other groups. Even greater variation was present in levels of estradiol, SHBG, and dihydrotestosterone. Group differences in body mass index did not explain most geographical differences. In addition, body mass index-independent racial differences were present; Black men had higher levels of estrogens (estradiol, estrone), and Asian men had lower levels of glucuronidated androgen metabolites.
Conclusions: On a global scale, there are important geographical and racial differences in the concentrations of serum sex steroids and SHBG in older men.
A large international study of older men reveals evidence for substantial geographical and racial differences in sex steroid levels.
Sex steroids have pleiotropic actions in men. In addition to their effects on reproductive tissues, they influence numerous biological systems including those that determine body composition, immunological function, glucose and lipid metabolism, and cardiovascular health. Aging is well known to result in a decline in sex hormone levels, and those changes have been linked to disorders such as frailty, muscle and skin atrophy, fat accumulation, osteoporosis, and prostate disease, as well as sexual and cognitive dysfunction.
Racial and geographical differences exist in some human phenotypes that are influenced by sex steroids (e.g. lean mass, bone density) and in the incidence of age-related disorders that are linked to sex steroid actions. For instance, the risk of developing prostate cancer is higher in Black men, whereas Asian men living in the Far East have rates considerably lower than those living in Western countries (1,2). These differences have stimulated interest in whether serum sex steroid concentrations or metabolism are affected by race and geography. Although some comparisons have suggested that there are racial differences in sex hormone levels (3,4,5), those findings are not consistent and are difficult to interpret because of small study sizes and the use of some sex steroid assay methods (RIA) with questionable specificity in lower concentration ranges. There are fewer studies of geographical variation, but some indicate that there may be environmental factors that affect sex hormone physiology (6,7).
To examine racial and geographic differences in sex hormone levels, we assembled a large international cohort of older men and measured the serum levels of the most biologically active estrogens and androgens as well as their precursors and metabolites.
Subjects and Methods
Study populations
We evaluated 5003 ambulatory, community-dwelling men at least 65 yr of age from five countries (Japan, Hong Kong, Sweden, Tobago, and the United States). All were participants in ongoing observational studies that used similar enrollment criteria and evaluation methods (Supplemental Table 1, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
Men in the United States, Sweden, and Hong Kong were participants in the Osteoporotic Fractures in Men Study (MrOS). Details of the U.S. MrOS cohort have been described (8). Self-defined racial/ethnic ancestry (Caucasian, Black, or Asian) was ascertained through questionnaire; fasting morning serum was collected. A random sample of MrOS Caucasian men and all Black and Asian men with sufficient stored serum were included. Samples were collected between 0800 and 1200 h, and serum was prepared and frozen for 6–7 yr in 1.0-ml cryovials at −80 C until assay. MrOS participants in Sweden (9) were Caucasian; all participants with sufficient morning, fasting serum were included. After phlebotomy, serum was prepared and stored for 2–4 yr in 0.5-ml microtubes at −70 C until assay. The MrOS Study in Hong Kong included Chinese men (10). All participants with sufficient stored serum from a morning, fasting blood draw were included. Serum was prepared and stored for 4–5 yr at −20 to −85 C in 0.6-ml microtubes. Vials were briefly thawed and refrozen once before assay.
In Tobago, the parent study was the Tobago Prostate Cancer Survey (11), a study of men of West African descent. A random sample of 500 participants at least 65 yr old was included. Fasting morning serum was obtained. After phlebotomy, serum was prepared and stored for 0.5–2.5 yr in 2.0-ml tubes at −80 C until assay.
In Japan, the parent study was the Research on Osteoarthritis and Osteoporosis Against Disability (ROAD) Study. Japanese participants were recruited from resident registration lists in two communities in the Wakayama District: a mountainous region in Hidakagawa and a coastal region in Taiji (12). Samples from all 367 men who were at least 65 yr old were analyzed; serum was collected between 0900 and 1500 h and stored for 1–2 yr in 2.0-ml vials at −80 C until assay.
The Institutional Review Board at each study center approved the protocol, and written informed consent was obtained from all participants.
Laboratory methods
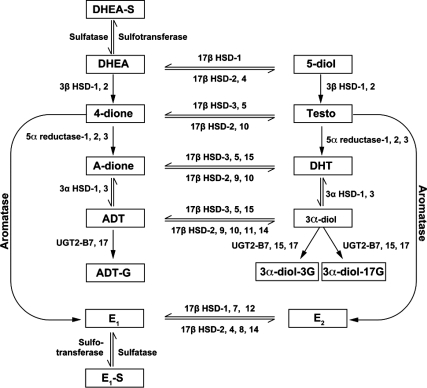
The steroids measured are depicted in Fig. 1. All hormone assays were performed at the Centre de Recherché du CHUL (University of Laval, Quebec, Canada) using gas chromatography/liquid chromatography/mass spectrometry (13). Serum was stored at −80 C until assay. Standard samples of several concentrations were included in all assay runs to ensure precision and accuracy. Moreover, blinded aliquots of a single serum pool included in all assay runs showed no longitudinal trend in any measurement. Ranges of detection for each steroid and mean coefficients of variation (CV) for pooled serum aliquots are in Supplemental Table 2.
Figure 1.
Measured steroids and their metabolic relationships. 5-diol, Androst-5-ene-3β,17β-diol; A-dione, androstanedione; E2, estradiol; E1, estrone; E1-S, estrone sulfate; HSD, hydroxysteroid dehydrogenase. [Adapted from Refs. 41,42,43.]
SHBG assays for Japan, the United States, and Tobago samples were performed at the Oregon Clinical and Translational Research Institute (Diagnostic Products Corp., Los Angeles, CA; CV, 7.1%), and Hong Kong and Sweden samples at the Clinical Pharmacology Lab, Sahlgrenska University Hospital, Gothenburg (Orion Diagnostics, Espoo, Finland; CV, 10.7%). Duplicate measures of 50 serum samples in each laboratory resulted in nearly identical mean values (40.3 vs. 40.4 nm) and sd values (14.2 vs. 13.2) and a very high correlation (r = 0.98). A Bland-Altman plot showed that 48 of 50 (96%) pairs fell within the 95% confidence limits of agreement (Supplemental Fig. 1). In addition, we tested a term for “laboratory” in the adjusted SHBG models and found that the term was not statistically significant (P = 0.25) and did not alter our estimates of mean SHBG levels across country or race. For these reasons, we assume any small differences in assay performance between the labs are not influential. Free fractions of testosterone and estradiol were calculated as described by Sodergard et al. (14). These calculated values appear to accurately reflect directly measured results (15,16,17,18).
Statistical methods
Men who reported the use of androgen or antiandrogen therapy had been orchidectomized or had testosterone or dihydrotestosterone (DHT) below the limit of detection (Supplemental Table 2) were excluded. Seasonal variation was not present in any steroid, and adjustment for season did not reduce group differences. Square root or natural log transformations were made to produce normally distributed variables. Age-adjusted least-squares means and 95% confidence intervals (CIs) were calculated for each group, and a P for ANOVA was presented. Age-group and BMI-group interactions were tested for each analyte; when significant (P < 0.10), the interaction term was retained to allow age and BMI adjustments to be group-specific. Means and CIs were back-transformed to facilitate the presentation of results in the original units of measure. Evaluation of patterns in mean concentrations across groups led to post hoc tests of differences after collapse of groups with similar means. Spearman correlations among untransformed sex steroid variables are shown in Supplemental Table 3.
For racial comparisons, we used three major groups (Caucasian, Black, and Asian). Country means were weighted equally within racial group to account for heterogeneity of sample sizes across countries. All analyses were performed using SAS version 9.1.3 (SAS Institute Inc., Cary, NC).
Results
The mean ages of the participants in the seven geographical/racial groups were very similar, but there were differences in indices of body size (Table 1).
Table 1.
Cohort characteristics (mean ± sd) and age-adjusted mean (95% CI) sex steroid concentrations
| Sweden | U.S. White | U.S. Asian | Hong Kong | Japan | Tobago | U.S. Black | Pa | |
|---|---|---|---|---|---|---|---|---|
| n | 1874 | 427 | 156 | 1479 | 364 | 482 | 221 | |
| Age (yr) | 75 ± 3 | 74 ± 6 | 73 ± 5 | 72 ± 5 | 74 ± 6 | 72 ± 6 | 72 ± 5 | |
| BMI (kg/m2) | 26 ± 4 | 27 ± 4 | 25 ± 3 | 23 ± 3 | 23 ± 3 | 27 ± 4 | 28 ± 4 | |
| SHBG (nmol/liter) | 42.95 (42.1–43.38) | 47.47 (45.6–49.4) | 47.47 (44.7–50.91) | 48.42 (47.47–49.40) | 72.97 (70.11–75.94) | 51.42 (49.9–53.52) | 50.40 (47.94–52.98) | <0.0001 |
| Precursors | ||||||||
| DHEA-S (μg/ml)b | 0.67 (0.66–0.71) | 0.55 (0.52–0.59) | 0.76 (0.69–0.83) | 0.85 (0.83–0.88) | 0.81 (0.76–0.86) | 0.94 (0.88–1.00) | 0.58 (0.52–0.62) | 0.003 |
| DHEA (ng/ml)b | 1.90 (1.88–1.96) | 1.21 (1.14–1.3) | 1.51 (1.37–1.66) | 1.72 (1.66–1.77) | 1.35 (1.25–1.44) | 1.39 (1.32–1.46) | 1.35 (1.23–1.46) | <0.0001 |
| 4-dione (ng/ml) | 0.82 (0.8–0.82) | 0.68 (0.65–0.7) | 0.68 (0.65–0.73) | 0.72 (0.7–0.72) | 0.68 (0.65–0.7) | 0.63 (0.6–0.65) | 0.72 (0.68–0.75) | <0.0001 |
| 5-diol (ng/ml)b | 0.59 (0.58–0.61) | 0.50 (0.48–0.53) | 0.53 (0.48–0.58) | 0.62 (0.59–0.64) | 0.71 (0.67–0.74) | 0.58 (0.55–0.61) | 0.58 (0.53–0.62) | <0.0001 |
| Androgens | ||||||||
| Testosterone (ng/ml) | 4.54 (4.45–4.62) | 4.54 (4.37–4.71) | 4.41 (4.12–4.67) | 5.29 (5.15–5.38) | 5.20 (4.97–5.38) | 4.54 (4.37–4.67) | 4.71 (4.45–4.93) | <0.0001 |
| Free T (ng/ml) | 9.52 (9.37–9.66) | 9.14 (8.86–9.42) | 8.82 (8.35–9.29) | 10.49 (10.34–10.65) | 8.17 (7.86–8.47) | 8.76 (8.49–9.03) | 9.06 (8.67–9.46) | <0.0001 |
| DHT (ng/ml) | 0.36 (0.36–0.37) | 0.36 (0.34–0.37) | 0.34 (0.31–0.36) | 0.45 (0.44–0.46) | 0.52 (0.49–0.53) | 0.46 (0.45–0.49) | 0.38 (0.36–0.41) | <0.0001 |
| Estrogens | ||||||||
| E1 (pg/ml)b | 35.28 (34.57–36) | 30.36 (29.27–31.58) | 29.38 (27.46–31.25) | 33.41 (32.72–34.11) | 25.00 (23.81–26.21) | 42.51 (41.22–43.82) | 39.82 (37.82–41.73) | 0.0002 |
| E1-S (ng/ml)b | 0.48 (0.45–0.49) | 0.51 (0.48–0.54) | 0.55 (0.51–0.62) | 0.57 (0.55–0.58) | 0.45 (0.42–0.48) | 0.7 (0.67–0.73) | 0.48 (0.43–0.52) | 0.07 |
| E2 (pg/ml)b | 20.7 (20.29–21.12) | 20.09 (19.49–20.91) | 18.73 (17.64–19.89) | 22.87 (22.2–23.34) | 18.17 (17.46–18.92) | 24.53 (23.57–25.28) | 23.10 (21.98–24.53) | 0.05 |
| Free E2 (pg/ml)b | 0.52 (0.51–0.54) | 0.49 (0.48–0.51) | 0.46 (0.43–0.49) | 0.55 (0.54–0.57) | 0.38 (0.36–0.39) | 0.58 (0.55–0.60) | 0.55 (0.52–0.57) | 0.02 |
| Metabolites | ||||||||
| ADT (ng/ml) | 0.17 (0.17–0.19) | 0.16 (0.15–0.16) | 0.17 (0.16–0.19) | 0.21 (0.21–0.22) | 0.16 (0.16–0.17) | 0.17 (0.17–0.19) | 0.16 (0.15–0.17) | <0.0001 |
| ADT-G (ng/ml) | 28.37 (27.79–29.27) | 24.28 (22.81–25.58) | 19.09 (17.54–20.98) | 22.10 (21.42–22.57) | 17.36 (16.29–18.49) | 28.37 (26.94–29.88) | 27.22 (25.31–29.27) | <0.0001 |
| 3α-Diol–3G (ng/ml) | 1.29 (1.27–1.34) | 1.27 (1.18–1.34) | 0.86 (0.75–0.95) | 0.82 (0.79–0.86) | 0.58 (0.54–0.65) | 1.23 (1.16–1.29) | 1.16 (1.08–1.27) | <0.0001 |
| 3α-Diol-17G (ng/ml) | 2.46 (2.39–2.53) | 2.60 (2.46–2.74) | 1.86 (1.66–2.06) | 1.92 (1.83–1.97) | 1.25 (1.14–1.36) | 2.42 (2.32–2.56) | 2.56 (2.35–2.78) | <0.0001 |
T, Testosterone; 5-diol, androst-5-ene-3β,17β-diol; E1, estrone; E1-S, estrone sulfate; E2, estradiol.
P for ANOVA.
Model includes age × group interaction to allow age adjustment to be group-specific.
Geographical variation in sex steroid and SHBG concentrations
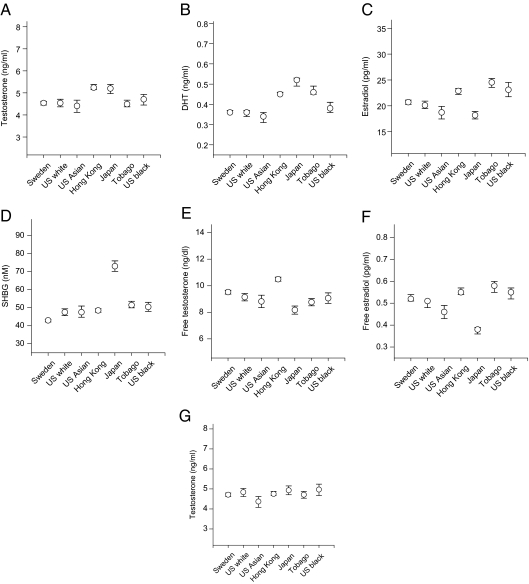
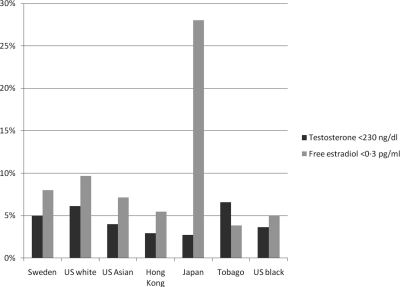
Androgen and estrogen levels varied among populations (Table 1). For example, whereas age-adjusted mean total testosterone levels were similar in men from Sweden, Tobago, and the United States, they were higher (16%; P < 0.0001) in men living in Hong Kong and Japan (Fig. 2A); Asian men living in the United States had levels similar to other U.S. residents. As a result of geographical heterogeneity, the proportion of men with total testosterone levels suggestive of hypogonadism (<230 ng/dl) (19) differed between populations (e.g. 5–6% in U.S. Caucasian and Swedish men; 3% in Hong Kong and Japanese men) (Fig. 3). There was also geographical variation in DHT concentrations, but the interpopulation pattern was distinct from that of testosterone. When compared with men in the United States and Sweden, age-adjusted levels were higher (33%; P < 0.0001) in Tobago and in Asia (Fig. 2B). Total estradiol levels were higher in men from Hong Kong and Tobago (22%) and among Black men in the United States (20%) (Fig. 2C) (Table 1). Free estradiol showed a similar pattern. We recently identified a level of free estradiol (0.3 pg/ml) below which fractures (including hip fracture) are more likely in older Caucasian men (20). The proportion of men with levels below that threshold varied considerably between cohorts (4 to 28%) (Fig. 3).
Figure 2.
Age-adjusted (A–F) and age- and BMI-adjusted (G) means (±95% CI) for selected steroids.
Figure 3.
Prevalence (±95% CI) of low total testosterone (<2.30 ng/ml) and low free estradiol (<0.3 pg/ml) in the cohorts studied.
Major geographical variation was also present in sex steroid precursors and metabolites (Table 1). For example, the age-adjusted mean dehydroepiandrosterone (DHEA) level was 44% higher, and the mean DHEA sulfate (DHEA-S) level was 20% higher in Sweden than in U.S. Caucasians. The concentrations of androgen metabolites varied across groups, primarily because androgen glucuronide levels were lower in Asian men (see Racial variation).
Whereas SHBG levels were similar in most groups, they were slightly lower (12%) in Swedish men and markedly higher (47%) in Japanese (Fig. 2D). As a result, mean free testosterone levels were significantly lower in Japanese (Fig. 2E). Mean free estradiol levels were higher in Hong Kong, U.S. Black, and Tobagonian men, but were lower in Japanese (Fig. 2F).
Adjustment for BMI somewhat altered the population differences in serum concentrations of steroids and SHBG, but significant heterogeneity generally remained or was enhanced (Supplemental Table 4). For instance, BMI adjustment had little effect on the cohort differences in SHBG, total estradiol, free estradiol, or free testosterone levels. On the other hand, total testosterone levels were similar among populations after BMI adjustment (Fig. 2G).
Racial variation
In addition to the effects of geography, two consistent racial patterns were found. First, Black men (from the United States and Tobago) had higher estrogen levels than Caucasians or Asians. Total and free estradiol levels were 10–16% higher and estrone levels 27–39% higher in Black men after age and BMI adjustments (Table 2). Moreover, in Blacks the ratios of total estradiol:total testosterone and estrone:androstenedione (4-dione) were increased compared with other groups (Table 2). Second, after age and BMI adjustments, Asian men (from the United States, Hong Kong, and Japan) had lower serum levels of glucuronidated androgen metabolites [androsterone-glucuronide (ADT-G), androstane-3α,17β-diol-3-glucuronide (3α-diol-3G), and androstane-3α,17β-diol-17-glucuronide (3α-diol-17G)] than did Blacks and Caucasians, and the ratios of these compounds to their precursors [DHT and androsterone (ADT)] were lower in Asian men (Table 2). Racial patterns in other compounds were not apparent.
Table 2.
Age- and BMI-adjusted mean (95% CI) levels for selected steroids and ratios
| White | Asian | Black |
P values for pairwise comparisons
|
|||
|---|---|---|---|---|---|---|
| White-Asian | White-Black | Asian-Black | ||||
| DHT (pg/ml)a,b | 0.38 (0.37, 0.39) | 0.39 (0.39, 0.40) | 0.46 (0.44, 0.48) | 0.03 | <0.0001 | <0.0001 |
| DHT:Ta | 0.29 (0.29, 0.29) | 0.28 (0.28, 0.29) | 0.31 (0.31, 0.31) | <0.0001 | <0.0001 | <0.0001 |
| E1 (pg/ml) | 32.15 (31.58–32.83) | 29.38 (28.84–29.81) | 40.70 (39.44–41.86) | <0.0001 | <0.0001 | <0.0001 |
| E2 (pg/ml)a | 20.09 (19.69–20.49) | 20.09 (19.69–20.49) | 23.34 (22.42–24.29) | 0.73 | <0.0001 | <0.0001 |
| Free E2 (pg/ml) | 0.49 (0.48–0.51) | 0.48 (0.48–0.49) | 0.54 (0.52–0.55) | 0.03 | <0.0001 | <0.0001 |
| E2:T | 1.40 (1.38–1.41) | 1.41 (1.40–1.42) | 1.49 (1.46–1.51) | 0.10 | <0.0001 | <0.0001 |
| Free E2:free T | 0.045 (0.043–0.046) | 0.047 (0.046–0.048) | 0.057 (0.055–0.060) | 0.04 | <0.0001 | <0.0001 |
| E1:4-dioneb | 10.58 (10.44–10.72) | 11.11 (10.99–11.23) | 13.01 (12.76–13.26) | <0.0001 | <0.0001 | <0.0001 |
| ADT-G (ng/ml)a | 26.11 (25.31–26.94) | 19.91 (19.29–20.33) | 27.79 (26.39–29.27) | <0.0001 | 0.06 | <0.0001 |
| 3α-diol-3G (ng/ml)a | 1.27 (1.23–1.32) | 0.80 (0.77–0.82) | 1.18 (1.12–1.25) | <0.0001 | 0.04 | <0.0001 |
| 3α-diol-17G (ng/ml)a | 2.39 (2.32–2.49) | 1.86 (1.8–1.92) | 2.39 (2.25–2.53) | <0.0001 | 0.93 | <0.0001 |
| 3α-diol-3G:DHT | 1.41 (1.37–1.44) | 1.00 (0.97–1.03) | 1.23 (1.17–1.29) | <0.0001 | <0.0001 | <0.0001 |
| 3α-diol-17G:DHTa | 2.05 (2.01–2.09) | 1.77 (1.73–1.80) | 1.89 (1.82–1.96) | <0.0001 | 0.0005 | 0.001 |
| ADT-G:ADTa | 26.85 (25.87–27.83) | 22.75 (21.87–23.64) | 27.35 (25.55–29.15) | <0.0001 | 0.67 | <0.0001 |
E1, Estrone; E2, estradiol; T, testosterone.
BMI × group interaction included in model to allow BMI adjustment to be group-specific.
Age × group interaction included in model to allow age adjustment to be group-specific.
Discussion
In this large, international study of older men, we found major variation between groups in the concentrations of sex steroids, in their precursors and metabolites, and in SHBG. Differences between populations transcend racial distinctions and thus strongly suggest that there are important geographical and environmental influences on sex steroid metabolism. However, in part, the variation was also the consequence of two fundamental racial differences in steroid metabolism-increased estrogen production by aromatase in Blacks and reduced androgen glucuronidation in Asians. As a result of overall population variation, there is considerable discrepancy between groups in the proportion of men who would be identified as hypogonadal or who would be considered at higher risk of fracture.
We describe evidence for global variation in steroid levels. The cohorts examined were sufficiently large and geographically diverse to provide strong evidence of an effect of locale. For instance, mean levels of total testosterone varied by 18%, free testosterone by 25%, and DHT by 42% (Table 1). There are few previous evaluations of geographical differences in sex steroid levels, possibly because the assembly of sufficiently large populations and the use of uniform experimental methods are challenging. Finally, there is controversy about the assessment of free testosterone and estradiol levels. Although we used commonly applied approaches for calculating free levels based on mass action equations, those methods may be influenced by geography, race, or other factors, and any such factors may in part underlie the differences we describe among populations. Although the populations are sufficiently large and the study designs sufficiently similar to strongly suggest geographical variation, that conclusion cannot be definitively drawn from these studies because our populations represent volunteers that may not be representative of the general populations in the regions studied. Certainly our results set the stage for more targeted studies with representative cohorts.
SHBG concentrations are a major determinant of sex steroid levels and action, and we also demonstrate geographical variation in SHBG concentrations. SHBG is considered to be important in human sex steroid physiology for several reasons, including its binding of steroids in the extracellular space. SHBG has strong binding affinity for testosterone and DHT and lesser affinity for other steroids (e.g. estradiol), but does not bind to steroids that lack the 17β-hydroxy group (e.g. 4-dione, estrone). Hence, SHBG may have various effects on sex steroid actions via these binding actions. Moreover, SHBG may have direct tissue effects via specific molecular mechanisms (21), and in large population studies SHBG concentrations are independently associated with important outcomes (20,21,22). Levels were considerably higher in two rural areas of Japan. Similar results have been reported in Japanese women (22,23). The significance of these findings is unclear, but high levels of SHBG in the Japanese were reflected in differences in testosterone and estradiol concentrations that could lead to alterations in androgenic and estrogenic action. Like sex steroids, SHBG may be affected by a wide variety of genetic as well as environmental factors including diet (7,24,25). Finally, there is controversy about the assessment of free sex steroid levels (15,26). Although we used commonly applied approaches for calculating free levels based on mass action equations, those methods may be influenced by geography, race, or other factors, and any such factors may in part underlie the differences we describe among populations.
The causation of differences among our populations is not yet clear. Ecological influences including diet, environmental chemicals, climate, physical activity, smoking, and social status (24,25,27,28) have been linked to effects on sex steroid physiology and could contribute to international variation. In addition, differences in the prevalence of reproductive disorders could contribute to our results. There were obvious differences in body mass index among the groups, and body composition is well known to influence sex steroid levels and physiology (29,30). In fact, adjustment for body mass index in our analyses altered the population differences, but in large part they persisted, suggesting that body mass index is at best a partial explanation for group variations.
International variation in sex steroid levels could have obvious implications for the diagnosis and treatment of hypogonadism. Our results show that use of a conventional criterion for age-related androgen insufficiency (<230 ng/dl) (19) would yield noteworthy population differences in the prevalence of hypogonadism that would in turn result in differences in the number of men considered for replacement therapy. In addition, population differences in sex steroid levels may be similarly relevant to the international epidemiology of common conditions that affect older men, including inflammation, prostate disease, neoplasia, frailty, and osteoporosis. For instance, serum estradiol levels are useful in estimating fracture risk in older men (20,31), at least in Caucasians, and we show that there appears to be substantial racial and geographical variation in the proportion of men considered at higher risk using this criterion. Variation in estrogen levels and evidence of increased aromatase activity have been linked to the risk of cardiovascular disease in older men and women (32). Finally, geographical and racial variation in sex steroid levels in older men may herald comparable variation in women, and if present during growth population variation could lead to developmental differences in sex steroid-dependent human phenotypes such as reproductive function, body composition, bone structure, and hair distribution.
Racial, presumably genetic, influences on sex steroid metabolism have long been sought. Some studies suggest the presence of higher estradiol levels in both Black men (33) (in a representative population in the United States) and women (34) but involved few participants and generally employed RIA methods that have limitations. Moreover, most comparisons involve subjects limited to one geographic area (28). Our findings considerably extend those reports and provide a global perspective. We demonstrate not only that Black men in the United States and Tobago have higher mean estradiol levels than populations of Caucasians and Asians but also provide evidence (higher estrone levels and reduced precursor/product ratios) that implicates increased aromatase activity as the likely explanation. These results may have important health implications. For instance, femoral cortical thickness and trabecular bone density are greater in older Black men (35), and Black men have lower hip fracture rates (36,37), findings that are consistent with higher estrogen levels. In our study, considerably fewer Black men had free estradiol levels below a threshold level associated with increased fracture risk (20,31).
We also show that androgen metabolism in Asian men is distinct. The levels of glucuronidated androgen metabolites (but not ADT) were clearly reduced in Asian men, as were the ratios of these compounds to their precursors, indicating that glucuronidation is likely affected. In support of these large population results, we previously reported that a proportion of Asians have a deletion in the uridine diphosphate-glucuronosyltransferase UGT2B17, consistent with lower rates of androgen glucuronidation. We and others have also shown that polymorphisms or copy number variation in the major enzymes responsible for glucuronidation of androgens (UGT2B15, UGT2B17, and UGT2B7) appear to have an impact on levels of glucuronidated androgens (38,39), suggesting that these genes are interesting candidates to explain racial differences in glucuronidated androgen levels. Of potential physiological importance, serum androgen glucuronide levels may be more strongly related to total androgen activity than are measures of individual androgens. Of practical concern, a reduction in testosterone glucuronidation with high serum and low urine testosterone levels in Asians can result in misinterpretations of common monitors for androgen use in athletes (40). Other studies have explored Asian-Caucasian differences in reproductive function (27) but have been small and rarely involved disparate geographies.
The study has considerable strengths. It represents a unique international collaboration that brings together well-characterized cohorts that were recruited and evaluated using similar methods. Large numbers of study participants provided adequate power to identify group differences, and we measured a large number of sex steroids, including potent androgens and estrogens as well as their precursors and metabolites. Importantly, all steroid measures were performed in a single laboratory employing rigorous quality control procedures, and the methods used avoid limitations of previous approaches. On the other hand, as mentioned above, the cohorts were recruited using very comparable strategies, and they are similar in many relevant respects, but they may not be completely representative of their broader populations. Our analyses are cross-sectional, and although the effects of sex steroids in older men are of considerable interest, we did not assess variation in women and younger people. The apparent racial differences we describe appear robust, but we classified race in broad racial categories and did not formally examine genetic admixture or racial subgroups. Moreover, whereas our results provide a new recognition of possible racial and geographical variation in sex steroid metabolism, we have not determined the mechanisms responsible. Finally, some variation may have been introduced by the approaches used for sample handling. For instance, whereas we attempted to standardize the methods for sample collection and storage, there were some minor differences that might have contributed to variation.
In summary, in a large, international study of older men we found evidence for substantial racial and geographical differences in the levels of major circulating androgens and estrogens, in their precursors and metabolites, and in SHBG. Such variation could result in diversity in important health outcomes, for instance in the proportion of older men with hypogonadism and in the risk of fracture attributable to estrogen deficiency. Understanding the causes of potential differences could yield new insights into racial and environmental influences on sex steroid regulation.
Supplementary Material
Acknowledgments
This study was made possible by the expertise of the research staffs at each of the study sites in this collaboration, the contributions of other investigators at each study site, and the generous provision of time and effort by each of the study participants. We are grateful to Patty Wang, M.S., for assistance with data management and statistics. Leslie Stonelake and Denise Duncan provided expert manuscript preparation.
Footnotes
Funding for this study was provided as follows: United States—National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institute on Aging, National Center for Research Resources, and National Institutes of Health Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 RR024140. Solvay Pharmaceuticals, Inc. provided additional funding. Canada—Endorecherche Inc., Quebec City, provided partial support for steroid assays. Sweden—The Swedish Research Council, the Swedish Foundation for Strategic Research, The ALF/LUA research grant in Gothenburg, Uppsala, and Lund; the Lundberg Foundation; the Torsten and Ragnar Söderberg′s Foundation; and the Novo Nordisk Foundation. Hong Kong—The Hong Kong Jockey Club Osteoporosis Research Fund. Tobago—Funding or in-kind services from the Division of Health and Social Services, Tobago House of Assembly, U.S. Department of Defense contract DAMD 17-99-1-9015, and grants R01 CA84950 and R25-CA57703 from the National Cancer Institute, and R01-AR049747 from the NIAMS. Japan—Grants-in-Aid for Scientific Research B20390182; Collaborating Research with NSF 08033011-00262 from the Ministry of Education, Culture, Sports, Science and Technology; H17-Men-eki-009, H18-Choujyu-037, and H20-Choujyu-009 from the Ministry of Health, Labor, and Welfare in Japan. Study sponsors had no role in the study or manuscript beyond funding.
Contributions: E.S.O., Study concept and design, funding, acquisition of data, data analysis, interpretation, writing, reviewing, final approval; C.M.N., data analysis, writing, critical review of manuscript; F.L., study concept and design, data analysis, writing, critical review of manuscript; S.R.C., funding, data analysis, reviewing; E.B.-C., study concept and design, acquisition of data, data analysis and interpretation, critical review of manuscript; J.A.C., study concept and design, acquisition of data, data analysis and interpretation, critical review of manuscript; M.L.S., study concept and design, acquisition of data, data analysis and interpretation, critical review of manuscript; K.E., acquisition of data, critical review of manuscript, and reviewing; E.L., funding and acquisition of data; Ö.L., study concept and design, acquisition of data; D.M., study concept and design, acquisition of data; M.K., study concept and design, acquisition of data; N.Y., establishing population-based cohorts in Japan, collecting serum samples of Japanese subjects, funding; P.C.L., study concept and design, funding, acquisition of data; A.L.P., study concept and design; K.N., acquisition of data and collecting serum samples of Japanese subjects; J.Z., study concept and design, acquisition of data, data analysis and interpretation, critical review of manuscript; L.V., data analysis, interpretation, critical review of manuscript, and reviewing; and C.O., study concept and design, funding, acquisition of data, interpretation, writing, reviewing, and critical review of manuscript.
Disclosure Summary: E.O. received research support from Solvay Pharmaceuticals, Inc.
First Published Online July 28, 2010
Abbreviations: ADT, Androsterone; ADT-G, ADT-glucuronide; CI, confidence interval; CV, coefficient of variation; DHEA, dehydroepiandrosterone; DHEA-S, DHEA sulfate; DHT, dihydrotestosterone; 3α-diol-3G, androstane-3α,17β-diol-3-glucuronide; 3α-diol-17G, androstane-3α,17β-diol-17-glucuronide; 4-dione, androstenedione.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ 2006 Cancer statistics, 2006. CA Cancer J Clin 56:106–130 [DOI] [PubMed] [Google Scholar]
- Cheng I, Yu MC, Koh WP, Pike MC, Kolonel LN, Henderson BE, Stram DO 2005 Comparison of prostate-specific antigen and hormone levels among men in Singapore and the United States. Cancer Epidemiol Biomarkers Prev 14:1692–1696 [DOI] [PubMed] [Google Scholar]
- Litman HJ, Bhasin S, Link CL, Araujo AB, McKinlay JB 2006 Serum androgen levels in black, Hispanic, and white men. J Clin Endocrinol Metab 91:4326–4334 [DOI] [PubMed] [Google Scholar]
- Santner SJ, Albertson B, Zhang GY, Zhang GH, Santulli M, Wang C, Demers LM, Shackleton C, Santen RJ 1998 Comparative rates of androgen production and metabolism in Caucasian and Chinese subjects. J Clin Endocrinol Metab 83:2104–2109 [DOI] [PubMed] [Google Scholar]
- Gapstur SM, Gann PH, Kopp P, Colangelo L, Longcope C, Liu K 2002 Serum androgen concentrations in young men: a longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiol Biomarkers Prev 11:1041–1047 [PubMed] [Google Scholar]
- Magee PJ, Rowland IR 2004 Phyto-oestrogens, their mechanism of action: current evidence for a role in breast and prostate cancer. Br J Nutr 91:513–531 [DOI] [PubMed] [Google Scholar]
- Longcope C, Feldman HA, McKinlay JB, Araujo AB 2000 Diet and sex hormone-binding globulin [see comment]. J Clin Endocrinol Metab 85:293–296 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K 2005 Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials 26:569–585 [DOI] [PubMed] [Google Scholar]
- Mellström D, Johnell O, Ljunggren O, Eriksson AL, Lorentzon M, Mallmin H, Holmberg A, Redlund-Johnell I, Orwoll E, Ohlsson C 2006 Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res 21:529–535 [DOI] [PubMed] [Google Scholar]
- Lau EM, Leung PC, Kwok T, Woo J, Lynn H, Orwoll E, Cummings S, Cauley J 2006 The determinants of bone mineral density in Chinese men—results from Mr. Os (Hong Kong), the first cohort study on osteoporosis in Asian men. Osteoporos Int 17:297–303 [DOI] [PubMed] [Google Scholar]
- Bunker CH, Patrick AL, Konety BR, Dhir R, Brufsky AM, Vivas CA, Becich MJ, Trump DL, Kuller LH 2002 High prevalence of screening-detected prostate cancer among Afro-Caribbeans: the Tobago Prostate Cancer Survey. Cancer Epidemiol Biomarkers Prev 11:726–729 [PubMed] [Google Scholar]
- Muraki S, Oka H, Akune T, Mabuchi A, En-yo Y, Yoshida M, Saika A, Suzuki T, Yoshida H, Ishibashi H 2008 Prevalence of radiographic lumbar spondylosis and its association with low back pain in the elderly of population-based cohorts: the ROAD study. BMJ 68:1401–1406 [DOI] [PubMed] [Google Scholar]
- Labrie F, Bélanger A, Bélanger P, Bérubé R, Martel C, Cusan L, Gomez J, Candas B, Castiel I, Chaussade V, Deloche C, Leclaire J 2006 Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J Steroid Biochem Mol Biol 99:182–188 [DOI] [PubMed] [Google Scholar]
- Södergård R, Bäckström T, Shanbhag V, Carstensen H 1982 Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J Steroid Biochem 16:801–810 [DOI] [PubMed] [Google Scholar]
- Matsumoto AM, Bremner WJ 2004 Serum testosterone assays—accuracy matters. J Clin Endocrinol Metab 89:520–524 [DOI] [PubMed] [Google Scholar]
- Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson Jr HE, Falk RT, Miller R, Schatzkin A, Allen DS, Fentiman IS, Key TJ, Wang DY, Dowsett M, Thomas HV, Hankinson SE, Toniolo P, Akhmedkhanov A, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Berrino F, Muti P, Micheli A, Krogh V, Sieri S, Pala V, Venturelli E, Secreto G, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Kuller LH, Cummings SR, Helzlsouer KJ, Alberg AJ, Bush TL, Comstock GW, Gordon GB, Miller SR, Longcope C; Endogenous Hormones Breast Cancer Collaborative Group 2003 Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst 95:1218–1226 [DOI] [PubMed] [Google Scholar]
- Endogenous Hormones and Breast Cancer Collaborative Group 2003 Free estradiol and breast cancer risk in postmenopausal women: comparison of measured and calculated values. Cancer Epidemiol Biomarkers Prev 12:1457–1461 [PubMed] [Google Scholar]
- Rinaldi S, Geay A, Déchaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, Shore RE, Riboli E, Toniolo P, Kaaks R 2002 Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev 11:1065–1071 [PubMed] [Google Scholar]
- Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morales A, Morley JE, Schulman C, Thompson IM, Weidner W, Wu FC 2009 Investigation, treatment and monitoring of late-onset hypogonadism in males. Int J Androl 32:1–10 [DOI] [PubMed] [Google Scholar]
- Mellström D, Vandenput L, Mallmin H, Holmberg AH, Lorentzon M, Odén A, Johansson H, Orwoll ES, Labrie F, Karlsson MK, Ljunggren O, Ohlsson C 2008 Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res 23:1552–1560 [DOI] [PubMed] [Google Scholar]
- Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA 2010 Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol 316:79–85 [DOI] [PubMed] [Google Scholar]
- Yasui T, Uemura H, Irahara M, Arai M, Kojimahara N, Okabe R, Ishii Y, Tashiro S, Sato H 2008 Associations of endogenous sex hormones and sex hormone-binding globulin with lipid profiles in aged Japanese men and women. Clin Chim Acta 398:43–47 [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Kasamatsu T, Sakata K, Hashimoto T, Cooper C 2002 The relationship between endogenous estrogen, sex hormone-binding globulin, and bone loss in female residents of a rural Japanese community: the Taiji Study. J Bone Miner Metab 20:303–310 [DOI] [PubMed] [Google Scholar]
- Hall SA, Esche GR, Araujo AB, Travison TG, Clark RV, Williams RE, McKinlay JB 2008 Correlates of low testosterone and symptomatic androgen deficiency in a population-based sample. J Clin Endocrinol Metab 93:3870–3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svartberg J, Jorde R 2007 Endogenous testosterone levels and smoking in men. The fifth Tromso study. Int J Androl 30:137–143 [DOI] [PubMed] [Google Scholar]
- Sartorius G, Ly LP, Sikaris K, McLachlan R, Handelsman DJ 2009 Predictive accuracy and sources of variability in calculated free testosterone estimates. Ann Clin Biochem 46:137–143 [DOI] [PubMed] [Google Scholar]
- van Houten ME, Gooren LJ 2000 Differences in reproductive endocrinology between Asian men and Caucasian men—a literature review. Asian J Androl 2:13–20 [PubMed] [Google Scholar]
- Wang C, Christenson P, Swerdloff R 2007 Clinical relevance of racial and ethnic differences in sex steroids. J Clin Endocrinol Metab 92:2433–2435 [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Kaufman JM, Giagulli VA 1996 Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. J Clin Endocrinol Metab 81:1821–1826 [DOI] [PubMed] [Google Scholar]
- Schneider G, Kirschner MA, Berkowitz R, Ertel NH 1979 Increased estrogen production in obese men. J Clin Endocrinol Metab 48:633–638 [DOI] [PubMed] [Google Scholar]
- LeBlanc ES, Nielson CM, Marshall LM, Lapidus JA, Barrett- Connor E, Ensrud KE, Hoffman AR, Laughlin G, Ohlsson C, Orwoll ES, Osteoporotic Fractures in Men Study Group 2009 The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab 94:3337–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naessen T, Sjogren U, Bergquist J, Larsson M, Lind L, Kushnir MM 2010 Endogenous steroids measured by high-specificity liquid chromatography-tandem mass spectrometry and prevalent cardiovascular disease in 70-year-old men and women. J Clin Endocrinol Metab 95:1889–1897 [DOI] [PubMed] [Google Scholar]
- Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek N, Yager JD, Platz EA 2007 Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab 92:2519–2525 [DOI] [PubMed] [Google Scholar]
- Setiawan VW, Haiman CA, Stanczyk FZ, Le Marchand L, Henderson BE 2006 Racial/ethnic differences in postmenopausal endogenous hormones: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev 15:1849–1855 [DOI] [PubMed] [Google Scholar]
- Marshall LM, Zmuda JM, Chan BK, Barrett-Connor E, Cauley JA, Ensrud KE, Lang TF, Orwoll ES, Osteoporotic Fractures in Men Research G 2008 Race and ethnic variation in proximal femur structure and BMD among older men. J Bone Miner Res 23:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JA, Barrett J, Malenka D, Fisher E, Kniffin W, Bubolz T, Tosteson T 1994 Racial differences in fracture risk. Epidemiology 5:42–47 [DOI] [PubMed] [Google Scholar]
- Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA 1990 Hip fracture incidence among the old and very old: a population-based study of 745,435 cases. Am J Public Health 80:871–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson C, Mellström D, Lorentzon M, Vandenput L, Jakobsson J, Rane A, Karlsson M, Ljunggren O, Smith U, Eriksson AL, Bélanger A, Labrie F, Ohlsson C 2007 The uridine diphosphate glucuronosyltransferase 2B15 D85Y and 2B17 deletion polymorphisms predict the glucuronidation pattern of androgens and fat mass in men. J Clin Endocrinol Metab 92:4878–4882 [DOI] [PubMed] [Google Scholar]
- Swanson C, Lorentzon M, Vandenput L, Labrie F, Rane A, Jakobsson J, Chouinard S, Bélanger A, Ohlsson C 2007 Sex steroid levels and cortical bone size in young men are associated with a uridine diphosphate glucuronosyltransferase 2B7 polymorphism (H268Y). J Clin Endocrinol Metab 92:3697–3704 [DOI] [PubMed] [Google Scholar]
- Schulze JJ, Lundmark J, Garle M, Skilving I, Ekström L, Rane A 2008 Doping test results dependent on genotype of uridine diphospho-glucuronosyl transferase 2B17, the major enzyme for testosterone glucuronidation [see comment]. J Clin Endocrinol Metab 93:2500–2506 [DOI] [PubMed] [Google Scholar]
- Luu-The V, Tremblay P, Labrie F 2006 Characterization of type 12 17β-hydroxysteroid dehydrogenase, an isoform of type 3 17β-hydroxysteroid dehydrogenase responsible for estradiol formation in women. Mol Endocrinol 20:437–443 [DOI] [PubMed] [Google Scholar]
- Bélanger A, Pelletier G, Labrie F, Barbier O, Chouinard S 2003 Inactivation of androgens by UDP-glucuronosyltransferase enzymes in humans. Trends Endocrinol Metab 14:473–479 [DOI] [PubMed] [Google Scholar]
- Luu-The V, Bélanger A, Labrie F 2008 Androgen biosynthetic pathways in the human prostate. Best Pract Res Clin Endocrinol Metab 22:207–221 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.